Introduction
Infertility is defined as the inability of a
sexually active couple to achieve pregnancy within one year of
unprotected regular sexual intercourse (1). Worldwide, ~15% of couples experience
infertility, with ~50% of cases attributed to male factor
infertility (2). The aetiology of
male infertility can be known or undetermined, with 30-50% of cases
being idiopathic (3). Idiopathic
oligoasthenoteratozoospermia (iOAT) is defined as an unexplained
decrease in the semen parameters of a male; such as a sperm count
of <15x106 cells/ml, abnormal sperm morphology >4%
and motility <40%, with no possible cause found upon physical
and endocrine examinations (4).
Oxidative stress is one of the main factors implicated in the
pathogenesis of iOAT and in most patients with iOAT, oxidative
stress manifests as an increase in reactive oxygen species (ROS)
with a reduction in the total antioxidant capacity in seminal
plasma (5,6).
The pineal gland secretes melatonin
(N-acetyl-5-methoxytryptamine), an antioxidant sleep-inducing
hormone, in a cyclical rhythm that is affected by the light/dark
cycle (7). Melatonin release is
inhibited by bright light and stimulated by darkness and sleep
(8). Additionally, the brain,
ovaries, spinal cord, skin, gastrointestinal tract, testis, retina
and lymphocytes produce melatonin (9).
Melatonin exerts its antioxidant mechanism by
scavenging free radicals, thus decreasing levels of ROS and
preventing the depletion of antioxidant enzymes (10). Melatonin improves the motility of
human spermatozoa via protection from apoptosis and DNA
fragmentation induced by ROS, thus maintaining sperm viability in
the reproductive tract during transit (11,12). The
present study aimed to evaluate serum and seminal plasma melatonin
levels in patients with iOAT compared with those in normal fertile
males and to assess the effects of light exposure at night on semen
parameters.
Materials and methods
Study design and participants
The present cross-sectional, case-controlled study
included 50 iOAT infertile laboratory workers who were classified
into a group of 34 males (exposed to light at night; night working
hours) and a second group of 16 males (not exposed to light at
night; day working hours). The shift rotation schedule system was a
7-day cycle composed of daytime, evening and night shifts. There
were 2 days off for every 5 days of work. The working times for the
day, evening and night shifts were 8:00 a.m.-4:00 p.m., 4:00
p.m.-12:00 a.m. and 12:00 a.m-8:00 a.m., respectively. The overall
duration of exposure for patients was 6 working nights daily for 5
days/week over the course of 1 year and 4 months.
A total of 50 normal male subjects served as the
control fertile group. Each patient of this group exhibited
normozoospermia and had fathered at least one child with no history
of infertility. Controls worked the same job as patients with iOAT
and were thus exposed to the same type of light with the same
intensity and wavelength for the same duration to avoid any
confounding factors that could affect serum and seminal plasma
melatonin levels. Controls were categorized into 19 males (night
light-exposed subgroup) and 31 males (non-exposed subgroup).
The control subjects were recruited from a
population of men accustomed to going to bed early (10:00 p.m.) and
not exposed to light at night. All subjects were randomly selected
from the outpatient clinic of Dermatology, Venereology and
Andrology, Qena University Hospitals, Egypt, between December 2015
and March 2016. The subjects were selected during winter to avoid
seasonal variations in melatonin secretion as a confounding factor.
The exclusion criteria were as follows: i) History of
epididymo-orchitis, prostatitis, urinary tract infection or
varicocele; ii) Abnormal hormonal profiles of thyroid stimulating
hormone (normal range, 0.30-6.00 mIU/ml), free triiodothyronine
(normal range, 1.80-4.60 pg/ml) and thyroxine (normal range,
0.93-1.70 ng/ml), follicle stimulating hormone (normal range,
1.5-12.4 µIU/ml), leuteinizing hormone (normal range, 1.70-8.60
mIU/ml), prolactin (normal range, 2.5-21.5 ng/ml) and free
testosterone (normal range, 2.0-95.0 pg/ml); iii) presence of any
endocrine disorders; iv) use of cytotoxic drugs, immune
suppressants or anti androgens; v) leukocytospermia
(>1x106 white blood cells/ml); vi) smoking; vii)
chronic alcohol intake and viii) hepatobiliary or renal
disorders.
All subjects were informed of the study procedures
and signed written informed consent prior to the start of the
study. The current study was approved by the Local Scientific and
Ethical Committee of Qena University Hospitals, Egypt (approval no.
09/2015).
Cases and controls were matched in terms of age and
body mass index (BMI). The study included 50 infertile patients
with iOAT [age range, 23-44 years; mean ± standard deviation (SD)
age, 31.56±5.36 years; BMI range, 19-29.6 kg/m2; mean ±
SD BMI, 24.69±3.59 kg/m2). The parameters of patients
with iQAT were comparable to those of the 50 age-matched healthy
fertile subjects who were included in the control group (age range,
23-49 years; mean ± SD age, 31.94±5.19 years; BMI range, 18-28.7
kg/m2; mean ± SD BMI, 24.38±2.93 kg/m2).
