Introduction
Approximately 1.5 million children in China have
congenital heart diseases (CHDs) and more than 0.2 million children
with CHD are born annually (1).
These cases include several types of complex CHD, such as trilogy
of Fallot, tetralogy of Fallot, double outlet right ventricle,
transposition of the great arteries, pulmonary artery atresia and
common arterial trunk (2). It has
been reported that 5-10% of these children require pulmonary artery
reconstruction for survival (3,4).
Using a valved conduit to reconstruct the pulmonary
artery could save the lives of children, greatly improve the
effectiveness of surgical treatments for such CHDs and increase the
long-term quality of life (5-10).
To date, several materials, including autologous pericardium,
xenograft materials, allogeneic valved aortic conduit and
artificial valved conduit, have been used, but conduits constructed
from ideal materials have yet to become available. Currently,
several valved right ventricle conduits, such as the Synergraft
valve, bovine Contegra valved conduit, bicuspid valved
polytetrafluoroethylene conduit and BIOVALVE, are used in clinical
practice in other countries, but they remain unavailable in China
(5-10).
Considering the limited lifespan of the current
bioprosthetics and shortage of heart valve donors, alternatives
such as animal tissues have been suggested as attractive options
because animals could provide an unlimited source of tissues for
xenotransplantation (11-13).
Bovine pericardium has been widely used as a supplementary
implantation material for repair in cardiovascular surgeries and it
has withstood over 40 years of testing (14,15).
Bovine pericardium is more convenient to use, inexpensive, safer,
more reliable and widely available, compared with other artificial
repair materials.
Bioprosthetic valves made of bovine pericardium with
chemical modifications have become widely accepted as artificial
bioprosthetic valves because of their good durability (5,6,9). Nevertheless, there is a risk of
hyperacute and acute rejection or vascular injury of the xenogeneic
tissues. Therefore, complete removal, or at least inactivation of
the antigens and nucleic acid remnants of the original resident
cells, is crucial. To do so, tissues must be decellularized using a
combination of physical agents, detergents, enzymes and chemical
compounds (16). Nevertheless, the
extracellular matrix (ECM), which is essential for correct tissue
function, should be left as intact as possible in terms of
architecture, ultrastructure, mechanical integrity and biological
activity. Indeed, the ultrastructure and composition of the ECM
influence cell mitogenesis, chemotaxis and differentiation, and
play important roles for integration into the host organ (17,18).
Studies performed in the liver (19), respiratory tract (20), nerves (21), adipose tissue (22) and mammary glands (23) have shown that such integration is
possible.
A new valved pulmonary arterial conduit, constructed
entirely of biomaterials and developed by Jiahe Zhongbang
Biotechnology Co., Ltd. is made of bovine pericardium that has
undergone decellularization by glutaraldehyde, de-immunogenicity
and a series of anti-calcium modifications, followed by bionic
structural designing and suturing. Its testing requires large
animals that can tolerate cardiopulmonary bypass surgery. Sheep
have hemodynamic and blood coagulation system characteristics and
laboratory indicators similar to those of humans. They are easy to
manage and not susceptible to infection following surgery.
Long-term feeding is relatively easy and their long-term survival
rate is high. Therefore, sheep were selected as the experimental
animal for use in the present study, which aimed to conduct a
pre-clinical assessment of this new conduit by transplanting it in
the outflow tract of the sheep right ventricle in order to assess
its safety profile and hemodynamics.
Materials and methods
Biomaterial valved pulmonary arterial
conduit. Design
On the basis of collected clinical data on heart
valve dynamic parameters, the rudimentary appearance of the
bioprosthetic valve was derived by analyzing the natural form of
the human heart valve. A bioprosthetic valve parametric design
platform was then constructed and computer-aided industrial design
parametric software (self-designed) was used to create the spatial
form that fulfilled the spatial geometry equations. Consequently, a
series of dimensional parameters with higher accuracy was obtained
for valve cusp parametric model construction, and finite element
analysis software ABAQUS 6.13 (Dassault Systemes SE) was used to
perform stress analysis on the changes in valve cusp parameters
after applying various configurations. The valved conduit was
generated by mimicking adult pulmonary artery and valve using a
bionic structural design consisted of proximal and distal conduits,
with the internal diameter being identical in various segments from
the entry point to the exit point (8-22 mm). A bovine jugular vein
containing a trileaflet venous valve was utilized for the proximal
conduit, while bovine pericardium that had undergone
decellularization, removal of immunogenicity and a series of
anti-calcification modifications was used for the segment from the
superior margin of the valve to the distal conduit.
Decellularization
Bovine pericardium obtained from a normal cow within
2 h of slaughter was placed in D-Hanks solution at 4˚C and stripped
of fat and muscle tissue. Subsequently, the pericardium was washed
twice with PBS, soaked in 0.3% glutaraldehyde (GA) at room
temperature for 48 h and stored in 0.5% GA solution at 4˚C before
further processing.
Chemical anti-calcification
treatment
The GA-treated bovine pericardium was washed twice
with PBS, treated with 8% epichlorohydrin for 48 h at 25˚C, washed
twice again with PBS, treated with 2,3-butanediol at 25˚C for
120-240 h and stored in 0.5% GA solution at 4˚C before further
use.
Stitching
A custom-made elastic band cutting knife was used to
cut the bovine pericardium into strips along a direction parallel
to the pericardial fibers. For each strip, thickness at three fixed
points was measured using a leather thickness gauge. Pre-punched
holes created by laser drilling ensured a uniform stitching pitch,
uniform load and minimum stress concentration. The pericardium was
stitched using polyester sutures; no synthetic fibers were used in
the stitching process. Once the pericardium had softened, it could
be cut into strips of various sizes. In addition, standardization,
efficiency and stability of product quality during the stitching
process were enhanced.
