Introduction
Diabetic nephropathy (DN) is a serious and harmful
chronic complication caused by diabetes and it is a common cause of
end-stage renal disease (1).
Diabetes can cause glomerular damage, accompanied by proteinuria
and subsequent tubulointerstitial lesions, which ultimately
culminates in end-stage renal disease (2-4). At
present, DN occurs in 20-40% of patients with diabetes (5). The development of DN takes years and
involves numerous characteristic pathological changes, such as the
excessive accumulation of the extracellular matrix, glomerular
sclerosis, tubular dilatation and atrophy, and interstitial
fibrosis (6). A major pathological
feature of DN is the abnormal apoptosis of podocytes. In a previous
study, it was identified that the apoptosis of podocytes was
associated with the decreased expression levels of multiple
proteins, including podocin, nephrin and Slit-associated proteins
(7), which resulted in considerable
proteinuria in DN (8). Therefore, it
is important to prevent podocyte apoptosis and cure DN at an early
stage.
Baicalin (BA; 5,6,7-trihydroxyflavone), a herbal
medicine found in the Chinese Pharmacopoeia, is one of the main
flavonoids purified from the roots of Astragalus membranaceus
(9). It has been reported that BA
exhibits significant anticancer effects in a variety of malignant
tumors, including colorectal (10),
breast (11) and lung cancer
(12), where it served an important
role in regulating cell growth. In addition, in a previous study,
it was identified that BA treatment improved the renal function of
patients with DN and delayed the progression of DN through both
anti-inflammatory and antioxidation mechanisms (13). However, to the best of our knowledge,
there are currently no existing studies on the effect of BA on high
glucose (HG)-induced podocyte apoptosis.
Sirtuin 1 (SIRT1) is an important nicotinamide
adenine dinucleotide-dependent nuclear histone deacetylase that has
been identified to serve a crucial role in the vascular system
through regulating cell proliferation and the cell cycle (14). It was previously demonstrated that
SIRT1 served an important role in the pathogenesis of kidney
disease (15). In fact, the
activation of SIRT1 has been suggested to be a novel target for the
treatment of patients with chronic kidney disease, including DN
(16).
NF-κB, one of the most important transcription
factors in macrophages, extensively regulates cell proliferation,
apoptosis and inflammatory cytokine expression (17), and it is also identified to be
activated in diabetic conditions (18). The activated and phosphorylated form
of NF-κB subunits (p65 and p50) translocate to the nucleus, which
indicates the activation of the NF-κB signaling pathway (19). Numerous reports have revealed that
hyperglycemia-induced DN and the associated inflammation is induced
by NF-κB; the majority of proinflammatory cytokines are regulated
by NF-κB transcription (20-22).
Notably, NF-κB was identified to be involved in regulating
apoptosis in podocytes (21,22).
The present study aimed to investigate the effects
of BA treatment on podocytes in vitro and to determine
whether BA protected against DN through inhibiting HG-induced
podocyte apoptosis. Furthermore, the underlying molecular
mechanisms were analyzed to reveal the potential role and value of
BA in DN. The results of the present study provided a theoretical
basis, as well as treatment strategies for the treatment of DN.
Materials and methods
Cell culture and HG-induced podocyte
injury model
The conditionally immortalized mouse podocyte MPC-5
cell line (cat no. C1389) were obtained from Shanghai Guandao
Biological Engineering Co., Ltd. Cells were cultured in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc.), supplemented with
10% FBS (Invitrogen; Thermo Fisher Scientific, Inc.) and 1% (v/v)
penicillin-streptomycin (Gibco; Thermo Fisher Scientific, Inc.),
and maintained in a humidified atmosphere containing 5%
CO2 at 37̊C.
For HG-induction, following 12 h of serum
starvation, podocytes were cultured at 37̊C for 48 h under
conditions of HG (30 mM D-glucose; Sigma-Aldrich; Merck KGaA) or
normal glucose (NG; 5 mM D-glucose; Sigma-Aldrich; Merck KGaA)
(23).
MTT assay
Cell viability was analyzed using an MTT assay
(Beijing Solarbio Science & Technology Co., Ltd.) according to
the manufacturer's protocol. For MTT detection, after 12 h of serum
starvation, podocytes were cultured for 48 h under conditions of HG
(30 mM D-glucose) or NG (5 mM D-glucose). Podocytes
(5x104 per well) were seeded into 96-well plates and
treated with 6.25, 12.5 or 25 µM BA (Sigma-Aldrich; Merck KGaA) at
37̊C for 24 h. Following the treatment, 20 µl MTT reagent was added
into each well and incubated for 4 h at 37̊C. Subsequently, 150 µl
DMSO was added into each well and shaken at 37̊C for 15 min.
Finally, the optical density values were measured at 490 nm using a
microplate reader.
