Introduction
Intensive Care Units (ICUs) treat critically ill
patients in hospitals and tracheal intubation is typically required
for assisted ventilation in ICU wards. Tracheal intubation
primarily consists of mechanical ventilation, to which many
patients develop intolerance (1,2).
Sedative and analgesic adjuvant therapy is often required during
clinical treatment, which reduces the discomfort and stress
responses of patients and increases the effectiveness of mechanical
ventilation (3). Moreover, studies
have shown that appropriate sedation can improve the clinical
outcomes of patients (4). The most
commonly used sedative in the clinic is dexmedetomidine (5). Dexmedetomidine, as a highly selective
α2-adrenergic receptor agonist, inhibits the release of thyroxine
by activating α2-adrenergic receptors, reducing sympathetic nervous
system activity (6). Patients
receiving dexmedetomidine anesthesia do not develop respiratory
depression, which is conducive for those with difficulties weaning
from mechanical ventilation (7). As
a short-acting alkylphenol sedative and hypnotic drug, propofol
acts on GABA receptors in the central nervous system and has the
characteristics of rapid onset, a short half-life, fast metabolism
and a lack of accumulation in the body (8). However, patients may experience memory
loss and anti-convulsive effects after propofol use (9).
Ventilator-associated pneumonia (VAP) is a common
cause of pulmonary infection in ICU wards. The incidence of
pulmonary infections during mechanical ventilation in the ICU is
much higher than that in other hospital departments (10). Artificial mechanical ventilation
provides a direct route for pathogens to enter the body and damages
the physical barrier of the airway, leading to reduced immune
resistance, further increasing the risk of infection (11). When patients develop pulmonary
infections, their condition deteriorates, affecting the treatment
of primary diseases. Dexmedetomidine combined with propofol reduces
extubation times, however, to the best of our knowledge, whether
combination therapy reduces VAP occurrence during mechanical
ventilation has not previously been studied (12,13).
In the present study the sedative effects of
dexmedetomidine combined with propofol in patients in the ICU
undergoing mechanical ventilation were explored and the risk
factors of VAP were assessed, in order to provide relevant
references for clinicians.
Materials and methods
Patient data
The present study is a retrospective analysis of 322
patients that received mechanical ventilation in the ICU ward of
Shanghai Fengxian District Central Hospital from May 2016 to June
2018. According to the use of anesthetics, patients were divided
into a combined and monotherapy group. A total of 212 patients were
included in the combined group, including 130 males and 82 females,
and 110 patients were included in the monotherapy group, including
59 males and 51 females. The current study was approved by the
Medical Ethics Committee of Shanghai Fengxian District Central
Hospital and the family members of patients signed informed consent
forms.
Inclusion and exclusion criteria
The patient inclusion criteria were as follows: i)
>18 years old; ii) required mechanical ventilation according to
a clinician's diagnosis; iii) complete clinical data; iv)
mechanical ventilation time <12 h or with an estimated
mechanical ventilation time of >24 h; v) received sedative
adjuvant therapy. Patient exclusion criteria were as follows: i)
Pregnancy; ii) died within 3 days of mechanical ventilation or were
transferred to other hospital within 3 days of mechanical
ventilation; iii) had undergone digestive tract surgery or had a
medical history of digestive disease, immune deficiency, central
nervous system disease or cognitive impairment; iv) required renal
replacement therapy; v) heart rate <55 beats/min.
Diagnostic criteria for VAP
Patients were diagnosed with VAP if they met the
Guidelines for the Diagnosis and Treatment of Hospital-acquired
Pneumonia at the Society of Respiratory Diseases of the Chinese
Medical Association (14), but also
met one of the following laboratory testing conditions: i)
Leukocyte counts >1*1011/l; ii) leukocyte
counts <4*1010/l; iii) body temperature
>37.5˚C; iv) purulent secretions in respiratory tract; v)
isolation of pathogenic bacteria from the airway.
Sedation schemes
Both groups were treated with fentanyl (Jiangsu Nhwa
Pharmaceutical Co., Ltd.; cat. no. H20143315; 0.3 µg/kg/h) for
continuous intravenous analgesia. In the combined group,
dexmedetomidine (Jiangsu Hengrui Medicine Co., Ltd; cat. no.
