Introduction
Alzheimer's disease (AD) is a common
neurodegenerative disease in the elderly, mainly manifested as
cognitive dysfunction, memory decline, social disorders, and
behavioral abnormalities (1,2). AD patients account for more than 80% of
dementia cases among people aged over 65 years in the world
(3). According to previous reports,
it was estimated that 47.5 million people worldwide would suffer
from dementia in 2015, and the incidence rate of AD shows an
increasing trend with changes in lifestyle (4,5).
Previous studies showed that the loss of cholinergic nerve cells in
basal forebrain and the increase of acetylcholinesterase (ACh E)
activity in AD patients lead to the decrease of neurotransmitter
acetylcholine (ACh). Therefore, the therapeutic purpose was mainly
achieved by inhibiting ACh E activity (6). Donepezil, one of acetylcholinesterase
inhibitors, is a commonly used drug for the treatment of AD and has
a good effect, which can significantly improve the cognitive
function of AD patients (7).
At present, many researchers are looking for
biomarkers that can detect the disease and monitor the course of
the disease, especially before symptoms appear or in early stages
(8,9). In recent years, the exploration of
peripheral blood microRNA (miRNA) has become a research hot-spot.
Some studies have reported that there are some changes in miRNA
levels in postmortem brain studies and changes in miRNA in whole
blood, plasma or serum. Therefore, the detection of the changes of
miRNA in AD has high clinical value for the early diagnosis and
curative effect evaluation of the disease (10-13).
miRNA belongs to an endogenous non-coding protein RNA gene (with
the length of about 18-24 nucleotides), which can play an important
role in a variety of diseases. For example, it mediates the
regulation of protein production by interacting with target
messenger RNA (mRNA) (14).
miR-28-3p is a miRNA that targets a variety of cancer-related genes
and can participate in epithelial-mesenchymal transition (EMT),
cell proliferation, invasion and migration (15,16).
Moreover, studies have showed that miR-28-3p was significantly
downregulated in nasopharyngeal carcinoma tissues and could
accelerate the invasion and migration of nasopharyngeal carcinoma
cells (16). Hong et al
(17) showed that miR-28-3p was
significantly upregulated in AD APP/PS1 transgenic mouse model.
Paltsev et al (18) reported
that miR-132 was involved in the pathogenesis of senile dementia by
detecting the serum miR-132 level of dementia patients, and it
could be used as a detection index for early diagnosis and
treatment of senile dementia. However, there is no research report
on whether miR-28-3p plays the same role in AD patients.
In this study, miR-28-3p in serum of AD patients
before and after treatment was determined, and its correlation with
clinical indicators was analyzed, to provide certain reference for
early diagnosis and treatment of AD.
Patients and methods
Clinical general data
Altogether 68 AD patients admitted to The People's
Hospital of Shouguang (Weifang, China) were collected as the AD
group, including 31 males and 37 females, with an average age of
70.12±2.09 years, with the MoCA score of 14.67±2.01 and MMSE score
of 15.48±1.68. Further 70 patients with early cognitive impairment
were treated as the MCI group, including 32 males and 38 females,
with an average age of 69.68±2.11 years, with the MoCA score of
20.76±1.69 and MMSE score of 22.67±0.73. Additionally 75 healthy
people who underwent physical examination in The People's Hospital
of Shouguang during the same period were selected as the normal
group, including 34 males and 41 females, with an average age of
69.47±1.98 years, with the MoCA score of 27.82±1.42 and MMSE score
of 28.03±1.52. This study was approved by the Ethics Committee of
The People's Hospital of Shouguang. The patients and their families
were informed in advance and signed a complete informed consent
form.
Inclusion criteria: Patients with good compliance
and complete clinical data; the educational level of the patients
was primary school or above; all patients received routine
examinations on urine routine, blood routine, electrocardiogram,
liver and kidney functions after admission; patients were
accompanied by their family when they were admitted to
hospital.
Exclusion criteria: Patients with previous history
of mental illness, liver dysfunction, severe organ lesions,
craniocerebral trauma, and autoimmune system defects; patients who
had a history of drug dependence and had taken antidepressants;
patients who had cognitive impairments.
Treatment methods
Patients with AD were treated with donepezil as
basic therapy. After admission, patients were given drugs to
improve cerebral blood circulation and promote brain cell
metabolism. According to the specific conditions of the patients,
symptomatic treatment was given, and the rest condition and diet of
the patients were adjusted. Patients should take moderate
rehabilitation exercise and get adequate sleep. At the same time,
donepezil hydrochloride tablets (Chinese Eisai Pharmaceutical Co.,
Ltd., SFDA approval no. H20050978) were given on this basis, once a
day and 5 mg for the first time. After one month of continuous
treatment, the stable blood drug concentration and clinical
response to the drug were evaluated. According to the specific
situation, the dosage was increased to 10 mg once a day for one
year.
Detection index
A total of 4 ml of fasting elbow venous blood were
collected from the three groups of patients after admission and
from AD patients after discharge. After coagulation for 60 min
(20-25˚C), each sample was centrifuged at 1,006.2 x g for 10 min to
collect the upper serum. The centrifugation radius was 10 cm and
the centrifugation temperature was 4˚C. The collected serum was
placed in a refrigerator at -80˚C for testing. The level of
homocysteine (Hcy) was detected by cyclic enzyme method (19). The level of miR-28-3p was detected by
real-time fluorescence quantitative PCR (qRT-PCR). Total RNA in
serum was extracted according to the instructions of TRIzol kit
(Shanghai Sangon Biotech). The template RNA was digested and
treated with DNase I (Shanghai Sangon Biotech) to eliminate DNA
contamination. The ultraviolet spectrophotometer (Beijing Up
General Technology Co., Ltd.) was used to measure the purity and
concentration, and OD 260/280 value more than 1.8 was considered
usable. The RNA sample was then reverse transcribed into cDNA, and
the operation was strictly carried out in accordance with the
instructions of cDNA reverse transcription kit (Takara Bio).
qRT-PCR was used for detection. The qRT-PCR reaction was carried
out on ABI 7500 system (Applied Biosystems) using SYBR-Green PCR
Master Mix (Thermo Fisher Scientific). PCR reaction conditions were
as follows: pre-denaturation at 95˚C for 10 min, denaturation at
95˚C for 10 sec, and annealing and extension at 60˚C for 60 sec,
for a total of 40 cycles. Primers for this experiment were designed
by Premier 5.0 (Premier) and generated by Tianjin Saierbio Co.,
Ltd. U6 was used as internal reference. The specific primer
sequences are shown in Table I. The
above system configuration was strictly in accordance with the
instructions. The level of miR-28-3p was calculated by
2-ΔCt.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene | Upstream | Downstream |
|---|
| miR-28-3p |
CGCGCACTAGATTGTGAGCT |
AGTGCAGGGTCCGAGGTATT |
| U6 |
CGACAAGACGATCCGGGTAAA |
GGTTGAGGAGTGGGTCGAAG |
Observation indicators
i) The miR-28-3p levels of patients in MCI group,
normal group and AD group were compared. ii) The relationship
between miR-28-3p level and clinical data in AD patients was
compared. iii) Montreal cognitive assessment scale (MoCA) (20) and mini mental state examination scale
(MMSE) (21) were used to evaluate
the cognitive function and mental state of MCI group, normal group
and AD group before treatment and 3 months after treatment, with a
total score of 30 points. The high score was closely related to the
good cognitive function. The levels of activity of daily life (ADL)
and Hcy were observed before treatment and 3 months after treatment
in AD group. ADL was used to evaluate the patients' activity of
life. The high score was closely related to poor activity of life
(22). iv) The correlation among
serum miR-28-3p level, score, and Hcy level in AD patients was
analyzed. v) The clinical value of miR-28-3p in diagnosing AD
patients was explored.
