Introduction
The incidence of breast cancer is increasing and
shifting to younger populations worldwide, becoming the main cause
of cancer-associated death in females (1). In 2012, there were an estimated 1.66
million new breast cancer cases (25% of all cancer cases) and
521,000 breast cancer-associated deaths (2). In 2015, breast cancer accounted for
~15% of newly diagnosed cancer cases in China (3). In Western countries, breast cancer
primarily occurs in 55-60-year-old women, but the trend of onset in
China is significantly earlier, occurring primarily in
45-55-year-old middle-aged and young women (4). The occurrence of breast cancer in
younger women has threatened the work, quality of life and health
of those affected (5). Currently,
the main treatment strategies for breast cancer are surgery,
radiotherapy, chemotherapy, endocrine therapy and
molecular-targeted therapy, which are all auxiliary (6). However, the side effects of
chemotherapy in patients remain serious, with 39.7% of patients
suffering from neutropenia and infection (7). The cardiotoxicity of chemotherapeutic
drugs is a well-known major adverse reaction affecting the quality
of life and mortality of patients with cancer (8). Anticancer drugs have been reported to
cause various types of cardiac toxicity, including heart failure,
bradycardia, prolonged QT intervals, myocardial ischemia and
cardiomyopathy (9). Neurotoxicity is
characterized by numbness and tingling of the fingers and toes, as
well as myalgia and myasthenia (10). Due to the aforementioned toxic and
adverse effects of chemotherapeutic drugs, a number of patients
have to reduce their dosage and prolong the chemotherapy cycle
(11). Furthermore, chemotherapy
cessation leads to treatment failure (11). While prolonged survival remains the
primary goal of chemotherapy, symptom relief and quality of life
are also important treatment considerations (12). Therefore, bioactive peptides are
rapidly being developed as potential anticancer drugs, which may
reduce the side effects and increase the sensitivity of traditional
chemotherapeutic drugs without altering their anticancer effect
(13).
A wide range of bioactive peptides exist in natural
resources, including specific small molecular protein fragments
that, although inactive within the protein sequence, can be
released during proteolysis or fermentation and are activated by
the digestive, endocrine, cardiovascular, immune and nervous
systems, which serve as important roles in human health (14). Several peptides released from animal
proteins have been reported to have different health effects in
vitro and in vivo, including antimicrobial properties,
blood pressure reduction, cholesterol reduction, antithrombotic and
antioxidant activity and opioid-like activity (14-16).
These peptides have also been reported to enhance mineral
absorption and bioavailability, exhibit cellular and
immunomodulatory effects and exhibit antiobesity and antigenotoxic
activities (14-16).
Bioactive peptides have low immunogenicity, excellent tissue
penetration, low production costs and are easy to modify to enhance
their stability and biological activity within the body, making
these molecules ideal candidates for cancer therapy (17). The anticancer bioactive peptide
(ACBP) used in the present study is a low molecular weight
bioactive substance extracted from goat spleen following induced
immunization (relative molecular weight, <8000 Da; patent no.
ZL961222236.0), which is a novel method of anticancer biological
preparation. A previous study has reported that ACBP inhibits tumor
angiogenesis, regulates protein degradation, interferes with DNA
synthesis, regulates the cell cycle, induces apoptosis and
influences further antitumor mechanisms (18). A large number of previous cell- and
animal-based experiments reported that ACBP served an inhibitory
effect on the BGC-823 and MGC-803 human gastric cancer cell lines,
the MKN-45 leukemia cell line, the H-22 hepatoma cell line, the CNE
nasopharyngeal carcinoma cell line and the GBC-SD gallbladder
cancer cell line (19-21).
Collectively, these aforementioned studies suggested that ACBP may
be a potential tumor stem cell-targeted drug and when combined with
chemotherapeutic drugs, ACBP may effectively improve their
therapeutic efficacy and reduce their toxicity in patients
(22).
Docetaxel (DTX) is an effective anticancer agent
that is widely used and has demonstrated extensive anticancer
activity against breast, lung, pancreatic, prostate, ovarian and
head and neck cancer (23-26).
DTX is one of the most commonly used chemotherapy drugs for breast
cancer (23). DTX binds to the
β-subunit of microtubule proteins, leading to stable and
non-functional microtubule formation by promoting polymerization
and inhibiting decomposition, ultimately resulting in mitosis
arrest and apoptosis induction (10). Therefore, the present study
investigated the effect of the intermittent short-term application
of ACBP and ACBP combined with DTX, on the quality of life of nude
mice bearing human breast cancer tumors. Furthermore, the
expression of p53, p21 and Ki67 were assessed. The effect of ACBP
on the human breast cancer cell line MDA-MB-231 in nude mice, as
well as the toxicity-reducing and sensitivity-increasing mechanisms
of ACBP were also studied.
Materials and methods
Cell lines and mice
All animal experiments were approved by the Ethics
Committee for Animal Experiments of Inner Mongolia Medical College
(approval no. YKD2016152). A total of 40 female Balb/c-Nu nude mice
(age, 4-6 weeks; weight, 16±2 g) of specific pathogen-free grade
were used. The animals were purchased from Beijing Weitong Lihua
Experimental Animal Technology Co., Ltd. [license no. SCXK
(Beijing) 2012-0001]. The human breast cancer cell line MDA-MB-231
was purchased from the China Infrastructure of Cell Line Resources,
Institute of Basic Medical Sciences, Chinese Academy of Medical
Sciences.
Establishment of an
axillary-transplanted tumor model using the human breast cancer
cell line MDA-MB-231 in nude mice
The human breast cancer cell line, MDA-MB-231, was
cultured in DMEM (Thermo Fisher Scientific, Inc.) supplemented with
10% fetal calf serum (HyClone; GE Healthcare Life Sciences) and 1%
penicillin-streptomycin solution (Thermo Fisher Scientific, Inc.)
in a humidified atmosphere of 5% CO2 at 37˚C. The cells
in the logarithmic growth phase were selected for further
experiments. A single-cell suspension of 5x107/ml was
used for injection.
Mice were maintained at 20-25˚C with 40-70% humidity
and free access to food and drinking water. Animals were also
housed under a 12 h light/dark cycle. Eating, feeding and operating
procedures strictly followed aseptic principles. After 3 days of
free access to food in nude mice, a 0.1 ml single-cell suspension
was inoculated into the right axilla of the 40 nude mice. The
activity and tumorigenesis of the nude mice were observed daily. At
6 days post-injection, rice-sized nodules were identified at the
injection site of the nude mice and the tumor formation rate was
100%. Therefore, the breast cancer model was successfully
established in nude mice.
