Introduction
Gastric cancer is currently the fifth most common
tumor type (with 1,000,000 newly diagnosed cases in 2012 alone) and
is the third highest cause of cancer-related deaths worldwide
(accounting for 74,000 deaths in 2012) (1,2). Current
biomarkers used for the early diagnosis of the disease such as
carcinoembryonic antigen and carbohydrate antigen 19-9 are
unsatisfactory due to their low sensitivity and specificity
(3,4). Therefore, the majority of patients
suffering from the disease are diagnosed at the advanced stages,
often accompanied with malignant proliferation, extensive invasion,
lymph node and distant metastasis (5), with surgery and chemotherapy thus being
the main methods of treatment. However, >50% of patients with
advanced gastric cancer experience metastasis and relapse after
treatment, leading to a high mortality rate in patients (6), a 5-year overall survival rate of ~15%
and a median overall survival time of <1 year (7). Therefore, it is necessary to develop
new therapeutic strategies and to explore effective biomarkers for
the early diagnosis of gastric cancer.
LYN kinase (LYN), a member of the Src family
tyrosine kinases that functions as a pro-oncogene in tumor
progression, is reported to be frequently overexpressed in numerous
tumor types, including chronic myelogenous leukemia, renal cancer,
cervical cancer, head and neck squamous cell carcinoma, gastric
cancer and prostate cancer (8-12).
For example, Liu et al (9)
reported that LYN promotes tumor proliferation and metastasis in
cervical cancer both in vitro and in vivo, and
activates the interleukin-6/STAT3 pathway (9). Moreover, a raised expression level of
LYN has been shown to be associated with a poor prognosis in
non-small cell lung cancer, as well as promoting tumor progression
(13).
It has been previously found, however, that
dasatinib, a LYN-targeting molecular drug, has the potential to
inhibit tumor growth in LYN-positive adenocarcinoma cell lines and
xenografts (13). Aira et al
(14) reported that LYN has the
potential to inhibit cell apoptosis in tumors by inhibiting the
pro-apoptotic protein Bim in the mitochondrial apoptosis pathway
(14). In regards to gastric cancer,
a previous study has shown that LYN expression is downregulated by
DNA methylation, and that altered DNA methylation of LYN is
associated with tumorigenesis, invasion and metastasis (15); however, the relationship between LYN
and gastric cancer progression is solely based on the
epidemiological analyses of tumor and non-tumor samples, with
direct evidence still being required to confirm this
relationship.
Thus, in the present study, the aim was to
investigate the effect of LYN on the proliferation, survival and
metastasis of human gastric cancer using RNA interference
technology. The mitochondrial apoptosis pathway, the Wnt/β-catenin
pathway, and the AKT/mTOR pathway were also investigated to
elucidate the potential regulatory mechanism of action of LYN in
gastric cancer. This current research therefore identified a novel
therapeutic target for the treatment of human gastric cancer.
Materials and methods
Ethics approval and consent to
participate
Tissue samples, including gastric cancer (n=73) and
paracarcinoma tissues (n=73) were collected from the Beijing
Friendship Hospital of the Capital Medical University of China
between January 2015 and December 2017 for immunohistochemistry
(IHC) analysis. These patients included 55 males and 18 females
with a median age of 53.8 years (age range, 31-78).
Clinicopathological parameters of each tumor was classified
according to the tumor-node-metastasis (TNM) classification system
recommended by the Union for International Cancer Control (16). Ten pairs of gastric cancer tissues
and paracarcinoma tissues were collected for reverse
transcription-quantitative PCR (RT-qPCR) and western blotting
analysis. The present study was approved by the ethics committee of
the Beijing Friendship Hospital of the Capital Medical University
of China. All patients provided written informed consent.
Bioinformatics
Gene Expression Profiling Interactive Analysis
(GEPIA; http://gepia.cancer-pku.cn/) is an
online server used to analyze the RNA expression and survival
correlation of genes in different tumor types, its data having been
extracted from the Cancer Genome Atlas and Genotype-Tissue
Expression dataset (16). The GEPIA
database was used to analyze the expression of LYN in gastric
cancer and normal tissues according to a previous study (17).
IHC analysis
Gastric tumor or paracarcinoma tissues were fixed
with 10% formalin at room temperature for 24 h, embedded in
paraffin and sectioned (5 µm thick) for immunohistochemical
staining. The tissue sections were then deparaffinized in xylene at
room temperature for 20 min and rehydrated in graded ethanol.
Following incubation in 3% H2O2 for 5-10 min,
the sections were incubated in PBS by heating to boil for 5-10 min
for antigen retrieval. After blocking with normal goat serum
(20-fold dilution with PBS; Beijing Solarbio Science &
Technology Co., Ltd.) for 1 h at room temperature, sections were
treated with anti-LYN rabbit antibodies (cat. no. 2796; 1:10; Cell
Signaling Technology, Inc.) at 4˚C overnight and incubated with
horseradish peroxidase-conjugated goat anti-rabbit IgG secondary
antibodies (cat. no. ab205718; 1:500; Abcam) at 4˚C for 2 h, then
visualized using a 3,3'-diaminobenzidine tetrahydrochloride
developer (Dako; Agilent Technologies, Inc.) at room temperature
for 5-20 min. A total of four random fields were then chosen at x40
magnification under a light microscope (Nikon Corporation) and
images were captured, and LYN staining was evaluated.
Under the x40 objective, staining was scored as
follows: ‘0’, no staining; ‘1’, weakly positive; ‘2’, moderately
positive; and ‘3’ (strongly positive). The percentage of positively
stained cells was scored as follows: ‘0’=0%; ‘1’=1-25%; ‘2’=26-50%;
‘3’=51-75%; and ‘4’=76-100%. Two independent pathologists were
involved in the evaluation of slides. Low expression was identified
when the sum of the score was <6, the cut-off derived from
X-tile analysis (18), otherwise the
samples were defined as high expression (19).
Cell culture and transfection
Human gastric cancer AGS cells were purchased from
the Cell Bank of The Chinese Academy of Sciences, and maintained in
DMEM (Sigma-Aldrich; Merck KGaA) supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) at 37˚C in 5% CO2.
The short hairpin RNA (shRNA) targeting LYN (sh-LYN)
and negative control (NC) shRNA (scrambled; shNC) were designed and
provided by Shanghai GenePharma Co., Ltd., and cloned into the
pcDNA3.1 (+) vector (Invitrogen; Thermo Fisher Scientific, Inc.)
following the manufacturer's protocol. Cells were seeded in a
6-well plate (1x105 cells/well) and incubated at 37˚C
overnight. When cell confluence reached 40-60%, sh-LYN (50 nM) and
shNC (50 nM) were transfected into AGS cells using
Lipofectamine® 6000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocols. Cells
were then cultured for 6-8 h at 37 ˚C. Subsequently, the medium
containing the transfection reagent was removed and cells were
cultured in DMEM for 24-48 h prior to further experimentation. The
sequences of the shRNA were as follows: shNC,
5'-TTCTCCGAACGTGTCACGT-3'; sh1-LYN, 5'-GTCTGA TGTGTGGTCCTTT-3';
sh2-LYN, 5'-GCACTACAAAATT AGA AGT-3'; sh3-LYN,
5'-GGAACTCGAGTTCCCATAG-3'.