Semen analysis
Once patient history was recorded and
general/genital examinations were performed, all semen samples were
collected by masturbation at 9:00 p.m. after 3-5 days of abstinence
from sexual activity, and kept at 37˚C for 30 min for liquefaction,
followed by semen analysis. The criteria for oligozoospermia,
asthenozoospermia and teratozoospermia were based on the World
Health Organization 2010 guidelines (13). Samples were observed via light
microscopic examination (magnification, x100) of sperm motility
(%), count (1x106 cells/ml) and abnormal head or tail
forms of sperms (%) using a haemocytometer. The remaining semen
samples were washed with nutrient mixture F-10 Ham medium
(Sigma-Aldrich; Merck KGaA) and centrifuged at 12,000 x g for 5 min
at 4˚C. The upper layer of the seminal plasma was
collected and stored at -80˚C until sample collection was complete.
A peroxidase test (Leucoscreen test kit; FertiPro N.V.) was used to
exclude leukocytospermia.
Hormone assays
A total of 5 ml of venous blood was collected in a
plain vacutainer tube at 9:00 p.m. for all subjects following a 12
h fast. Blood was left to clot for 10-20 min at room temperature
before centrifugation at a speed of 3,000 x g for 5 min at 4˚C.
Subsequently, the serum was removed, aliquoted and stored at -80˚C
for further biochemical analysis of melatonin, which was performed
within 3 months of sample collection. Seminal plasma and serum
samples were centrifuged after thawing as previously stated before
melatonin analysis. Hormonal investigations were performed to
exclude subjects according to the aforementioned exclusion
criteria. Commercially available ELISA kits were used to measure
levels of the following serum hormones according to the
manufacturer's protocol: Free triiodothyronine (FT3; cat. no.
F3106T; Calbiotech, Inc.), thyroxine (FT4; cat. no. F4107T;
Calbiotech, Inc.), follicle stimulating hormone (FSH; cat. no.
FS046F; Calbiotech, Inc.), leutenizing hormone (LH; cat. no.
500720; Cayman Chemical Company), prolactin (cat. no. PR234F;
Calbiotech, Inc.) and free testosterone (cat. no. 11-FTSHU-E01;
ALPCO). Serum and seminal plasma melatonin levels were determined
using a human melatonin ELISA kit (cat. no. SL1169Hu; Sunlong
Biotech Co., Ltd) according to the manufacturer's protocol, using
an assay range of 2-70 ng/l. ELISA was performed on the EMR-500
clinical microplate reader (Labomed, Inc.) The sensitivity and
inter- and intra-assay coefficient of variations were 7.5 and 9.5%,
respectively.
Radiologiocal investigation was performed using
scrotal color Doppler ultrasonography to confirm the presence or
absence of varicocles or inflammatory conditions of testicles
and/or epididymis.
Statistical analysis
SPSS (v20.0; IBM Corp.) was used for data analysis.
The Shapiro-Wilk test was performed to assess whether the data were
normally distributed (parametric vs. non-parametric, respectively).
Parametric data are presented as the mean ± SD and non-parametric
data are presented as the median and inter-quartile range. The
Mann-Whitney U test was used for comparison between two
quantitative variables. The correlation between melatonin levels in
seminal plasma and serum with sperm parameters were assessed by
Spearman's rank correlation coefficient to measure correlations
between quantitative variables in non-parametric data.
Results
Serum and seminal plasma melatonin
levels
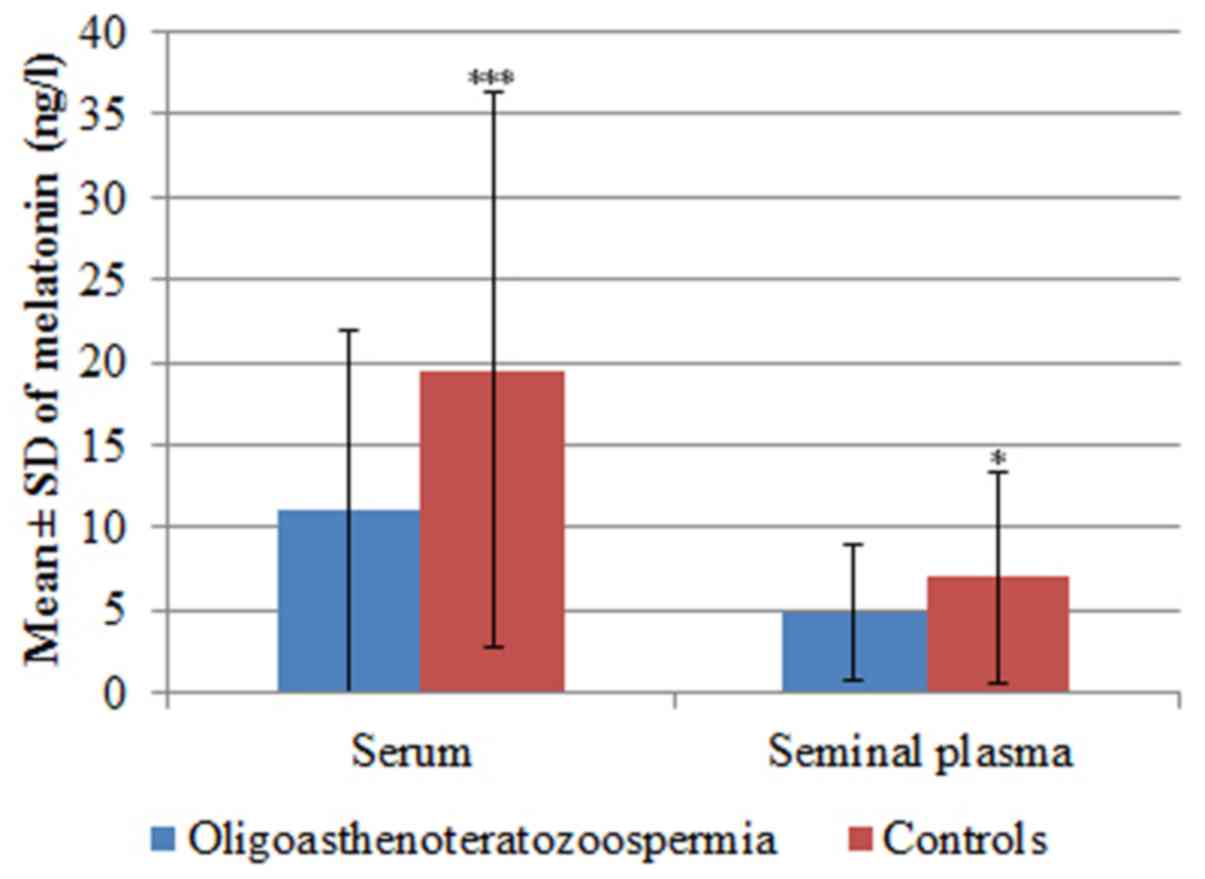
In patients with iOAT, mean serum melatonin levels
were 11.01±10.99 ng/l and seminal plasma levels were4.84±4.06 ng/l.
