Introduction
With the technological advancement of stem cell and
tissue engineering, regenerative medicine has become a hot topic in
the field of biological medicine (1). The self-renewal and multidirectional
differentiation of stem cells enables them to serve as seed cells
for tissue engineering, facilitating the repair of damaged tissues
or organs (2). In tissue
engineering, ≥2 conditions are required to optimize the application
of stem cells, including the presence of an effective inducer and
tolerance to immunological assault (3). A number of biomaterials have been
previously found to induce the osteogenic differentiation of bone
marrow mesenchymal stem cells (BMSCs) (4), such as hydrolyzed fish collagen (HFC)
(5). Since it is readily available
and accessible, HFC is a material that warrants further
investigations in this field.
The biological activity of HFC has become a notable
focus of research. Blanco et al (6) revealed that HFC possessed significant
antioxidant properties, whilst Liu and Sun (7) observed that HFC induced the osteogenic
differentiation of human periodontal ligament cells in another
study. HFC was found to induce adipose-derived stromal cell
chondrogenesis as effectively as transforming growth factor
(TGF)-β1(8), whereas the
anti-inflammatory properties of HFC has also been previously
documented (9). Collectively, these
findings suggested that HFC possesses a number of biological
activities with the potential for future clinical application.
Immunological rejection remains to be the primary
limitation for the transplantation of allogenic stem cells and
their derivatives (10). Stem cells
and their associated scaffolds are known to face acute immune
rejection mediated by host macrophages, hindering the migration of
reparative cells and weakening the ability of new tissues to
propagate to their surroundings, in turn leading to failure in
tissue regeneration (11). Although
previous research has largely focused on inhibiting the activation
of host immunity (12,13), macrophages have also attracted
increased attention for their substantial plasticity and
phenotype-switching capacity (14).
Owing to their prominent phenotypic plasticity, macrophages have
been discovered to mediate both proinflammatory rejection and
anti-inflammatory tissue remodeling (15). The exposure of M1 macrophages to M2
signals and vice versa, has been discovered to induce the
re-polarization of differentiated macrophages, which demonstrates
their high functional plasticity and potential therapeutic use
(16). Therefore, modulating
macrophage plasticity may provide a novel strategy for combating
immune rejection in tissue engineering.
Mesenchymal stem cells (MSCs) possess unique
immunoregulatory properties. A previous study reported that MSC
transplantation modulated the immune response against allografts
and alleviated transplant rejection, prolonging allograft survival
(17). In addition, macrophages
co-cultured with MSCs were found to consistently express high
levels of CD206, a marker of alternatively-activated macrophages
(18). In addition, the secretion
levels of interleukin (IL)-10 and IL-12 were found to be increased
and reduced, respectively, which is characteristic of
alternatively-activated macrophages (19). In another previous study, macrophages
co-cultured with MSCs were revealed to express lower levels of
tumor necrosis factor α (TNF-α) compared with macrophages cultured
alone, suggesting that MSCs modulate the inflammatory response by
inducing M2 macrophage differentiation (20,21).
Although HFC has been revealed to induce the osteogenic
differentiation of MSCs as aforementioned, the effects of HFC on
the immunomodulatory functions of MSCs remain unknown.
In the present study, a cell-cell contact co-culture
model between BMSCs and macrophages was established to determine
the regulatory effects of HFC-induced BMSCs on the crucial
inflammatory factors associated with macrophages. Additionally, the
immunomodulatory mechanism of BMSCs was investigated, providing a
foundation for the application of HFC and BMSCs in tissue
engineering.
Materials and methods
Materials
HFC was supplied by the Shanghai Fisheries Research
Institute (Shanghai, China).
RAW264.7 cell culture
The murine macrophage RAW264.7 cell line was
obtained from The Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences. The macrophages were cultured in DMEM
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.), 50 µg/ml streptomycin and
100 IU/ml penicillin, which were maintained at 37˚C (5%
CO2) in a humidified atmosphere.
Isolation and in vitro culture of
BMSCs
The present study was approved by the Ethics
Committee of Shanghai Ninth People's Hospital, affiliated with the
School of Medicine, Shanghai Jiao Tong University (Shanghai,
China). The BMSCs originated from bone marrow mononuclear cells,
which have the potential to differentiate into a number of
different cell types, including osteoblasts, adipocytes,
chondrocytes and neural cells (22).
BMSCs have garnered considerable research attention due to their
simplicity of preparation, ethical considerations, accessibility
and low immunogenicity (23). In the
present study, 10 male Sprague Dawley rats (age at sacrifice, 4
weeks; weight, 62.3±2.5 g) were sacrificed by cervical dislocation
and the body was soaked in 75% ethanol for 5 min at room
temperature. All rats were housed in a temperature-controlled room
(21±2˚C) with relative air humidity of 40-60%, under a 12-h
light/dark cycle, with free access to food and water. The tibia and
femur of the rats were then obtained under sterile conditions at
room temperature, where a 5 ml syringe and a 25-gauge needle was
used to flush the bone marrow from the femur and tibia of rats by
injecting 0.5 ml DMEM supplemented with 10% FBS, 100 IU/ml
penicillin and 50 µg/ml streptomycin, into the bone marrow cavity
three times. The washing fluid was collected and centrifuged at 200
x g at room temperature for 10 min, following which the supernatant
was discarded. Subsequently, the pelleted cells were dispersed and
centrifuged at 200 x g at room temperature for 10 min again and the
supernatant was discarded.
BMSCs were subsequently cultured in low glucose DMEM
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.) at 37˚C in 5% CO2.
thereafter, with the medium being changed every day for the first 3
days. At 80% confluence, the cells were passaged into fresh plates
by trypsinization, where third generation BMSCs were collected for
follow-up experiments. The morphology of the primary BMSCs was
observed under a phase contrast microscope (Magnification, x10;
Nikon Corporation).
For osteogenic and adipogenic differentiation,
1x105 BMSCs/ml were seeded into six-well plates in the
low glucose DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.).
After reaching 80% confluence, the cells were treated with
osteogenic differentiation medium (DMEM supplemented with 10% FBS,
50 ng/ml ascorbic acid, 100 µmol/l dexamethasone and 10 mmol/l
β-glycerophosphate (All from Sigma-Aldrich; Merck KGaA) or
adipogenic differentiation medium [DMEM supplemented with 10% FBS,
0.5 mM 3-isobutyl-1-methylxanthine (Sigma-Aldrich; Merck KGaA), 50
µM indomethacin (Sigma-Aldrich; Merck KGaA), 10 µM dexamethasone
Sigma-Aldrich; Merck KGaA) and 10 µg/ml insulin Sigma-Aldrich;
Merck KGaA)] for 14 days at 37 ˚C, where the culture medium was
changed every 3 days.
Establishment of the cell co-culture
system
BMSCs were treated with either 0.2 mg/ml HFC for 7
days at 37˚C or with 0.2 mg/ml HFC for 7 days followed by 10 µM
NS-398 (Tocris Bioscience), a specific cyclooxygenase 2 (COX-2)
inhibitor, for 1 day at 37˚C. The cells were then plated into
24-well plates at a density of 5x104/ml. Following 24 h
of incubation at 37˚C, RAW264.7 macrophages were pre-stimulated
with lipopolysaccharide (1 µg/ml; Sigma-Aldrich; Merck KGaA) for 30
min at 37˚C and then added to the plates containing the BMSCs at a
density of 1x104 cells/ml. RAW264.7 macrophages cultured
alone were used as a control. The cells and supernatants were
collected following incubation for 24 h at 37˚C in 5%
CO2.
MTT assay
At 24 h after co-culture initiation, cell viability
was determined for all experimental groups using an MTT assay.
Briefly, 400 µl MTT solution was added to each well and incubated
for 4 h at 37˚C. Following the removal of medium, 200 µl DMSO was
added to each well and incubated for a further 10 min at room
temperature. The optical density value of each well was measured
using a plate reader at 570 nm.
Determination of nitrous oxide (NO)
concentration in cell supernatants
The supernatants from each group were collected,
where the concentration of NO in the supernatant was determined
using Griess' method (cat. no. S0021; Beyotime Institute of
Biotechnology), according to the manufacturer's protocol.
ELISAs
The concentrations of IL-1β (cat. no. SMLB00C), IL-6
(cat. no. SM6000B), TGF-β (cat. no. DY1679) and IL-10 (cat. no.