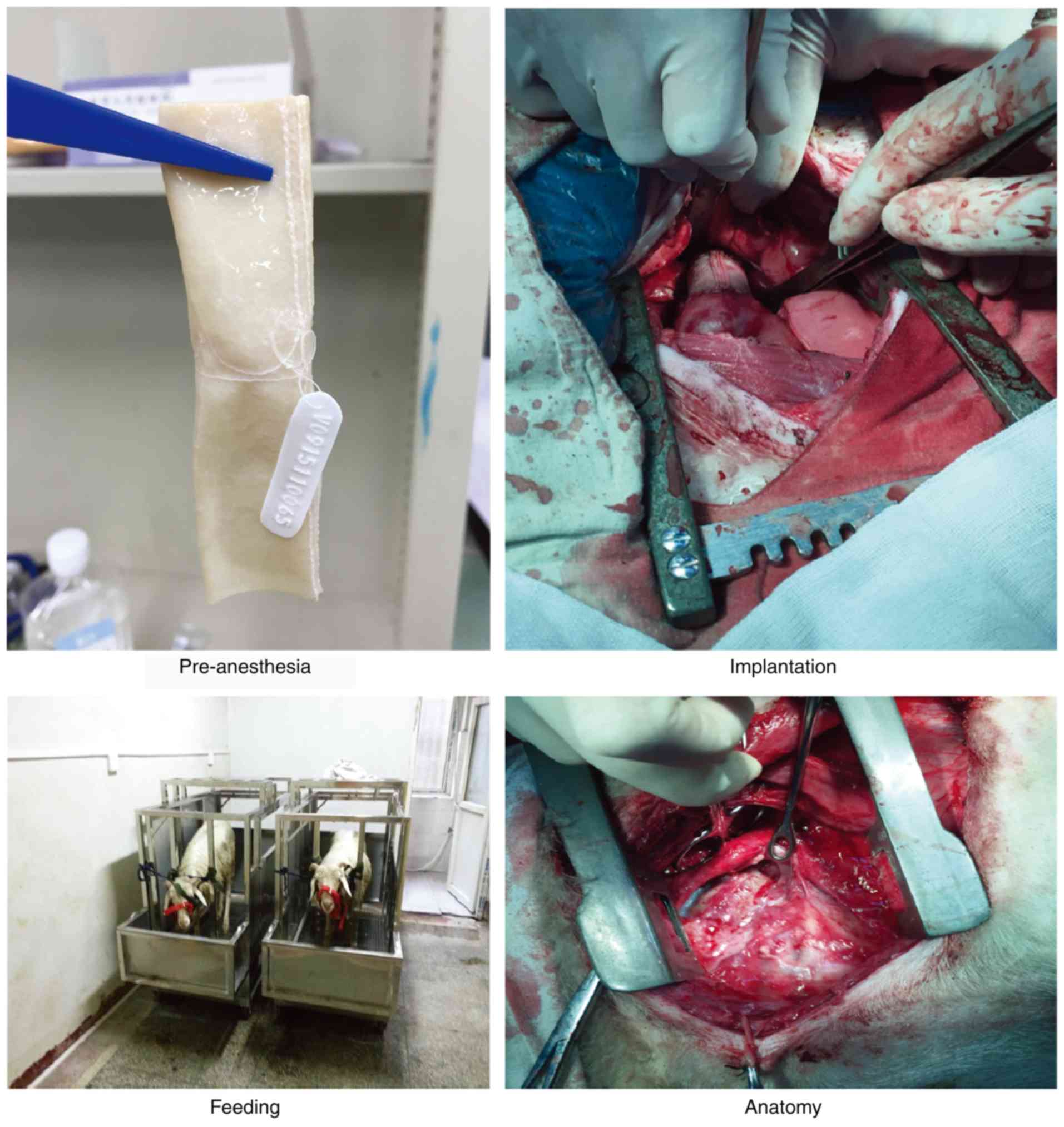
Animal model
A total of 14 healthy adult male sheep (age, 10-12
months; weight, 51±5.4 kg; Xi'an Dilepu Biological Resource
Development, Co., Ltd.) were used in the study, and kept at the
Animal Center of the Experimental Surgery Department, Xijing
Hospital, under normal temperature conditions (25˚C and 70%
humidity). The sheep fold was cleaned twice daily. Regular feeding
was provided; the sheep had free access to food and water. All
sheep were quarantined for 7 days before the experiments and their
activities and feeding behaviors were monitored during this period.
All sheep were eligible for the experiments after approval from a
veterinarian. Animals with abnormalities were handled under the
guidance of the clinical veterinarian and director of this study.
The sheep were fasted for 12 h pre-operatively. Selection, feeding
and monitoring of the sheep, as well as the study protocol, were
approved by the Animal Ethics Committee of the Fourth Military
Medical University. Two of the sheep were used as sham
controls.
Anesthesia
General anesthesia was induced by the muscular
injection of 8 mg/kg ketamine and 0.6-0.9 mg scopolamine and an
intravenous line was established. Then, 1-2 mg/kg propofol and 1-2
µg/kg fentanyl were intravenously injected (24). Tracheal intubation was conducted to
establish an artificial airway and a ventilator was used with the
following respiratory parameters: Tidal volume, 10-12 ml/kg and
frequency, 10-16 bpm. Isoflurane (1-2%) was used for anesthesia
maintenance. Femoral artery puncture was conducted to monitor
arterial blood pressure. Electrocardiogram monitoring was
performed.
