Introduction
Ulcerative colitis (UC) is one of the typical
complex inflammatory bowel diseases (1). Major clinical manifestations of UC
include abdominal pain, diarrhea, vomiting and weight loss, with
the hallmark clinical symptom of UC being bloody diarrhea (2). UC is influenced by genetic,
environmental, immunoregulatory and microbial factors (3). Unlike Crohn's disease (CD), UC is a
mucosal disease that always affects the rectum and could spread up
to the cecum with a continuous retrograde distribution (4). UC is characterized by chronic relapsing
intestinal inflammation that eventually leads to extensive tissue
fibrosis and a stiff colon that is unable to perform peristalsis or
resorb fluids (5,6). In early fibrotic UC cases, fibrosis
affects the muscularis mucosae and submucosa, while the muscularis
propria is not affected. In advanced fibrotic UC cases, fibrosis
extends to affect the muscle layers and the myenteric plexus
(7).
The establishment of an animal model is required for
efficient study of etiology, diagnosis, treatment and novel drug
discovery in UC. Previous studies have induced UC in animals by a
variety of methods including acetic acid, carrageenan, dextran
sodium sulfate (DSS) and dinitrochlorobenzene (8,9). In
particular, the DSS-induced murine model has been widely used in
UC-related experimental investigations (10,11). In
these previous studies, the pathological alterations were
characterized as epithelial erosion and ulceration, submucosal
edema and infiltration of neutrophils into the lamina propria and
submucosa, which is similar to what occurs in human UC (12). While the histological and
ultrastructural features in the DSS-induced UC model remain to be
elucidated, improving the understanding of pathological
characteristics of the model is likely to offer further insight
into UC research (13). Histological
and ultrastructural investigations were performed in a DSS-induced
colitis murine model in the present study. Changes in the
microstructure of the colon tissue in response to early stages of
experimental colitis were also assessed.
Materials and methods
Mice
A total of 30 specific pathogen-free female
C57BL/10J wild type mice (age, 10-12 weeks; weight, 25-35 g) were
obtained from the Model Animal Research Center of Nanjing
University. The mice were kept at the animal housing facilities
(24±1˚C; 12-h light/dark cycle; 55% humidity and ad libitum access
to food and water) at Tongji University. All experimental
procedures were performed according to international guidelines for
the care and use of laboratory animals (14) and approved by the Animal Ethics
Committee of Tongji University School of Medicine, Shanghai, China
(approval no. TJLAC-014-015).
Experimental colitis
The mice were randomly divided into two groups: One
healthy control group and one experimental UC group (n=15 mice in
each group). DSS (36-50 kDa; cat. no. 160110; MP Biomedicals) was
added to tap water at a concentration of 4%. Mice in the
experimental UC group were exposed to DSS for 7 days (15). Healthy control animals drank tap
water alone. The fresh stools of each mouse were collected each day
for a total of 7 days. Stool scores varied from 0 (normal) to 3
(diarrhea) points based on stool properties such as shape,
moisture, and viscosity (15).
Colitis score
Colonic damage was assessed according to the
macroscopic score (MS). According to Li et al (15) and Kimball et al (16), the MS encompassed: i) Weight loss
score; ii) colon length shortening score; and iii) the occult blood
score. Each scoring system had four points in total. Thus, the MS
was the sum of the three scores (0 points, most healthy; 12 points,
least healthy).
Specimen preparation
Mice were sacrificed by cervical dislocation on the
seventh day of colitis induction. The intestines were excised and
carefully rinsed with saline. A 30-mm section of colon, which was
considered to begin at a point 10 mm away from the caecum, was cut
out and weighed. The colon was then dissected into two portions,
with 10 mm (sample 1) allotted for histological analysis and 5 mm
(sample 2) allotted for transmission electron microscopy (TEM).
Sample 1 was fixed in 4% paraformaldehyde (Sigma-Aldrich; Merck
KGaA) in 4˚C for 24 h, while sample 2 was fixed in 2.5%
paraformaldehyde and 2.5% glutaraldehyde in 0.1 M Na-Cacodylate
buffer (pH 7.4; all from Sigma-Aldrich; Merck KGaA) in 4˚C for 24
h. In total, 15 control and 15 UC tissue samples were collected to
undergo histological analysis and TEM evaluation.
Histology
For histological investigation, sample 1 was washed
with PBS and dissected into two portions. One of the portions was
embedded in paraffin and sectioned at 5-µm thickness on a Leica
RM2126 microtome (Leica Biosystems). The sections were first
deparaffinized and then stained with hematoxylin and eosin
(H&E; Abcam) to assess the degree of inflammation according to
the instructions from the manufacturer. Sections were stained with
Masson's trichrome (Sigma-Aldrich; Merck KGaA) and Verhoeff's
elastic staining (Abcam) to visualize the connective tissue
according to the manufacturer's instruction. The other portion was
embedded in optimal cutting temperature compound (Leica Biosystems)
and sectioned at 10-µm thickness on a Leica CM1860 microtome (Leica
Biosystems). The sections were first washed with PBS and
permeabilized in 0.025% Triton X-100 and 1% BSA in TBS buffer for
20 min at room temperature. Then the sections were incubated
overnight at 4˚C with anti-α smooth muscle actin antibody (1:100;
Abcam; cat. no. ab5694) in TBS buffer with 1% BSA, followed by
labeling with Alexa 488-conjugated goat anti-rabbit IgG H&L
(1:300; Abcam; cat. no. ab150077) in TBS buffer with 1% BSA at room
temperature for 1 h. The sections were washed with PBS before using
the mounting medium with DAPI (Abcam; cat. no. ab104139) in the
dark. Tissue pathophysiology was characterized by the presence of
ulcerations, inflammatory cells (such as neutrophils, macrophages,
lymphocytes and plasma cells), signs of edema, crypt loss, surface
epithelial cell hyperplasia, goblet cell reduction and signs of
epithelial regeneration. The histopathological score (HS) was used
as a method for evaluating the degree of UC lesions. The HS
evaluation included: i) Crypt architecture damage score (0-2
points); ii) edema in submucosa score (0-3 points); and iii)
inflammatory cell infiltration score (0-3 points). HS was the sum
of the three scores (0 points, most healthy; 8 points, least
healthy), based on previously published reports by Li et al
(15) and Engel et al
(17).
