Introduction
Alveolar epithelial cells type Ⅱ (AEC Ⅱ), also known
as granule alveolar cells, are generally located in depressions on
the alveolar surface, alveolar and alveolar junctions, and alveolar
angles. Some studies have revealed that AEC Ⅱ are the main
precursor cells for alveolar damage repair during infection and
tissue damage, and have strong immune function (1,2). AEC Ⅱ
are involved in the immune response through various ways (3), i.e., secreting a variety of
antibiotics, such as lysozyme, expressing a variety of receptors,
such as Toll-like receptors (TLRs) (4) and receptors for advanced glycation
endproducts, and secreting pulmonary surfactant proteins (5,6).
However, the specific mechanism by which AEC Ⅱ generates immunity
through these methods is not fully understood. Therefore, an
antiviral immunity study of AEC Ⅱ would be beneficial for both the
basic medicine and clinical treatment.
TLRs belong to the family of pattern recognition
receptors (PRRs). At present, 11 TLRs [TLR1-TLR10(7) and TLR14] have been identified in the
human genome. TLRs can be stimulated by microorganisms, recruiting
specific adaptor proteins, and activating a series of signal
cascades that trigger the body's immune response. As a type of
PRRs, TLRs can recognize lipids, peptides and carbohydrates
expressed by various pathogenic microorganisms (8-11).
According to previous studies, there are two major signal
transduction pathways in the cascade amplification reaction; one is
the myeloid differentiation factor 88 (MyD88)-dependent pathway
(12,13), and the other is the MyD88-independent
pathway (14). The MyD88-dependent
pathway is a transduction pathway in which all TLRs are involved,
apart from TLR3. TLR4 is widely distributed in the immune cells,
other than B cells, T cells, and NK cells. Cyr et al
(15) have shown that pneumonia
immune response to respiratory syncytial virus (RSV) infection is
dependent on the complete TLR4 or MyD88 signaling pathway. Zhou
et al (16) have indicated
that TLR4 could mediate the production of cellular inflammatory
factors, such as tumor necrosis factor-α (TNF-α) and
interleukin-1β, and could be involved in the transduction of
inflammatory signals and responses. Yan (17), and Akira and Takeda (18) have demonstrated that the MyD88
signaling pathway could activate the NF-κB signaling pathway, which
could activate NF-κB and IFN-regulated factor 3 (IRF3). The
activity of NF-κB is enhanced in viral myocarditis. The use of
NF-κB blocker can effectively block the condition of myocarditis
and inhibit the expression of inflammatory cytokine TNF-α in
cardiomyocytes (19). The
aforementioned studies suggest that TLR4 may be involved in the
body's immune response and may be regulated by the TLR4/MyD88/NF-κB
signaling pathway.
RSV is a single-stranded negative RNA virus that
belongs to pneumoviruses, and is the most common pathogen of lower
respiratory tract infections in infants and young children. Of all
the TLRs found in the human body, TLR3, TLR4 and TLR7 are the ones
most closely related to RSV (20).
TLR4 is mainly expressed on the surface of cell membranes. When RSV
invades, TLR4 can recognize the F protein of RSV and induce the
excessive activation of MyD88 and NF-κB in the downstream, leading
to the production of cellular inflammatory factors (TNF-α and IL-2)
(21), thus causing diseases, such
as pneumonia and asthma. However, it is not entirely clear which
signal factors are involved in the transduction and expression
level downstream of the immune process. Studies have shown that
glucocorticoids (GCs) can act on innate immune cells (22) and protect the body from the damage of
lipopolysaccharides (23).
Therefore, it is not clear whether TLR4 induces an immune response,
and whether glucocorticoid receptor (GR) is involved, in the
process of antiviral immunity is not yet fully understood.
In the present study, RSV-infected human AEC Ⅱ A549
were used, and RSV-infected GR agonist methylprednisolone (MP) was
utilized to pre-intervene human AEC Ⅱ A549 and establish an in
vitro cell model, in order to evaluate the effect of RSV on the
proliferation of A549 cells by MTT. ELISA, reverse
transcription-quantitative PCR (RT-qPCR) and western blot analysis
were carried out to investigate the effect of MP pre-intervention
on the expression of TLR4, MyD88, NF-κB p65, TNF-α and GR. The
present study aimed to investigate the effect of MP on the
expression of cytokines in lung epithelial cells infected with RSV
and explore the TLR4-mediated immune mechanism in human AEC Ⅱ
during antiviral immunity.
Materials and methods
Virus and cells
Respiratory virus (international standard strain
long) was purchased from Wuhan Procell Life Science Co., Ltd.,
human AEC Ⅱ A549 were purchased from Shanghai Institute of
Biochemistry and Cell Biology, Chinese Academy of Sciences, and
both gene knocked out and overexpressed TLR4 human AEC Ⅱ A549 cells
were purchased from Wuhan Servicebio Technology Co., Ltd. The study
was approved by the Ethics Committee of Xuzhou Children's Hospital
Affiliated to Xuzhou Medical University (Xuzhou, China).
