Introduction
Open spina bifida (OSB) is a severe congenital
anomaly of the central nervous system associated with a wide range
of neurological handicaps (1). In
the last 10 years, there is an increasing interest in diagnosing
OSB at 11-13 weeks of gestation. This interest has been promoted by
advances of the ultrasound equipment and progressive understanding
of the abnormal arrangement of the posterior fossa because of the
continuous leakage of cerebrospinal fluid (CSF) (2,3). The
local pressure gradient leads to caudal displacement of the
brainstem (BS) with the obliteration of the cisterna magna
(4,5).
Consequently, different secondary markers have been
described to improve the early detection of spina bifida. They
reflect specific characteristics of this displacement but have yet
to be introduced into practice. In the mid-sagittal view of the
head, the most extensively researched markers for OSB were
intracranial translucency (IT) (6-8), BS
diameter, brainstem to occipital bone (BSOB) distance, BS/BSOB
ratio and the junction between the midbrain and the BS below the
maxilla-to-occipital line (5,9,10). OSB markers in the axial view of the
head are less studied: ratio of choroid plexus size to head size
(3), biparietal diameter (BPD)
(11), posterior displacement of the
midbrain (4,12) and the distance between the occipital
bone and the aqueduct of Sylvius (AoS) (12,13).
Recently there is an increasing interest in the
evaluation of the fetal head in the axial view through description
of simple signs, which can be easily recognized at the time of BPD
measurement: the dry brain and the crash sign (3,4).
However, the posterior displacement of the midbrain and its impact
with the occipital bone could vary and may not be present in all
cases in the first trimester (4).
Therefore, we found that there is a need for a quantitative
description of the normal position of the fetal midbrain. This
study aimed to describe the reference ranges for the distance from
the mesencephalon to the occipital bone in our population, in the
axial plane, at 11 to 13+6 weeks of gestation.
Patients and methods
This is a prospective study that included women
attending for aneuploidy risk assessment at 11-13 gestational
weeks, between 2018 and 2019, at a prenatal diagnostic center
(private practice). It was part of a protocol approved by the
Institutional Board of the ‘Euromedicenter’ Medical Centre (Iasi,
Romania) and all patients provided signed informed consent for
fetal examination. The patients were informed about the limitations
of the first-trimester ultrasound in detecting anomalies and were
invited for a second-trimester fetal anomaly scan. All viable,
singleton pregnancies with a crown-rump length (CRL) of 45-84 mm
and without fetal structural or chromosomal abnormalities were
included. The examinations were performed by experienced
sonographers, accredited by Fetal Medicine Foundation (FMF) for
first-trimester screening. The scans were started transabdominally,
and if the visualization of the markers was inadequate, a
transvaginal ultrasound was offered. Ultrasound examinations were
performed with Voluson E10 and E8 machines, equipped with
transabdominal RM-6C and RAB4-8D transducers (GE Medical
Systems).
The GA was checked according to the CRL. Baseline
maternal characteristics and ultrasound data, such as nuchal
translucency (NT) measurement and presence of fetal anomalies, were
recorded within the Astraia database (Astraia Software Gmbh) as a
part of routine clinical practice.
On the mid-sagittal view of the fetal profile, the
IT was also assessed, using the method described by Chaoui et
al (6). BPD was measured on an
axial view of the fetal cranium, with a symmetrical section of the
brain, that included the midline echo and choroid plexuses, the
calipers placed outer-to-inner borders of the skull (14).
