1. Introduction
Lysyl oxidase proteins (LOXs) are secretory amine
oxidases that aid in the formation of the extracellular matrix
(ECM) in a copper-dependent manner (1). The LOX precursor (50 kDa), secreted
mainly by smooth muscle cells and fibroblasts, is hydrolyzed into
the catalytically active, matured form of LOX (30 kDa) and the
non-catalytic active peptide (18 kDa) (2,3). The
extracellular roles of activated LOX include promoting the
crosslinking between ECM collagen type I, collagen type III and
elastin, through catalyzing the lysine residue, which subsequently
transforms collagens and elastin into a non-soluble state (1).
Since their initial discovery, the role of LOXs in
collagen and elastin crosslinking has been confirmed by numerous
studies (1,2,4,5), and additional LOX-like proteins
(LOXLs)1-4 were subsequently discovered. LOXL1, LOXL2, LOXL3 and
LOXL4 have been demonstrated to share 85, 58, 65 or 62% sequence
similarity with the LOX protein in the conserved regions and,
altogether, these enzymatic proteins form the LOX family (6).
The regulation of LOXs varies depending on the
tissue and on the developmental stage of a specific organ; these
enzymes have been found to serve a role in oncogenesis or tumor
metastasis through dysregulating tumor microenvironment homeostasis
and have also been suggested to be biomarkers for cancer prognosis
and survival (7). In other studies,
LOXs were found to be important in regulating ECM metabolism, which
promoted alterations in the tumor tissue tensile strength;
consequently, the ECM properties may influence embryogenesis and
organ development substantially (5,7,8). Tumorigenesis and embryogenesis involve
similar cellular morphological changes, migrations and adjustments
in the mechanical stiffness and structural integrity of the tissues
(8,9). Previous studies have reported
contradictory effects of LOXs on the phases of tumor
differentiation (5). These
contradictions may indicate the spaciotemporal specificities for
LOXs response and regulations, which suggest that the response of
LOXs may vary at various stages of organ development. A similar
phenomenon was observed during embryogenesis, which suggested that
LOXs may serve a significant role in the process of ECM structural
stabilization and organ development (10,11).
In addition, aberrant ECM metabolism has been
identified in several different diseases, including
atherosclerosis, liver cirrhosis, aneurysms and Menkes syndrome,
and the effects of LOXs were observed to be increased during
organogenesis and development (5).
ECM remodeling does not only form the foundations for stromal and
interstitial stability, it also ensures the proper cellular
anchorage to facilitate stabilized cell-cell adhesions, which is
essential for maintaining functional stromal polarity (12). In this process, numerous proteins
involved in ECM remodeling, including the LOXs, are required to be
correctly functioning in the phases of organ development.
2. Roles of members of the LOX family in
organ development
The ECM is crucial in regulating extracellular
functions. Not only does it maintain tissue mechanical rigidity and
stiffness, it is also responsible for manipulating cellular
functions and regulating cell migrations (8,13).
Amongst the hundreds of components identified in the ECM, fibrous
proteins and proteoglycans are the two main constituents (14,15). As
these proteins are mainly fibrous in form, the correct crosslinks
between these macromolecules are essential for proper tissue
development (6,15-18).
Furthermore, ECM metabolic processes and remodeling contribute to
the complex extracellular mesh, which relies heavily on the
catalyzing function of LOXs (19).
During embryogenesis and organ development, LOXs
promote infrastructural integration, in addition to facilitating
the proper cell stiffness and rigidity required for organogenesis
and subsequent morphological maintenance (17,20-23).
That is, the cells can be secured and maintained properly in the
organic mesh and are able to maintain their functional polarity
(12). Owing to the enzymes being
secreted locally, the expression levels of LOXs and their
subsequent activity have been correlated with the modulation and
transformation of the cells. In this manner, cells can detect
microenvironmental changes directly or indirectly, and serve as
both participants and regulators in response to these changes
(13,24). Again, as the mechanical force is
becoming increasingly recognized in inducing organ development and
tissue remodeling (25,26), further research embryogenesis should
not be limited to biochemical factors, but to biomechanical factors
as well.
Along with tissue adjustment, cells respond to
external stress through the mediation of the ECM, which is also
under the influence of the activity of LOXs (25,27-29).
In simpler terms, LOXs have been demonstrated to function as
mediators between cells and the ECM during development. Thus, this
family of enzymes is not only responsible for the overall
architecture of cells, but also for the transduction of signals and
responses between external forces and parenchymal/stromal cells
during the processes of proliferation and remodulation.
Establishment of mechanical
strength.
The crosslinking of fibrous macromolecules is
crucial for tissue formation because the properly formed ECM
provides mechanical rigidity and stiffness, which is essential for
maintaining the structural correctness and the proper functioning
of organs (8,30,31).
With regards to the respiratory system, bronchial formation and
alveolarization are the main processes that occur during lung
development. In a previous study analyzing the expression levels of
LOX in the pulmonary parenchyma and pleural membrane during
development, it was observed that LOX expression levels in prenatal
and postnatal rabbits were increased, and subsequently reduced to
50% within 4-10 weeks (17). In the
same study, a one-side pneumonectomy was performed in hamsters to
determine LOX expression levels during the compensatory lung
growth; an instant elevation in LOX expression levels were noted,
alongside compensatory growth of the lung tissue prior to cellular
proliferation. Thus, a chronologic-specific pattern of LOX
expression levels was observed during lung growth and development.
Conversely, by inhibiting LOX activity, either through
downregulating its expression or using the enzymatic inhibitor,
β-aminopropionitrile (BAPN), the newly formed lungs are observed
with impaired bronchial morphogenesis and alveolarization (32,33).
Notably, Maki et al (20)
investigated the LOX-/- and
LOXL1-/- knockout mice, and it was discovered
that the improperly developed airways were associated with
abnormally formed elastin and collagen fiber, which resembled the
human embryo sample observed in a study by Kumarasamy et al
(28). Although the direct evidence
that abnormal embryogenesis contributes to pulmonary diseases is
lacking, the pulmonary emphysema identified alongside alveolar
enlargement and structural distortion were identified to be
correlated to ECM abnormalities and LOX downregulation in a
previous study (34). Consequently,
these findings suggested that the development of the lung
mesenchyme, bronchus and the pulmonary artery may be strongly
associated with LOX modulation and properly regulated ECM formation
may be substantial in maintaining the structural and functional
mechanical load of bronchi and alveoli for ventilation and gas
exchange.
In LOX-/- mice, it was
reported that the mice died perinatally due to aortic aneurysms,
cardiovascular dysfunction and diaphragmatic ruptures (20). This result is logical because the
structural stability of the cardiovascular system is vital, as it
endures the most constant and relative mechanical pressure compared
with other organ systems, thus any structural incompetence will
lead to fatal consequences. With regards to development, Tsuda
et al (35) discovered
increased expression levels of LOX mRNA during embryogenic
myocardium development in mice on the 11th and 13th day of
embryonic development. Similarly, Behmoaras et al (15) revealed that LOX and LOXL1 activities
were at their highest during the first 15 days of development,
which suggested that both LOXs may be required for elastin and
collagen remodeling in the aorta of rats. In addition, the
insufficient activity of both enzymes was found to render Brown
Norway rats susceptible to spontaneous artery rupture, which
further indicated the pivotal role of LOXs in ECM regulation,
especially in stabilizing collagen and elastin crosslinks (14,15).
Complementary research similarly confirmed that LOXs were observed
to reside in the aortic arch vessel, amongst other sites as
myocardial, endocardial, epicardium (35).