Drugs and main reagents
Fetal bovine serum and DMEM (both from Gibco; Thermo
Fisher Scientific, Inc.); MTT kit (cat. no. 11465007001; Roche
Diagnostics); MP (cat. no. BP249; Sigma-Aldrich; Merck KGaA);
TRIzol® total RNA extraction kit (cat. no. T9424;
Sigma-Aldrich; Merck KGaA). Reagents for RT-qPCR experiments, such
as Taq DNA polymerase, were purchased from Wuhan Servicebio
Technology Co., Ltd. Human TLR4 (cat. no. E0753h), MyD88 (cat. no.
E1707h), NF-κB p65 (cat. no. E1824h), TNF-α (cat. no. E0133h) and
ELISA kits were purchased from Wuhan EIAab Science Co, Ltd. Murine
NF-κB subunit p65 monoclonal antibody (cat. no. 13752-1; Cayman
Chemical Company) and murine TLR4 antibody (cat. no. 3251-100)
(both from BioVision, Inc.); murine MyD88 antibody (cat. no.
ant-464; ProSpec); GR antibody (cat. no. BS6617; Bioworld
Technology, Inc.); HRP-labeled goat anti-mouse IgG secondary
antibody (cat. no. SE131; Solarbio Science & Technology Co.,
Ltd.).
Main instruments
Multiskan FC microplate reader (Thermo Fisher
Scientific, Inc.); StepOne™ fluorescence Real-Time PCR (Applied
Biosystems; Thermo Fisher Scientific, Inc.); Mini Trans-Blot Cell
protein transfer instrument, electrophoresis instrument, and
VersaDoc Mode 5000 gel imaging analysis system (all from Bio-Rad
Laboratories, Inc.).
Determination of viral infection
The viral solution was serially diluted 10 times in
a centrifuge tube from 10-1-10-10 of the
original solution. The diluted virus was inoculated into a 96-well
plate, and 6 wells were set at each dilution, and 100 µl were
inoculated per well. Cell suspension was added to each well for a
final concentration of 2-3x105 cells/ml. The normal cell
group (100 µl growth solution + 100 µl cell suspension) was set,
the number of cytopathic effects was observed for 6 consecutive
days, and the experimental results were recorded. The median tissue
culture infective dose (TCID50) of the virus was
calculated according to the Reed-Muench method (24). TCID50 was the
concentration of the RSV used in the subsequent experiments.
Cell culture and grouping
Human AEC Ⅱ A549 were cultured in a
25-cm2 cell culture flask after trypsin digestion. After
24 h, the cell adherent growth state was observed. Human AEC Ⅱ A549
were collected, digested by trypsin and inoculated in a
25-cm2 cell culture bottle. The adherent growth status
of cells was observed after 24 h. The study was divided into two
parts: a) The knocked out and overexpressed TLR4, as well as the
normal expressed A549 cells were divided into three groups: i)
TLR4-/- group, ⅱ) normal group, ⅲ) TLR4+
group. All three groups were cultured with DMEM containing 100 µl
RSV for 24 h. b) The normally expressed A549 cells were divided
into three groups: i) Control group: A549 cells cultured in DMEM;
ⅱ) RSV group: A549 cells cultured in DMEM containing 100 µl RSV for
24 h; and ⅲ) RSV+MP group: A549 cells cultured for 4 h in DMEM
containing 600 ng/ml MP, and then cultured in DMEM containing 600
ng/ml MP + 100 µl RSV for 24 h.
Cell viability determination by MTT
asssay
A549 cells of the TLR4-/- group, normal
group and TLR4+ group were trypsinized and inoculated
into 96-well plates, and then cultured for 24 h. RSV (100 µl) was
added to each group of cells, and 6 duplicate wells were set as the
drug group. At the same time, the control group was set with the
addition of 100 µl of DMEM. After cell incubation for 24 h in a
CO2 incubator, 10 µl of MTT (5 mg/ml) were added to each
well, and the cells were cultured for 4 h with the addition of 150
µl dimethyl sulfoxide (DMSO) to each well. A microplate reader was
used to measure the absorbance value (A value) at 570 nm. The cell
survival rate was calculated as: Survival rate (%) = (A value of
drug group)/(A value of control group) x100%.
A549 cells with normal TLR4 expression were digested
with trypsin and inoculated in 96-well plates, and then cultured
for 24 h. MP of different concentrations (0, 100, 200, 300, 400,
500, 600, 700 and 800 ng/ml) was added to each well for incubation
for 4 h, and 100 µl RSV were added to the A549 cells that had been
incubated by MP. Six complex wells were set in each well as the
drug group, and the control group was set at the same time. A549
cells were incubated in a CO2 incubator of constant
temperature for 24 h and then 10 µl MTT (5 mg/ml) were added to
each well. A total of 150 ml DMSO were added to each well after
further culture for 4 h. The A value was determined at 570 nm using
ELISA. The cell survival rate was calculated as: Survival rate (%)
= (A value of drug group)/(A value of control group) x100%.
ELISA
A549 cells in the TLR4-/- group, normal
group, and TLR4+ group were trypsinized and then
inoculated into 24-well plates for 24 h. The cells were digested
with trypsin, and then collected with centrifugation at 25˚C for 4
min at 1,000 x g. The collected cells were washed 3 times with cold
PBS, and were disrupted by ultrasonic wave. Cells were frozen and
thawed 3 times repeatedly to break the cells as much as possible.
