Introduction
The pathological basis of pre-eclampsia is generally
considered to be an abnormal placental vasculature that is caused
by endothelial dysfunction (1).
Pre-eclampsia and fetal growth restriction are late pregnancy
complications that are associated with insufficient uterine
vascular density and suboptimal placental perfusion (2). Furthermore, vessel formation is driven
by progenitor cells and leads to vascularization and
vasculogenesis, which is distinguished by the presence or absence
of pre-existent vessels (3,4). Damaged endothelial cells cannot be
repaired by already differentiated endothelial cells, but can be
repaired by endothelial progenitor cells (EPCs) (5).
EPCs are precursors of vascular endothelial cells
and originate from bone marrow, similar to angioblast and umbilical
vein endothelial cells, which together belong to a subgroup of
hematopoietic stem cells (6). There
are two sources of EPCs that can be detected in vitro,
immature and mature EPCs. While early immature EPCs display a
linear growth structure and are spindle-shaped, late mature EPCs
form cobblestone-like, oval shaped structures (7). EPCs take part in vascularization during
embryonic development, and also participate in postnatal
vascularization and reparative processes post-trauma (8). Therefore, EPCs hold extensive prospects
for vascular tissue engineering and potential clinical application
in coronary artery disease and wound healing (9). Some studies have indicated that the
number of EPCs in patients with pre-eclampsia is reduced, and the
function of EPCs is diminished (10,11).
Available evidence demonstrates that neovascularization may be due
to impaired availability or function of EPCs (12,13).
Therefore, EPCs may be a diagnostic tool and a direct target for
medical intervention during pre-eclampsia.
microRNAs (miRNAs or miRs) are conserved non-coding
RNAs that are 19-26 nucleotides in length and that regulate gene
expression by binding to specific sites in the 3'-untranslated
region (UTR) of mRNAs. miRNAs are associated with a variety of
in vivo physiological processes, including angiogenesis and
embryonic development (14,15). miR-646 is a newly discovered miRNA,
and is a common miRNA isolated from vascular endothelial cells
(16). miR-646 has been demonstrated
to serve a key role in many aspects of angiogenesis, including in
vascularization and wound healing (16). Li et al (17) revealed that miR-646 is not only
expressed in normal cells, but also has low expression in renal
cell carcinoma, and serves an important role in renal cell
angiogenesis, which is associated with the NOB1/MAPK pathway
(17). A growing number of studies
have demonstrated that miRNAs are abundantly expressed in
pre-eclampsia during pregnancy, and the dysregulation of miRNAs is
associated with the pathogenesis of pre-eclampsia (18).
Although a few studies have identified a functional
relationship between miR-646, angiogenesis and endothelial cells,
the biological functions and mechanisms of miR-646 and EPCs in
pregnancy-associated vascular complications remain to be
determined. In the current study, the number of EPCs, the
expression of miR-646 and its relationship to blood in patients
with and without pre-eclampsia, was assessed. Additionally, the
effects of miR-646 on proliferation, angiogenesis and migration of
EPCs were investigated. The current study aimed to reveal the
regulatory mechanisms of miR-646 function in EPCs, and to provide
molecular evidence for miR-646 regulation of vascular endothelial
growth factor (VEGF)-A and hypoxia inducible factor-1α (HIF1α)
expression, and reveal the role of this regulation in the
pathogenesis of pre-eclampsia.
Materials and methods
Patients and blood samples
This research was conducted at the department of
Obstetrics and Gynecology, Zhongnan Hospital of Wuhan University
(Wuhan, China) from August 2017 to July 2018. The study group
included 20 women without pre-eclampsia (control group) and 20
women with pre-eclampsia. The inclusion criteria were as follows:
i) Women with single pregnancy; ii) women aged <35 years old and
>18 years old; iii) The diagnostic criteria for pre-eclampsia
were a systolic blood pressure ≥140 mmHg and/or diastolic blood
pressure ≥90 mmHg after 20 weeks of gestation, whereas women with
previous normal blood pressure had proteinuria ≥1+ (300 mg/24 h)
after pregnancy (19). 4) Patients
did not receive any previous or ongoing treatment. Exclusion
criteria: Patients with chromosomal abnormalities, anatomical
variations, hormonal disorders and infectious diseases were
excluded. The formal medical history was obtained for all of the
women. The patients underwent obstetric examination, ultrasound
examination of the abdomen, hematology and urine protein analysis.
Peripheral blood was collected during regular review (every 4
weeks) of the pregnant women. To compare the expression of miR-646
in the placenta of the control group to the patients with
pre-eclampsia, umbilical cord blood was also collected at birth.
Baseline characteristics were recorded for all pregnant women
enrolled (Table I). The ethical
approval of the current study's protocol was gained from the Ethics
Committee of the Zhongnan Hospital of Wuhan University, and
detailed written consent was obtained from all enrolled
subjects.
 | Table IDemographic of all pregnant women
enrolled in the study. |
Table I
Demographic of all pregnant women
enrolled in the study.
|
Characteristics | Control (n
=20) | Preeclampsia (n
=20) | P-value |
|---|
| Age (years) | 29.7±2.3 | 31.7±3.2 | NS |
| Gestational age
(weeks) | 11.5±2.1 | 12.3±2.8 | NS |
| Pregnancy time
(weeks) | 10.6±1.7 | 11.6±1.5 | NS |
| Median maternal
weight (kg) | 53.9±4.3 | 55.2±3.9 | NS |
| BMI
(kg/m2) | 23.4±3.1 | 28.4±3.8 | NS |
| Proteinuria | 20% (4/20) | 100% (20/20) | <0.05 |
| S/D ratio of
umbilical artery | 2.7±0.8 | 3.6±0.7 | <0.05 |
| Birth weight
(g) | 2316±632 | 3034±517 | <0.05 |
| Apgar score | 9.0±0.87 | 8.3±0.76 | NS |
miRNA and target gene expression assay
and reverse transcription-quantitative (RT-q) PCR
A TRIzol kit (Qiagen GmbH) was used to extract total
RNA from peripheral blood and umbilical cord blood of pregnant
women. cDNA was subsequently synthesized from total mRNA (8 µg)
using miScript II RT kit (Qiagen GmbH). The reverse transcription
procedure includes incubation at 37˚C for 1 h, followed by
inactivation by briefly incubating at 95˚C. The expression of
miRNAs was quantified using the miRNA-specific TaqMan miRNA assay
kit (Applied Biosystems; Thermo Fisher Scientific, Inc.). qPCR was
performed using a FastStart Univepreeclampsial SYBR Green Master
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The
expression of miR-646 was normalizes to the relative expression of
U6, and the expression of the target genes VEGF-A and HIF-1α were
normalized to β-actin for quantification. The RT-PCR primers used
were as follows: VEGF-A forward, 5'-GAGGAGCAGTTACGGTCTGTG-3' and
reverse, 5'-TCCTTTCCTTAGCTGACACTTGT-3'; HIF-1α forward,
5'-ATGCGGTCAGCAAGAGCATC-3' and reverse,
5'-AGACGATACTCTCCGACTGGG-3'; Akt forward,
5'-CTACCCACACAGCAGTACGC-3' and reverse, 5'-AAGTCGCTGGTGTTAAGCCG-3';
β-actin forward, 5'-CGGAGTGAGCGATCTTACAGG-3' and reverse,
5'-TCATCAGCGACTCTGACCACA-3'. SYBR Premix Ex TaqTM was then used on
an Applied Biosystem 7300 Real-Time PCR System (Applied Biosystems;
Thermo Fisher Scientific, Inc.), and each reaction was repeated
three times in succession. Immediately after the initial
denaturation step (95˚C; 2 min), the program was set up to consist
of 40 cycles (95˚C, 15 sec; 62˚C, 15 sec; 72˚C, 45 sec). Finally,
the resulting PCR was subjected to melting curve analysis. The
relative gene expression of miR-646 and the target gene were
analyzed using the 2-ΔΔCt method (20).