In more concrete organs, such as the teeth or bones,
the ECM is found to be mostly mineralized, with other constituents
namely being collagen, elastin and other fibrous proteins (18). The development of teeth has been
found to involve ECM condensation and odontogenic stabilization
(36), of which both processes can
be observed through densely packed collagen fibers and orderly
polarized picrosirius red staining. Through investigating
odontogenesis, Tjaderhane et al (22) identified no significant differences
among the teeth of LOX-/-,
LOX+/- and wild-type mice under the light
microscope; however, following histochemical examination, the teeth
of the LOX-/- and
LOX+/- mice were observed to be thinner and
unpolarized, which indicated that these effects may be due to the
dysregulation of LOX. Kim et al (37) demonstrated that both LOX and LOXLs
were essential for organizing periodontal ECM fibrogenesis and
promoting the differentiation of dental pulp cells from
odontoblasts. Accordingly, both investigations favor the
substantial effect of LOX in promoting the thickening of teeth and
matrix collagen filling during development.
Similar to bones, the differentiation and
mineralization of stromal cells and ECM are reported to be under
LOX regulation. For example, during osteoblastogenesis, a parallel
expression pattern between collagen and LOX was found in isolated
mice clavicle cells (38,39). This result suggested that LOX may
promote osteocyte stabilization and bone ECM remodeling.
Furthermore, in LOX-/- mice, Pischon et
al (40) found reduced
osteoblast differentiation and insufficient mineral
crystallization. Similarly, Turecek et al (41) inhibited LOX expression with BAPN in
cultured osteoblasts and discovered that not only were the collagen
crosslinks dysregulated, but the expression and activity of
osteoblasts were also undermined. These results further suggested
an essential role of LOX in bone matrix formation. The
collagen-based mesh weights more crucial than is instinctively
comprehend in bones and teeth development; however, the importance
of LOXs in regulating development in these organs remains
relatively unclear.
The musculoskeletal system accounts for nearly half
of the body weight (42). The main
constituent of muscles is myofibers, which are coated with muscle
connective tissue (MCT) formed from muscle ECM, and muscles and
tendons are formed from muscle fibers, fasciculi and other myogenic
progenitors (43). Resembles to the
ECM in other tissues, the homeostatic metabolism of the MCT is
crucial; not only does it provide the supporting forces that bind
the muscle fibers together, but the myofiber-MCT cross-talk is
crucial during myogenesis (43).
Kutchuk et al (44) revealed
that muscle fibers formed in LOX-/- mice were
shorter, smaller and decreased in number. Also, the more
undeveloped embryonic limbs formed in these mice exhibited a
disorganized MCT and the dysregulated deposition of collagen
fibrils (44). Thus, it was
indicated that insufficient LOX activity may underlie the deformed
growth of embryonic limbs, which may be potentially correlated with
the origin of Duchenne muscular dystrophy (23).
Attached to the skeletal muscles are the more ECM
abundant structures, such as tendons and ligaments. In chick
embryos, LOX expression levels in tendons were found to be elevated
during their development (26).
Moreover, the tendons were found with minor elastic modulus
following the application of BAPN during embryogenesis. These
results may partially explain the defective healing capability
discovered in the anterior cruciate ligament; differential LOX
expression levels and collagen crosslinking in the three ligaments
of the knee has been found to predispose different healing
capabilities (45,46).
The dynamic structural and functional changes during
the maturation of the central nervous system (CNS), namely neuronal
plasticity, is a critical process of both pre- and postnatal
development (47). The involvement
of neurogenesis, programmed cell death and ECM remodeling in
neuronal plasticity following the adaption to environmental changes
is markedly enhanced during brain development (48). In the maturation of the CNS, various
components have been identified to be involved in cerebrum and
cerebellum ECM remodeling (49). As
one of these components, the dendritic extensions of neurons have
been observed not only to determine brain function, but also are
indicative of a neuron's development and its regeneration. A
previous study identified the presence of LOXs in the cerebrum and
immunohistochemical analysis further revealed that cells in the
pyramid layer in the hippocampus of LOXL-null mice exhibited
decreased diameters (50). These
results revealed a potential essential role of LOXLs in inducing
cellular differentiation; for example, it has been discovered that
the increase in intranuclear LOX-propeptide facilitated microtubule
stability and dendritic cell development (51).
Similarly, as the genetic deficiency of vacuolar
protein sorting protein 18 (VPS18) is reported to facilitate the
lysosomal degradation of LOX, the impaired dendritogenesis in VPS18
knockout mice was suggested to be correlated with the accumulation
of LOX (52). That study revealed
that other than ECM remodeling, LOX may also be capable of
regulating organ development. Additionally, in studying amputated
mice, the white matter and gray matter in the spinal cord were both
found to be atrophied (53).
Meanwhile, the decreased myelination and downregulated ECM
regulatory factors were depressed along with LOX expression levels
in another study (53). Furthermore,
in superoxide dismutase 1-induced neurodegenerative model rats,
neurons in the developed amyotrophic lateral sclerosis were found
to express increased LOX expression levels and exhibit increased
enzyme activity (54). This evidence
suggested that, as the principle form of ECM remodulation in the
CNS, synaptic remodeling may be regulated by both LOX and neuronal
signaling transmissions. Thus, proper regulation with a spatial and
conditional specialty is fundamental for CNS development.
In a previous study, LOXs were found widely
distributed cutaneously and subcutaneously, and were discovered to
be correlated with aging (55). In
fact, LOX expression was abundant in the epidermal basal layer, the
basal keratinocytes and dermal fibroblasts, dermal vascular
endothelial cells, hair follicles, sebaceous glands, sweat glands
and hair (55,56). Cenizo et al (57) demonstrated that LOX expression levels
in skin fibroblasts in adults were decreased compared with
children, whereas, Langton et al (58) identified higher LOX activity in the
elderly compared with young people. In epidermal keratinocytes,
further research identified an important role for LOX in regulating
cellular keratinization, whereas LOXL2 was found to interact with
cell-matrix interactions (59-61).
Le Provost et al (60) have
thus suggested that finely regulated LOX expression is crucial for
maintaining epidermal homeostasis.
LOX mediates the ECM response to
environmental change.
During organogenesis, the interaction between cells
and the ECM in the adapting environment change is crucial (8). Physiological and biochemical changes in
the environment, namely hypoxia, glycation, hormonal changes and
deposition of metabolites, were discovered to trigger alterations
in ECM formation and enzyme expression (17,62-65).
These adaptations in turn required the responses of the stroma and
ECM to initiate the post-translational tissue remodeling process
(7,8,13).
Pneumonectomy and hypoxia treatment are the
conventional methods used to investigating tissue alterations
during lung organogenesis (17). As
an ECM modulating enzyme, it was observed that hypoxia triggered
cellular responses directly through increasing the expression
levels of LOX (66). Cells are known
to be sensitive to and promptly react to tissue oxygen saturation
(StO2), as the O2 in the ECM is directly
exchanged through passive diffusion extra-intracellularly (67). Low StO2 directly hinders
hypoxia induced factors (the HIF) from degrading, hence its
accumulation triggers subsequent signaling cascades in response to
hypoxia (68). In fact, low
O2 levels were ubiquitously observed to increase LOX
expression levels and mediate ECM remodeling among different
organogenesis and tissue development (29,35,66,69). In
a model of pulmonary arterial hypertension, it was demonstrated
that the vascular smooth muscle cells in the pulmonary arteries
responded to hypoxia through increased LOX expression levels and
subsequent enzymatic activity (64,70).