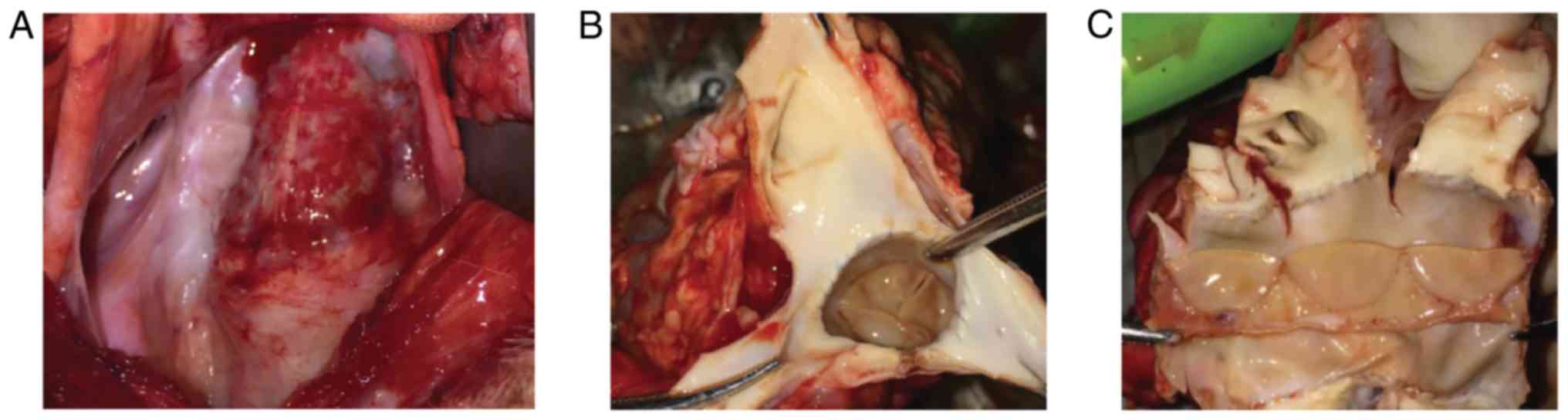
Surgery
An incision was made at the left side of the chest
and the left fourth rib was removed. Vena cava catheterization was
conducted through the right auricle. The arterial catheter was
sutured to the ascending aorta. A drainage tube was inserted into
the right side of the heart at 1 cm below the pulmonary valve ring.
Heparin (3-4 mg/kg) was injected to adjust the whole blood
activated clotting time to >480 sec. Extracorporeal circulation
was established after systemic heparinization. The main pulmonary
artery space was isolated and the main pulmonary artery diameter
was measured. The valved conduits were rinsed with 500 ml 0.9%
normal saline three times (5 min/time). A vascular clamp was used
to block the distant pulmonary artery at the site close to the
pulmonary artery bifurcation. Then, the pulmonary artery was
resected at 1 cm above the pulmonary artery valve, the three
pulmonary artery valve leaflets were removed and an artificial
blood vessel with the same diameter as that of the main pulmonary
artery was selected. The extracorporeal circulation machine was
removed after circulation stabilization. A drainage tube was placed
at the left fifth intercostal space and the thoracic cavity was
closed layer by layer. Tracheal extubation was performed after
anesthetic recovery (Fig. 1).
Post-operative care and follow-up
Animals were fed at 6 h after tracheal extubation.
Daily behaviors of the sheep, namely food and water intake and
activities, and their psychological status with regards to stress
and dysphoria were monitored postoperatively and recorded. Body
weights were measured every 2 weeks. Sheep were sacrificed at 30
(n=2), 90 (n=2) and 180 (n=8) days postoperatively. Changes in
hemodynamics, outflow tract of the right ventricle and pulmonary
artery structure were assessed.
From the second day after the surgery, there was no
risk of bleeding. Aspirin tablets were mixed into the food for
anticoagulation at a dose of 100 mg/day.
Valve conduit function evaluation
Closing and opening of the valves were assessed by
ultrasonography and the pressure gradient was also measured. An
ultrasound device (Vivid-7; GE Healthcare) was used to monitor
right ventricular pressure and distal pulmonary artery pressure
before and after implantation. At 30, 60 and 180 days
post-implantation, sheep were administered an muscular injection of
8 mg/kg ketamine under quiet conditions. Right ventricular pressure
and distal pulmonary artery pressure were monitored and
transpulmonary valve pressure difference was calculated.
To observe the effect of the biomaterial valved
pulmonary arterial conduit on the heart after implantation, the
pressure and hemodynamic changes of each heart chamber were
assessed. The heart was exposed and the central venous and arterial
pressures, pulmonary arterial pressure at the distal artificial
blood pressure of the right side of the heart, and pressure of the
outflow tract of the right ventricle were measured. Pressure
gradient over the artificial blood vessel was calculated using the
following equation: Pressure gradient (mmHg) = systolic pressure of
the outflow tract of the right ventricle-systolic pressure of the
distal pulmonary artery.
Valved conduit calcification
evaluation
Calcium levels in the artificial biomaterial blood
vessel and valves were measured. The samples were dried at 75˚C
until the weight remained constant and then sealed tightly in dry
containers. The samples were placed in a 25-ml conical flask with 5
ml mixed acids (nitric acid:perchloric acid, 8:2) and the flask was
shaken at 150-180˚C. After the solution cleared, the sample was
washed with 1% nitric acid solution and transferred to a 10-ml
scale test tube with a lid. The solution was mixed and the calcium
levels were then measured with a graphite furnace atomic absorption
spectrometer. The results were recorded as calcium levels by weight
(mg) of dry tissue.
Statistical analysis
SPSS 11.10 (SPSS, Inc.) was used for statistical
analysis. All data are presented as means ± standard deviation.
Analysis of variance and Tukey's post hoc test were used for
comparisons. P<0.05 was considered statistically
significant.
Results
Surgical outcomes
In situ implantation of the artificial
biomaterial valved blood vessel was successfully conducted in all
14 sheep with extracorporeal circulation and a beating heart under
general anesthesia. The mean operative time was 131±18 min and mean
extracorporeal circulation time was 35±8 min. The sheep were able
to stand by themselves at 2 h postoperatively and drink water
within 4 h postoperatively. The drainage tube was removed within 24
h postoperatively. The mean drainage volume was 70±30 ml. Although
the drainage volume was >400 ml in one sheep, the animal was in
good condition and showed stable respiration and circulation; the
drainage tube in this sheep was removed 1 day later.
Heart structure and hemodynamics are
not affected by post-valved pulmonary arterial conduit
implantation
Echocardiography through the heart surface was
conducted intraoperatively following the removal of extracorporeal
circulation. The biomaterial pulmonary arterial valves of all 14
sheep could open and close freely, surfaces were smooth and no
abnormal echo, valve position or activity was detected. The
artificial pulmonary artery was patent and the lumen was clear.
Color Doppler flow imaging showed normal blood flow velocity
through the pulmonary arterial valve, with no evident backflow. No
evident accelerated blood flow at the anastomosis of the artificial
blood vessel and pulmonary artery was detected. These results
suggest that the implantation of the completely biomaterial valved
pulmonary arterial conduit did not noticeably affect the heart
structure and hemodynamics.
