Introduction
Epilepsy is quite a common nervous system disease
even in adults. However, epilepsy has different incidence in
different age groups, and its seizure frequency in children is
obviously higher than that in adults (1). Epilepsy may be attributed to multiple
factors, mainly including genetic abnormality, interruption of
normal development and perinatal injury (2). According to the studies on the mental
symptoms of children and young people experiencing epileptic
seizures, epilepsy patients have a very high risk of mental
disorder (3), including depression
and anxiety (4). It was reported
that the use of barbiturates can contribute to depression in
childhood, but little is known about the overall incidence rate of
depression and anxiety in epilepsy children and the determinants,
which have not yet been deeply researched (5). Rutter et al (6) reported that mental disorder occurs in
as many as 33% of epilepsy children, which was, however, not
elaborated. Hoare (7) found that the
incidence rate of behavior difficulty in epilepsy children is
higher than that in diabetes children, but the incidence rates of
depression and anxiety remain to be identified. Therefore, the
cognitive disorders in epileptic child patients need to be further
studied.
Valproate (VPA) and its derivants are widely applied
in antiepileptic drugs (AEDs). As recommended by the International
League Against Epilepsy, VPA is a potential candidate initial
monotherapy for childhood absence epilepsy (8). The concerns regarding the use of VPA in
reproductive women enable the shift of the prescription to novel
AEDs, especially levetiracetam (LEV), lamotrigine and topiramate.
LEV, a new generation of AED, has widely recognized efficacy and
tolerance in treating epilepsy. Synaptic vesicle protein 2A is an
intracellular protein that serves as the binding site of LEA in the
brain (9,10). Prenatal exposure to lamotrigine has
been confirmed to be able to significantly heighten the risk of
neuropsychological function impairment compared to that in VPA in
children, whether they are aged 4 or 5 years or of school age.
However, there is little evidence for the risk caused by the
exposure to LEV or topiramate. The infants exposed to LEV have
consistent neurodevelopment with controls, and better development
at the age of 1 and 3 years, compared with that of infants exposed
to VPA (11,12), suggesting that LEV has fewer adverse
effects on epileptic children. In recent years, the importance of
intellectual function in the judgment of agnosia and prognosis in
epileptic patients has been better understood, and there have been
increasingly more relevant research. Long-term use of AEDs is one
of the important factors influencing cognitive function (13).
Aiming at the clinical problems, the
electroencephalograms (EEGs) of the potential epilepsy patients are
evaluated for several different purposes. Firstly, for the patients
who recently had paroxysmal episodes, EEG evaluation may help
identify whether these episodes are derived from epilepsy. Then for
those newly diagnosed with epilepsy or suspected of having
epilepsy, it is likely to raise the certainty of diagnosis,
demonstrate the risk of epilepsy recurrence and conduce to
confirming the presence of epilepsy syndrome. Moreover, EEG is used
to verify the nature of epilepsy in the existing seizures, and more
importantly, probably to confirm the precise presence of epilepsy
syndrome in patients with drug-refractory epilepsy (14). Lastly, in drug-refractory focal
epilepsy, EEG remains the gold standard for the preoperative
diagnosis, which can be used to localize irritative zone and
epileptic seizure zone, as the symbol of the extension of
epileptogenic zone. In such a case, postoperative epileptic seizure
freedom can be predicted using EEG as well. EEG has high time
resolution and provides a very good overview, thus potently
supporting the diagnosis of pediatric epilepsy (15).
The present study explored the efficacy of sodium
valproate combined with LVE in pediatric epilepsy and its
influences on neuron-specific enolase (NSE), interleukin-6 (IL-6)
and high-sensitivity C-reactive protein (hs-CRP) as well as EEG
improvement. The advantages of this regimen were comprehensively
evaluated by observing the efficacy in patients, quality of life
(QOL) score, serum indicators such as NSE, IL-6 and hs-CRP, and EEG
improvement, so as to provide a theoretical foundation and
experimental basis for the treatment of pediatric epilepsy
patients.