The shRNAs were used in the following functional and
mechanistic investigations: Proliferation assays, colony formation
assays, apoptosis detection, Transwell assays and signaling pathway
testing. Insulin like growth factor (IGF)-1 (MedChemExpress) was
dissolved in deionised water and then diluted to a final
concentration (200 ng/ml) with DMEM. Cells were treated with IGF-1
(200 ng/ml) for 24 h.
RT-qPCR
After transfection for 48 h, mRNA expression levels
of LYN in AGS cells were detected using RT-qPCR. In brief, total
RNA of the AGS cells was collected using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) and utilized to
synthesize complementary DNA using M-MLV Reverse transcriptase kit
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturers' protocol. The temperature protocol was as follows:
60 min of incubation at 37˚C. The RT-qPCR reaction was conducted
using a SuperReal PreMix (SYBR-Green) RT-qPCR kit (Tiangen Biotech
Co., Ltd.) according to the manufacturers' protocol. mRNA
expression levels were analyzed using an FTC-3000 real-time
quantitative thermal cycler (Funglyn Biotech, Inc.) using GAPDH as
an internal control. The thermocycling conditions were as follows:
95˚C for 30 sec, 95˚C for 10 sec, 60˚C for 30 sec and 72˚C for 15
sec, for a total of 45 cycles. The
2-ΔΔCq method was used for gene
expression quantification (20). The
primers used for qPCR were as follows: LYN forward,
5'-TGTGGCCAAACTCAACACCT-3' and reverse, 5'-TGCTGCAGGGTCTTCATGAG-3';
GAPDH forward, 5'-TGTTCGTCATGGGTGTGAACC-3' and reverse,
5'-ATGGACTGTGGTCATGAGTCC-3'.
Proliferation assay
For the CCK-8 assays, a CCK-8 kit (Beijing Solarbio
Science & Technology Co., Ltd.) was used in accordance with the
manufacturer's protocol. Briefly, the shNC or sh-LYN transfected
AGS cells were seeded at a density of 3,000 cells/well in 96-well
plates. At a series of time points (0, 24, 48 and 72 h), CCK-8
reagent was added into each well and the cells were incubated for
1.5 h at 37˚C. Cell viability was represented by the OD value at
450 nm.
For the colony formation assays, the shNC or sh-LYN
transfected AGS cells (~500 cells) were seeded into a 10-cm culture
dish and cultured with the aforementioned protocol until large
colonies were formed (~10 days). The cells were then fixed using
methanol at room temperature for 20 min, washed twice by PBS, and
stained using 0.1% crystal violet at room temperature for 30 min.
The colonies were then imaged and counted using a Bio-Rad ChemiDoc
XRS imaging system (Bio-Rad Laboratories, Inc.) and Image Lab
software (version 3.0; Bio-Rad Laboratories, Inc.).
Cell apoptosis analysis
After transfection for 24 h, cells were harvested
and washed twice using 1X PBS. Cells (1-5x105) were then
stained using Annexin V and propidium iodide (BD Biosciences)
supplemented with 1 µg/ml RNase (Sigma-Aldrich; Merck KGaA) at 37˚C
in the dark for 30 min. The percentage of cells undergoing
apoptosis was analyzed using a FACScalibur flow cytometer (BD
FACSCanto II; BD Biosciences) using CellQuestPro software (version
5.1; Becton-Dickinson and Company).
Western blotting
Cells were lysed with RIPA lysis buffer (Beyotime
Institute of Biotechnology) for 30 min on ice. Following
centrifugation at 12,000 x g for 15 min at 4˚C, a BCA protein assay
kit (Beyotime Institute of Biotechnology) was used to detect
protein concentration. Total cell extracts (20 µg) were subjected
to 10% SDS-PAGE, with separated proteins then being blotted to PVDF
membranes. The membranes were blocked with 5% non-fat dry milk for
1 h at room temperature, probed overnight at 4˚C with primary
antibodies, and incubated with the horseradish
peroxidase-conjugated secondary antibodies (1:3,000; cat. no.
SA00001-2; ProteinTech Group, Inc.) at room temperature for 1 h.
Protein bands were then visualized using an ECL developing system,
and analyzed using ImageJ software (version 1.52s; National
Institutes of Health). The antibodies anti-Bcl-2 (1:1,000; cat. no.
15071), anti-Bax (1:1,000; cat. no. 2772), anti-cleaved caspase-9
(1:1,000; cat. no. 7237), anti-cleaved caspase-3 (1:1,000; cat. no.
9661), anti-GAPDH (1:1,000; cat. no. 2118), anti-Wnt3 (1:1,000;
cat. no. 2391), anti-β-catenin (1:1,000; cat. no. 9562),
anti-E-cadherin (1:1,000; cat. no. 14472), anti-N-cadherin
(1:1,000; cat. no. 4061), anti-vimentin (1:1,000; cat. no. 49636),
anti-snail family transcriptional repressor (Snail)1 (1:1,000; cat.
no. 3879), anti-Snail2 (1:1,000; cat. no. 9585), anti-AKT (1:1,000;
cat. no. 9272), anti-phosphorylated (p)-AKT (Ser473) (1:1,000; cat.
no. 4060), anti-mTOR (1:1,000; cat. no. 2972), anti-p-mTOR
(Ser2448) (1:1,000; cat. no. 2971), and anti-p70S6 kinase (p70S6K;
1:1,000; cat. no. 9204) were all purchased from Cell Signaling
Technology, Inc.
Cell invasive and migratory
analysis
After AGS cells were transfected with shNC or sh-LYN
for 48 h, cell invasion and migration were assessed using Transwell
assays. For cell invasion, Transwell inserts were precoated with
Matrigel® (Corning, Inc.) at 37˚C for 30 min. A total of
2x105 cells were seeded into the upper chamber with 500
µl serum-free medium, while the lower chamber contained 500 µl
medium containing 10% FBS as an inducer. After 24 h of culture at
37˚C, the residual cells on the upper surface of the membrane were
removed, and the cells on the lower surface of the membrane were
fixed using methanol for 30 min at room temperature, stained with
0.1% crystal violet for 10 min at room temperature and then counted
at x100 magnification using a light microscope (Nikon
Corporation).
The process for the cell migration assay was the
same as for the cell invasion assay, except that the Transwell
membranes were not precoated with Matrigel.
Statistical analysis
Data are presented as the mean ± standard deviation.
All statistical analyses were performed using Prism 7 (GraphPad
Software, Inc.). One-way or two-way ANOVAs followed by post-hoc
Bonferroni tests were used when multiple comparisons were made.
Comparisons between two groups were analyzed using
independent-samples t-tests. P<0.05 was considered to indicate a
statistically significant difference. All experiments were
performed in triplicate.