Flow cytometric analysis of
apoptosis
Following the treatment of podocytes with 6.25, 12.5
or 25 µM BA, apoptosis was analyzed using flow cytometry. Briefly,
the podocytes were digested from 6-well plates using trypsin,
resuspended in fresh medium and centrifuged at 500 x g for 5 min at
room temperature. The supernatant was discarded and the pellet was
resuspended in 1X binding buffer. The cells were subsequently
stained with Annexin V-FITC solution and PI at 4̊C for 15 min using
the Annexin V-FITC Apoptosis Detection kit (Sigma-Aldrich; Merck
KGaA) according to the manufacturer's protocol. Apoptotic cells
were analyzed using a flow cytometer and the flow cytometry data
were analyzed by FlowJo software (version 7.6.1; FlowJo LLC) to
determine the percentage of apoptotic cells.
Western blotting
Following the treatment of podocytes with 6.25, 12.5
or 25 µM BA, total protein was extracted from the conditionally
immortalized mouse podocytes using RIPA lysis buffer (Beyotime
Institute of Biotechnology). Total protein was quantified using a
bicinchoninic acid assay kit (Beyotime Institute of Biotechnology)
and 30 µg protein/lane was separated by 10% SDS-PAGE. The separated
proteins were subsequently transferred onto a PVDF membrane (EMD
Millipore) and blocked at room temperature with 5% fat-free
powdered milk dissolved in tris-buffered saline (TBS) containing
0.1% Tween for 1.5 h. The membrane was incubated with the following
primary antibodies at 4̊C overnight: Anti-SIRT1 (1:1,000; Cell
Signaling Technology, Inc.), anti-cleaved caspase-3 (1:1,000; Santa
Cruz Biotechnology, Inc.), anti-pro-caspase-3 (1:1,000; Santa Cruz
Biotechnology, Inc.), anti-GAPDH (1:5,000; Cell Signaling
Technology, Inc.), anti-p65 (1:1,000; Abcam) and
anti-phosphorylated (p)-p65 (1:1,000; Abcam). Following the primary
antibody incubation, the membranes were incubated with a
horseradish peroxidase-conjugated secondary antibody (cat no.
ab7090; 1:2,000; Abcam) at room temperature for 2 h. The protein
bands were visualized using an enhanced chemiluminescence reagent
(EMD Millipore) and GAPDH served as a loading control for
normalization.
Reverse transcription-quantitative PCR
(RT-qPCR)
Following the treatment of podocytes with 6.25, 12.5
or 25 µM BA, total RNA was extracted from podocytes using
TRIzol® reagent on ice (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Following the RNA extraction, the concentration of each sample was
measured using an ultraviolet spectrophotometer. Total RNA was
reverse transcribed into cDNA using a HiScipt II 1st Strand cDNA
Synthesis Kit (Vazyme Biotech Co., Ltd.), according to the
manufacturer's protocol. The following RT temperature protocol was
used: 70̊C for 5 min, 37̊C for 5 min and 42̊C for 60 min. qPCR was
subsequently performed using a HiScript II One Step qRT-PCR SYBR
Green kit (Vazyme Biotech Co., Ltd.) according to the
manufacturer's protocol. The following thermocycling conditions
were used for the qPCR: 95̊C for 3 min; followed by 40 cycles of
95̊C for 30 sec, 56̊C for 30 sec and 72̊C for 30 sec. GAPDH served
as the internal loading control for normalization. Primer sequences
for PCR were listed as following: SIRT1 forward,
5'-AATCCAGTCATTAAAGGTCTACAA-3' and reverse,
5'-TAGGACCATTACTGCCAGAGG-3'; p65 forward, 5'-CATGCGCTTCCGCTACAAG-3'
and reverse, 5'-GGTCCCGCTTCTTTACACAC-3' and GAPDH forward,
5'-CCATGGGGAAGGTGAAGGTC-3' and reverse,
5'-GAAGGGGTCATTGATGGCAAC-3'. Data were quantified using the
2-∆∆Cq method (24).
