Introduction
Thyroid-associated ophthalmopathy (TAO), also
referred to as Graves' ophthalmopathy, is a chronic autoimmune
orbital disease whose annual incidence rate approximates 16 females
and 3 males per 100,000 individuals of the population in the United
States (1). TAO represents the most
common cause of orbital disease in adults; it is associated with
thyroid dysfunction and constitutes the most common extrathyroidal
sign of Graves' disease, featuring hyperthyroidism, diffuse goiter,
ophthalmopathy, and, rarely, dermopathy (2).
Given its unclear pathogenesis and complex clinical
manifestations, current treatment options for TAO, including
glucocorticoids, radiotherapy and immunosuppressive agents, are not
standardized, and their therapeutic effects remain largely elusive
(3). This is particularly the case
for TAO treatment at the inflammatory and unstable stage. At
present, glucocorticoids are widely used to improve ocular symptoms
by initiating anti-inflammatory responses (4).
The efficacy of glucocorticoids may be affected by
several factors, particularly the administration route. For
instance, systemically administered glucocorticoids may have
multiple complications and side effects (5). However, retrobulbar or subconjunctival
injection of corticosteroids for TAO treatment may avoid systemic
complications (6). Previous studies
have revealed the benefits of periocular injection of
methylprednisolone and triamcinolone in improving the symptoms of
TAO (6,7).
At present, studies evaluating the effectiveness of
various corticosteroids used locally to treat Chinese patients with
TAO are scarce. Therefore, the present study aimed to
retrospectively analyze the clinical outcomes of periorbital
injection of triamcinolone acetonide (TA) combined with
dexamethasone (DEX) in 386 Chinese patients with TAO and also
assess factors that affect therapeutic effectiveness.
Patients and methods
Study design and subjects
Patients diagnosed with TAO at the Ophthalmology
Department of West China Hospital, Sichuan University (Chengdu,
China) between November 2015 and August 2018 were enrolled in this
retrospective cohort study. The inclusion criteria were as follows:
i) First diagnosis of TAO; ii) clinical activity score (CAS) ≥3
(active TAO). The following exclusion criteria were applied: i)
Contraindication to steroids (diabetes or systemic hypertension,
gastritis, psychosis or pregnancy); ii) previous treatment of TAO
with steroids or radiation. Patients were included regardless of
their endocrine status.
This retrospective study was performed according to
the Declaration of Helsinki, and approved by the review board of
West China Hospital, Sichuan University (Chengdu, China). Patients
provided written informed consent for the publication of the images
and data in this retrospective study.
Periorbital injection, clinical
assessment and follow-up
Patients included in the present study were treated
with injections of TA (40 mg) combined with DEX (2.5 mg) divided
equally for injection to the supra- and infra-orbital foramen at
4-week intervals (monthly) until no further symptom improvement was
observed. The dose of TA was determined referring to previous
studies (6). The patient was placed
in the supine position and sterile local anesthetic drops were
placed in the eye. Subsequently, the eyelids were cleaned with
povidone-iodine solution. Mixed corticosteroids were injected into
the interior lateral and superior inner quadrants of the orbit,
vertically and slowly by the same physician using a 26-gauge
disposable needle, avoiding the eyeball and surrounding vessels.
The eye was closed, with light pressure immediately placed on the
periorbital area for 10-15 min after needle withdrawal in order to
spread the steroids and prevent hematoma formation. The patient's
pulse and ocular condition were also observed.
All patients were followed up and assessed one month
after each injection. At each visit, the following parameters were
evaluated by the same ophthalmologist (8,9):
Best-corrected visual acuity (BCVA; logMAR), eyelid width (mm),
downward movement of the lids (mm), proptosis (mm; measured by a
Hertel exophthalmometer), clinical activity score of TAO (CAS)
(9,10), modified NOSPECS class (6,11),
assessment of TAO severity (Table I)
(12), limited eye motility (defined
as ≥5˚ reduction of eye movement in any direction assessed by the
Hess chart) (13), intraocular
pressure (IOP) and fundus features. Ocular complications linked to
glucocorticoid injection and systemic adverse reactions were
recorded. All patients underwent a monthly follow-up for half a
year, followed by quarterly follow-up for at least another
year.
 | Table IAssessment of the severity of
thyroid-associated ophthalmopathy. |
Table I
Assessment of the severity of
thyroid-associated ophthalmopathy.
| | Parameter |
|---|
| Severity |
Proptosisa
(mm) | Diplopiab | Visual acuity
(logMAR) |
|---|
| Mild | 15-17 | Intermittent | Normal |
| Moderate | 18-20 | Inconstant | 0.09-0.3 |
| Markedc | >20 | Constant | >0.3 |
Criteria for evaluating treatment
effectiveness
Treatment effectiveness was defined as follows
(12,14): a) Improvement in major outcome
measures: Improvement in downward lid movement ≥2 mm; amelioration
in eye motility (≥5˚ increase in eye movement in any direction);
reduction in proptosis ≥2 mm (measured by Hertel exophthalmometer);
b) improvement in auxiliary outcome measures: Reduction in eyelid
width ≥2 mm; mitigation of eyelid edema, conjunctival hyperemia and
chemosis; c) Improvement in subjective symptoms.