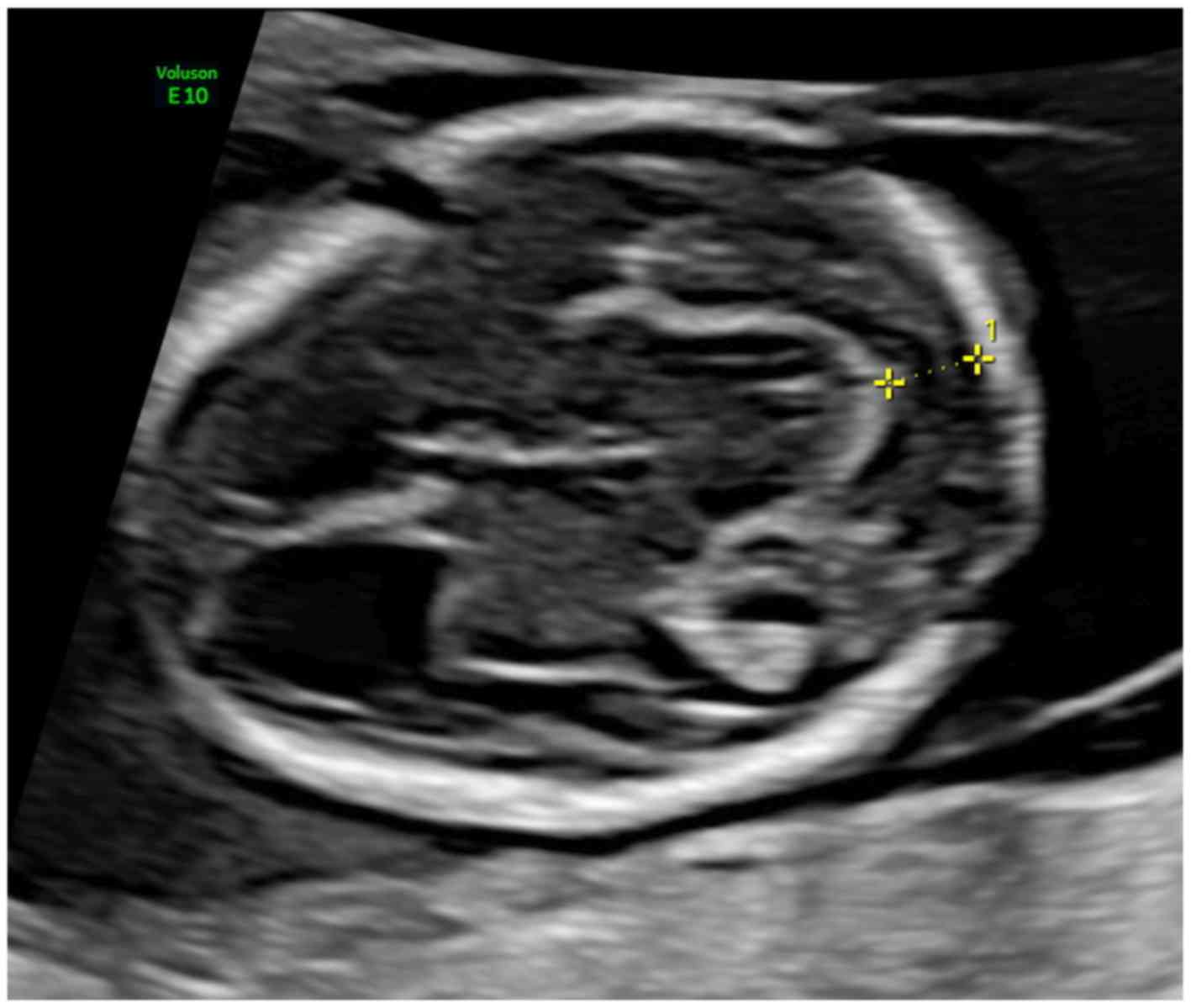
The distance between the posterior limit of the
mesencephalon and the occipital bone was measured in an axial view
of the head, as recommended by the International Society of
Ultrasound in Obstetrics and Gynecology (ISUOG) for BPD
measurements at this gestational age (GA) (15). This plane, also described by Finn
et al (12), was acquired
through a cranial sweep in the axial plane, excluding almost all
the choroid plexus in the lateral ventricles. Here, ‘the
mesencephalon is visualized as a semicircular structure in the
posterior brain and appears as a continuation of the thalami’,
presenting the cerebral aqueduct of Sylvius (AoS) centrally
(Ushakov et al) (4). The
image was magnified, so as the fetal head occupy almost the whole
screen. The calipers were placed on the posterior border of the
mesencephalon and the anterior border of the occiput, on the lines
that define the borders, using the same technique as for NT
measurement. The bony occiput was carefully differentiated from the
occipital cortex (Fig. 1).