Statistical analysis
Experiments were repeated three times. Statistical
analysis was performed using SPSS 22.0 software (IBM Corp.) and
data are presented as the mean ± SD. Statistical differences
between groups were determined using one-way ANOVA with a
Bonferroni post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of BA on podocyte viability
and apoptosis
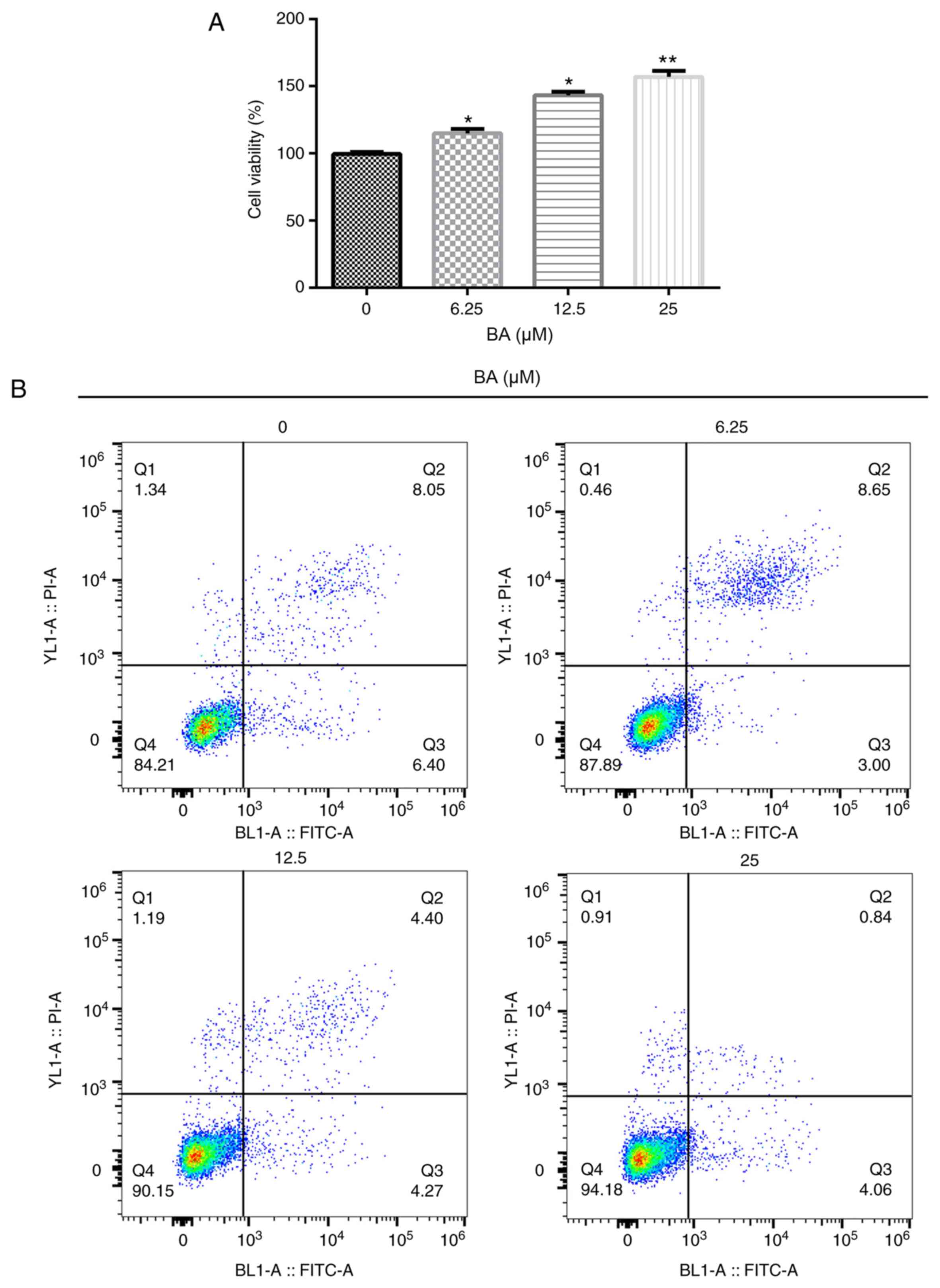
To investigate the effects of BA, an MTT assay and
flow cytometry were performed to determine the cell viability and
rate of apoptosis, respectively. Compared with the untreated group,
BA significantly increased the viability of podocytes in a
dose-dependent manner (Fig. 1A). In
addition, compared with the untreated group, BA significantly
inhibited cell apoptosis in a dose-dependent manner (Fig. 1B and C). The results from the western blotting
also revealed that BA treatment reduced the
cleaved-caspase-3/pro-caspase-3 ratio compared with that in the
untreated cells (Fig. 1D and
E). Taken together, these results
suggested that BA treatment may increase cell viability and
decrease the rate of apoptosis.