Substantial improvement (very good effectiveness)
was defined as changes in all major and auxiliary outcome measures
and improvement in subjective symptoms. Minor improvement (good
effectiveness) was defined as changes in one or more major and/or
auxiliary outcome measures irrespective of subjective symptom
changes. No change was defined as no improvement in any outcome
measure after periorbital injection.
Statistical analysis
All data were analyzed with SPSS 22 (IBM Corp.).
Continuous variables with a normal distribution were expressed as
the mean ± standard deviation and assessed by a paired t-test.
Variables with a skewed distribution were tested by the Wilcoxon
signed-rank test or the Kruskal-Wallis H test. Fisher's exact test
or the χ2 test was used to compare categorical
variables. The significance level was adjusted to α'=0.017 or
α'=0.0125, considering multiple comparisons. Bivariate analysis was
performed by the non-parametric Mann-Whitney U-test or
Kruskal-Wallis test followed by Dunn's test to examine the
associations of effectiveness with predictive factors.
In the present study, the ordinal logistic
regression model conforming to the proportional odds assumption
(P=0.29) was employed to evaluate the odds ratios (ORs) of factors
potentially affecting the effectiveness of periorbital injection of
corticosteroids. Accordingly, ordinal response variables (the
overall effectiveness of periorbital injection) were defined as
follows: i) 0, ‘no change’; ii) 1, ‘good’ efficacy (minor
improvement); and iii) 2, ‘very good’ efficacy (substantial
improvement). The ORs between ‘no change’ vs. ‘good’/‘very good’
efficacy and ‘no change’/‘good’ vs. ‘very good’ efficacy were
assumed to be identical (15-18).
The relevant independent variables were as follows: Demographic
characteristics, including gender and age; lifestyle parameters
such as smoking and alcohol consumption; a thyroid
function-associated factor, i.e., treatment response of thyroid
dysfunction; and TAO-associated predictors, including CAS score,
NOSPECS class and course of TAO. Variable selection was based on a
previous study assessing the potential of variables for predicting
the clinical outcome (10). A
two-tailed P<0.05 was considered to indicate statistical
significance.
Results
Characteristics of the study
population
A total of 386 patients (515 eyes) were included.
There were 146 males (37.8%) and 240 females (62.2%), aged between
11 and 80 years (mean, 45.14±12.77 years). The average duration of
ocular signs and TAO symptoms was 14.72±6.63 months (range, 8.5-21
months). A total of 262 patients (67.9%) presented with
hyperthyroidism, including 89 cases of unilateral and 173 cases of
bilateral TAO; 89 individuals (23.0%) had euthyroid, including 53
unilateral and 36 bilateral cases (P>0.05). Furthermore, 35
cases (9.1%) presented with primary hypothyroidism. Among the 222
patients treated with anti-thyroid drug, 98 recovered to have
normal thyroid function. Of the 40 cases receiving 131I
therapy, 32 (80%) had hypothyroid. Those patients with thyroid
dysfunction were advised to receive medical endocrinology treatment
during the therapy for TAO.
According to the severity grades of NOSPECS, there
were 173 cases of TAO (45.33%) defined as grade 2, 79 (20.47%) as
grade 3, 96 (24.87%) as grade 4 and 38 (9.84%) as grade 5. A total
of 41 (34.25%) of the 123 patients (including 118 males) with a
smoking history ranging from 2 to 40 years successfully quit
smoking during the treatment. All patients had varying degrees of
eyelid swelling, retraction and sluggishness. Furthermore, 38
patients suffered from corneal epithelial defects without optic
nerve involvement. The clinicopathological characteristics of the
patients are summarized in Table
II.
 | Table IITreatment effectiveness according to
patient characteristics. |
Table II
Treatment effectiveness according to
patient characteristics.