H20090248; 0.5 µg/kg) was injected 10 min prior to fentanyl
administration and continuously injected through a micro-injection
pump (0.1-0.2 mg/kg/h). Propofol (0.1-0.3 mg/kg/h) was also
continuously pumped intravenously. In the monotherapy group,
dexmedetomidine (0.5 µg/kg) was slowly injected 10 min prior to
fentanyl administration. Dexmedetomidine (0.3-0.6 mg/kg/h) was
continuously intravenously injected through a micro-injection
pump.
Pathogenic bacteria cultures
An aseptic sputum aspirator was used to collect
lower respiratory tract secretions and pharyngeal swabs were used
to collect pharyngeal wall specimens every 24 h. Pathogenic
bacteria were detected using a BacT/Alert 3d60 automatic
bacterial/mycobacterial culture monitoring system (Institut
Mérieux).
Observation indicators
The main observation indicator was the number of
patients with VAP in both the monotherapy and the combined group.
According to VAP occurrence, patients were divided into VAP and
non-VAP groups. Independent risk factors for the development of VAP
were analyzed using multivariate analysis, and significance
indicators were plotted using receiver operating characteristic
(ROC) curves.
Secondary observation indicators, including clinical
data such as sex, age, body mass index, past medical history,
alcohol abuse, smoking history and acute physiology and chronic
health evaluation II (APACHE II) scores (15) as well as sedative effects (onset
time, waking time and extubation time), were compared between the
groups. After extubation, the Ramsay scores (16) of the two groups were compared. The
patients were scored as follows: A score of 1 was given if the
patient was anxious, restless or, uneasy; a score of 2 was given if
the patient was quiet, cooperative and had good directional force;
a score of 3 was given if the patient was quiet and only responded
to instructions; a score of 4 was given if the patient was in a
state of sleep and only responding to light elastic stimulation
between the eyebrows; a score of 5 was given if the patient was in
a state of sleep and the response to light elastic stimulation
between the eyebrows was slow; a score of 6 was given if the
patient was in a state of sleep and did not respond to
stimulation.
Statistical analysis
SPSS 20.0 software was used to analyze the collected
data. GraphPad Prism 7 (GraphPad Software, Inc.) was used for image
processing. Counting data were expressed as number (percentage) and
assessed through the χ2 method. Kolmogorov-Smirnov tests
were used to analyze data distributions. Measurement data were
expressed as the mean ± standard deviation. Independent sample
t-tests were used to compare normal distribution data between the
two groups. Using VAP as an independent variable and indicators
with differences in a single factor as dependent variables, the
independent risk factors of VAP were analyzed using the forward
stepwise (Wald) method of multi-factor logistic regression. ROC
curves were drawn to analyze indicators that had differences in
multiple factors. P<0.05 was considered to indicate a
statistically significant difference.
Results
Comparison of clinical data
Through comparison of the clinical data between the
groups, no significant differences in sex, age, body mass index,
past medical history, history of alcoholism, history of smoking,
residence and APACHE II scores were observed (P>0.05; Table I).
 | Table IClinical information. |
Table I
Clinical information.
| Characteristic | Monotherapy group
(n=110) | Combined group
(n=212) | χ2/t | P-value |
|---|
| Sex | | | 1.764 | 0.184 |
|
Male | 59 (53.64) | 130 (61.32) | | |
|
Female | 51 (46.36) | 82 (38.68) | | |
| Age | 57.20 (7.30) | 59.00 (8.40) | 1.842 | 0.067 |
| BMI
(kg/m2) | 22.44 (1.55) | 22.69 (1.95) | 1.167 | 0.244 |
| Past medical
history | | | | |
|
Hypertension | 27 (24.55) | 60 (28.30) | 0.518 | 0.472 |
|
Diabetes | 12 (10.91) | 27 (12.74) | 0.227 | 0.634 |
|
COPD | 16 (14.55) | 35 (16.51) | 0.210 | 0.647 |
| History of
alcoholism | | | 1.179 | 0.278 |
|
Yes | 10 (9.09) | 28 (13.21) | | |
|
No | 100 (90.91) | 184 (86.79) | | |
| History of
smoking | | | 0.151 | 0.698 |
|
Yes | 65 (59.09) | 130 (61.32) | | |
|
No | 45 (40.91) | 82 (38.68) | | |
| Residence | | | 0.348 | 0.555 |
|
City | 69 (62.73) | 140 (66.04) | | |
|
Country | 41 (37.27) | 72 (33.96) | | |
|
APACHE II
score | 28.36 (4.25) | 27.86 (5.22) | 0.939 | 0.323 |
Comparison of sedative effects
No significant differences in onset times were
observed, but the waking and extubation times of the combined group
were shorter than those of the monotherapy group. After extubation,
the Ramsay score of the combined group was lower than that of the
monotherapy group (Table II).