Statistical analysis
SPSS 20.0 (IBM Corp.) was used for statistical
analysis. GraphPad Prism 7 (GraphPad Software Co., Ltd.) was used
to illustrate the collected data. The count data was expressed as n
(%), and chi-square test was used for inter-group comparison. The
measurement data was expressed as mean ± standard deviation (mean ±
SD). The t-test was used for comparison between the two groups,
one-way ANOVA was used for comparison among multiple groups, which
was expressed as F. LSD-t-test was used for post-event analysis,
Pearson's analysis was used for bivariate correlation analysis, and
receiver operating characteristic curve (ROC) was used for
diagnosis of AD patients. P<0.05, was considered a statistically
significant difference.
Results
Comparison of general clinical data of
three groups
There was no significant difference in sex, average
age, smoking and drinking, body mass index, complications,
triglyceride (TG) or total cholesterol (TC) among AD, MCI and
normal groups (P>0.05). The high density lipoprotein cholesterol
(HDL-C), MoCA score and MMSE score of AD group were significantly
lower than those of the other two groups, while the low density
lipoprotein cholesterol (LDL-C) of AD group was higher than that of
the normal group (P<0.05) (Table
II).
 | Table IIComparison of general clinical data
of three groups [mean ± SD, n (%)]. |
Table II
Comparison of general clinical data
of three groups [mean ± SD, n (%)].
| Clinical data | AD group
(n=68) | MCI group
(n=70) | Normal group
(n=75) | F/χ2
value | P-value |
|---|
| Sex | | | | 0.002 | 0.999 |
|
Male | 31 (45.59) | 32 (45.71) | 34 (45.33) | | |
|
Female | 37 (54.41) | 38 (54.29) | 41 (54.67) | | |
| Average age
(years) | 70.12±2.09 | 69.68±2.11 | 69.47±1.98 | 1.832 | 0.163 |
| Smoking | | | | 0.273 | 0.873 |
|
Yes | 24 (35.29) | 22 (31.43) | 24 (32.00) | | |
|
No | 44 (64.71) | 48 (68.57) | 51 (68.00) | | |
| Drinking | | | | 3.496 | 0.174 |
|
Yes | 47 (69.12) | 48 (68.57) | 42 (56.00) | | |
|
No | 21 (30.88) | 22 (31.43) | 33 (44.00) | | |
| Body mass index
(kg/m2) Complication | 23.49±3.27 | 24.07±3.31 | 23.95±4.06 | 0.505 | 0.604 |
|
Diabetes | 27 (39.71) | 25 (35.71) | 22 (29.33) | 1.736 | 0.420 |
|
Hypertension | 36 (52.94) | 33 (47.14) | 37 (49.33) | 0.473 | 0.790 |
| TG (mmol/l) | 1.68±0.72 | 1.61±0.67 | 1.57±0.77 | 0.420 | 0.657 |
| TC (mmol/l) | 5.29±1.12 | 5.15±1.04 | 5.10±0.99 | 0.620 | 0.5391 |
| HDL-C (mmol/l) | 1.30±0.56 |
1.53±0.62a |
1.91±0.87a,b | 13.460 | <0.001 |
| LDL-C (mmol/l) | 2.98±1.13 | 2.82±1.07 |
2.54±1.10a | 2.951 | 0.055 |
| MoCA score | 14.67±2.01 |
20.76±1.69a |
27.82±1.42a,b | 1.055 | <0.001 |
| MMSE score | 15.48±1.68 |
22.67±0.73a |
28.03±1.52a,b | 1.491 | <0.001 |
Comparison of miR-28-3p levels in
three groups
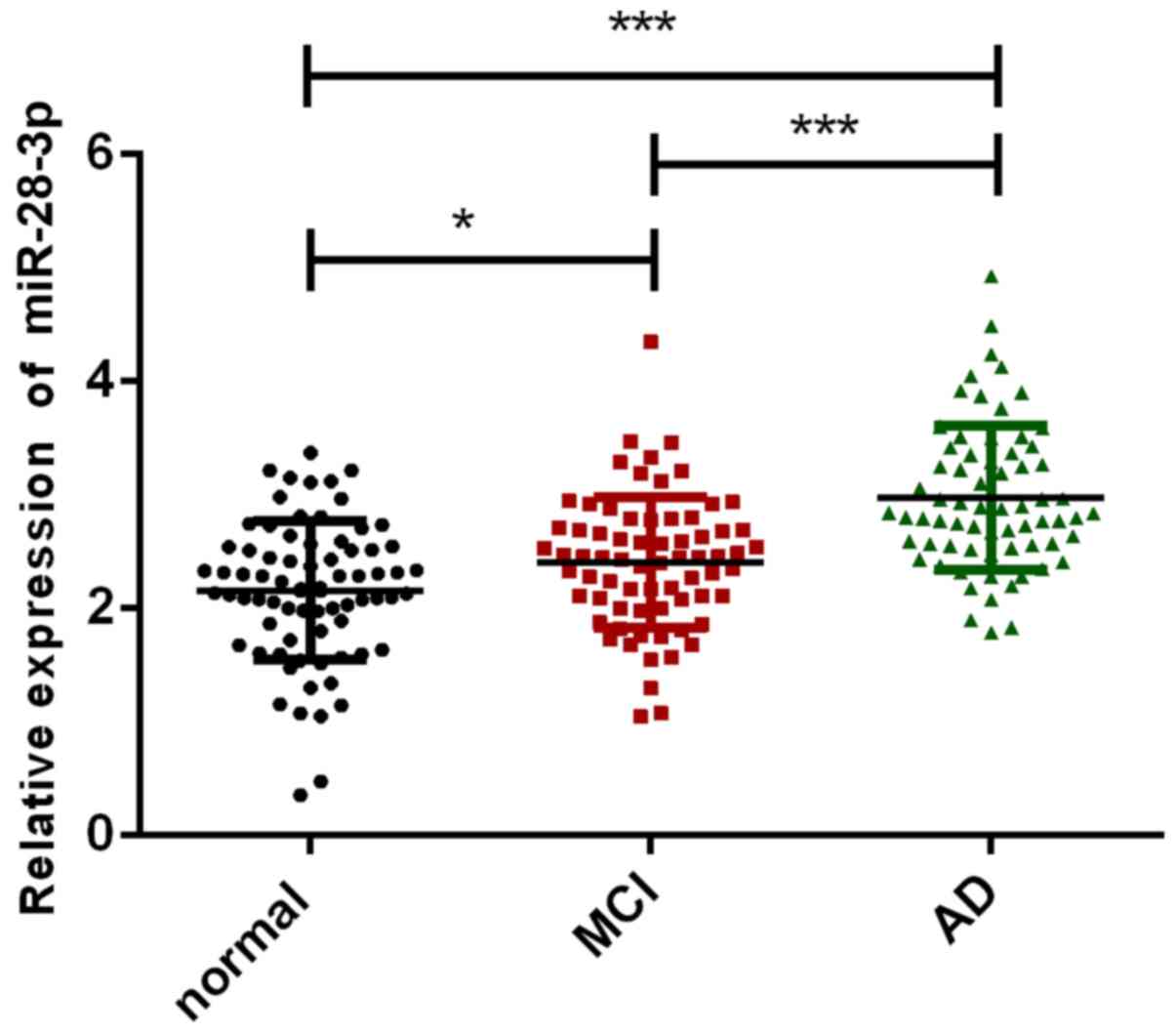
Comparing the serum miR-28-3p levels of AD group,
MCI group and normal group, as shown in Fig. 1, it was clear that the miR-28-3p
content of normal group was significantly lower than that of AD
group and MCI group (P<0.05), while the serum miR-28-3p level of
MCI group was significantly lower than that of AD group patients
(P<0.001).