The disrupted cells were centrifuged at 4˚C, at 1,500 x g for 10
min, and the supernatant was collected for later use. For each test
substance, the experimental procedures were carried out according
to the manufacturer's instructions of the specific ELISA kit. The
optical density value of each well at 450 nm was measured using a
microplate reader. A standard curve was drawn to calculate the
concentration value of each substance according to the curve
equation.
RT-qPCR
Total RNA was extracted from A549 cells of the
TLR4-/- group, normal group, TLR4+ group,
control group, RSV group and RSV+MP group using TRIzol®
reagent, and the absorbance value at 260 and 280 nm was measured by
a micro-spectrophotometer for the content and purity determination
of RNA. After incubating at 37˚C for 50 min and heating at 70˚C for
15 min, total RNA was reverse transcribed into cDNA, and PCR
amplification was carried out. PCR reaction conditions were 95˚C
for 30 sec, 95˚C for 15 sec, 60˚C for 30 sec, with a total of 40
cycles. β-actin was used as the internal reference. Three replicate
wells were set in each group, and the relative expression of each
gene was calculated by 2-ΔΔCq (25). The primer sequences of β-actin, TLR4,
MyD88, NF-κB p65, RSV and GR are presented in Table I.
 | Table IqPCR primers. |
Table I
qPCR primers.
| Target | Forward
primers | Reverse
primers |
|---|
| β-actin |
5'-GGAGCGAGATCCCTCCAAAAT-3' |
5'-GGCTGTTGTCATACTTCTCATGG-3' |
| TLR4 |
5'-AGACCTGTCCCTGAACCCTAT-3' |
5'-CGATGGACTTCTAAACCAGCCA-3' |
| MyD88 |
5'-GGCTGCTCTCAACATGCGA-3' |
5'-CTGTGTCCGCACGTTCAAGA-3' |
| NF-κB p65 |
5'-ATGTGGAGATCATTGAGCAGC-3' |
5'-CCTGGTCCTGTGTAGCCATT-3' |
| RSV |
5'-ACCGGCTGTCTCGTATGAAT-3' |
5'-CTGCCAACATCCGATCAGTG-3' |
| GR |
5'-CCAAGCTTCGATTCAGCAGGCCACTACA-3' |
5'-GGGGTACCTCACTTTTGAAACAGATTTTTG-3' |
Western blot analysis
A549 cells in control group, RSV group and RSV+MP
group were digested with trypsin and inoculated into
25-cm2 cell culture flasks according to the requirements
of each group. Total protein was extracted from A549 cells using
RIPA protein extraction buffer (G2002; Wuhan Servicebio Technology
Co., Ltd.). RIPA lysate and protease inhibitor were prepared at
100:1 dilution). The total protein concentration in the supernatant
was determined by BCA protein assay. A solution containing 70 µg of
protein was subjected to sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (7.5%), and the separated protein was transferred
to a polyvinylidene fluoride membrane with the protein transfer
instrument and blocked with 5% skim milk for 2 h at 25˚C. After
washing, a murine TLR4 antibody diluted at 1:1,200, or a murine
MyD88 antibody, or a murine NF-κB subunit p65 antibody was added
and incubated overnight at 4˚C. After washing again, HRP-labeled
rabbit anti-mouse IgG secondary antibody diluted at 1:2,000 was
added and incubated at 37˚C for 2 h on a shaker. The reference
protein GAPDH (1:1,000, 60004-1-Ig) was provided by Proteintech
Group, Inc. Protein bands were visualized with
electrochemiluminescence reagent (G2020-1; Wuhan Servicebio
Technology Co., Ltd.) and the image strips were scanned with gray
scale using VersaDoc Mode 5000 gel imaging analysis system. The
relative level of each target protein was quantified by the gray
value ratio of target protein/GAPDH using Image-Pro Plus 6.0
software (Media Cybernetics, Inc.).
Statistical analysis
SPSS 22.0 software (IBM Corp.) was used for the
statistical analysis of the data. The experimental data were
expressed as the mean ± standard error of the mean (mean ± SEM).
One-way ANOVA was used to compare multiple groups of data and SNK-q
was the post hoc test used. P<0.05 was considered to indicate a
statistically significant difference.
Results
Titration results of the median
infective dose of RSV
Reed-Muench method was used to calculate the
distance ratio as: [(infection percentage >50% - 50%)/(infection
percentage >50% - infection percentage <50%)] xlog dilution,
and then the following formula was used: log(TCID50) =
dilution of infection percentage >50% + distance ratio. The
experimental results showed that TCID50 was
10-6.5/0.1 ml.