The images were retrospectively assessed, and only
high-quality measurements were included for subsequent analysis and
development of reference ranges. When more than two images were
available, we selected two of the highest quality.
All patients had a normal spine and brain structures
scan in the second trimester according to the ISUOG guidelines
(15), and pregnancy outcomes were
retrieved from the hospital database and by direct questioning of
the parents.
Statistical analysis
Continuous variables were reported as medians and
interquartile ranges, while categorical variables as numbers and
percentages. The normality of data was assessed using the
Kolmogorov-Smirnov test. The distribution of the measurements was
made close to Gaussian through logarithmic transformation. The
maternal weight and maternal body mass index (BMI) distributions
were made Gaussian after reciprocal transformation. The multiple of
median (MoM) values for IT and mesencephalon to occiput (MO)
measurements were calculated using the expected values from the
regression equations described by Kappou et al (16) and our developed formula,
respectively. Finally, the MoMs distributions were made Gaussian
trough logarithmic transformation. The relationship between MO
distance MoM and other maternal and fetal parameters was
investigated using Pearson's correlation coefficient. Continuous
variables were compared using Student's t-test. The interobserver
variability was evaluated using the intraclass correlation
coefficient (ICC).
The mean and standard deviation polynomial models
were evaluated for the measured data using the least-squares
regression. Regression coefficients for the standard deviation were
obtained by regressing the scaled standardized absolute residuals
on studied independent variables (13). We identified and excluded from the
initial model significant outliers with standardized residuals. The
normal distribution of the residuals and model premises was
verified through histograms, probability plots, and
quantile-quantile (Q-Q) plots of standardized residuals. Quantile
regression was used to compute the different percentiles of the
studied measurements.
Statistical analysis was performed with the SPSS
25.0 software (SPSS Inc.) and R software with quantreg and ggplot2
packages for quantile regression and graphical representation of
the data. A P-value of <0.05 was considered statistically
significant.
Results
In total, 385 pregnant women were included in our
study, for whom we performed 428 measurements of the mesencephalon
to occipital distance. The median maternal age was 32 years
(interquartile 29-35), and 100% of the women were Caucasians.
Maternal weight had a median of 62 kg (interquartile 56-69), and
the median BMI was 22.7 kg/m2 (interquartile 20.7-25.8).
The median GA at examination was 12.7 weeks (interquartile
12.3-13.1). At the same time, CRL and BPD had a median of 65 mm
(interquartile 60-71) and 22 mm (interquartile 20-24),
respectively. The NT had a median of 1.8 mm (interquartile
1.5-2.1), and that for IT was 1.8 mm (interquartile 1.6-2). There
were 188 (55%) of nullipara and 27 (7.9%) smokers in the study
group. The conception was spontaneous in 306 (89.5%) of the
patients, the rest had received in vitro fertilization (24
cases, 7%), or ovulation induction (11 cases, 3.2%), 278 (83.5%) of
patients received folic acid throughout the first trimester of
gestation. In 5.8% of the examinations, the measurements were
performed on axial views obtained through a transvaginal
ultrasound.