In the control group, mean serum melatonin levels were 19.51±16.82
ng/l, and mean seminal plasma levels were 6.96±6.35 ng/l. Serum
melatonin levels (P=0.0004) and seminal plasma levels (P=0.01) of
the iOAT group were significantly lower compared with the control
group (Table I and Fig. 1).
 | Table ISerum and seminal plasma melatonin
levels in patients and controls. |
Table I
Serum and seminal plasma melatonin
levels in patients and controls.
| Parameter |
Oligoasthenoteratozoospermia (n=50) | Controls (n=50) | P-value |
|---|
| Serum melatonin
(ng/l) | | | 0.0004 |
|
Mean ±
SD | 11.01±10.99 | 19.51±16.82 | |
|
Median
(range) | 5.38
(2.62-42.11) | 12.33
(2.52-63.78) | |
| Seminal plasma
melatonin (ng/l) | | | 0.01 |
|
Mean ±
SD | 4.84±4.06 | 6.96±6.35 | |
|
Median
(range) | 2.82 (0.90-9.02) | 4.80
(1.83-24.66) | |
Effect of light exposure on serum and
seminal plasma melatonin levels and semen parameters
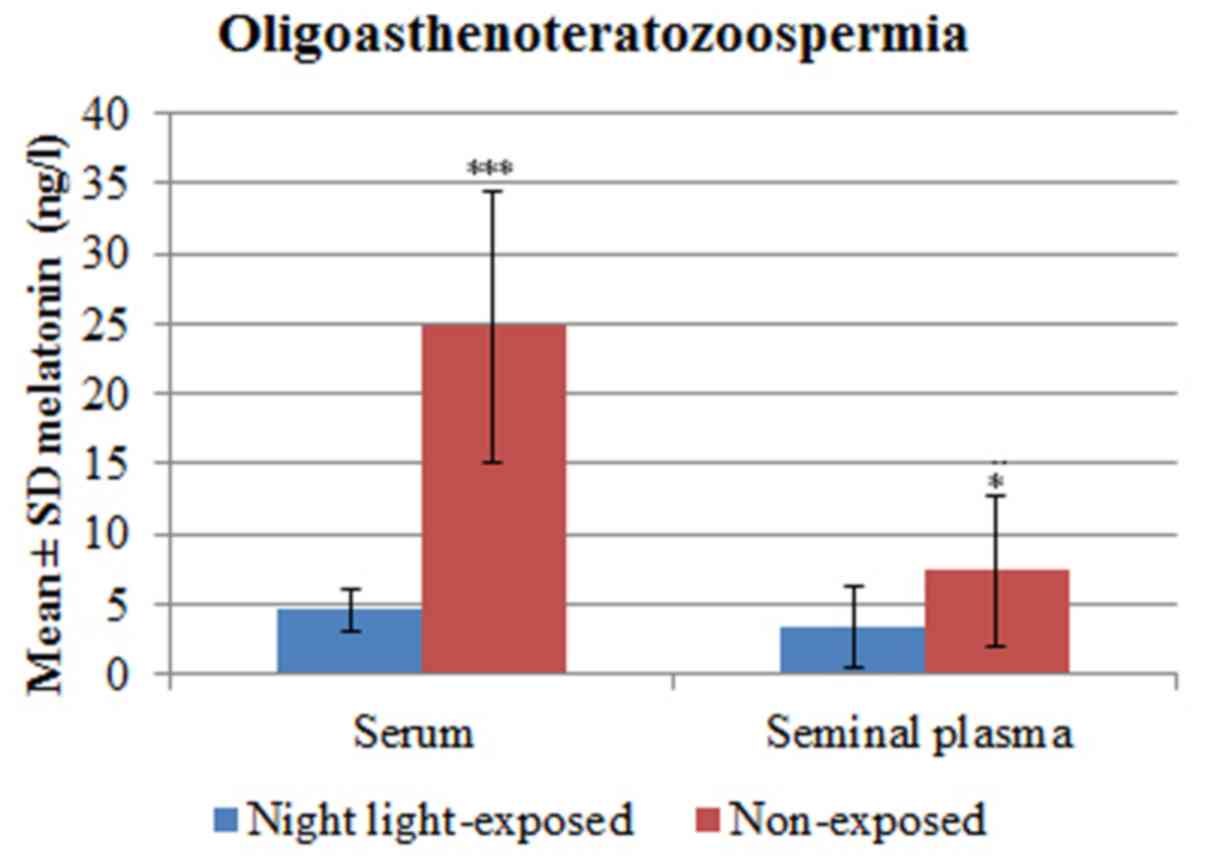
Patients with iOAT exposed to light at night
presented with melatonin serum levels ranging from 1.6-7.57 ng/l
(4.54±1.48 ng/l) and seminal plasma levels ranging from 0.90-9.02
ng/l (3.36±2.94 ng/l). Non-exposed patients with iOAT presented
with serum melatonin levels ranging from 11.26-42.11 ng/l (mean ±
SD, 24.77±9.65 ng/l) and seminal plasma melatonin levels ranging
from 1.58-16.89 ng/l (7.34± 5.29 ng/l). Serum melatonin levels
(P<0.0001) and seminal plasma levels (P=0.02) in patients with
iOAT exposed to light at night were significantly lower compared
with non-exposed cases (Table II
and Fig. 2).