Results
LYN is upregulated in gastric cancer
tissues
The GEPIA database was used to analyze the
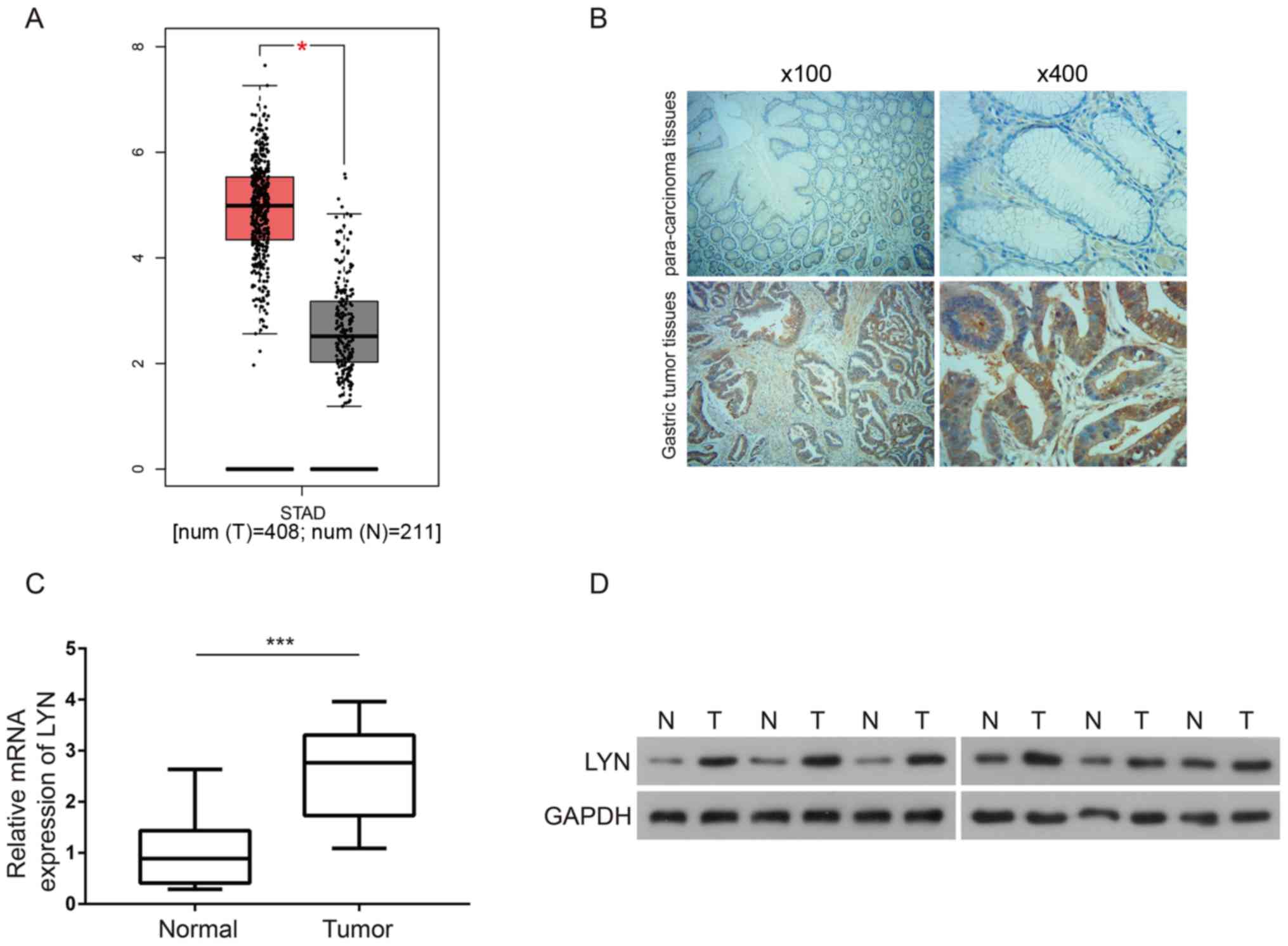
expression of LYN in gastric cancer tissues. As shown in Fig. 1A, by inputting ‘LYN’ and ‘Stomach
adenocarcinoma (STAD)’, it was found that LYN expression was
significantly upregulated in gastric tumor tissues compared to
normal controls (P<0.05). IHC analysis was then used to validate
the expression profile of LYN in gastric tumor tissues compared
with normal tissues. Both the images of tissue sections (Fig. 1B) and statistical analysis (Table I) suggested a significant increase in
LYN expression levels in gastric tumor tissue (P<0.05). The
association between LYN expression and clinicopathological
parameters was thereafter analyzed. As shown in Table II, it was suggested that LYN
expression levels were significantly associated with the
pathological grades and T stages of gastric cancer patients
(P<0.05), but that there was no significant association with
patient sex, age or tumor diameter. Moreover, RT-qPCR analysis
showed that LYN mRNA was upregulated in gastric cancer tissue
compared with the paracancerous normal tissue (P<0.01; Fig. 1C). As indicated by western blotting,
LYN protein was also highly expressed in gastric cancer tissue
(Fig. 1D). Therefore, the present
data suggested that LYN was upregulated in gastric cancer and may
be associated with gastric cancer progression.
 | Table ILYN expression in gastric cancer
tissues compared with paracarcinoma tissues. |
Table I
LYN expression in gastric cancer
tissues compared with paracarcinoma tissues.
| | | LYN expression | |
|---|
| Group | n | Low, n (%) | High, n (%) | P-value |
|---|
| Gastric cancer
tissues | 73 | 15 (20.5) | 58 (79.5) | 0.001a |
| Paracarcinoma | 73 | 45 (61.6) | 28 (38.4) | |
 | Table IIAssociations between LYN expression
and clinicopathological parameters in gastric cancer patients. |
Table II
Associations between LYN expression
and clinicopathological parameters in gastric cancer patients.