No renal or liver function impairment
occur following valved pulmonary arterial conduit implantation
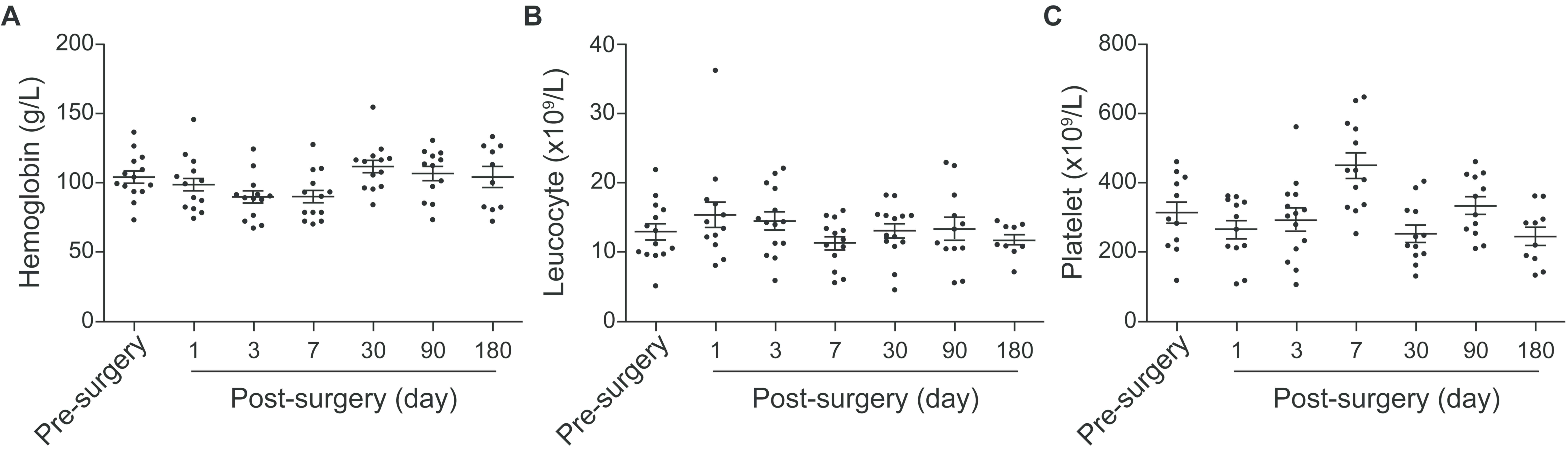
Before and following the surgery, no changes in the
levels of hemoglobin (103±16 vs. 104±24 g/l; Fig. 2A), white blood cells
(11.4±2.2x109 vs. 9.8±2.2x109 cells/l;
Fig. 2B) or platelets
(317±89x109 vs. 292±71x109 platelets/l;
Fig. 2C) caused by hemolysis were
found, indicating that the implantation of the biomaterial valved
pulmonary arterial conduit did not result in adverse effects, such
as evident blood cell destruction or inflammatory responses.
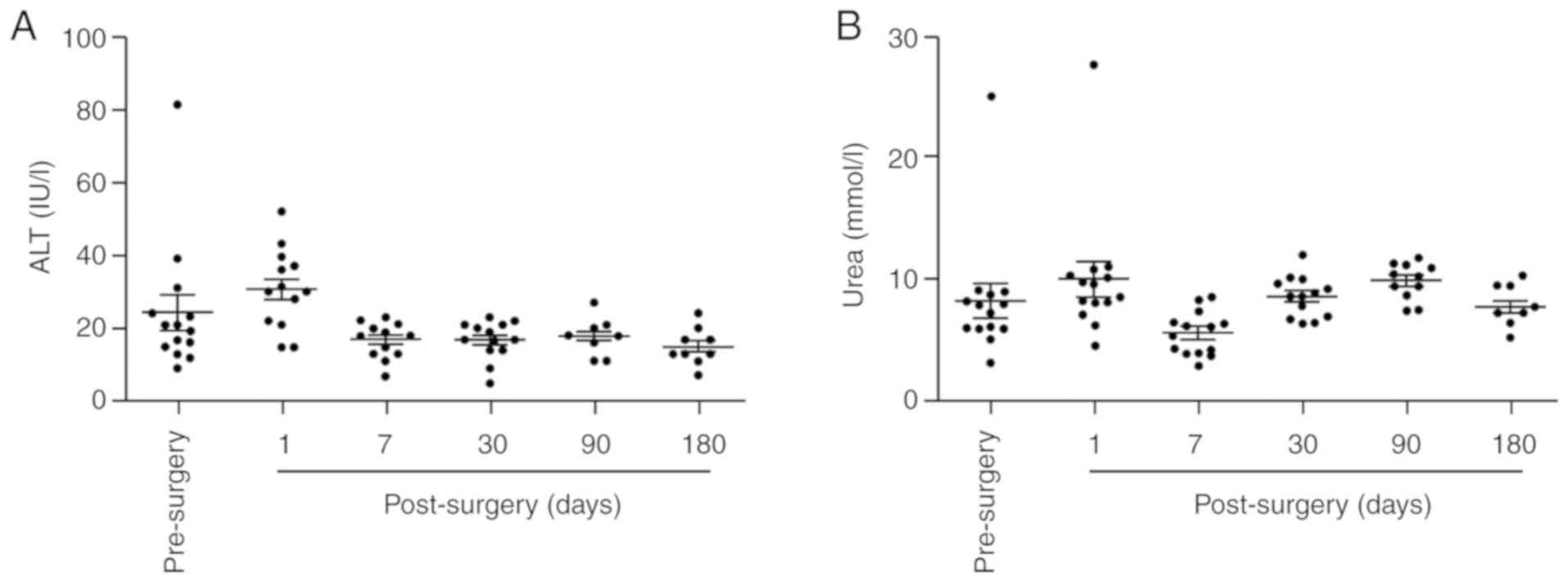
Alanine transaminase (ALT) levels of the sheep
increased temporarily on the day after the surgery, but gradually
returned to normal. The mean ALT levels 180 days postoperatively
were 15±5 IU/l (Fig. 3A). Blood urea
nitrogen levels did not change significantly postoperatively
compared with the preoperative level (Fig. 3B). These results indicate that the
biomaterial valved pulmonary arterial conduit implantation did not
result in evident renal or liver function impairment.