Relationship between serum miR-28-3p
level and clinical data in AD patients
The clinical data of AD patients were collected as
shown in Table III. The miR-28-3p
level had no significant correlation with sex, age, smoking or
drinking (P>0.05), however, miR-28-3p level had a significant
correlation with disease course and severity (P<0.05).
 | Table IIIRelationship between serum miR-28-3p
level and clinical characteristics in AD patients (mean ± SD). |
Table III
Relationship between serum miR-28-3p
level and clinical characteristics in AD patients (mean ± SD).
| Clinical
characteristics | n | miR-28-3p | F/t value | P-value |
|---|
| Sex | | | 0.487 | 0.628 |
|
Male | 37 | 3.11±0.65 | | |
|
Female | 31 | 3.04±0.51 | | |
| Age | | | 0.785 | 0.435 |
|
<65
years | 20 | 3.02±0.45 | | |
|
≥65
years | 48 | 3.12±0.49 | | |
| Course of
disease | | | 3.058 | 0.003 |
|
<2
years | 42 | 2.89±0.44 | | |
|
≥2
years | 26 | 3.25±0.49 | | |
| Smoking | | | 0.405 | 0.687 |
|
Yes | 24 | 3.10±0.46 | | |
|
No | 44 | 3.05±0.50 | | |
| Drinking | | | 1.101 | 0.275 |
|
Yes | 47 | 3.15±0.51 | | |
|
No | 21 | 3.01±0.42 | | |
| Severity | | | 10.280 | <0.001 |
|
Mild | 22 | 2.75±0.37 | | |
|
Medium | 31 | 3.09±0.42 | | |
|
Severe | 15 | 3.37±0.47 | | |
Comparison of serum miR-28-3p levels
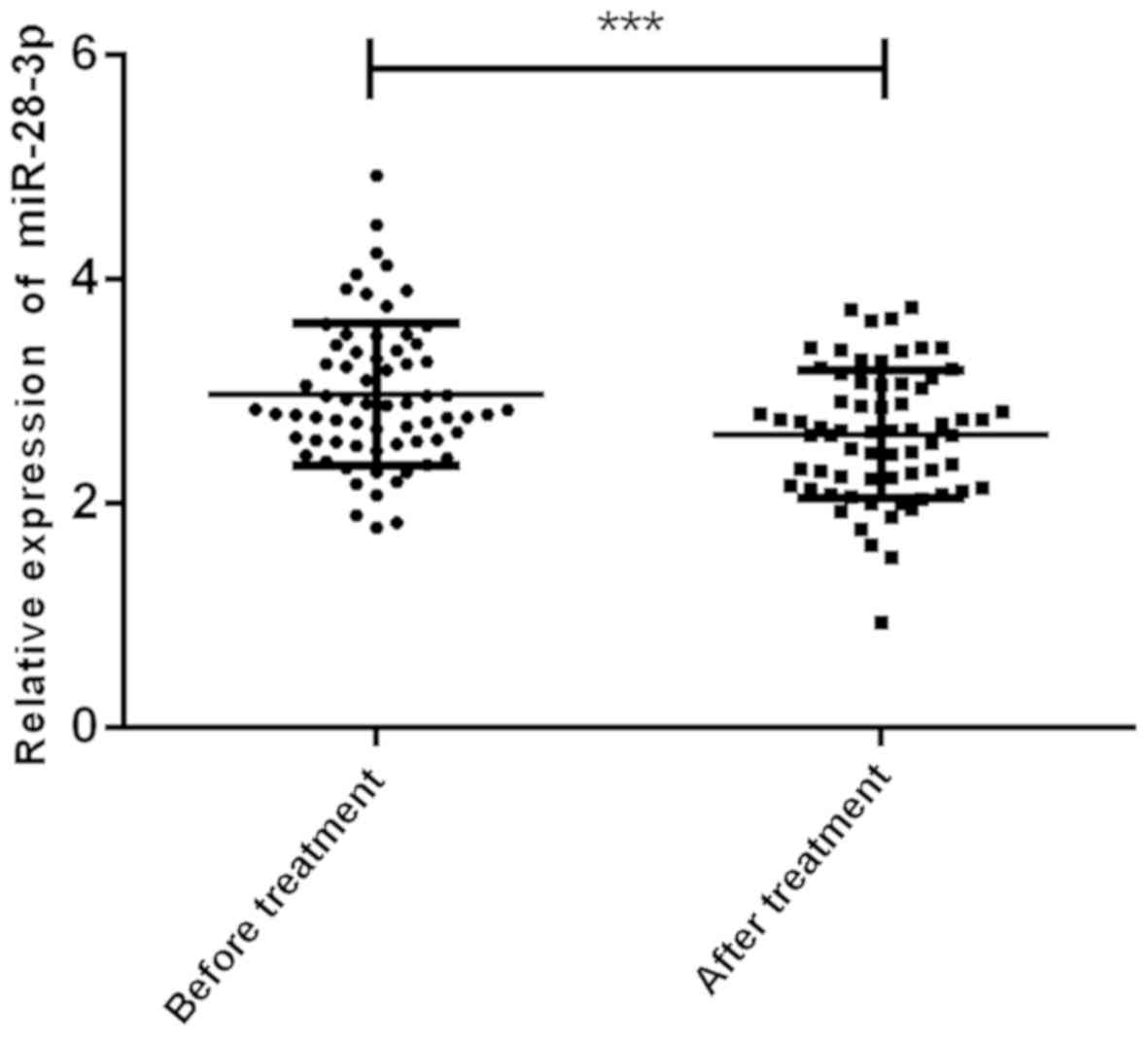
of AD patients before and after treatment
Comparison of the serum miR-28-3p level of AD
patients before and after treatment in Fig. 2, indicated that the serum miR-28-3p
level of AD patients before treatment was 3.07±0.71, the serum
miR-28-3p level after treatment was 2.55±0.61, and the serum
miR-28-3p level after treatment was significantly lower than that
before treatment (P<0.001).