TEM
For TEM experiments, sample 2 was first washed with
PBS, then fixed in 1% osmic acid in room temperature for 2 h,
dehydrated by acetone and embedded in Spurr Embedding medium in
60˚C for 48 h (Sangerbio). Subsequently, 70-nm sections were cut,
stained with uranyl acetate for 20 min and lead citrate for 5 min
in room temperature, and examined using JEOL-1010 transmission
electron microscope (original magnification, x20,000; JEOL,
Ltd.).
Statistical analysis
Experimental data are presented as the mean ± SD.
Shapiro-Wilk and Kolmogorov-Smirnov tests were used to determine
data normality. Comparisons of the quantitative values between the
control and UC groups were performed using Student's t-test.
Mann-Whitney U test was used to analyze the ordinal data. P<0.05
was considered to indicate a statistically significant difference.
Statistical analyses were performed using SPSS 21.0 statistical
software (IBM Corp.).
Results
Mouse UC model
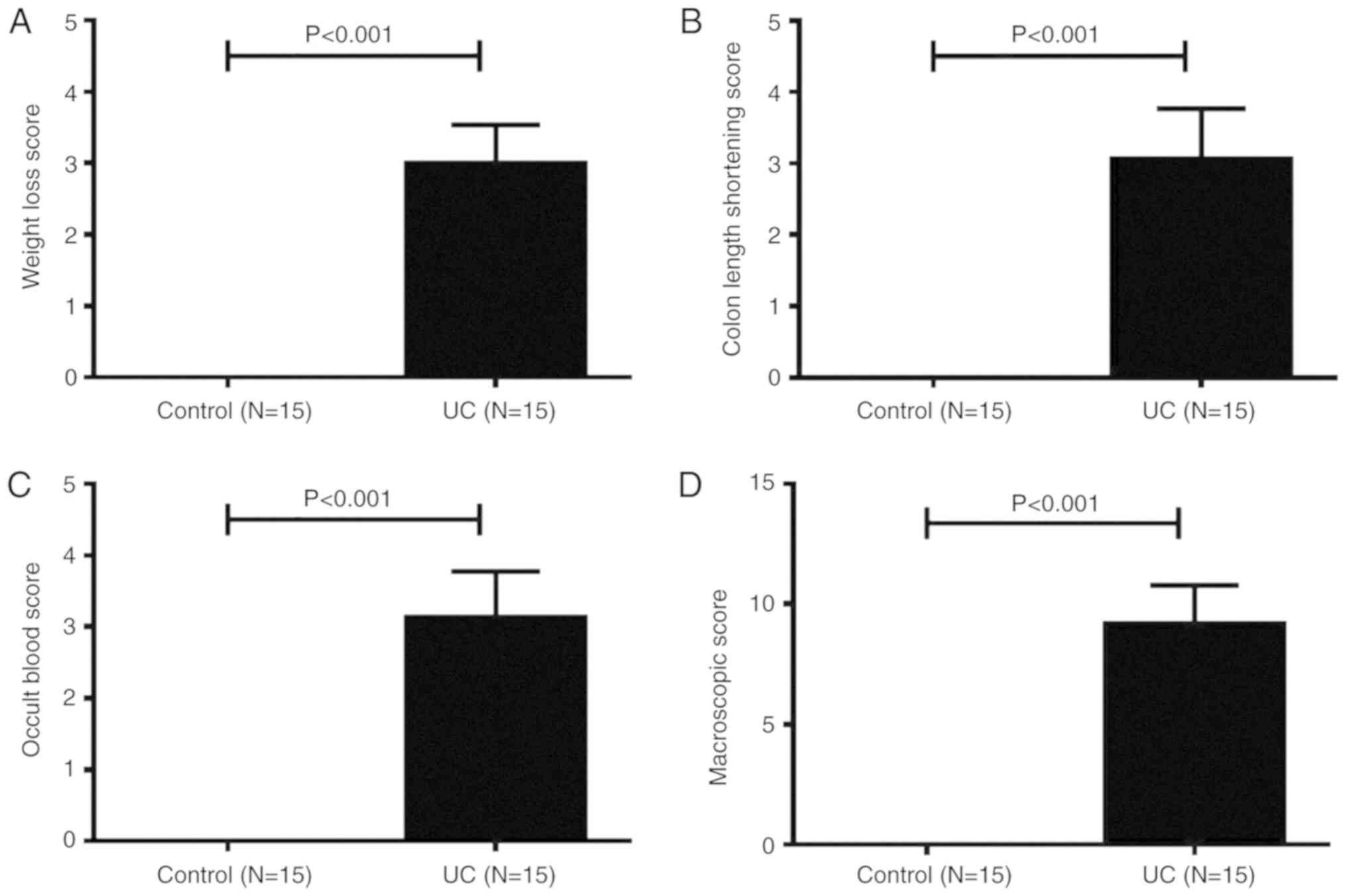
During DSS feeding days, clinical symptoms of UC
mice, such as loss of body weight, loose feces/watery diarrhea and
fecal blood were noted. Macroscopically, the body weight loss of
mice with UC was observed from day 2, and on day 7, the body weight
loss score of UC mice was significantly higher compared with
control mice (Fig. 1A). On day 3,
several mice in the UC group began to experience diarrhea, followed
by hematochezia. All UC mice had diarrhea and hematochezia on day
6. The length of the colon was measured at necropsy. As shown in
Fig. 1B, the colon length shortening
scores of UC mice were significantly higher compared with healthy
mice. The occult blood scores of UC mice were significantly higher
compared with healthy controls (Fig.