Pressure gradient over the artificial
blood vessel is comparable at pre- or post-valved arterial conduit
implantation
The 12 sheep with artificial blood vessels were
sacrificed at various different time points after tracheal
intubation under general anesthesia. Evident tissue adhesion and
substantial bleeding from the right auricle were found in one sheep
after the heart was exposed. This sheep was therefore not included
in the following statistical analyses.
The central venous and arterial pressures of the
sheep were all normal. The highest pressures of the outflow tract
of the pulmonary artery systolic pressure in all sheep were <30
mmHg. No significant changes in the pressure gradient over the
artificial blood vessel were detected (Table I). No significant abnormality was
identified in pulmonary artery systolic pressure, right ventricle
systolic pressure or pressure gradient over the artificial blood
vessel after implantation compared with the values before
implantation (P>0.05).
 | Table IHemodynamic changes after
reconstruction of the right ventricular artery (n=12; mmHg). |
Table I
Hemodynamic changes after
reconstruction of the right ventricular artery (n=12; mmHg).
| Index | Before surgery | After surgery |
|---|
| Pulmonary artery
systolic pressure | 23±6 | 24±5 |
| Right ventricle
systolic pressure | 36±5 | 39±4 |
| Pressure gradient
over artificial blood vessel | 13±2 | 12±4 |
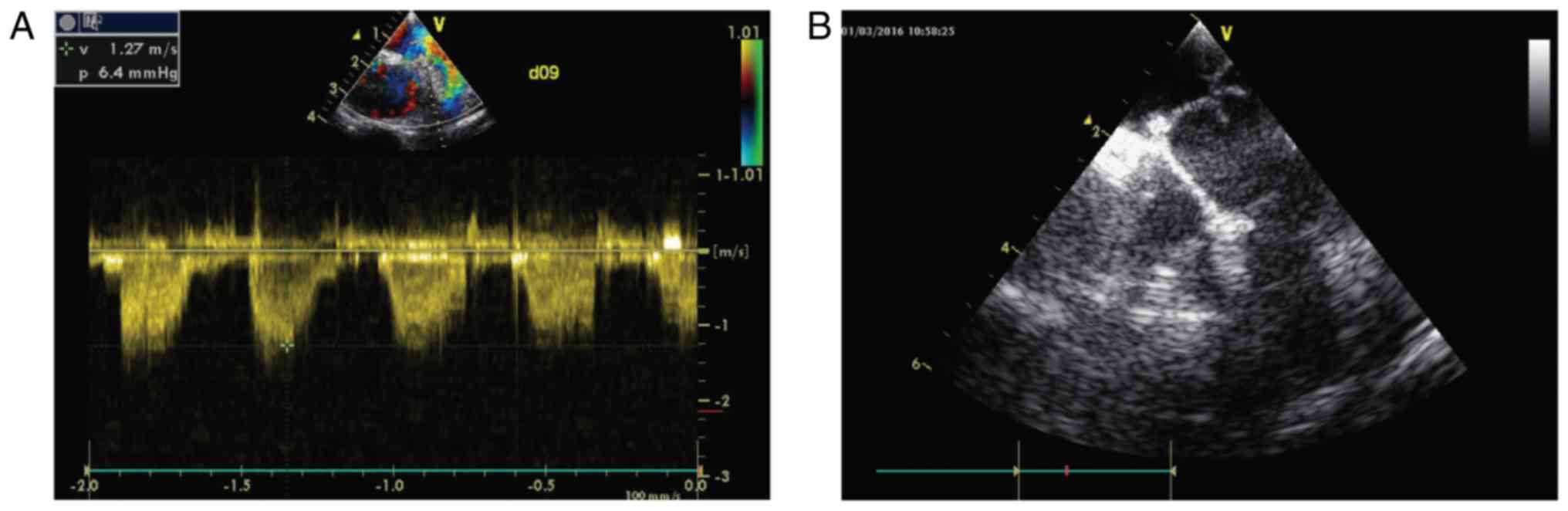
Ultrasound examinations conducted 30 and 90 days
postoperatively showed that the biomaterial valves of the pulmonary
arteries in all animals could open and close freely, had a smooth
surface and were without abnormal echo, position or activity. The
artificial pulmonary artery was patent and the lumen was clear.
Color Doppler flow imaging revealed normal blood flow velocity
through the pulmonary arterial valve, with no evident backflow. No
accelerated blood flow at the anastomosis of the artificial blood
vessel and pulmonary artery was detected. Ultrasound imaging
results are shown in Fig. 4.
Examinations on postoperative day 180 showed that
the biomaterial valve of the pulmonary artery in one animal could
not close and open freely. In addition, valve thickening and
enhanced echo were detected. Blood flow velocity through the
pulmonary arterial valve was slightly increased and mild backflow
through the pulmonary arterial valve was detected. No pulmonary
arterial valve abnormalities were observed in the other 7 sheep
(Fig. 5). These results showed that
completely biomaterial valved pulmonary arterial conduit
implantation did not result in long-term adverse effects on heart
structure and hemodynamic.
No adverse effects, bleeding or
infarction are evident following valved pulmonary arterial conduit
implantation
The wool of the 12 sheep recovered well. A white
sticky liquid was detected in a subcutaneous mass in one sheep,
which was not connected to the thoracic cavity. The heart sizes and
shapes of all 12 sheep were normal. No evident bleeding or pale
areas were observed on the heart surface. Heart chamber sizes were
normal. Signs of myocardial infarction, thrombus or mass were not
observed. Artificial blood vessel positions and sizes were normal.
The artificial vessels were soft and tissue adhesion was detected.
Following resection of the longitudinal artificial vessel, it was
observed that the valves in one sheep had hardened slightly and
exhibited calcium deposition, so the valve activities were
suboptimal. By contrast, the valves and blood vessel walls in all
other 11 sheep were free from thrombus, mass or calcium deposition.