Effects of BA on the HG-induced
podocyte injury model
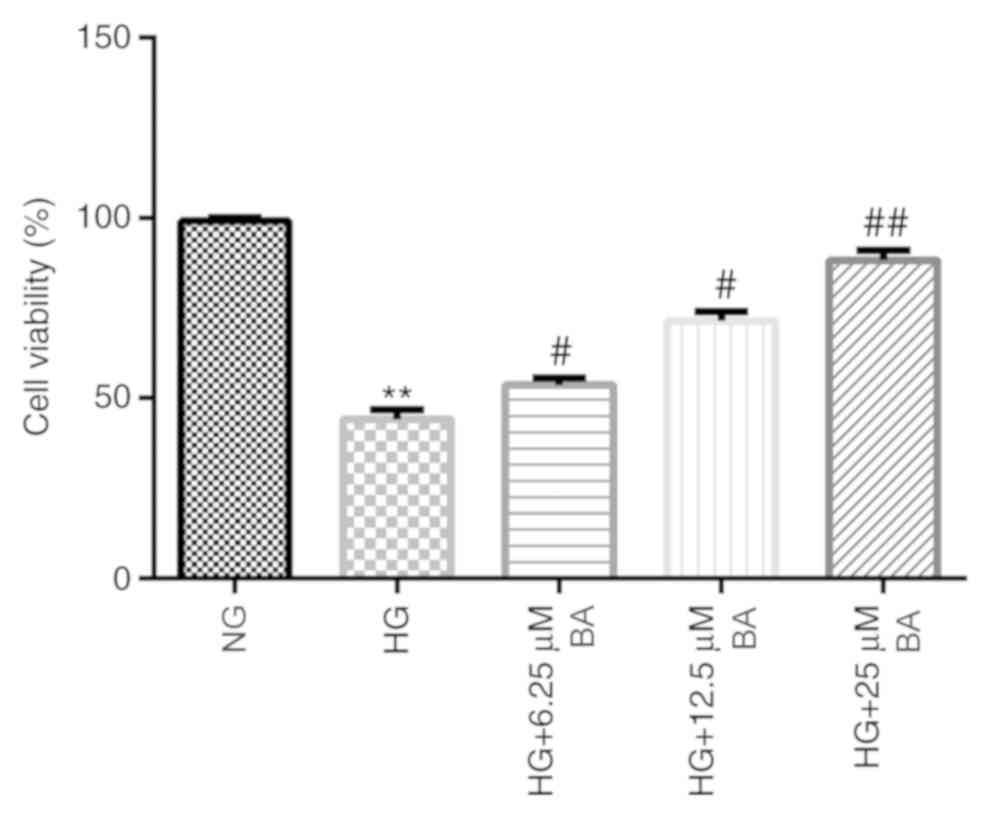
To investigate the effects of BA on the HG-induced
podocyte injury model, podocytes were cultured for 48 h under
conditions of HG (30 mM D-glucose) or NG (5 mM D-glucose) following
serum starvation for 12 h. Then, the cells were treated with
different concentrations of BA for 24 h. The MTT assay demonstrated
that HG stimulation significantly decreased the viability of
podocyte cells, whereas the treatment of HG-induced podocytes with
BA significantly weakened the effects of HG in a dose-dependent
manner; a significantly increased cell viability was observed in
the HG + BA-treated groups compared with the HG group (Fig. 2).
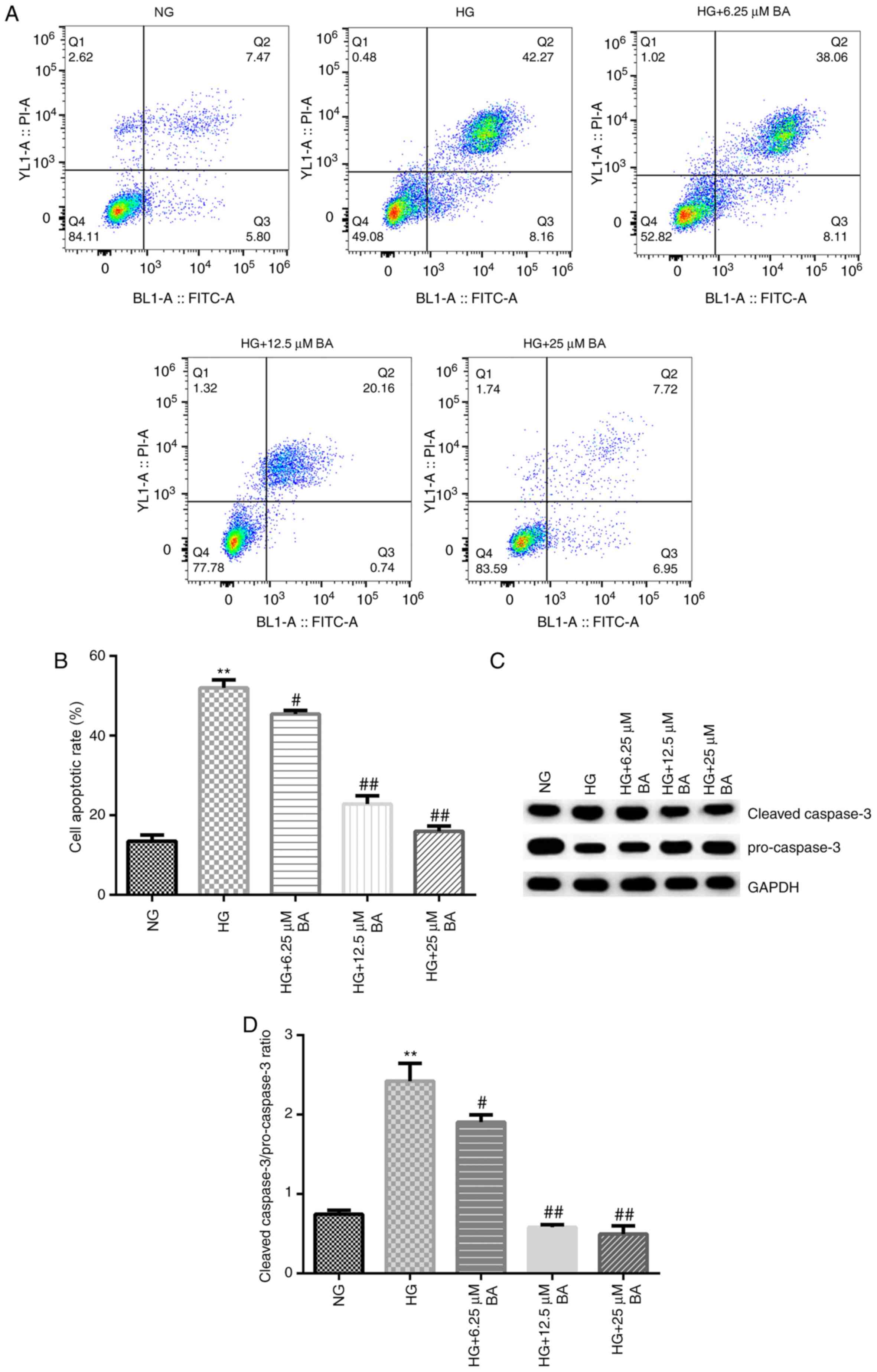
Flow cytometry and western blotting were
subsequently performed to determine the rate of apoptosis and the
expression levels of apoptosis-associated proteins, respectively.