SM1000B) in the co-culture medium were measured in the supernatants
using corresponding ELISA kits (R&D Systems, Inc.) according to
the manufacturer's protocols. To analyze the concentration of
prostaglandin E2 (PGE2), the supernatants of the treated BMSCs were
collected before the RAW264.7 macrophages were added, where the
concentration was determined using an ELISA kit (cat. no.
MBS262150; MyBioSource, Inc.), according to the manufacturer's
protocol.
Reverse transcription-quantitative PCR
(RT-qPCR)
The cells were separated using magnetic beads as
previously described (24). CD34 (10
µg; cat. no. ab187282; Abcam), CD45 (10 µg; cat. no. ab25078;
Abcam) and Dynabeads™ Goat Anti-Mouse IgG beads (cat. no. 11033;
Invitrogen; Thermo Fisher Scientific, Inc.) were used according to
the manufacturer's protocol. In RAW264.7 macrophages, the
expression levels of IL-1β, IL-6, CD206, resistin-like α (FIZZ1)
and prostaglandin E2 receptor 4 (EP4) were analyzed. In BMSCs,
IL-1β, IL-6, CD206, FIZZ1, runt-related transcription factor 2
(RUNX2), osteocalcin (OCN), alkaline phosphatase (ALP), lipoprotein
lipase (LPL), adipose differentiation related protein (ADRP) and
peroxisome proliferator-activated receptor γ (PPARγ) expression
levels were assessed. Total RNA was extracted from RAW264.7
macrophages or BMSCs using RNAeasy™ Animal RNA Isolation Kit (cat.
no. R0024FT; Beyotime Institute of Biotechnology) according to the
manufacturer's protocol, the total RNA (10 µg) was then reverse
transcribed into cDNA using the PrimeScript™ RT Reagent kit (Takara
Bio, Inc.), according to the manufacturer's protocol, the reverse
transcription reaction condition was as follows: 37˚C for 15 min,
85˚C for 5 sec and 4˚C for 2 min. qPCR was subsequently performed
using the Power SYBR™ Green Master Mix according to manufacturer's
protocol (cat. no. 4368577; Thermo Fisher Scientific, Inc.), The
thermal cycling conditions consisted of an initial denaturation at
95˚C for 3 min, followed by 40 cycles of denaturation at 95˚C, 15
sec, annealing 60˚C for 30 sec and elongation 72˚C for 30 sec. The
primer sequences used for qPCR are listed in Table I. Target gene expression was
quantified using the 2-ΔΔCq method
and normalized to that of the GAPDH gene (25).
 | Table ISequences of primers used for reverse
transcription-quantitative PCR. |
Table I
Sequences of primers used for reverse
transcription-quantitative PCR.
| Gene | Primer sequence
(5'→3') |
|---|
| Runt-related
transcription factor 2 | F:
GCCGGGAATGATGAGAACTA |
| | R:
GGACCGTCCACTGTCACTTT |
| Osteocalcin | F:
TGCATTCTGCCTCTCTGACC |
| | R:
ACCACCTTACTGCCCTCCTG |
| Alkaline
phosphatase | F:
AAGGCTTCTTCTTGCTGGTG |
| | R:
GCCTTACCCTCATGATGTCC |
| Peroxisome
proliferator-activated receptor γ | F:
CCAAGTGACTCTGCTCAAGTATGG |
| | R:
CATGAATCCTTGTCCCTCTGATATG |
| Lipoprotein
lipase | F:
TGAAGACACAGCTGAGGACA |
| | R:
GATCACCACAAAGGTTTTGC |
| Adipose
differentiation related protein | F:
ATTCTGGACCGTGCCGATT |
| | R:
CTGCTACTGATGCCATTTTTCCT |
| IL-1β | F:
GGACAGAATATCAACCAACAAGTGATA |
| | R:
GTGTGCCCGTCTTTCATTACACAG |
| IL-6 | F:
CCAGAAACCGCTATGAAGTTCCT |
| | R:
CACCAGCATCAGTCCCAAGA |
| CD206 | F:
GTCTGAGTGTACGCAGTGGTTGG |
| | R:
TCTGATGATGGACTTCCTGGTAGCC |
| Resistin-like
α | F:
TGCTGGGATGACTGCTACTG |
| | R:
TGCTGGGATGACTGCTACTG |
| Prostaglandin E2
receptor 4 | F:
TCTACTTGCTCCCAGTGGACATAGATGG |
| | R:
GAACAGACTCCTGAACTGGGTATGGTTC |
| GAPDH | F:
AGGTGAAGGTCGGAGTCAACG |
| | R:
CCTGGAAGATGGTGATGGGAT |
Western blotting
The cells were separated using the magnetic beads
method as aforementioned and previously described (24). Total protein was extracted from
RAW264.7 macrophages or BMSCs using RIPA lysis buffer (Beyotime
Institute of Biotechnology) with a protease inhibitor cocktail
(Roche Diagnostics). Total protein was quantified using a
bicinchoninic acid assay kit (Pierce; Thermo Fisher Scientific,
Inc.) and 20 µg protein/lane was separated via 12% SDS-PAGE. The
separated proteins were subsequently transferred onto a
polyvinylidene fluoride membrane and blocked with 5% skim milk for
2 h at room temperature. The membranes were further incubated
overnight at 4˚C with the following mouse primary antibodies for
the RAW264.7 protein samples: Anti-cyclic AMP-responsive
element-binding protein (CREB) (1:1,000; cat. no. ab31387; Abcam),
anti-phosphorylated (p)-CREB (1:500; cat. no. ab32096; Abcam),
anti-CCAAT/enhancer-binding protein (C/EBP) (1:1,000; cat. no.
ab40764; Abcam), anti-arginase 1 (Arg-1; 1:500; cat. no. 93668;
Cell Signaling Technology, Inc.), anti-inducible nitric oxide
synthase (iNOS; 1:1,000; cat. no. ab178945; Abcam), and
anti-β-actin (1:1,000; cat. no. ab8227; Abcam). The membranes were
incubated with the following rat primary antibodies for the BMSC
protein samples at room temperature for 1 h: Anti-CD29 (1:500; cat.
no. AF2405, R&D Systems, Inc.), anti-CD90 (1:1,000; cat. no.
ab92574; Abcam), anti-CD34 (1:1,000 dilution; cat. no. AF4117;
R&D Systems, Inc.), anti-CD45 (1:1,000; cat. no. ab10558;
Abcam) and anti-β-actin (1:1,000; cat. no. ab8227; Abcam).
Following the primary antibody incubation, the membranes were
washed with 0.1% TBS-Tween 3 times for 10 min each prior to
incubation with a horseradish peroxidase-conjugated secondary
antibodies at room temperature for 1 h [(cat. no. ab6802; 1:5,000;
Abcam) or (cat. no. ab6885; 1:5,000; Abcam)]. The protein bands
were visualized using ECL reagents (EMD Millipore) and quantified
using ImageJ software (version 1.48; National Institutes of
Health).
Statistical analysis
All experiments were repeated three times and all
data in this study were presented as the mean ± SD. Statistical
analysis was performed using SPSS 22.0 software (IBM Corp.).
Statistical comparisons were made using one-way ANOVA and a Tukey's
post hoc test for multiple comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
Characterization of BMSCs
The isolated cells were confirmed to be BMSCs based
on their spindle-shaped morphology and adherence properties
(Fig. 1A). In addition, western
blotting confirmed the expression of CD29 and CD90 and the lack of
CD34 and CD45 expression of BMSCs (Fig.
1B), consistent with the previous studies that BMSCs express
CD29 and CD90, but not CD34 and CD45 (26,27). The
expression levels of osteogenic (RUNX2, ALP and OCN) and adipogenic
(LPL, ADRP and PPARγ) markers were also significantly increased in
osteogenic- or adipogenic-induced BMSCs in vitro compared with
those in unstimulated BMSCs (Fig. 1C
and D).