The valves in these 11 sheep were soft and smooth and demonstrated
good activity. The blood vessel walls were also soft and smooth,
with no evidence of calcification. The lung size and shape of all
12 sheep were normal. Adhesion was found in operative areas in all
animals; no edema, bleeding or infarction was detected in other
parts of the lungs. Gross shapes of the livers, kidneys and spleens
in all 12 sheep were normal; the surfaces of these organs were all
smooth, with no signs of bleeding or infarction (Fig. 5). These results showed that the
implantation of the biomaterial arterial conduit did not result in
evident thermogenesis, embolism or infarction in the organs, as
demonstrated by the autopsies.
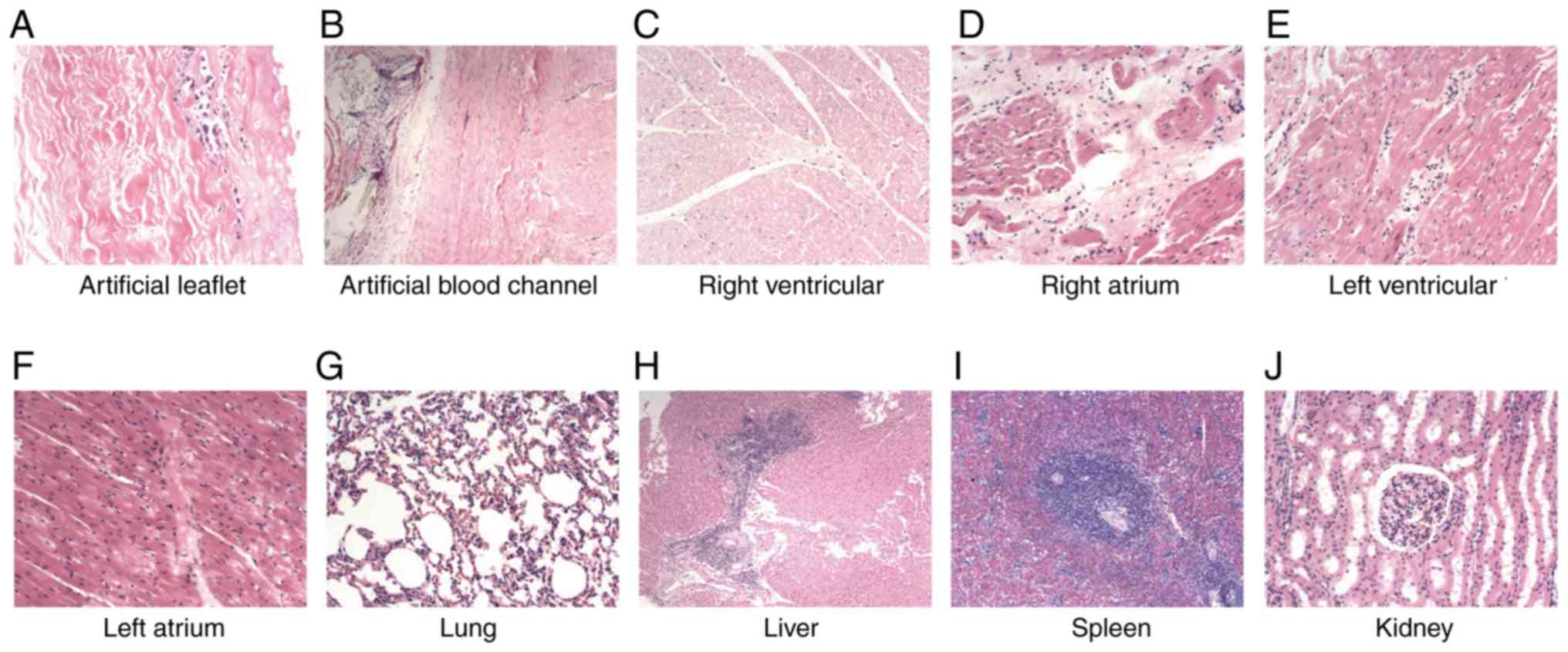
Histopathological examinations showed partial
calcification of the collagenous fiber in one sheep, but no
necrosis or degeneration of the fiber tissues of the valves was
evident. Histopathological examinations of the heart revealed no
evident fibrogenesis or infarction in the heart tissues in all
sheep. Examinations of the pulmonary tissues showed that the
alveolar structure was generally normal. The tissues of the livers,
kidneys and spleens were generally normal, with no evident bleeding
or necrosis (Fig. 6). These results
showed that the conduit implantation did not result in evident
adverse effects on the organs, or evident bleeding or
infarction.
Artificial conduit remains free from
calcification
The calcium levels in the artificial blood vessels
in the 12 sheep are shown in Table
II: The calcium levels in the sheep sacrificed on postoperative
days 30 and 90 were all <10 µg/mg. For the sheep sacrificed on
postoperative day 180, the calcium levels in the valve were
elevated in two of the sheep, at 204 and 27.2 µg/mg. Nevertheless,
no evident valve stenosis or insufficiency was found in the
artificial blood vessels of these two sheep. The highest pressure
gradient over the artificial blood vessel was 22.5 mmHg, which did
not result in adverse effects on hemodynamics. The calcium levels
in all the other valves were <10 µg/mg. These results showed
that the artificial conduit was free from calcification.
 | Table IICalcium levels in the artificial blood
vessels. |
Table II
Calcium levels in the artificial blood
vessels.