Changes of score and Hcy level of AD
patients before and after treatment
ADL score, MMSE score and Hcy level of AD after
treatment were compared. As shown in Table IV, ADL score and Hcy level of AD
patients after treatment were significantly lower than those before
treatment (P<0.05). MMSE score and MoCA score after treatment
were significantly higher than those before treatment
(P<0.05).
 | Table IVComparison of clinical indexes of AD
patients before and after treatment (mean ± SD). |
Table IV
Comparison of clinical indexes of AD
patients before and after treatment (mean ± SD).
| Treatment
stage | ADL score | MMSE score | MoCA score | Hcy (µmol/l) |
|---|
| Before
treatment | 47.76±5.13 | 15.48±1.68 | 14.67±2.01 | 19.21±7.68 |
| After
treatment | 43.23±4.58 | 20.76±1.87 | 19.89±1.88 | 11.52±8.02 |
| t value | 5.432 | 17.320 | 15.640 | 5.711 |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 |
Correlation of serum miR-28-3p level
with score and Hcy level and clinical indicators of AD patients
before treatment
Pearson's analysis showed that serum miR-28-3p level
was significantly positively correlated with ADL score and Hcy
level before treatment (r=0.6837, 0.6861, P<0.05), and miR-28-3p
level was significantly negatively correlated with MMSE score and
MoCA score (r=-0.6142, -0.6198, P<0.05) (Fig. 3).
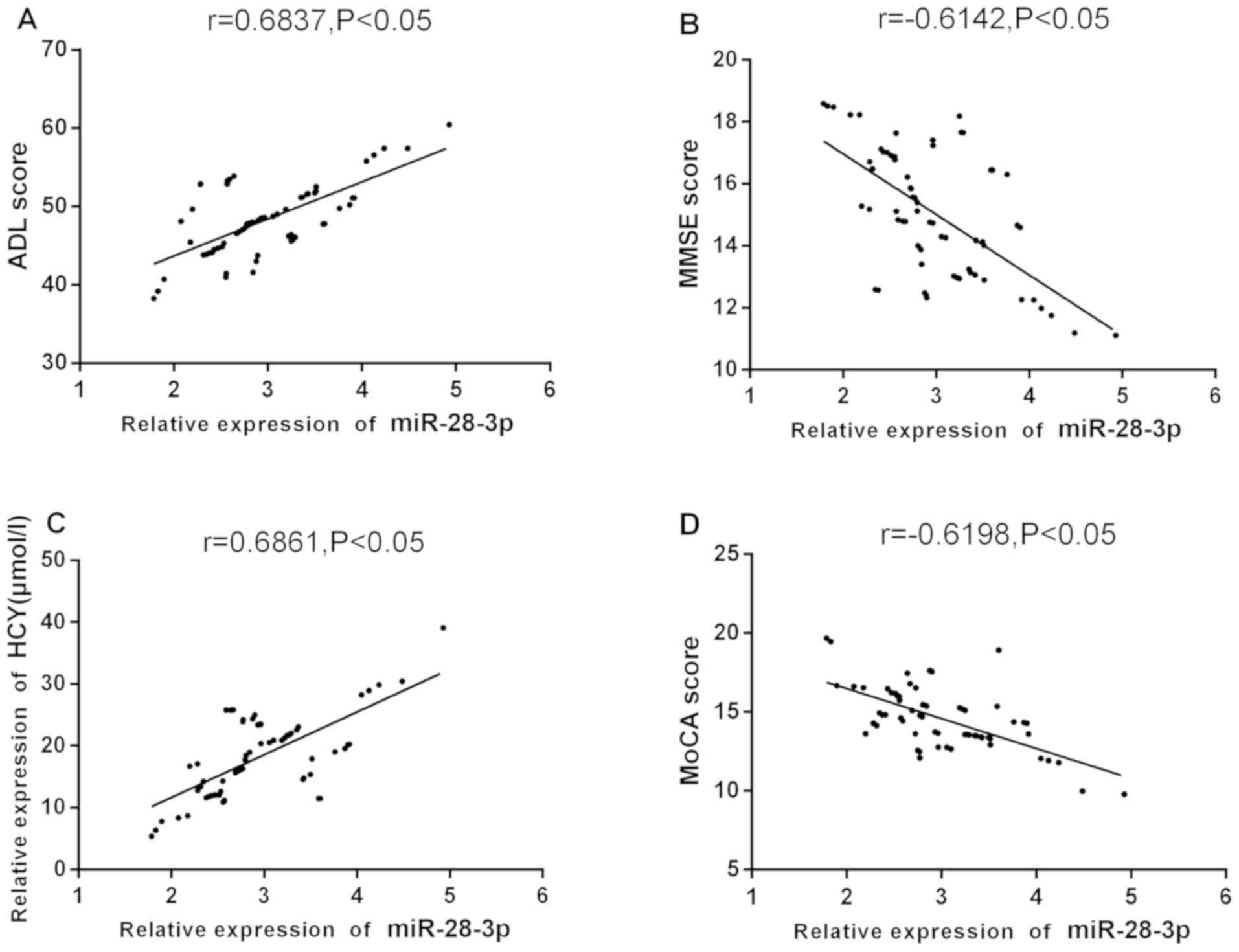 | Figure 3Correlation between serum miR-28-3p
level and clinical indexes in AD patients before treatment.
Pearson's analysis showing that, (A) The serum miR-28-3p level of
AD patients before treatment was significantly positively
correlated with ADL score (r=0.6837, P<0.05); (B) the serum
miR-28-3p level of AD patients before treatment was significantly
negatively correlated with MMSE score (r=-0.6142, P<0.05); (C)
before treatment, the serum miR-28-3p level of AD patients was
significantly positively correlated with Hcy level (r=0.6861,
P<0.05); (D) miR-28-3p level was significantly negatively
correlated with MoCA score (r=-0.6198, P<0.05). AD, Alzheimer's
disease; ADL, daily living scale; MoCA, Montreal cognitive
assessment scale; Hcy, homocysteine; MMSE, mini mental state
examination scale. |
Correlation of serum miR-28-3p level with score and
Hcy correlation between serum miR-28-3p level and clinical
indicators in AD patients after treatment. Pearson's analysis
showed that serum miR-28-3p level was significantly positively
correlated with ADL score and Hcy level after treatment (r=0.6464,
0.6824, P<0.05), and miR-28-3p level was significantly
negatively correlated with MMSE score and MoCA score (r=-0.6598,
-0.6524, P<0.05) (Fig. 4).