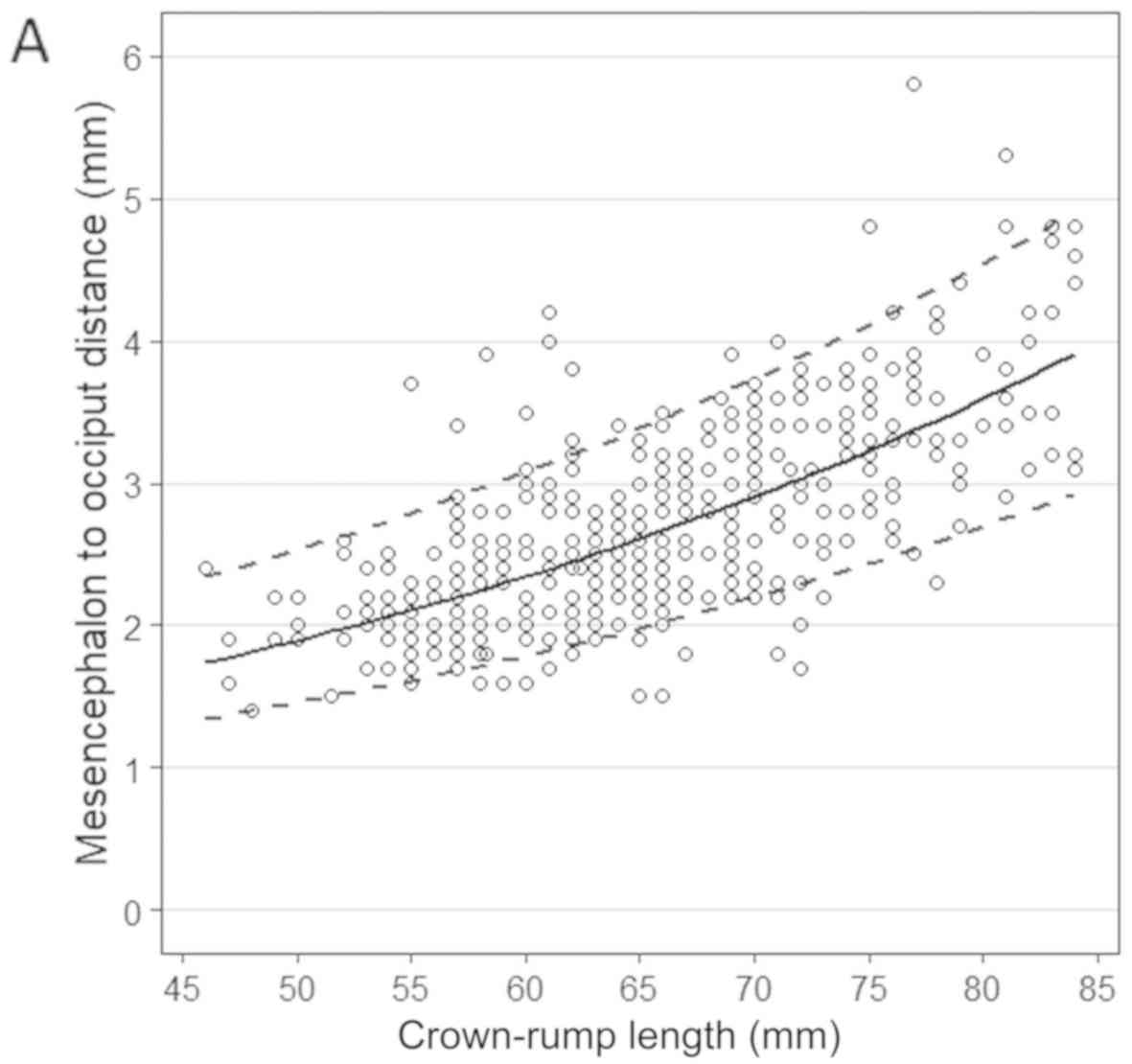
A good, positive correlation was observed between
the MO distance and BPD, CRL or GA (Pearson's correlation
coefficient of 0.719, 0.701 and 0.654, P<0.01 for all). A simple
polynomial regression can best describe the relationship between
the mean of MO distance and these parameters. Standard deviation
also had a linear correlation with BPD, CRL, or GA. The regression
coefficients of the models are presented in Table I. The scatter plots with the median
and the 5th and 95th percentiles are presented in Fig. 2. Table
II shows the 1st, 5th, 50th, 95th and 99th percentiles for the
fetal MO measurements, according to CRL, BPD, and GA in the first
trimester.
 | Table IRegression coefficients for the mean
and standard deviation of the logarithm of mesencephalon to
occipital bone distance (log10 MO) with respect to CRL,
BPD and GA in days. |
Table I
Regression coefficients for the mean
and standard deviation of the logarithm of mesencephalon to
occipital bone distance (log10 MO) with respect to CRL,
BPD and GA in days.