Experimental grouping, administration
and observation records
The 40 nude mice were randomly divided into five
groups (n=8) as follows: Normal saline group (NS), anticancer
bioactive peptide group (ACBP), docetaxel group (DTX), ACBP
combined DTX treatment group (MIX) and ACBP combined with low dose
DTX treatment group (L-MIX). The treatments were administered by
tail vein injection twice a week for a total of 3 weeks. The NS
group was administered 0.4 ml saline, the ACBP group was
administered 0.4 ml ACBP (70 mg/ml; Prepared by The Clinical
Medical Research Center, Affiliated Hospital of Inner Mongolia
Medical University), the DTX group was administered 5 mg/kg DTX
(0.5 ml/20 mg; Prepared by Jiangsu Hengrui Pharmaceutical Co.,
Ltd.; Chinese medicine standard no. H20020543), the MIX group was
administered 0.4 ml ACBP combined with 5 mg/kg DTX, and the L-MIX
was administered 0.4 ml ACBP combined with 2.5 mg/kg DTX. The
activity status of the nude mice was observed daily. The weight and
tumor boundaries were measured every other day to calculate the
tumor volume (V) using the following formula: V=ab2/2,
where a is tumor length and b is the shortest tumor diameter. Food
and water intake were measured every 2 days to calculate the
quantity consumed. After 3 weeks, the retro-orbital blood of nude
mice were collected following anesthesia and the nude mice were
subsequently euthanized by cervical dislocation. The tumor tissues,
livers and spleens of the nude mice were isolated and weighed. The
isolated tissues were divided into two parts: One was fixed in 4%
paraformaldehyde for 24 h at 25˚C, and the other was stored at
-80˚C until further analysis.
Determination of the liver and spleen
coefficients
Following sacrifice, the body weights of the nude
mice were measured. The livers and spleens were removed from the
mice, dried with filter paper and were then weighed. The liver and
spleen coefficients were calculated as follows: Liver
coefficient=liver mass/nude mouse body mass and spleen
coefficient=spleen weight/body mass of nude mice.
Paraffin-embedded tissue sections and
hematoxylin & eosin staining
Tissues fixed in 4% paraformaldehyde were trimmed to
a size of 5x5x3 mm3, placed in an embedding box, labeled
and slowly flushed with flowing water overnight at 25˚C. The tumor
tissue was dehydrated with an ascending ethanol series in 30 min
increments as follows: 50% ethanol, 60% ethanol, 70% ethanol, 80%
ethanol, 95% ethanol I, 95% ethanol II, 95% ethanol I and anhydrous
ethanol II at 25˚C. The liver tissue was dehydrated with an
ascending ethanol series as follows: 50% ethanol for 15 min, 60%
ethanol for 15 min, 70% ethanol for 15 min, 80% ethanol for 15 min,
95% ethanol I for 30 min, 95% ethanol II for 30 min, 95% ethanol I
for 30 min and anhydrous ethanol II for 30 min at 25˚C.
Furthermore, the spleen tissue was dehydrated with an ascending
ethanol series as follows: 50% ethanol for 10 min, 60% ethanol for
10 min, 70% ethanol for 10 min, 80% ethanol for 10 min, 95% ethanol
I for 30 min, 95% ethanol I for 30 min, anhydrous ethanol II for 30
min and anhydrous ethanol II again for 30 min at 25˚C. The tissue
sections were deparaffinized using xylene I for 20 min and xylene
II for 20 min at 37˚C. Subsequently, the wax block was cut into
4-µm-thick slices for hematoxylin & eosin staining for 2 h at
25˚C.
p53, p21 and Ki67 protein expression
in transplanted tumor tissues as detected by
immunohistochemistry
Streptavidin-peroxidase immunohistochemistry was
performed to analyze the protein expression of p53, p21 and Ki67 in
transplanted tumor tissues. For dewaxing (xylene I for 10 min and
xylene II for 10 min), hydration and antigen repair (1,000 ml
citrate buffer), the fragments were heated at 60˚C for 1 h until
the wax melted. Paraffin embedded sections were then rehydrated
using a descending alcohol series. Subsequently, the endogenous
peroxidase/phosphatase activity was blocked by incubating sections
with 3% H2O2 at room temperature for 9 min.
Sections were then treated with the following primary antibodies,
washed three times for 3 mins with PBS and placed in a wet box
overnight at 4˚C: p53 (1:50; Abcam; cat. no. ab32049), p21 (1:50;
Cell Signaling Technology, Inc.; cat. no. 2946) and Ki-67 (1:150;
Abcam; cat. no. sc-9976). Subsequently, secondary antibodies (1:50;
Abcam; cat. no. ab205718) were added to the slides for 30 min at
room temperature. PBS was added to the sections for 3 min at 25˚C
to wash away the DAB (Abcam; cat. no. ab64238) chromogenic agent
and subsequently, the slides were counterstained with hematoxylin
& eosin for 5 min at 25˚C. The sections were slowly rinsed with
running water for 5 sec, and 1% ethanol hydrochloride was mixed 10
times for cell differentiation. The samples were then rinsed with
tap water for 30 sec. Subsequently, the samples were observed under
a light microscope (magnification, x100) for statistical analysis
by a semiquantitative scoring system. The staining intensity was
scored as follows: No staining, 0; light yellow, 1; brown, 2; or
tan, 3. Four high power visual fields were randomly selected and
the percentage of positive cells was determined as follows: No
positive cells, 0; positive cells <25%, 1; positive cells
<25-49%, 2; and positive cells >50%, 3. The final score was
calculated as staining intensity x percentage of positive cells.
Scores <3 were defined as negative and scores ≥3 were defined as
positive (27).
Detection of p53, p21 and Ki67 mRNA
expression in transplanted tumor tissues by reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from the tumor tissues using
TRIzol® reagent (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Pectrophotometric
quantification was utilized to determine RNA purity using an OD
ratio of 260 nm/280 nm and a BeekmanDU 800 UV spectrophotometer. A
total of 1 mg total RNA was reverse transcribed into first-strand
cDNA using the Revert Aid First Strand cDNA Synthesis kit (Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
qPCR was performed using the Taq-Man™ Gene Expression assay
(Applied Biosystems; Thermo Fisher Scientific, Inc.) on an Mx3000P
real-time PCR system (Agilent Technologies, Inc.) according to the
manufacturer's instructions. The primer pairs used in the present
study were designed and synthesized by Sangon Biotech Co., Ltd.