Patients and methods
General information
A total of 100 patients with pediatric epilepsy, who
were admitted to and treated in Xiantao First People's Hospital
Affiliated to Yangtze University (Xiantao, China) from December
2015 to 2018, were enrolled as the subjects in this study and
divided into observation group (n=50) and control group (n=50) by
the randomized controlled method. Inclusion criteria: Patients
diagnosed based on the diagnosis criteria that conform to the
epilepsy syndrome classification in 2014 National Standardized
Diagnosis,Treatment and Scientific Research of Epilepsy, those who
voluntarily participated in the present study, those who signed the
informed consent, those who received no treatment previously and
those without allergy history of medications used in this study.
Exclusion criteria: Patients who recently took drugs that affect
growth and development as well as glucose and lipid metabolisms,
those who used glucocorticoids for a long time, those with severe
electrolyte disorder, or those with severe dysfunctions of the
liver or kidney. All the clinical specimens in this experiment were
collected with the approval of the Ethics Committee of the hospital
and the patients as well as their family members, and the clinical
trial regimen was approved by the Ethics Committee of Xiantao First
People's Hospital Affiliated to Yangtze University. Table I shows the patients' clinical
information collected at admission, including age, sex, weight,
physical conditions and course of disease. There were no
statistically significant differences in the general clinical
information of patients between the two groups.
 | Table IClinical information of the
patients. |
Table I
Clinical information of the
patients.
| Clinical
information | Control group | Observation
group |
|---|
| n | 50 | 50 |
| Male | 25 | 26 |
| Mean age (years) | 2±1.1 | 2±1.3 |
| Mean weight (kg) | 15.5±3.6 | 16.1±3.2 |
| BMI
(kg/m2) | 20.9±1.0 | 21.2±1.3 |
| Course of disease
(month) | 4 | 4.5 |
Treatment methods
Patients in the control group were given sodium
valproate sustained-release tablets normally at the dose of 40
mg/kg/d, which was then gradually adjusted to 50 mg/kg/d, for 3
courses with 30 days as a course. In addition to the procedures in
the control group, LEV treatment was performed in the observation
group as follows: The initial dose was 20 mg/kg/d and the dose was
increased once every 5-7 days and maintained within 30 mg/kg/d. In
case of epileptic seizure, the dose was increased by no more than 2
g/days, and the medications were taken twice for 3 consecutive
months. During the whole medication, the administration was
appropriately adjusted according to the disease changes in
patients, and all the indicators were detected after the course of
treatment.
Observation of efficacy in the two
groups of patients
Efficacy was evaluated based on the following
criteria: Control: After treatment, clinical symptoms and signs
completely disappear without acute epileptic seizures in the
epileptic children. Marked effectiveness: After treatment, the
frequency of epileptic seizures declines by over 75%, and the acute
epileptic seizures can be controlled by drugs in epileptic
children. Effectiveness: After treatment, the frequency of
epileptic seizures is decreased by 50-70% in epileptic children.
Ineffectiveness: After treatment, the frequency of epileptic
seizures is decreased by <50% in epileptic children. The number
of patients at each grade was counted and recorded in detail.
Observation of adverse reactions in
the two groups of patients
The adverse reactions were observed and recorded in
the two groups of patients as follows: the adverse reactions,
sleepiness, rash, gastrointestinal reaction and nausea, in the two
groups of patients were recorded by specially-arranged medical
staff, and the specific types of adverse reactions were counted and
recorded in detail. Finally, the adverse reactions after treatment
in the two groups of patients were summarized.
Cognitive function scoring
After treatment, the cognitive function of patients
was assessed in the two groups using the Wechsler Memory
Scale-Revised in China (WMS-RC). At 3 months after treatment, the
Mini-Mental State Examination (MMSE) was performed by specialized
neurologists, with the main examination items of orientation,
memory, ability of mental arithmetic, short-term ability to listen
and memorize and language and imitation abilities, and the higher
the score is, the better the cognitive function will be (100 points
in total). The cognitive function of the patients was assessed
using the Montreal cognitive assessment (MoCA) scale with the total
score of 30 points, and the higher scores correspond to better
cognitive function.
QOL scoring
The QOL of the patients was scored using the QOL in
epilepsy-31 inventory (QOLIE-31) scale which was properly revised
according to Chinese cultural backgrounds and features. At the same
time, their daily living abilities were evaluated using Barthel
Index with the total score of 100 points, and the higher score
denotes stronger daily living abilities and suggests higher QOL in
patients. The scoring was conducted and elaborated in the two
groups of patients by at least three medical staff members.