| | Effectiveness, n
(%) | |
|---|
| Variable | Cases (n) | Very good | Good | No change | P-value |
|---|
| Gender | | | | | 0.001a |
|
Male | 146 | 41 (28.08) | 63 (43.15) | 42 (28.76) | |
|
Female | 240 | 135 (56.25) | 82 (34.17) | 23 (9.60) | |
| Age (years) | | | | | 0.150b |
|
15-30 | 21 | 12 (57.14) | 7 (33.33) | 2 (9.52) | |
|
31-45 | 96 | 55 (57.29) | 29 (30.21) | 12 (12.50) | |
|
46-60 | 152 | 64 (42.11) | 56 (38.84) | 32 (21.05) | |
|
≥61 | 117 | 46 (39.32) | 52 (44.43) | 19 (16.24) | |
| TOTD | | | | | 0.001a |
|
R/E | 212 | 129 (60.85) | 63 (29.72) | 20 (9.43) | |
|
R/No | 174 | 53 (30.46) | 76 (43.68) | 45 (25.86) | |
| TAO duration | | | | | 0.001a |
|
<1
year | 284 | 145 (51.06) | 102 (35.92) | 37 (13.03) | |
|
≥1 year | 102 | 31 (30.39) | 43 (42.16) | 28 (27.45) | |
| Smoking | | | | | 0.001a |
|
Yes | 58 | 9 (15.52) | 16 (27.59) | 33 (56.90) | |
|
No | 328 | 171 (52.13) | 125 (38.11) | 32 (9.76) | |
| Alcohol use | | | | | 0.080a |
|
Yes | 109 | 49 (44.96) | 36 (33.03) | 24 (22.02) | |
|
No | 277 | 123 (44.40) | 113 (40.79) | 41 (14.80) | |
| CAS scores | | | | | 0.001b |
|
3 | 135 | 36 (26.67) | 58 (42.96) | 41 (30.37) | |
|
4 | 130 | 54 (41.54) | 59 (45.38) | 17 (13.08) | |
|
5 | 121 | 92 (76.03) | 22 (18.18) | 7 (5.79) | |
| NOSPECS class | | | | | 0.001b |
|
2 | 173 | 119 (68.79) | 48 (27.75) | 6 (3.47) | |
|
3 | 79 | 42 (53.16) | 29(36.71) | 8 (10.13) | |
|
4 | 96 | 29 (30.21) | 36 (37.50) | 31 (32.29) | |
|
5 | 38 | 5 (13.16) | 13 (34.21) | 20 (52.63) | |
| Number of
injections | | | | | 0.02b |
|
2 | 135 | 27 (20.00) | 43 (31.85) | 65 (48.15) | |
|
3 | 160 | 97
(60.62)c | 63 (39.38) | 0 (0.00) | |
|
4 | 91 | 56
(61.54)d | 35 (38.46) | 0 (0.00) | |
| Disease
severity | | | | | 0.023b |
|
Mild | 251 | 116 (46.22) | 87 (34.66) | 48 (19.12) | |
|
Moderate | 75 | 18
(24.00)e | 36 (48.00) | 21 (28.00) | |
|
High | 60 | 14
(23.33)f | 17
(28.33)g | 29 (48.33) | |
Therapeutic evaluation of periorbital
injection
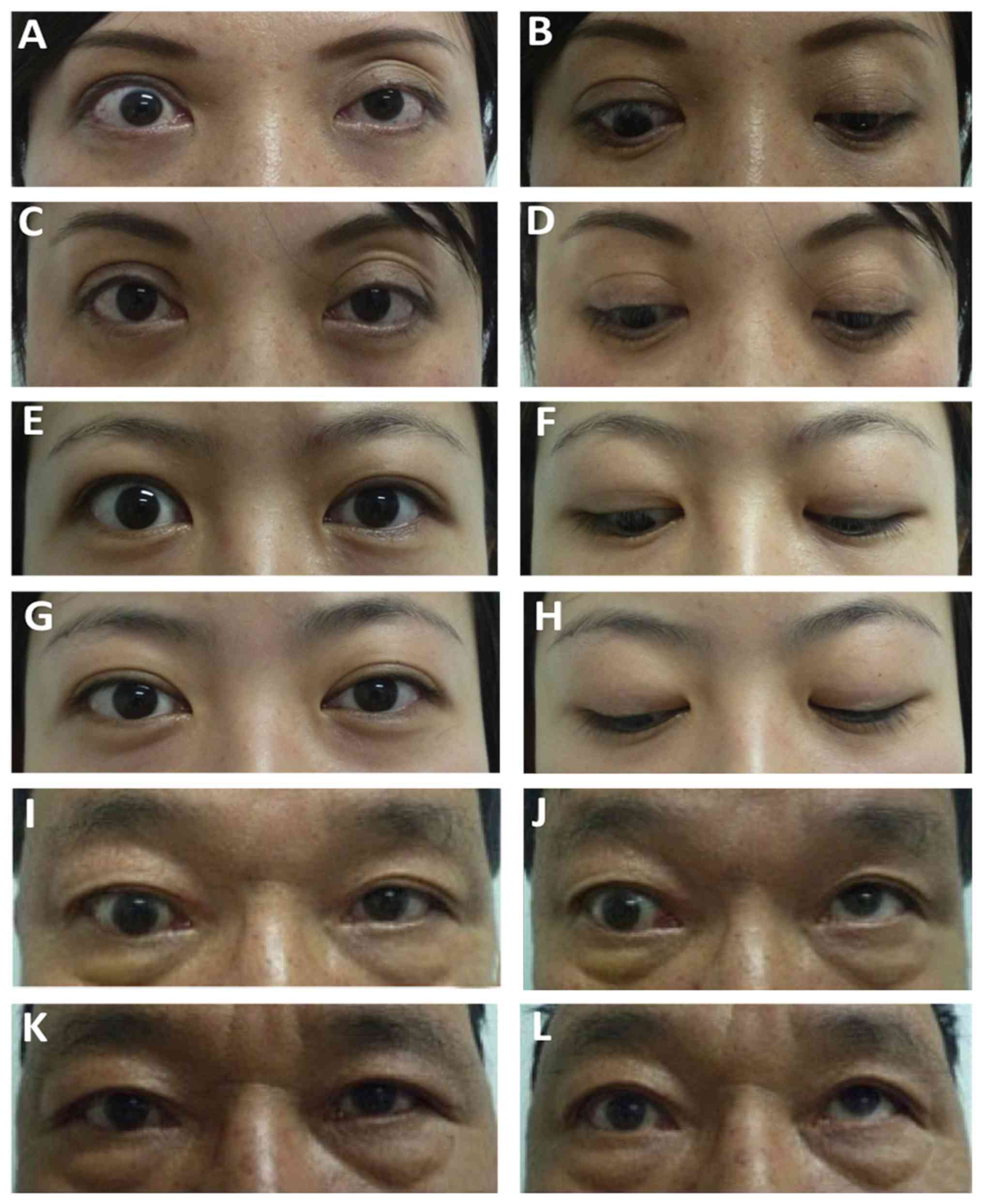
Fig. 1 provides
images of three representative cases prior to and after
treatment.
Symptomatic improvement
A total of 386 patients received periorbital
injection therapy with a 3-5-month follow-up period. Among the 156
patients who experienced tearing, photophobia and blurred vision,
127 (81.41%) exhibited improvement to varying degrees; the BCVA was
significantly improved after treatment (0.16±0.18 vs. 0.35±0.12,
P=0.023). Pain due to eye movement was relieved in 31 of the 43
cases (72.09%).
Sign improvement
Among the 276 cases with eyelid swelling, 225
(81.52%) exhibited improvement; all conjunctival hyperemia cases
were improved after treatment. Eyelid retraction was improved in
167 (62.08%) of the 269 cases (representative cases showed in
Fig. 1A and C). Upper eyelid sluggishness in 133