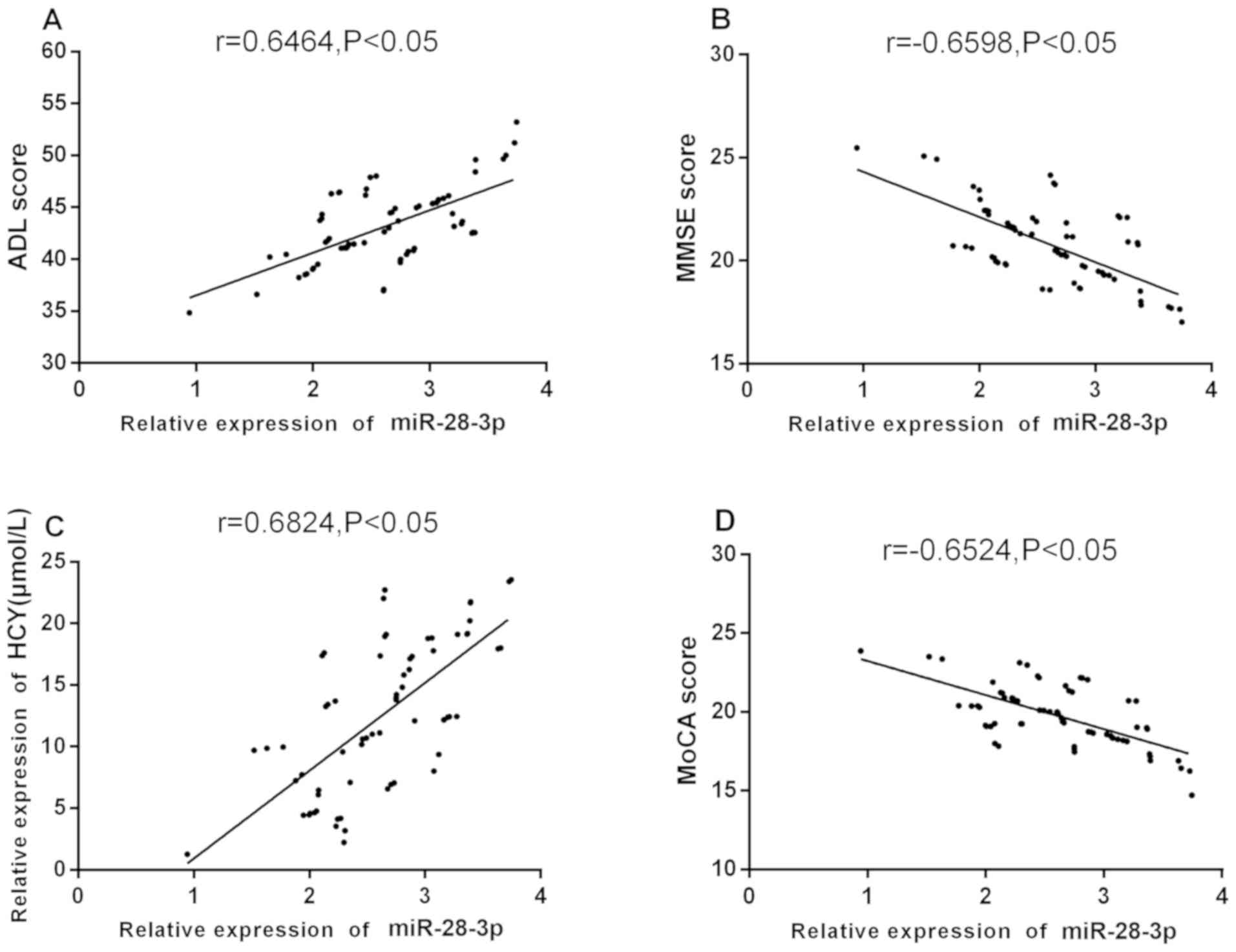 | Figure 4Correlation between serum miR-28-3p
level and clinical indexes in AD patients after treatment.
Pearson's analysis showing that, (A) after treatment, the serum
miR-28-3p level of AD patients was significantly positively
correlated with ADL score (r=0.6464, P<0.05); (B) miR-28-3p
level was significantly negatively correlated with MMSE score
(r=-0.6598, P<0.05); (C) after treatment, the serum miR-28-3p
level of AD patients was significantly positively correlated with
Hcy level (r=0.6824, P<0.05); (D) miR-28-3p level was
significantly negatively correlated with MoCA score (r=-0.6524,
P<0.05). AD, Alzheimer's disease; ADL, daily living scale; MoCA,
Montreal cognitive assessment scale; Hcy, homocysteine; MMSE, mini
mental state examination scale. |
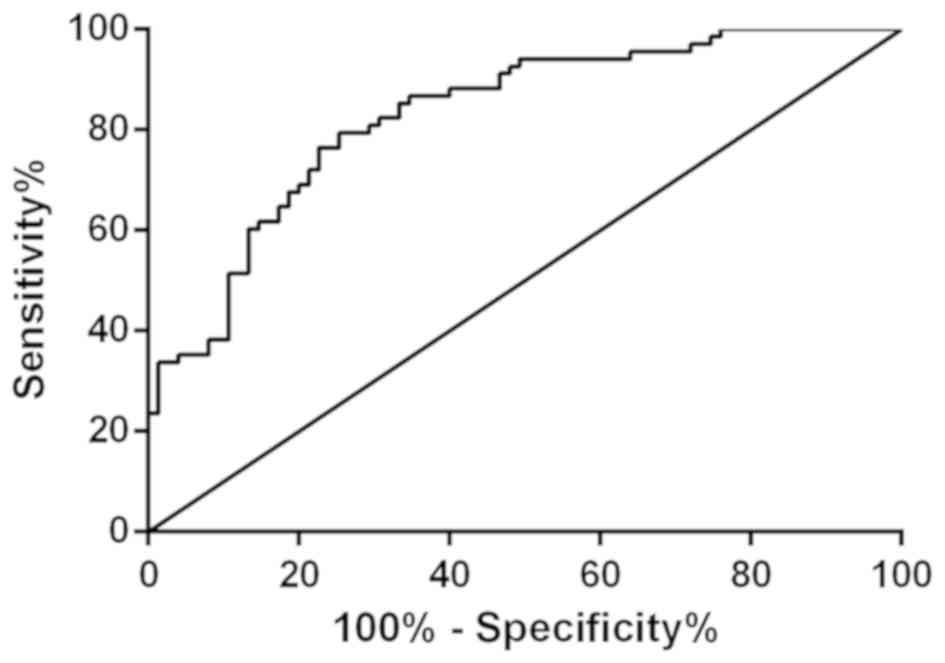
Value of miR-28-3P in diagnosis of AD
patients
AD group and normal group were selected to observe
the diagnostic value of miR-28-3p, as shown in Fig. 5. The sensitivity of miR-28-3p for
distinguishing AD patients from normal subjects was 79.41%, the
specificity was 74.67%, the AUC was 0.8306 (0.7649-0.8963), and the
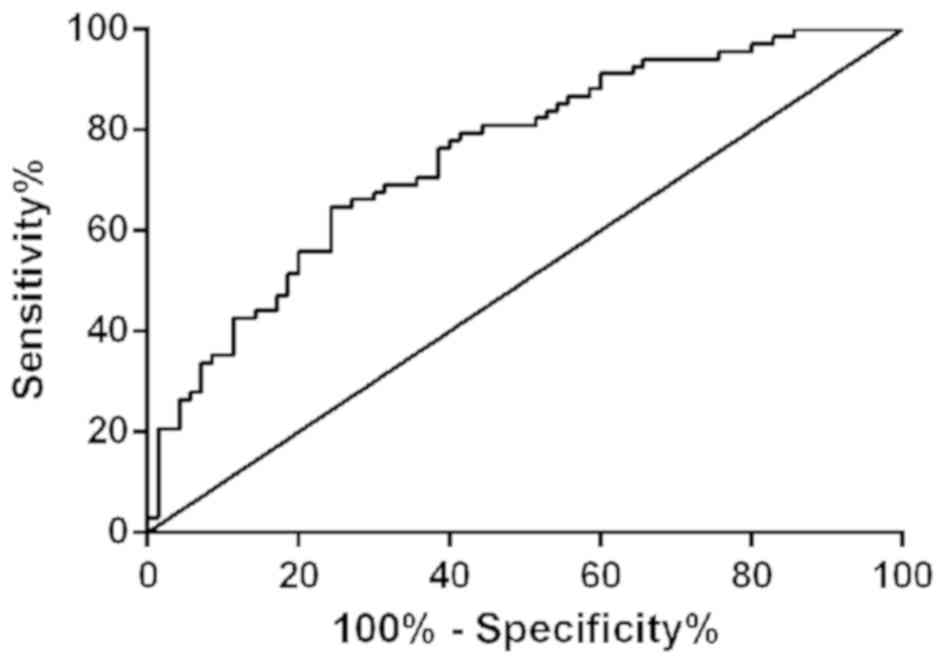
cut-off value was 2.52. Patients in AD group and MCI group were
selected, as shown in Fig. 6. As
shown miR-28-3p had sensitivity of 64.71%, specificity of 75.71%,
AUC of 0.7506 (0.6704-0.8309), and the cut-off value of 2.72 for
distinguishing AD patients from MCI patients.