Furthermore, this contributed to the increased deposition of
collagen and elastin, alongside enhanced cellular proliferation
during the progression of pulmonary vascular remodeling. Further
studies have also identified the participation of other LOXLs, such
as LoxL1, LoxL2, LoxL3 and LoxL4, contributing to idiopathic
pulmonary arterial hypertension with response to hypoxia (71). Although this was observed in a
pathological model, a similar mechanism is presumably employed
during tissue development. Similarly, LOX-mediated ECM remodeling
in response to hypoxia has been reported in myocardial ischemia,
liver fibrosis and in patients with obstructive sleep apnea
(72,73). In addition, through investigating
tendon and cartilage structure, the tenocytes were found to respond
to hypoxia following increased LOX expression levels (29,74,75); as
a consequence, these tenocytes were found with enhanced expansion
capacity and proliferative potential. These results demonstrated
that monitoring LOX expression, as the direct cellular response to
hypoxia, is potentially useful as a biomarker and as a tissue
engineering target to control tissue development.
Similar to hypoxia, dysregulated glycation induced
tissue pathophysiology with altered ECM formation is a feature of
metabolic diseases (76). It is
reported that collagen crosslinking pathways involve both
non-enzymatic glycation and LOX-mediated oxidative deamination of
lysine and hydroxylysine (77).
Commonly, dysregulated glycation is a result of diabetes, which has
been indicated to induce altered enzymatic crosslinks and collagen
physicochemical properties of the ECM in multiple systems (76). In the skin, patients with diabetes
are more likely to appear aged and have a skin infection or foot
ulcers, which is accompanied by fragmented collagens and
dysregulated dermal connective tissues (78). In the retina, diabetes resulted in
structural abnormalities in the retinopathy, which featured as
thickened retinal capillary basement membranes with upregulated
levels of ECM fibroproteins (79,80).
These findings may seem contradictory. A plausible explanation for
this phenomenon is that the structural changes in the oculus tissue
were the consequence of excessive microvasculature permeability,
whereas the abnormal collagen crosslinking change was similar to
other hyperglycemia developed lesions (77). These results indicated that the
glycation that contributes to ECM formation through LOX may be
regulated at the metabolic level, as well as at the vascular and
oxidative levels. For example, high glucose levels were discovered
to directly increase the expression levels of LOX and its
subsequent enzymatic activation in the dermis or endothelial layer,
which resulted in excessive crosslinking and disruption in the
formation of collagen fibrils and hindered ECM integrity (80). Furthermore, increased expression
levels of matrix metalloproteinase (MMP)-1 and MMP-2 were
identified in glycation-induced LOX dysregulation; this result
revealed the existence of indirect regulation between LOX and
glycation (78).
Native low-density lipoprotein and alcohol have also
been reported to reduce LOX expression, collagen and elastin
crosslinking in endothelial cells and the formation of scar tissue
(65,81). Though these results were obtained
following pathogenic studies, the implication of these results in
manipulating organogenesis requires further investigations.
Similarly, in age-related macular degeneration
(AMD), pathological alterations involve choroidal
neovascularization, choroidal capillary proliferation and aberrant
basement membrane architecture (82). Upon investigating environmental
tobacco smoke-induced AMD, it was observed that the side stream
smoke may directly suppress LOX expression in choroidal endothelial
cells (83).
Thus, ECM formation and its regulation are complex
issues involving complex mechanisms in developing tissues. In those
organs or systems in which their structural or mechanical
properties mainly determine the function, the appropriate
regulation of the formation of the ECM is substantial for the
mechanical strength required for structural maintenance and tension
upholding, but also in providing the foundations for initiating
growth. Nonetheless, studies have also revealed that LOXs are able
to manipulate the transformation of the cellular phenotype in
response to environmental changes during organogenesis (35,37,38,84).
These results suggested that the role of LOXs in regulating organ
development remain to be fully determined (see also Table I).
 | Table IRole of LOX and LOXL in organ
development. |
Table I
Role of LOX and LOXL in organ
development.
| Authors, year | Tissue | Member | Role | Refs. |
|---|
| Rauch, 2004 | Cerebrum | LOX | Elevated in
superoxide dismutase 1-induced neurodegeneration | (49) |
| Li et al,
2010 | |
LOX-pro-peptide | Interfered with
NF-κB RelA signaling and microtubule stability | (51) |
| Peng et al,
2012 | | LOX | VPS18 gene
knockdown impairs dendritogenesis following the accumulation of
LOX | (52) |
| Chelyshev et
al, 2014 | Spinal cord | LOX | Decreased
expression alongside decreased myelination | (53) |
| Brody et al,
1979 | Lung | LOX | Newborn rabbit
exhibited transient increased expression levels compared with adult
rabbits, and were reduced within 4-10 weeks after birth | (17) |
| Tsuda et al,
2003 | Heart | LOX | Positively
correlated with embryogenic myocardium development | (35) |
| Hornstra et
al, 2003 | Aorta | LOX | Genetic deletion in
mice caused aneurysms and diaphragmatic rupture | (14) |
| Voloshenyuk et
al, 2011 | | LOX | Facilitated
vascular ECM hardening and remodeling | (94) |
| Tjaderhane et
al, 2013 | Tooth | LOX and LOXLs | Promoted pulp
medulla dentinal cellular differentiation, ECM augmentation and
mineral nodule formation | (22) |
| Kaku et al,
2016 | | LOX | Responded to
mechanical stress and promoted odontogenic differentiation | (36) |
| Tjaderhane et
al, 2013 | | LOX | Promoted tooth
thickening and matrix collagen filling | (22) |
| Vora et al,
2010 | Bone | LOX and LOXLs | Promoted osteoblast
differentiation and bone matrix mineralization | (39) |
| Pischon et
al, 2009 | | LOX |
β-aminopropionitrile or genetic knockout
reduced osteoblast differentiation and osteoblast deactivation | (40) |
| Vora et al,
2010 | |
LOX-pro-peptide | Inhibited
osteoblast proliferation and differentiation | (39) |
| Makris et
al, 2013 | Cartilage | LOX | Strengthened
cartilage by hypoxia induction | (29) |
| Marturano et
al, 2014 | Muscle and
tendon | LOX | Promoted collagen
fibril formation in muscle and tendons | (74) |
| Xie et al,
2012; Kato et al, 2015 | | LOX | Promote collagen
maturation in cruciate ligament | (45) (46) |
| Szauter et
al, 2005 | Skin | LOX and LOXL2 | Deactivated with
aging, dynamically expressed by fibroblasts with the cellular
response | (55) |
| Jiang et al,
2014 | Uterus | LOX | Maintained by
estrogen and downregulated during aging | (62) |
3. Signaling pathways involved in the
modulation of LOX during tissue development
Numerous factors have been identified that regulate
LOX; for example, the stimulation of fibroblasts or myofibroblasts
prompts the secretion of the LOX precursor or promotes LOX
hydroxylation and direct enzymatic activation (30). It was reported that hypoxia-inducible
factor (HIF)1α, advanced glycation end-products-dependent
transcription factor, transforming growth factor (TGF)-β, tolloid
protein-1 (TLD1) and fibronectin all promoted either the expression
or activation of LOX (33,84-89).
The opposite effect occurred following the stimulation from
prostaglandin E2 and homocysteine (11,16).
BAPN is extensively applied in in vivo and in vitro
experiments as it selectively and non-reversibly inhibits LOX
activity (5).
During the branching of airways and pulmonary
vasculogenesis, hypoxia is one of the deciding promoters that
triggers their development (66,86).
Therefore, hypoxia treatment or lateral-pneumonectomy are the most
common animal model methods used for studying the issues of the
respiratory system (17). In tissues
with high expression levels of HIF1α and HIF2, the tissue exhibited
increased fibrogenesis and collagen deposition, alongside increased
expression levels of LOX (69).