These results indicated that compared with the NG group, HG
stimulation significantly increased the apoptotic rate of podocytes
(Fig. 3A and B) and enhanced the
cleaved-caspase-3/pro-caspase-3 ratio (Fig. 3C and D). Moreover, compared with that in the HG
group, BA treatment significantly decreased the rate of apoptosis
in podocytes in a dose-dependent manner and the reduced
cleaved-caspase-3/pro-caspase-3 ratio (Fig. 3C and D).
Effects of BA on the expression levels
of SIRT1 and p-p65 in HG-induced podocytes
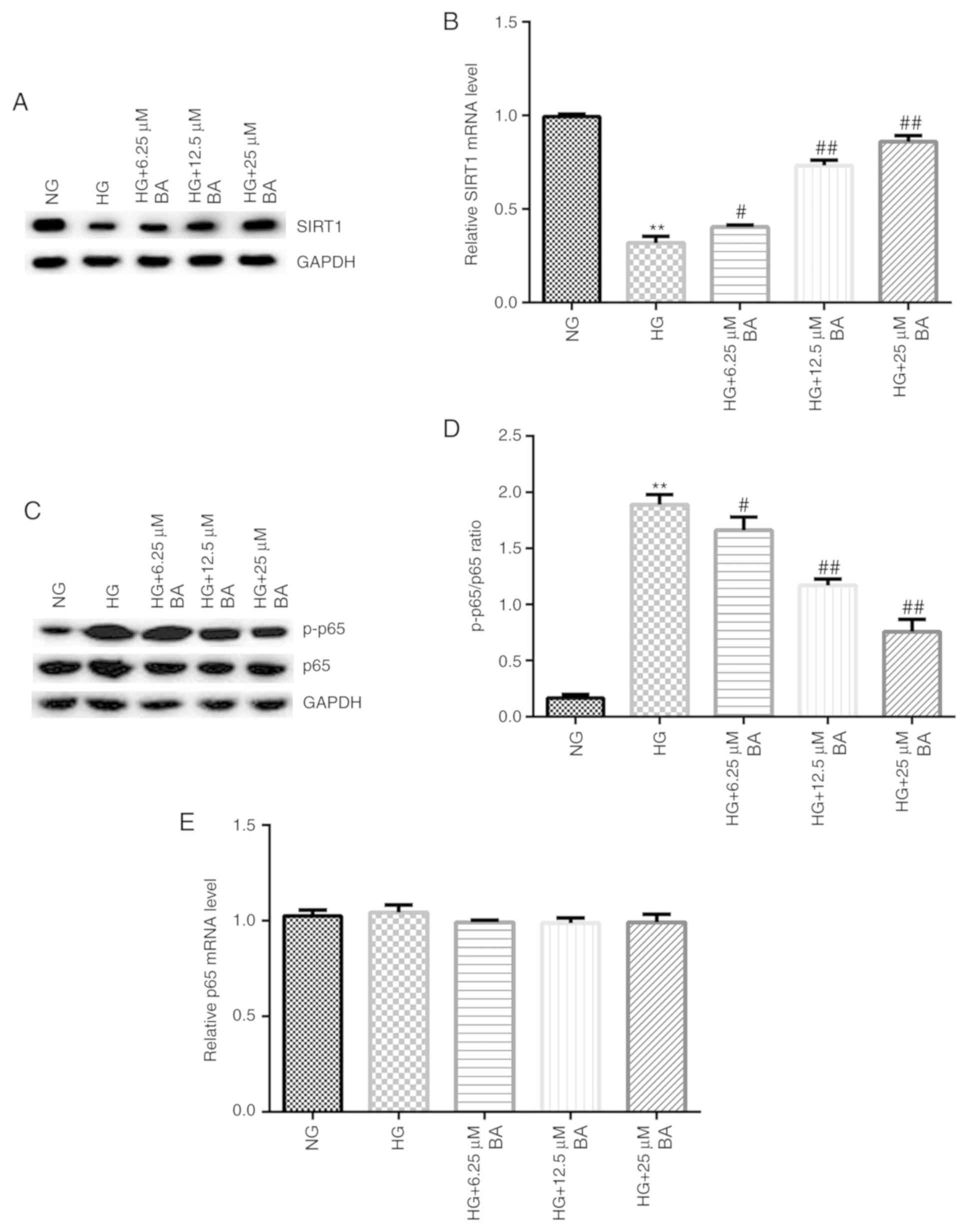
RT-qPCR and western blotting assays were performed
to detect the expression of SIRT1 and p-p65 mRNA and protein,
respectively. Compared with the NG group, HG-induced podocytes
exhibited decreased SIRT1 protein expression levels (Fig. 4A) and significantly decreased SIRT1
mRNA expression levels (Fig. 4B).