| | | Mean | Standard
deviation |
|---|
| Independent
variable | R2 | Intercept | Coefficient | Intercept | Coefficient |
|---|
| CRL | 0.479 | -0.1833513 | 0.0092027 | 0.568432 | 0.003407 |
| BPD | 0.507 | -0.30942 | 0.03309 | 0.866793 | -0.003963 |
| GA | 0.421 | -1.0494882 | 0.0164975 | 0.7289963 | 0.0006472 |
 | Table IIPercentiles (1st, 5th, 50th, 95th, and
99th) for mesencephalon to occipital bone distance, with respect to
CRL, BPD and GA, derived through quantile regression in a
population of 385 fetuses without spina bifida. |
Table II
Percentiles (1st, 5th, 50th, 95th, and
99th) for mesencephalon to occipital bone distance, with respect to
CRL, BPD and GA, derived through quantile regression in a
population of 385 fetuses without spina bifida.
| | Percentiles |
|---|
| Variable | 1st | 5th | 50th | 95th | 99th |
| CRL (mm) |
|
45 | 1.31 | 1.36 | 1.7 | 2.3 | 3.22 |
|
50 | 1.43 | 1.45 | 1.89 | 2.53 | 3.45 |
|
55 | 1.51 | 1.61 | 2.11 | 2.79 | 3.7 |
|
60 | 1.6 | 1.79 | 2.34 | 3.08 | 3.96 |
|
65 | 1.69 | 1.98 | 2.61 | 3.39 | 4.25 |
|
70 | 1.78 | 2.2 | 2.9 | 3.74 | 4.55 |
|
75 | 1.88 | 2.43 | 3.23 | 4.12 | 4.88 |
|
80 | 1.99 | 2.7 | 3.59 | 4.54 | 5.22 |
|
84 | 2.08 | 2.93 | 3.91 | 4.91 | 5.52 |
| BPD (mm) |
|
16 | 1.22 | 1.26 | 1.62 | 2.2 | 2.9 |
|
18 | 1.42 | 1.43 | 1.9 | 2.56 | 3.29 |
|
20 | 1.59 | 1.67 | 2.22 | 2.97 | 3.73 |
|
22 | 1.78 | 1.95 | 2.6 | 3.45 | 4.23 |
|
24 | 2 | 2.27 | 3.05 | 4.01 | 4.8 |
|
26 | 2.24 | 2.65 | 3.57 | 4.66 | 5.45 |
|
28 | 2.52 | 3.1 | 4.18 | 5.41 | 6.18 |
|
30 | 2.83 | 3.62 | 4.89 | 6.28 | 7.01 |
| GA (days) |
|
77 | 1.29 | 1.31 | 1.61 | 2.23 | 3.17 |
|
80 | 1.4 | 1.43 | 1.82 | 2.49 | 3.42 |
|
83 | 1.5 | 1.59 | 2.05 | 2.79 | 3.7 |
|
86 | 1.6 | 1.76 | 2.31 | 3.12 | 4 |
|
89 | 1.72 | 1.96 | 2.6 | 3.49 | 4.32 |
|
92 | 1.84 | 2.17 | 2.93 | 3.9 | 4.67 |
|
95 | 1.97 | 2.42 | 3.3 | 4.36 | 5.05 |
|
98 | 2.11 | 2.68 | 3.72 | 4.88 | 5.46 |
We found a significant increase in the MO MoM in the
patients who did not receive folic acid in the first trimester of
pregnancy [1.056 vs. 1.008 MoM, t(115) =-2.49, P=0.014]. There were
no significant associations between MO distance MoM, and maternal
demographic characteristics (age, parity, weight, BMI, smoking
status, mode of conception, MoM IT). The Pearson's correlation
coefficients (cc) were, respectively: age (cc=0.01, P=0.43),
maternal weight (cc=0.01, P=0.49), maternal BMI (cc=0.03, P=0.31)
and IT MoM (cc=0.1, P=0.04). MO measurements MoMs were not
significantly different between parous versus nulliparous (P=0.25),
smokers versus non-smokers (P=0.56), and spontaneously conceived
pregnancies versus pregnancies resulted from assisted reproduction
(P=0.24).
The intraobserver reproducibility for MO
measurement, assessed in a separate group of 42 fetuses, was good,
with an ICC of 0.89 (95% confidence interval 0.81-0.94,
P<0.001).