 | Table IISerum and seminal plasma melatonin
levels in night light-exposed and non-exposed
oligoasthenoteratozoospermia cases. |
Table II
Serum and seminal plasma melatonin
levels in night light-exposed and non-exposed
oligoasthenoteratozoospermia cases.
| Parameter | Night light-exposed
(n=34) | Non-exposed
(n=16) | P-value |
|---|
| Serum melatonin
(ng/l) | | | <0.0001 |
|
Mean ±
SD | 4.54±1.48 | 24.77±9.65 | |
|
Median
(range) | 4.11
(1.62-7.57) | 25.60
(11.26-42.11) | |
| Seminal plasma
melatonin (ng/l) | | | 0.02 |
|
Mean ±
SD | 3.36±2.94 | 7.34±5.29 | |
|
Median
(range) | 2.31
(0.90-9.02) | 5.94
(1.58-16.89) | |
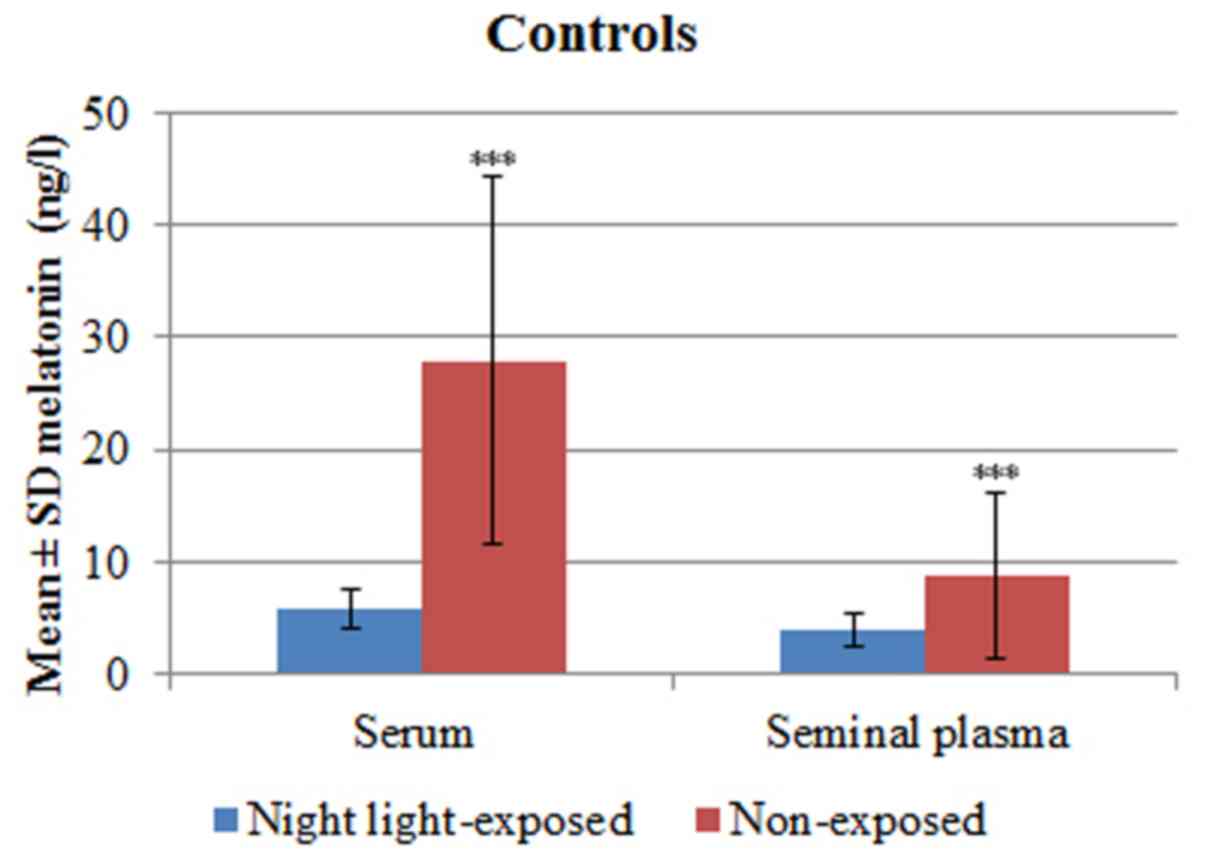
Fertile controls exposed to light at night presented
with melatonin serum levels ranging between 2.52-8.88 ng/l
(5.83±1.76 ng/l) and seminal plasma levels ranging between
1.83-6.60 ng/l (3.85±1.45 ng/l). In non-exposed controls, serum
melatonin levels ranged from 7.96-63.78 ng/l (27.89±16.40 ng/l) and
seminal plasma melatonin levels ranged from 1.95-24.66 ng/l,
(8.86±7.40 ng/l). Serum melatonin levels (P<0.0001) and seminal
plasma levels (P=0.0006) of fertile controls exposed to light at
night were significantly compared with non-exposed controls
(Table III and Fig. 3).