(Table I). The following
thermocycling conditions were used: Initial denaturation at 95˚C
for 5 min; 35 cycles of denaturation at 95˚C for 10 sec, annealing
at 60˚C for 30 sec, elongation at 60˚C for 30 sec and a final
extension step at 60˚C for 40 sec. mRNA levels were normalized to
the internal reference gene GAPDH. ∆∆CT values of each
group were calculated separately=[(CT experimental target gene - CT
internal reference target gene)-(CT control target gene-CT internal
reference control gene)]. mRNA relative expression was calculated
using 2-∆∆Cq values (28).
 | Table IPrimer sequences used for reverse
transcription-quantitative PCR. |
Table I
Primer sequences used for reverse
transcription-quantitative PCR.
| | Sequence
(5'→3') |
|---|
| Gene | Forward | Reverse |
|---|
| p53 |
TCAACAAGATGTTTTGCCAACTG |
ATGTGCTGTGACTGCTTGTAGATG |
| p21 |
AAACTTTGGAGTCCCCTCAC |
AAAGGCTCAACACTGAGACG |
| Ki67 |
CTTGCCTCCTAATACGCCTCTC |
CCTGACTCTTGTTTTCCTGATGGT |
| GAPDH |
TCCACCACCCTGTTGCTGTA |
ACCACAGTCCATGCCATCAC |
Statistical analysis
Statistical analyses were performed using SPSS
software (version 19.0; IBM Corp.). Data are presented as the mean
± standard deviation. Data containing two samples were analyzed
using a Student's t-test. Comparisons in datasets containing >3
groups were evaluated by one-way ANOVA followed by Bonferroni post
hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
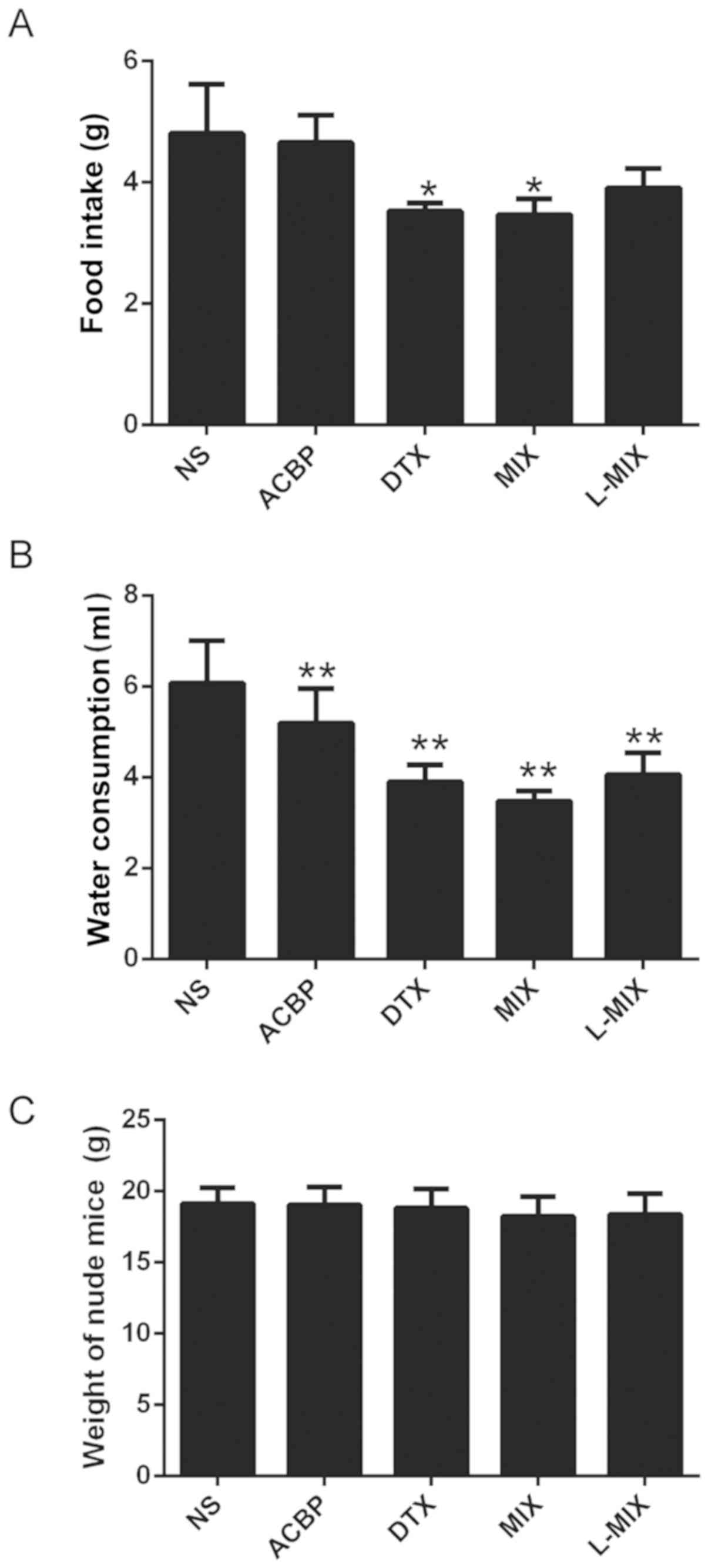
Food intake, water consumption and
body weight of tumor-bearing nude mice
The levels of food intake and water consumption of
the nude mice in each group exhibited significant differences. The
ACBP group exhibited no significant dietary intake (P>0.05) and
decreased drinking water consumption (P<0.01) compared with the
NS group (Fig. 1A and B). In addition, the dietary intake and
drinking water consumption were significantly reduced in the DTX
and MIX groups compared with the NS group (P<0.05; Fig. 1A and B). Furthermore, there was a statistically
significant decrease in water consumption in the L-MIX group
compared with the NS group (P<0.05). Each treatment group
exhibited no significant difference in body weight compared with
the NS group (Fig. 1C).