Moreover, compared with the results in the control group, HG
stimulation increased p-p65 protein expression levels (Fig. 4C) and the p-p65/p65 ratio (Fig. 4D). Notably, BA treatment increased
the expression levels of SIRT1 protein and mRNA in a dose-dependent
manner (Fig. 4A and B), whilst decreasing p-p65 protein
expression levels (Fig. 4C) and the
ratio between p-p65/p65 compared with the HG group (Fig. 4D). There were no significant
differences observed in the expression levels of p65 at the protein
or mRNA level between the groups (Fig.
4C and E).
Discussion
At present, the main risk of diabetes is the
occurrence of chronic complications, for example, high levels of
blood glucose can lead to damage in the retina, nerves and kidneys
(25). Therefore, it is important to
prevent the occurrence of such complications. DN is the most
important chronic complication of patients with diabetes and it
demonstrates a complex pathogenesis, including the involvement of
genetic factors and the activation of polyol signaling pathways,
the inflammatory response and the oxidative stress response
(1).
BA, a natural molecule, is a major bioactive
flavonoid (26). Multiple studies
have demonstrated that BA serves a variety of biological functions,
including antibacterial (27),
antiviral (28-30),
antitumor (31) and
anti-inflammatory effects (32,33). For
example, Lu et al (34)
identified that BA promoted osteoclast maturation and function
through the MAPK/microphthalmia-associated transcription factor
signaling pathway; Zheng et al (35) suggested that BA may serve as a
potential therapeutic against leukemia; and Ju et al
(36) revealed that BA protected
against thrombin-induced cell damage by inhibiting the expression
of proteinase-activated receptor 1 and NF-κB activation. However,
the role of BA in DN remains relatively unclear. Therefore, the
present study investigated the effects of BA on HG-induced
podocytes. The results demonstrated that BA significantly improved
podocyte viability and decreased the rate of cell apoptosis.
Following the establishment of a HG-induced podocyte injury model,
it was also revealed that BA significantly increased the viability
of HG-induced podocyte cells and decreased the rate of cell
apoptosis.
Previous studies have reported that SIRTs were
highly expressed and activated in the kidney, liver, spleen, lung,
heart and muscle (37,38). In addition, SIRT was identified to be
heterogeneous, serving different physiological and pathological
roles in different cells and tissues under certain stress
conditions (39). SIRT1 is the most
studied member of the SIRT family, which is most likely due to its
ability to regulate the cell cycle, mitochondrial metabolism,
energy homeostasis, inflammation, oxidative stress and apoptosis
(38). SIRT1 was found to serve a
crucial role in systemic metabolic homeostasis; the downregulation
of SIRT1 expression in visceral adipose tissue led to metabolic
abnormalities associated with visceral obesity in diabetic and
obese women (40). In the present
study, BA treatment increased SIRT1 expression levels in a
dose-dependent manner in HG-induced podocytes. In addition, BA
treatment decreased the p-p65/p65 ratio, whilst there were no
significant differences observed in the expression levels of total
p65 at both the protein and mRNA level between BA-treated cells and
the control cells. Thus, these data indicated that BA may inhibit
the HG-induced activation of the NF-κB signaling pathway. Previous
studies have demonstrated that the high expression levels of SIRT1
decreased the acetylation of NF-κB and decreased the side effects
of cisplatin on renal tubular toxicity (41). Taken together, it was suggested that
BA may suppress HG-induced podocyte apoptosis in DN. However, it is
worth noting that in the present study, the apoptotic rate of the
NG group was >10%, which may just represent the normal rate of
programmed cell death in podocytes or it may be due to the cell
culture environment; thus, further confirmation is required.
Another interesting result was that in contrast with our study, a
previous study reported that BA induced apoptosis in cancer cells
(10-12).