1C). The total MS of mice in the UC group was 9.3±0.3 points,
while the control group total MS was 0 points (Fig. 1D). Thus, acute UC was successfully
induced in C57BL/10J mice by oral administration of 4% DSS for 7
days.
Histology
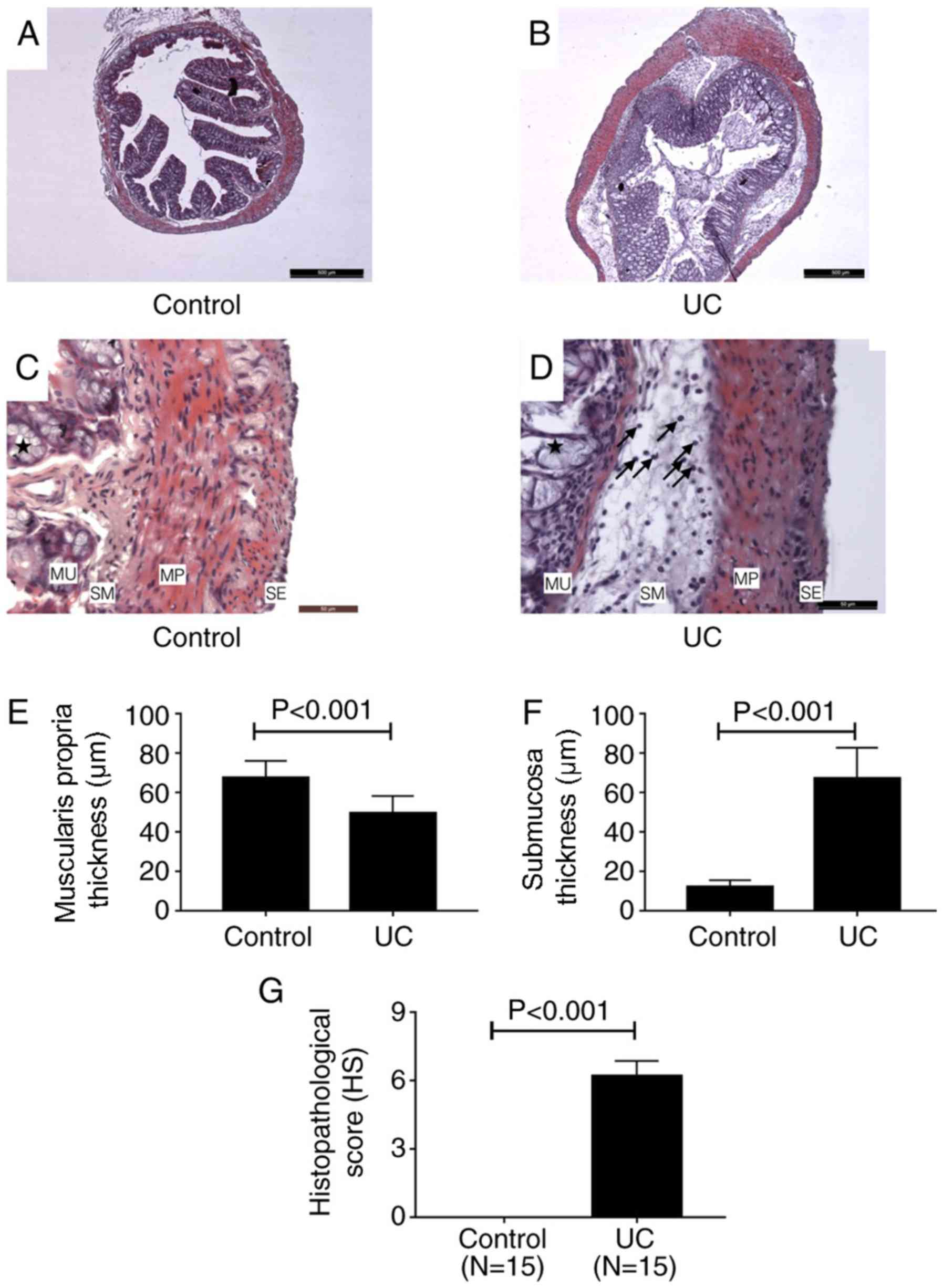
H&E staining. H&E staining revealed
structural changes of colon tissues of mice in control and UC
groups (Fig. 2A-D). A series of
pathological changes occurred in the UC group (Fig. 2B and D) compared with the control group (Fig. 2A and C). In the mucosa, the goblet cell
architecture of the colon tissue was damaged (as indicated by the
stars in Fig. 2D). A number of
inflammatory cells infiltrated into the stroma and the structure of
the gland was destroyed (Fig. 2D).
In the submucosa, the tissue presented with significant edema,
which was mainly due to inflammatory cell infiltration (indicated
by the arrows in Fig. 2D). In the
muscularis propria, the smooth muscle cell structure was altered;
the nuclei became rounder and the boundaries of the cells became
indistinct. However, inflammatory cells were not found in this
layer (Fig. 2D). The thickness of
the muscularis propria of UC cells was significantly reduced
compared with the control group (Fig.