 | Table IIComparison of sedative effects. |
Table II
Comparison of sedative effects.
| Group | Monotherapy group
(n=110) | Combined group
(n=212) | t value | P-value |
|---|
| Onset time (s) | 16.25 (4.88) | 17.54 (6.24) | 1.889 | 0.060 |
| Waking time
(min) | 20.31 (5.22) | 8.51 (4.33) | 20.349 | P<0.001 |
| Extubation time
(h) | 7.84 (0.59) | 5.36 (0.38) | 39.977 | P<0.001 |
| Ramsay score after
extubation | 3.35±0.68 | 2.62±0.49 | 11.054 | P<0.001 |
Occurrence of VAP and the distribution
of pathogenic bacteria
A total of 54 patients with VAP were identified, 30
in the monotherapy group (27.27%) and 24 in the combined group
(11.32%). The incidence of VAP was significantly higher in the
monotherapy group (χ2=16.565; P=0.003; Table III). According to the distribution
of pathogenic bacteria detected through microorganisms, 72
pathogenic bacteria were detected in 54 patients. Amongst them, 38
strains (52.78%) were Gram-negative bacteria, 32 strains (44.44%)
were Gram-positive and 2 strains (2.78%) were fungi (Table IV).
 | Table IIIComparison of ventilator-associated
pneumonia incidence. |
Table III
Comparison of ventilator-associated
pneumonia incidence.
| Group | Occurred | Not occurred | χ2
value | P-value |
|---|
| Monotherapy group
(n=110) | 30 | 80 | 16.565 | 0.003 |
| Combined group
(n=212) | 24 | 188 | | |
 | Table IVComparison of distribution of
pathogenic bacteria in VAP patients. |
Table IV
Comparison of distribution of
pathogenic bacteria in VAP patients.
| Pathogenic
bacteria | n | Constituent ratio
(%) |
|---|
| Gram-negative
bacterium |
|
Staphylococcus
aureus | 27 | 37.50 |
|
Staphylococcus
epidermidis | 8 | 11.11 |
|
Enterococcus | 3 | 4.17 |
| Gram-positive
bacterium |
|
Pseudomonas
aeruginosa | 23 | 31.94 |
|
Klebsiella
pneumoniae | 4 | 5.56 |
|
Escherichia
coli | 3 | 4.17 |
|
Acinetobacter
baumannii | 2 | 2.78 |
| Fungus |
|
Candida
albicans | 1 | 1.39 |
|
Candida
tropicalis | 1 | 1.39 |
Univariate analysis of patients with
VAP
Patients were re-grouped into VAP (n=54) and non-VAP
groups (n=268). Univariate analysis of their clinical data revealed
no differences in sex, body mass index, past medical history,
history of alcoholism, history of smoking, residence or onset time
(P>0.05), whilst differences in age, APACHE II score,
consciousness disorders, invasive operations, sedation programs,
waking time, and extubation time were significant (P<0.001;
Table V).
 | Table VUnivariate analysis. |
Table V
Univariate analysis.