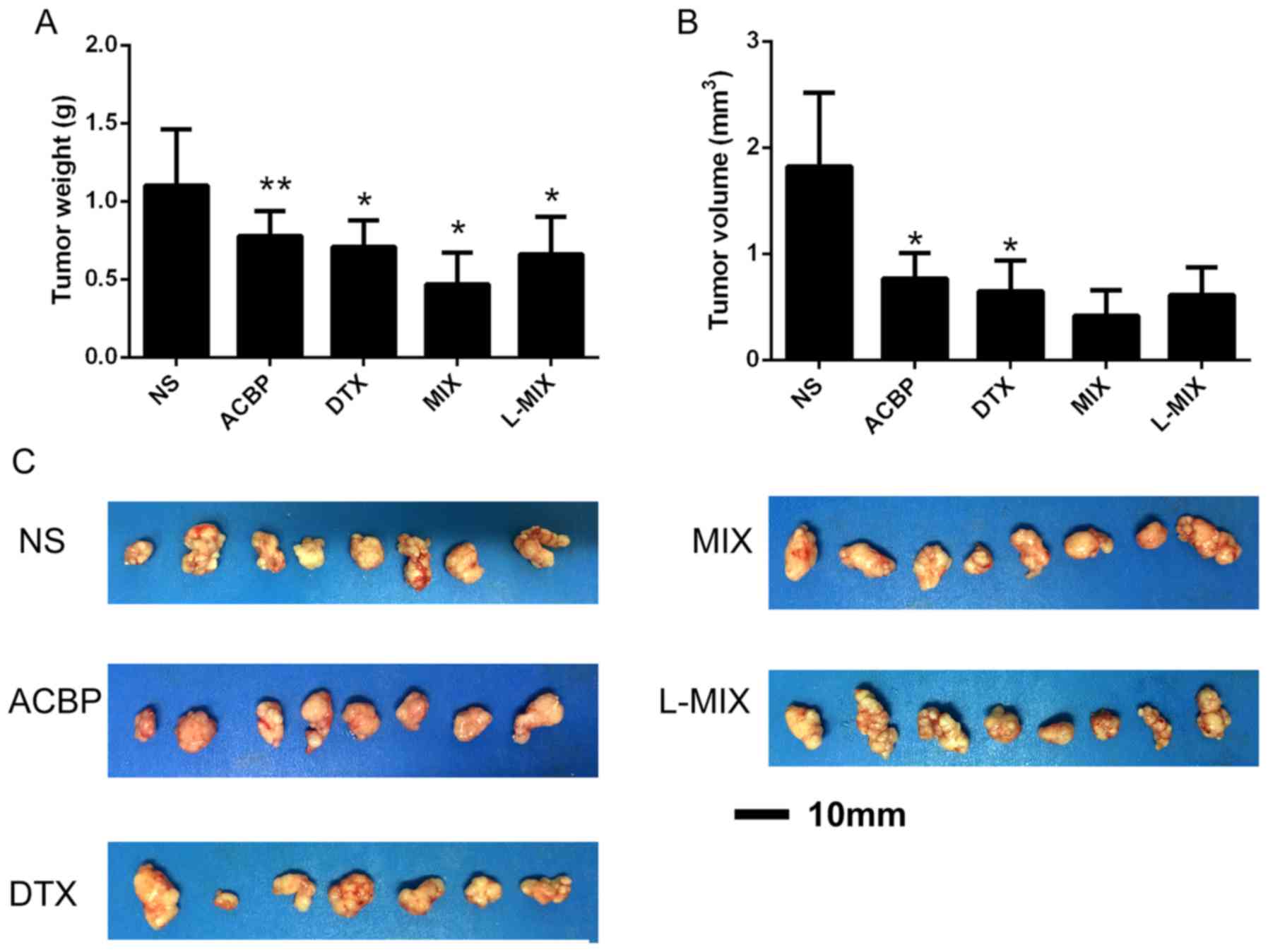
Tumor growth in nude mice
The treatment groups exhibited significantly
decreased tumor weights and volumes compared with the NS group,
indicating that the treatments had an inhibitory effect (Fig. 2A and B). The tumor weight of the NS, ACBP, DTX,
MIX and L-MIX groups were 1.10±0.36, 0.78±0.16, 0.71±0.17,
0.47±0.20 and 0.66±0.24 g, respectively (Fig. 2A). The tumor weights were
significantly decreased in the DTX, MIX, L-MIX (P<0.05) and ACBP
groups (P<0.01) compared with the NS group (Fig. 2A). Furthermore, the tumor volumes of
the NS, ACBP, DTX, MIX and L-MIX groups were 1.82±0.70, 0.77±0.24,
0.65±0.29, 0.42±0.24 and 0.61±0.26 mm3, respectively
(Fig. 2B). The tumor volumes in the
ACBP and DTX groups were significantly reduced compared with the NS
group (P<0.05; Fig. 2B).
Additionally, the tumor weights and volumes were lower in the MIX
group, although not significantly different, compared with the ACBP
and DTX groups, which suggested that ACBP not only exerted an
inhibitory effect on the tumors alone, but also acted
synergistically with DTX (Fig. 2A).
The maximum tumor diameter observed was ~10 mm and the maximum
tumor volume observed was ~2.5 mm3, both in the NS group
(Fig. 2C). Therefore, the results
suggested that ACBP inhibited tumor growth in breast cancer.
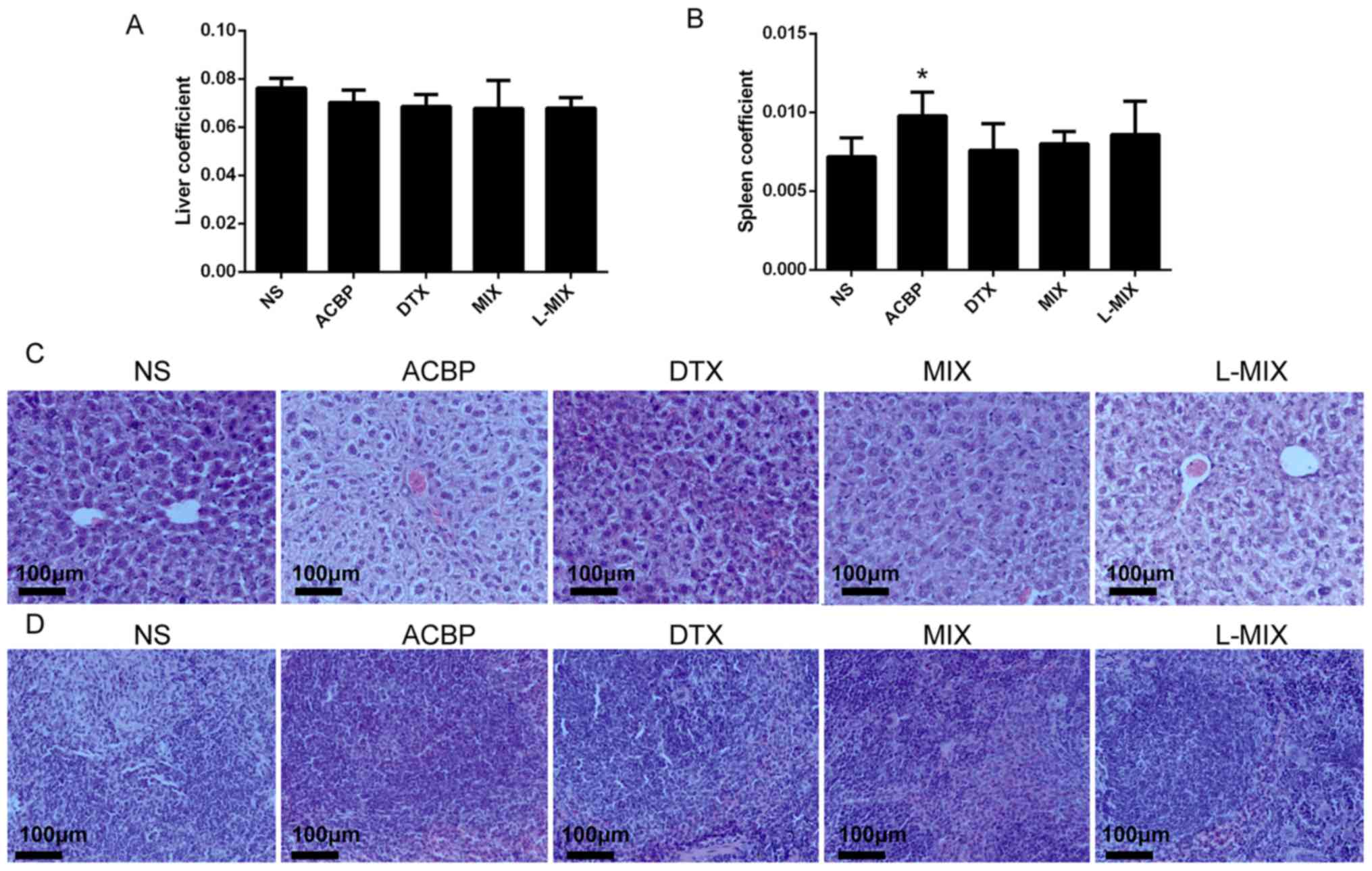
Liver and spleen tissue structure and
coefficients in nude mice
The liver coefficients of the treatment groups
exhibited no significant differences compared with the NS group
(Fig. 3A). However, the ACBP group
displayed a significantly higher spleen coefficient compared with
the NS group (P=0.041; Fig. 3B).