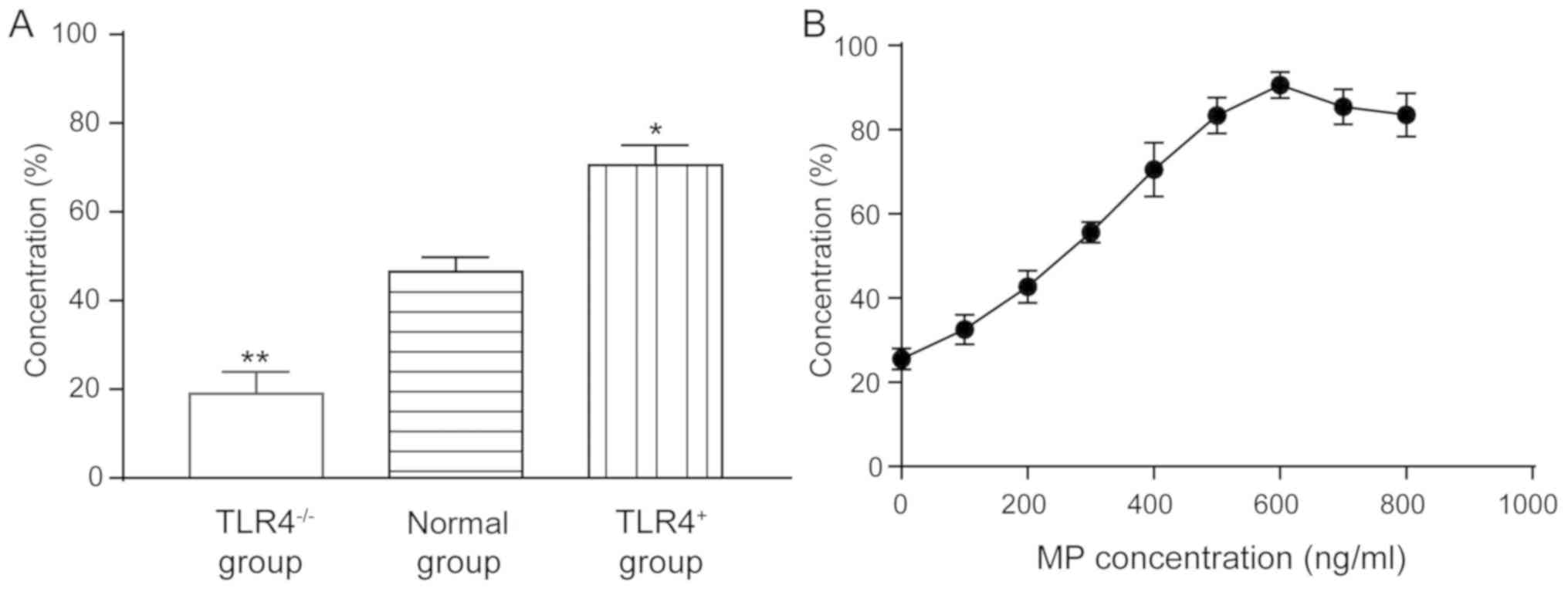
Effect of RSV on proliferation of
human AEC Ⅱ
In the MTT assay of TLR4 knocked out and
overexpressed A549 cells, the cell viability of the
TLR4-/- group was 19.58±3.54%, which was significantly
lower than that of the normal group (47.86±2.58%) (P<0.01). The
survival rate of cells in the TLR4+ group (71.25±3.86%)
was significantly higher than that in the normal group (P<0.05)
(Fig. 1A), indicating that the
expression of TLR4 can enhance the survival of cells after
infection with the virus.
MP has a proliferation-promoting effect on
RSV-incubated A549 cells, in the normal expression of TLR4 cells
(Fig. 1B). In 100 µl of incubated
cells, when the MP concentration was 0, 100, 200, 300, 400, 500,
600, 700 and 800 ng/ml, the promotion rate was 25.53±2.53,
32.56±3.48, 42.67±3.86, 55.62±2.40, 70.56±6.40, 83.35±4.25,
90.61±3.13, 85.45±4.21 and 83.52±5.21%, respectively, indicating
that MP can play an antiviral role.
ELISA detection
The mass concentration of TLR4 in the
TLR4-/- group was 0.0568±0.0153, in the normal group was
0.853±0.034, and in the TLR4+ group was 2.4369±0.0541.
Compared with the normal group, TLR4 mass concentration in the
TLR4-/- group was significantly decreased (P<0.001),
whereas in the TLR4+ group was significantly increased
(P<0.001) (Fig. 2A). The mass
concentration of MyD88 in the TLR4-/- group was
0.5835±0.204, in the normal group was 2.326±0.113, and in the
TLR4+ group was 4.056±0.159. Compared with the normal
group, the mass concentration of MyD88 in the TLR4-/-
group was significantly decreased (P<0.01), whereas in the
TLR4+ group was significantly increased (P<0.01)
(Fig. 2B). The mass concentration of
NF-κB p65 in the TLR4-/- group was 0.732±0.0864, in the
normal group was 2.552±0.095, and in the TLR4+ group was
3.892±0.105. Compared with the normal group, the mass concentration
of NF-κB p65 in the TLR4-/- group was significantly
decreased (P<0.01), whereas in the TLR4+ group was
significantly increased (P<0.01) (Fig. 2C). The mass concentration of TNF-α in
the TLR4-/- group was 7.206±0.586, in the normal group
was 18.308±0.534, and in the TLR4+ group was
30.582±0.489. Compared with the normal group, the mass
concentration of the TNF-α in the TLR4-/- group was
significantly decreased (P<0.01), whereas in the
TLR4+ group was significantly increased (P<0.01)
(Fig. 2D). The mass concentration of
GR in the TLR4-/- group was 30.892±2.62, in the normal
group was 61.389±1.351, and in the TLR4+ group was
69.25±2.158. Compared with the normal group, the mass concentration
of GR in the TLR4-/- group was significantly decreased
(P<0.01), whereas in the TLR4+ group was significant
increased (P<0.05) (Fig. 2E). The
results revealed that the expression of TLR4 could promote the
expression of its downstream regulatory factors MyD88, NF-κB p65,
TNF-α and GR.