| Factor | VAP group
(n=54) | Non-VAP group
(n=268) | χ2/t
value | P-value |
|---|
| Sex | | | 2.977 | 0.084 |
|
Male | 26 (48.15) | 163 (60.82) | | |
|
Female | 28 (51.85) | 105 (39.18) | | |
| Age | 65.54 (3.25) | 58.41 (3.65) | 13.326 | P<0.001 |
| BMI
(kg/m2) | 22.54 (1.52) | 22.69 (1.84) | 0.561 | 0.575 |
| Past medical
history | | | | |
| Hypertension | 13 (24.07) | 74 (27.61) | 0.182 | 0.671 |
| Diabetes | 8 (14.81) | 31 (11.57) | 0.445 | 0.505 |
| COPD | 10 (18.52) | 41 (15.30) | 0.350 | 0.554 |
| History of
alcoholism | | | 0.084 | 0.772 |
|
Yes | 7 (12.96) | 31 (15.57) | | |
|
No | 47 (87.04) | 237 (88.43) | | |
| History of
smoking | | | 1.013 | 0.314 |
|
Yes | 36 (66.67) | 159 (59.33) | | |
|
No | 18 (33.33) | 109 (40.67) | | |
| Place of
residence | | | 0.850 | 0.357 |
|
City | 38 (70.37) | 171 (63.81) | | |
|
Country | 16 (29.63) | 97 (36.19) | | |
|
APACHE II
score | 31.55 (2.58) | 25.33 (3.84) | 11.389 | P<0.001 |
| Disturbance of
consciousness | | | 22.381 | P<0.001 |
|
Yes | 22 (40.74) | 38 (13.67) | | |
|
No | 32 (59.26) | 240 (86.33) | | |
| Invasive
operation | | | 30.719 | P<0.001 |
|
Yes | 29 (53.70) | 49 (18.28) | | |
|
No | 25 (46.30) | 219 (81.72) | | |
| Sedation
scheme | | | 19.822 | P<0.001 |
|
Monotherapy | 30 (55.56) | 70 (25.18) | | |
|
Combined
therapy | 24 (44.44) | 208 (74.82) | | |
|
Onset time
(sec) | 17.25 (4.48) | 18.54 (5.44) | 1.634 | 0.103 |
|
Recovery
time (min) | 18.54 (4.25) | 12.58 (3.98) | 9.925 | P<0.001 |
|
Extubation
time (h) | 8.54 (0.74) | 5.32 (1.25) | 18.281 | P<0.001 |
Multivariate analysis and ROC
curves
Single factors with significance were included and
assigned (Table VI) for
multivariate logistic regression analysis. Age, APACHE II score,
consciousness disorders, invasive operations, waking time,
extubation time and sedation regimens were independent risk factors
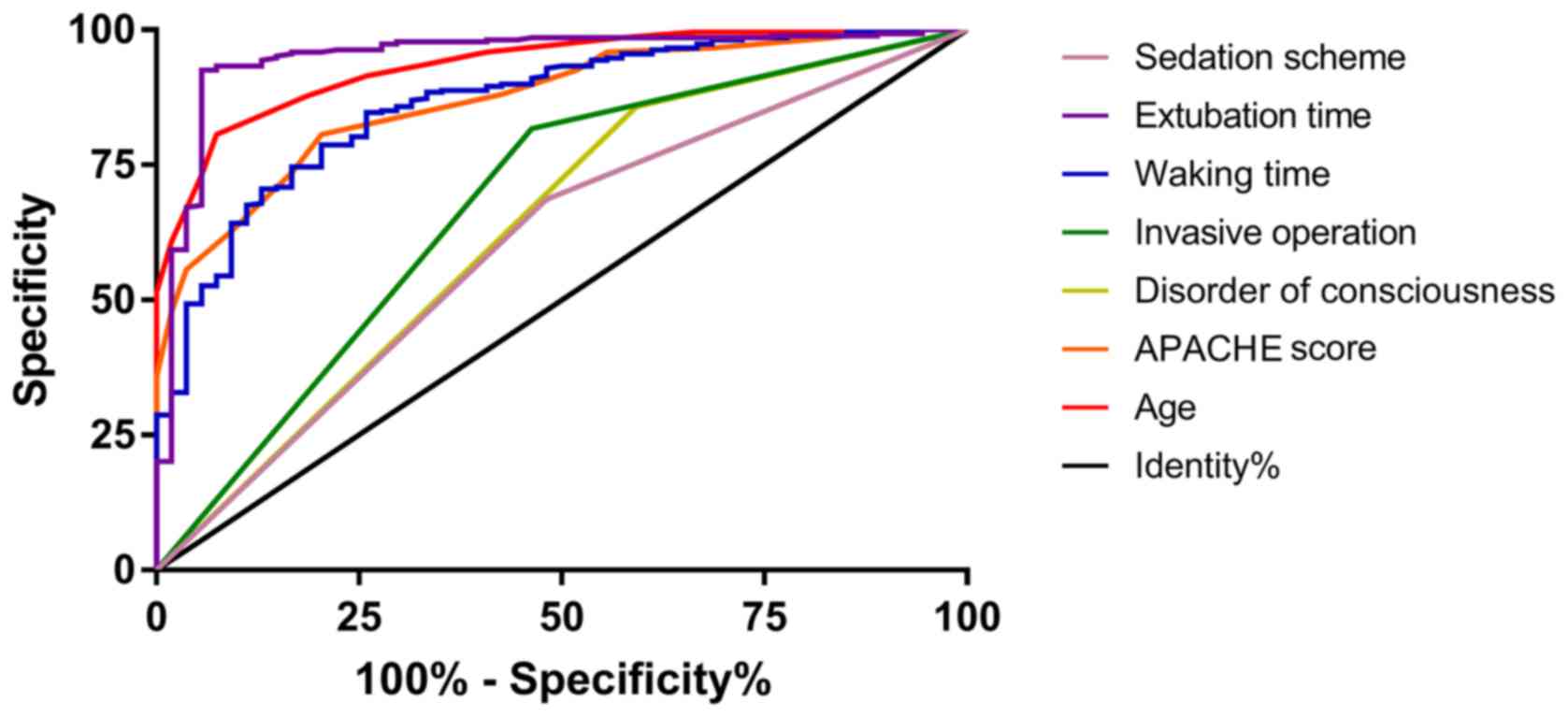