Furthermore, the spleen coefficients of the DTX, MIX and L-MIX
groups were not significantly different compared with the NS group
(Fig. 3B). Hematoxylin & eosin
staining of the liver and spleen revealed no marked cell necrosis,
edema or inflammatory cell infiltration in all groups (Fig. 3C and D).
p21, p53 and Ki67 immunohistochemical
scores in tumor tissues from nude mice
Immunohistochemical staining displayed lower Ki67
protein expression in the ACBP, DTX, MIX and L-MIX groups compared
with the NS group (Fig. 4A). p21
immunohistochemical scores were significantly lower in the MIX and
L-MIX groups compared with the NS group (P<0.01) (Fig. 4B). p21 immunohistochemical scores
were also decreased in the ACBP and DTX groups, but the decrease
was not significantly different compared with the NS group
(Fig. 4B). The p21
immunohistochemical score was significantly reduced in the MIX and
L-MIX groups compared with the NS and ACBP groups, but no
significant difference was identified between the MIX and L-MIX
groups (Fig. 4B).
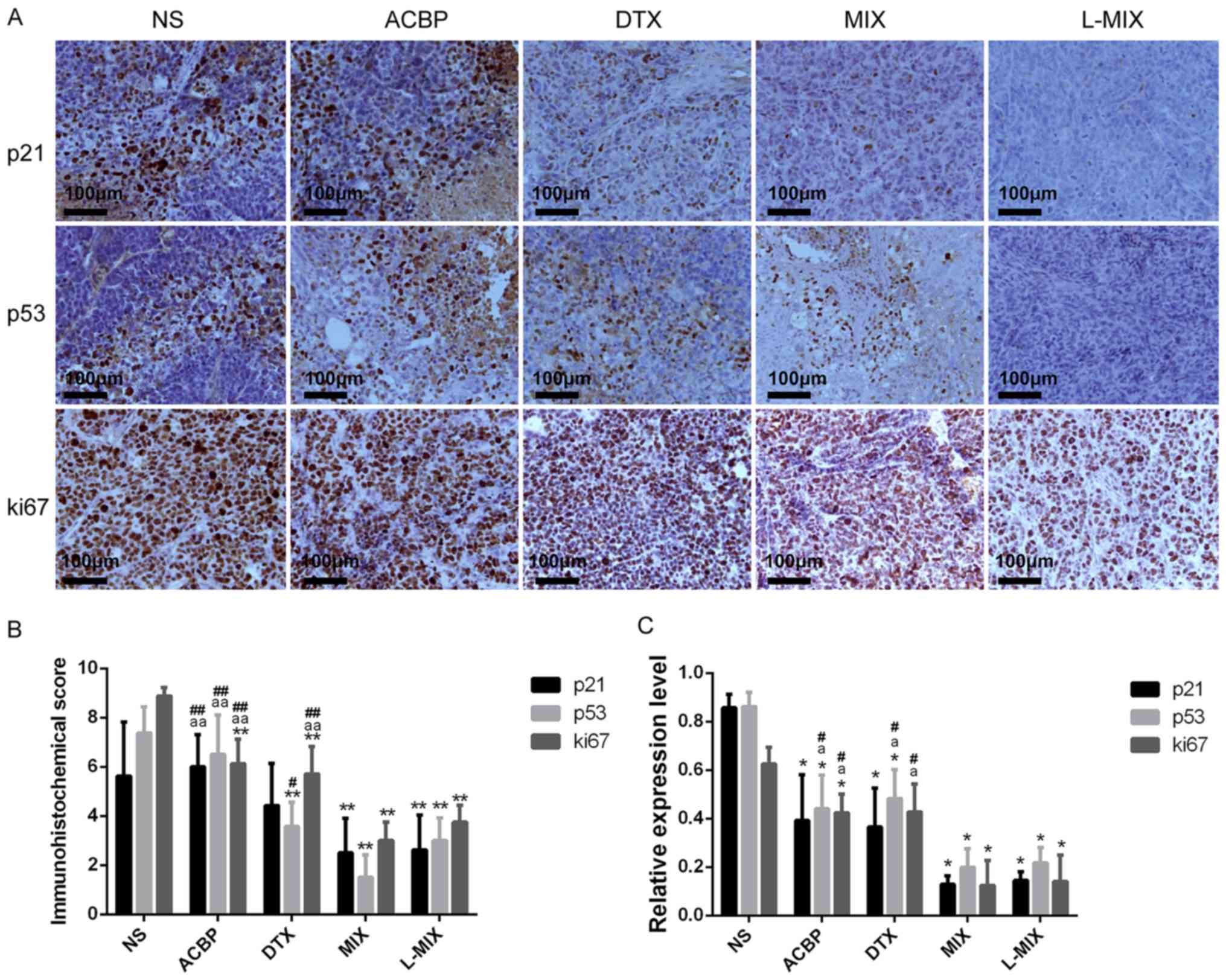 | Figure 4Expression of p21, p53 and Ki67 in
tumor tissues. Expression of p21, p53 and Ki67 in tumor tissues by
(A) immunohistochemical staining (magnification, x100) and (B)
immunohistochemical scoring. (C) Relative expression of p21, p53
and Ki67 in tumor tissues, measured by reverse
transcription-quantitative PCR. *P<0.05 and
**P<0.01 vs. the NS group. aP<0.05 and
aaP<0.01 vs. the MIX group. #P<0.05 and
##P<0.01 vs. the L-MIX group. NS, normal saline;
ACBP, anticancer bioactive peptide; DTX, docetaxel; MIX, ACBP
combined with DTX; L-MIX, ACBP combined with low dose DTX. |
The p53 immunohistochemical score was significantly
decreased in the L-MIX, DTX and MIX groups compared with the NS
group (P<0.01; Fig. 4B), but the
ACBP group displayed a score that was not significantly different
compared with the NS group (P<0.05; Fig. 4B). The MIX group exhibited a
significantly decreased p53 immunohistochemical score compared with
the ACBP (P<0.01; Fig. 4B).