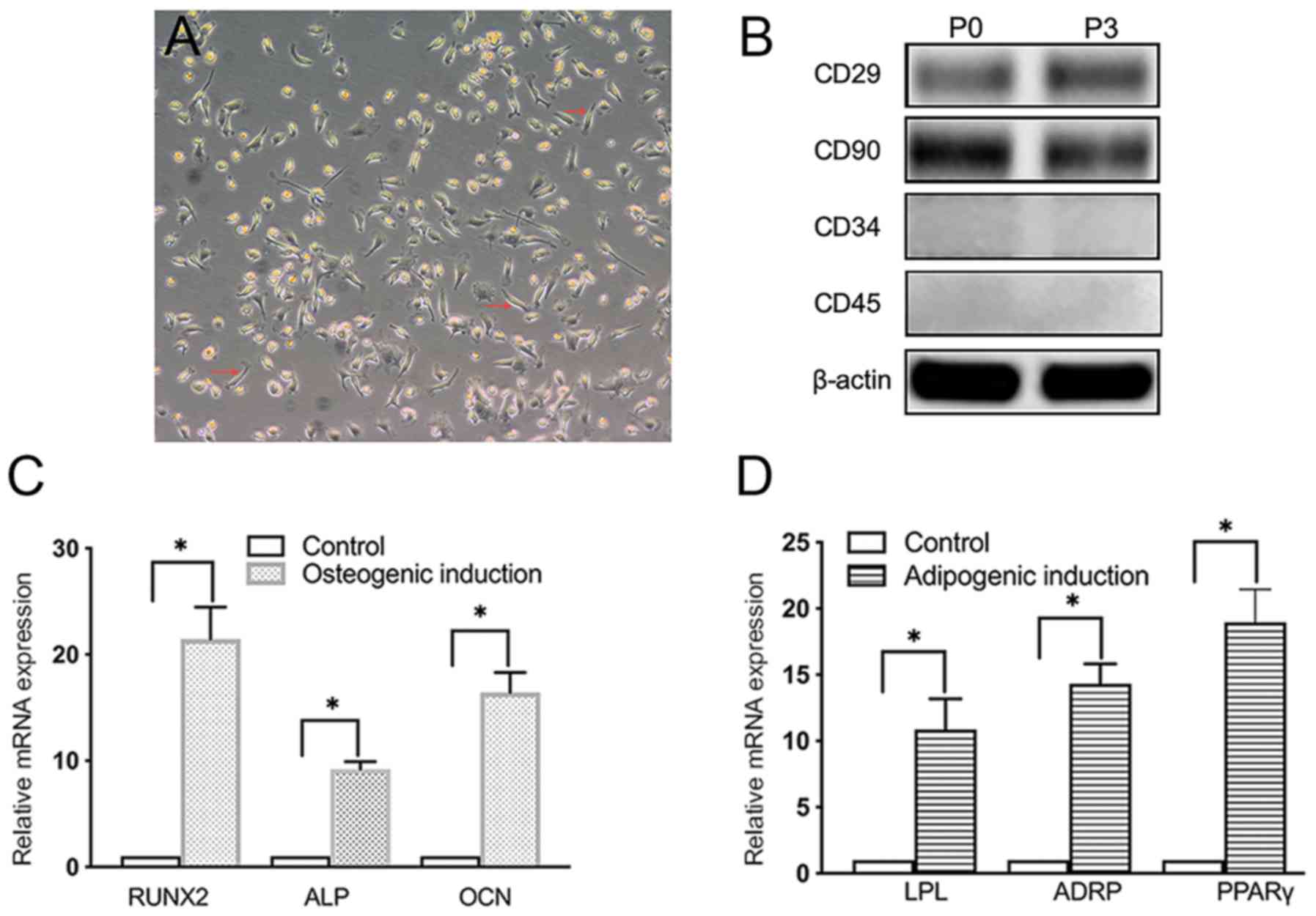 | Figure 1Presentation of data of morphology,
identification, osteogenic and adipogenic differentiation of BMSCs.
(A) Morphology of primary BMSCs. Magnification, x10. Red arrows
indicate the location of exemplary BMSCs. (B) Western blot analysis
of the expression levels of CD29, CD90, CD34 and CD45 in primary
BMSCs at passages 0 and 3. (C) RT-qPCR analysis of the expression
levels of osteogenic markers RUNX2, ALP and OCN in BMSCs 14 days
after osteogenic induction. (D) RT-qPCR analysis of the expression
levels of adipogenic markers LPL, ADRP and PPARγ in BMSCs 14 days
after adipogenic induction. *P<0.05. BMSCs, bone
marrow mesenchymal stem cells; RUNX2, runt-related transcription
factor 2; ALP, alkaline phosphatase; OCN, osteocalcin; LPL,
lipoprotein lipase; ADRP, adipose differentiation related protein;
PPARγ, peroxisome proliferator-activated receptor γ; P, passage;
RT-qPCR, reverse transcription-quantitative PCR. |
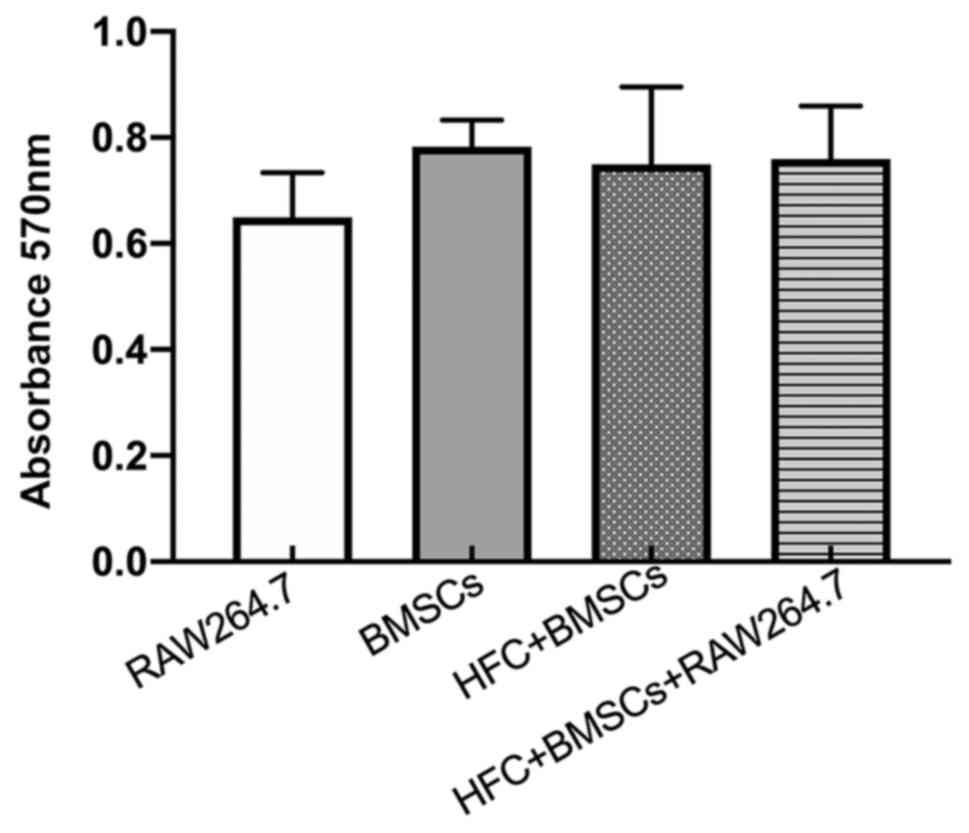
Cell viability
Viability in the co-culture system was found to be
comparable compared with that observed for RAW264.7 macrophages or
BMSCs when either were cultured alone, since no significant
differences were observed in cell viability between the co-culture
and the monocultures (Fig. 2),
suggesting that co-culturing or HFC does not negatively influence
the viability of RAW264.7 macrophages and BMSCs.