Discussion
Pathogenesis of AD is relatively complex and
correlated with inheritance, age, cytoskeleton changes,
inflammatory response, nerve transmission obstruction and other
factors. Most patients cannot receive timely treatment after
suffering from the illness, which will lead to cognitive impairment
of different degrees. The occurrence of the disease will bring
economic burden and seriously affect the quality of life of
patients (18). The pathological
features of AD are mainly vascular amyloidosis, aggregation of
abnormally phosphorylated tau protein in cells, formation of
neurofibrillary tangles (NFTs), senile plaques formed by deposition
of β-amyloid protein (Aβ), loss of neuronal cells in hippocampus
and cerebral cortex (23,24). Donepezil can specifically hinder the
degradation of ACh in the brain, reduce the atrophy of brain
tissue, and increase the level of choline in the cerebral cortex,
thus improving the cognitive function of patients. Donepezil is
widely used because of its low hepatotoxicity, long half-life and
high safety (25,26).
Recent studies found that there are abundant miRNA
in human brain, and about 20-25% of miRNA are significantly
upregulated in AD brain, which is of great significance for
identifying and/or diagnosis (27).
Most miRNA are highly expressed or specifically expressed in the
nervous system, participating in the processes of memory formation,
synaptic plasticity and nerve differentiation, and regulating
protein synthesis (28-30).
Studies have found that miRNA plays an important role in the
occurrence and development of AD, and miRNA interference may become
a new target for the treatment of AD (31). miRNA has good stability and can be
found in plasma and serum samples (32). Jia and Liu (33) detected the expression of miR-223 and
miR-519 in serum of AD patients, and found that miR-223 was
downregulated and miR-519 was upregulated. miRNA combined diagnosis
can be used to predict the occurrence of AD disease. Therefore,
detecting the number and types of miRNA in serum samples can
effectively reflect the types and severity of the disease, and
serum detection is convenient and non-invasive, thus, an ideal
early screening method (34,35).
Relevant studies reported that miR-28-3p is a cell
limiter, which can inhibit human T cell leukemia virus, type 1
(HTLV-1) replication and virus infection. miR-28-3p can target a
sequence located in the mRNA of viral gag/pol genomic virus and
reduce virus replication and gene expression in transient
transgenic cells of HTLV-1 molecular clone (36). miR-28-3p can be detected in various
diseases and participates in the occurrence and development of
diseases (37-39).
Related studies showed that miR-28-5p and miR-28-3p are
downregulated in colorectal cancer samples compared with normal
colon samples (15). In addition,
the differential expression of 27 miRNA between AD patients and
normal controls was identified, suggesting that miR-28-3p was
significantly upregulated in AD patients (40). This indicated that miR-28-3p is
expressed differently in different diseases. The results of this
study showed that the miR-28-3p level in normal group was
significantly lower than that in AD group and MCI group, while the
serum miR-28-3p level in MCI group was significantly lower than
that in AD group (P<0.001). It is suggested that the cognitive
impairment of patients might be related to the upregulation of
miR-28-3p expression and the occurrence of AD was related to the
regulation of miRNA. Therefore, combined with previous literature,
it was speculated that the upregulation of miR-28-3p in AD might be
related to cell survival and cell communication. For example,
PI3K-Akt signal transduction, ECM receptor and adhesion spot
interaction may be induced and activated by compensatory response
to extensive neurodegeneration of AD brain, but more basic
experiments are needed to explore the specific action pathway of
miR-28-3p (40). The expression of
various miRNA in cerebrospinal fluid, the serum of patients with AD
and Parkinson's disease, and its relationship with pathological
features were detected. It was found that the expression of miRNA
was significantly correlated with the severity of the disease,
Braak stage, dementia status, plaque and entanglement density, and
Louis body (41). However, there is
scarce research on the relationship between miR-28-3p and
pathological characteristics of AD patients. The present study
found that the level of miR-28-3p had no significant correlation
with sex, age, smoking or drinking, and the level of miR-28-3p was
significant different in different disease courses and severity
grades (P<0.05). It was suggested that the level of miR-28-3p
increased gradually with the increase of course and severity, which
was significantly correlated with the severity of the disease. Wang
et al (42) suggested that
miR-206-3p participated in the anti-dementia effect of donepezil
and it might be a new pharmacological target for the treatment of
AD. Rosignolo et al (43)
showed that the level of miR-28-3p in patients with papillary
thyroid cancer before operation was significantly higher than that
in postoperative and healthy control groups. This study found that
the serum miR-28-3p level of AD patients after treatment was
significantly lower than that before treatment. This indicated that
the combination of donepezil and basic therapy could significantly
reduce the miR-28-3p level.
Hcy is produced by metabolism of methionine, which
will produce oxidative stress, lead to cell damage and blood-brain
barrier damage, and increase the level of brain Aβ. Many studies
have shown that the increase of Hcy level in peripheral blood of AD
patients was considered to be a risk factor for the occurrence of
AD (44,45). Research by Gu et al (46) showed that Dihuang Yizhi recipe
combined with donepezil hydrochloride could significantly improve
ADL score, MMSE score and MoCA score of dementia patients with
Parkinson's disease. Moreover, it was reported that donepezil could
significantly reduce the serum Hcy level of AD patients and had
good therapeutic effect (47). Our
study found that ADL score and Hcy level of AD patients after
treatment were significantly lower than those before treatment, and
MMSE score and MoCA score after treatment were significantly higher
than those before treatment (P<0.05). It was suggested that Hcy
might be involved in the occurrence of AD, and ADL score, MMSE
score and MoCA score could effectively reflect the therapeutic
effect. Related studies found that serum miR-223 in AD patients was
significantly positively correlated with MMSE score, while serum
miR-519 was not significantly positively correlated with MMSE score
(33). Correlation analysis showed
that serum miR-132 level was negatively correlated with Hcy level,
and serum miR-132 level was positively correlated with atrial
natriuretic peptide (ANP) level and MMSE score (18). It was presumed that miR-28-3p had the
same effect. After analysis, it was found that serum miR-28-3p
level in AD patients was significantly positively correlated with
ADL score and Hcy level, while miR-28-3p level was significantly
negatively correlated with MMSE score and MoCA score. The results
showed that with the increase of mental state and quality of life
score and the decrease of serum Hcy level, the level of serum
miR-28-3p decreased gradually, which effectively reflected the
severity of cognitive impairment in AD patients and miR-28-3p level
could be used as a therapeutic evaluation of AD disease. Reports
showed that the AUC of miR-28-3p was 0.792 (95% confidence
interval: 0.689-0.896), and the high expression of miR-28-3p in
plasma could be used as a non-invasive and stable biomarker for
detecting pulmonary embolism (48).