Furthermore, the L-MIX group displayed a significantly decreased
p53 immunohistochemical score compared with the ACBP group
(P<0.01; Fig. 4B). The L-MIX
group also exhibited significantly reduced p53 immunohistochemical
scores compared with the ACBP, and DTX (P<0.05).
Ki67 immunohistochemical scores were significantly
lower in the four treatment groups compared with the NS group
(P<0.01; Fig. 4B). The MIX and
L-MIX groups exhibited significantly decreased Ki67
immunohistochemical scores compared with, ACBP and DTX groups
(P<0.01), but there was no significant differences between the
MIX and L-MIX groups (Fig. 4B).
Expression of the tumor-associated
genes p21, p53 and Ki67
Using GAPDH as an internal reference gene, the
relative mRNA levels of p21, p53 and Ki67 were detected by RT-qPCR.
The relative mRNA expression of p21 and p53 was significantly lower
in the four treatment groups compared with the NS group
(P<0.05). Regarding p21, the four treatment groups displayed
decreased expression levels compared with the NS group (P<0.05),
but there was no significant difference between the ACBP and DTX
groups and the MIX, L-MIX groups. The expression of p53 was
significantly reduced in the four treatment groups compared with
the NS group (P<0.05; Fig. 4C).
Furthermore, p53 expression levels were significantly reduced in
the MIX and L-MIX groups compared with the ACBP and DTX groups
(P<0.05; Fig. 4C), but there was
no significant difference between the MIX and L-MIX groups. The
ACBP, MIX and L-MIX groups exhibited significantly reduced Ki67
gene expression compared with the NS group (P<0.05; Fig. 4C). However, there was no significant
difference in the expression of Ki67 between the DTX and NS groups
(Fig. 4C). The MIX and L-MIX groups
displayed significantly decreased Ki67 levels compared with the
ACBP and DTX groups (P<0.05; Fig.
4C). However, there was no significant difference in Ki67
expression between the MIX and L-MIX groups (Fig. 4C).
Discussion
Neoadjuvant and adjuvant chemotherapy have become
routine treatment strategies for breast cancer. Approximately 81.4%
of patients with invasive breast cancer are treated with
chemotherapy (29). At present,
chemotherapy is not effective and is often limited as the majority
of tumors have pre-existing resistance mediators (30). Furthermore, treatment prior to
chemotherapy is often ineffective and initial chemotherapeutic
drugs decrease in effectiveness over the course of the treatment,
eventually leading to disease progression and tumor recurrence
(30). Common complications of
chemotherapeutic drugs are nausea, vomiting, phlebitis, alopecia,
oral mucositis and bone marrow suppression (31). A large number of studies have
reported ovarian failure (mainly menstrual atresia) and cognitive
impairment caused by breast cancer chemotherapy, resulting in
physical and mental complications in young and middle-aged female
patients (31-33).
Therefore, the present study aimed to identify a novel drug that
enhanced the sensitivity and reduced the associated toxic and
adverse effects of chemotherapeutic drugs.
In addition to the more commonly used methods of
clinical surgery, radiotherapy, chemotherapy and endocrine therapy,
the use of biotherapy as a cancer treatment is increasing (34). It has been reported that ACBP
treatment can effectively inhibit the human breast cancer cell line
MDA-MB-231, inhibit tumor cell proliferation, induce apoptosis and
block cell cycle progression (35).
Furthermore, ACBP can activate the immune system to improve the
immune function of the human body and achieve an antitumor effect
(35). In addition, numerous studies
have suggested that ACBP regulates energy consumption and
alleviates digestive tract toxicity (18-20,22).
Therefore, ACBP treatment may be potentially decrease the toxic
side effects of DTX.
According to the hematoxylin & eosin staining in
the present study, the hepatocytes in each treatment group were
neatly arranged and even in size and there was no obvious
inflammatory cell infiltration, fibrosis or necrosis in the
hepatocytes. However this may be due to the short period of
intermittent drug administration. Additionally, there were no
significant differences in the liver coefficients between the
groups of tumor-bearing nude mice, which also indicated that the
short-term intermittent use of ACBP did not have a toxic effect on
the liver. The spleen is the largest immune organ and is primarily
involved in humoral immunity (36).
The spleen coefficient can reflect the immune status of the
individual; the higher the spleen coefficient is, the stronger the
immunity of the individual in the absence of other diseases
(36). The spleen coefficient was
significantly higher in the ACBP group compared with control groups
(P<0.05). The results indicated that ACBP may enhance the
immunity of tumor-bearing nude mice.
Breast cancer is a highly heterogeneous disease,
which can be divided into four types according to the expression of
the estrogen receptor, progesterone receptor, human epidermal
growth factor receptor-2 (HER2) and Ki-67, detected by
immunohistochemistry. The four types are luminal A, luminal B,
HER2-positive and triple-negative breast cancer (TNBC) (37). These classifications provide reliable
guidance for the individualized treatment of breast cancer and the
prediction of prognosis at the molecular level (37). The p53 gene is the most widely
studied tumor suppressor gene (38).
The p21 gene is a product of the Ras proto-oncogene and its
upregulation is closely associated with the development of
colorectal cancer (39). However,
the relationship between p21 and breast cancer remains unclear.