2E), however, the whole thickness of the colon wall of UC mice
significantly increased compared with the control group due to
edema of the submucosa (Fig. 2F).
There was no visible difference between the control and UC groups
in the serosa. As shown in Fig. 2G,
the HS for the UC group was 6.2±0.2 points, while the HS of the
control group was 0 points. This result indicated that the lesion
of colon in DSS-induced UC mice was significant.
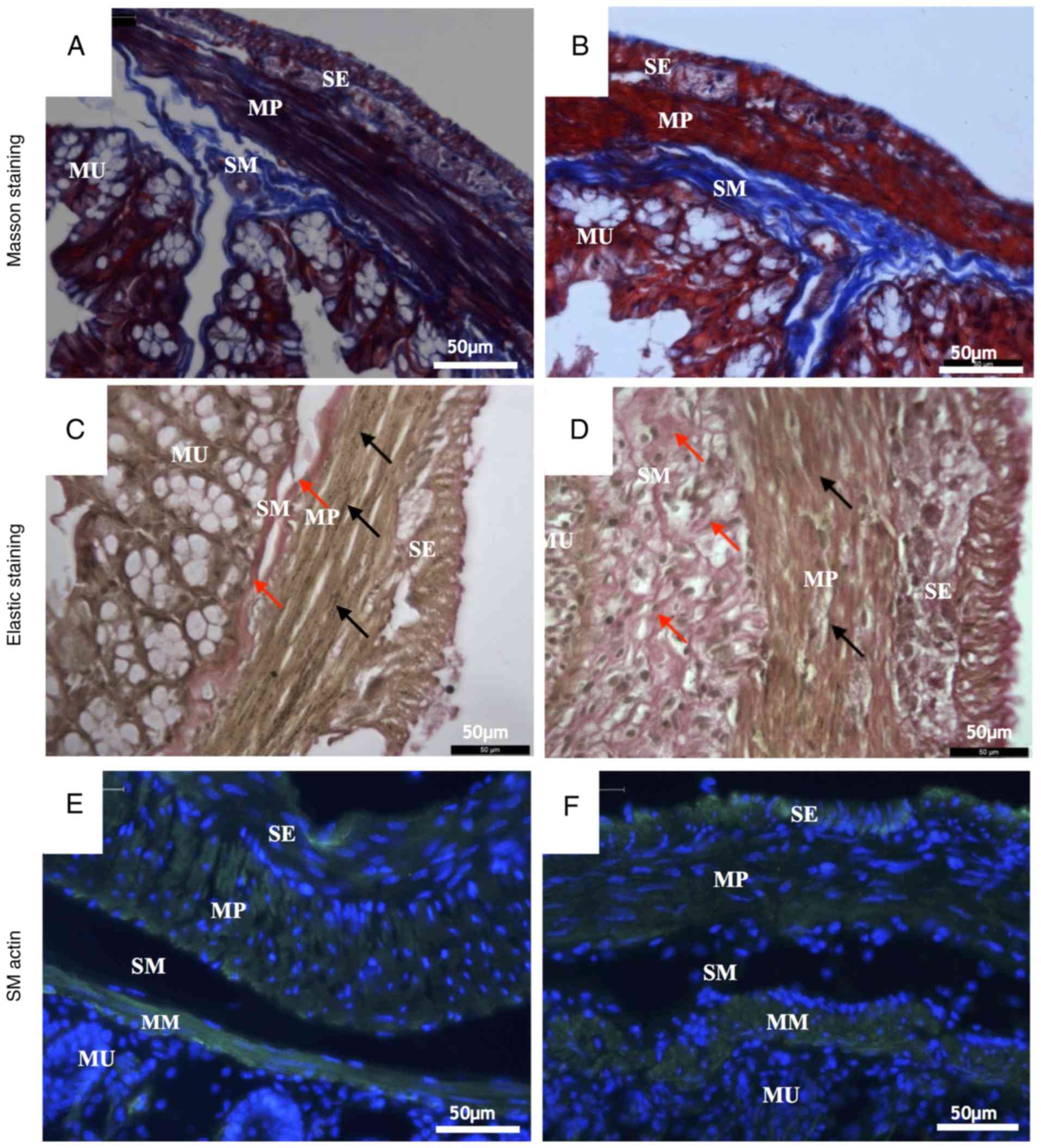
Masson's trichrome staining and
Verhoeff's elastic staining
Masson's trichrome staining and Verhoeff's elastic
staining showed changes in the microscopic structure of the mouse
colon wall. Masson's staining dyed the collagen fibers blue, the
muscle cells red and the nuclei dark blue. The elastic staining
dyed the elastic fibers black, the collagen fibers red, the muscle
cells yellow and the nuclei blue to black.
Compared with the control group (Fig. 3A), a number of pathological changes
occurred in the microstructure of the UC group (Fig. 3B) as revealed by Masson's trichrome
staining. In the submucosa, the collagen fibers were scarce in the
UC group but tightly packed into bundles in the control group.
Collagen fibers in the UC submucosa evidently presented with
hyperplasia and the fiber alignment was more disordered compared
with control. In the muscularis propria, collagen fibers in the
control group surrounded the smooth muscle cells regularly.
However, numerous collagen fibers in the UC muscular layer were
disrupted and fiber bundles became thinner compared with the
control group (Fig. 3A and B).