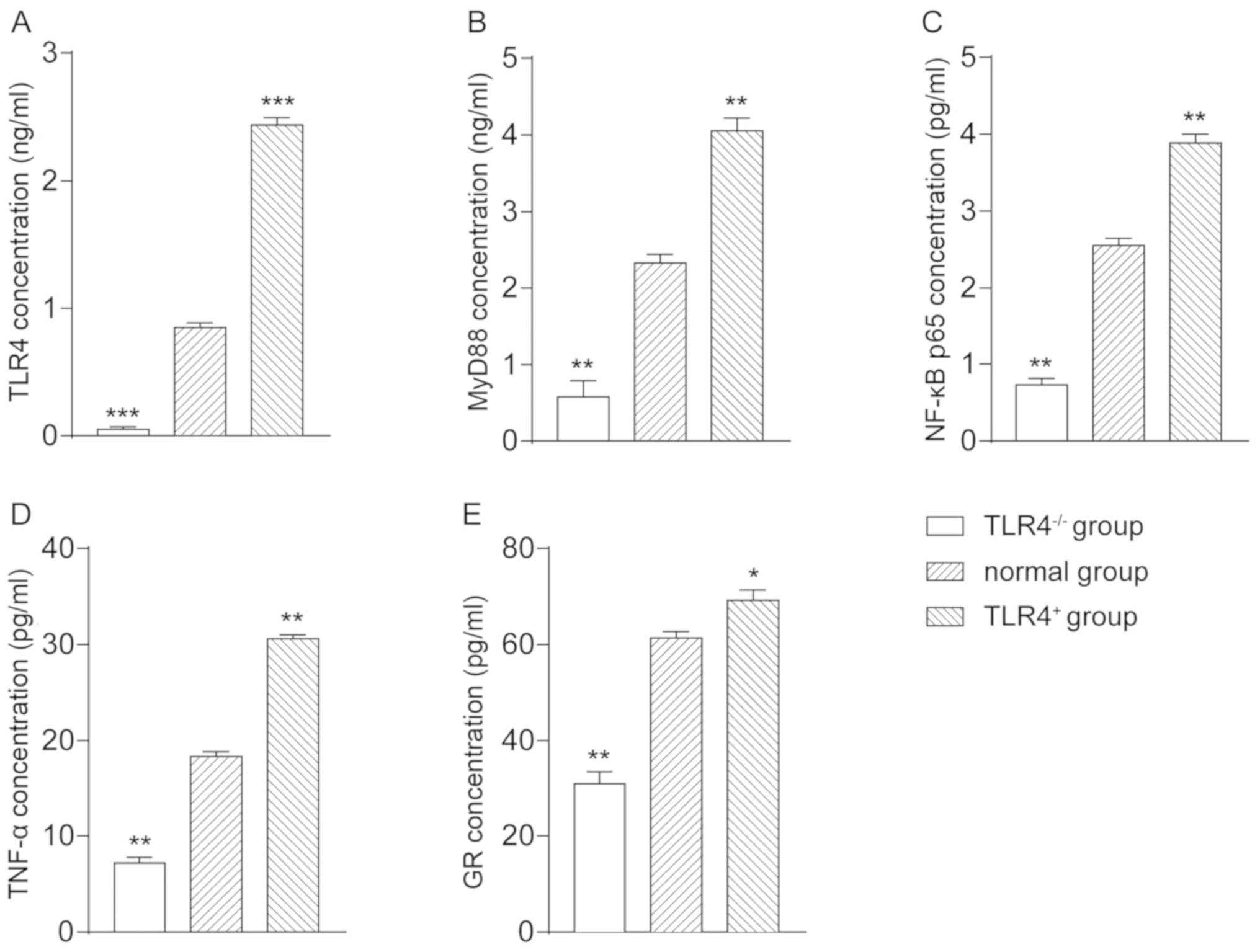 | Figure 2Determination of mass concentration of
TLR4, MyD88, NF-κB p65, TNF-α, and GR in each group of A549 cells
via ELISA. Mass concentration of (A) TLR4, (B) MyD88, (C) NF-κB
p65, (D) TNF-α, and (E) GR in each group of cells.