Ki67 is recognized as a breast cancer proliferation factor as Ki67
expression and breast cancer cell proliferation, malignancy degree,
invasiveness and distant metastasis are positively correlated
(40). The MDM2-p53-p21 signaling
pathway is one of the most important signaling pathways involving
the p53 gene. Alterations in the function or structure of any gene
in this signaling pathway can result in tumor formation (41). p21 is located downstream of p53 and
depends primarily on p53 activity during cell senescence, cell
cycle regulation and apoptosis (41). The human breast cancer cell line
MDA-MB-231 used in the present study is a TNBC with a high
tumor-formation rate, high degree of malignancy, strong
invasiveness and poor prognosis (42).
In the present study, p53 and p21 levels were not
consistent at the level of protein and mRNA expression. The mRNA
expression of p53 and p21 was significantly lower in tumor-bearing
nude mice treated with ACBP and DTX compared with NS treated mice.
From the results, it may be speculated that the anticancer effect
of ACBP may occur via the regulation of p53 and p21 gene
expression. Therefore, ACBP may increase the rate of apoptosis
induced by tumor cells and regulate cell cycle progression in
breast cancer cells. High expressions of p53, p21 and Ki67 has been
associated with poor prognosis in a number of studies (40,43,44). The
expression of the three genes in nude mice treated with ACBP was
significantly decreased compared with the NS group. While p53 and
Ki67 was significantly lower in the MIX group compared with the
ACBP and DTX groups. As a result, ACBP alone had an antitumor
effect, but when combined with DTX, ACBP enhanced DTX sensitivity.
Regarding quality of life parameters, although the activity levels
and weights of the nude mice were not significantly different
between the groups, the water consumption in the ACBP group was
decreased compared with the NS group (P<0.01). The tumor weights
of the MIX and L-MIX groups were significantly lower compared with
the NS groups (P<0.05), which also suggested that ACBP had an
antitumor effect and enhanced DTX sensitivity. The present study
identified a novel combined therapy for breast cancer.
To conclude, ACBP effectively inhibited the growth
of the human breast cancer cell line MDA-MB-231 in tumor-bearing
mice, which not only improved the quality of life of animals but
also increased DTX sensitivity. Short-term use of ACBP did not
elicit toxic and adverse effects associated with hepatocyte injury,
and the increase in the spleen coefficient suggested that the
antitumor effect of ACBP might be associated with improvements in
immunity of nude mice. The expression of p53, p21 and Ki67 further
suggested an antitumor effect for ACBP and indicated that ACBP
combined with chemotherapy to reduce DTX toxicity. Therefore, the
p53-p21 signaling pathway might serve a key role in the treatment
of human breast cancer. The present study suggested a novel
approach for the clinical treatment of breast cancer. Further
investigation into the mechanisms of action of the antitumor and
chemotherapy sensitization effects of ACBP are required.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81660468), the
Natural Science Foundation of Inner Mongolia Autonomous region
(grant no. 2017BS0812) and the General Project of Clinical Medical
Research Center, Affiliated Hospital of Inner Mongolia Medical
University (grant no. NYFY YB044).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XS conceived and designed the present study. BG and
XL acquired, analyzed and interpreted the data. XL drafted the
manuscript. XS agrees to be accountable for the work in ensuring
that questions related to the integrity of any part of the work are
appropriately investigated and resolved. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Ethics
Committee for Animal Experiments of Inner Mongolia Medical College
(approval no. YKD2016152).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Song CG, Hu Z, Wu J, Luo JM, Shen ZZ,
Huang W and Shao ZM: The prevalence of BRCA1 and BRCA2 mutations in
eastern Chinese women with breast cancer. J Cancer Res Clin Oncol.
132:617–626. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Schild SE and Vokes EE: Pathways to
improving combined modality therapy for stage III nonsmall-cell
lung cancer. Ann Oncol. 27:590–599. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ye H, Xu HL, Shen Q, Zheng Q and Chen P:
Palliative resection of primary tumor in metastatic nonfunctioning
pancreatic neuroendocrine tumors. J Surg Res. 243:578–587.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ghafoor A, Jemal A, Ward E, Cokkinides V,
Smith R and Thun M: Trends in breast cancer by race and ethnicity.
Ca Cancer J Clin. 53:342–355. 2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Feng RM, Zong YN, Cao SM and Xu RH:
Current cancer situation in China: Good or bad news from the 2018
global cancer statistics? Cancer Commun (Lond).
39(22)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Culakova E, Thota R, Poniewierski MS,
Kuderer NM, Wogu AF, Dale DC, Crawford J and Lyman GH: Patterns of
chemotherapy-associated toxicity and supportive care in US oncology
practice: A nationwide prospective cohort study. Cancer Med.
3:434–444. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Verbelen H, Tjalma W, Meirte J and
Gebruers N: Long-term morbidity after a negative sentinel node in
breast cancer patients. Eur J Cancer Care (Engl).
28(e13077)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Le DL, Cao H and Yang LX: Cardiotoxicity
of molecular-targeted drug therapy. Anticancer Res. 34:3243–3249.
2014.PubMed/NCBI
|
|
9
|
Feng QJ, Zhang F, Huang XY and Wu ZX:
Effectiveness and complications of anthracycline and taxane in the
therapy of breast cancer: A meta-analysis. Pathol Oncol Res.
20:179–184. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rivera E and Cianfrocca M: Overview of
neuropathy associated with taxanes for the treatment of metastatic
breast cancer. Cancer Chemother Pharmacol. 75:659–670.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Andreopoulou E and Sparano JA:
Chemotherapy in patients with anthracycline- and taxane-pretreated
metastatic breast cancer: An overview. Curr Breast Cancer Rep.
5:42–50. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kayl AE and Meyers CA: Side-effects of
chemotherapy and quality of life in ovarian and breast cancer
patients. Curr Opin Obstet Gynecol. 18:24–28. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yavari B, Mahjub R, Saidijam M, Raigani M
and Soleimani M: The potential use of peptides in cancer treatment.
Curr Protein Pept Sci. 19:759–770. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Albenzio M, Santillo A, Caroprese M, Della
Malva A and Marino R: Bioactive peptides in animal food products.
Foods. 6(E35)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bhat ZF, Kumar S and Bhat HF:
Antihypertensive peptides of animal origin: A review. Crit Rev Food
Sci Nutr. 57:566–578. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cicero AFG, Fogacci F and Colletti A:
Potential role of bioactive peptides in prevention and treatment of
chronic diseases: A narrative review. Br J Pharmacol.
174:1378–1394. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hilchie AL, Hoskin DW and Power Coombs MR:
Anticancer activities of natural and synthetic peptides. Adv Exp
Med Biol. 1117:131–147. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xing Z, Yu L, Li X and Su X: Anticancer
bioactive peptide-3 inhibits human gastric cancer growth by
targeting miR-338-5p. Cell Biosci. 6(53)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Su X, Dong C, Zhang J, Su L, Wang X, Cui H
and Chen Z: Combination therapy of anti-cancer bioactive peptide
with cisplatin decreases chemotherapy dosing and toxicity to
improve the quality of life in xenograft nude mice bearing human
gastric cancer. Cell Biosci. 4(7)2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li X, Xia L, Ouyang X, Suyila Q, Su L and
Su X: Bioactive peptides sensitize cells to anticancer effects of
oxaliplatin in human colorectal cancer xenografts in nude mice.