According to the results of elastic staining, the
most notable difference between the control group (Fig. 3C) and the UC group (Fig. 3D) was the completeness and continuity
of elastic fibers. In the muscularis propria, elastic fibers are
produced by smooth muscle cells (18). In the submucosa, the collagen fibers
of the UC group were proliferated and disordered (indicated by red
arrows; Fig. 3D). In muscularis
propria, the elastic fibers were continuous and arranged between
the smooth muscle cells equally (indicated by black arrows;
Fig. 3C) in the control group.
However, in the UC group, the elastic fibers were rare, and the
arrangement was irregular (Fig.
3D).
Immunohistochemistry staining
Staining with α smooth muscle actin antibody
specifically stains the smooth muscle cells. There are two muscle
layers in the mouse colon wall: Muscularis mucosa and muscularis
propria. The latter muscle cells are divided in two groups:
Longitudinal muscle and circular muscle (19). In the control group (Fig. 3E), the smooth muscle cells in the
muscularis mucosa and muscularis propria exhibited normal
morphology. The cells were long and spindle-shaped, the size was
uniform, the cell membrane was clear, the cell edge was distinct
and the cell nucleus was spindle-shaped (Fig. 3E). By contrast, smooth muscle cells
in the UC group were rounder and shorter and the membranes were not
as distinct as those in the control group. Additionally, the
muscularis mucosa was thicker, but the muscularis propria was
thinner and the nuclei were smaller compared with the control group
(Fig. 3F).
Ultrastructure
TEM was performed to observe the colon wall
ultrastructure. In the control group (Fig. 4A), the smooth muscle cells were long,
spindle-shaped and surrounded by collagen fibers (indicated by the
black arrows; Fig. 4A). However, in
the UC group (Fig. 4B), the cell
shape was abnormal, and the cells appeared shorter and rounder.
Additionally, the arrangement of the cells was irregular, and the
collagen fibers (indicated by the black arrows; Fig. 4B) were rare. Collagen fibers
surrounding the smooth muscle cells were tied into a compact bundle
in the control group (indicated by the star; Fig. 4C), but in the UC group, the collagen
fibers were looser, and the arrangement was irregular (indicated by
the star; Fig. 4D). Changes in the
smooth muscle cells were also observed. In the control group
(Fig. 4E), the nuclear envelope of
smooth muscle cells was complete (indicated by black arrows;
Fig. 4E) and the nucleus was long
and spindle-shaped with a small but clear nucleolus (indicated by
the triangle; Fig. 4E). Conversely,
in the UC group (Fig. 4F), the
smooth muscle cell was edematous, the nuclear envelope was unclear,
and the shape of the nucleus was irregular and edematous.
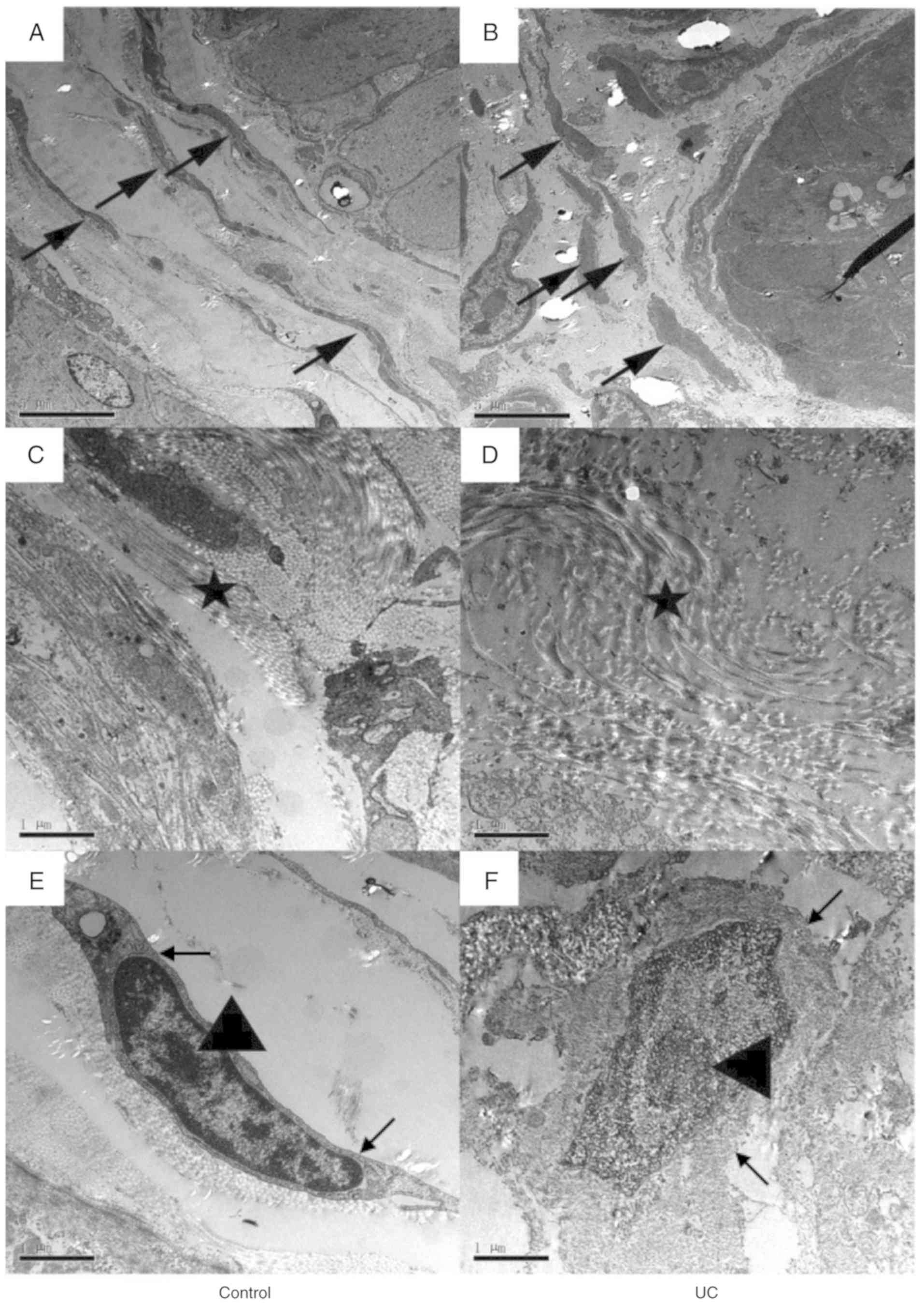 | Figure 4Representative transmission electron
microscopy images of colon walls. Images of (A) control and (B) UC
group colon samples at x5,000 magnification. Smooth muscle cells
are indicated by the arrows. The smooth muscle cells were long
spindle-shaped and surrounded by the collagen fibers in the control
group, while they were shorter and rounder in the UC group. Scale
bar, 5 µm. Images of (C) control and (D) UC group samples at
x20,000 magnification. Collagen fibers are indicated by the stars.