| Clinicopathological
parameters | n | LYN low n (%) | LYN high n (%) | P-value |
|---|
| Sex |
|
Male | 55 | 9 (16.4) | 46 (83.6) | 0.122 |
|
Female | 18 | 6 (33.3) | 12 (66.7) | |
| Age, years |
|
<60 | 31 | 9 (29.0) | 22 (71.0) | 0.123 |
|
≥60 | 42 | 6 (14.3) | 36 (85.7) | |
| Tumor diameter,
cm |
|
<5 | 30 | 6 (20.0) | 24 (80.0) | 0.923 |
|
≥5 | 43 | 9 (20.9) | 34 (79.1) | |
| Pathological
grading |
|
I-II | 20 | 8 (40.0) | 12 (60.0) | 0.012a |
|
III-IV | 53 | 7 (13.2) | 46 (86.8) | |
| T stage |
|
Stage
1-2 | 12 | 7 (58.3) | 5 (41.7) | 0.036a |
|
Stage
3-4 | 59 | 50 (84.7) | 9 (15.3) | |
Inhibition of AGS cell proliferation
by downregulating LYN
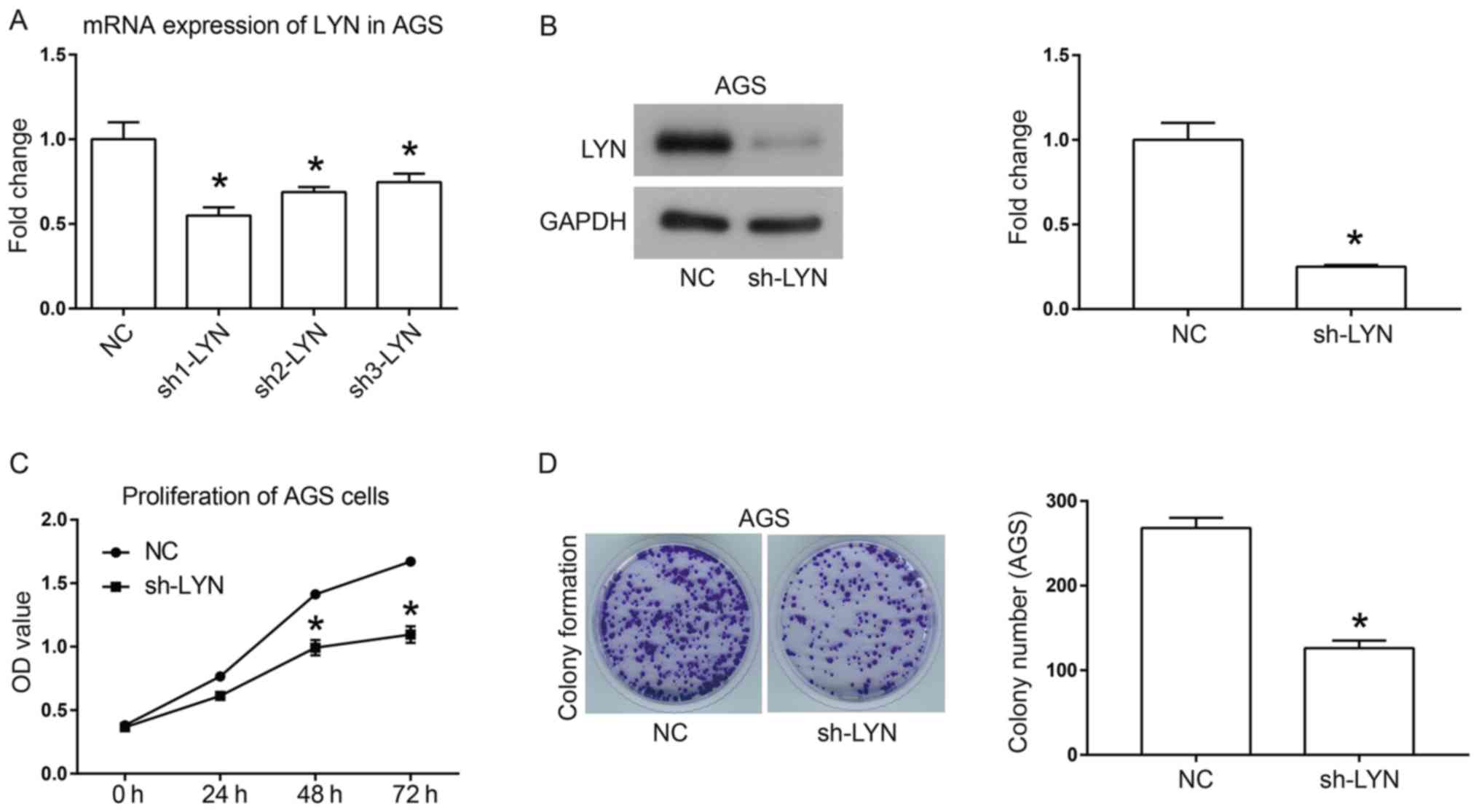
To investigate the function of LYN in human gastric
cancer, a loss-of-function experiment was performed. Three shRNAs
that targeted different loci of LYN were introduced into human
gastric cancer AGS cells. As shown in Fig. 2A, sh1-LYN exhibited the highest
efficiency for knocking down LYN, and was thus selected for
functional evaluation. Furthermore, western blotting demonstrated
the interference effect that sh-LYN had on the protein expression
of LYN (Fig. 2B). Cell growth was
analyzed using a CCK-8 assay (Fig.
2C) and colony formation assay (Fig.
2D). The results of both these analyses suggested that
downregulation of LYN inhibited the cell proliferative and colony
formation abilities of AGS gastric cancer cells. The colony number
was decreased from 270±12 in the NC group to 120±10 in the sh-LYN
group (Fig. 2D; P<0.05).
Downregulation of LYN induces AGS cell
apoptosis by activating the apoptosis pathway
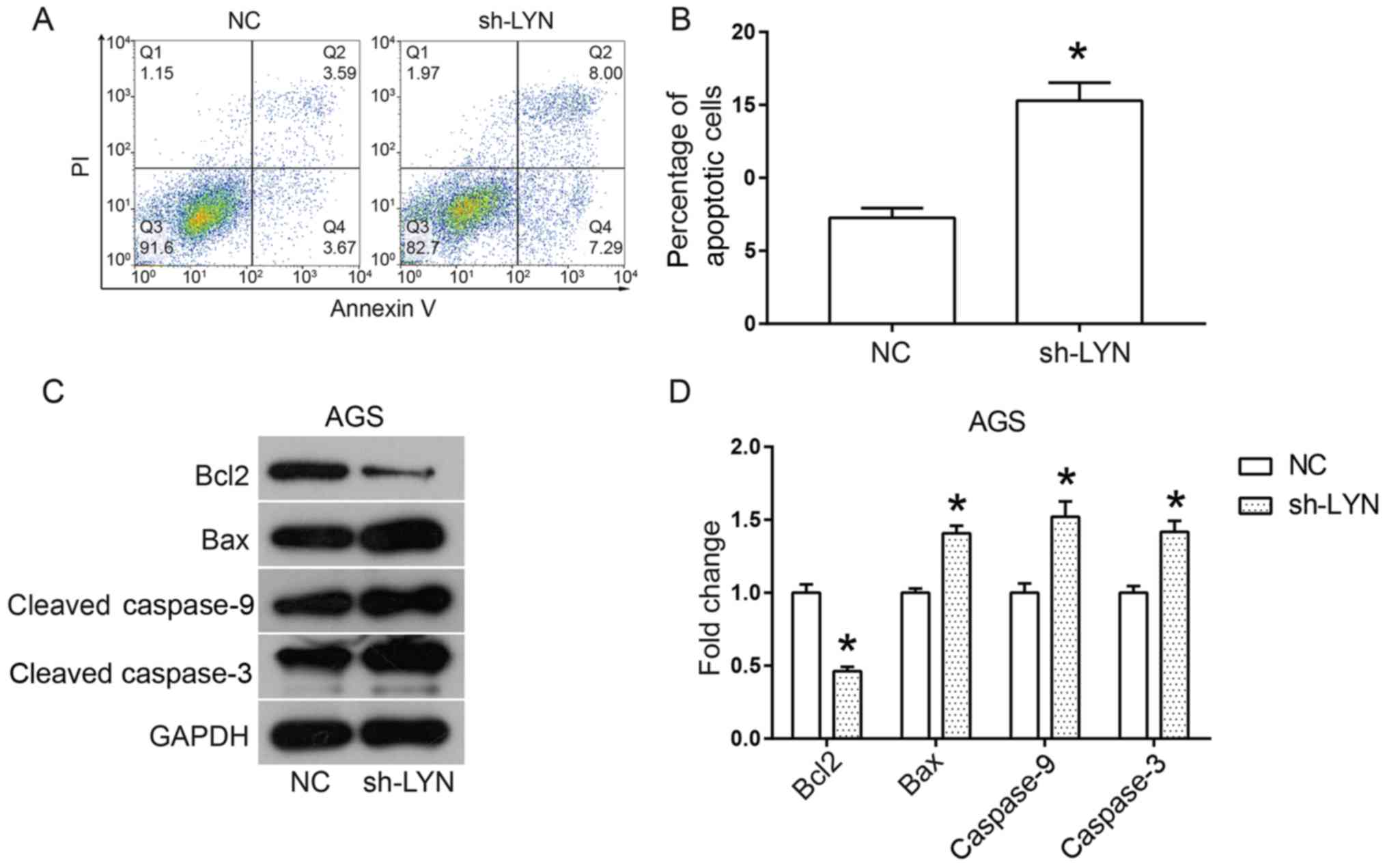
Induction of cell apoptosis is an important
mechanism behind the inhibition of cell viability, therefore the
current study analyzed cell apoptosis in LYN-silenced AGS cells
using flow cytometry. As shown in Fig.