Yu et al (49) used qRT-PCR
and then analyzed the operating characteristics of the subjects,
and found that the expression of miR-28-3p, miR-143-3p, miR-151a-3p
and miR-148a-3p were closely related to Helicobacter pylori
infection. The above four plasma miRNA groups were expected to be
used as non-invasive biomarkers for Helicobacter pylori
infection. The present study found that the detection of serum
miR-28-3p level could be used for early diagnosis of AD patients,
but due to the small sample size and few related studies, a large
number of studies are needed to further prove the research
results.
This study provided references for the early
diagnosis and treatment of AD patients by detecting the level of
miR-28-3p in the serum of AD patients. However, no long-term
follow-up of AD patients were carried out to observe their
prognosis and whether the expression of miR-28-3p could be used as
a prediction of long-term curative effect. Thus, the exact
mechanism of miR-28-3p in the occurrence and development of AD
needs further research.
In conclusion, donepezil therapy may reduce
miR-28-3p level to improve the symptoms of AD patients, and
miR-28-3p level can be used as early diagnosis and prognosis
evaluation of AD patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ wrote the manuscript, interpreted and analyzed
the patient data. SW designed the study and performed the
experiments. WS was responsible for the analysis and discussion of
the data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The People's Hospital of Shouguang (Weifang, China). Patients who
participated in this research had complete clinical data. Signed
informed consents were obtained from the patients and/or
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hampel H, Mesulam MM, Cuello AC, Farlow
MR, Giacobini E, Grossberg GT, Khachaturian AS, Vergallo A, Cavedo
E, Snyder PJ, et al: The cholinergic system in the pathophysiology
and treatment of Alzheimer's disease. Brain. 141:1917–1933.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kelly SC, He B, Perez SE, Ginsberg SD,
Mufson EJ and Counts SE: Locus coeruleus cellular and molecular
pathology during the progression of Alzheimer's disease. Acta
Neuropathol Commun. 5(8)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Reitz C and Mayeux R: Alzheimer disease:
Epidemiology, diagnostic criteria, risk factors and biomarkers.
Biochem Pharmacol. 88:640–651. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Alzheimer's Disease International: World
Alzheimer Report. Alzheimer's Disease International, London,
2015.
|
|
5
|
Vijayan M and Reddy PH: Stroke, vascular
dementia, and Alzheimer's disease: Molecular links. J Alzheimers
Dis. 54:427–443. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Morelli A, Sarchielli E, Guarnieri G,
Coppi E, Pantano D, Comeglio P, Nardiello P, Pugliese AM, Ballerini
L, Matucci R, et al: Young human cholinergic neurons respond to
physiological regulators and improve cognitive symptoms in an
animal model of Alzheimer's disease. Front Cell Neurosci.
11(339)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kim SH, Kandiah N, Hsu JL, Suthisisang C,
Udommongkol C and Dash A: Beyond symptomatic effects: Potential of
donepezil as a neuroprotective agent and disease modifier in
Alzheimer's disease. Br J Pharmacol. 174:4224–4232. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fagan AM, Xiong C, Jasielec MS, Bateman
RJ, Goate AM, Benzinger TL, Ghetti B, Martins RN, Masters CL,
Mayeux R, et al: Dominantly Inherited Alzheimer Network.
Longitudinal change in CSF biomarkers in autosomal-dominant
Alzheimer's disease. Sci Transl Med. 6(226)2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jack CR Jr and Holtzman DM: Biomarker
modeling of Alzheimer's disease. Neuron. 80:1347–1358.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Absalon S, Kochanek DM, Raghavan V and
Krichevsky AM: MiR-26b, upregulated in Alzheimer's disease,
activates cell cycle entry, tau-phosphorylation, and apoptosis in
postmitotic neurons. J Neurosci. 33:14645–14659. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hébert SS, Wang WX, Zhu Q and Nelson PT: A
study of small RNAs from cerebral neocortex of pathology-verified
Alzheimer's disease, dementia with lewy bodies, hippocampal
sclerosis, frontotemporal lobar dementia, and non-demented human
controls. J Alzheimers Dis. 35:335–348. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lugli G, Cohen AM, Bennett DA, Shah RC,
Fields CJ, Hernandez AG and Smalheiser NR: Plasma exosomal miRNAs
in persons with and without Alzheimer disease: altered expression
and prospects for biomarkers. PLoS One. 10(e0139233)2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kumar P, Dezso Z, MacKenzie C, Oestreicher
J, Agoulnik S, Byrne M, Bernier F, Yanagimachi M, Aoshima K and Oda
Y: Circulating miRNA biomarkers for Alzheimer's disease. PLoS One.
8(e69807)2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Long JM, Ray B and Lahiri DK: MicroRNA-153
physiologically inhibits expression of amyloid-β precursor protein
in cultured human fetal brain cells and is dysregulated in a subset
of Alzheimer disease patients. J Biol Chem. 287:31298–31310.
2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Almeida MI, Nicoloso MS, Zeng L, Ivan C,
Spizzo R, Gafà R, Xiao L, Zhang X, Vannini I, Fanini F, et al:
Strand-specific miR-28-5p and miR-28-3p have distinct effects in
colorectal cancer cells. Gastroenterology. 142:886–896.e9.
2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lv Y, Yang H, Ma X and Wu G:
Strand-specific miR-28-3p and miR-28-5p have differential effects
on nasopharyngeal cancer cells proliferation, apoptosis, migration
and invasion. Cancer Cell Int. 19(187)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hong H, Li Y and Su B: Identification of
circulating miR-125b as a potential biomarker of Alzheimer's
disease in APP/PS1 transgenic mouse. J Alzheimers Dis.
59:1449–1458. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Paltsev MA, Zuev VA, Kozhevnikova EO,
Linkova NS, Kvetnaia TV, Polyakova VO and Kvetnoy IM: Molecular
markers of Alzheimer disease early diagnostic: Investigation
perspectives of peripheral tissues. Adv Gerontol. 30:809–817.
2017.(In Russian). PubMed/NCBI
|
|
19
|
Ma Y, Zhang Z, Chen R, Shi R, Zeng P, Chen
R, Leng Y and Chen AF: NRP1 regulates HMGB1 in vascular endothelial
cells under high homocysteine condition. Am J Physiol Heart Circ
Physiol. 316:H1039–H1046. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Matías-Guiu JA, Valles-Salgado M, Rognoni
T, Hamre-Gil F, Moreno-Ramos T and Matías-Guiu J: Comparative
diagnostic accuracy of the ACE-III, MIS, MMSE, MoCA, and RUDAS for
screening of Alzheimer disease. Dement Geriatr Cogn Disord.