The arrangement of each collagen fibers bundled along with the
smooth muscle's axle in the control group, while the collagen
fibers were much looser in the UC group. Scale bar, 1 µm. Images of
(E) control and (F) UC samples at x20,000 magnification. The
nuclear envelope of smooth muscle cell was complete in the control
group, and the nucleus was long and spindle-shaped with a small but
clear nucleolus. However, in the UC group, the smooth muscle cell
was edematous, the nuclear envelope was unclear, and the shape of
the nucleus was irregular and edematous. Scale bar, 1 µm. UC,
ulcerative colitis. |
Discussion
DSS is regarded as the most effective way to
generate a UC mouse model (13). The
DSS-induced colitis model has certain advantages relative to other
animal models of colitis. An acute, chronic or relapsing model can
be easily produced by altering the concentration of DSS
administered (20). Among these
protocols, oral administration of DSS is regarded as a simple,
economical and effective method in mice. The proposed and most
accepted mechanism by which DSS induces intestinal inflammation is
associated with the disruption of the intestinal epithelial
monolayer lining, leading to the entry of luminal bacteria and
associated antigens into the mucosa and allowing the dissemination
of proinflammatory intestinal contents into underlying tissue
(21). Histological investigation
was performed on a mouse model in the present study to elucidate
changes in the microstructure of colon tissue in response to
DSS-induced early-stage UC.
One way to diagnose and monitor inflammatory bowel
disease (IBD) in the clinical setting, which mainly includes UC and
CD, is by recording the clinical symptoms of the patient. Symptoms
often related to UC include rectal bleeding or bloody diarrhea
(22). The manifestations observed
in DSS-induced mice used in the current study include weight loss,
diarrhea, occult blood in stools and anemia, which were similar to
those previously reported in the human UC case (23).
Histological features typical of IBD compared with
other mucosal inflammations are epithelial distortions, such as
crypt branching and shortening and decreased crypt density, and
severe infiltration of inflammatory cells to the intestinal wall
(20,24). Furthermore, a reduction in goblet
cell numbers is typical for UC, but not for CD (25,26).
Another typical histological feature found in patients with IBD is
the severe infiltration of mononuclear cells and plasma cells into
the basal lamina propria of the inflamed intestinal wall (25,26). The
present study found irregular epithelial formation with crypt
distortion and goblet cell depletion (data not shown), similar to
changes found in patients with UC (27,28). In
addition, a high number of inflammatory cells infiltrated into the
stroma and the structure of the gland was destroyed. The tissue in
the submucosa showed significant edema due to inflammatory cell
infiltration.
Fibrosis in UC is characterized by increased
deposits of collagen in the submucosa and lamina propria (29). In the animal model used in the
present study, an extensive deposition of collagen was detected in
the mucosa and submucosa of acutely inflamed colons, resembling
fibrosis in UC. In the current study, it was observed that the
collagen fibers proliferated into the submucosa without a
regulatory arrangement at the early stage of UC. They were loose,
forming a disorganized fiber net instead of a fiber bundle. In the
muscularis propria, the fibers, including both collagen and elastic
fibers, were reduced and fractured. The above changes lead to
insufficient intestinal motility.
In human UC, ultrastructure alterations of the
epithelium have been observed, including microvilli depletion,
shattering of the epithelial junctions, cytoplasmic vacuolization,
dilatation of the endoplasmic reticulum, pyknotic nuclei and
altered structuring of the mitochondria and Golgi complexes
(30,31). However, to the best of our knowledge,
the ultrastructure of the muscularis propria has not been fully
elucidated. The results of the present study found an irregular
arrangement of the smooth muscle cells in the muscularis propria of
the DSS-induced mouse model. These cells were rounder and shorter,
and surrounded by looser and more irregular collagen fibers. Severe
alterations of the cell membrane and nucleus were also observed.
The changes observed among smooth muscle cells and collagen fibers
indicated that the colon wall in UC was less resistant to external
forces, as previously reported by the authors of the current study
(32). Changes in the morphology of
these cells may indicate epithelial cell injury.
Chemical reagents used for inducing colitis in
animal models mainly include 2,4,6-trinitrobenzene sulfonic acid
(TNBS), DSS and oxazolone (13,33,34).