 | Table IIISerum and seminal plasma melatonin
levels in night light-exposed and non-exposed controls. |
Table III
Serum and seminal plasma melatonin
levels in night light-exposed and non-exposed controls.
| Parameter | Night light-exposed
(n=19) | Non-exposed
(n=31) | P-value |
|---|
| Serum melatonin
(ng/l) | | | <0.0001 |
|
Mean ±
SD | 5.83±1.76 | 27.89±16.40 | |
|
Median
(range) | 5.68
(2.52-8.88) | 24.49
(7.96-63.78) | |
| Seminal plasma
melatonin (ng/l) | | | 0.0006 |
|
Mean ±
SD | 3.85±1.45 | 8.86±7.40 | |
|
Median
(range) | 3.50
(1.83-6.60) | 5.82
(1.95-24.66) | |
Sperm parameters of patients with iOAT exposed to
light at night exhibited sperm concentrations ranging between
0.5-7x106 cells/ml (3.30±1.87x106 cells/ml).
Non-exposed patients with iOAT exhibited sperm concentrations
ranging between 10-14x106 cells/ml
(11.97±1.25x106 cells/ml). The percentage of sperm
motility in exposed cases ranged from 0-50% (18.29±13.09%). In
non-exposed cases, the percentage of sperm motility ranged from
0-50% (27.81±16.43%). The percentage of abnormal sperm forms in
exposed cases ranged from 30-80% (59.26±17.97%). In non-exposed
cases, the percentage of abnormal sperm forms ranged from 20-85%
(46.62±32.45%).
A significant difference in sperm concentration
(P<0.0001), sperm motility (P=0.04) and abnormal sperm form
percentage (P=0.04) was found between exposed and non-exposed
groups (Table IV).
 | Table IVSemen parameters in night
light-exposed and non-exposed oligoasthenoteratozoospermia. |
Table IV
Semen parameters in night
light-exposed and non-exposed oligoasthenoteratozoospermia.
| Parameter | Night light-exposed
(n=34) | Non-exposed
(n=16) | P-value |
|---|
| Sperm concentration
(1x106 cells/ml) | | | <0.0001 |
|
Mean ±
SD | 3.30±1.87 | 11.97±1.25 | |
|
Median
(range) | 3 (0.5-7) | 12 (0.50) | |
| Motility (%) | | | 0.04 |
|
Mean ±
SD | 18.29±13.09 | 27.81±16.43 | |
|
Median
(range) | 18 (0-50) | 30 (0-50) | |
| Abnormal form
(%) | | | 0.04 |
|
Mean ±
SD | 59.62±17.97 | 46.62±23.45 | |
|
Median
(range) | 65 (30-80) | 39 (20-85) | |
Correlation between serum and seminal
plasma melatonin levels and semen parameters
Spearman's correlation revealeda significantly
positive correlation between serum melatonin and sperm motility
(r=0.614; P<0.0001) among all patients with iOAT. Additionally,
there was a significant positive correlation between the serum
melatonin and seminal plasma melatonin levels in non-exposed
patients with iOAT (r=0.753; P=0.0008). No other significant
correlations were identified between seminal plasma or serum
melatonin levels and semen parameters (Table V). Additionally, no significant
correlations were identified between seminal plasma or serum
melatonin levels with semen analysis parameters among controls
(Table VI).
 | Table VCorrelation between serum and seminal
melatonin levels with semen parameters in patients with iOAT. |
Table V
Correlation between serum and seminal
melatonin levels with semen parameters in patients with iOAT.