Protein Pept Lett. 26:512–522. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ma J, Hu X, Li J, Wu D, Lan Q, Wang Q,
Tian S and Dong W: Enhancing conventional chemotherapy drug
cisplatin-induced anti-tumor effects on human gastric cancer cells
both in vitro and in vivo by thymoquinone targeting PTEN gene.
Oncotarget. 8:85926–85939. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yu L, Yang L, An W and Su X: Anticancer
bioactive peptide-3 inhibits human gastric cancer growth by
suppressing gastric cancer stem cells. J Cell Biochem. 115:697–711.
2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang S, Guan J, Sun M, Zhang D, Zhang H,
Sun B, Guo W, Lin B, Wang Y, He Z, et al: Self-delivering
prodrug-nanoassemblies fabricated by disulfide bond bridged oleate
prodrug of docetaxel for breast cancer therapy. Drug Deliv.
24:1460–1469. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chi CL, Li FW, Liu HB, Feng SY, Zhang YJ,
Zhou D and Zhang RG: Docetaxel-loaded biomimetic nanoparticles for
targeted lung cancer therapy in vivo. J Nanoparticle Res.
21(144)2019.
|
|
25
|
Li C, Wang Z, Wang Q, Ka Yan Ho RL, Huang
Y, Chow MSS, Kei Lam CW and Zuo Z: Enhanced anti-tumor efficacy and
mechanisms associated with docetaxel-piperine combination- in vitro
and in vivo investigation using a taxane-resistant prostate cancer
model. Oncotarget. 9:3338–3352. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Grover A, Hirani A, Pathak Y and Sutariya
V: Brain-targeted delivery of docetaxel by glutathione-coated
nanoparticles for brain cancer. AAPS PharmSciTech. 15:1562–1568.
2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Stacul F, Bertolotto M, De Gobbis F,
Calderan L, Cioffi V, Romano A, Zanconati F and Cova MA: US
colour-Doppler US and fine-needle aspiration biopsy in the
diagnosis of thyroid nodules. Radiol Med. 112:751–762.
2007.PubMed/NCBI View Article : Google Scholar : (In English,
Italian).
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Fan L, Strasser-Weippl K, Li JJ, St Louis
J, Finkelstein DM, Yu KD, Chen WQ, Shao ZM and Goss PE: Breast
cancer in China. Lancet Oncol. 15:e279–e289. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Demichele A, Yee D and Esserman L:
Mechanisms of resistance to neoadjuvant chemotherapy in breast
cancer. N Engl J Med. 377:2287–2289. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Morarji K, Mcardle O, Hui K, Gingras-Hill
G, Ahmed S, Greenblatt EM, Warner E, Sridhar S, Ali AMF, Azad A and
Hodgson DC: Ovarian function after chemotherapy in young breast
cancer survivors. Curr Oncol. 24:e494–e502. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Witlox L, Schagen SB, de Ruiter MB,
Geerlings MI, Peeters PHM, Koevoets EW, van der Wall E, Stuiver M,
Sonke G, Velthuis MJ, et al: Effect of physical exercise on
cognitive function and brain measures after chemotherapy in
patients with breast cancer (PAM study): Protocol of a randomised
controlled trial. BMJ Open. 9(e028117)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gokal K: Effects of physical activity on
cognitive and psychosocial functioning in breast cancer patients
undergoing chemotherapy: A randomised controlled trial (unpublished
PhD thesis). Loughborough University, 2015.
|
|
34
|
Gan X and Wang L: Current situation and
prospect of biotherapy of metastatic renal cell carcinoma. Chin J
Cancer Biother, 2018 (In Chinese).
|
|
35
|
Jia Q, Wang W and Su X: Inhibitory effect
of anticancer bioactive peptides on proliferation of human breast
cancer nm 231 cell. J 270-275, 2007.
|
|
36
|
Xu M, Lu JG and Ma QJ: Research on splenic
nerve and spleen Immune function. Prog Mod Biomed. 10:2177–2179.
2010.
|
|
37
|
Cheang MC, Chia SK, Voduc D, Gao D, Leung
S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, et al: Ki67
index, HER2 status, and prognosis of patients with luminal B breast
cancer. J Natl Cancer Inst. 101:736–750. 2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li Q, Zhu Y, Hou L, Wang J, Hu G, Fang X,
Hu Y, Tao T, Wei X, Tang H, et al: C23 promotes tumorigenesis via
suppressing p53 activity. Oncotarget. 7:58274–58285.
2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Bai S, Feng Q, Pan XY, Zou H, Chen HB,
Wang P, Zhou XL, Hong YL, Song SL and Yang JL: Overexpression of
wild-type p21Ras plays a prominent role in colorectal cancer. Int J
Mol Med. 39:861–868. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Koopman T, Buikema HJ, Hollema H, de Bock
GH and van der Vegt B: Digital image analysis of Ki67 proliferation
index in breast cancer using virtual dual staining on whole tissue
sections: Clinical validation and inter-platform agreement. Breast
Cancer Res Treat. 169:33–42. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Morrison CD, Allington TM, Thompson CL,
Gilmore HL, Chang JC, Keri RA and Schiemann WP: c-Abl inhibits
breast cancer tumorigenesis through reactivation of p53-mediated
p21 expression. Oncotarget. 7:72777–72794. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wang K, Xie SM, He JJ, Ren Y, Xia HB and
Zhang XW: Establishment of a bioluminescent MDA-MB-231 cell line
for in vivo imaging of human triple-negative breast cancer
xenograft. Nan Fang Yi Ke Da Xue Xue Bao. 31:1812–1818.
2011.PubMed/NCBI(In Chinese).
|
|
43
|
Dumay A, Feugeas JP, Wittmer E,
Lehmann-Che J, Bertheau P, Espié M, Plassa LF, Cottu P, Marty M,
André F, et al: Distinct tumor protein p53 mutants in breast
cancers subgroups. Int J Cancer. 132:1227–1231. 2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Curtis C, Shah SP, Chin SF, Turashvili G,
Rueda OM, Dunning MJ, Speed D, Lynch AG, Samarajiwa S, Yuan Y, et
al: The genomic and transcriptomic architecture of 2,000 breast
tumours reveals novel subgroups. Nature. 486:346–352.
2012.PubMed/NCBI View Article : Google Scholar
|