Detection of changes in the
neurological function indicators NSE and glial fibrillary acidic
protein (GFAP)
Before and after course of treatment, 5 ml of venous
blood was drawn from the arm of the patients, respectively, placed
in an Eppendorf (EP) tube containing anticoagulant and centrifuged
at 2,000 x g and room temperature for 15 min. The supernatant was
collected to detect the changes in the content of serum
neurological function indicators NSE and GFAP according to the
instructions of the enzyme-linked immunosorbent assay (ELISA) kit
(Nanjing Jiancheng Bioengineering Institute). The absorbance in
each group was measured using a microplate reader.
Evaluation of changes in the content
of serum IL-2, IL-6 and hs-CRP
After treatment, 5 ml of venous blood was drawn from
the arm of the patients, placed in an EP tube containing
anticoagulant and centrifuged at 2,000 x g at room temperature for
15 min. The supernatant was collected to detect serum inflammatory
factors IL-2, IL-6 and hs-CRP in accordance with the operation
instructions of the ELISA kit (Nanjing Jiancheng Bioengineering
Institute). Finally, the absorbance in each group was measured
using the microplate reader.
Detection of EEG improvement
After the third course of drug administration, EEG
examination was performed. The electrodes were placed according the
international 10-20 system, with the monitoring duration no shorter
than 4 h, including wake-up and sleep periods. With the number of
spike waves per minute, namely spike wave index, as the statistical
indicator, the mean frequency of α wave and the number of β, θ and
δ waves was observed and recorded.
Statistical analysis
The raw experimental data recorded were processed
using SPSS 20.0 analysis software, and subjected to multiple
comparisons. χ2 test was employed for the percentage.
The experimental results obtained were expressed as mean ± standard
deviation (mean ± SD), and P<0.05 indicated statistically
significant differences. Histograms were plotted using GraphPad
Prism 6.0.
Results
Clinical efficacy of patients
As shown in Table
II, there was a statistically significant difference in the
total effective rate of clinical treatment between the observation
group and the control group (96 vs. 70%) (P<0.05).
 | Table IIEfficacy. |
Table II
Efficacy.
| Group | Control | Marked
effectiveness | Effectiveness | Ineffectiveness | Total effective
rate |
|---|
| Control group | 11 | 14 | 10 | 15 | 70 |
| Observation
group | 16a | 20a | 12a | 2a | 96a |
Adverse reactions in the two groups of
patients
According to the statistical results (Table III), the cases of sleepiness, rash,
gastrointestinal reaction and nausea declined markedly in the
observation group (P<0.05).
 | Table IIIAdverse reactions in the two groups of
patients. |
Table III
Adverse reactions in the two groups of
patients.
| Group | Sleepiness | Rash | Gastrointestinal
reaction | Nausea | Adverse reaction rate
(%) |
|---|
| Control group | 3 | 2 | 4 | 5 | 28 |
| Observation
group | 1 | 1 | 2 | 1 | 10a |
Cognitive function of patients in both
groups
Observation group had notably higher WMS-RC, MMSE
and MoCA scores than the control group (P<0.05) (Table IV), suggesting that the cognitive
function was significantly improved.
 | Table IVCognitive function of patients in both
groups. |
Table IV
Cognitive function of patients in both
groups.