| A, Serum melatonin
(ng/l) |
|---|
| | iOAT patients
(n=50) | Night light-exposed
iOAT patients (n=34) | Non-exposed iOAT
patients (n=16) |
|---|
| Parameter | r-value | P-value | r-value | P-value | r-value | P-value |
|---|
| Seminal melatonin
(ng/l) | 0.259 | 0.069 | 0.143 | 0.417 | 0.753 | 0.0008a |
| Sperm concentration
(1x106 cells/ml) | 0.191 | 0.183 | 0.096 | 0.590 | 0.324 | 0.221 |
| Motility (%) | 0.614 |
<0.0001a | 0.232 | 0.187 | 0.089 | 0.743 |
| Abnormal form
(%) | -0.021 | 0.887 | -0.199 | 0.259 | -0.215 | 0.424 |
| B, Seminalmelatonin
(ng/l) |
| | iOAT patients
(n=50) | Night light-exposed
iOAT patients (n=34) | Non-exposed
iOATpatients (n=16) |
| Parameter | r-value | P-value | r-value | P-value | r-value | P-value |
| Sperm concentration
(1x106 cells/ml) | 0.250 | 0.080 | 0.121 | 0.497 | 0.112 | 0.680 |
| Motility (%) | 0.196 | 0.172 | 0.166 | 0.349 | 0.088 | 0.747 |
| Abnormal form
(%) | -0.168 | 0.244 | -0.226 | 0.120 | -0.052 | 0.848 |
 | Table VICorrelation between serum and seminal
melatonin levels with semen parameters in control subjects. |
Table VI
Correlation between serum and seminal
melatonin levels with semen parameters in control subjects.
| A, Serum melatonin
(ng/l) |
|---|
| | Controls
(n=50) | Night light-exposed
patients (n=19) | Non-exposed
patients (n=31) |
|---|
| Parameter | r-value | P-value | r-value | P-value | r-value | P-value |
|---|
| Seminal melatonin
(ng/l) | 0.220 | 0.601 | 0.250 | 0.207 | 0.209 | 0.5128 |
| Sperm concentration
(1x106 cells/ml) | 0.013 | 0.976 | 0.254 | 0.160 | 0.476 | 0.233 |
| Motility (%) | 0.048 | 0.060 | 0.245 | 0.558 | 0.527 | 0.180 |
| Abnormal form
(%) | -0.476 | 0.233 | -0.095 | 0.823 | -0.048 | 0.911 |
| B, Seminal
melatonin (ng/l) |
| | Controls
(n=50) | Night light-exposed
patients (n=19) | Non-exposed
patients (n=31) |
| Parameter | r-value | P-value | r-value | P-value | r-value | P-value |
| Sperm concentration
(1x106 cells/ml) | 0.220 | 0.601 | 0.233 | 0.420 | 0.114 | 0.788 |
| Motility (%) | 0.071 | 0.867 | 0.024 | 0.955 | 0.323 | 0.435 |
| Abnormal form
(%) | -0.048 | 0.911 | -0.235 | 0.394 | -0.571 | 0.139 |
Discussion
Melatonin has important effects on testicular
function and male reproduction, particularly in patients with
idiopathic infertility, due to its anti-proliferative and
anti-inflammatory effects on testicular macrophages and its
protective effects against oxidative stress in testicular mast
cells (14). Melatonin is involved
in the modulation of inflammatory and oxidant/antioxidant states in
testicular pathology (14). In the
current study, significantly lower mean seminal plasma and serum
melatonin levels were measured in the iOAT group compared with the
normal fertile control group. These results are consistent with a
previous study by Awad et al (15), which demonstrated an association
between a reduction in sperm motility and low melatonin levels.
The observed antioxidant scavenger effect of
melatonin, which is important for the neutralization of ROS and
reactive nitrogen species, has been revealed to improve semen
quality in animal and human studies (16). Sharbatoghli et al (17) demonstrated that seminal plasma
melatonin is positively correlated with sperm DNA damage in
infertile patients but is also not associated with certain sperm
parameters such as concentration, motility and morphology. Bejarano
et al (18) also determined
that orally-administered melatonin improved sperm quality, which is
important for successful natural and assisted pregnancy outcomes.
Furthermore, Shang et al (19) demonstrated that melatonin protected
sperm mitochondria from ROS-induced damage through its antioxidant
effects. Lewis et al (20)
also revealed that low antioxidant levels were observed in the
seminal plasma of infertile men compared with fertile men,
particularly in those with poor semen motility. These results might
explain the decreased levels of melatonin in all infertile groups
of the current study. In contrast to previous results, Shang et
al (21) did not identify any
significant difference in the serum and seminal plasma levels of
melatonin between fertile and infertile men, and the mean seminal
plasma melatonin concentration was lower compared with serum
melatonin. Yie et al (22)
demonstrated that seminal plasma melatonin hormone levels were
higher in oligozoospermic and azoospermicpatients compared with
normozoospermic men. A negative correlation between progressive
sperm motility and seminal plasma melatonin hormone levels was also
demonstrated. These differences in results may be attributed to
different inclusion criteria of the studied subjects.
In the present study, significantly lower serum and
seminal plasma levels of melatonin were revealed in cases exposed
to light at night (night shift work) compared with non-exposed
cases. There were also significantly higher sperm concentrations
and motilities and a significantly lower abnormal sperm form
percentage in non-exposed cases compared with cases exposed to
light at night in the iOAT group. In humans, melatonin is secreted
during the dark phase of the light/dark cycle (23,24).
Daytime melatonin levels are much lower compared with night-time
levels (23,24). There is a delay and a slowing of
melatonin secretion, even with the low-intensity light emitted by
recent technologies, such as LEDs, computer screens, televisions,
mobile phones and tablets (25).