EPCs cell culture and
characterization
Peripheral blood mononuclear cells (PBMCs) were
isolated from peripheral blood using the methods described by
Schildberger et al (21).
PBMCs were isolated using Ficoll-Paque density gradient
centrifugation (300 x g; 25 min; 15˚C) in a lymphocyte separation
solution (Applied Biosystems; Thermo Fisher Scientific, Inc.).
Cells were then washed 3 times with PBS then resuspended in
endothelium-based medium-II (Applied Biosystems; Thermo Fisher
Scientific, Inc.) containing 5 EGM-2-MV-SingleQuots (Lonza Group,
Ltd.) at a concentration of 5x105 cells/ml. Medium
contained 10% FBS, 40 ng/ml human VEGF (Sigma-Aldrich; Merck; KGaA;
cat. no. 127464-60-2), 40 ng/ml human insulin-like growth factor-1
(IGF-1; Sigma-Aldrich; Merck; KGaA; cat. no. 67763-96-6), 50 ng/ml
human epidermal growth factor (Sigma-Aldrich; Merck; KGaA; cat. no.
62253-63-8), 120 µg/ml penicillin and 120 µg/ml streptomycin. PBMCs
(5x106) were seeded on a fibronectin-coated six-well
culture dish. After 4 days of cell-induced culture at 37˚C,
adherent cells were observed to form small round EPC clusters under
Olympus BX50 (Olympus Corporation; Magnification, x400). After 6-8
days of cell-induced culture, the spindle cells gradually turned
into a cluster of round cells in which a plurality of
spindle-shaped cells were observed to germinate from the central
core and designated as colony forming units (CFU). The method of
identification of EPCs was as follows: The attached cells were then
incubated with DiI-Ac-LDL (2.4 mg/ml) for 4 h at 37˚C, then fixed
with 2% paraformaldehyde at room temperature for 10 min, and
finally incubated with FITC-UEA-1 (10 mg/ml) at room temperature
for 1.5 h. Adherent cells were observed by a microscope
(magnification, x400; Olympus IX81; Olympus Corporation), and the
cells co-stained with DiI-Ac-LDL and FITC-UEA-1 were identified as
differentiated mature EPCs.
Transfection in vitro
The miR-646 mimics and miR-646 inhibitors
oligonucleotides (Applied Biosystems; Thermo Fisher Scientific,
Inc.) were diluted in PBS to a concentration of 30 µM. Cells were
transfected with Superfect (Invitrogen; Thermo Fisher Scientific,
Inc.) using miR-646 mimics (2.5 nM), miR-646 inhibitor (2.5 nM) or
a VEGF-A shRNA plasmid (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The control (siR-RibTM; 2.5 nM) was then
transfected with the Cy3-small interfering (si)RNA kit (Tiangen
Biotech Co., Ltd.) to determine miRNA transfection efficiency.
Efficiency was determined by comparison to the miRNA mimic negative
control (miR-NC; NC) siRNA, which was provided by Guangzhou RiboBio
Co., Ltd. A period of 48 h after transfection, harvested cells were
used for subsequent analysis.
Protein extraction and western blot
analysis
Proteins were extracted from EPCs with different
transfection conditions with RIPA lysis buffer (2 mM Tris HCl; pH
7.5; 15 mM NaCl; 0.1 mM Na2EDTA; 0.1 mM EGTA; 0.1% Triton; 0.25 mM
sodium pyrophosphate; 0.1 mM β-glycerophosphate; 0.1 mM Na3VO4; 0.1
µg/ml leupeptin; Tiangen Biotech Co., Ltd.). Protein concentration
was detected using the BCA method. Proteins (10 µg) were resolved
in 15% SDS-PAGE. They were then transferred onto an Immobilon-P
membrane (EMD Millipore). The membrane was blocked with 5% non-fat
milk in TBST for 1 h at room temperature. The membrane was
subsequently probed with primary antibodies against VEGF-A
(1:2,000; cat. no. ab46160; Abcam) or β-actin (1:5,000; cat. no.
sc-58673; Santa Cruz Biotechnology, Inc.), and incubated overnight
at 4˚C. After extensive washing with PBST, the membrane was
incubated with secondary antibody conjugated with horseradish
peroxidase (1:10,000; Santa Cruz Biotechnology, Inc.) for 1 h at
room temperature. An Odyssey Infrared Imaging System (LI-COR
Biosciences) was used to visualize protein bands. The relative
intensity of protein bands compared with β-actin was quantified
using Image J software version 1.47 (National Institutes of
Health).
Proliferation and colony
formation
MTT assay was used to determine the cell
proliferation rate. Cells were inoculated into 96-well plates
(1x103 cells per well) and MTT reagent was added (25 µl;
5 mg ml) to each well. Supernatant was removed from the medium and
DMSO reagent was added to each well. The absorbance was measured at
520 nm using a microplate reader (595 nm). For colony formation
experiments, cells were resuspended at 1x103 cells/well
and cultured at 37˚C for 5-6 days until macroscopic colonies
appeared. Finally, the colonies were stained with crystal violet at
room temperature for 30 min.
Angiogenesis experiment
EPCs formed capillary structures as previously
described. Pre-registration 96-well plates were coated with
Matrigel at 37˚C 30 min prior to the experiment. EPCs
(1x103 cells per well) were then cultured at 37˚C in 250
ml of Ham's F12K medium (Applied Biosystems; Thermo Fisher
Scientific, Inc.). A total of 1x106 tumor cells were
then transfected with miR-646 mimics or inhibitors, and cultured
for 48 h under the same conditions. An image capturing
capillary-like structure formation was obtained at 12 h using an
inverted microscope (Olympus Corporation; magnification, x50).
Finally, the tubular structure was evaluated.
EPCs cell migration assay
Transwell assay was used to examine EPC migration.
Transfected cells were harvested at 24 h after transfection and
resuspended in cell culture medium (Eagle's Minimum Essential
Medium; Thermo Fisher Scientific, Inc.) supplemented with 5% FBS. A
total of 7x103 cells were added in the upper-chamber of
the transwells, and the lower-chambers were filled with 600 µl of
cell culture medium (Eagle's Minimum Essential Medium; Thermo
Fisher Scientific, Inc.). Cells were then stored in a 37˚C, 5%
CO2 atmosphere for 48 h. After 48 h, the unmigrated
cells were gently removed from the upper side of the transwell
membrane using a cotton swab. The migrated cells attached to the
lower side of the membrane were then fixed in methanol and stained
with crystal violet at room temperature for 30 min. Finally, the
average number of cells was counted manually. Images of the samples
were then captured using an inverted microscope (magnification,
x400; Olympus IX81; Olympus Corporation).
Luciferase reporter assay
To ascertain whether miR-646 inhibited VEGF-A
expression, TargetScan was used, which is an online publicly
available algorithm, to analyze miR-646 sequence 3'-UTR
(NM_001025369) of VEGF-A. Sequence-combined index comprehensive
ranking was performed to confirm that the combined prediction score
for the miR-646 binding site in the 3'UTR was the highest scoring
site. Once it was established that miR-646 could target VEGF-A,
subsequent experiments were performed. EPCs were transfected with
either wild type or mutant VEGF-A constructs without the miR-646
binding site. This part of the experiment allowed EPC cells to grow
to 70-80% confluence in 24-well plates and
LipofectamineTM 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used with a luciferase reporter vector (200
ng) (Promega Corporation), and appropriate miRNA (50 nM, mimics and
inhibitors) were co-transfected. Following the experimental
protocol, after 72 h, the cells were washed and lysed with passive
lysis buffer (Sigma-Aldrich; Merck KGaA). The relative activity of
luciferase was then assessed using a dual luciferase vector assay
(Thermo Fisher Scientific, Inc.). Finally, relative reporter gene
activity was determined by normalizing to Renilla luciferase
activity.