for VAP in ICU patients undergoing mechanical ventilation (Table VII). Subsequently, ROC curves were
plotted for the indicators of significant difference. The results
demonstrated that the area under the curve of age, APACHE II score,
consciousness disorders, invasive operations, waking time,
extubation time and sedation regimens were 0.934, 0.870, 0.632,
0.677, 0.865, 0.950 and 0.603, respectively (Fig. 1 and Table VIII).
 | Table VILogistic regression factor assignment
table. |
Table VI
Logistic regression factor assignment
table.
| Factor | n |
|---|
| Age (X) | <50 years old=1,
51-55 years old=2, 56-60 years old=3, >61 years old=4 |
| APACHE scores
(X) | Using original data
as it belongs to continuous variable |
| Conscious
disturbance (X) | Yes=1, No=2 |
| Invasive
operation | Yes=1, No=2 |
| Onset time (X) | Using original data
as it belongs to continuous variable |
| Extubation time
(X) | The average
extubation time is 5.85; ≤5.85=1, >5.85=2 |
| Sedation scheme
(X) | Monotherapy=1,
combined therapy=2 |
| VAP situation
(Y) | VAP patient=1,
non-VAP patient=2 |
 | Table VIIMultivariate logistic regression
analysis. |
Table VII
Multivariate logistic regression
analysis.
| Factor | β | SEM | Wals | P-value | Exp (β) | 95% CI of Exp
(β) |
|---|
| Low limit | Upper limit |
|---|
| Age | -2.172 | 0.571 | 14.459 | <0.001 | 0.114 | 0.037 | 0.349 |
| APACHE II
scores | -0.449 | 0.130 | 11.889 | 0.001 | 0.638 | 0.494 | 0.824 |
| Disorder of
consciousness | 2.630 | 1.066 | 6.090 | 0.014 | 13.870 | 1.718 | 111.973 |
| Invasive
operation | 2.950 | 1.003 | 8.655 | 0.003 | 19.099 | 2.677 | 136.289 |
| Waking time | -0.302 | 0.128 | 5.571 | 0.018 | 0.740 | 0.576 | 0.950 |
| Extubation
time | -5.451 | 1.540 | 12.394 | <0.001 | 0.004 | 0.000 | 0.089 |
| Sedation
scheme | 1.825 | 0.818 | 4.983 | 0.026 | 6.204 | 1.249 | 30.805 |
 | Table VIIIROC parameters. |
Table VIII
ROC parameters.