| Group | WMS-RC
scorea | MMSE score | MoCA score |
|---|
| Control group | 78.4±2.4 | 71.7±2.3 | 21.5±1.9 |
| Observation
group | 90.8±2.6a | 89.5±2.1a | 27.9±2.0a |
QOL score
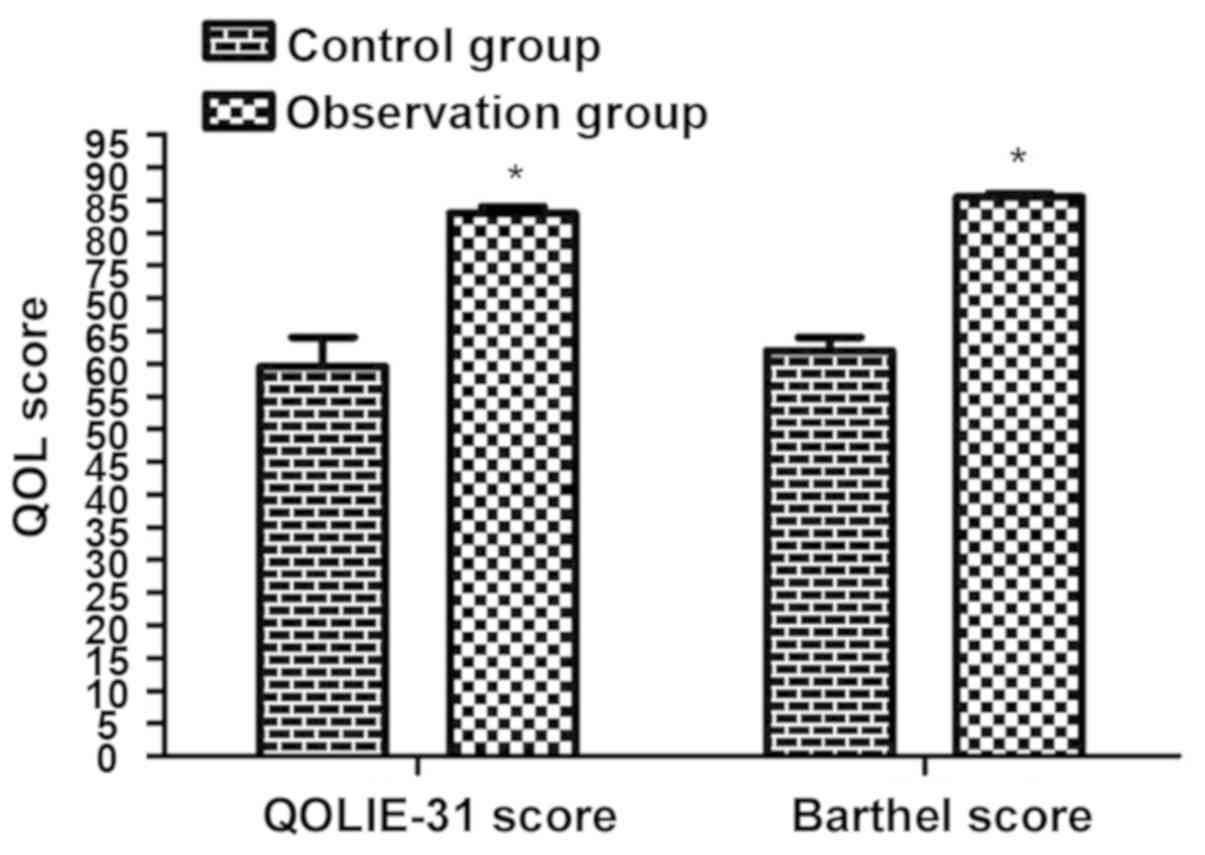
As shown in Fig. 1,
the QOL score in the observation group was remarkably higher than
that in the control group (P<0.05).
Serum NSE and GFAP content
According to the detection results (Table V), there was no statistically
significant difference in the content ratio of NSE/GFAP before
treatment (p>0.05), and after treatment, the content of NSE and
GFAP was obviously lowered in both groups of patients, but the
decline in the observation group was dramatically greater than that
in the control group (P<0.05).
 | Table VSerum and GFAP content. |
Table V
Serum and GFAP content.