Statistical analysis
All experiments were performed in triplicate, and
the results are presented as the mean ± standard deviation of 3
independent experiments. Statistical comparisons were performed
using a one-way ANOVA. Statistical analyses were performed using R
software (R version 3.3.2), GraphPad Prism Software (7.0; GraphPad
Software, Inc.), and the SPSS 17.0 statistical software package
(IBM Corp). P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinical data of enrolled
patients
The clinical data of the patients enrolled in the
current study are presented in Table
I. Patients with pre-eclampsia and control subjects were
matched by their age, body mass index (BMI) and gestational age,
respectively. The umbilical cord blood vessels and placenta
indicators of the two groups of pregnant women were observed using
ultrasound examination, and some data had significant gaps. In
patients with clinical pre-eclampsia, lower placental microvessel
density (MVD) indicated abnormal vasculogenesis in the placenta
(Table I). The data indicated that
the fetuses of patients with pre-eclampsia have an elevated risk of
preterm birth (Table I).
Increased expression of miR-646 in
patients with preeclampsia
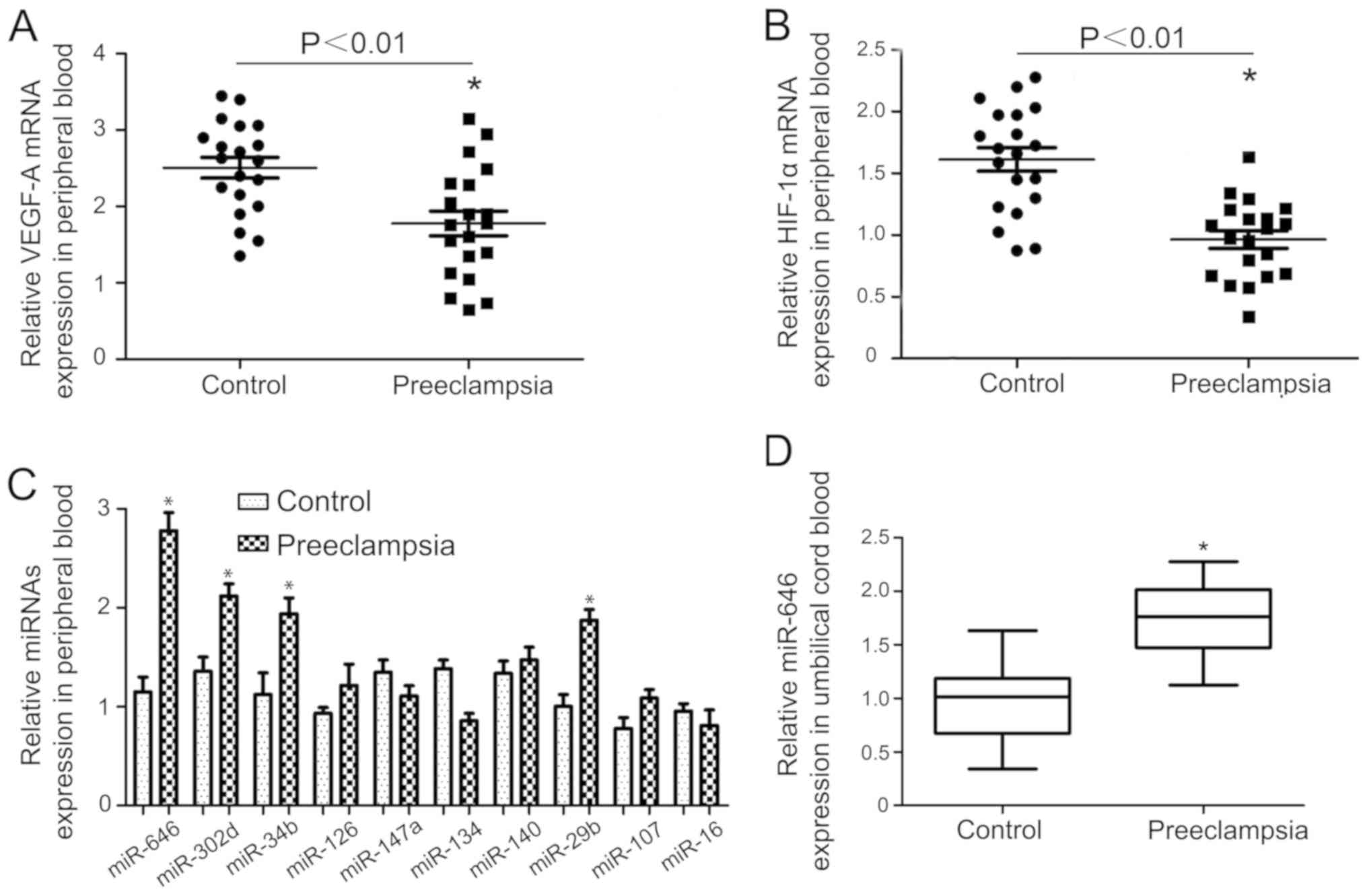
A previous clinical study demonstrated that VEGF-A
and HIF-1α expression were lower in the peripheral blood of
patients with preeclampsia compared with the controls (Fig. 1A and B). To investigate whether specific miRNAs
regulate VEGF-A or HIF-1α expression in preeclampsia, the total of
20 potential miRNAs that targeted the VEGF-A gene were determined.
The expressions of these 20 miRNAs were determined using RT-qPCR.
The expression of the first 10 of these miRNAs is presented in
Fig. 1C. The expression of miR-646
in the preeclampsia group was significantly higher compared with
control pregnant women (Fig. 1C).
The higher expression of miR-646 was confirmed in umbilical cord
blood of preeclampsia cases, suggesting that the upregulation of
miR-646 may be associated with preeclampsia (Fig. 1D). Therefore, the data indicated that
miR-646 was upregulated in patients with pre-eclampsia.
The number of EPCs was significantly
lower in patients with preeclampsia, accompanied by morphological
differences in EPCs
Microscopic images of EPCs from patients with
preeclampsia and control groups are presented in Fig. 2. Compared with the control group, the
number of EPCs in patients with pre-eclampsia decreased
significantly, and the CFUs in patients with pre-eclampsia were
~1.5 times larger. Fig. 2A and
B demonstrated that pre-eclampsia
significantly reduced EPC and CFU counts. EPC number: 132±47 vs.
186±51; P<0.05; CFU count: 3.3±3.7 vs. 11.43±10.6, P<0.01
(data not shown). As presented in Fig.
2C, the number of EPCs was negatively correlated with miR-646
levels in the pre-eclampsia group (R2=0.26; P =0.0028),
suggesting that miR-646 levels may be important for the regulation
of the amount of EPCs in pre-eclampsia. Protein levels of VEGF-A,
HIF-1α and p-Akt (Akt phosphorylation level), which are three
important regulators of angiogenesis, also decreased in
pre-eclampsia, relative to β-actin (Fig.
2D). The ratio of phosphorylated AKT/total AKT ratio was
significantly decreased in the pre-eclampsia group. Specifically,
this supports the hypothesis that miR-646 is involved in the
pathogenesis and regulation of pre-eclampsia by regulating
EPCs.