Ortiz et al (26)
demonstrated the positive effects of melatonin on sperm motility
and attributed the antioxidant effects of melatonin, as it
maintained the efficiency of oxidative phosphorylation and
stimulated synthesis of ATP while protecting the mitochondria from
oxidative damage. Additionally, Arendt (27) reported higher sperm concentration
levels during winter compared with during the summer. The authors
explained their results as being due to less exposure to light
during winter and increased melatonin secretion, which confirmed
the negative effect of light on melatonin. Sletten et al
(28) demonstrated that the
administration of 0.5 mg melatonin combined with behavioural
factors involving sleep/wake scheduling improved sleep in patients
with delayed sleep-wake phase disorder.
The pineal hormone, melatonin, participates in
reproductive processes over the course of the day through its
stimulation of gonadotropin-releasing hormone and the consequent
secretion of follicle stimulating hormone and luteinizing hormone
(29). In addition, melatonin
regulates testosterone secretion by Leydig cells and Sertoli cell
function (29). However, other
studies have reported differing results, suggesting that regulation
by melatonin is not entirely light-dependent and concluding that
exposure to light at night may have no effect on melatonin hormone
secretion (29,30).
Correlations between serum or seminal melatonin
levels and various semen parameters are still a matter of
speculation. In the present study, significantly positive
correlations were identified between serum melatonin levels and
sperm motility in the entire iOAT patient cohort and between
seminal plasma and serum melatonin levels in non-exposed iOAT
patients, while no significant correlation was identified between
seminal and serum melatonin levels and sperm count or abnormal form
percentage in the entire patient cohort and in the exposed or
non-exposed iOAT patient subgroups. These results are in agreement
with those of Awad et al (15) and Pitout et al (31). Bornman et al (32) reported a positive correlation between
plasma and seminal melatonin levels but did not identify any
significant correlation between melatonin levels and various semen
variablessuch as sperm concentration, motility percentage and
percentage of abnormal sperm forms. Several mechanisms have been
postulated regarding the effect of melatonin on sperm motility,
including the influencing role of melatonin on the microtubular
sliding mechanism of the sperm axoneme, the involvement of
melatonin in the cAMP protein phosphorylation cascade that is
involved in sperm motility and the presence of melatonin binding
sites in spermatozoa that are regulated by sialic acid (31,33,34).
In conclusion, serum and seminal levels of melatonin
are lower in patients with iOAT compared with normal controls and
exposure to light at night can negatively affect these levels,
consequently affecting semen parameters. Therefore, regular
night-time darkness is important to improve semen quality in
patients with iOAT.
Acknowledgements
Part of the abstract of this manuscript has been
presented as a poster presentation at the American Urological
Association (AUA-2018) conference, May 18-21 2018 in San Francisco,
CA and published as an abstract in the Journal of Urology.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MHH, HMI and MAET conceived and designed the study.
HMI and MAET clinically evalutated patients. NNF, MHH and HMF
collected the samples. MHH and HMF performed laboratory assays.
HMI, MAET, RT, MHH and HMF analysed the data. HMI, MAET, RT, MHH,
NNF and HMF performed literature search. MHH drafted the
manuscript. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of the Faculty of Medicine, South Valley University,
Qena, Egypt and was performed in accordance with the Declaration of
Helsinki. Informed written consent was obtained from each
patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Neschlag E, Behre HM and Nieschlag S
(eds.): Diseases of the Hypothalamus and the Pituitary Gland. In:
Andrology. Male reproductive health and dysfunction. 3rd edition.
Springer Verlag, Berlin, Heidelberg, pp169-192, 2010.
|
|
2
|
Alkhedaide A, Alshehri ZS, Sabry A,
Abdel-Ghaffar T, Soliman MM and Attia H: Protective effect of grape
seed extract against cadmium-induced testicular dysfunction. Mol
Med Rep. 13:3101–3109. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Farhi J and Ben-Haroush A: Distribution of
causes of infertility in patients attending primary fertility
clinics in Israel. Isr Med Assoc J. 13:51–54. 2011.PubMed/NCBI
|
|
4
|
Jungwirth A, Dohle GR, Diemer T, Giwercman
A, Kopa Z and Krausz C: Guidelines of male infertility. European
Association of Urology guidelines, pp1-64, 2010.
|
|
5
|
Agarwal A, Virk G, Ong C and du Plessis
SS: Effect of oxidative stress on male reproduction. World J Mens
Health. 32:1–17. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Du Plessis SS, Agarwal A, Halabi J and
Tvrda E: Contemporary evidence on the physiological role of
reactive oxygen species in human sperm function. J Assist Reprod
Genet. 32:509–520. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Du Plessis SS, Hagenaar K and Lampiao F:
The in vitro effects of melatonin on human sperm function and its
scavenging activities on NO and ROS. Andrologia. 42:112–116.
2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Reiter RJ, Tan DX and Fuentes-Broto L:
Melatonin: A multitasking molecule. Prog Brain Res. 181:127–151.
2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gonzalez-Arto M, Hamilton TR, Gallego M,
Gaspar-Torrubia E, Aguilar D, Serrano-Blesa E, Abecia JA, Pérez-Pé
R, Muiño-Blanco T, Cebrián-Pérez JA and Casao A: Evidence of
melatonin synthesis in the ram reproductive tract. Andrology.