| Group | NSE (ng/ml) | GFAP (pg/ml) |
|---|
| Control group |
|
Before
treatment | 15.4±1.2 | 88.1±2.7 |
|
After
treatment |
8.2±2.1a |
30.7±2.1a |
| Control group |
|
Before
treatment | 14.9±1.7 | 91.7±1.9 |
|
After
treatment |
5.7±2.7a,b |
19.4±1.3a,b |
Serum inflammatory factors detected
via ELISA
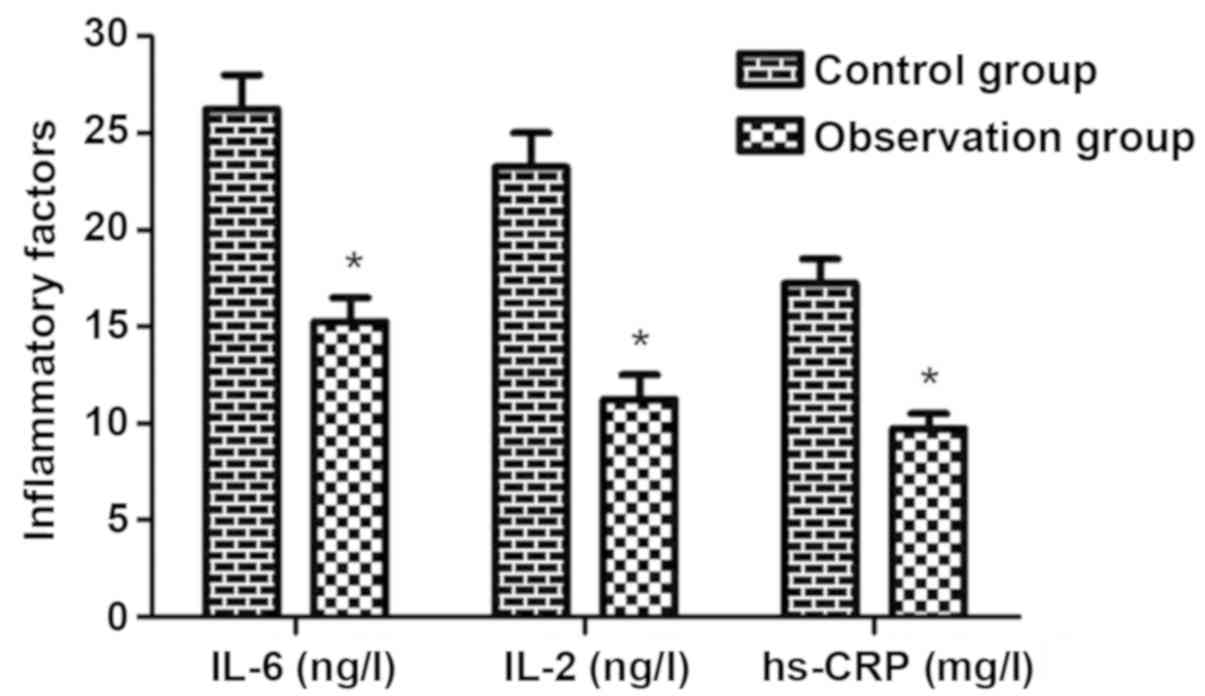
The content of IL-2, IL-6 and hs-CRP was notably
reduced in observation group (P<0.05) (Fig. 2), implying that the treatment with
sodium valproate combined with LEV can significantly inhibit the
production of inflammatory factors.
EEG findings
As shown in Table
VI, observation group exhibited substantially decreased α wave
(P<0.05), but notably increased θ and δ waves (P<0.05).
 | Table VIEEG findings. |
Table VI
EEG findings.
| Group | α | θ | β | δ |
|---|
| Control group | 18.4±2.4 | 21.7±2.3 | 11.5±1.9 | 17.5±1.9 |
| Observation
group |
12.8±2.6a |
29.5±2.1a | 10.9±2.0 |
25.5±1.9a |
Discussion
Epilepsy is a common neurological syndrome
characterized by complex etiology and recurrent seizures in
childhood, which also refers to the convulsive seizure caused by
paroxysmal and temporary brain dysfunction. It can be classified
into primary and secondary types according to etiology, with the
clinical manifestations of recurrent jerk and transient
abnormalities in consciousness, sense and emotion (16,17). In
the present study, the efficacy of sodium valproate combined with
LEV in pediatric epilepsy and its influences on NSE, IL-6 and
hs-CRP as well as EEG improvement were observed. Based on the
results, there was a statistically significant difference in the
total effective rate of clinical treatment between observation
group and control group (96 vs. 70%), and observation group had
substantially reduced number of cases of sleepiness, rash,
gastrointestinal reaction and nausea. A study found that the
cognitive ability of epilepsy children of school age exposed to
LEV, topiramate or sodium valproate can be improved dramatically
(18), and it was found in the
present study that the WMS-RC, MMSE and MoCA scores in the
observation group were notably higher than those in the control
group, suggesting that cognitive function was remarkably improved.
Additionally, the QOL score in observation group was remarkably
higher than that in the control group. The above findings in this
study are similar to those in the previous study (19).