3A and B, the percentage of
apoptotic AGS cells was significantly increased by the
downregulation of LYN when compared to the NC group. Thus, in order
to further investigate the pro-apoptotic mechanism of action behind
LYN knockdown in AGS, the expression levels of proteins involved in
the apoptosis pathway were analyzed using western blotting.
Overall, Bax binds to the membrane of mitochondria in order to
induce the opening of the permeability transition (PT) pore. This
in turn leads to the release of cytochrome c in to cytoplasm
(21), which activates the caspase
cascade and induces apoptosis (21).
Bcl2 plays an anti-apoptotic role throughout the process by
blocking the opening of the PT pore through competitive
interactions with Bax (21).
Downregulation of LYN significantly decreased the expression levels
of Bcl2 and increased the expression levels of Bax, cleaved
caspase-9 and cleaved caspase-3 (Fig.
3C and D). Taken together, these
data suggested that downregulation of LYN significantly promotes
AGS cell apoptosis by activating the apoptosis pathway.
Inhibition of AGS cell migration and
invasion by down-regulation of LYN
In addition to a state of almost constant
proliferation, migration and invasion are also important features
of cancer cells that often trigger tumor metastasis (22,23). In
order to assess the effects of LYN knockdown on these two features
of AGS, Transwell assays were performed. As shown in Fig. 4A and B, knockdown of LYN significantly decreased
the number of invasive and migratory cells, suggesting that cell
invasion and migration were significantly inhibited by the loss of
LYN. Thus, in order to determine the underlying mechanism of action
behind the mobility in AGS cells, the status of the Wnt/β-catenin
signaling pathway was investigated. The Wnt/β-catenin signaling
pathway has been reported to promote epithelial-mesenchymal
transition (EMT) in tumors, which is commonly the initial event of
tumor invasion and metastasis (24).
When the pathway is triggered, Wnt3a binds to its cell surface
receptors, which then leads to the accumulation of cytoplasmic
β-catenin. This in turn causes cytoplasmic β-catenin to transfer to
the nucleus, activating the transcription of targeted genes such as
constitutively expressed MYC and cyclin D1(24). In the present study, LYN knockdown
led to a decrease in the expression levels of Wnt3a, β-catenin,
Snail1 and Snail2 (Fig. 4C and
D) as well as the EMT mesenchymal
markers N-cadherin and vimentin, whereas E-cadherin increased
(Fig. 4C and D). Taken together, these results suggested
that downregulation of LYN inhibits the cell mobility of gastric
cancer through the inactivation of the Wnt/β-catenin signaling
pathway.
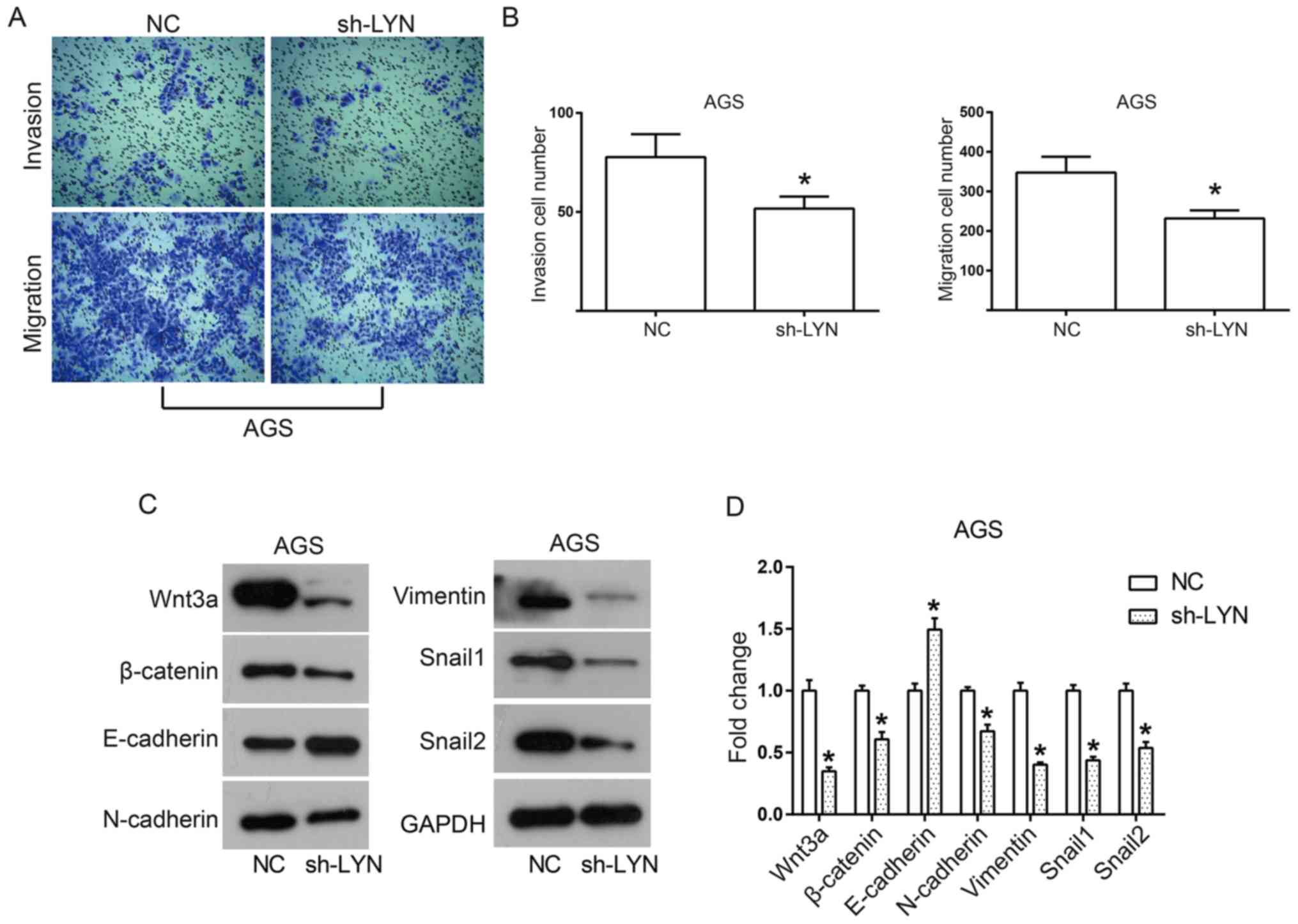 | Figure 4Downregulation of LYN inhibits both
cell mobility and the Wnt/β-catenin signaling pathway in AGS cells.