| Sheep no. | Calcium levels of the
valve (µg/mg) | Calcium levels of the
conduit (µg/mg) |
|---|
| 1 | 0.79 | 1.12 |
| 2 | 1.09 | 1.01 |
| 3 | 1.08 | 0.81 |
| 4 | 2.99 | 1.61 |
| 5 | 1.43 | 4.02 |
| 6 | 27.2 | 2.4 |
| 7 | 1.07 | 0.95 |
| 8 | 1.06 | 1.08 |
| 9 | 0.91 | 0.96 |
| 10 | 8.05 | 1.2 |
| 11 | 204 | 4.5 |
| 12 | 2.51 | 4.21 |
Discussion
The present study investigated the performance of a
new, valved pulmonary arterial conduit prepared using a proprietary
method. The conduit has the following characteristics: It consists
of a stentless bioprosthetic pulmonary valve and bioprosthetic
great vessels, and can be transplanted during cardiovascular
surgery for the surgical reconstruction of the right ventricular
outflow tract or replacement of non-functioning previously
transplanted tracts to treat patients with right ventricular
outflow tract anomalies or lesions. Given that it has undergone
acellular and immunogenicity removal and serial anti-calcification
modification treatment, the conduit is strong and durable. It is
sewn with polyester suture from the bovine pericardium without any
synthetic fiber, thereby preventing thrombus formation and
non-bacterial inflammation caused by the porous structure of
synthetic fiber. The results showed that this artificial valved
conduit effectively replaced the original pulmonary artery and
pulmonary arterial valves of sheep.
Clinicians are looking for graft biomaterials that
do not require long-term anticoagulant therapy. With the expansion
of the surgical indications of complex CHD, the insufficiency of
the available materials for reconstruction of the right ventricular
outflow tract has become more evident and is regarded as an
important factor restricting the clinical application of such
materials, especially for correction surgeries in neonates.
Currently, several studies have reported the use of valved bovine
jugular veins (25-28),
but such conduits have several disadvantages: They are difficult to
harvest, easily calcify and tend to be prone to aneurysm-like
dilation, severely affecting the surgical outcomes.
In the present study, the new valve pulmonary
arterial conduit constructed entirely from biomaterials was used to
replace the original pulmonary artery and pulmonary arterial
valves. The valves were found to close and open freely, with no
severe stenosis or valve insufficiency detected. Additionally, the
hemodynamics all met the physiological requirements of the sheep.
Furthermore, the calcium levels in the valves of artificial blood
vessels were >10 µg/mg in only two sheep at 180 days
postoperatively, but without any evident valve stenosis or
insufficiency. In addition, the highest pressure gradient over the
artificial blood vessel was 22.5 mmHg, which did not result in
adverse effects on hemodynamics. No thrombogenesis or associated
adverse events were observed, despite the fact that no or low-dose
anticoagulant therapy was applied. No evident thrombogenesis or
embolism was identified in the organs during autopsy. These results
show that this product has high blood compatibility. The results of
the present study are consistent with other studies of valved
pulmonary arterial conduit implantation in large animal models,
albeit using different valved pulmonary arterial conduits (8,29). The
conduit has more outstanding anti-calcification characteristics and
is biologically safe.
In conclusion, the 6-month follow-up data showed
that the implantation of this novel biosynthetic vascular graft
into animals was safe and could meet the safety and effectiveness
requirements for clinical application. However, as no large-animal
model with pulmonary artery stenosis was used, the long-term effect
of the valved conduit in such conditions could not be evaluated in
the present study. The present authors plan to conduct another
study to evaluate the effect of the new valved pulmonary arterial
conduit in animal models of diseases, such as pulmonary stenosis
and pulmonary regurgitation. For children with complex CHDs
requiring pulmonary artery reconstruction to achieve a radical
cure, this pulmonary arterial conduit has certain clinical
application significance. Due to time constraints and the lack of
clinically relevant mature products, a sham control group was used
in the present study; however, the number of animals was small and
this is recognized as a limitation of this study. The long-term
effects and safety of this biological conduit require further study
with longer observation periods along with rigorous testing before
clinical trials or routine clinical use.
Acknowledgements
The authors acknowledge the help of Dr Liang Cheng
of the Department of Cardiovascular Surgery, Xijing Hospital, The
Fourth Military Medical University, Xi'an, Shaanxi for the
conception and design of the study.
Funding
This study was supported by the Project of the
National Natural Science Foundation of China (grant no.
31370996).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TC conceived and supervised the study. ZJ designed
the experiments. DY, SY and XW performed the experiments. BY and YZ
analyzed the data. KR, WD and LL wrote the manuscript and acquired,
analyzed and interpreted the data. All authors reviewed the results
and read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Animal Ethics
Committee of the Air Force Medical University.
Patient consent for publication
Not applicable.
Competing interests
YZ is affiliated with Hangzhou Jiahe Zhongbang
Biotechnology Co., Ltd., Hangzhou, Zhejiang, the company that
developed the valved pulmonary arterial conduit used in the study.
No patent or available publication is involved.
References
|
1
|
Pei L, Kang Y, Zhao Y and Yan H:
Prevalence and risk factors of congenital heart defects among live
births: A population-based cross-sectional survey in Shaanxi
province, Northwestern China. BMC Pediatr. 17(18)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Qu Y, Liu X, Zhuang J, Chen G, Mai J, Guo
X, Ou Y, Chen J, Gong W, Gao X, et al: Incidence of congenital
heart disease: The 9-year experience of the guangdong registry of
congenital heart disease, China. PloS One.
11(e0159257)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Monge MC, Mainwaring RD, Sheikh AY, Punn
R, Reddy VM and Hanley FL: Surgical reconstruction of peripheral
pulmonary artery stenosis in Williams and Alagille syndromes. J
Thorac Cardiovasc Surg. 145:476–481. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rao PS and Chugh R: A comprehensive
approach to congenital heart diseaes. Vijayalakshmi IB, editor. Nel
Delhi: Jaypee Brothers Medical Publichers;. 2013.
|
|
5
|
Breymann T, Thies WR, Boethig D, Goerg R,
Blanz U and Koerfer R: Bovine valved venous xenografts for RVOT
reconstruction: results after 71 implantations. Eur J Cardiothorac
Surg. 21:703–710; discussion, 710. 2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wu L, Wang ZH, Wang WD, Liu WY and Jin F:
Experimental study on construction of valved conduits by
decellularized bovine jugular vein in vivo. Prog Mod Biomed.
2:246–248. 2011.
|
|
7
|
Takewa Y, Yamanami M, Kishimoto Y, Arakawa
M, Kanda K, Matsui Y, Oie T, Ishibashi-Ueda H, Tajikawa T, Ohba K,
et al: In vivo evaluation of an in-body, tissue-engineered,
completely autologous valved conduit (biovalve type VI) as an
aortic valve in a goat model. J Artif Organs. 16:176–184.