(46.02%) of 289 patients showed improvement (Fig. 1B and D). A total of 49 (41.52%) of 118 cases
exhibited amelioration of proptosis (Fig. 1I and K). Eye motility disturbance was improved in
29 (30.21%) of the 96 cases (Fig. 1J
and L) and corneal epithelial
involvement was alleviated in 9 (23.68%) of the 38 cases
affected.
Therapeutic effect profile
The patients received at least two injections and
stopped when no significant effect was observed after the last
injection. The number of patients exhibiting substantial
improvement after 3 or 4 injections was higher than that of cases
administered 2 injections (P<0.017). No significant difference
in treatment effectiveness was observed between the 3 and 4
injection groups. These results indicated that the rate of patients
with substantial improvement increased with the number of
injections (Table II).
The patients were stratified into groups of mild to
severe TAO based on the degree of proptosis, diplopia and visual
acuity impairment, according to the assessment criteria of TAO
severity (Table I). A total of 116
cases (46.22%) of mild TAO were substantially improved, which was a
higher rate compared with that in patients with moderate and severe
TAO. Significantly more patients with moderate TAO had minor
improvements after treatment compared with the severe TAO group
(Table II).
Of the patients with TAO administered radioactive
iodine (131I) treatment for Graves' hyperthyroidism, 80%
presented with hypothyroid. Thus, thyroid function influenced the
response to treatment. After periorbital injection of
corticosteroids, significantly more patients in the primarily
euthyroid group had ‘very good’ effectiveness in comparison with
that in the abnormal after medical treatment (MT) group (P=0.008;
Table III). Compared with the
group of primary euthyroid or patients with normal thyroid function
after MT, the group of hypothyroidism after treatment with
131I therapy had a significantly lower proportion of
patients with ‘very good’ effectiveness (P=0.001 and P=0.006,
respectively; Table III).
 | Table IIIEffects of thyroid dysfunction on
therapeutic effectiveness. |
Table III
Effects of thyroid dysfunction on
therapeutic effectiveness.
| | Effectiveness, n
(%) |
|---|
| Thyroid
function | Very Good | Good | No change |
|---|
| Primarily euthyroid
(n=89) | 37 (41.57) | 44 (49.43) | 8 (10.10) |
| Normal (after MT)
(n=98) | 32 (32.65) | 55 (56.12) | 11 (11.22) |
| Abnormal (after MT)
(n=124) | 27
(21.77)c | 67 (54.03) | 30 (24.19) |
| Hypothyroidism
(after I131) (n=32) | 5
(15.62)a,d | 11
(34.38)b | 16 (50.0) |
Management of adverse effects of
periorbital injection
A total of 5 subjects developed subcutaneous
ecchymosis, which was relieved gradually within two weeks during
follow-up by pressure manipulation using swabs to hemostasis.