*P<0.05, **P<0.01,
***P<0.001 vs. normal group. TLR4, Toll-like receptor
4; MyD88, myeloid differentiation factor 88; TNF-α, tumor necrosis
factor-α; GR, glucocorticoid receptor. |
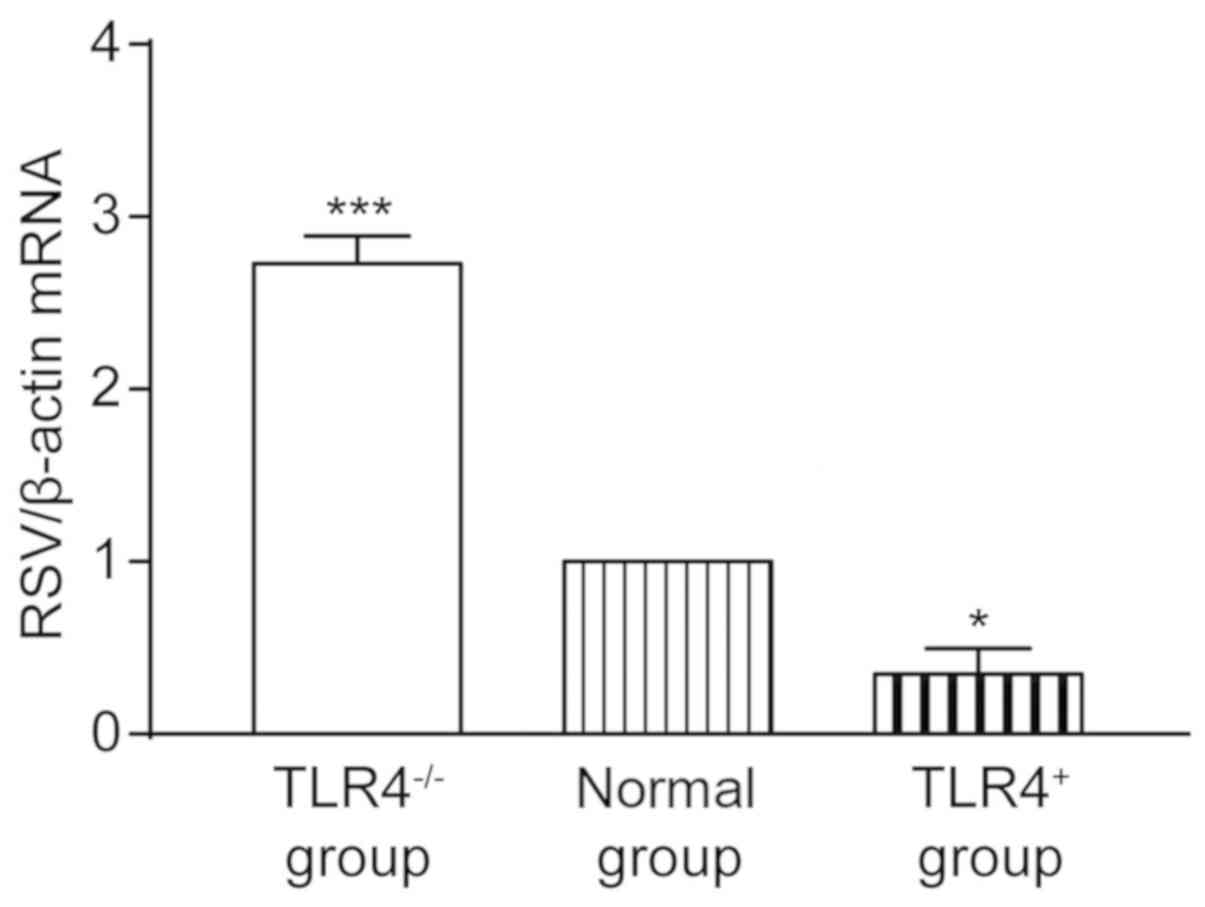
Gene expression of TLR4, MyD88, NF-κB
p65, GR and mRNA expression of RSV in human AEC Ⅱ
The relative expression of RSV in TLR4-/-
group was 2.729±0.159, which was significantly higher than that in
the normal group (P<0.001). The relative expression of RSV in
the TLR4+ group was 0.348±0.345, which was lower than
that in the normal group, with a statistically significant
difference (P<0.05) (Fig. 3). The
results indicated that the expression of TLR4 could inhibit the
replication of RSV in A549 cells.
The gene expression of TLR4 in the RSV group was
5.234±0.104 and in the RSV+MP group was 2.935±0.312, both of which
were increased to different degrees compared with the TLR4
expression in the control group, presenting statistically
significant differences (P<0.001 and <0.01, respectively).
The degree of increase in the RSV+MP group was lower than that in
the RSV group, with a statistically significant difference
(P<0.01) (Fig. 4A). The gene
expression of MyD88 in the RSV group was 3.123±0.241 and in the
RSV+MP group was 1.678±0.192, both of which were increased to
different degrees compared with the MyD88 expression in the control
group, presenting statistically significant differences (P<0.001
and <0.05, respectively). The degree of increase in the RSV+MP
group was lower than that in the RSV group, with a statistically
significant difference (P<0.05) (Fig.
4B). The gene expression of NF-κB p65 in the RSV group was
2.951±0.235 and in the RSV+MP group was 1.791±0.157, both of which
were increased to different degrees compared with the NF-κB p65
expression in the control group, presenting statistically
significant differences (P<0.001 and <0.01, respectively).