Both TNBS and oxazolone-mediated colitis are induced by intrarectal
administration of the reagents and the pathogenesis primarily
involves a T cell-mediated response against autologous proteins or
luminal antigens (10). However, in
the DSS-induced colitis model, mice are treated with water
supplemented with DSS for several days (10,20,24). DSS
seems to play a directly toxic role in colonic epithelial cells of
the basal crypts, and several immunological responses also play a
role in the pathogenesis of UC (33). TNBS-induced colitis is thought to be
a T helper (Th) cell-mediated disease, while Th2-relevant cytokine
levels are increased in DSS- and oxazolone-induced colitis
(33). Although the exact mechanisms
differ, these three colitis-inducing chemical reagents in murine
models share a number of similarities in terms of histology
alterations (35,36). The infiltration of neutrophils and
macrophages is observed as early as on the first day after chemical
stimulation, and the infiltration is increased over time (10). By day 3, ulcerations, goblet cell
depletion and fibrosis are present in the colon (33,34).
Farkas et al (37) observed
leucocyte rolling, sticking and extravasation under an electron
microscope. The present work further extended the understanding of
ultrastructure alterations in DSS-induced colitis. The
ultrastructure alterations found in the current DSS-induced model
might also be found in other reagent-induced colitis models, since
the three chemical-induced colitis murine models share similar
pathological injuries under light microscopy (35). It is worth further investigating the
ultrastructure of other reagent-induced colitis models in order for
the characteristics of the three models to be compared.
Additionally, colitis research can be improved through choosing
more suitable colitis models, such as congenital and adaptive cell
transferred models (38).
In conclusion, UC was successfully induced with 7
consecutive days of DSS oral administration in mice. The
ultrastructure changes of DSS-induced UC colon were examined.
Experimental DSS-induced colitis in mice shared most features with
human UC. These features may contribute to improved understanding
of the pathogenesis and mechanism of UC.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National
Natural Science Foundation of China (grant nos. 31571181 and
11702197) and the Fundamental Research Funds for the Central
Universities (grant nos. 22120180077 and 1500219128).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XX and YL conceived and designed the study. XX, SL,
JT, YY, XG and KL performed the experiments and analyzed the data.
XX, SL, JT and YL wrote and revised the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures were performed according
to International Guidelines for the Care and Use of Laboratory
Animals and were approved by the Animal Ethics Committee of Tongji
University School of Medicine, Shanghai, China (approval no.
TJLAC-014-015).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ungaro R, Mehandru S, Allen PB,
Peyrin-Biroulet L and Colombel JF: Ulcerative colitis. Lancet.
389:1756–1770. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
da Silva BC, Lyra AC, Rocha R and Santana
GO: Epidemiology, demographic characteristics and prognostic
predictors of ulcerative colitis. World J Gastroenterol.
20:9458–9467. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Adams SM and Bornemann PH: Ulcerative
colitis. Am Fam Physician. 87:699–705. 2013.PubMed/NCBI
|
|
4
|
Di Sabatino A, Biancheri P, Rovedatti L,
Macdonald TT and Corazza GR: Recent advances in understanding
ulcerative colitis. Intern Emerg Med. 7:103–111. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Maul J and Zeitz M: Ulcerative colitis:
Immune function, tissue fibrosis and current therapeutic
considerations. Langenbecks Arch Surg. 397:1–10. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rieder F and Fiocchi C: Intestinal
fibrosis in inflammatory bowel disease - Current knowledge and
future perspectives. J Crohn's Colitis. 2:279–290. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Manetti M, Rosa I, Messerini L and
Ibba-Manneschi L: Telocytes are reduced during fibrotic remodelling
of the colonic wall in ulcerative colitis. J Cell Mol Med.
19:62–73. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chassaing B and Darfeuille-Michaud A: The
commensal microbiota and enteropathogens in the pathogenesis of
inflammatory bowel diseases. Gastroenterology. 140:1720–1728.
2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rijnierse A, Nijkamp FP and Kraneveld AD:
Mast cells and nerves tickle in the tummy: Implications for
inflammatory bowel disease and irritable bowel syndrome. Pharmacol
Ther. 116:207–235. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Randhawa PK, Singh K, Singh N and Jaggi
AS: A review on chemical-induced inflammatory bowel disease models
in rodents. Korean J Physiol Pharmacol. 18:279–288. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Whittem CG, Williams AD and Williams CS:
Murine Colitis modeling using Dextran Sulfate Sodium (DSS). J Vis
Exp. 35(1652)2010.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Okayasu I, Hatakeyama S, Yamada M, Ohkusa
T, Inagaki Y and Nakaya R: A novel method in the induction of
reliable experimental acute and chronic ulcerative colitis in mice.
Gastroenterology. 98:694–702. 1990.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chassaing B, Aitken JD, Malleshappa M and
Vijay-Kumar M: Dextran sulfate sodium (DSS)-induced colitis in
mice. Curr Protoc Immunol 104. Unit. 15(25)2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Alleva E and Santucci D: Guide for the
care and use of laboratory animals. Ethology. 103:1072–1073.
1997.
|
|
15
|
Li YY, Yuece B, Cao HM, Lin HX, Lv S, Chen
JC, Ochs S, Sibaev A, Deindl E, Schaefer C, et al: Inhibition of
p38/Mk2 signaling pathway improves the anti-inflammatory effect of
WIN55 on mouse experimental colitis. Lab Invest. 93:322–333.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kimball ES, Wallace NH, Schneider CR,
D'Andrea MR and Hornby PJ: Vanilloid receptor 1 antagonists
attenuate disease severity in dextran sulphate sodium-induced
colitis in mice. Neurogastroenterol Motil. 16:811–818.