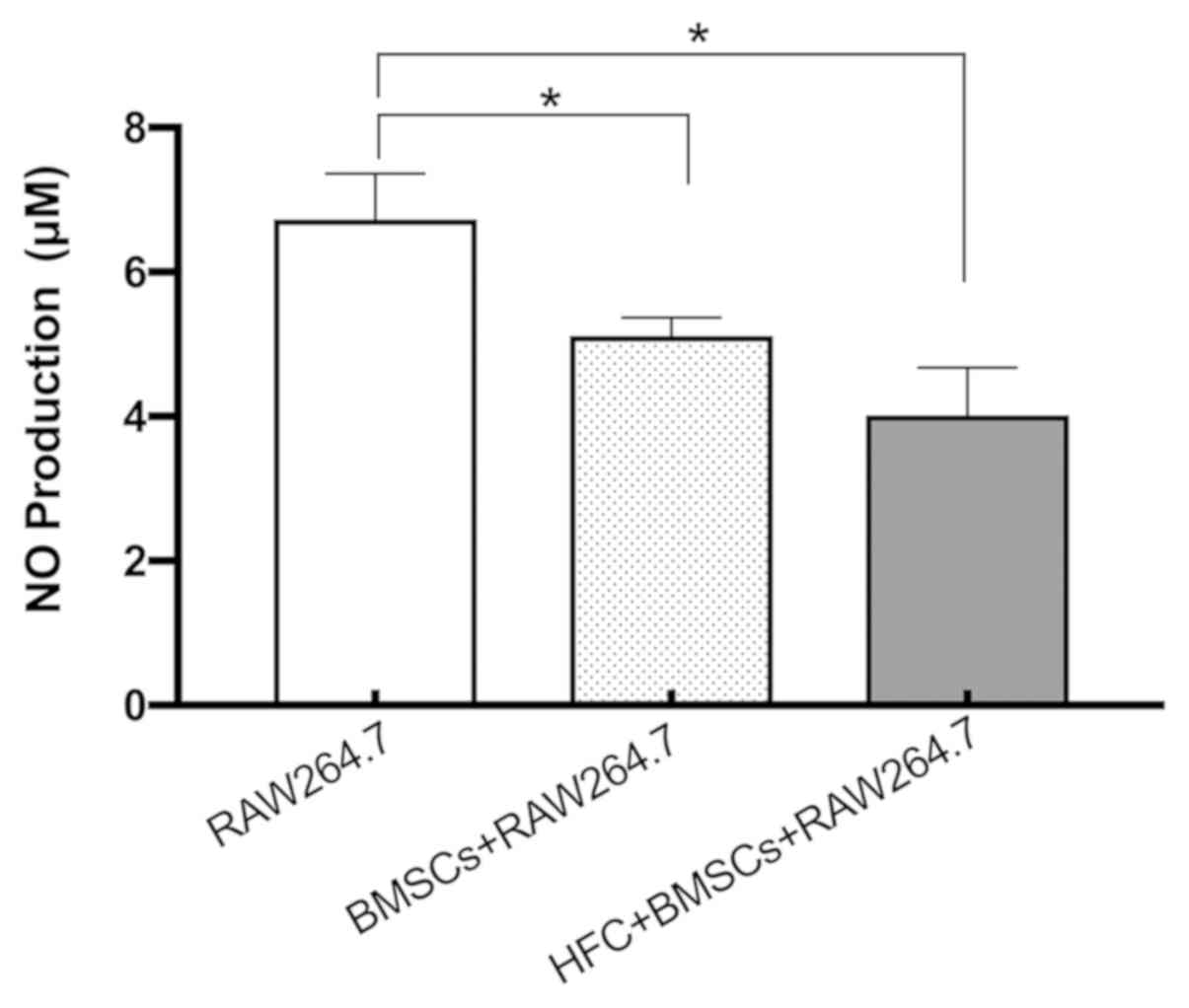
NO production
Co-culturing with HFC-induced BMSCs was revealed to
significantly reduce NO production by RAW264.7 macrophages compared
with that by RAW264.7 macrophages when cultured alone (Fig. 3). NO production in the co-culture
system consisting of untreated BMSCs and RAW264.7 macrophages was
also found to be significantly decreased compared with that by
RAW264.7 macrophages alone (Fig. 3).
However, no significant difference was observed between the
RAW264.7 co-cultured with unstimulated BMSCs and RAW264.7
co-cultured with HFC-induced BMSCs (Fig.
3).
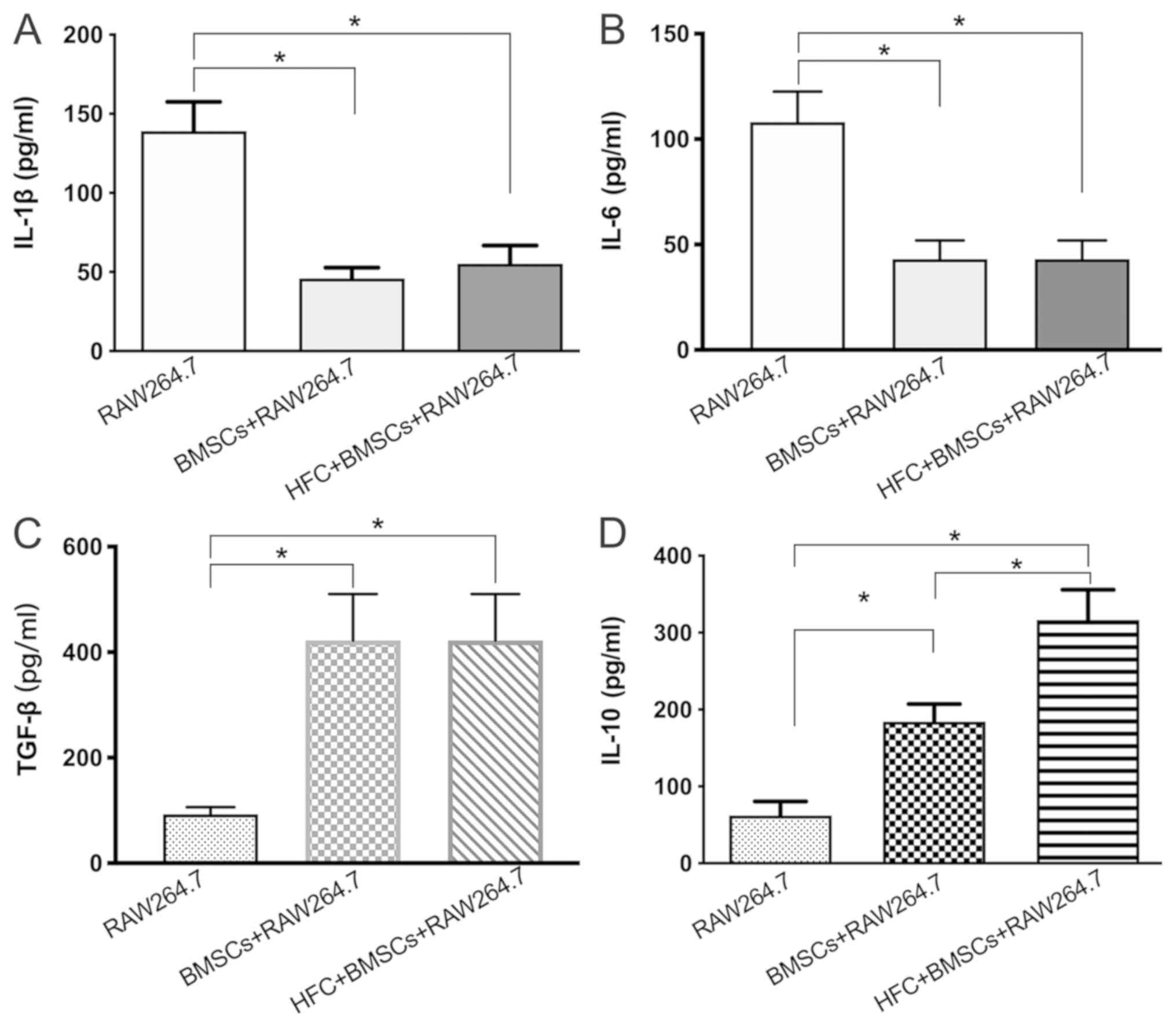
Cytokine secretion
The concentrations of IL-1β, IL-6, TGF-β and IL-10
in the RAW264.7 macrophage supernatants were measured using ELISAs.
The secretion of IL-1β and IL-6 from the RAW264.7 macrophages
co-cultured with HFC-induced BMSCs was found to be significantly
decreased compared with that from the RAW264.7 group when cultured
alone (Fig. 4A and B). By contrast, the concentrations of TGF-β
and IL-10 in the supernatants of RAW264.7 macrophages co-cultured
with HFC-induced BMSCs were significantly increased compared with
those found in those of the RAW264.7 monoculture (Fig. 4C and D). Notably, the concentration of IL-10 in
the supernatants of the HFC-induced BMSC + RAW264.7 group was also
significantly increased compared with that in the untreated BMSC +
RAW264.7 group (Fig. 4D). However, a
significant difference in the concentrations of IL-1β, IL-6 or
TGF-β between the untreated or HFC-induced BMSC + RAW264.7 groups
was not observed.