Furthermore, 3 cases exhibited conjunctival edema that was eased
after applying swabs with erythromycin ointment to return the
prolapsed conjunctiva back into the eyelid. In addition, 1 case
developed a self-limiting subconjunctival hemorrhage. A total of 31
cases (8.03%) exhibited high IOP (24.45±2.06 mmHg) after injection.
After stopping the injections and administering ocular hypotensive
agents, 3 cases had no improvement and 2 had increased fundus C/D
values and visual field defects, as well as symptoms and signs of
corticosteroid-induced glaucoma. Furthermore, 2 post-menopausal
females (49 and 55 years old) had a menstruation after treatment.
In addition, menstrual disorders occurred in 11 out of 90 (16.67%)
pre-menopausal females and the menstrual cycle became regular after
stopping or completing the treatment. A total of 2 patients had
moon face symptoms. No other ocular or systemic adverse reactions,
including infection, penetrating eye, eye muscle motility
disturbance (restriction of eye movement) and cerebral vascular
events were observed (9).
Furthermore, the rate at which patients experienced an effective
outcome significantly increased with the number of periorbital
injections.
Factors associated with the
effectiveness of periorbital injection
According to the univariate analysis (Table II), clinical outcome was associated
with patient gender, treatment outcomes of thyroid dysfunction
(TOTD), duration of TAO, smoking habit, number of injections, CAS
and disease severity (NOSPECS; P<0.05). However, age and
drinking habits were not significantly associated with the
effectiveness of periorbital injection.
According to the multivariate analyses, the adjusted
factors that significantly and favorably associated with treatment
efficacy in the ordinal logistic regression were female (vs. male)
gender, no smoking, stable thyroid function, low NOSPECS class and
high CAS (Table IV). Specifically,
male patients were less likely to exhibit a substantial improvement
compared with females (OR=0.32, P=0.001). Compared with patients
with a history of smoking, non-smokers had a higher odds of good
effectiveness (OR=4.62, P=0.008). Although patients with ocular
sign for ≤1 year had a higher odds of effective treatment than
those with a longer disease course, the course of TAO had no
significant association with the clinical outcome (P=0.22). In
terms of NOSPECS grades, patients with a grade of ≤3 had a higher
odds of substantial improvement compared with those with grade 5
(all P<0.05). Patients with grade 4 tended to have a higher
effectiveness than those with grade 5, but with no statistical
significance (P>0.05). Finally, patients with a relapse of
thyroid dysfunction had poorer therapeutic effectiveness compared
with those with primarily euthyroid or stable thyroid function
(OR=0.41, P=0.002).
 | Table IVResults of ordinal logistic
regression analysis regarding effective treatment. |
Table IV
Results of ordinal logistic
regression analysis regarding effective treatment.
| | 95% CI | |
|---|
| Item | OR | Lower | Upper | P-value |
|---|
|
Sex (male
vs. female) | 0.32 | 0.16 | 0.62 | 0.001 |
|
TOTD (R/E
vs. R/No) | 2.43 | 1.39 | 4.17 | 0.002 |
|
Smoking (yes
vs. no) | 0.22 | 0.07 | 0.67 | 0.008 |
|
TAO duration
(>1 vs. ≤1 year) | 0.72 | 0.42 | 1.21 | 0.220 |
| CAS |
|
3 vs. 4 and
5 | 0.10 | 0.04 | 0.20 | <0.001 |
|
3 and 4 vs.
5 | 0.20 | 0.09 | 0.42 | <0.001 |
| NOSPECS class |
|
2 vs. 3, 4
and 5 | 8.59 | 3.78 | 19.69 | <0.001 |
|
2 and 3 vs.
4 and 5 | 4.06 | 1.52 | 10.80 | 0.005 |
|
2, 3 and 4
vs. 5 | 1.95 | 0.89 | 4.31 | 0.097 |
Discussion
The present study demonstrated the effectiveness of
periorbital injection of corticosteroids, namely TA and DEX in
combination. This clinical outcome was affected by several factors,
including gender, smoking, as well as disease severity and
activity.