Decreased HIF expression levels were found to lead to vascular
alveolar hypoplasia, neonatal respiratory distress and
bronchopulmonary dysplasia that featured alongside insufficiently
deposited and crosslinked collagens (66). It is therefore suggested that LOX may
serve an important role in ECM modeling under HIF regulation, as
Pez et al (90) revealed that
HIF and LOX synergistically promote the growth of newly formed
tissue and increased cellular proliferation in these tissues.
Fibrosis serves an important role in structural
reconfiguration during development. Although LOX and LOXLs are
reported to significantly promote fibrotic alterations in tissues,
certain stimuli are required to stimulate fibroblasts or
myofibroblasts to secrete their precursors (91,92). In
addition, the increased hydroxylation of LOX and its direct
enzymatic activation are required (30). The presence of TGF-β is required to
facilitate the growth of embryos and fetal myoblast fibroblasts;
Cusella-De Angelis et al (85) and Leonard et al (93) both discovered that TGF-β triggered
the proliferation of fetal myoblasts and fibroblasts. This may in
turn stimulate primary limb bud formation, which is essential for
the secondary level of limb development, and ECM and stromal
enrichment. In addition, in neonatal rat aorta smooth muscle cells,
TGF-β1 was found to significantly promote LOX expression levels
(35). A similar result was reported
in cardiac fibroblasts treated with TGF-β; it was observed that LOX
expression levels were increased at both the mRNA and protein
level, and this may be prevented by inhibitors of the TGF-β cascade
(94). These findings suggested that
the presence of TGF-β may be essential for maintaining LOX
stability.
Within in the TGF-β superfamily, the bone
morphogenetic proteins (BMPs) have also been demonstrated to
influence organogenesis in the brain, eye, hair follicles, kidney,
lung, liver, skin and teeth in a pleiotropic manner (89). In regulating the morphogenesis of
each organ, BMP was found to activate LOX and LOXL1 from
pro-enzymes, thus it can be suggested that they are both critical
for the ECM crosslinking that determines the biomechanical features
of the tissue (95). Additionally,
BMP-1 was found to promote the efficiency of pro-LOX activity
between 1- and 20-fold compared with mammalian TLD (mTLD) or
mammalian TLD-like (mTLL)-1 and mTLL-2(89). Thus, it was suggested that BMP may
regulate embryogenic fibroblasts and control LOX activity in an
mTLD and mTTD assisted manner (96).
Similar to the crosslinking between collagens, the
elastin-collagen crosslinking determines the stability of the ECM
(15,84). Elastin stabilization requires the
catalyzation of LOX, whereas, elastin and collagen crosslinking
require the catalyzation of fibronectins (FNs), a type of
glycoprotein that is abundantly expressed in the ECM (88). FN receptors are located in every
tissue that originates from the three primary germ layers and are
therefore considered to modulate embryogenic cellular migration and
anchoring (24), which suggested the
FN signaling may mediate the cellular-ECM signal transduction that
may, in turn, modulate LOX secretion. Fogelgren et al
(97) revealed that the decreased
LOX proteolytic processing in FN-null mouse embryonic fibroblasts
was associated with lower LOX activity; however, whilst their
enzymatic activity data did not determine the role of cellular FN
(cFN) in regulating LOX, it was hypothesized that the FN matrix may
activate LOX in a comprehensive manner. Specifically, FN was
hypothesized to be expressed differently in various stages of organ
development with the alteration of tissue microenvironment.
In LOXL1 knockout mice, the malformed pelvic floor,
weakened vaginal wall and tendencies of pelvic organ prolapse were
observed (98). A similar phenomenon
was also observed in estrogen-deficient mice (63). Furthermore, it was noticed that
estrogen promoted LOX upregulation and enzymatic maturation through
direct and indirect means, by which such a mechanism is deemed
beneficial in maintaining pelvic wall stability and skin elasticity
(63,99). Notably, the pro-LOX effect of
estrogen was found to be inhibited following the administration of
SB431542, a TGF-β1 receptor inhibitor, revealing the participation
of TGF-β signaling in the estrogenic effect (63). Similarly, the pro-LOX effect was also
identified with androgens, as Harlow et al (100) and Slee et al (101) identified that
5α-dihydrotestosterone significantly increased LOX expression
levels both in vitro and in vivo. In addition, the
follicle-stimulating hormone was observed to reduce both LOX
expression levels and its activity (100). These results revealed the
complicated regulation of LOX by the sex hormones and indicated a
potential target in manipulating organogenesis through LOX
regulation.
Multiple signaling pathways are involved in LOX and
LOXLs regulation at different levels, including pathways involved
in genetic transcription, enzymatic synthesis and activation both
intracellularly and extracellularly. ECM maturation facilitates
cellular transformation, which results in embryogenic progression
and organ development. Fibroblasts and SMCs are both considered as
primary regulators and messengers. For they were observed not only
receiving shifting signaling and extracellular feedbacks (91). In turn, they stimulate alterations
that contribute to the stromal changes and tissue biomechanical
alterations (such as changes in the rigidity) in response to
organ-specific needs and parenchymal requirements (33). The response of LOX to multiple
factors in organ development is summarized in Fig. 1.
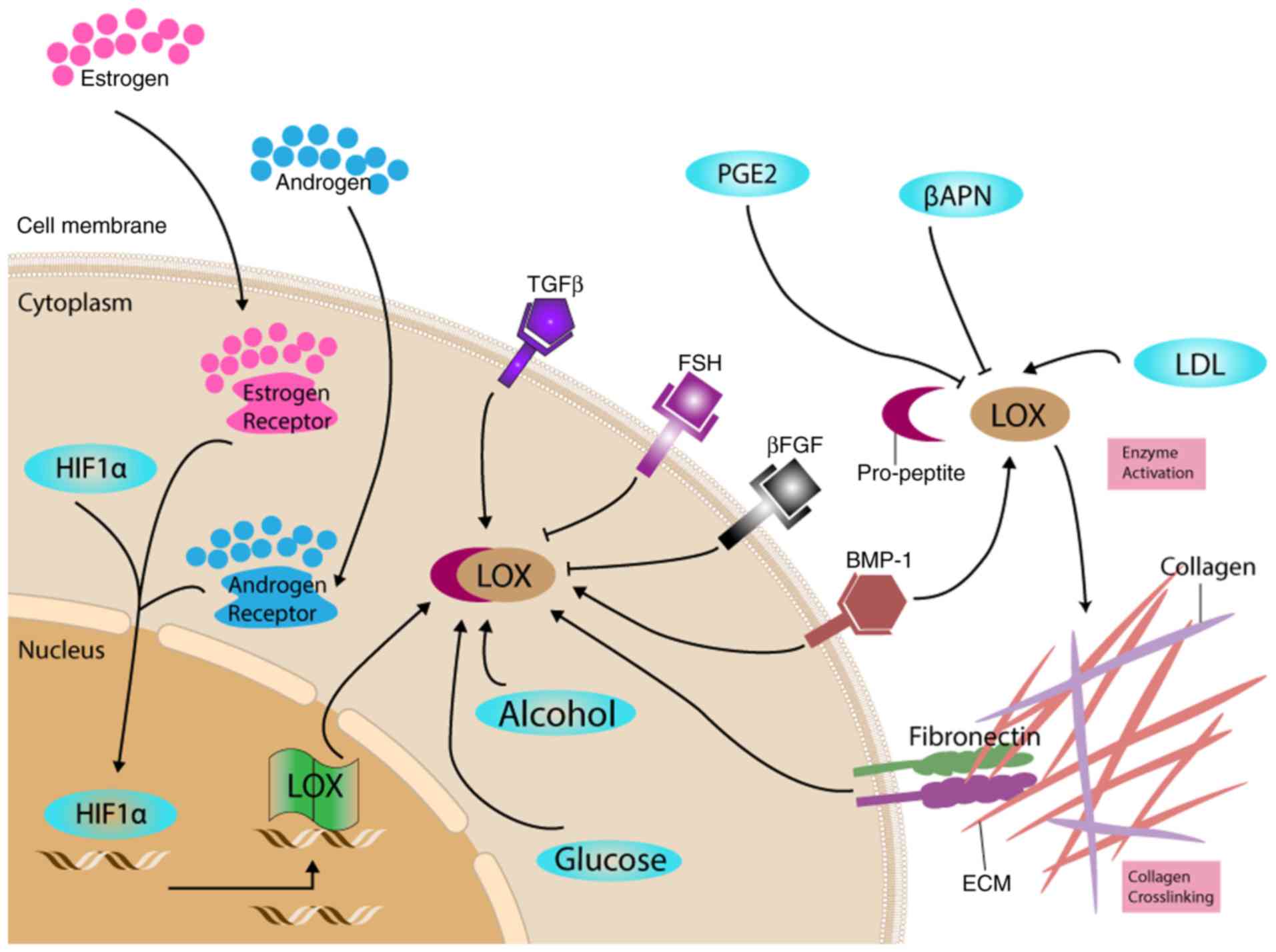 | Figure 1.Proposed roles of LOX in response to
different factors. LOX was expressed and secreted in its proenzyme
form. The regulation of LOX occurs at multiple levels, including at
the transcriptional and translational level, and following both
intracellular and extracellular enzyme activation. These
regulations and reactions are correlated with the ECM
transformation during embryogenesis and leads to changes in cell
adhesion and proliferation, which correspond to organ development.