The degree of increase in the RSV+MP group was lower than that in
the RSV group, with a statistically significant difference
(P<0.05) (Fig. 4C). The gene
expression of GR in the RSV group was 0.4811±0.086, which was
significantly decreased compared with that in the control group
(P<0.001), and in the RSV+MP group was 0.891±0.057, which was
significantly increased compared with that in the RSV group
(P<0.01) (Fig. 4D). The results
showed that the infection with RSV could lead to increased
expression of TLR4, MyD88 and NF-κB p65, and to a decreased
expression of GR, whereas MP could reverse this phenomenon.
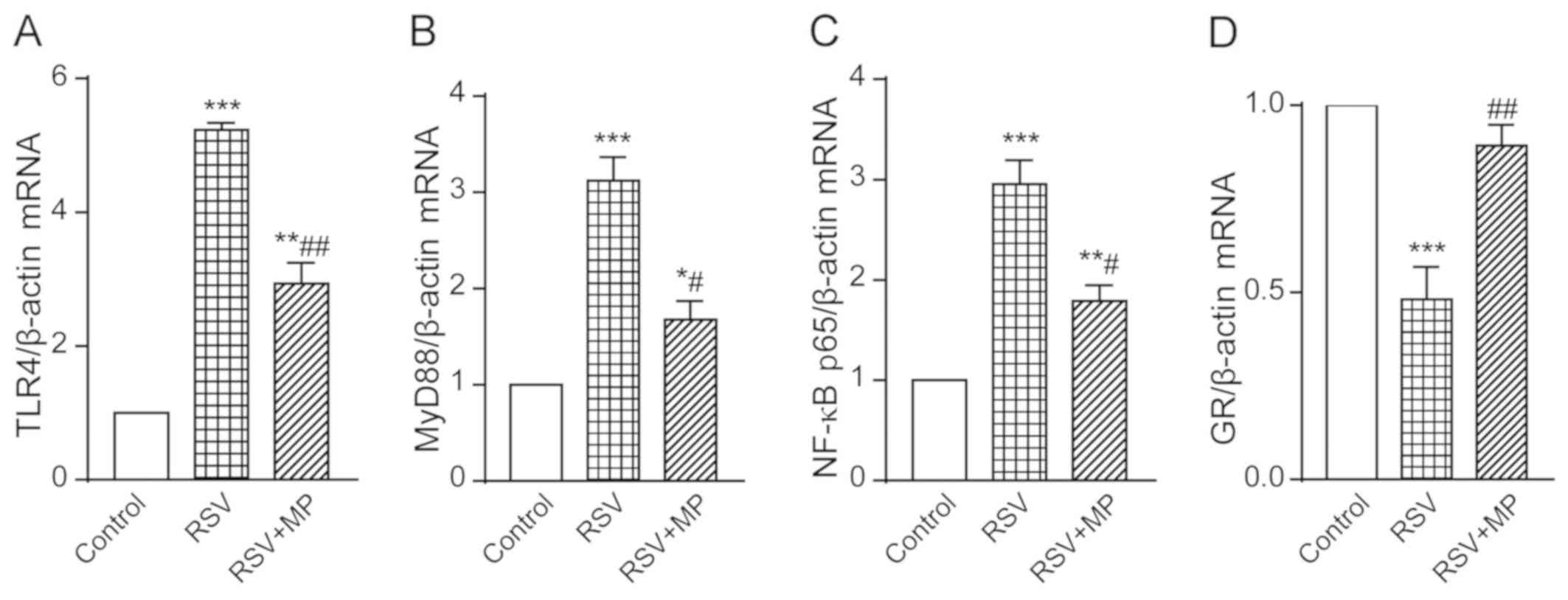 | Figure 4Determination of TLR4, MyD88, NF-κB
p65, and GR gene expression in A549 cells of the control group, RSV
group and RSV+MP group using RT-qPCR. Gene expression levels of (A)
TLR4, (B) MyD88, (C) NF-κB, and (D) GR in each group.
*P<0.05, **P<0.01,
***P<0.001 vs. control; #P<0.05,
##P<0.01 vs. RSV. TLR4, Toll-like receptor 4; MyD88,
myeloid differentiation factor 88; GR, glucocorticoid receptor;
RSV, respiratory syncytial virus; MP, methylprednisolone. |
Protein expression of TLR4, MyD88,
NF-κB p65 and GR in human AEC Ⅱ
The protein expression of TLR4 in the control group
was 0.161±0.026, in the RSV group was 0.568±0.048, and in the
RSV+MP group was 0.332±0.037. The protein expression of MyD88 in
the control group was 0.104±0.056, in the RSV group was
0.538±0.046, and in the RSV+MP group was 0.304±0.054. The protein
expression of NF-κB p65 was 0.055±0.284 in the control group,
0.432±0.059 in the RSV group, and 0.097±0.013 in the RSV+MP group.
The protein expression of GR in the control group was 0.868±0.125,
in the RSV group was 0.147±0.052, and in the RSV+MP group was
0.488±0.086. The protein expression levels of TLR4, MyD88 and NF-κB
p65 in the RSV and RSV+MP groups were significantly higher than
those in the control group, with statistically significant
differences (P<0.05 and <0.001). The increase in the RSV+MP
group was lower than that in the RSV group, with statistically
significant differences (P<0.05 and <0.01). The protein
expression of GR in the RSV and RSV+MP groups was significantly
lower than that in the control group (P<0.05 and <0.001).