In conclusion, the results of the present study
suggested that BA treatment may inhibit podocyte apoptosis in a HG
environment and serve a protective role in DN. In addition, the
mechanism of action of BA may be associated with its regulation
over SIRT1/NF-κB signaling pathway activation. However, this study
is only a preliminary in vitro study of the role of BA in
DN. To further validate the role of BA in DN, more in-depth studies
are required; for example, determining how BA affects the
SIRT1/NF-κB signaling pathway. In addition, the effect of BA in DN
needs to be verified in vivo, which is our aim in future
studies.
Acknowledgements
Not applicable.
Funding
The present study was partly supported by Research
Foundation of Taizhou People's Hospital (grant no. ZL201951).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL designed the study, collected the data, performed
the statistical analysis, interpreted the data and prepared the
manuscript. YL, SYi, SYa and MK contributed to the data collection
and helped perform the statistical analysis. ZL contributed to the
data collection, statistical analysis and helped prepare the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yang M, Kan L, Wu L, Zhu Y and Wang Q:
Effect of baicalin on renal function in patients with diabetic
nephropathy and its therapeutic mechanism. Exp Ther Med.
17:2071–2076. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Akhtar M, Taha NM, Nauman A, Mujeeb IB and
Al-Nabet ADMH: Diabetic kidney disease: Past and present. Adv Anat
Pathol. 27:87–97. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fu J, Lee K, Chuang PY, Liu Z and He JC:
Glomerular endothelial cell injury and cross talk in diabetic
kidney disease. Am J Physiol Renal Physiol. 308:F287–F297.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nath KA: Tubulointerstitial changes as a
major determinant in the progression of renal damage. Am J Kidney
Dis. 20:1–17. 1992.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kowalski A, Krikorian A and Lerma EV:
Diabetic nephropathy for the primary care provider: New
understandings on early detection and treatment. Ochsner J.
14:369–379. 2014.PubMed/NCBI
|
|
6
|
Papadopoulou-Marketou N, Kanaka-Gantenbein
C, Marketos N, Chrousos GP and Papassotiriou I: Biomarkers of
diabetic nephropathy: A 2017 update. Crit Rev Clin Lab Sci.
54:326–342. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ying Q and Wu G: Molecular mechanisms
involved in podocyte EMT and concomitant diabetic kidney diseases:
An update. Ren Fail. 39:474–483. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chen J, Chen JK and Harris RC: EGF
receptor deletion in podocytes attenuates diabetic nephropathy. J
Am Soc Nephrol. 26:1115–1125. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Luo J, Dong B, Wang K, Cai S, Liu T, Cheng
X, Lei D and Chen Y, Li Y, Kong J and Chen Y: Baicalin inhibits
biofilm formation, attenuates the quorum sensing-controlled
virulence and enhances Pseudomonas aeruginosa clearance in a mouse
peritoneal implant infection model. PLoS One.
12(e0176883)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jia Y, Chen L, Guo S and Li Y: Baicalin
induced colon cancer cells apoptosis through miR-217/DKK1-mediated
inhibition of wnt signaling pathway. Mol Biol Rep. 46:1693–1700.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang XF, Zhou QM, Du J, Zhang H, Lu YY and
Su SB: Baicalin suppresses migration, invasion and metastasis of
breast cancer via p38MAPK signaling pathway. Anticancer Agents Med
Chem. 13:923–931. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cao HJ, Yan YB, Dai JY, et al: Effect of
baicalin on human lung cancer A549 cell line. Chin J Exp Tradit Med
Formul. 19:216–220. 2013.(In Chinese).
|
|
13
|
Yi QQ, Xia YY, Chen J, et al: Baicalin
improves diabetic nephropathy in mice by activating Sirt1/Nrf2
signal through miR-141 inhibition. Med J Wuhan Uni. 40:186–191.
2019.(In Chinese).
|
|
14
|
Sun QR, Zhang X and Fang K: Phenotype of
vascular smooth muscle cells (VSMCs) is regulated by miR-29b by
targeting sirtuin 1. Med Sci Monit. 24:6599–6607. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Morigi M, Perico L and Benigni A: Sirtuins
in renal health and disease. J Am Soc Nephrol. 29:1799–1809.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kitada M, Kume S, Takeda-Watanabe A,
Kanasaki K and Koya D: Sirtuins and renal diseases: Relationship
with aging and diabetic nephropathy. Clin Sci (Lond). 124:153–164.
2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shen HM and Tergaonkar V: NF-κB signaling
in carcinogenesis and as a potential molecular target for cancer
therapy. Apoptosis. 14:348–363. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Deng X, Sun L, Lai X, Xiang L, Li Q, Zhang
W, Zhang L and Sun S: Tea polypeptide ameliorates diabetic
nephropathy through RAGE and NF-κB signaling pathway in type 2
diabetes mice. J Agric Food Chem. 66:11957–11967. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu T, Zhang L, Joo D and Sun SC: NF-κB
signaling in inflammation. Signal Transduct Target Ther.