βAPN, β-aminopropionitrile; βFGF, fibroblast growth factor β;
BMP-1, bone morphogenetic protein-1; ECM, extracellular matrix;
FSH, follicle-stimulating hormone; HIF1α, hypoxia-inducible factor
1α; LDL, low density lipoprotein; LOX, lysyl oxidase; TGF-β1,
transforming growth factor-β1; PGE2, prostaglandin E2. |
However, how to utilize these signaling effects and
the ECM-cellular responses described above to manipulate ECM
formation and organogenesis with LOXs requires further
investigation; the prevention of abnormal development through
targeting LOX requires a more thorough understanding of the
regulation of LOXs and their spatiotemporal specificity, which
currently remains relatively unknown.
4. Conclusion
ECM metabolism serves a critical role in tissue
development; not only is it an essential mechanical structure for
cells to maintain normal organ function and transducing
extracellular mechanical signals to stimulate cellular responses,
but it also forms the microenvironment that enables stromal and
parenchymal interactions. Apart from triggering inter-collagen
crosslinking that determines tissue stiffness, LOXs are
demonstrated to be involved in multiple physiological or
pathological pathways, both in extracellular modulation and
intracellular signaling. The findings of the present review
suggested that LOX may be comprehensively involved in the
organogenesis of all systems. Nevertheless, regulating mechanical
homeostasis should be considered as the pivotal role of LOX.
In order to decipher this pivotal role, efforts
should focus on investigating how LOXs modulates the ECM by
maintaining the mechanical properties, which are essential in
maintaining organ integrity and organ functional properties. During
this process, direct and indirect influences of LOX on the stromal
and parenchymal interactions that guide cellular phenotypic
adaptations is also worth of further investigation.
A number of methods have proven effective in
targeting LOX; however, research remains far from declaring a
plausible and credible method for regulating LOX expression. The
complexity and spatiotemporal specificity of LOX requires further
investigations, followed by its modulatory role in physiological
and pathophysiological states. Studies on LOXs have been conducted
for decades; however, there are currently no clinical trials on
LOXs for disease prevention and treatment, which suggested that the
current understanding of LOXs is primitive. Thus, the application
of LOX genes and proteins on disease diagnosis, treatment and
prognosis are required to be further investigated.
Acknowledgements
Not applicable.
Funding
This study was supported by the Natural Science
Foundation of China (grant nos. 81671453, 81270691, and 81170565),
Science and Technology Project of the Health Planning Committee of
Sichuan (grant no. 18PJ487) and Thousand Talent Plan of Sichuan
Province.
Availability of data and materials
Not applicable.
Authors' contributions
LG, JY, FQ and CW performed the conception and
design of the study. JY and FQ provided administrative support. SW,
LG and JY aided with the provision of study materials or patients.
LG, JY, SW and CW collected and assembled the data. SW, LG, YW and
CW performed the data analysis and interpretation. SW, LG, JY and
CW wrote the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Finney J, Moon HJ, Ronnebaum T, Lantz M
and Mure M: Human copper-dependent amine oxidases. Arch Biochem
Biophys. 546:19–32. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lucero HA and Kagan HM: Lysyl oxidase: An
oxidative enzyme and effector of cell function. Cell Mol Life Sci.
63:2304–2316. 2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Papadantonakis N, Matsuura S and Ravid K:
Megakaryocyte pathology and bone marrow fibrosis: The lysyl oxidase
connection. Blood. 120:1774–1781. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Smith-Mungo LI and Kagan HM: Lysyl
oxidase: Properties, regulation and multiple functions in biology.
Matrix Biol. 16:387–398. 1998.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Barker HE, Cox TR and Erler JT: The
rationale for targeting the LOX family in cancer. Nat Rev Cancer.
12:540–552. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Chen L, Li S and Li W: LOX/LOXL in
pulmonary fibrosis: Potential therapeutic targets. J Drug Target.
27:790–796. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Amendola PG, Reuten R and Erler JT:
Interplay Between LOX Enzymes and Integrins in the Tumor
Microenvironment. Cancers (Basel). 11(11)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: Emt: 2016. Cell. 166:21–45. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dongre A and Weinberg RA: New insights
into the mechanisms of epithelial-mesenchymal transition and
implications for cancer. Nat Rev Mol Cell Biol. 20:69–84.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Polgar N, Fogelgren B, Shipley JM and
Csiszar K: Lysyl oxidase interacts with hormone placental lactogen
and synergistically promotes breast epithelial cell proliferation
and migration. J Biol Chem. 282:3262–3272. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rosenbloom J, Ren S and Macarak E: New
frontiers in fibrotic disease therapies: The focus of the Joan and
Joel Rosenbloom Center for Fibrotic Diseases at Thomas Jefferson
University. Matrix Biol. 51:14–25. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lewis PL, Yan M, Su J and Shah RN:
Directing the growth and alignment of biliary epithelium within
extracellular matrix hydrogels. Acta Biomater. 85:84–93.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Al Ameri W, Ahmed I, Al-Dasim FM, Ali
Mohamoud Y, Al-Azwani IK, Malek JA and Karedath T: Cell
Type-Specific TGF-β Mediated EMT in 3D and 2D Models and Its
Reversal by TGF-β Receptor Kinase Inhibitor in Ovarian Cancer Cell
Lines. Int J Mol Sci. 20(20)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hornstra IK, Birge S, Starcher B, Bailey
AJ, Mecham RP and Shapiro SD: Lysyl oxidase is required for
vascular and diaphragmatic development in mice. J Biol Chem.