2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Engel MA, Kellermann CA, Rau T, Burnat G,
Hahn EG and Konturek PC: Ulcerative colitis in AKR mice is
attenuated by intraperitoneally administered anandamide. J Physiol
Pharmacol. 59:673–689. 2008.PubMed/NCBI
|
|
18
|
Ippolito C, Colucci R, Segnani C, Errede
M, Girolamo F, Virgintino D, Dolfi A, Tirotta E, Buccianti P, Di
Candio G, et al: Fibrotic and Vascular Remodelling of Colonic Wall
in Patients with Active Ulcerative Colitis. J Crohn's Colitis.
10:1194–1204. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lai S, Yu W, Wallace L and Sigalet D:
Intestinal muscularis propria increases in thickness with corrected
gestational age and is focally attenuated in patients with isolated
intestinal perforations. J Pediatr Surg. 49:114–119.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Melgar S, Karlsson A and Michaëlsson E:
Acute colitis induced by dextran sulfate sodium progresses to
chronicity in C57BL/6 but not in BALB/c mice: Correlation between
symptoms and inflammation. Am J Physiol Gastrointest Liver Physiol.
288:G1328–G1338. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rath HC, Schultz M, Freitag R, Dieleman
LA, Li F, Linde HJ, Schölmerich J and Sartor RB: Different subsets
of enteric bacteria induce and perpetuate experimental colitis in
rats and mice. Infect Immun. 69:2277–2285. 2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Baumgart DC and Sandborn WJ: Inflammatory
bowel disease: Clinical aspects and established and evolving
therapies. Lancet. 369:1641–1657. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Perše M and Cerar A: Dextran sodium
sulphate colitis mouse model: Traps and tricks. J Biomed
Biotechnol. 2012(718617)2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Taghipour N, Molaei M, Mosaffa N,
Rostami-Nejad M, Asadzadeh Aghdaei H, Anissian A, Azimzadeh P and
Zali MR: An experimental model of colitis induced by dextran
sulfate sodium from acute progresses to chronicity in C57BL/6:
Correlation between conditions of mice and the environment.
Gastroenterol Hepatol Bed Bench. 9:45–52. 2016.PubMed/NCBI
|
|
25
|
Tanaka M, Riddell RH, Saito H, Soma Y,
Hidaka H and Kudo H: Morphologic criteria applicable to biopsy
specimens for effective distinction of inflammatory bowel disease
from other forms of colitis and of Crohn's disease from ulcerative
colitis. Scand J Gastroenterol. 34:55–67. 1999.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Geboes K and Dalle I: Influence of
treatment on morphological features of mucosal inflammation. Gut.
50 (Suppl 3):III37–III42. 2002.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Allison MC, Hamilton-Dutoit SJ, Dhillon AP
and Pounder RE: The value of rectal biopsy in distinguishing
self-limited colitis from early inflammatory bowel disease. Q J
Med. 65:985–995. 1987.PubMed/NCBI
|
|
28
|
Theodossi A, Spiegelhalter DJ, Jass J,
Firth J, Dixon M, Leader M, Levison DA, Lindley R, Filipe I and
Price A: Observer variation and discriminatory value of biopsy
features in inflammatory bowel disease. Gut. 35:961–968.
1994.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Matthes H, Herbst H, Schuppan D, Stallmach
A, Milani S, Stein H and Riecken EO: Cellular localization of
procollagen gene transcripts in inflammatory bowel diseases.
Gastroenterology. 102:431–442. 1992.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rumessen JJ: Ultrastructure of
interstitial cells of Cajal at the colonic submuscular border in
patients with ulcerative colitis. Gastroenterology. 111:1447–1455.
1996.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Fratila OC and Craciun C: Ultrastructural
evidence of mucosal healing after infliximab in patients with
ulcerative colitis. J Gastrointestin Liver Dis. 19:147–153.
2010.PubMed/NCBI
|
|
32
|
Gong X, Xu X, Lin S, Cheng Y, Tong J and
Li Y: Alterations in biomechanical properties and microstructure of
colon wall in early-stage experimental colitis. Exp Ther Med.
14:995–1000. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Alex P, Zachos NC, Nguyen T, Gonzales L,
Chen TE, Conklin LS, Centola M and Li X: Distinct cytokine patterns
identified from multiplex profiles of murine DSS and TNBS-induced
colitis. Inflamm Bowel Dis. 15:341–352. 2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wirtz S, Popp V, Kindermann M, Gerlach K,
Weigmann B, Fichtner-Feigl S and Neurath MF: Chemically induced
mouse models of acute and chronic intestinal inflammation. Nat
Protoc. 12:1295–1309. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Goyal N, Rana A, Ahlawat A, Bijjem KR and
Kumar P: Animal models of inflammatory bowel disease: A review.
Inflammopharmacology. 22:219–233. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sartor RB: Review article: How relevant to
human inflammatory bowel disease are current animal models of
intestinal inflammation? Aliment Pharmacol Ther. 11 (Suppl
3):89–96; discussion 96-97. 1997.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Farkas S, Herfarth H, Rössle M, Schroeder
J, Steinbauer M, Guba M, Beham A, Schölmerich J, Jauch KW and
Anthuber M: Quantification of mucosal leucocyte endothelial cell
interaction by in vivo fluorescence microscopy in
experimental colitis in mice. Clin Exp Immunol. 126:250–258.
2001.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Mizoguchi E, Low D, Ezaki Y and Okada T:
Recent updates on the basic mechanisms and pathogenesis of
inflammatory bowel diseases in experimental animal models. Intest
Res. 18:151–167. 2020.PubMed/NCBI View Article : Google Scholar
|