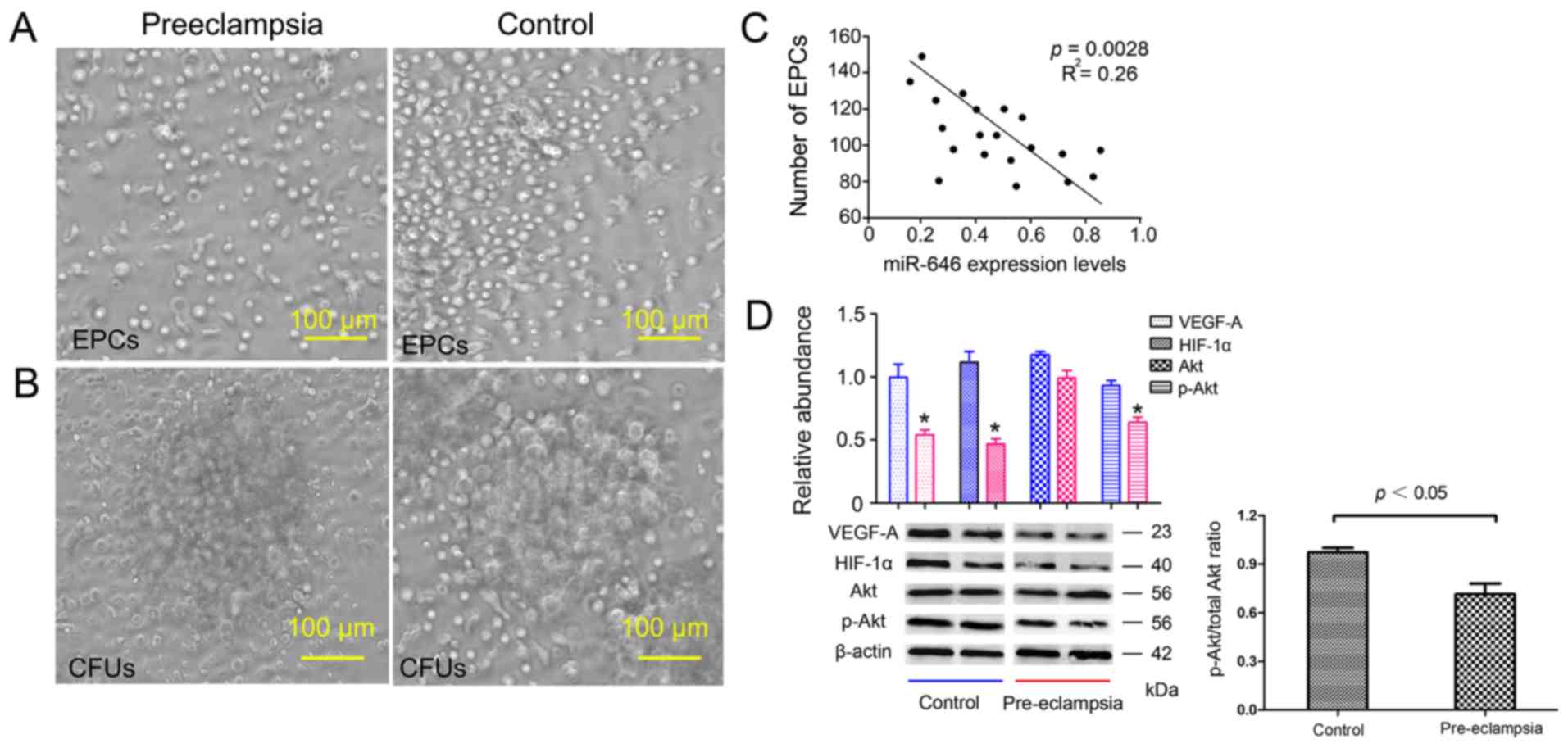 | Figure 2Morphology of EPCs and CFUs
representative of one patient with preeclampsia and one with a
normal pregnancy following 7 days of cell culture. (A) The number
of EPCs decreased in the patient with preeclampsia compared with
the control (magnification, x200). (B) The numbers of CFUs were
lower in the patient with preeclampsia, and the diameter was
significantly reduced. Only typical CFUs with spindle cells
gradually turning into a cluster of round cells and germinating
from the central core were counted using light microscopy
(magnification, x200). (C) Pearson's correlation analysis indicated
a correlation between EPC number and miR-646 expression in patients
with preeclampsia; significant negative correlation,
R2=0.26, P=0.0028. (D) Protein levels of VEGF-A, HIF-1α,
Akt and p-Akt in patients with preeclampsia and healthy controls,
VEGF-A, HIF-1α and p-Akt level in the preeclampsia group decreased,
and the difference was statistically significant. Blue and red
represent control and pre-eclampsia group respectively.
*P<0.05 vs. control group. EPC, endothelial
progenitor cells; CFU, colony forming unit; miR, microRNA; VEGF,
vascular endothelial growth factor; HIF, hypoxia inducible factor;
p, phosphorylated. |
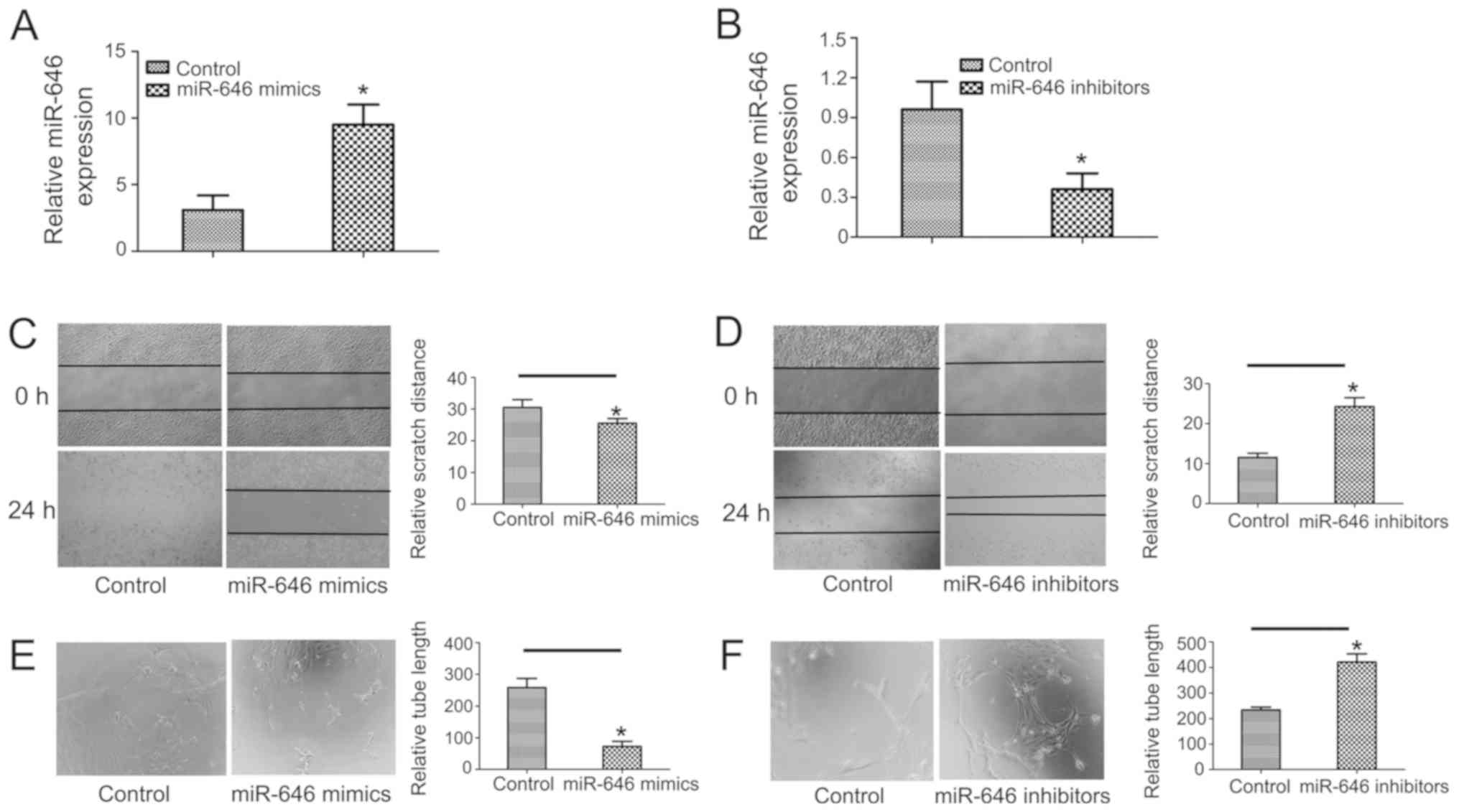
miR-646 inhibition and overexpression
in primary cultured EPC cells
To investigate the mechanism of action by which
miR-646 regulates EPCs, miR-646 overexpression or suppression was
performed in normal non-preeclampsia EPCs. A period of 48 h after
transfection, the expression efficiency was determined using a
Cy3-siRNA transfection control. The cells exhibited intense and
extensive cytosolic delivery by Cy3-siRNA (Fig. 3A and B). The expression of miR-646 was confirmed
in different transfection groups using RT-qPCR. The results
confirmed that miR-646 was expressed in these groups, and the
increase in miR-646 mimic transfected cells was higher (1.3±0.9 vs.