Treatment options for different TAO stages remain
controversial, as systemic administration of glucocorticoids may
exert numerous side effects (5). The
present study demonstrated that periorbital injection of TA
combined with DEX improved the symptoms of TAO with limited
systemic adverse effects and side effects. TA, an insoluble
long-acting synthetic fluorinated corticosteroid, is slowly
absorbed into the eye; its potency is 5 times higher than that of
cortisol, with effects sustained for 2-3 weeks or even longer
(19). Thus, TA injection is
required every 3-4 weeks. DEX is also a long-acting corticosteroid
with a potency 25 times that of short-acting products (20), and combination with TA requires
reduced injection dosages and times in patients with mild to
moderate TAO compared with each corticosteroid applied as a
monotherapy. According to our own experience, combination therapy
of TA and DEX for patients with TAO may also achieve a prolonged
local steroid concentration and higher potency to inhibit the
inflammation compared with the use of TA only. In addition, a
previous study indicated that periorbital injection of a
combination of TA and DEX may improve lid retraction and limitation
of eye movement in patients with TAO (21).
After periorbital injection, the symptoms of TAO
exhibited various levels of improvement. Among TAO signs,
substantial remissions in eyelid swelling, upper eyelid lag and
retraction were achieved in the present study. Proptosis,
involvement of eye muscles and corneal epithelial defects were also
improved to varying degrees. Next, patients with active TAO were
divided into groups based on disease severity (mild to severe)
according to the degree of proptosis, diplopia and visual acuity
impairment, which referred to the assessment criterion of TAO
severity (13). This assessment can
compensate for the subjective index of NOSPECS classification.
Although substantial improvement was achieved in patients with mild
to moderate proptosis, eye muscles were not successfully improved.
These results indicated that periorbital injection of
corticosteroids was more effective in patients with mild to
moderate TAO.
The results also indicated that patients with TAO
injected 3 or 4 times had a more pronounced improvement than those
who received two injections. This suggests that the effectiveness
of periorbital injection of these corticosteroids is
cumulative.
Next, factors potentially predicting clinical
outcome were assessed. The present analysis indicated that gender,
smoking habits, CAS and NOSPECS class significantly affected the
effectiveness of the treatment. Indeed, the current treatment was
more effective in female patients compared with males, while
smoking decreased the therapeutic effectiveness. A previous
meta-analysis revealed a strong association of cigarette smoking
with TAO progression and deterioration (22). In addition, smoking was previously
reported to attenuate the effectiveness of systemic glucocorticoids
as well as orbital radiotherapy (23). However, the pathogenic mechanisms
underlying the effects of smoking in TAO remain to be fully
elucidated. Thus, it is imperative for patients with TAO to quit
smoking. The present study also demonstrated that patients with
mild or moderate TAO were more likely to exhibit improvement after
treatment compared with cases of severe TAO. These results
indicated that periorbital injection of corticosteroids alone in
cases of severe TAO may not suffice and should be combined with
systemic administration of glucocorticoids or orbital radiotherapy.
Furthermore, patients with a shorter course of TAO responded better
to treatment than those with a longer course, although the duration
of TAO was not significantly associated with the effectiveness of
the treatment. Therefore, early treatment is also recommended for
TAO. Previous studies indicated that progressive exacerbation of
TAO characterized by lymphocyte infiltration may occur over 6-24
months and is considered the active progressive phase when
anti-inflammatory therapy is indicated (24). This is followed by a weakened
inflammatory activity and a static phase, which may lead to orbital
tissue fibrosis. Cases of active TAO with high CAS scores were also
likely to respond better to periorbital injection of steroids
compared with the low CAS score group. The severity of TAO reflects
the degree of orbital inflammation, which is remedied by the
anti-inflammatory function of glucocorticoids (24). In addition, thyroid dysfunction may
increase the risk of aggravation of TAO and reduce the
effectiveness of periorbital injection. Previous studies revealed
that thyroid-stimulatory hormone receptor antibody and thyroxine or
triiodothyronine levels are associated with the worsening of TAO
(25-27).
However, further investigation is required to confirm the roles of
the above factors in the development of TAO. It is known that
131I administration triggers hypothyroidism and
autoimmune inflammation, leading to the deterioration of TAO
(28). The risk of radioactive
iodine therapy is higher in comparison with that of anti-thyroid
drugs (29,30). As indicated above, patients with TAO
and thyroid dysfunction had a poorer response to periorbital
injection of corticosteroids than those with stable thyroid
function. Therefore, thyroid function is considered the key factor
influencing TAO treatment and 131I should be cautiously
considered in hyperthyroid patients with ocular symptoms.
High intraocular pressure occurred in 8.03% of
patients with complications caused by periorbital injection of
corticosteroids and 83% of these patients recovered after
discontinuation of treatment. A total of three patients had
corticosteroid-induced glaucoma. A previous study demonstrated that
local application of corticosteroids may lead to high IOP (31). While the associated pathogenesis
remains elusive, a range of classical theories have been suggested.
Steroid application has been previously found to reduce cellularity
of the trabecular meshwork and increase extracellular matrix
deposition, leading to increased aqueous outflow resistance and
rise in IOP (32,33). In the present study, after treatment,
menstrual disorders arose in 16.67% of pre-menopausal patients,
while menstruation occurred in two post-menopausal patients, whose
condition returned to normal after treatment discontinuation.