2(17023)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kuhad A and Chopra K: Attenuation of
diabetic nephropathy by tocotrienol: Involvement of NF-kB signaling
pathway. Life Sci. 84:296–301. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhu L, Han J, Yuan R, Xue L and Pang W:
Berberine ameliorates diabetic nephropathy by inhibiting TLR4/NF-κB
pathway. Biol Res. 51(9)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xu L, Zhang P, Guan H, Huang Z, He X, Wan
X, Xiao H and Li Y: Vitamin D and its receptor regulate
lipopolysaccharide-induced transforming growth factor-β,
angiotensinogen expression and podocytes apoptosis through the
nuclear factor-κB pathway. J Diabetes Investig. 7:680–688.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang SZ, Qiu XJ, Dong SS, Zhou LN, Zhu Y,
Wang MD and Jin LW: MicroRNA-770-5p is involved in the development
of diabetic nephropathy through regulating podocyte apoptosis by
targeting TP53 regulated inhibitor of apoptosis 1. Eur Rev Med
Pharmacol Sci. 23:1248–1256. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kawada T: Risk factors for developing
prediabetes. Diabetes Res Clin Pract. 135(232)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Peng XD, Dai LL, Huang CQ, He CM and Chen
LJ: Correlation between anti-fibrotic effect of baicalin and serum
cytokines in rat hepatic fibrosis. World J Gastroenterol.
15:4720–4725. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Roy MK, Nakahara K, Na TV, Trakoontivakorn
G, Takenaka M, Isobe S and Tsushida T: Baicalein, a flavonoid
extracted from a methanolic extract of oroxylum indicum inhibits
proliferation of a cancer cell line in vitro via induction of
apoptosis. Pharmazie. 62:149–153. 2007.PubMed/NCBI
|
|
28
|
Li HY, Hu J, Zhao S, Yuan ZY, Wan HJ, Lei
F, Ding Y, Xing DM and Du LJ: Comparative study of the effect of
baicalin and its natural analogs on neurons with oxygen and glucose
deprivation involving innate immune reaction of TLR2/TNF-α. J
Biomed Biotechnol. 2012(267890)2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Moghaddam E, Teoh BT, Sam SS, Lani R,
Hassandarvish P, Chik Z, Yueh A, Abubakar S and Zandi K: Baicalin,
a metabolite of baicalein with antiviral activity against dengue
virus. Sci Rep. 4(5452)2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Nayak MK, Agrawal AS, Bose S, Naskar S,
Bhowmick R, Chakrabarti S, Sarkar S and Chawla-Sarkar M: Antiviral
activity of baicalin against influenza virus H1N1-pdm09 is due to
modulation of NS1-mediated cellular innate immune responses. J
Antimicrob Chemoth. 69:1298–1310. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen J, Li Z, Chen AY, Ye X, Luo H, Rankin
GO and Chen YC: Inhibitory effect of baicalin and baicalein on
ovarian cancer cells. Int J Mol Sci. 14:6012–6025. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Novy P, Urban J, Leuner O, Vadlejch J and
Kokoska L: In vitro synergistic effects of baicalin with
oxytetracycline and tetracycline against Staphylococcus aureus. J
Antimicrob Chemoth. 66:1298–1300. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lee W, Ku SK and Bae JS: Anti-Inflammatory
effects of baicalin, baicalein, and wogonin in vitro and in vivo.
Inflammation. 38:110–125. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lu L, Rao L, Jia H, Chen J, Lu X, Yang G,
Li Q, Lee KKH and Yang L: Baicalin positively regulates osteoclast
function by activating MAPK/Mitf signaling. J Cell Mol Med.
21:1361–1372. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zheng J, Asakawa T, Chen Y, Zheng Z, Chen
B, Lin M, Liu T and Hu J: Synergistic effect of baicalin and
adriamycin in resistant HL-60/ADM leukaemia cells. Cell Physiol
Biochem. 43:419–430. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ju XN, Mu WN, Liu YT, Wang MH, Kong F, Sun
C and Zhou QB: Baicalin protects against thrombin induced cell
injury in SH-SY5Y cells. Int J Clin Exp Pathol. 8:14021–14027.
2015.PubMed/NCBI
|
|
37
|
Morigi M, Perico L and Benigni A: Sirtuins
in renal health and disease. J Am Soc Nephrol. 29:1799–1809.
2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chang HC and Guarente L: SIRT1 and other
sirtuins in metabolism. Trends Endocrinol Metab. 25:138–145.
2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Dai H, Sinclair DA, Ellis JL and Steegborn
C: Sirtuin activators and inhibitors: Promises, achievements, and
challenges. Pharmacol Ther. 188:140–154. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bible E: Diabetic nephropathy: Sirt1
attenuates diabetic albuminuria. Nat Rev Nephrol.
9(696)2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sakao Y, Kato A, Tsuji T, Yasuda H, Togawa
A, Fujigaki Y, Kahyo T, Setou M and Hishida A: Cisplatin induces
Sirt1 in association with histone deacetylation and increased
Werner syndrome protein in the kidney. Clin Exp Nephrol.
15:363–372. 2011.PubMed/NCBI View Article : Google Scholar
|