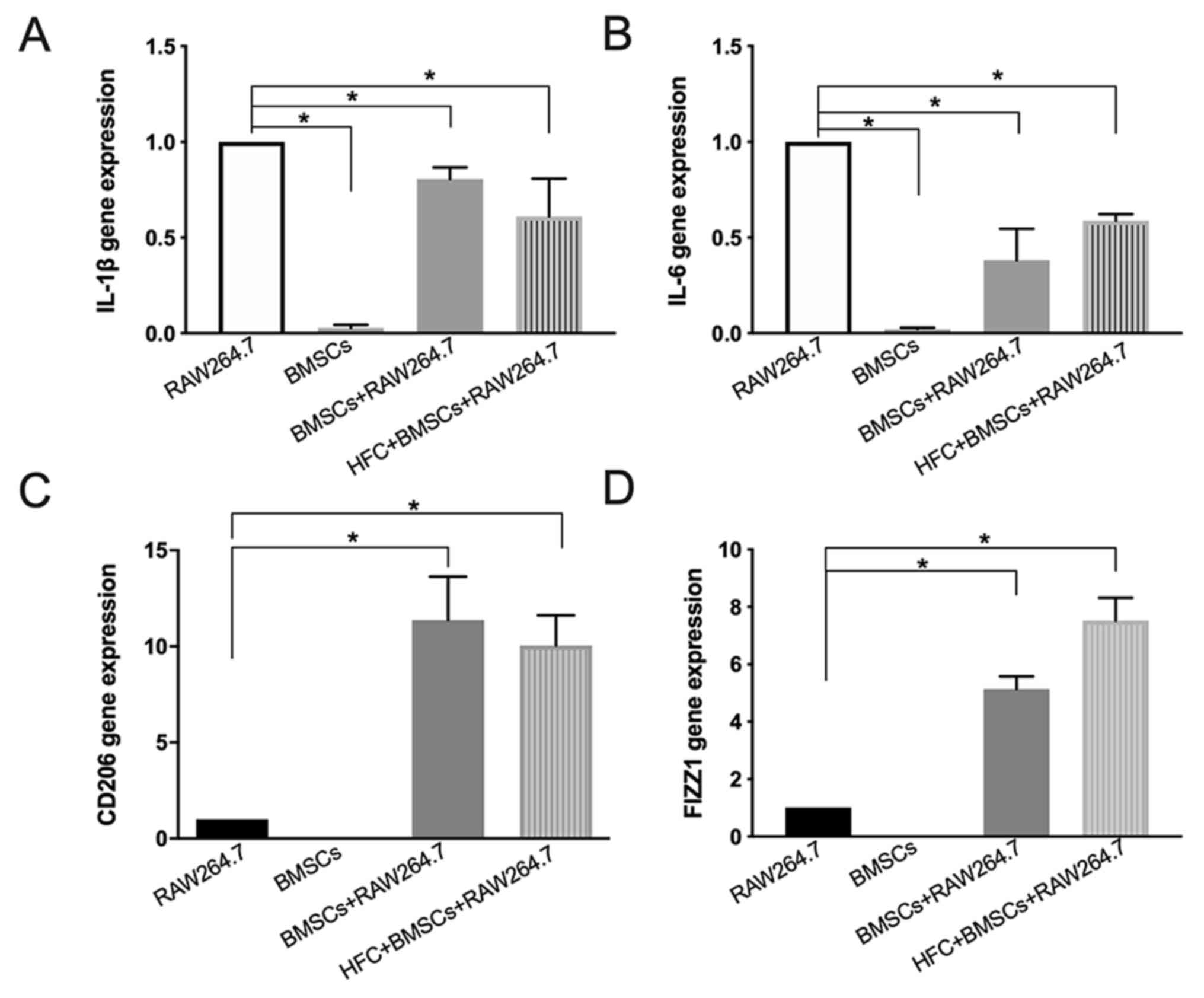
Expression of genes associated with
inflammation in RAW264.7 macrophages and BMSCs
IL-1β and IL-6 are important M1-type macrophage
markers (28). RT-qPCR results
demonstrated that HFC-induced BMSCs significantly reduced the
expression levels of IL-1β and IL-6 mRNA in RAW264.7 macrophages
compared with those in the RAW264.7 macrophage monoculture. In
addition, there were no significant differences in RAW264.7
macrophages co-cultured with either HFC-induced BMSCs or untreated
BMSCs (Fig. 5A and B). Conversely, HFC-induced BMSCs increased
the expression levels of CD206 and FIZZ1 mRNA in RAW264.7
macrophages compared with those in the RAW264.7 macrophages alone
(Fig. 5C and D), which are important M2-type macrophage
markers (29). Notably, the mRNA
expression levels of IL-1β and IL-6 in BMSCs alone were found to be
significantly decreased compared with those in RAW264.7 macrophages
alone (Fig. 5A and B). No CD206 and FIZZ1 mRNA expression could
be detected in BMSCs (Fig. 5C and
D).
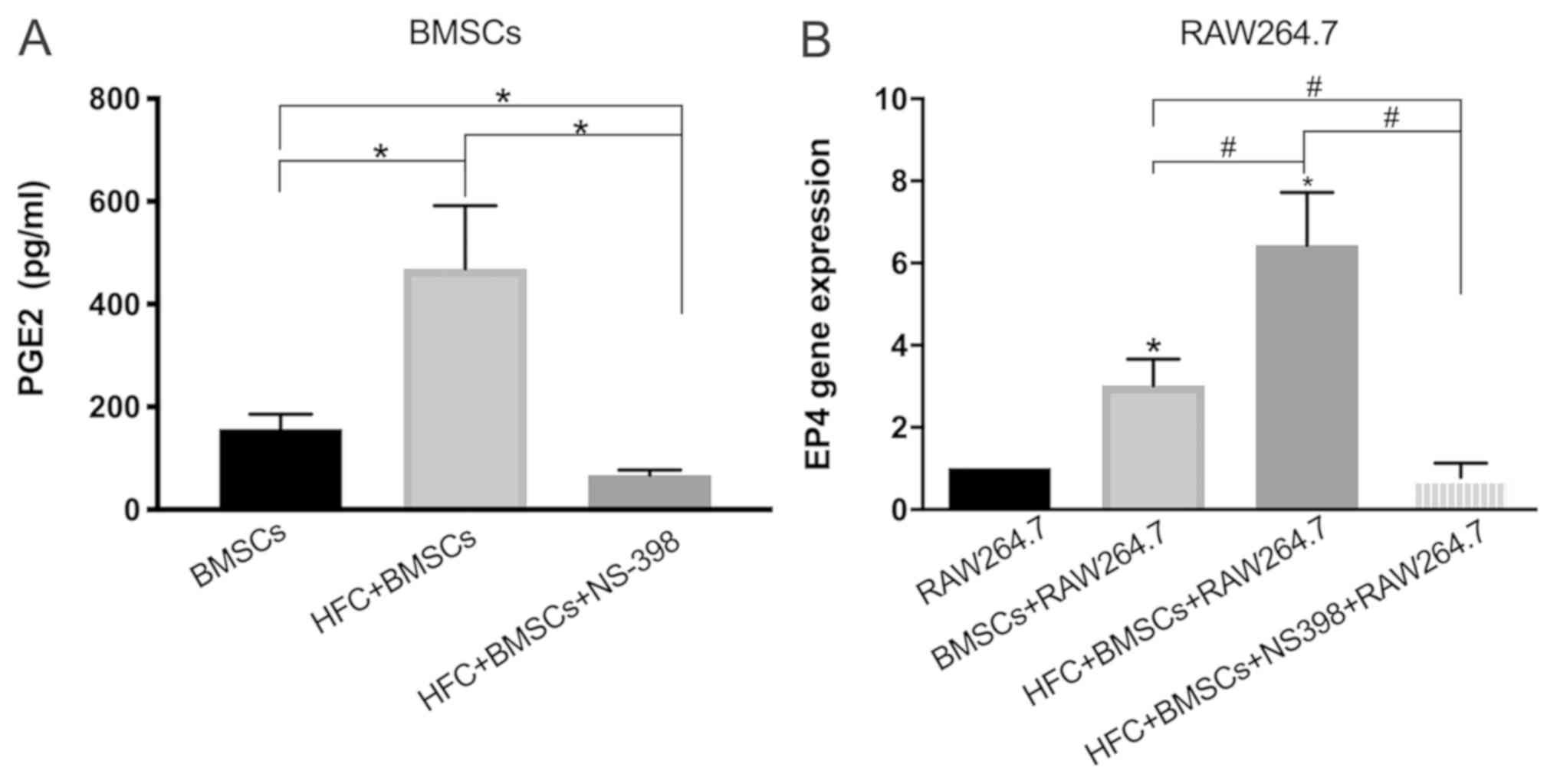
Measurement of PGE2 secretion by BMSCs
and EP4 expression in RAW264.7 macrophages
ELISA results revealed that HFC treatment
significantly increased the secretion of PGE2 in BMSCs compared
with that by untreated BMSCs, which was antagonized by NS-398, a
specific inhibitor of COX-2, which is responsible for PGE2
production (Fig. 6A) (30). EP4 is the receptor for PGE2, an
important transmembrane G protein-coupled receptor in macrophages
(31). The results of the RT-qPCR
analysis demonstrated that HFC-induced BMSCs significantly
increased the expression levels of EP4 mRNA compared with RAW264.7
alone. The stimulatory effect of increased EP4 expression following
co-culture with HFC-induced BMSCs was significantly greater
compared with those in the co-culture consisting of macrophages and
untreated BMSCs (Fig. 6B). Notably,
the effects induced by HFC-induced BMSCs were significantly
reversed in the presence of NS-398 (Fig.