2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wu H, Xu ZW, Liu XM, Gong D, Wan JY, Xu
XF, Zhou ZF and Li WB: An in vivo model of in situ implantation
using pulmonary valved conduit in large animals under off-pump
condition. Chin Med J (Engl). 126:4540–4544. 2013.PubMed/NCBI
|
|
9
|
Yuan SM: The Contegra valved bovine
conduit: A biomaterial for the surgical treatment of congenital
heart defects. Arq Bras Cardiol. 99:1159–1166. 2012.PubMed/NCBI View Article : Google Scholar : (In English,
Portuguese).
|
|
10
|
Zhang J and Liu Y: Establishment of rabbit
carotid artery homograft valved conduits transplantation model.
Chin J Exp Surg. 2000:568–569. 2000.
|
|
11
|
Piazza N, de Jaegere P, Schultz C, Becker
AE, Serruys PW and Anderson RH: Anatomy of the aortic valvar
complex and its implications for transcatheter implantation of the
aortic valve. Circ Cardiovasc Interv. 1:74–81. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ong K, Boone R, Gao M, Carere R, Webb J,
Kiess M and Grewal J: Right ventricle to pulmonary artery conduit
reoperations in patients with tetralogy of fallot or pulmonary
atresia associated with ventricular septal defect. Am J Cardiol.
111:1638–1643. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Boethig D, Thies WR, Hecker H and Breymann
T: Mid term course after pediatric right ventricular outflow tract
reconstruction: A comparison of homografts, porcine xenografts and
contegras. Eur J Cardiothorac Surg. 27:58–66. 2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pires AC, Saporito WF, Cardoso SH and
Ramaciotti O: Bovine pericardium used as a cardiovascular patch.
Heart Surg Forum. 2:60–69. 1999.PubMed/NCBI
|
|
15
|
Neethling WM, Strange G, Firth L and Smit
FE: Evaluation of a tissue-engineered bovine pericardial patch in
paediatric patients with congenital cardiac anomalies: Initial
experience with the ADAPT-treated CardioCel(R) patch. Interact
Cardiovasc Thorac Surg. 17:698–702. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Keane TJ, Swinehart IT and Badylak SF:
Methods of tissue decellularization used for preparation of
biologic scaffolds and in vivo relevance. Methods. 84:25–34.
2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dohmen PM, da Costa F, Yoshi S, Lopes SV,
da Souza FP, Vilani R, Wouk AF, da Costa M and Konertz W:
Histological evaluation of tissue-engineered heart valves implanted
in the juvenile sheep model: Is there a need for in-vitro seeding?
J Heart Valve Dis. 15:823–829. 2006.PubMed/NCBI
|
|
18
|
Gallo M, Naso F, Poser H, Rossi A, Franci
P, Bianco R, Micciolo M, Zanella F, Cucchini U, Aresu L, et al:
Physiological performance of a detergent decellularized heart valve
implanted for 15 months in Vietnamese pigs: Surgical procedure,
follow-up, and explant inspection. Artif Organs.
36(E138-E150)2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sellaro TL, Ranade A, Faulk DM, McCabe GP,
Dorko K, Badylak SF and Strom SC: Maintenance of human hepatocyte
function in vitro by liver-derived extracellular matrix gels.
Tissue Eng Part A. 16:1075–1082. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Petersen TH, Calle EA, Zhao L, Lee EJ, Gui
L, Raredon MB, Gavrilov K, Yi T, Zhuang ZW, Breuer C, et al:
Tissue-engineered lungs for in vivo implantation. Science.
329:538–541. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Karabekmez FE, Duymaz A and Moran SL:
Early clinical outcomes with the use of decellularized nerve
allograft for repair of sensory defects within the hand. Hand (N
Y). 4:245–249. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Flynn LE: The use of decellularized
adipose tissue to provide an inductive microenvironment for the
adipogenic differentiation of human adipose-derived stem cells.
Biomaterials. 31:4715–4724. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wicha MS, Lowrie G, Kohn E, Bagavandoss P
and Mahn T: Extracellular matrix promotes mammary epithelial growth
and differentiation in vitro. Proc Natl Acad Sci USA. 79:3213–3217.
1982.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Salama AK: Comparison between ketamine and
hyoscine for the management of postoperative catheter-related
bladder discomfort: A randomized controlled double-blind study. J
Anaesthesiol Clin Pharmacol. 33:76–80. 2017. View Article : Google Scholar
|
|
25
|
Brown JW, Ruzmetov M, Rodefeld MD, Vijay P
and Darragh RK: Valved bovine jugular vein conduits for right
ventricular outflow tract reconstruction in children: An attractive
alternative to pulmonary homograft. Ann Thorac Surg. 82:909–916.
2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bove T, Demanet H, Wauthy P, Goldstein JP,
Dessy H, Viart P, Devillé A and Deuvaert FE: Early results of
valved bovine jugular vein conduit versus bicuspid homograft for
right ventricular outflow tract reconstruction. Ann Thorac Surg.
74:536–541; discussion 541. 2002.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Herijgers P, Ozaki S, Verbeken E, Van
Lommel A, Meuris B, Lesaffre E, Daenen W and Flameng W: Valved
jugular vein segments for right ventricular outflow tract
reconstruction in young sheep. J Thorac Cardiovasc Surg.
124:798–805. 2002.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Meyns B, Van Garsse L, Boshoff D, Eyskens
B, Mertens L, Gewillig M, Fieuws S, Verbeken E and Daenen W: The
Contegra conduit in the right ventricular outflow tract induces
supravalvular stenosis. J Thorac Cardiovasc Surg. 128:834–840.
2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Leyh RG, Wilhelmi M, Rebe P, Ciboutari S,
Haverich A and Mertsching H: Tissue engineering of viable pulmonary
arteries for surgical correction of congenital heart defects. Ann
Thorac Surg. 81:1466–14670; discussion 1470-1471. 2006.PubMed/NCBI View Article : Google Scholar
|