7.2±1.4; P<0.01; Fig. 3A), while
miR-646 expression was suppressed by miR-646 inhibitor (0.9±0.37
vs. 0.46±0.32; P<0.01; Fig.
3B).
The function of miR-646 on EPCs
proliferation, migration, colony formation and tube formation
Based on the correlation between miR-646 and VEGF-A
expression, and the important role of VEGF-A in angiogenesis,
nutritional maintenance and homeostasis of the placenta, the
antiangiogenic effect of miR-646 was assessed by transfecting EPCs
with a miR-646 mimic or inhibitor in vitro. Important
processes for angiogenesis include cell proliferation and
migration. Therefore, the effect of miR-646 on VEGF-A induced EPC
proliferation and migration was assessed. The data demonstrated
that the overexpression of miR-646 in EPCs significantly inhibited
their proliferation and migration. The inhibition of miR-646
expression significantly promoted the proliferation and migration
of EPCs (Fig. 3B and C). By calculating the length of the formed
tubules using inverted phase contrast microscopy, the ability of
EPCs to form tubular structures was revealed. Additionally, miR-646
overexpression inhibited EPC tube formation, while miR-646
inhibitor transfection increased tube formation, clonal formation
experiments indicated a similar role for miR-646 (Fig. 3D-F).
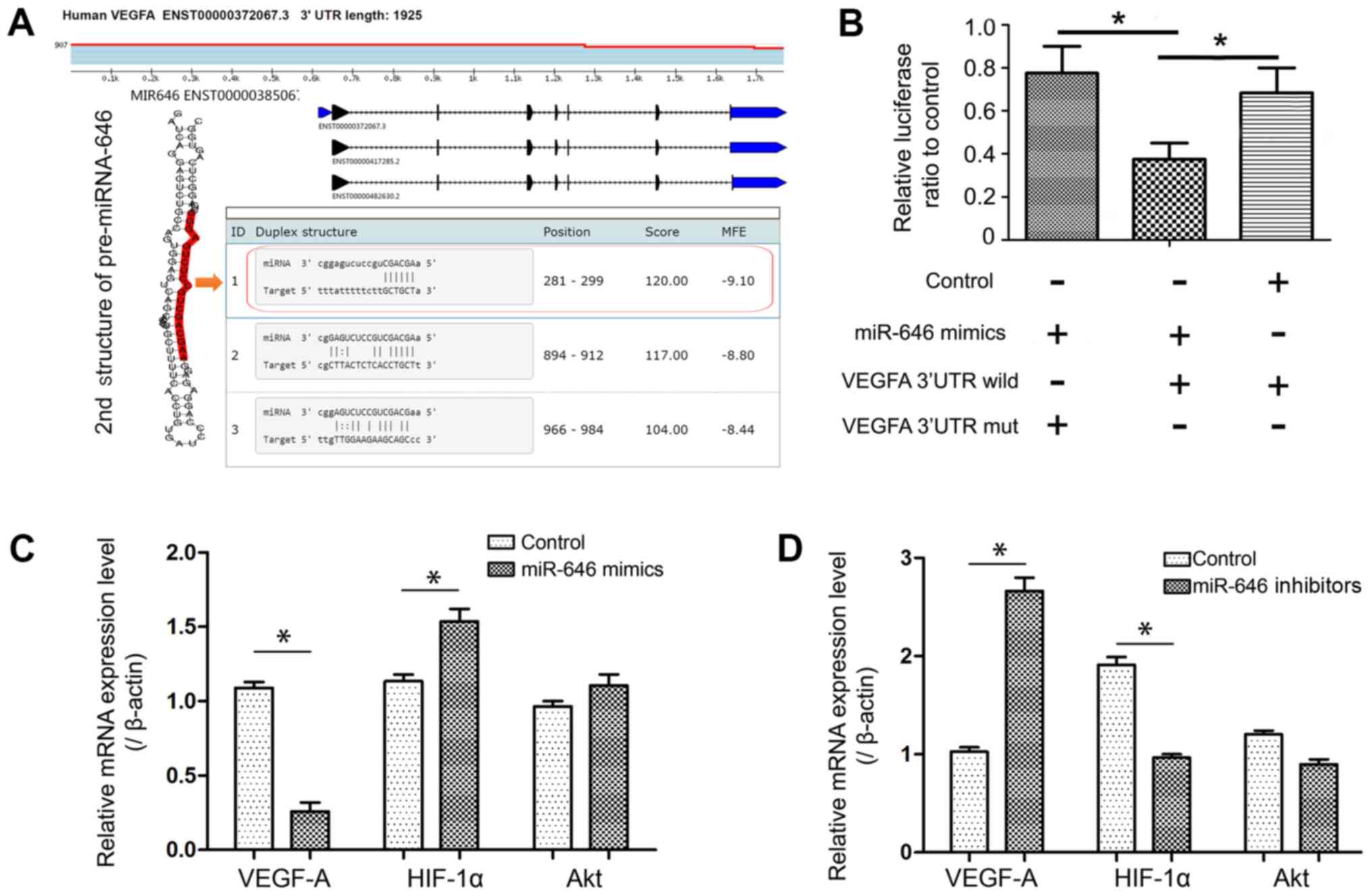
miR-646 directly targeted VEGF-A
3'-UTR and downregulated its expression, regulating the
VEGF-HIFα-Akt signaling axis
VEGF has been identified as a major cellular
molecule that regulates angiogenesis (22). Using online microRNA target databases
(miRBase, http://www.mirbase.org; miRWalk,
http://www.ma.uni-heidelberg.de/apps/zmf/mirwalk/; and
TargetScan, http://www.targetscan.org/), it was demonstrated that
VEGF-A is a potential target of miR-646 (Table II). To ascertain whether miR-646
inhibited VEGF-A expression, the online publicly available
algorithm TargetScan was used to analyze miR-646 target sequences
in the 3'-UTR of VEGF-A. With reference to sequence information,
the wild type or mutant sequence was cloned, which contained the
full-length genomic transcript of VEGF and its miR-646 binding
target site, visually indicating the secondary structure of miR-646
(Fig. 4A). These fragments
containing the binding sequence were used for luciferase reporter
assays (Fig. 4B). Additionally,
RT-qPCR was used to measure VEGF-A expression in these different
groups with and without the miR-646 binding site. The results
demonstrated that the lack of a binding site in the 3'UTR
attenuated the miR-646 mediated degradation of VEGF-A mRNA
(Fig. 4B). HIF-1α and AKT are two
important factors in VEGF/AKT signaling, which regulates multiple
key forms of angiogenesis and vascular homeostasis (23). The results indicated that HIF-1α mRNA
was increased after transfection with miR-646 mimics (Fig. 4C). Although the mRNA level of AKT did
not change significantly, it was indicated that the p-AKT protein
level was significantly decreased in combination with the previous
western blotting results, demonstrating that the phosphorylation
level of AKT changed under the action of miR-646. It was
hypothesized that this phenomenon may be due to exogenously
increasing the expression of miR-646, which can directly target the
expression of VEGF-A, resulting in decreased VEGF-A expression. The
consequence of decreased VEGF-A expression is inhibition of
placental angiogenesis. The hypoxic-ischemic internal environment
eventually leads to an increase in the expression of
hypoxia-inducible factor-HIF-1α. Therefore, in the miR-646
inhibitor group, HIF-1α and AKT expression levels were
significantly lower compared with the control group (P<0.05;
Fig. 4D).