Discussion
Main findings
We measured the MO distance prospectively in the
axial view and constructed its reference ranges at 11 to 13+6 weeks
of gestation. These measurements were not significantly associated
with the maternal demographic characteristics (age, weight, BMI,
parity, smoking status, mode of conception), nor IT. We found a
significant increase in the MO distance in the patients who did not
receive folic acid in the first trimester of pregnancy. To the best
of our knowledge, there are no other studies in the literature
evaluating this type of measurement.
Comparison with previous studies
The posterior fossa was evaluated at 11 to 13+6
weeks, mainly by transabdominal ultrasound, in the midsagittal view
of the fetal head. This was linked with the measurement of the
indirect signs for OSB, such as IT, BS, and BSOB distance (5-7,9).
The reasons behind this were multiple (7). First, this view was used in early
screening for aneuploidies; therefore, it was considered that the
posterior brain region could be viewed easier, more successfully,
in the same mid-sagittal plane. Second, experts believed that an
axial view of the posterior fossa is more challenging to achieve by
transabdominal ultrasound and needs a transvaginal approach, which
cannot be considered as a routine tool for screening. Third,
experts thought that the transvaginal ultrasound reveals many
details and needs some expertise to understand.
Few studies have evaluated the indirect OSB markers
in the axial plane. They mainly described the ultrasound aspects of
the posterior displacement of the mesencephalon and its impact with
the occipital bone, named recently as a ‘crash sign’ (2,4,12,13,17).
Only two studies reported an objective assessment of the midbrain
shifting, measuring the distance between the AoS to the occiput
(12,13). This aspect could be significant in
OSB detection because up to 9% of fetuses with OSB could have a
negative ‘crash sign’ (4).
In our study, the MO measurement was easily
obtainable in an axial plane and did not require extra scanning
time, as the required view is the same to the one needed for the
BPD measurement (Fig. 1), at this GA
(15). We believe that the axial
view is less demanding compared with the sagittal one, and it can
be especially useful when the fetus has a dorsal-anterior or
lateral position. The border of the mesencephalon is more clearly
defined, compared with the measurement of the AoS to occiput
distance, especially at low GAs.
Moreover, in our prospective study, the need for a
transvaginal measurement was significantly lower compared with that
found by two retrospective studies that evaluated the AoS to
occiput distance (5.8 vs. 30%) (12,13).
The MO distance, like the AoS-to-occiput distance,
had a good correlation with CRL and GA (12,13).
Furthermore, the relationship between the means of the markers and
the CRL was also linear, which supports the argument that posterior
fossa measurements in the axial plane are reproducible and could
have clinical use. Like other indirect markers for OSB measured in
the sagittal plane (IT or cisterna magna) (18), the MO distance does not correlate
with maternal demographic characteristics. Also, we found a good,
comparable, intraobserver variability with other studies that
evaluated the posterior fossa (12).
Strengths and limitations
Our study described a new measurement in the
posterior fossa, accounting for its variation in pregnancy by
developing reference ranges in healthy fetuses. The regression
models provided a good fit, and we can use them in the assessment
of future pregnancies. The main advantage of our study is its
prospective nature, the measurements being performed during the
patient examination. However, our study failed to include
measurements from cases with OSB; therefore, we cannot evaluate the
efficacity of MO assessment.
Interestingly, in those patients who did not receive
folic acid during the first trimester of pregnancy, we found a
significant rise in the MO distance. This increase has not been
reported previously, it could appear due to increased production of
cerebrospinal fluid, and its significance needs to be confirmed by
future studies.
Clinical implications
At 11-13 weeks, the fetuses with OSB shows a
reduction of the cerebrospinal fluid, due to its leakage at the
level of the spinal defect (3).
Consequently, others have proposed that there is a reduction of
pressure in the spinal cord, cisterna magna, and fourth ventricle,
compared with choroid ventricles, which produces a posterior and
caudal displacement of the mesencephalon (4,8). We can
evaluate this, in an axial view, as an impact and deformation of
the mesencephalon on the occipital bone. Ushakov et al
(4) described this as a ‘crash
sign’, a simple qualitative marker for diagnosing spina bifida.