Menstrual irregularities and amenorrhea are known side effects of
DEX (34,35). Therefore, female patients should be
informed of the possibility of menstrual disorders during
treatment.
The present study had certain strengths, namely that
the effectiveness of periocular injection of corticosteroids in the
treatment of Chinese patients with TAO was assessed and factors
affecting the clinical outcome were reported to guide clinical
practice.
However, there were also certain limitations. First,
selection bias could not be ruled out, as the recruited patients
visited the hospital for ocular symptoms. Furthermore, the sample
size was relatively small and should be increased in future
investigations. These issues limit the generalizability of the
present results. However, since West China Hospital (Chengdu,
China), the largest hospital in Western China, encounters a large
number of patients with TAO, some of whom have also come from
another greater area. The present study may be considered to be
representative of the population of patients with TAO in Southwest
China.
In conclusion, the present study demonstrated that
peribulbar injection of TA combined with DEX is an effective and
safe treatment option for active TAO, particularly for mild to
moderate active TAO. In addition, periorbital injection is a
low-cost treatment and easy to administer, with almost no systemic
side effects. However, patients with severe TAO may not achieve a
satisfying response to periorbital injection of corticosteroids
alone. Furthermore, in patients with TAO and thyroid dysfunction,
appropriate endocrine therapy must be considered when applying
periorbital injection of corticosteroids.
Acknowledgements
Not applicable.
Funding
The present study was supported by the 1.3.5 Project
for Disciplines of Excellence-Clinical Research Incubation Project,
West China Hospital, Sichuan University (grant no. 2018HXFH024) and
the Post-Doctoral Research Project, West China Hospital, Sichuan
University (grant no. 2019HXBH051) and Sichuan provincial science
and technology program (18ZDYF1977).
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YJW and WMH designed the study. MY, YYZ and BXD
performed experiments and recorded the data. YJW and MY were
responsible for statistical analysis and data interpretation. YJW,
BXD and WMH prepared the manuscript. All authors read and approved
the final manuscript.
Ethical approval and consent to
participate
All procedures performed in this retrospective study
involving human participants were in accordance with and approved
by the review board of West China Hospital of Sichuan University
and conformed to the Declaration of Helsinki.
Patient consent for publication
Patients provided written informed consent for the
publication of their images and data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bahn RS: Graves' ophthalmopathy. N Engl J
Med. 362:726–738. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Barrio-Barrio J, Sabater AL, Bonet-Farriol
E, Velázquez-Villoria Á and Galofré JC: Graves' ophthalmopathy:
VISA versus EUGOGO classification, assessment, and management. J
Ophthalmol. 2015(249125)2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bartalena L, Baldeschi L, Boboridis K,
Eckstein A, Kahaly GJ, Marcocci C, Perros P, Salvi M and Wiersinga
WM: European Group on Graves' Orbitopathy (EUGOGO). The 2016
European Thyroid Association/European Group on Graves' Orbitopathy
Guidelines for the management of Graves' Orbitopathy. Eur Thyroid
J. 5:9–26. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dibas A and Yorio T: Glucocorticoid
therapy and ocular hypertension. Eur J Pharmacol. 787:57–71.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gao G, Dai J, Qian Y and Ma F:
Meta-analysis of methylprednisolone pulse therapy for Graves'
ophthalmopathy. Clin Exp Ophthalmol. 42:769–777. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ebner R, Devoto MH, Weil D, Bordaberry M,
Mir C, Martinez H, Bonelli L and Niepomniszcze H: Treatment of
thyroid associated ophthalmopathy with periocular injections of
triameinolone. Br J Ophthalmol. 88:1380–1386. 2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Alkawas AA, Hussein AM and Shahien EA:
Orbital steroid injection versus oral steroid therapy in management
of thyroid-related ophthalmopathy. Clin Exp Ophthalmol. 38:692–697.
2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Frueh BR: Why the NO SPECS classification
of Graves' eye disease should be abandoned, with suggestions for
the characterization of this disease. Thyroid. 2:85–88.
1992.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mourits MP, Koornneef L, Wiersinga WM,
Prummel MF, Berghout A and van der Gaag R: Clinical criteria for
the assessment of disease activity in Graves' ophthalmopathy: A
novel approach. Br J Ophthalmol. 73:639–644. 1989.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mourits MP, Prummel MF, Wiersinga WM and
Koornneef L: Clinical activity score as a guide in the management
of patient with Graves' ophthalmopathy. Clin Endocrinol. 47:9–14.
1997.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wiersinga WM, Prummel MF, Mourits MP,
Koornneef L and Buller HR: Classification of the eye changes of
Graves' disease. Thyroid. 1:357–360. 1991.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bartalena L, Pinchera A and Marcocci C:
Management of Graves' Ophthalmopathy: Reality and perspectives.