 | Table IIBioinformatics prediction
differential expression miRNAs (top 20). |
Table II
Bioinformatics prediction
differential expression miRNAs (top 20).
| ID | Species
(miRNA) | Species
(Target) | miRNA | Target | Sum |
|---|
| MIRT061535 | Homo sapiens | Homo sapiens | hsa-miR-646 | VEGFA | 2 |
| MIRT000722 | Homo sapiens | Homo sapiens |
hsa-miR-302d-3p | VEGFA | 2 |
| MIRT002466 | Homo sapiens | Homo sapiens | hsa-miR-34b-5p | VEGFA | 3 |
| MIRT003428 | Homo sapiens | Homo sapiens | hsa-miR-126-3p | VEGFA | 4 |
| MIRT003810 | Homo sapiens | Homo sapiens | hsa-miR-147a | VEGFA | 2 |
| MIRT003811 | Homo sapiens | Homo sapiens | hsa-miR-134-5p | VEGFA | 5 |
| MIRT003812 | Homo sapiens | Homo sapiens | hsa-miR-140-5p | VEGFA | 2 |
| MIRT003813 | Homo sapiens | Homo sapiens | hsa-miR-29b-3p | VEGFA | 3 |
| MIRT003814 | Homo sapiens | Homo sapiens | hsa-miR-107 | VEGFA | 2 |
| MIRT003890 | Homo sapiens | Homo sapiens | hsa-miR-16-5p | VEGFA | 4 |
| MIRT004055 | Homo sapiens | Homo sapiens | hsa-miR-93-5p | VEGFA | 2 |
| MIRT004271 | Homo sapiens | Homo sapiens | hsa-miR-17-5p | VEGFA | 3 |
| MIRT004272 | Homo sapiens | Homo sapiens | hsa-miR-150-5p | VEGFA | 2 |
| MIRT004273 | Homo sapiens | Homo sapiens | hsa-miR-195-5p | VEGFA | 2 |
| MIRT004274 | Homo sapiens | Homo sapiens | hsa-miR-15b-5p | VEGFA | 4 |
| MIRT004275 | Homo sapiens | Homo sapiens | hsa-miR-15a-5p | VEGFA | 5 |
| MIRT004276 | Homo sapiens | Homo sapiens |
hsa-miR-520g-3p | VEGFA | 6 |
| MIRT004277 | Homo sapiens | Homo sapiens |
hsa-miR-378a-3p | VEGFA | 2 |
| MIRT004278 | Homo sapiens | Homo sapiens | hsa-miR-330-3p | VEGFA | 3 |
| MIRT004443 | Homo sapiens | Homo sapiens | hsa-miR-383-5p | VEGFA | 3 |
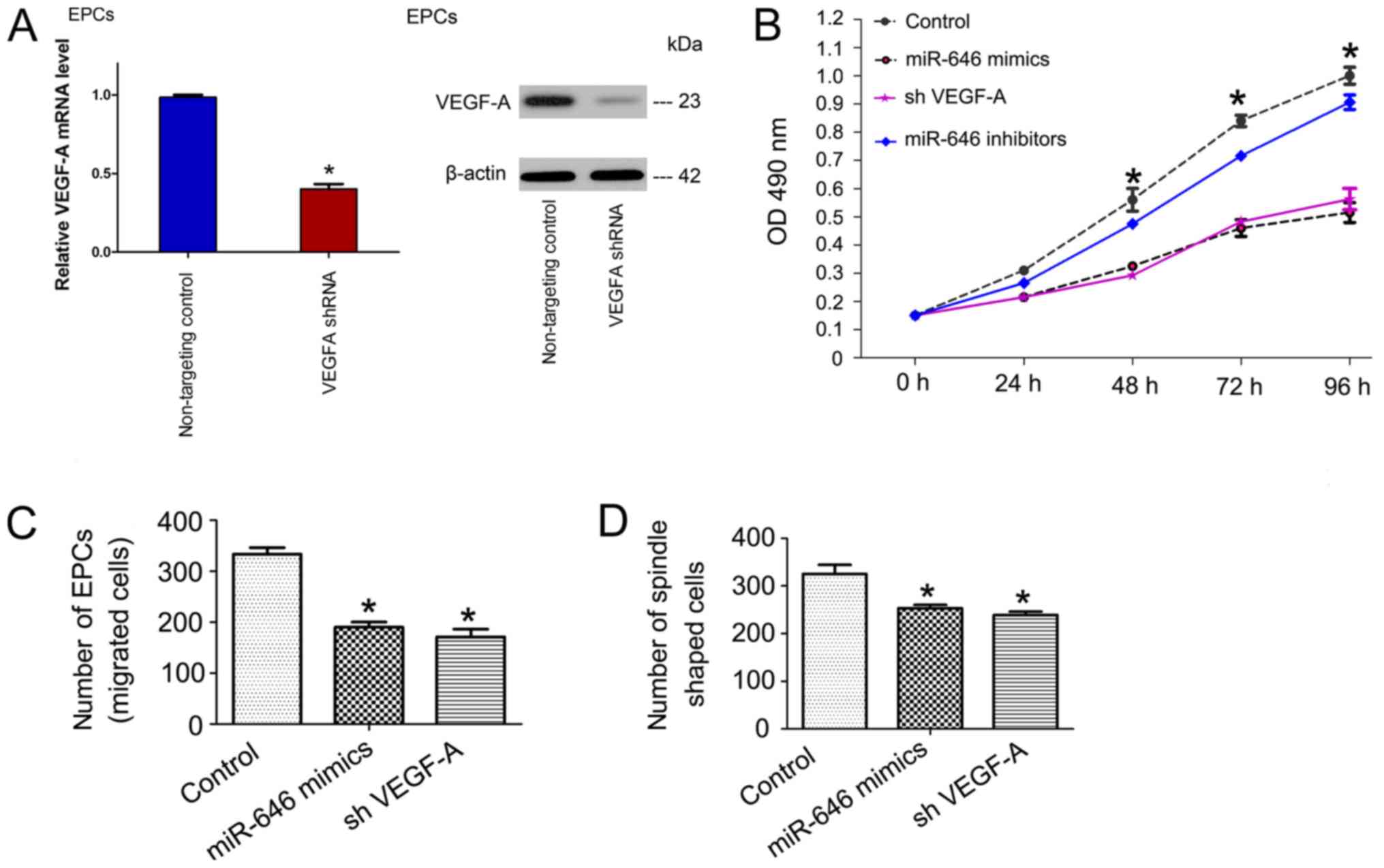
To clarify whether the inhibitory effect of miR-646
on EPC proliferation is regulated by the negative regulation of
VEGF-A, a study of gene function loss was performed. Specifically,
whether the knockdown of VEGF-A mimics the effect of miR-646 via
silencing VEGF-A was assessed by performing an MTT analysis,
counting the number of EPCs and their spindle-like morphology. EPCs
were transfected with shVEGF-A and EPCs proliferation,
differentiation and migration capabilities were detected. As
indicated in Fig. 5, silencing of
VEGF-A significantly inhibited cell growth, differentiation and
migration, similar to that induced by the miR-646 mimetic. The data
demonstrated that miR-646 directly targeted the angiogenic gene
VEGF-A, and is associated with the regulation of the VEGF-Akt
signaling pathway.