(A) Images of (magnification, x100), and (B) quantification of cell
invasion and migration of AGS cells, detected using Transwell
assays. (C) Proteins involved with the Wnt/β-catenin signaling
pathway, including Wnt3a, β-catenin, N-cadherin, E-cadherin,
vimentin, Snail1 and Snail2 were detected using western blotting,
and (D) the density of protein bands was quantified using ImageJ
software. All experiments were performed in triplicate.
*P<0.05 vs. respective NC. LYN, LYN kinase; NC,
negative control; sh, short hairpin; Snail, snail family
transcriptional repressor. |
Downregulation of LYN inhibits the
proliferation and invasion of AGS cells via the AKY/mTOR signaling
pathway
The AKT/mTOR signaling pathway is an important
pathway in regulating numerous physiological processes, including
the promotion of cell proliferation, survival and movement
(25). In general, when the AKT/mTOR
signaling pathway is activated, the phosphorylation levels of AKT
are increased, resulting in the phosphorylation and activation of
the downstream signaling protein mTOR (26). In turn, mTOR regulates its downstream
effectors that are important in cellular growth such as p70S6K,
resulting in the enhanced translation of a subset of genes that are
required for protein synthesis, cell growth, inhibition of
apoptosis, acceleration of cell proliferation and tumorigenesis
(26). In the present study, the
effect of LYN knockdown on the AKT/mTOR pathway was investigated.
As shown in Fig. 5A and B, downregulation of LYN resulted in the
decreased expression levels of p-AKT (Ser473), p-mTOR and p70S6K,
while the total expression of AKT and mTOR did not appear to be
influenced. These data suggest that the AKT/mTOR signaling pathway
is inactivated by the downregulation of LYN in AGS gastric cancer
cells. Furthermore, to investigate whether changes in cell
phenotype induced by LYN knockdown occurred through the AKT/mTOR
pathway, a rescue experiment using an AKT pathway activator, IGF-1,
was performed. As shown in Fig. 5C
and D, the CCK-8 and Transwell
invasion assays demonstrated that IGF-1 had the potential to
reverse the inhibitory effects of LYN knockdown on the
proliferation, migration and invasion of AGS cells compared with
the LYN-silenced group (P<0.05). Taken together, these data
suggested that the pro-oncogenic function of LYN in human gastric
cancer cells was mediated through the AKT/mTOR pathway.
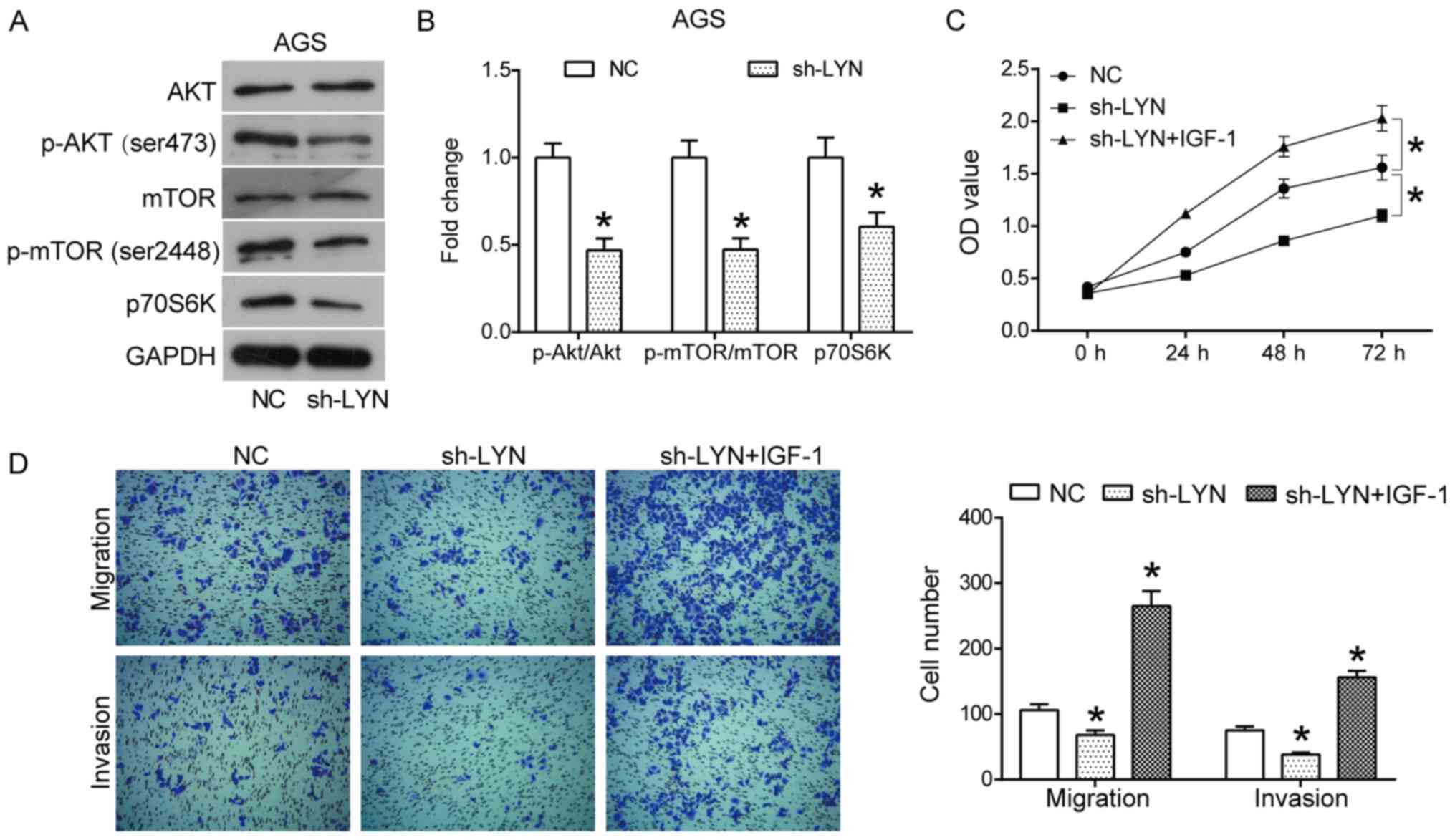 | Figure 5Downregulation of LYN inhibits the
AKT/mTOR signaling pathway in AGS cells. (A) Proteins involved in
the AKT/mTOR pathway, including AKT, p-AKT (Ser473), mTOR, p-mTOR
(Ser2448) and p70S6K were detected using western blotting, and (B)
the density of protein bands was quantified using ImageJ software.
(C) A CCK8 assay was used to measure the proliferation of AGS cells
transfected with sh-LYN or sh-LYN+IGF-1. (D) A Transwell assay was
used to measure the migration (magnification, x100) and invasion
(magnification, x100) of AGS cells transfected with sh-LYN or
sh-LYN+IGF-1. All experiments were performed in triplicate.
*P<0.05 vs. respective NC. IGF, insulin like growth
factor; LYN, LYN kinase; NC, negative control; p,
phosphorylated. |
Discussion
Increasing evidence shows that the Src-related
protein kinase LYN plays important roles in a variety of
tumor-related pathological processes, including proliferation,
differentiation, autophagy, survival and metastasis (11,27-29).
For example, LYN regulates the localization and stability of Snail
family proteins through the Vav-Rac1-p21-activated kinase pathway,
promoting EMT and metastasis in primary tumors (30). In a previous study, it was found that
in nutrient-deprived conditions, raised expression levels of LYN
had the potential to both promote tumor growth and autophagy, as
well as to inhibit cell death in human glioblastoma tumors in
vitro and in vivo (31).