278:14387–14393. 2003.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Behmoaras J, Slove S, Seve S, Vranckx R,
Sommer P and Jacob MP: Differential expression of lysyl oxidases
LOXL1 and LOX during growth and aging suggests specific roles in
elastin and collagen fiber remodeling in rat aorta. Rejuvenation
Res. 11:883–889. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tang SS, Trackman PC and Kagan HM:
Reaction of aortic lysyl oxidase with beta-aminopropionitrile. J
Biol Chem. 258:4331–4338. 1983.PubMed/NCBI
|
|
17
|
Brody JS, Kagan H and Manalo A: Lung lysyl
oxidase activity: Relation to lung growth. Am Rev Respir Dis.
120:1289–1295. 1979.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mammoto T, Mammoto A, Jiang A, Jiang E,
Hashmi B and Ingber DE: Mesenchymal condensation-dependent
accumulation of collagen VI stabilizes organ-specific cell fates
during embryonic tooth formation. Dev Dyn. 244:713–723.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gartland A, Erler JT and Cox TR: The role
of lysyl oxidase, the extracellular matrix and the pre-metastatic
niche in bone metastasis. J Bone Oncol. 5:100–103. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mäki JM, Sormunen R, Lippo S,
Kaarteenaho-Wiik R, Soininen R and Myllyharju J: Lysyl oxidase is
essential for normal development and function of the respiratory
system and for the integrity of elastic and collagen fibers in
various tissues. Am J Pathol. 167:927–936. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Eberson LS, Sanchez PA, Majeed BA,
Tawinwung S, Secomb TW and Larson DF: Effect of lysyl oxidase
inhibition on angiotensin II-induced arterial hypertension,
remodeling, and stiffness. PLoS One. 10(e0124013)2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tjäderhane L, Vered M, Pääkkönen V, Peteri
A, Mäki JM, Myllyharju J, Dayan D and Salo T: The expression and
role of Lysyl oxidase (LOX) in dentinogenesis. Int Endod J.
46:581–589. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Heinemeier KM, Olesen JL, Haddad F,
Langberg H, Kjaer M, Baldwin KM and Schjerling P: Expression of
collagen and related growth factors in rat tendon and skeletal
muscle in response to specific contraction types. J Physiol.
582:1303–1316. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Duband JL, Rocher S, Chen WT, Yamada KM
and Thiery JP: Cell adhesion and migration in the early vertebrate
embryo: Location and possible role of the putative fibronectin
receptor complex. J Cell Biol. 102:160–178. 1986.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Athanasiou KA, Responte DJ, Brown WE and
Hu JC: Harnessing biomechanics to develop cartilage regeneration
strategies. J Biomech Eng. 137(020901)2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pan XS, Li J, Brown EB and Kuo CK: Embryo
movements regulate tendon mechanical property development. Philos
Trans R Soc Lond B Biol Sci. 373(373)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Adam O, Theobald K, Lavall D, Grube M,
Kroemer HK, Ameling S, Schäfers HJ, Böhm M and Laufs U: Increased
lysyl oxidase expression and collagen cross-linking during atrial
fibrillation. J Mol Cell Cardiol. 50:678–685. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kumarasamy A, Schmitt I, Nave AH, Reiss I,
van der Horst I, Dony E, Roberts JD Jr, de Krijger RR, Tibboel D,
Seeger W, et al: Lysyl oxidase activity is dysregulated during
impaired alveolarization of mouse and human lungs. Am J Respir Crit
Care Med. 180:1239–1252. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Makris EA, Hu JC and Athanasiou KA:
Hypoxia-induced collagen crosslinking as a mechanism for enhancing
mechanical properties of engineered articular cartilage.
Osteoarthritis Cartilage. 21:634–641. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
López B, González A, Hermida N, Valencia
F, de Teresa E and Díez J: Role of lysyl oxidase in myocardial
fibrosis: From basic science to clinical aspects. Am J Physiol
Heart Circ Physiol. 299:H1–H9. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Magnusson SP, Heinemeier KM and Kjaer M:
Collagen Homeostasis and Metabolism. Adv Exp Med Biol. 920:11–25.
2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mammoto T, Jiang E, Jiang A and Mammoto A:
Extracellular matrix structure and tissue stiffness control
postnatal lung development through the lipoprotein receptor-related
protein 5/Tie2 signaling system. Am J Respir Cell Mol Biol.
49:1009–1018. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hashimoto S, Nakano H, Suguta Y, Irie S,
Jianhua L and Katyal SL: Exogenous fibroblast growth factor-10
induces cystic lung development with altered target gene expression
in the presence of heparin in cultures of embryonic rat lung.
Pathobiology. 79:127–143. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Besiktepe N, Kayalar O, Ersen E and Oztay
F: The copper dependent-lysyl oxidases contribute to the
pathogenesis of pulmonary emphysema in chronic obstructive
pulmonary disease patients. J Trace Elem Med Biol. 44:247–255.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tsuda T, Pan TC, Evangelisti L and Chu ML:
Prominent expression of lysyl oxidase during mouse embryonic
cardiovascular development. Anat Rec A Discov Mol Cell Evol Biol.
270:93–96. 2003.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kaku M, Rosales Rocabado JM, Kitami M, Ida
T, Akiba Y, Yamauchi M and Uoshima K: Mechanical Loading Stimulates
Expression of Collagen Cross-Linking Associated Enzymes in
Periodontal Ligament. J Cell Physiol. 231:926–933. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kim EC, Lee HJ and Kim Y: Lysyl oxidase
and the lysyl oxidase-like protein modulate odontoblastic
differentiation of human dental pulp cells. J Endod. 38:769–773.
2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Sharma-Bhandari A, Park SH, Kim JY, Oh J
and Kim Y: Lysyl oxidase modulates the osteoblast differentiation
of primary mouse calvaria cells. Int J Mol Med. 36:1664–1670.
2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Vora SR, Palamakumbura AH, Mitsi M, Guo Y,
Pischon N, Nugent MA and Trackman PC: Lysyl oxidase propeptide
inhibits FGF-2-induced signaling and proliferation of osteoblasts.
J Biol Chem. 285:7384–7393. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Pischon N, Mäki JM, Weisshaupt P, Heng N,
Palamakumbura AH, N'Guessan P, Ding A, Radlanski R, Renz H,
Bronckers TA, et al: Lysyl oxidase (lox) gene deficiency affects
osteoblastic phenotype. Calcif Tissue Int. 85:119–126.
2009.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Turecek C, Fratzl-Zelman N, Rumpler M,
Buchinger B, Spitzer S, Zoehrer R, Durchschlag E, Klaushofer K,
Paschalis EP and Varga F: Collagen cross-linking influences
osteoblastic differentiation. Calcif Tissue Int. 82:392–400.
2008.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Janssen I, Heymsfield SB, Wang ZM and Ross
R: Skeletal muscle mass and distribution in 468 men and women aged
18-88 yr. J Appl Physiol. 89:81–88. 2000.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Hasson P: ‘Soft’ tissue patterning:
Muscles and tendons of the limb take their form. Dev Dyn.
240:1100–1107. 2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kutchuk L, Laitala A, Soueid-Bomgarten S,
Shentzer P, Rosendahl AH, Eilot S, Grossman M, Sagi I, Sormunen R,
Myllyharju J, et al: Muscle composition is regulated by a Lox-TGFβ
feedback loop. Development. 142:983–993. 2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Xie J, Jiang J, Zhang Y, Xu C, Yin L, Wang
C, Chen PC and Sung KL: Up-regulation expressions of lysyl oxidase
family in Anterior Cruciate Ligament and Medial Collateral Ligament
fibroblasts induced by Transforming Growth Factor-Beta 1. Int
Orthop. 36:207–213. 2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kato S, Saito M, Funasaki H and Marumo K:
Distinctive collagen maturation process in fibroblasts derived from
rabbit anterior cruciate ligament, medial collateral ligament, and
patellar tendon in vitro. Knee Surg Sports Traumatol Arthrosc.