Endocr Rev. 21:168–199. 2000.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Watts P, Nayak H, Lim MK, Ashcroft A,
Madfai HA and Palmer H: Validity and ease of use of a computerized
Hess chart. J AAPOS. 15:451–454. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nakamura T, Wakakura M and Ishikawa S:
Exophthalmometric values in contemporary Japanese population. Jpn J
Clin Ophthalmol. 46:1031–1035. 1992.
|
|
15
|
McCullagh P: Regression models for ordinal
data. J R Stat Soc. 42:109–142. 1980.
|
|
16
|
Ananth CV and Kleinbaum DG: Regression
model for ordinal responses: A review of methods and applications.
Int J Epidemiol. 26:1323–1332. 1997.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bender R and Grouven U: Using binary
logistic regression models for ordinal data with non-proportional
odds. J Clin Epidemiol. 51:809–816. 1998.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Adeleke KA and Adepoju AA: Ordinal
logistic regression model: An application to pregnancy outcomes. J
Math Stat. 6:279–285. 2010.
|
|
19
|
Xu D, Liu Y, Xu H and Li H: Repeated
triamcinolone acetonide injection in the treatment of upper lid
retraction in patients with thyroid-associated ophthalmopathy. Can
J Ophthalmol. 47:34–41. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zoorob RJ and Cender D: A different look
at corticosteroids. Am Fam Physician. 58:443–450. 1998.PubMed/NCBI
|
|
21
|
Bagheri A, Abbaszadeh M and Yazdani S:
Intraorbital steroid injection for active thyroid ophthalmopathy. J
Ophthalmic Vis Res. 15:69–77. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Vestergaard P: Smoking and thyroid
disorders-A meta-analysis. Eur J Endocrinol. 146:153–161.
2002.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hegediüs L, Brix TH and Vestergaard P:
Relationship between cigarette smoking and Graves' ophthalmopathy.
J Endocrinol Invest. 27:265–271. 2004.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hiromatsu Y, Eguchi H, Tani J, Masataka K
and Yasuo T: Graves' Ophthalmopathy: Epidemiology and natural
history. Intern Med. 53:353–360. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wall JR and Lahooti H: Pathogenesis of
thyroid eye disease-does autoimmunity against the TSH receptor
explain all cases. Endokrynol Pol. 61:222–227. 2010.PubMed/NCBI
|
|
26
|
Stan MN and Bahn RS: Risk factors for
development or deterioration of graves' ophthalmopathy. Thyroid.
20:777–783. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Träisk F, Tallstedt L, Abraham-Nordling M,
Andersson T, Berg G, Calissendorff J, Hallengren B, Hedner P, Lantz
M, Nyström E, et al: Thyroid-associated ophthalmopathy after
treatment for Graves' hyperthyroidism with antithyroid drugs or
iodine-131. J Clin Endocrinol Metab. 94:3700–3707. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bartalena L, Marcocci C, Bogazzi F,
Manetti L, Tanda ML, Dell'Unto E, Bruno-Bossio G, Nardi M,
Bartolomei MP, Lepri A, et al: Relation between therapy for
hyperthyroidism and the course of Graves' ophthalmopathy. N Engl J
Med. 338:73–78. 1998.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang R, Tan J, Zhang G, Zheng W and Li C:
Risk factors of hepatic dysfunction in patients with Graves'
hyperthyroidism and the efficacy of 131 iodine treatment. Medicine.
96(e6035)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Louvet C, De Bellis A, Pereira B, Bournaud
C, Kelly A, Maqdasy S, Roche B, Desbiez F, Borson-Chazot F,
Tauveron I and Batisse-Lignier M: Time course of Graves'
orbitopathy after total thyroidectomy and radioiodine therapy for
thyroid cancer. Medicine (Baltimore). 95(e5474)2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Jones R III and Rhee DJ:
Corticosteroid-induced ocular hypertension and glaucoma: A brief
review and update of the literature. Curr Opin Ophthalmol.
17:163–167. 2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Skuta GL and Morgan RK: Corticosteroid
induced glaucoma. In: The glaucoma. Ritch R, Shields MB and Krupin
T (eds). 2nd edition. Mosby, St. Louis, MO, pp17721188, 1996.
|
|
33
|
Razeghinejad MR and Katz LJ:
Steroid-induced iatrogenic glaucoma. Ophthalmic Res. 47:66–80.
2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Mahajan VK, Sharma NL, Sharma RC and Garg
G: Twelve-year clinico-therapeutic experience in pemphigus: A
retrospective study of 54 cases. Int J Dermatol. 44:821–827.
2005.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Abramavicius S, Velickiene D and
Kadusevicius E: Methimazole-induced liver injury overshadowed by
methylprednisolone pulse therapy. Medicine.
96(e8159)2017.PubMed/NCBI View Article : Google Scholar
|