However, up to 9% of fetuses with confirmed OSB did not have such a
definite sign. Therefore, an objective assessment of the distance
from the mesencephalon and the occiput is necessary here, to
improve the diagnosis. This measurement has the advantage of being
performed in the same axial thalamic plane as the one needed for
the BPD measurement (Fig. 1).
Measurement of BPD is a good practice recommendation at this GA,
according to the ISUOG guidelines (15).
In our study, the first percentile of the computed
normal range varies from 1.31 mm at a CRL of 45 mm to 2.08 mm at a
CRL of 84 mm. There are also available MO percentiles for BPD and
GA. These should not be used in practice as a diagnosis of OSB, but
as a guide from which one should consider the evaluation for the
possibility of a neural tube defect. Recognizing a displaced
mesencephalon could alert the operator to the need for a
supplementary evaluation. Therefore, a search for other indirect
markers in axial and sagittal planes and direct visualization of
the spine in axial view by an expert, using a higher resolution
with transvaginal probe and 3D volume reconstruction is advised
(7,19). Also, the patient could be asked to
have a review after two weeks to confirm the diagnosis (12).
In our opinion, the evaluation of the MO distance
could be easily integrated into the routine first-trimester
screening scan at 11 to 13+6 gestational weeks. Implementation of
this measurement into the routine first-trimester practice could
lead to an early diagnosis of OSB, which gives the parents time for
an informed decision. They could have the option for a fetal
surgery with the intra-uterine closure of the spinal defect, with
promising results, but available only in specialized centers
(20). Otherwise, early termination
of pregnancy is safer and less traumatic for the affected
parents.
In conclusion, we described a simple measurement
between the mesencephalon and the occipital bone, obtained in the
same axial view as the one required for the BPD measurement, in the
first-trimester screening. Its reference values have a good
correlation with CRL, BPD, and GA. Integration into the routine
ultrasound screening in association with the ‘crash sign’ and
recognizing the lower extreme values could lead to an early
diagnosis of OSB. Also, the distance seems to increase in those
patients who did not received folic acid during the first trimester
of pregnancy. We hope that future studies will specify the role of
this measurement in early ultrasound.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DN and MEZ designed the study. DN performed the
measurements within the study. AMA, IAT and DS acquired and
assembled the data. DN, REB, DBN and MEZ analyzed and interpreted
the data. DN, AMA, IAT, DS, REB, DBN and MEZ were involved in
drafting the manuscript and revising it critically for important
intellectual content. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the World Medical Association Declaration of Helsinki and was
approved by the Institutional Board of the ‘Euromedicenter’ Medical
Centre (Iasi, Romania). The pregnant women included in the study
provided written informed consents.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing
interests.
References
|
1
|
Avagliano L, Massa V, George TM, Qureshy
S, Bulfamante GP and Finnell RH: Overview on neural tube defects:
From development to physical characteristics. Birth Defects Res.
111:1455–1467. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Loureiro T, Ushakov F, Montenegro N,
Gielchinsky Y and Nicolaides KH: Cerebral ventricular system in
fetuses with open spina bifida at 11-13 weeks' gestation.
Ultrasound Obstet Gynecol. 39:620–624. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chaoui R, Benoit B, Entezami M, Frenzel W,
Heling KS, Ladendorf B, Pietzsch V, Sarut Lopez A and Karl K: Ratio
of fetal choroid plexus to head size: Simple sonographic marker of
open spina bifida at 11-13 weeks' gestation. Ultrasound Obstet
Gynecol. 55:81–86. 2020. View Article : Google Scholar
|
|
4
|
Ushakov F, Sacco A, Andreeva E, Tudorache
S, Everett T, David AL and Pandya PP: Crash sign: New
first-trimester sonographic marker of spina bifida. Ultrasound
Obstet Gynecol. 54:740–745. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lachmann R, Chaoui R, Moratalla J,
Picciarelli G and Nicolaides KH: Posterior brain in fetuses with
open spina bifida at 11 to 13 weeks. Prenat Diagn. 31:103–106.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Chaoui R, Benoit B, Mitkowska-Wozniak H,
Heling KS and Nicolaides KH: Assessment of intracranial
translucency (IT) in the detection of spina bifida at the
11-13-week scan. Ultrasound Obstet Gynecol. 34:249–252.