| Parameter | AUC | 95% CI | Specificity % | Sensitivity % | Youden index % | Cut-off |
|---|
| Age | 0.934 | 0.904-0.964 | 92.59 | 80.59 | 73.19 | <61.00 |
| APACHE II
scores | 0.870 | 0.826-0.915 | 79.63 | 80.59 | 60.23 | <29.00 |
| Disorder of
consciousness | 0.632 | 0.545-0.721 | 59.26 | 85.82 | 26.56 | 1 |
| Invasive
operation | 0.677 | 0.592-0.762 | 53.70 | 81.72 | 35.42 | 1 |
| Waking time | 0.865 | 0.814-0.915 | 74.07 | 84.70 | 58.78 | <16.47 |
| Extubation
time | 0.950 | 0.914-0.986 | 94.44 | 92.54 | 86.98 | <7.11 |
| Sedation
scheme | 0.603 | 0.518-0.687 | 51.85 | 68.66 | 20.51 | 1 |
Discussion
The ICU treats an array of critically ill patients
(17). Due to their serious
condition, many patients in the ICU have difficulties breathing. In
the clinic, mechanical ventilation can assist breathing to keep the
airways open, improving ventilation and oxygenation, to prevent
hypoxia and carbon dioxide accumulation (18). However, patients in the ICU often
suffer from intolerance to mechanical ventilation, as a result,
sedatives are commonly used for assisted ventilation (19). The side effects of opioid use in the
ICU include prolonged ventilation, psychiatric effects (such as
irritability or hallucination), asceticism and urinary retention,
which can reduce the patient compliance to treatment (20). According to the guidelines on
analgesia, sedation and delirium issued by the American Society of
Critical Care Medicine in 2013, patients in the ICU should try to
treated to ‘shallow sedation’, where the patient is still
responsive to language instructions, but where cognitive functions
and coordination abilities are affected, without impacting on the
breathing and circulation. Regardless of the sedation duration,
dexmedetomidine or propofol are recommended for sedation, whilst
benzodiazepines are not recommended (21).
As a common short-acting intravenous anesthetic,
propofol provides anesthesia and sedation by acting on GABA
receptors. However, propofol has clear inhibitory effects on the
circulation and respiration, leading to lower blood pressure and
slower heart rates in patients (22). As a highly selective α2 receptor
agonist, dexmedetomidine has analgesic, sedative and anti-anxiety
effects by promoting the secretion of GABA; compared with propofol,
dexmedetomidine was not observed not cause respiratory depression,
which was conducive to extubation (23). The sedative effects of
dexmedetomidine used alone or in combination with propofol were
compared in the present study. The results indicated that, although
the dose of the two drugs was lower in the combined group, the two
groups had similar onset times of anesthesia with the combined
group showing significantly shorter waking times and extubation
times compared with the monotherapy group. In studies by Xia et
al (24), the sedative effects
of propofol and dexmedetomidine alone on mechanical ventilation in
the ICU were compared. The recovery and extubation times following
dexmedetomidine treatment alone were significantly shorter than
those of propofol. In the present study, shorter recovery and
extubation times in response to the combination of dexmedetomidine
and propofol were also observed, suggesting a good sedative effect.
It can be speculated that the reason for this may be that the dose
of the two drugs is reduced compared with that of the single drug,
resulting in a shortening of the recovery time.
VAP is a common complication of mechanical
ventilation in the ICU. The incidence of VAP in ICU patients after
mechanical ventilation is high, which not only aggravates the
condition, but also significantly increases mortality rates, which
prolongs ICU residence times and increases costs (22,23). The
incidence of VAP was compared between monotherapy and combined
groups and a significantly lower incidence of VAP was observed in
the combined group compared with the monotherapy group, indicating
that the sedation impacted the occurrence of VAP after mechanical
ventilation in the ICU. However, to the best of our knowledge, no
relevant studies on different sedation regimens as independent
factors for VAP have been performed. In the present study clinical
data was collected and were patients divided into VAP and non-VAP
groups. Multivariate analysis indicated that age, APACHE II score,
the disturbance of consciousness, invasive operations, waking time,
extubation time and sedation programs were independent factors
affecting VAP after mechanical ventilation in ICU patients. With
increased patient age, body functions gradually weaken, and older
patients have more basic diseases, such as hypertension,
hyperlipidemia and diabetes, which often aggravates the condition
(25). This is likely to be the main
reason for the increased incidence of VAP after mechanical
ventilation.
APACHE II scores are important comprehensive scores
to assess the severity of the patients' condition. Higher scores
indicate a higher risk of VAP. Invasive manipulation provides a
direct channel for pathogens to enter the body. Moreover, invasive
manipulations damage the respiratory mucosa and increase VAP
incidence (11). In the different
sedation schemes, the waking and extubation times of the
monotherapy group were lower compared with the combined group. If
the patient exhibited no consciousness disturbance after awakening,
the machine was withdrawn ahead of schedule. Extubation times have
been shown to be directly related to the incidence of VAP, mainly
due to the fact tracheal insertion establishes a channel that
increases infections by pathogenic bacteria infection. The shorter
the extubation time, the lower the incidence of VAP (26).