23:1384–1392. 2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Takesian AE and Hensch TK: Balancing
plasticity/stability across brain development. Prog Brain Res.
207:3–34. 2013.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ismail FY, Fatemi A and Johnston MV:
Cerebral plasticity: Windows of opportunity in the developing
brain. Eur J Paediatr Neurol. 21:23–48. 2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Rauch U: Extracellular matrix components
associated with remodeling processes in brain. Cell Mol Life Sci.
61:2031–2045. 2004.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Bronson NW, Hamilton JS, Han M, Li PA,
Hornstra I, Horowitz JM and Horwitz BA: LOXL null mice demonstrate
selective dentate structural changes but maintain dentate granule
cell and CA1 pyramidal cell potentiation in the hippocampus.
Neurosci Lett. 390:118–122. 2005.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Li J, Gu X, Ma Y, Calicchio ML, Kong D,
Teng YD, Yu L, Crain AM, Vartanian TK, Pasqualini R, et al: Nna1
mediates Purkinje cell dendritic development via lysyl oxidase
propeptide and NF-κB signaling. Neuron. 68:45–60. 2010.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Peng C, Yan S, Ye J, Shen L, Xu T and Tao
W: Vps18 deficiency inhibits dendritogenesis in Purkinje cells by
blocking the lysosomal degradation of Lysyl Oxidase. Biochem
Biophys Res Commun. 423:715–720. 2012.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Chelyshev YA, Muhamedshina YO, Povysheva
TV, Shaymardanova GF, Rizvanov AA, Nigmetzyanova MV, Tiapkina OV,
Bondarenko NI, Nikolskiy EE and Islamov RR: Characterization of
spinal cord glial cells in a model of hindlimb unloading in mice.
Neuroscience. 280:328–339. 2014.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Li PA, He Q, Cao T, Yong G, Szauter KM,
Fong KS, Karlsson J, Keep MF and Csiszar K: Up-regulation and
altered distribution of lysyl oxidase in the central nervous system
of mutant SOD1 transgenic mouse model of amyotrophic lateral
sclerosis. Brain Res Mol Brain Res. 120:115–122. 2004.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Szauter KM, Cao T, Boyd CD and Csiszar K:
Lysyl oxidase in development, aging and pathologies of the skin.
Pathol Biol (Paris). 53:448–456. 2005.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Yamazaki Y, Mikami Y, Yuguchi M, Namba Y
and Isokawa K: Development of collagen fibres and lysyl oxidase
expression in the presumptive dermis of chick limb bud. Anat Histol
Embryol. 41:68–74. 2012.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Cenizo V, André V, Reymermier C, Sommer P,
Damour O and Perrier E: LOXL as a target to increase the elastin
content in adult skin: A dill extract induces the LOXL gene
expression. Exp Dermatol. 15:574–581. 2006.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Langton AK, Griffiths CE, Sherratt MJ and
Watson RE: Cross-linking of structural proteins in ageing sk in: An
in situ assay for the detection of amine oxidase activity.
Biogerontology. 14:89–97. 2013.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Fujimoto E and Tajima S: Reciprocal
regulation of LOX and LOXL2 expression during cell adhesion and
terminal differentiation in epidermal keratinocytes. J Dermatol
Sci. 55:91–98. 2009.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Le Provost GS, Debret R, Cenizo V, Aimond
G, Pez F, Kaniewski B, André V and Sommer P: Lysyl oxidase
silencing impairs keratinocyte differentiation in a
reconstructed-epidermis model. Exp Dermatol. 19:1080–1087.
2010.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Noblesse E, Cenizo V, Bouez C, Borel A,
Gleyzal C, Peyrol S, Jacob MP, Sommer P and Damour O: Lysyl
oxidase-like and lysyl oxidase are present in the dermis and
epidermis of a skin equivalent and in human skin and are associated
to elastic fibers. J Invest Dermatol. 122:621–630. 2004.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Jiang Y, Zong W, Luan H, Liu JH, Zhang AZ,
Li XL, Liu SY, Zhang SQ and Gao JG: Decreased expression of elastin
and lysyl oxidase family genes in urogenital tissues of aging mice.
J Obstet Gynaecol Res. 40:1998–2004. 2014.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Zong W, Jiang Y, Zhao J, Zhang J and Gao
JG: Estradiol plays a role in regulating the expression of lysyl
oxidase family genes in mouse urogenital tissues and human Ishikawa
cells. J Zhejiang Univ Sci B. 16:857–864. 2015.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Zimnicka AM, Tang H, Guo Q, Kuhr FK, Oh
MJ, Wan J, Chen J, Smith KA, Fraidenburg DR, Choudhury MS, et al:
Upregulated copper transporters in hypoxia-induced pulmonary
hypertension. PLoS One. 9(e90544)2014.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Ranzer MJ, Chen L and DiPietro LA:
Fibroblast function and wound breaking strength is impaired by
acute ethanol intoxication. Alcohol Clin Exp Res. 35:83–90.
2011.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Shimoda LA and Semenza GL: HIF and the
lung: Role of hypoxia-inducible factors in pulmonary development
and disease. Am J Respir Crit Care Med. 183:152–156.
2011.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Semenza GL: HIF-1: Mediator of
physiological and pathophysiological responses to hypoxia. J Appl
Physiol. 88:1474–1480. 2000.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Adams JM, Difazio LT, Rolandelli RH, Luján
JJ, Haskó G, Csóka B, Selmeczy Z and Németh ZH: HIF-1: A key
mediator in hypoxia. Acta Physiol Hung. 96:19–28. 2009.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Higgins DF, Kimura K, Bernhardt WM,
Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler
M, Cohen CD, et al: Hypoxia promotes fibrogenesis in vivo via HIF-1
stimulation of epithelial-to-mesenchymal transition. J Clin Invest.
117:3810–3820. 2007.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Xia XD, Lee J, Khan S, Ye L, Li Y and Dong
L: Suppression of Phosphatidylinositol 3-Kinase/Akt Signaling
Attenuates Hypoxia-Induced Pulmonary Hypertension Through the
Downregulation of Lysyl Oxidase. DNA Cell Biol. 35:599–606.
2016.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Nave AH, Mižíková I, Niess G, Steenbock H,
Reichenberger F, Talavera ML, Veit F, Herold S, Mayer K, Vadász I,
et al: Lysyl oxidases play a causal role in vascular remodeling in
clinical and experimental pulmonary arterial hypertension.
Arterioscler Thromb Vasc Biol. 34:1446–1458. 2014.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Xiao Y, Nie X, Han P, Fu H and James Kang
Y: Decreased copper concentrations but increased lysyl oxidase
activity in ischemic hearts of rhesus monkeys. Metallomics.
8:973–980. 2016.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Mesarwi OA, Shin MK, Drager LF,
Bevans-Fonti S, Jun JC, Putcha N, Torbenson MS, Pedrosa RP,
Lorenzi-Filho G, Steele KE, et al: Lysyl Oxidase as a Serum
Biomarker of Liver Fibrosis in Patients with Severe Obesity and
Obstructive Sleep Apnea. Sleep (Basel). 38:1583–1591.
2015.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Marturano JE, Xylas JF, Sridharan GV,
Georgakoudi I and Kuo CK: Lysyl oxidase-mediated collagen
crosslinks may be assessed as markers of functional properties of
tendon tissue formation. Acta Biomater. 10:1370–1379.