2009.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Chaoui R and Nicolaides KH: Detecting open
spina bifida at the 11-13-week scan by assessing intracranial
translucency and the posterior brain region: Mid-sagittal or axial
plane? Ultrasound Obstet Gynecol. 38:609–612. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lachmann R, Picciarelli G, Moratalla J,
Greene N and Nicolaides KH: Frontomaxillary facial angle in fetuses
with spina bifida at 11-13 weeks' gestation. Ultrasound Obstet
Gynecol. 36:268–271. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Chen FC, Gerhardt J, Entezami M, Chaoui R
and Henrich W: Detection of spina bifida by first trimester
screening - Results of the prospective multicenter Berlin IT-Study.
Ultraschall Med. 38:151–157. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ramkrishna J, Araujo Júnior E, Peixoto AB,
Da Silva Costa F and Meagher S: Maxillo-occipital line: A
sonographic marker for screening of open spina bifida in the first
trimester of pregnancy. J Matern Fetal Neonatal Med. 32:4073–4079.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Karl K, Benoit B, Entezami M, Heling KS
and Chaoui R: Small biparietal diameter in fetuses with spina
bifida on 11-13-week and mid-gestation ultrasound. Ultrasound
Obstet Gynecol. 40:140–144. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Finn M, Sutton D, Atkinson S, Ransome K,
Sujenthiran P, Ditcham V, Wakefield P and Meagher S: The aqueduct
of Sylvius: A sonographic landmark for neural tube defects in the
first trimester. Ultrasound Obstet Gynecol. 38:640–645.
2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wertaschnigg D, Ramkrishna J, Ganesan S,
Tse C, Scheier M, Volpe N, Ghi T, Meagher S and Rolnik DL: Cranial
sonographic markers of fetal open spina bifida at 11 to 13 weeks of
gestation. Prenat Diagn. 40:365–372. 2020. View Article : Google Scholar
|
|
14
|
Verburg BO, Steegers EA, De Ridder M,
Snijders RJ, Smith E, Hofman A, Moll HA, Jaddoe VW and Witteman JC:
New charts for ultrasound dating of pregnancy and assessment of
fetal growth: Longitudinal data from a population-based cohort
study. Ultrasound Obstet Gynecol. 31:388–396. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Salomon LJ, Alfirevic Z, Bilardo CM,
Chalouhi GE, Ghi T, Kagan KO, Lau TK, Papageorghiou AT,
Raine-Fenning NJ, Stirnemann J, et al: ISUOG practice guidelines:
Performance of first-trimester fetal ultrasound scan. Ultrasound
Obstet Gynecol. 41:102–113. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kappou D, Papastefanou I, Pilalis A,
Kavalakis I, Kassanos D and Souka AP: Towards detecting open spina
bifida in the first trimester: The examination of the posterior
brain. Fetal Diagn Ther. 37:294–300. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Buisson O, De Keersmaecker B, Senat MV,
Bernard JP, Moscoso G and Ville Y: Sonographic diagnosis of spina
bifida at 12 weeks: Heading towards indirect signs. Ultrasound
Obstet Gynecol. 19:290–292. 2002.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Papastefanou I, Souka AP, Pilalis A,
Panagopoulos P and Kassanos D: Fetal intracranial translucency and
cisterna magna at 11 to 14 weeks: Reference ranges and correlation
with chromosomal abnormalities. Prenat Diagn. 31:1189–1192. 2011.
View Article : Google Scholar
|
|
19
|
Scheier M, Lachmann R, Pětroš M and
Nicolaides KH: Three-dimensional sonography of the posterior fossa
in fetuses with open spina bifida at 11-13 weeks' gestation.
Ultrasound Obstet Gynecol. 38:625–629. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Sacco A, Simpson L, Deprest J and David
AL: A study to assess global availability of fetal surgery for
myelomeningocele. Prenat Diagn. 38:1020–1027. 2018. View Article : Google Scholar
|