The results of the present study indicated that a
combination of drugs can shorten the waking and extubation times of
patients with mechanical ventilation in the ICU. Multivariate
analysis also suggests that different sedation schemes are
independent risk factors for VAP. However, there are some
limitations to the present study. As a retrospective analysis,
selection bias can occur. Additionally patients treated with
propofol only anesthesia were not included in the current study and
their impact on the study findings requires assessment. Finally,
statistics were not gathered regarding the adverse reactions of
patients to the drugs. It is not clear whether the two schemes
affect the adverse reactions of patients. Further prospective
randomized controlled trials and other sedation regimens are now
required to confirm these results.
In conclusion, dexmedetomidine combined with
propofol may shorten the recovery and extubation time of patients
with mechanical ventilation in the ICU compared with
dexmedetomidine treatment alone. Different sedation schemes are
also suggested to be independent risk factors for VAP in patients
with mechanical ventilation in the ICU.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets use and/or anlayzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HD was responsible for the research and analyzed the
data;FH drafted the manuscript and analyzed the data.; WW and LL
collected cases; DW and FL collected data.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of Shanghai Fengxian District Central Hospital and all
patients signed informed consent forms.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hu ZL, Zhang ZC, Li DW, Shuai WZ and Zou
JF: People's Liberation Army. Med J Chin. 40(6)2015.
|
|
2
|
Jabaley CS, Groff RF, Sharifpour M,
Raikhelkar JK and Blum JM: Modes of mechanical ventilation vary
between hospitals and intensive care units within a university
healthcare system: A retrospective observational study. BMC Res
Notes. 11(425)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
de Jong B, Schuppers AS,
Kruisdijk-Gerritsen A, Arbouw ME, van den Oever HL and van Zanten
AR: The safety and efficacy of nicotine replacement therapy in the
intensive care unit: A randomised controlled pilot study. Ann
Intensive Care. 8:70. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sieber FE, Neufeld KJ, Gottschalk A,
Bigelow GE, Oh ES, Rosenberg PB, Mears SC, Stewart KJ, Ouanes JP,
Jaberi M, et al: Effect of depth of sedation in older patients
undergoing hip fracture repair on postoperative delirium: The
STRIDE randomized clinical trial. JAMA Surg. 153:987–995.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Aouad MT, Zeeni C, Al Nawwar R,
Siddik-Sayyid SM, Barakat HB, Elias S and Yazbeck Karam VG:
Dexmedetomidine for improved quality of emergence from general
anesthesia: A dose-finding study. Anesth Analg. 129:1504–1511.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Weerink MA, Struys MM, Hannivoort LN,
Barends CR, Absalom AR and Colin P: Clinical pharmacokinetics and
pharmacodynamics of dexmedetomidine. Clin Pharmacokinet.
56:893–913. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Su X, Meng ZT, Wu XH, Cui F, Li HL, Wang
DX, Zhu X, Zhu SN, Maze M and Ma D: Dexmedetomidine for prevention
of delirium in elderly patients after non-cardiac surgery: A
randomised, double-blind, placebo-controlled trial. Lancet.
388:1893–1902. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Qiu Q, Choi SW, Wong SS, Irwin MG and
Cheung CW: Effects of intra-operative maintenance of general
anaesthesia with propofol on postoperative pain outcomes-a
systematic review and meta-analysis. Anaesthesia. 71:1222–1233.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kreienbühl L, Elia N, Pfeil-Beun E, Walder
B and Tramèr MR: Patient-controlled versus clinician-controlled
sedation with propofol: Systematic review and meta-analysis with
trial sequential analyses. Anesth Analg. 127:873–880.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Schurink CA, Nieuwenhoven CA, Jacobs JA,
Rozenberg-Arska M, Joore HC, Buskens E, Hoepelman AI and Bonten MJ:
Clinical pulmonary infection score for ventilator-associated
pneumonia: Accuracy and inter-observer variability. Intensive Care
Med. 30:217–224. 2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Karakuzu Z, Iscimen R, Akalin H, Kelebek
Girgin N, Kahveci F and Sinirtas M: Prognostic risk factors in
ventilator-associated pneumonia. Med Sci Monit. 24:1321–1328.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
López-Aguilar J, Bassi GL, Quílez ME,
Martí JD, Ranzani OT, Xiol EA, Rigol M, Luque N, Guillamat R,
Ferrer I, et al: Hippocampal damage during mechanical ventilation
in trendelenburg position: A secondary analysis of an experimental
study on the prevention of ventilator-associated pneumonia. Shock.