4:163–171. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Reiter RJ, Tan DX and Galano A: Melatonin:
Exceeding expectations. Physiology (Bethesda). 29:325–333.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Espino J, Ortiz Á, Bejarano I, Lozano GM,
Monllor F, García JF, Rodríguez AB and Pariente JA: Melatonin
protects human spermatozoa from apoptosis via melatonin receptor
and extracellular signal regulated kinase-mediated pathways. Fertil
Steril. 95:2290–2296. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Reiter R, Rosales-Corral S, Manchester L
and Tan DX: Peripheral reproductive organ health and melaton in:
Ready for prime time. Int J Mol Sci. 14:7231–7272. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cooper TG, Noonan E, von Eckardstein S,
Auger J, Baker HW, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT
and Vogelsong KM: World Health Organization reference values for
human semen characteristics. Hum Reprod Update. 16:231–245.
2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Frungieri MB, Calandra RS and Rossi SP:
Local actions of melatonin in somatic cells of the testis. Int J
Mol Sci. 18(E1170)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Awad H, Halawa F, Mostafa T and Atta H:
Melatonin hormone profile in infertile males. Int J Androl.
29:409–414. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fernado S and Rombauts L: Melatonin:
Shedding light on infertility? A review of the recent literature. J
Ovarian Res. 7(98)2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sharbatoghli M, Rezazadeh Valojedi M and
Rashli Galeno L: The correlation of seminal melatonin with sperm
conventional parameters, DNA frag. And nuclear maturity in
candidate male for ICSI treatment. Iran J Reprod Med.
12(112)2014.
|
|
18
|
Bejarano I, Monllor F, Marchena AM, Ortiz
A, Lozano G, Jiménez MI, Gaspar P, García JF, Pariente JA,
Rodríguez AB and Espino J: Exogenous melatonin supplementation
prevents oxidative stress-evoked DNA damage in human spermatozoa. J
Pineal Res. 57:333–339. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Shang X, Ye Z, Yu X and Huang Y: Detection
of melatonin in the serum and seminal plasma of fertile and
infertile men. Zhonghua Nan Ke Xue. 10:293–294. 2004.PubMed/NCBI(In Chinese).
|
|
20
|
Lewis SE, Boyle PM, McKinney KA, Young IS
and Thompson W: Total antioxidant capacity of seminal plasma is
different in fertile and infertile men. Fertil Steril. 64:868–870.
1995.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shang X, Huang Y, Ye Z, Yu X and Gu W:
Protection of melatonin against damage of sperm mitochondrial
function induced by reactive oxygen species. Zhonghua Nan KeXue.
10:604–607. 2004.PubMed/NCBI(In Chinese).
|
|
22
|
Yie SM, Daya S, Brown GM, Deys L and
YoungLai EV: Melatonin and aromatase stimulating activity or human
seminal plasma. Andrologia. 23:227–231. 1991.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Burgess HJ, Revell VL and Eastman CI: A
three pulse phase response curve to three milligrams of melatonin
in humans. J Physiol. 586:639–647. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Duffy JF, Cain SW, Chang AM, Phillips AJ,
Münch MY, Gronfier C, Wyatt JK, Dijk DJ, Wright KP Jr and Czeisler
CA: Sex difference in the near-24-hour intrinsicperiod of the human
circadian timing system. Proc Natl Acad Sci USA. 108 (Suppl
3):S15602–S15608. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chang AM, Aeschbach D, Duffy JF and
Czeisler CA: Evening use of light-emitting Readers negatively
affects sleep, circadian timing, and next-morning alertness. Proc
Natl Acad Sci USA. 112:1232–1237. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ortiz A, Espino J, Bejarano I, Lozano GM,
Monllor F, García JF, Pariente JA and Rodríguez AB: High endogenous
melatonin concentrations enhance sperm quality and short-term in
vitro exposure to melatonin improves aspects of sperm motility. J
Pineal Res. 50:132–139. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Arendt J: Melatonin and human rhythms.
Chronobiol Int. 23:21–37. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sletten TL, Magee M, Murray JM, Gordon CJ,
Lovato N, Kennaway DJ, Gwini SM, Bartlett DJ, Lockley SW, Lack LC,
et al: Efficacy of melatonin with behavioural sleep wake scheduling
for delayed sleep-wake phase disorder: A double-blind, randomised
clinical trial. PLoS Med. 15(e1002587)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yu K, Deng SL, Sun TC, Li YY and Liu YX:
Melatonin regulates the synthesis of steroid hormones on male
reproduction: A Review. Molecules. 23(E447)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bedrosian TA, Herring KL, Walton JC,
Fonken LK, Weil ZM and Nelson RJ: Evidence for feedback control of
pineal melatonin secretion. Neurosci Lett. 542:123–125.
2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Pitout MJ, Van Vuuren RJ, Van Aswegen CH
and Theron JJ: Melatonin and sperm motility. S Afr Med J.
79(683)1991.PubMed/NCBI
|
|
32
|
Bornman MS, Oosthuizen JM, Barnard HC,
Schulenburg GW, Boomker D and Reif S: Melatonin and sperm motility.
Andrologia. 21:483–485. 1989.PubMed/NCBI
|
|
33
|
Guraya SS: Biology of spermatogenesis and
spermatozoa in mammals. Springer-Verlag, Berlin, pp.286-302,
1987.
|
|
34
|
Van Vuuren RJ, Pitout MJ, Van Aswegen CH
and Theron JJ: Putative melatonin receptor in human spermatozoa.
Clin Biochem. 25:125–127. 1992.PubMed/NCBI View Article : Google Scholar
|