Currently, NSE is a commonly used indicator for
evaluating cranial nerve function impairment in epileptic children,
and it is a key enzyme with enolase activity in glucose metabolism,
which is specifically present in neurons. When cerebral neurons are
damaged, the activity of NSE will be remarkably enhanced, so the
elevation of serum NSE can be taken as a highly specific and
sensitive biochemical indicator reflecting neuronal damage
(20). GFAP, a specific astrocyte
marker, is expressed in the central nervous system. The high
expression of GFAP can damage various aspects of the central
nervous system (21), and the
regulation of cognitive function, namely information reception,
integration and transmission, is associated with the functions of
the neurons and astrocytes in the cerebral cortex. According to the
findings in the present study, before treatment, there was no
statistically significant difference in the content ratio of
NSE/GFAP, and after treatment, the content of NSE and GFAP was
obviously reduced in both groups of patients, but the decline in
the observation group was much greater than that in the control
group. As an acute-phase protein, hs-CRP was confirmed to be
increased in diabetes patients (22). However, whether its content is raised
in patients with pediatric epilepsy remains to be further studied.
The activation of inflammatory factors and generation of cytokines
as well as increase in oxidative stress may cause tissue damage,
and are related to the evolution of epilepsy, namely inflammation
probably promotes the worsening of disease in epileptic patients
(23). In this study, it was
discovered that the content of IL-2, IL-6 and hs-CRP declined
notably in observation group, implying that the treatment with
sodium valproate combined with LEV can significantly inhibit the
production of inflammatory factors. A study demonstrated that the
increases in the absolute power and relative power (RP) of δ and θ
waves are observed in EEGs of epilepsy children, while the RP of α
wave is decreased. Besides, routine EEGs reveal interictal
discharge such as spikes, multiple spikes and sharp waves (24), which is a rare activity in children
with normal development (25). The
results of this study showed that α wave declined substantially,
but θ and δ waves were increased notably in the observation group,
similarly to previous studies. The present study proved through a
series of experiments that sodium valproate combined with LEV has
favorable efficacy in pediatric epilepsy, and can substantially
lower the levels of NSE, IL-6 and hs-CRP, and improve EEG
indicators.
In conclusion, it was found through the various
experiments in this study that sodium valproate combined with LEV
can notably improve the pathology, cognitive function and QOL of
patients, with a favorable overall effect. The present study
provides a theoretical basis for the prevention and treatment of
pediatric epilepsy and a novel idea for the forthcoming further
research.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL wrote the manuscript. ZL and JiL collected and
analyzed the general data of patients. FY and YH were responsible
for the observation of efficacy. JuL and HH assisted with the
cognitive function scoring and QOL scoring. ZL and WS detected EEG
improvement. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Xiantao First People's Hospital Affiliated to Yangtze University
(Xiantao, China) and informed consents were signed by the patients
and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stafstrom CE, Moshé SL, Swann JW, Nehlig
A, Jacobs MP and Schwartzkroin PA: Models of pediatric epilepsies:
Strategies and opportunities. Epilepsia. 47:1407–1414.
2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Harvey AS, Cross JH, Shinnar S and Mathern
GW: ILAE Pediatric Epilepsy Surgery Survey Taskforce: Defining the
spectrum of international practice in pediatric epilepsy surgery
patients. Epilepsia. 49:146–155. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ettinger AB, Weisbrot DM, Nolan EE, Gadow
KD, Vitale SA, Andriola MR, Lenn NJ, Novak GP and Hermann BP:
Symptoms of depression and anxiety in pediatric epilepsy patients.
Epilepsia. 39:595–599. 1998.PubMed/NCBI
|
|
4
|
Becker AJ, Blümcke I, Urbach H, Hans V and
Majores M: Molecular neuropathology of epilepsy-associated
glioneuronal malformations. J Neuropathol Exp Neurol. 65:99–108.
2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cross JH, Jayakar P, Nordli D, Delalande
O, Duchowny M, Wieser HG, Guerrini R and Mathern GW: International
League against Epilepsy Subcommission for Paediatric Epilepsy
Surgery; Commissions of Neurosurgery and Paediatrics: Proposed
criteria for referral and evaluation of children for epilepsy
surgery: Recommendations of the Subcommission for Pediatric
Epilepsy Surgery. Epilepsia. 47:952–959. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rutter M, Graham P and Yule WA: A
neuropsychiatric study in childhood. Arch Dis Child.
46(577)1971.
|
|
7
|
Hoare P: The development of psychiatric
disorder among schoolchildren with epilepsy. Dev Med Child Neurol.
26:3–13. 1984.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ackers R, Besag FM, Wade A, Murray ML and
Wong IC: Changing trends in antiepileptic drug prescribing in girls
of child-bearing potential. Arch Dis Child. 94:443–447.