52:75–82. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Louie J, Lonardo N, Mone M, Stevens V,
Deka R, Shipley W and Barton R: 1576: Does the addition of
dexmedetomidine to propofol sedation reduce duration of mechanical
ventilation? Crit Care Med. 46(772)2018. View Article : Google Scholar
|
|
14
|
Chinese Medical Association Respiratory
Diseases Infectious Group. Guidelines for the diagnosis and
treatment of acquired pneumonia and ventilator-associated pneumonia
in Chinese adult hospitals (2018 edition). Chinese Journal of
Tuberculosis and Respiratory Diseases (Zhong Hua Jie He He Hu Xi Za
Zhi). 4:255–280. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Qiu J, Wang C, Pan X, Pan L, Huang X, Xu
J, Ji X and Mao M: APACHE-II score for anti-tuberculosis tolerance
in critically ill patients: A retrospective study. BMC Infect Dis.
19(106)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rasheed AM, Amirah MF, Abdallah M, P-J P,
Issa M and Alharthy A: Ramsay sedation scale and richmond agitation
sedation scale: A cross-sectional study. Dimens Crit Care Nurs.
38:90–95. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Krinsley JS, Maurer P, Holewinski S, Hayes
R, McComsey D, Umpierrez GE and Nasraway SA: Glucose control,
diabetes status, and mortality in critically Ill patients: The
continuum from intensive care unit admission to hospital discharge.
Mayo Clin Proc. 92:1019–1029. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dres M, Dubé BP, Mayaux J, Delemazure J,
Reuter D, Brochard L, Similowski T and Demoule A: Coexistence and
impact of limb muscle and diaphragm weakness at time of liberation
from mechanical ventilation in medical intensive care unit
patients. Am J Respir Crit Care Med. 195:57–66. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Venn RM and Grounds RM: Comparison between
dexmedetomidine and propofol for sedation in the intensive care
unit: Patient and clinician perceptions. Br J Anaesth. 87:684–690.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rømsing J, Møiniche S, Mathiesen O and
Dahl JB: Reduction of opioid-related adverse events using
opioid-sparing analgesia with COX-2 inhibitors lacks documentation:
A systematic review. Acta Anaesthesiol Scand. 49:133–142.
2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Barr J, Fraser GL, Puntillo K, Ely EW,
Gélinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM, et
al: Clinical practice guidelines for the management of pain,
agitation, and delirium in adult patients in the intensive care
unit. Crit Care Med. 41:263–306. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fogarty M, Kuck K, Orr JA, Sakata D,
Brewer L, Johnson KB and Fang JC: Sa1040 a comparison of adequacy
of ventilation with a non-invasive ventilator system vs standard
O2 with a nasal cannula for colonoscopy with moderate
sedation using propofol and fentanyl. Gastrointest Endosc.
85(AB164-AB165)2017. View Article : Google Scholar
|
|
23
|
Singh G, Kaur H, Aggarwal S, Sharda G,
Singh A, Jha A and Aggarwal H: Intravenous dexmedetomidine vs
lignocaine in attenuating the hemodynamic responses during
laryngoscopy and endotracheal intubation: A randomized double blind
study. Anaesthesia Pain Intensive Care. 21:181–186. 2017.
|
|
24
|
Xia ZQ, Chen SQ, Yao X, Xie CB, Wen SH and
Liu KX: Clinical benefits of dexmedetomidine versus propofol in
adult intensive care unit patients: A meta-analysis of randomized
clinical trials. J Surg Res. 185:833–843. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mulugeta W, Xue H, Glick M, Min J, Noe MF
and Wang Y: Disease burdens and risk factors for diabetes,
hypertension, and hyperlipidemia among refugees in Buffalo, New
York, 2004-2014. J Health Care Poor Underserved. 30:1119–1131.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Isik A, Karavas E and Firat D: Spontaneous
milk fistula from an axillary accessory breast. Breast J.
25(154)2019. View Article : Google Scholar
|