6B).
Effect of HFC-induced BMSCs on the
expression levels of proteins associated with the CREB pathway in
RAW264.7 macrophages
Western blotting data suggested that HFC-induced
BMSCs significantly increased CREB phosphorylation in the
macrophages compared with that in all other groups, especially when
compared with that in the BMSCs + RAW264.7 group (Fig. 7A and B), resulting in the increased expression
levels of the C/EBPβ protein (Fig.
7A and C), upregulation of the
Arg-1 protein and the inhibition of iNOS expression compared with
those in all other groups, especially when compared with those in
the BMSCs + RAW264.7 group (Fig. 7A
and D). These aforementioned effects
were all found to be significantly reversed following the
application of NS-398 (Fig. 7).
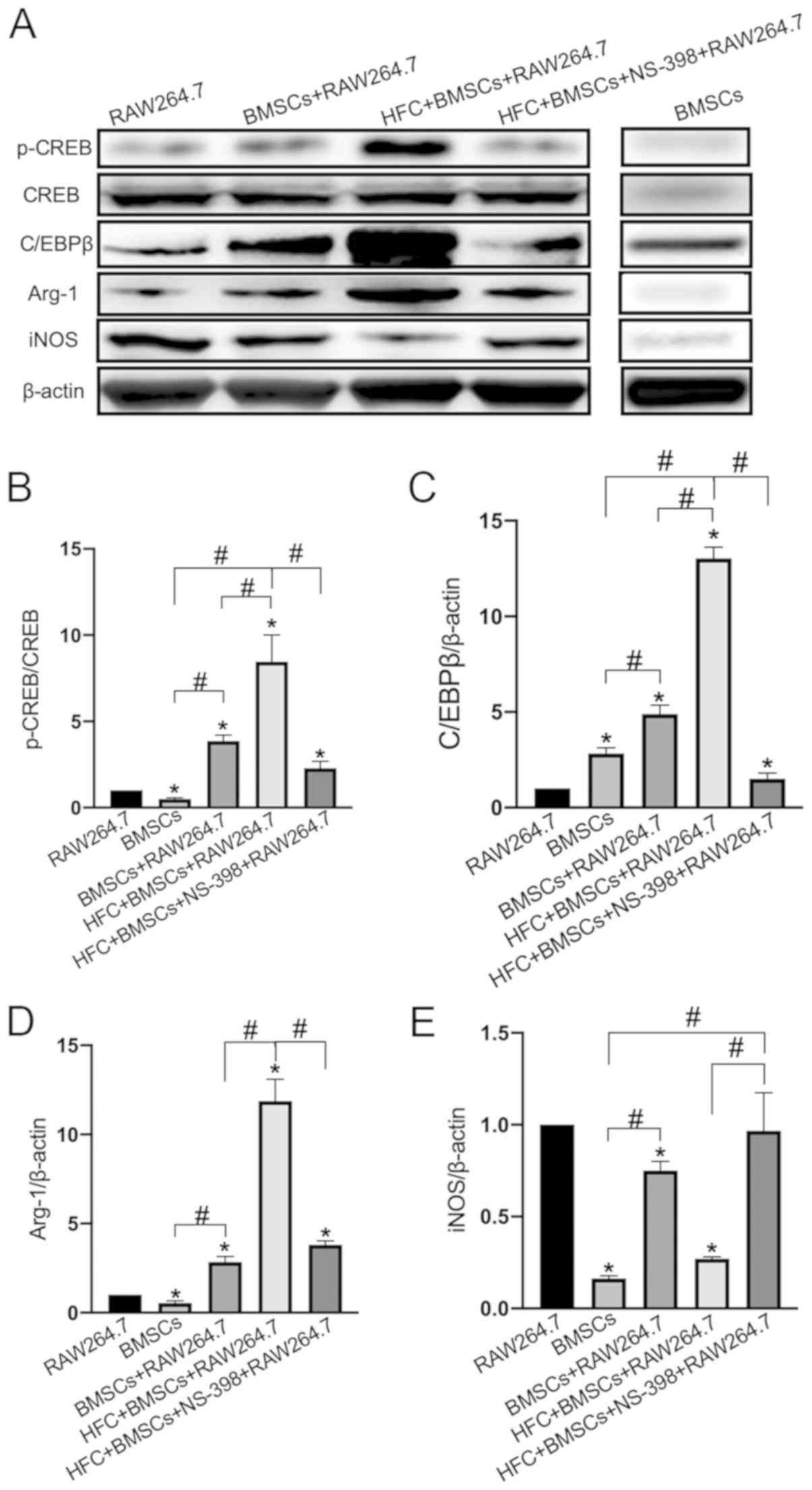 | Figure 7CREB/C/EBPβ signaling pathway
mediates the immunomodulatory role of HFC-induced BMSCs. (A) Levels
of CREB phosphorylation, in addition to C/EBPβ, Arg-1 and iNOS
protein expression were determined using western blotting.
Densitometric analysis of (B) p-CREB, (C) C/EBPβ, (D) Arg-1 and (E)
iNOS expression levels. Data are presented as the mean ± SD from
three independent experiments. *P<0.05 vs. RAW264.7;
#P<0.05. CREB, cyclic AMP-responsive element-binding
protein; p-, phosphorylated; C/EBPβ, CCAAT/enhancer-binding protein
β; Arg-1, arginase 1; iNOS, inducible nitric oxide synthase; BMSCs,
bone marrow mesenchymal stem cells; HFC, hydrolyzed fish collagen;
NS-398, a specific cyclooxygenase 2 inhibitor. |
Discussion
In close proximity, cells communicate via paracrine
signaling or cell-cell contact. Direct-contact co-culture systems
cover both of these aspects; therefore, they can be considered to
be more representative of the cellular microenvironment in vivo
compared with monoculture systems, which can be used to study
cell-cell interactions effectively in vitro (32). To date, studies on the
immunomodulatory effects of BMSCs have primarily focused on
interactions with T and B lymphocytes, natural killer and dendritic
cells (33-36),
but those on macrophages remain insufficient.
Since NO is considered the most sensitive and
efficient indicator of inflammatory macrophages (37), it was analyzed in the present study.
HFC-induced BMSCs were found to significantly inhibit the
production of NO in the co-culture system, with this effect was
comparable with the untreated BMSCs, suggesting that the
HFC-induced BMSCs had retained their immunomodulatory
functions.
Macrophages serve an important role in
immunomodulation by secreting inflammatory factors, where the
polarization state of the macrophages can be identified by changes
to the cytokine profile (38). For
example, M1 macrophages primarily secrete proinflammatory cytokines
IL-1β, IL-6 and TNF-α (39), whilst
M2 macrophages secrete vascular endothelial growth factor, TGF-β,
endothelial growth factor and IL-10, all of which are involved in
anti-inflammatory responses and tissue regeneration (16). To confirm the effect of HFC-induced
BMSCs on macrophage polarization in the present study, the
secretion of the relevant inflammatory factors was also analyzed.