Furthermore, it has been found that the specific inhibition of LYN,
using small interfering RNA, in Ewing's sarcoma significantly
decreases primary tumor growth and lytic activity while also
reducing lung metastases in vivo (27).
LYN is also reported to be involved in the
regulation of tumor-related immune responses. For example, it has
been found that the inhibition of LYN decreases the number of
myeloid-derived suppressor cells in head and neck cancer,
suggesting that LYN may be a potential therapeutic for tumor
immunotherapy (10). Another study
has shown that LYN is involved in mediating both the chemokine
induction activity of LL-37 as well as synthetic cationic peptides
in monocytic cells (32). In the
present study, it was demonstrated that LYN was upregulated in
human gastric cancer tissue, being significantly associated with
pathological grading and the T stage of gastric cancer patients.
Moreover, it was found that knockdown of LYN inhibited
proliferation, migration and invasion, while also inducing
apoptosis in human gastric cancer AGS cells, exposing a
pro-oncogenic role for LYN in gastric cancer. The current results
may extend the understanding of the function of LYN in human
gastric cancer, providing a basis for the clinical application of
LYN.
The underlying mechanism of action behind the
activity of LYN in human gastric cancer was further investigated by
focusing on several key pathways in tumor progression, primarily
the mitochondrial apoptotic, Wnt/β-catenin and AKT/mTOR signaling
pathways. The results indicated that LYN knockdown decreased the
expression of anti-apoptotic protein Bcl2 and activated the
Bax/caspase cascade. When apoptosis is activated, Bax oligomerizes,
and translocates to the mitochondrial outer membrane in order to
increase its permeability. This in turn leads to the release of
cytochrome c and further activation of the caspase cascade
(33,34). Bcl2 functions as an anti-apoptotic
protein by competitively binding to Bax (33,34).
Furthermore, a previous study showed that LYN has the potential to
both phosphorylate the pro-apoptotic protein Bim at Tyr92 and
Tyr161, and to further promote the binding of Bim to the
anti-apoptotic protein Bcl-xL. This, in turn, inhibits the
permeability of the mitochondrial outer membrane and subsequent
activation of the caspase cascade (14).
The AKT/mTOR pathway is a key pathway in the
regulation of multiple biological processes, including the
promotion of proliferation, survival and gene expression (35,36). A
previous study suggested that LYN induces the activation of EGFR in
lung adenocarcinoma cells, which further promotes the activation of
the PI3K/AKT signaling pathway (37). Further studies have shown how LYN is
involved in regulating the activation of the PI3K/AKT signaling
pathway in murine bone marrow-derived macrophages (38), human myeloma (39) and colon cancer (40). The current study suggested that LYN
knockdown resulted in the inactivation of the AKT/mTOR pathway,
including a reduction in the phosphorylation levels of AKT and
mTOR, as well as a reduction in the expression levels of p70S6K.
Furthermore, IGF-1, an AKT pathway activator, could reverse the
inhibitory effects of LYN knockdown on the proliferation, migration
and invasion of AGS cells. When the AKT signal is activated, the
Wnt/β-catenin signaling pathway can be promoted by glycogen
synthase kinase 3β. The Wnt/β-catenin signaling pathway is also an
important signaling pathway which mediates tumor cell proliferation
and metastasis in gastric cancer. The current results showed that
the Wnt/β-catenin pathway was inactivated in LYN-silenced AGS
gastric cancer cells. Moreover, LYN knockdown also led to decreased
expression of the EMT mesenchymal markers N-cadherin and vimentin,
and increased the expression of the epithelial marker E-cadherin.
These results are consistent with previous studies that found that
LYN had the potential to promote metastasis and EMT in tumors by
regulating the stability of the Snail family, while also decreasing
the expression of E-cadherin (30).
In addition, the inactivation of the AKT/mTOR pathway also induces
mitochondrial apoptosis through regulation of the Bax/Bcl2 ratio.
Taken together, the present study provides a novel understanding of
the regulatory mechanism of action of LYN in gastric cancer,
particularly through the activation of the mitochondrial apoptotic
pathway, and the inactivation of the Wnt/β-catenin and AKT/mTOR
signaling pathways. However, the current study only demonstrated
the oncogenic function of LYN in AGS cell, which limits its
application. Further studies should investigate the function of LYN
function in other tumor cell lines.
In conclusion, the current results revealed that LYN
knockdown both inhibited the proliferation and mobility of human
gastric cancer AGS cells, and induced apoptosis through the
regulation of the mitochondrial apoptotic, Wnt/β-catenin and
AKT/mTOR pathways. Furthermore, the current results suggested that
LYN may be a potential therapeutic target for future
treatments.
Acknowledgements
Not applicable.
Funding
The current study was supported by the Beijing
Health System of High level Health Technical Personal Training
Project (grant no. 2013-3-067) and the Beijing Municipal Science
& Technology Commission (grant no. D171100006517003).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
RS and JZ designed the current study, and RS
performed the experiments. All authors collaborated to interpret
results and develop the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Beijing Friendship Hospital of the Capital Medical
University of China. All patients provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhang Y-Z, Zhang L-H, Gao Y, Li CH, Jia
SQ, Liu N, Cheng F, Niu DY, Cho WC, Ji JF, et al: Discovery and
validation of prognostic markers in gastric cancer by genome-wide
expression profiling. World J Gastroenterol. 17:1710–1717.
2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cui L, Zhang X, Ye G, Zheng T, Song H,
Deng H, Xiao B, Xia T, Yu X, Le Y, et al: Gastric juice MicroRNAs
as potential biomarkers for the screening of gastric cancer.
Cancer. 119:1618–1626. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fang XY, Pan HF, Leng RX and Ye DQ: Long
noncoding RNAs: Novel insights into gastric cancer. Cancer Lett.
356:357–366. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Karimi P, Islami F, Anandasabapathy S,
Freedman ND and Kamangar F: Gastric cancer: Descriptive
epidemiology, risk factors, screening, and prevention. Cancer
Epidemiol Biomarkers Prev. 23:700–713. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kim SJ, Wang YG, Lee HW, Kang HG, La SH,
Choi IJ, Irimura T, Ro JY, Bresalier RS and Chun KH: Up-regulation
of neogenin-1 increases cell proliferation and motility in gastric
cancer. Oncotarget. 5:3386–3398. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Delaunoit T: Latest developments and
emerging treatment options in the management of stomach cancer.
Cancer Manag Res. 3:257–266. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Roseweir AK, Qayyum T, Lim Z, Hammond R,
MacDonald AI, Fraser S, Oades GM, Aitchison M, Jones RJ and Edwards
J: Nuclear expression of Lyn, a Src family kinase member, is
associated with poor prognosis in renal cancer patients. BMC
Cancer. 16(229)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu S, Hao X, Ouyang X, Dong X, Yang Y, Yu
T, Hu J and Hu L: Tyrosine kinase LYN is an oncotarget in human
cervical cancer: A quantitative proteomic based study. Oncotarget.