2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Vigevano F: Levetiracetam in pediatrics. J
Child Neurol. 20:87–93. 2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
De Smedt T, Raedt R, Vonck K and Boon P:
Levetiracetam: Part II, the clinical profile of a novel
anticonvulsant drug. CNS Drug Rev. 13:57–78. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wen X, Meador KJ and Hartzema A:
Antiepileptic drug use by pregnant women enrolled in Florida
Medicaid. Neurology. 84:944–950. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Baker GA, Bromley RL, Briggs M, Cheyne CP,
Cohen MJ, García-Fiñana M, Gummery A, Kneen R, Loring DW, Mawer G,
et al: Liverpool and Manchester Neurodevelopment Group: IQ at 6
years after in utero exposure to antiepileptic drugs: A controlled
cohort study. Neurology. 84:382–390. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Morrow J, Russell A, Guthrie E, Parsons L,
Robertson I, Waddell R, Irwin B, McGivern RC, Morrison PJ and Craig
J: Malformation risks of antiepileptic drugs in pregnancy: A
prospective study from the UK Epilepsy and Pregnancy Register. J
Neurol Neurosurg Psychiatry. 77:193–198. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rosenow F and Lüders H: Presurgical
evaluation of epilepsy. Brain. 124:1683–1700. 2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tao JX, Ray A, Hawes-Ebersole S and
Ebersole JS: Intracranial EEG substrates of scalp EEG interictal
spikes. Epilepsia. 46:669–676. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Goldlust IS, Hermetz KE, Catalano LM,
Barfield RT, Cozad R, Wynn G, Ozdemir AC, Conneely KN, Mulle JG,
Dharamrup S, et al: Unique Rare Chromosome Disorder Support Group:
Mouse model implicates GNB3 duplication in a childhood obesity
syndrome. Proc Natl Acad Sci USA. 110:14990–14994. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sakkalis V, Doru Giurc Neanu C,
Xanthopoulos P, Zervakis ME, Tsiaras V, Yang Y, Karakonstantaki E
and Micheloyannis S: Assessment of linear and nonlinear
synchronization measures for analyzing EEG in a mild epileptic
paradigm. IEEE Trans Inf Technol Biomed. 13:433–441.
2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bromley RL, Calderbank R, Cheyne CP,
Rooney C, Trayner P, Clayton-Smith J, García-Fiñana M, Irwin B,
Morrow JI, Shallcross R, et al: UK Epilepsy and Pregnancy Register:
Cognition in school-age children exposed to levetiracetam,
topiramate, or sodium valproate. Neurology. 87:1943–1953.
2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tumay Y, Altun Y, Ekmekci K and Ozkul Y:
The effects of levetiracetam, carbamazepine, and sodium valproate
on P100 and P300 in epileptic patients. Clin Neuropharmacol.
36:55–58. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Akcan A, Akyildiz H, Deneme MA, Akgun H
and Aritas Y: Granulomatous lobular mastitis: A complex diagnostic
and therapeutic problem. World J Surg. 30:1403–1409.
2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Takeda K, Sawamura S, Tamai H, Sekiyama H
and Hanaoka K: Role for cyclooxygenase 2 in the development and
maintenance of neuropathic pain and spinal glial activation.
Anesthesiology. 103:837–844. 2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Karantza MV, Mittelman SD, Dorey F, Samie
S, Kaiserman K, Halvorson M and Kaufman FR: Relationship of highly
sensitive C-reactive protein and lipid levels in adolescents with
type 1 diabetes mellitus. Pediatr Diabetes. 9:122–126.
2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Vezzani A: Inflammation and epilepsy.
Epilepsy Curr. 5:1–6. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cantor DS and Chabot R: QEEG studies in
the assessment and treatment of childhood disorders. Clin EEG
Neurosci. 40:113–121. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Porras-Kattz E, Harmony T, Ricardo-Garcell
J, Galán L, Fernández T, Prado-Alcalá R, Avecilla-Ramírez G,
Sánchez-Moreno L, Barrera-Reséndiz J, Corsi-Cabrera M, et al:
Magnesium valproate in learning disabled children with interictal
paroxysmal EEG patterns: Preliminary report. Neurosci Lett.
492:99–104. 2011.PubMed/NCBI View Article : Google Scholar
|