Discussion
To the best of our knowledge, the current study
revealed, for the first time, that miR-646 is expressed in EPCs in
patients with pre-eclampsia and an important mechanism of miR-646
is associated with regulating the angiogenesis characteristics of
early EPCs. The present study revealed that the expression of
miR-646 in the peripheral or cord blood of the patients with
pre-eclampsia is higher compared with control patients, the change
in the number of EPCs and the level of miR-646 in the pre-eclampsia
group demonstrated a negative correlation and miR-646 inhibited
proliferation, differentiation and migration of EPCs by directly
targeting the angiogenesis-related gene VEGF-A. The mechanism of
action of miR-646 on EPCs is via the VEGF-Akt signaling
pathway.
miRNAs have been considered to serve important
regulatory roles in a variety of gynaecological and obstetric
diseases, including leiomyoma, endometriosis and preterm birth
(24). Additionally, studies have
demonstrated the differential expression of miRNAs in peripheral or
cord blood of patients with pre-eclampsia. This significantly
different expression miRNAs can affect pregnancy via regulating
vascular events, including angiogenesis (25). Additionally, a number of miRNAs can
cause premature birth via regulating genes involved in angiogenesis
(26). One study revealed that
miR-646 inhibits angiogenesis, trophoblast proliferation and
migration (17). The current study
indicated that miR-646 may serve a key role in placental
angiogenesis. However, these novel findings require further
investigation.
In the current study, patients with pre-eclampsia
and control patients were indicated to exhibit significant
differences in the expression of miR-646 in peripheral blood, which
is a point not previously assessed. The standardization of data
indicated that miR-646 in the cord blood of patients with
pre-eclampsia was significantly higher. In vitro data
indicated that miR-646 overexpression in EPC inhibited cell
proliferation, migration and angiogenesis, suggesting that miR-646
may be a novel inhibitor of angiogenesis. Consistent with these
findings, a recent study demonstrated that miR-646 inhibited the
proliferation, migration and angiogenesis potential of mesenchymal
stem cells (27). The further
clinical value of the present study is that miRNAs and their
targets may be used as biomarkers for pre-eclampsia in early
pregnancy. These biomarkers may aid in the prediction of
pre-eclampsia to avoid adverse outcomes in pregnant women and
perinatal children. The current study also indicated that in
patients with pre-eclampsia, the serum levels of miR-646 and VEGF-A
continued to be abnormal and may be associated with changes in
uterine artery Doppler energy. The genetic basis of pre-eclampsia,
and the determination of biomarkers with a predictive value for
this condition requires study in the future. Further research will
allow the identification of the best combination of biomarkers for
clinical use. In the current study, miR-646 was identified as a
biomarker that serves as an indicator of preeclampsia and may be a
good target for drug intervention. It was demonstrated that the
protein levels of VEGF-A and HIF were decreased in the
pre-eclampsia group, and the phosphorylated AKT (p-AKT) level was
also significantly decreased (Fig.
2). Furthermore, the level of VEGF-A mRNA was decreased
following the overexpression of miR-646, but the HIF-1α mRNA
expression was increased (Fig. 4).
It was hypothesize that this phenomenon may be due to exogenously
increasing the expression of miR-646, which can directly target the
expression of VEGF-A, resulting in decreased VEGF-A expression. The
consequence of decreased VEGF-A expression is the inhibition of
placental angiogenesis. A hypoxic-ischemic internal environment
eventually leads to an increase in the expression of
hypoxia-inducible factor HIF-1α. Although the mRNA level of Akt did
not change significantly in the transfection group, it was
indicated that the p-Akt level was significantly decreased in
combination with previous western blotting analysis (Fig. 2), indicating that the phosphorylation
level of AKT (p-AKT) changed under the action of miR-646,
suggesting that miR-646 may serve a role in the regulation of
VEGF/AKT pathway in patients with pre-eclampsia.
VEGF-A is an important regulator for new vessel
development and establishment, and serves an important role in an
angiogenic switch initiating new vessel formation against ischemia
(28). Through bioinformatics
analysis, it was revealed that miRNAs may regulate VEGF-A, and the
comprehensive score of miR-646 was identified to be the highest
scoring binding site (Table II).
Endothelial cells are able to adapt to the pathological environment
and produce pro-angiogenic and hypoxic regulators, including
VEGF-A, IGF-1 and HIF-1α (29-31).
A HIF-1α dependent pathway serves an important role in new vessel
development against hypoxic environment, including tissue hypoxia
(23). HIF-1 is a heterodimeric
transcription factor complex that binds to DNA. It is composed of
two basic helix-loop-helix domains. The α (1α or 2α) subunits are
regulated by oxygen and are the hypoxia inducible domains. In
contrast, 1β domain is a non-oxygen responsive subunit and is
expressed constitutively (32-34).
Previous studies have demonstrated that VEGF-A was first discovered
in decidual cells in early pregnancy, and is capable of regulating
angiogenesis in embryonic development and physiology of placenta in
early pregnancy (35). The
dysregulation of VEGF-A expression is important in the development
of placental lesions, including pre-eclampsia, preterm birth and
intrauterine growth restriction, and is closely associated with
multiple steps in the development and progression of pre-eclampsia
(36-38).
Previous studies have demonstrated that preterm birth may be
associated with low levels of VEGF-A expression, and changes in
VEGF-A expression may help uncover the specific cause of
pre-eclampsia (39,40).
In the current study, it was demonstrated that the
miR-646-mediated reduction of VEGF-A is likely to be a key event in
placental angiogenesis. Endovascular disorders can be attributed in
part to the overexpression of miR-646 in peripheral or cord blood
of patients with pre-eclampsia. Here, it is demonstrated that
miR-646 is an endogenous inhibitor of VEGF-A thereby modulating
EPC-mediated angiogenesis in vitro. mir-646 may be a
therapeutic target for cytokine regulation that interferes with
placental vascular growth in pre-eclampsia.
Acknowledgements
Not applicable.
Funding
The present study was supported by Hubei Provincial
Natural Science Foundation of China (grant no. 2018CFB765).
Availability of data and materials
The data and materials are available from the
corresponding author upon reasonable request.
Authors' contributions
DD conceived the idea, designed the study, performed
all the experiments, analyzed the data and wrote the manuscript.
YKh and YKo performed RT-qPCR, MTT and transwell assay. YZ carried
out the angiogenesis assay and the luciferase reporter assay and
helped in revising the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The ethical approval of the current study's protocol
was gained from the Ethics Committee of the Zhongnan Hospital of
Wuhan University, and detailed written consent was obtained from
all enrolled subjects.
Patients consent for publication
All patients provided written consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Al-Jameil N, Aziz Khan F, Fareed Khan M
and Tabassum H: A brief overview of preeclampsia. J Clin Med Res.
6:1–7. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kalafat E and Thilaganathan B:
Cardiovascular origins of preeclampsia. Curr Opin Obstet Gynecol.
29:383–389. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Anders HJ, Romagnani P and Mantovani A:
Pathomechanisms: Homeostatic chemokines in health, tissue
regeneration, and progressive diseases. Trends Mol Med. 20:154–165.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sipos PI, Rens W, Schlecht H, Fan X,
Wareing M, Hayward C, Hubel CA, Bourque S, Baker PN, Davidge ST, et
al: Uterine vasculature remodeling in human pregnancy involves
functional macrochimerism by endothelial colony forming cells of
fetal origin. Stem Cells. 31:1363–1370. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yoder MC: Endothelial progenitor cell: A
blood cell by many other names may serve similar functions. J Mol
Med (Berl). 91:285–295. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Silvestre JS, Smadja DM and Lévy BI:
Postischemic revascularization: From cellular and molecular
mechanisms to clinical applications. Physiol Rev. 93:1743–1802.