The results revealed that HFC-induced BMSCs inhibited the secretion
of IL-1β and IL-6, whilst promoting the secretion of TGF-β and
IL-10 by RAW246.7 macrophages. IL-1β and IL-6 are well-recognized
proinflammatory cytokines and important markers of M1 macrophages.
IL-1β is derived from macrophages and serve as the primary
regulator of innate immune and inflammatory responses (40). By contrast, IL-6, which is
predominantly secreted by T cells and macrophages, is an important
member of the inflammatory network (41). As a multifunctional cytokine, IL-6
has been discovered to regulate cellular immune responses,
inflammation and hematopoiesis (41). Since macrophages are one of the main
sources of TGF-β, they serve a broader role in cellular
proliferation, differentiation and immune functioning (42). TGF-β inhibits the proliferation of
immune effector cells and the generation of cytotoxic lymphocytes
from CD8+ cells, in addition to directly inhibiting T-helper cell
differentiation (43). IL-10 is an
anti-inflammatory cytokine that can directly inhibit the activation
of inflammatory cells, thereby reducing the production of
inflammatory cytokines (44). The
timely and moderate production of IL-10 has also been discovered to
relieve inflammation and protect normal tissues from inflammatory
injuries (45). In the present
study, HFC-induced BMSCs were found to inhibit the secretion of
IL-1β and IL-6, whilst increasing the levels of TGF-β and IL-10
secretion, suggesting that HFC treatment did not impair the
immunomodulatory functions of BMSCs.
The immunomodulatory role of HFC-induced BMSCs was
subsequently investigated on genetic level. HFC-induced BMSCs
inhibited the expression levels of IL-1β and IL-6 mRNA, whilst
increasing the expression levels of CD206 and FIZZ1 in the
macrophages. Both CD206 and FIZZ1 are M2 macrophage markers
(46), therefore, these results
indicated that HFC-induced BMSCs may regulate macrophage
polarization on transcriptional level.
A previous study demonstrated that PGE2 also
regulated macrophage polarization (47). To verify whether HFC-induced BMSCs
exerted their activity via PGE2, its secretion by BMSCs was
investigated in the present study. HFC treatment was found to
induce the production of PGE2 in BMSCs, which was reversed by the
specific PGE2 inhibitor NS-398. It has been suggested that the
regulatory role of PGE2 is mediated via the EP4 receptor on
macrophages (48). Consistent with
these findings, results of the current study revealed that the
expression levels of EP4 were increased in macrophages following
co-culture with HFC-induced BMSCs, which were reversed by the
presence of NS-398. These results suggested that the
immunomodulatory role of HFC-induced BMSCs may be mediated through
PGE2.
After identifying the important immunoregulatory
role of PGE2 in BMSCs, the question of how PGE2 inhibited
M1-polarization whilst promoting M2 polarization was raised. To
clarify the underlying mechanism of PGE2, potential pathways were
studied using western blotting. The results discovered that the
phosphorylation levels of CREB and the protein expression levels of
C/EBPβ were significantly increased in the presence of HFC-induced
BMSCs, which ultimately increased the expression of Arg-1 and
inhibited the production of iNOS. By contrast, in the presence of
NS-398, the expression levels of C/EBPβ, Arg-1 and CREB
phosphorylation were inhibited, whereas the expression levels of
iNOS were increased. These findings indicated that HFC may induce
the production of PGE2 by BMSCs, which may subsequently activate
the transcription of Arg-1 in macrophages by activating the
p-CREB/C/EBPβ pathway, resulting in polarization towards the M2
subtype.
It is important to differentiate the two different
cell types in the co-culture system, as both BMSCs and RAW264.7
macrophages produce NO and express a number of
inflammation-associated genes, including IL-1β and IL-6. In the
present study, a sufficient and suitable control group was used to
achieve this. The BMSC + RAW264.7 co-culture group was used as a
control for NO production, whereas the BMSC + RAW264.7 co-culture
and BMSC monoculture were used for mRNA expression, which confirmed
that HFC-induced BMSCs were influencing these effects.
It should be noted that BMSCs are the most widely
used cells in bone tissue engineering applications. The focus of
the present study was on bone tissue engineering, which was the
reason for BMSCs being used. In fact, co-culture of RAW264.7
macrophages with other types of cells, including hematopoietic stem
cells or embryonic stem cells, would serve as an excellent control
for comparative purposes to the effects of BMSCs, which will be
investigated in future studies.
In conclusion, the findings of the present study
suggested that HFC-induced MSCs may significantly inhibit the
expression of M1-macrophage markers and promote the expression of
M2 markers, indicating that HFC-induced BMSCs may exert significant
immunomodulatory activities. Mechanistically, the findings
indicated that HFC may promote the secretion of PGE2 from BMSCs,
which may activate the p-CREB/C/EBPβ pathway through binding to the
EP4 receptor on the macrophages, resulting in an increase in Arg-1
expression and a reduction in iNOS expression. The biological
functions of HFC were clarified, which may facilitate further
development of HFC-based biomaterials.
Acknowledgements
The authors would like to thank Professor Nanping
Wang (Shanghai Fisheries Research Institute, Shanghai, China) for
generously providing the HFC.
Funding
This work was supported by the National Natural
Science Foundation of China (grant no. 31600760).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CL performed all the experiments. JS interpreted and
analyzed the data. All authors read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Shanghai Ninth People's Hospital, affiliated with the
School of Medicine, Shanghai Jiao Tong University (approval no.
31600760; Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mantha S, Pillai S, Khayambashi P,
Upadhyay A, Zhang Y, Tao O, Pham HM and Tran SD: Smart hydrogels in
tissue engineering and regenerative medicine. Materials (Basel).
12(pii: E3323)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Costa-Almeida R, Calejo I and Gomes ME:
Mesenchymal stem cells empowering tendon regenerative therapies.
Int J Mol Sci. 2(pii: E3002)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Crupi A, Costa A, Tarnok A, Melzer S and
Teodori L: Inflammation in tissue engineering: The Janus between
engraftment and rejection. Eur J Immunol. 45:3222–3236.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Przekora A: The summary of the most
important cell-biomaterial interactions that need to be considered
during in vitro biocompatibility testing of bone scaffolds for
tissue engineering applications. Mater Sci Eng C Mater Biol Appl.
97:1036–1051. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liu C and Sun J: Potential application of
hydrolyzed fish collagen for inducing the multidirectional
differentiation of rat bone marrow mesenchymal stem cells.
Biomacromolecules. 15:436–443. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Blanco M, Vázquez JA, Pérez-Martín RI and
Sotelo CG: Hydrolysates of fish skin collagen: An opportunity for
valorizing fish industry byproducts. Mar Drugs. 15(pii:
E131)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu C and Sun J: Hydrolyzed tilapia fish
collagen induces osteogenic differentiation of human periodontal
ligament cells. Biomed Mater. 10(065020)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Raabe O, Reich C, Wenisch S, Hild A,
Burg-Roderfeld M, Siebert HC and Arnhold S: Hydrolyzed fish
collagen induced chondrogenic differentiation of equine adipose
tissue-derived stromal cells. Histochem Cell Biol. 134:545–554.
2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu C, Liu X, Xue Y, Ding TT and Sun J:
Hydrolyzed tilapia fish collagen modulates the biological behavior
of macrophages under inflammatory conditions. RSC Adv.
5:30727–30736. 2015.
|
|
10
|
Rackham CL and Jones PM: Potential of
mesenchymal stromal cells for improving islet transplantation
outcomes. Curr Opin Pharmacol. 43:34–39. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mukherjee S, Darzi S, Paul K, Werkmeister
JA and Gargett CE: Mesenchymal stem cell-based bioengineered
constructs: Foreign body response, cross-talk with macrophages and
impact of biomaterial design strategies for pelvic floor disorders.
Interface Focus. 9(20180089)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yang M and Liu L: MHC II gene knockout in
tissue engineering may prevent immune rejection of transplants. Med
Hypotheses. 70:798–801. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Weston LE, Geczy AF and Briscoe H:
Production of IL-10 by alloreactive sibling donor cells and its
influence on the development of acute GVHD. Bone Marrow Transplant.