43:237–246. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhou G, Liu S, Yu X, Zhao X, Ma L and Shan
P: High prevalence of sleep disorders and behavioral and
psychological symptoms of dementia in late-onset Alzheimer disease:
A study in Eastern China. Medicine (Baltimore).
98(e18405)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Milani P, Vincent Rajkumar S, Merlini G,
Kumar S, Gertz MA, Palladini G, Lacy MQ, Buadi FK, Hayman SR, Leung
N, et al: N-terminal fragment of the type-B natriuretic peptide
(NT-proBNP) contributes to a simple new frailty score in patients
with newly diagnosed multiple myeloma. Am J Hematol. 91:1129–1134.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cacabelos R, Torrellas C and López-Muñoz
F: Epigenomics of Alzheimer's disease. J Exp Clin Med. 6:75–82.
2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Long JM, Maloney B, Rogers JT and Lahiri
DK: Novel upregulation of amyloid-β precursor protein (APP) by
microRNA-346 via targeting of APP mRNA 5'-untranslated region:
Implications in Alzheimer's disease. Mol Psychiatry. 24:345–363.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cavedo E, Grothe MJ, Colliot O, Lista S,
Chupin M, Dormont D, Houot M, Lehéricy S, Teipel S, Dubois B, et
al: Hippocampus Study Group: Reduced basal forebrain atrophy
progression in a randomized Donepezil trial in prodromal
Alzheimer's disease. Sci Rep. 7(11706)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li Q, He S, Chen Y, Feng F, Qu W and Sun
H: Donepezil-based multi-functional cholinesterase inhibitors for
treatment of Alzheimer's disease. Eur J Med Chem. 158:463–477.
2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhao Y, Pogue AI and Lukiw WJ: MicroRNA
(miRNA) signaling in the human CNS in sporadic Alzheimer's disease
(AD) - novel and unique pathological features. Int J Mol Sci.
16:30105–30116. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hohjoh H and Fukushima T: Expression
profile analysis of microRNA (miRNA) in mouse central nervous
system using a new miRNA detection system that examines
hybridization signals at every step of washing. Gene. 391:39–44.
2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Schratt GM, Tuebing F, Nigh EA, Kane CG,
Sabatini ME, Kiebler M and Greenberg ME: A brain-specific microRNA
regulates dendritic spine development. Nature. 439:283–289.
2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Luo L: Actin cytoskeleton regulation in
neuronal morphogenesis and structural plasticity. Annu Rev Cell Dev
Biol. 18:601–635. 2002.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Satoh J: MicroRNAs and their therapeutic
potential for human diseases: Aberrant microRNA expression in
Alzheimer's disease brains. J Pharmacol Sci. 114:269–275.
2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Blondal T, Jensby Nielsen S, Baker A,
Andreasen D, Mouritzen P, Wrang Teilum M and Dahlsveen IK:
Assessing sample and miRNA profile quality in serum and plasma or
other biofluids. Methods. 59:S1–S6. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jia LH and Liu YN: Downregulated serum
miR-223 serves as biomarker in Alzheimer's disease. Cell Biochem
Funct. 34:233–237. 2016.
|
|
34
|
Brase JC, Wuttig D, Kuner R and Sültmann
H: Serum microRNAs as non-invasive biomarkers for cancer. Mol
Cancer. 9(306)2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K,
Guo J, Zhang Y, Chen J, Guo X, et al: Characterization of microRNAs
in serum: A novel class of biomarkers for diagnosis of cancer and
other diseases. Cell Res. 18:997–1006. 2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Bai XT and Nicot C: miR-28-3p is a
cellular restriction factor that inhibits human T cell leukemia
virus, type 1 (HTLV-1) replication and virus infection. J Biol
Chem. 290:5381–5390. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Pospisilova S, Pazourkova E, Horinek A,
Brisuda A, Svobodova I, Soukup V, Hrbacek J, Capoun O, Hanus T,
Mares J, et al: MicroRNAs in urine supernatant as potential
non-invasive markers for bladder cancer detection. Neoplasma.
63:799–808. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Argyropoulos C, Wang K, Bernardo J, Ellis
D, Orchard T, Galas D and Johnson JP: Urinary microRNA profiling
predicts the development of microalbumin uria in patients with type
1 diabetes. J Clin Med. 4:1498–1517. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Prats-Puig A, Ortega FJ, Mercader JM,
Moreno-Navarrete JM, Moreno M, Bonet N, Ricart W, López-Bermejo A
and Fernández-Real JM: Changes in circulating microRNAs are
associated with childhood obesity. J Clin Endocrinol Metab.
98:E1655–E1660. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Satoh J, Kino Y and Niida S: MicroRNA-Seq
data analysis pipeline to identify blood biomarkers for Alzheimer's
disease from public data. Biomark Insights. 10:21–31.
2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Burgos K, Malenica I, Metpally R,
Courtright A, Rakela B, Beach T, Shill H, Adler C, Sabbagh M, Villa
S, et al: Profiles of extracellular miRNA in cerebrospinal fluid
and serum from patients with Alzheimer's and Parkinson's diseases
correlate with disease status and features of pathology. PLoS One.
9(e94839)2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wang CN, Wang YJ, Wang H, Song L, Chen Y,
Wang JL, Ye Y and Jiang B: The anti-dementia effects of donepezil
involve miR-206-3p in the hippocampus and cortex. Biol Pharm Bull.
40:465–472. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Rosignolo F, Sponziello M, Giacomelli L,
Russo D, Pecce V, Biffoni M, Bellantone R, Lombardi CP, Lamartina
L, Grani G, et al: Identification of thyroid-associated serum
microRNA profiles and their potential use in thyroid cancer
follow-up. J Endocr Soc. 1:3–13. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kamath AF, Chauhan AK, Kisucka J, Dole VS,
Loscalzo J, Handy DE and Wagner DD: Elevated levels of homocysteine
compromise blood-brain barrier integrity in mice. Blood.
107:591–593. 2006.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Minagawa H, Watanabe A, Akatsu H, Adachi
K, Ohtsuka C, Terayama Y, Hosono T, Takahashi S, Wakita H, Jung CG,
et al: Homocysteine, another risk factor for Alzheimer disease,
impairs apolipoprotein E3 function. J Biol Chem. 285:38382–38388.
2010.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Gu C, Shen T, An H, Yuan C, Zhou J, Ye Q,
Liu T, Wang X and Zhang T: Combined therapy of Di-Huang-Yi-Zhi with
Donepezil in patients with Parkinson's disease dementia. Neurosci
Lett. 606:13–17. 2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Liu X, Zhang J, Xia M, Liu J and Jiang S:
Effect of donepezil on Hcy level in serum of Alzheimer's disease
patients and correlation analysis of Hcy and dyssomnia. Exp Ther
Med. 17:1395–1399. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Zhou X, Wen W, Shan X, Qian J, Li H, Jiang
T, Wang W, Cheng W, Wang F, Qi L, et al: MiR-28-3p as a potential
plasma marker in diagnosis of pulmonary embolism. Thromb Res.
138:91–95. 2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Yu J, Xu Q, Zhang X and Zhu M: Circulating
microRNA signatures serve as potential diagnostic biomarkers for
Helicobacter pylori infection. J Cell Biochem.
120:1735–1741. 2018.PubMed/NCBI View Article : Google Scholar
|