2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cheng CC, Chang SJ, Chueh YN, Huang TS,
Huang PH, Cheng SM, Tsai TN, Chen JW and Wang HW: Distinct
angiogenesis roles and surface markers of early and late
endothelial progenitor cells revealed by functional group analyses.
BMC Genomics. 14(182)2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Urbich C and Dimmeler S: Endothelial
progenitor cells: Characterization and role in vascular biology.
Circ Res. 95:343–353. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kikuchi K and Poss KD: Cardiac
regenerative capacity and mechanisms. Annu Rev Cell Dev Biol.
28:719–741. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gammill HS, Lin C and Hubel CA:
Endothelial progenitor cells and preeclampsia. Front Biosci.
12:2383–2394. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Attar A, Monabati A and Parsanezhad ME:
Endothelial progenitor cell subsets and preeclampsia: Findings and
controversies. J Chin Med Assoc. 80:615–622. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Crocker IP and Sipos PI: Review:
Endothelial progenitor cells in pregnancy and obstetric
pathologies. Placenta. 34 (Suppl):S62–S67. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Simmons DG: Postimplantation development
of the chorioallantoic placenta. In: The Guide to Investigation of
Mouse Pregnancy. Croy A, DeMayo FJ, Yamada AT and Adamson SL (eds).
Academic Press, Massachusetts, pp143-161, 2014.
|
|
14
|
Min W, Wang B, Li J, Han J, Zhao Y, Su W,
Dai Z, Wang X and Ma Q: The expression and significance of five
types of miRNAs in breast cancer. Med Sci Monit Basic Res.
20(97)2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ha M and Kim VN: Regulation of microRNA
biogenesis. Nat Rev Mol Cell Biol. 15:509–524. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Finnerty JR, Wang WX, Hébert SS, Wilfred
BR, Mao G and Nelson PT: The miR-15/107 group of microRNA genes:
Evolutionary biology, cellular functions, and roles in human
diseases. J Mol Biol. 402:491–509. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li W, Liu M, Feng Y, Xu YF, Huang YF, Che
JP, Wang GC, Yao XD and Zheng JH: Downregulated miR-646 in clear
cell renal carcinoma correlated with tumour metastasis by targeting
the nin one binding protein (NOB1). Br J Cancer. 111:1188–1200.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Choudhury M and Friedman JE: Epigenetics
and microRNAs in preeclampsia. Clin Exp Hypertens. 34:334–341.
2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chaiworapongsa T, Chaemsaithong P, Yeo L
and Romero R: Pre-eclampsia part 1: Current understanding of its
pathophysiology. Nat Rev Nephrol. 10:466–480. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Schildberger A, Rossmanith E, Eichhorn T,
Strassl K and Weber V: Monocytes, peripheral blood mononuclear
cells, and THP-1 cells exhibit different cytokine expression
patterns following stimulation with lipopolysaccharide. Mediators
Inflamm. 2013(697972)2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Raines AL, Berger MB, Patel N, Hyzy SL,
Boyan BD and Schwartz Z: VEGF-A regulates angiogenesis during
osseointegration of Ti implants via paracrine/autocrine regulation
of osteoblast response to hierarchical microstructure of the
surface. J Biomed Mater Res A. 107:423–433. 2019.
|
|
23
|
Ma Y, Xiu Z, Zhou Z, Huang B, Liu J, Wu X,
Li S and Tang X: Cytochalasin H inhibits angiogenesis via the
suppression of HIF-1α protein accumulation and VEGF expression
through PI3K/AKT/P70S6K and ERK1/2 signaling pathways in non-small
cell lung cancer cells. J Cancer. 10:1997–2005. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kobayashi H, Imanaka S, Nakamura H and
Tsuji A: Understanding the role of epigenomic, genomic and genetic
alterations in the development of endometriosis (review). Mol Med
Rep. 9:1483–1505. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li H, Ge Q, Guo L and Lu Z: Maternal
plasma miRNAs expression in preeclamptic pregnancies. Biomed Res
Int. 2013(970265)2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhu Y, Lu H, Huo Z, Ma Z, Dang J, Dang W,
Pan L, Chen J and Zhong H: MicroRNA-16 inhibits feto-maternal
angiogenesis and causes recurrent spontaneous abortion by targeting
vascular endothelial growth factor. Sci Rep.
6(35536)2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang P, Tang WM, Zhang H, Li YQ, Peng Y,
Wang J, Liu GN, Huang XT, Zhao JJ, Li G, et al: MiR-646 inhibited
cell proliferation and EMT-induced metastasis by targeting FOXK1 in
gastric cancer. Br J Cancer. 117:525–534. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cao Z (ed): VEGF-mediated Vascular
Functions in Health and Disease. Linköping University Electronic
Press, LiU-Tryck, Linköping, 2015.
|
|
29
|
Miranda E, Nordgren IK, Male AL, Lawrence
CE, Hoakwie F, Cuda F, Court W, Fox KR, Townsend PA, Packham GK, et
al: A cyclic peptide inhibitor of HIF-1 heterodimerization that
inhibits hypoxia signaling in cancer cells. J Am Chem Soc.
135:10418–10425. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang Z, Dabrosin C, Yin X, Fuster MM,
Arreola A, Rathmell WK, Generali D, Nagaraju GP, El-Rayes B,
Ribatti D, et al: Broad targeting of angiogenesis for cancer
prevention and therapy. Semin Cancer Biol. 35 (Suppl):S224–S243.
2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Nassiri SM and Rahbarghazi R: Interactions
of mesenchymal stem cells with endothelial cells. Stem Cells Dev.
23:319–332. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Masoud GN and Li W: HIF-1α pathway: Role,
regulation and intervention for cancer therapy. Acta Pharm Sin B.
5:378–389. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Semenza GL: HIF-1 mediates metabolic
responses to intratumoral hypoxia and oncogenic mutations. J Clin
Invest. 123:3664–3671. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Gilkes DM, Bajpai S, Chaturvedi P, Wirtz D
and Semenza GL: Hypoxia-inducible factor 1 (HIF-1) promotes
extracellular matrix remodeling under hypoxic conditions by
inducing P4HA1, P4HA2, and PLOD2 expression in fibroblasts. J Biol
Chem. 288:10819–10829. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Fisher SJ: Why is placentation abnormal in
preeclampsia? Am J Obstet Gynecol. 213 (4 Suppl):S115–S122.
2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Bidarimath M, Khalaj K, Wessels JM and
Tayade C: MicroRNAs, immune cells and pregnancy. Cell Mol Immunol.
11:538–547. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Tannetta D and Sargent I: Placental
disease and the maternal syndrome of preeclampsia: Missing links?
Curr Hypertens Rep. 15:590–599. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Fan X, Rai A, Kambham N, Sung JF, Singh N,
Petitt M, Dhal S, Agrawal R, Sutton RE, Druzin ML, et al:
Endometrial VEGF induces placental sFLT1 and leads to pregnancy
complications. J Clin Invest. 124:4941–4952. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Whitehead CL, Walker SP and Tong S:
Measuring circulating placental RNAs to non-invasively assess the
placental transcriptome and to predict pregnancy complications.
Prenat Diagn. 36:997–1008. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Laskowska M, Laskowska K and Oleszczuk J:
Elevated maternal serum sP-selectin levels in preeclamptic
pregnancies with and without intrauterine fetal growth restriction,
but not in normotensive pregnancies complicated by isolated IUGR.
Med Sci Monit. 19:118–124. 2013.PubMed/NCBI View Article : Google Scholar
|