37:207–212. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sica A and Mantovani A: Macrophage
plasticity and polarization: In vivo veritas. J Clin Invest.
122:787–795. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Ben-Mordechai T, Palevski D,
Glucksam-Galnoy Y, Elron-Gross I, Margalit R and Leor J: Targeting
macrophage subsets for infarct repair. J Cardiovasc Pharmacol Ther.
20:36–51. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shapouri-Moghaddam A, Mohammadian S,
Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi
A, Afshari JT and Sahebkar A: Macrophage plasticity, polarization,
and function in health and disease. J Cell Physiol. 233:6425–6440.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bernardo ME and Fibbe WE: Mesenchymal
stromal cells and hematopoietic stem cell transplantation. Immunol
Lett. 168:215–221. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Deng W, Chen W, Zhang Z, Huang S, Kong W,
Sun Y, Tang X, Yao G, Feng X, Chen W and Sun L: Mesenchymal stem
cells promote CD206 expression and phagocytic activity of
macrophages through IL-6 in systemic lupus erythematosus. Clin
Immunol. 161:209–216. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Abumaree MH, Al Jumah MA, Kalionis B,
Jawdat D, Al Khaldi A, Abomaray FM, Fatani AS, Chamley LW and Knawy
BA: Human placental mesenchymal stem cells (pMSCs) play a role as
immune suppressive cells by shifting macrophage differentiation
from inflammatory M1 to anti-inflammatory M2 macrophages. Stem Cell
Rev Rep. 9:620–641. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kim J and Hematti P: Mesenchymal stem
cell-educated macrophages: A novel type of alternatively activated
macrophages. Exp Hematol. 37:1445–1453. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ripoll CB, Flaat M, Klopf-Eiermann J,
Fisher-Perkins JM, Trygg CB, Scruggs BA, McCants ML, Leonard HP,
Lin AF, Zhang S, et al: Mesenchymal lineage stem cells have
pronounced anti-inflammatory effects in the twitcher mouse model of
Krabbe's disease. Stem Cells. 29:67–77. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Bianco P, Riminucci M, Gronthos S and
Robey PG: Bone marrow stromal stem cells: Nature, biology, and
potential applications. Stem Cells. 19:180–192. 2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Polymeri A, Giannobile WV and Kaigler D:
Bone marrow stromal stem cells in tissue engineering and
regenerative medicine. Horm Metab Res. 48:700–713. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wallace CS and Truskey GA: Direct-contact
co-culture between smooth muscle and endothelial cells inhibits
TNF-alpha-mediated endothelial cell activation. Am J Physiol Heart
Circ Physiol. 299:H338–H346. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Livak JK and Schmittgen TD: Analysis of
relative gene expression data using quantitative PCR and the 2-ΔΔCt
method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shujia J, Haider HK, Idris NM, Lu G and
Ashraf M: Stable therapeutic effects of mesenchymal stem cell-based
multiple gene delivery for cardiac repair. Cardiovasc Res.
77:525–533. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Secunda R, Vennila R, Mohanashankar AM,
Rajasundari M, Jeswanth S and Surendran R: Isolation, expansion and
characterisation of mesenchymal stem cells from human bone marrow,
adipose tissue, umbilical cord blood and matrix: A comparative
study. Cytotechnology. 67:793–807. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Climaco-Arvizu S, Domínguez-Acosta O,
Cabañas-Cortés MA, Rodríguez-Sosa M, Gonzalez FJ, Vega L and
Elizondo G: Aryl hydrocarbon receptor influences nitric oxide and
arginine production and alters M1/M2 macrophage polarization. Life
Sci. 155:76–84. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Arndt L, Dokas J, Gericke M, Kutzner CE,
Müller S, Jeromin F, Thiery J and Burkhardt R: Tribbles homolog 1
deficiency modulates function and polarization of murine bone
marrow-derived macrophages. J Biol Chem. 293:11527–11536.
2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Michelo CM, Fasse E, van Cranenbroek B,
Linda K, van der Meer A, Abdelrazik H and Joosten I: Added effects
of dexamethasone and mesenchymal stem cells on early Natural Killer
cell activation. Transpl Immunol. 37:1–9. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Albu DI, Wang Z, Huang KC, Wu J, Twine N,
Leacu S, Ingersoll C, Parent L, Lee W, Liu D, et al: EP4 Antagonism
by E7046 diminishes Myeloid immunosuppression and synergizes with
Treg-reducing IL-2-Diphtheria toxin fusion protein in restoring
anti-tumor immunity. Oncoimmunology. 6(e1338239)2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mattes B and Scholpp S: Emerging role of
contact-mediated cell communication in tissue development and
diseases. Histochem Cell Biol. 150:431–442. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Rahimzadeh A, Mirakabad FS, Movassaghpour
A, Shamsasenjan K, Kariminekoo S, Talebi M, Shekari A, Zeighamian
V, Ghalhar MG and Akbarzadeh A: Biotechnological and biomedical
applications of mesenchymal stem cells as a therapeutic system.
Artif Cells Nanomed Biotechnol. 44:559–570. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu C and Sun J: Osteogenically
differentiated mesenchymal stem cells induced by hydrolyzed fish
collagen maintain their immunomodulatory effects. Life Sci.
238(116970)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Abdelrazik H, Spaggiari GM, Chiossone L
and Moretta L: Mesenchymal stem cells expanded in human platelet
lysate display a decreased inhibitory capacity on T- and NK-cell
proliferation and function. Eur J Immunol. 41:3281–3290.
2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Duffy MM, Ritter T, Ceredig R and Griffin
MD: Mesenchymal stem cell effects on T-cell effector pathways. Stem
Cell Res Ther. 2(34)2011.PubMed/NCBI View
Article : Google Scholar
|
|
37
|
Mills CD: M1 and M2 Macrophages: Oracles
of health and disease. Crit Rev Immunol. 32:463–488.
2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Varela P, Sartori S, Viebahn R, Salber J
and Ciardelli G: Macrophage immunomodulation: An indispensable tool
to evaluate the performance of wound dressing biomaterials. J Appl
Biomater Funct Mater. 17(2280800019830355)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Mueller CK and Schultze-Mosgau S:
Histomorphometric analysis of the phenotypical differentiation of
recruited macrophages following subcutaneous implantation of an
allogenous acellular dermal matrix. Int J Oral Maxillofac Surg.
40:401–407. 2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Dinarello CA: Immunological and
inflammatory functions of the interleukin-1 family. Annu Rev
Immunol. 27:519–550. 2009.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Nishimoto N: Interleukin-6 as a
therapeutic target in candidate inflammatory diseases. Clin
Pharmacol Ther. 87:483–487. 2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Batlle E and Massagué J: Transforming
Growth Factor-β Signaling in Immunity and Cancer. Immunity.
50:924–940. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Gorelik L and Flavell RA: Immune-mediated
eradication of tumors through the blockade of transforming growth
factor-beta signaling in T cells. Nat Med. 7:1118–1122.
2001.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Steen EH, Wang X, Balaji S, Butte MJ,
Bollyky PL and Keswani SG: the role of the anti-inflammatory
cytokine interleukin-10 in tissue fibrosis. Adv Wound Care (New
Rochelle). 9:184–198. 2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Mittal SK and Roche PA: Suppression of
antigen presentation by IL-10. Curr Opin Immunol. 34:22–27.
2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ferrante CJ, Pinhal-Enfield G, Elson G,
Cronstein BN, Hasko G, Outram S and Leibovich SJ: The
adenosine-dependent angiogenic switch of macrophages to an M2-like
phenotype is independent of interleukin-4 receptor alpha (IL-4Rα)
signaling. Inflammation. 36:921–931. 2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Boehler RM, Kuo R, Shin S, Goodman AG,
Pilecki MA, Gower RM, Leonard JN and Shea LD: Lentivirus delivery
of IL-10 to promote and sustain macrophage polarization towards an
anti-inflammatory phenotype. Biotechnol Bioeng. 111:1210–1221.
2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ylöstalo JH, Bartosh TJ, Coble K and
Prockop DJ: Human mesenchymal stem/stromal cells cultured as
spheroids are self-activated to produce prostaglandin E2 that
directs stimulated macrophages into an anti-inflammatory phenotype.
Stem Cells. 30:2283–2296. 2012.PubMed/NCBI View Article : Google Scholar
|