7:75468–75481. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mao L, Deng W-W, Yu G-T, Bu LL, Liu JF, Ma
SR, Wu L, Kulkarni AB, Zhang WF and Sun ZJ: Inhibition of SRC
family kinases reduces myeloid-derived suppressor cells in head and
neck cancer. Int J Cancer. 140:1173–1185. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ingley E: Functions of the Lyn tyrosine
kinase in health and disease. Cell Commun Signal.
10(21)2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jin MS, Khang SK, Kim MS, Choi HS, Lee JE,
Kim KH, Jeon DG and Koh JS: Lyn expression in osteoblastic
osteosarcoma tissues and its correlation with clinicopathologic
factors. Korean J Pathol. 44:125–131. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kim YJ, Hong S, Sung M, Park MJ, Jung K,
Noh KW, Oh DY, Lee MS, Oh E, Shin YK, et al: LYN expression
predicts the response to dasatinib in a subpopulation of lung
adenocarcinoma patients. Oncotarget. 7:82876–82888. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Aira LE, Villa E, Colosetti P, Gamas P,
Signetti L, Obba S, Proics E, Gautier F, Bailly-Maitre B, Jacquel
A, et al: The oncogenic tyrosine kinase Lyn impairs the
pro-apoptotic function of Bim. Oncogene. 37:2122–2136.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mello AA, Leal MF, Rey JA, Pinto GR,
Lamarão LM, Montenegro RC, Alves AP, Assumpção PP, Borges BN, Smith
MC, et al: Deregulated expression of SRC, LYN and CKB kinases by
DNA methylation and its potential role in gastric cancer
invasiveness and metastasis. PLoS One. 10(e0140492)2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Patel MI, Rhoads KF, Ma Y, Ford JM, Visser
BC, Kunz PL, Fisher GA, Chang DT, Koong A, Norton JA, et al:
Seventh edition (2010) of the AJCC/UICC staging system for gastric
adenocarcinoma: Is there room for mprovement? Ann Surg Oncol.
20:1631–1638. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: a web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Camp RL, Dolled-Filhart M and Rimm DL:
X-tile: A new bio-informatics tool for biomarker assessment and
outcome-based cut-point optimization. Clin Cancer Res.
10:7252–7259. 2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tang J, Kong D, Cui Q, Wang K, Zhang D,
Gong Y and Wu G: Prognostic genes of breast cancer identified by
gene co-expression network analysis. Front Oncol.
8(374)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yi X, Yin XM and Dong Z: Inhibition of
Bid-induced apoptosis by Bcl-2. tBid insertion, Bax translocation,
and Bax/Bak oligomerization suppressed. J Biol Chem.
278:16992–16999. 2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Polacheck WJ, Zervantonakis IK and Kamm
RD: Tumor cell migration in complex microenvironments. Cell Mol
Life Sci. 70:1335–1356. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhao J, Liu Y, Zhang W, Zhou Z, Wu J, Cui
P, Zhang Y and Huang G: Long non-coding RNA Linc00152 is involved
in cell cycle arrest, apoptosis, epithelial to mesenchymal
transition, cell migration and invasion in gastric cancer. Cell
Cycle. 14:3112–3123. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liu X, Yun F, Shi L, Li ZH, Luo NR and Jia
YF: Roles of signaling pathways in the epithelial-mesenchymal
transition in cancer. Asian Pac J Cancer Prev. 16:6201–6206.
2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu T, Sun Q, Li Q, Yang H, Zhang Y, Wang
R, Lin X, Xiao D, Yuan Y, Chen L, et al: Dual PI3K/mTOR inhibitors,
GSK2126458 and PKI-587, suppress tumor progression and increase
radiosensitivity in nasopharyngeal carcinoma. Mol Cancer Ther.
14:429–439. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wu N, Du Z, Zhu Y, Song Y, Pang L and Chen
Z: The expression and prognostic impact of the PI3K/AKT/mTOR
signaling pathway in advanced esophageal squamous cell carcinoma.
Technol Cancer Res Treat: Jan 1, 2018 (Epub ahead of print). doi:
10.1177/1533033818758772.
|
|
27
|
Guan H, Zhou Z, Gallick GE, Jia SF,
Morales J, Sood AK, Corey SJ and Kleinerman ES: Targeting Lyn
inhibits tumor growth and metastasis in Ewing's sarcoma. Mol Cancer
Ther. 7:1807–1816. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Dong S and Byrd JC: A New role for Lyn in
the CLL microenvironment. Cancer Cell. 30:511–512. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Choi YL, Bocanegra M, Kwon MJ, Shin YK,
Nam SJ, Yang JH, Kao J, Godwin AK and Pollack JR: LYN is a mediator
of epithelial-mesenchymal transition and a target of dasatinib in
breast cancer. Cancer Res. 70:2296–2306. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Thaper D, Vahid S, Nip KM, Moskalev I,
Shan X, Frees S, Roberts ME, Ketola K, Harder KW, Gregory-Evans C,
et al: Targeting Lyn regulates Snail family shuttling and inhibits
metastasis. Oncogene. 36:3964–3975. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liu WM, Huang P, Kar N, Burgett M,
Muller-Greven G, Nowacki AS, Distelhorst CW, Lathia JD, Rich JN,
Kappes JC, et al: Lyn facilitates glioblastoma cell survival under
conditions of nutrient deprivation by promoting autophagy. PLoS
One. 8(e70804)2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Nijnik A, Pistolic J, Cho P, Filewod NC,
Falsafi R, Ramin A, Harder KW and Hancock RE: The role of the Src
family kinase Lyn in the immunomodulatory activities of
cathelicidin peptide LL-37 on monocytic cells. J Leukoc Biol.
91:599–607. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jeong SY and Seol DW: The role of
mitochondria in apoptosis. BMB Rep. 41:11–22. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Fulda S: Shifting the balance of
mitochondrial apoptosis: Therapeutic perspectives. Front Oncol.
2:121. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Franke TF, Hornik CP, Segev L, Shostak GA
and Sugimoto C: PI3K/Akt and apoptosis: Size matters. Oncogene.
22:8983–8998. 2003.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gao N, Flynn DC, Zhang Z, Zhong XS, Walker
V, Liu KJ, Shi X and Jiang BH: G1 cell cycle progression and the
expression of G1 cyclins are regulated by PI3K/AKT/mTOR/p70S6K1
signaling in human ovarian cancer cells. Am J Physiol Cell Physiol.
287:C281–C291. 2004.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sutton P, Borgia JA, Bonomi P and Plate
JM: Lyn, a Src family kinase, regulates activation of epidermal
growth factor receptors in lung adenocarcinoma cells. Mol Cancer.
212(76)2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Keck S, Freudenberg M and Huber M:
Activation of murine macrophages via TLR2 and TLR4 is negatively
regulated by a Lyn/PI3K module and promoted by SHIP1. J Immunol.
184:5809–5818. 2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Iqbal MS, Tsuyama N, Obata M and Ishikawa
H: A novel signaling pathway associated with Lyn, PI 3-kinase and
Akt supports the proliferation of myeloma cells. Biochem Biophys
Res Commun. 392:415–420. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Subramaniam V, Vincent IR, Gardner H, Chan
E, Dhamko H and Jothy S: CD44 regulates cell migration in human
colon cancer cells via Lyn kinase and AKT phosphorylation. Exp Mol
Pathol. 83:207–215. 2007.PubMed/NCBI View Article : Google Scholar
|