2014.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Zhang Y, Wang B, Zhang WJ, Zhou G, Cao Y
and Liu W: Enhanced proliferation capacity of porcine tenocytes in
low O2 tension culture. Biotechnol Lett. 32:181–187.
2010.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Matafome P, Rodrigues T and Seica R:
Glycation and Hypoxia: Two Key Factors for Adipose Tissue
Dysfunction. Curr Med Chem. 22:2417–2437. 2015.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Reiser KM, Crouch EC, Chang K and
Williamson JR: Lysyl oxidase-mediated crosslinking in granulation
tissue collagen in two models of hyperglycemia. Biochim Biophys
Acta. 1097:55–61. 1991.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Argyropoulos AJ, Robichaud P, Balimunkwe
RM, Fisher GJ, Hammerberg C, Yan Y and Quan T: Alterations of
Dermal Connective Tissue Collagen in Diabetes: Molecular Basis of
Aged-Appearing Skin. PLoS One. 11(e0153806)2016.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Chronopoulos A, Tang A, Beglova E,
Trackman PC and Roy S: High glucose increases lysyl oxidase
expression and activity in retinal endothelial cells: Mechanism for
compromised extracellular matrix barrier function. Diabetes.
59:3159–3166. 2010.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Yang X, Scott HA, Monickaraj F, Xu J,
Ardekani S, Nitta CF, Cabrera A, McGuire PG, Mohideen U, Das A, et
al: Basement membrane stiffening promotes retinal endothelial
activation associated with diabetes. FASEB J. 30:601–611.
2016.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Rodríguez C, Raposo B, Martínez-González
J, Casaní L and Badimon L: Low density lipoproteins downregulate
lysyl oxidase in vascular endothelial cells and the arterial wall.
Arterioscler Thromb Vasc Biol. 22:1409–1414. 2002.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Van Bergen T, Spangler R, Marshall D,
Hollanders K, Van de Veire S, Vandewalle E, Moons L, Herman J,
Smith V and Stalmans I: The Role of LOX and LOXL2 in the
Pathogenesis of an Experimental Model of Choroidal
Neovascularization. Invest Ophthalmol Vis Sci. 56:5280–5289.
2015.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Yang X, Scott HA, Ardekani S, Williams M,
Talbot P and Ghosh K: Aberrant cell and basement membrane
architecture contribute to sidestream smoke-induced choroidal
endothelial dysfunction. Invest Ophthalmol Vis Sci. 55:3140–3147.
2014.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Kothapalli CR and Ramamurthi A: Lysyl
oxidase enhances elastin synthesis and matrix formation by vascular
smooth muscle cells. J Tissue Eng Regen Med. 3:655–661.
2009.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Cusella-De Angelis MG, Molinari S, Le
Donne A, Coletta M, Vivarelli E, Bouche M, Molinaro M, Ferrari S
and Cossu G: Differential response of embryonic and fetal myoblasts
to TGF beta: A possible regulatory mechanism of skeletal muscle
histogenesis. Development. 120:925–933. 1994.PubMed/NCBI
|
|
86
|
Groenman FA, Rutter M, Wang J, Caniggia I,
Tibboel D and Post M: Effect of chemical stabilizers of
hypoxia-inducible factors on early lung development. Am J Physiol
Lung Cell Mol Physiol. 293:L557–L567. 2007.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Hong HH and Trackman PC: Cytokine
regulation of gingival fibroblast lysyl oxidase, collagen, and
elastin. J Periodontol. 73:145–152. 2002.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Huang G, Zhang Y, Kim B, Ge G, Annis DS,
Mosher DF and Greenspan DS: Fibronectin binds and enhances the
activity of bone morphogenetic protein 1. J Biol Chem.
284:25879–25888. 2009.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Kishigami S and Mishina Y: BMP signaling
and early embryonic patterning. Cytokine Growth Factor Rev.
16:265–278. 2005.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Pez F, Dayan F, Durivault J, Kaniewski B,
Aimond G, Le Provost GS, Deux B, Clézardin P, Sommer P, Pouysségur
J, et al: The HIF-1-inducible lysyl oxidase activates HIF-1 via the
Akt pathway in a positive regulation loop and synergizes with HIF-1
in promoting tumor cell growth. Cancer Res. 71:1647–1657.
2011.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Boak AM, Roy R, Berk J, Taylor L, Polgar
P, Goldstein RH and Kagan HM: Regulation of lysyl oxidase
expression in lung fibroblasts by transforming growth factor-beta 1
and prostaglandin E2. Am J Respir Cell Mol Biol. 11:751–755.
1994.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Xie J, Huang W, Jiang J, Zhang Y, Xu Y, Xu
C, Yang L, Chen PC and Sung KL: Differential expressions of lysyl
oxidase family in ACL and MCL fibroblasts after mechanical injury.
Injury. 44:893–900. 2013.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Leonard CM, Fuld HM, Frenz DA, Downie SA,
Massagué J and Newman SA: Role of transforming growth factor-beta
in chondrogenic pattern formation in the embryonic limb:
Stimulation of mesenchymal condensation and fibronectin gene
expression by exogenenous TGF-beta and evidence for endogenous
TGF-beta-like activity. Dev Biol. 145:99–109. 1991.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Voloshenyuk TG, Landesman ES, Khoutorova
E, Hart AD and Gardner JD: Induction of cardiac fibroblast lysyl
oxidase by TGF-β1 requires PI3K/Akt, Smad3, and MAPK signaling.
Cytokine. 55:90–97. 2011.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Borel A, Eichenberger D, Farjanel J,
Kessler E, Gleyzal C, Hulmes DJ, Sommer P and Font B: Lysyl
oxidase-like protein from bovine aorta. Isolation and maturation to
an active form by bone morphogenetic protein-1. J Biol Chem.
276:48944–48949. 2001.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Uzel MI, Scott IC, Babakhanlou-Chase H,
Palamakumbura AH, Pappano WN, Hong HH, Greenspan DS and Trackman
PC: Multiple bone morphogenetic protein 1-related mammalian
metalloproteinases process pro-lysyl oxidase at the correct
physiological site and control lysyl oxidase activation in mouse
embryo fibroblast cultures. J Biol Chem. 276:22537–22543.
2001.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Fogelgren B, Polgár N, Szauter KM,
Ujfaludi Z, Laczkó R, Fong KS and Csiszar K: Cellular fibronectin
binds to lysyl oxidase with high affinity and is critical for its
proteolytic activation. J Biol Chem. 280:24690–24697.
2005.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Liu X, Zhao Y, Pawlyk B, Damaser M and Li
T: Failure of elastic fiber homeostasis leads to pelvic floor
disorders. Am J Pathol. 168:519–528. 2006.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Sanada H, Shikata J, Hamamoto H, Ueba Y,
Yamamuro T and Takeda T: Changes in collagen cross-linking and
lysyl oxidase by estrogen. Biochim Biophys Acta. 541:408–413.
1978.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Harlow CR, Rae M, Davidson L, Trackman PC
and Hillier SG: Lysyl oxidase gene expression and enzyme activity
in the rat ovary: Regulation by follicle-stimulating hormone,
androgen, and transforming growth factor-beta superfamily members
in vitro. Endocrinology. 144:154–162. 2003.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Slee RB, Hillier SG, Largue P, Harlow CR,
Miele G and Clinton M: Differentiation-dependent expression of
connective tissue growth factor and lysyl oxidase messenger
ribonucleic acids in rat granulosa cells. Endocrinology.
142:1082–1089. 2001.PubMed/NCBI View Article : Google Scholar
|