There was a significant difference in the expression of GR protein
between the RSV+MP and RSV groups (P<0.05) (Fig. 5).
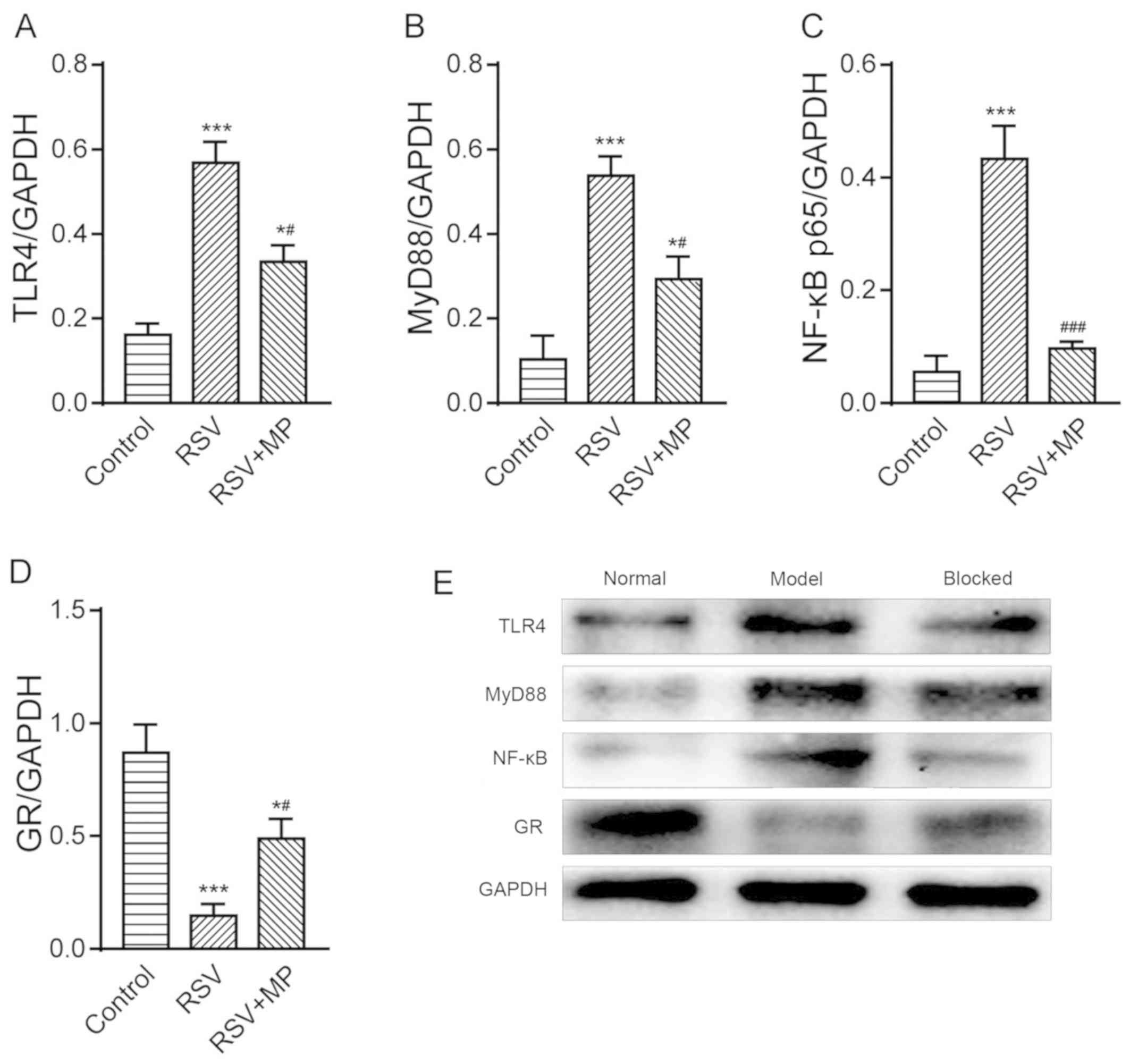 | Figure 5Determination of TLR4, MyD88, NF-κB
p65 and GR protein expression in A549 cells of the control group,
RSV group and RSV+MP group using western blot analysis. Protein
expression levels of (A) TLR4, (B) MyD88, (C) NF-κB, and (D) GR in
each group. (E) Western blots. *P<0.05 and
***P<0.001 vs. control; #P<0.05,
###P<0.01 vs. RSV. TLR4, Toll-like receptor 4; MyD88,
myeloid differentiation factor 88; GR, glucocorticoid receptor;
RSV, respiratory syncytial virus; MP, methylprednisolone. |
Discussion
Viral pneumonia is a common respiratory disease, and
RSV is the most important pathogen of the viral lower respiratory
tract infections in infants and young children. Delgado et
al (26) have suggested that
infants lack protection, probably due to low antibody affinity for
protective epitopes, and low antibody affinity results in lack of
TLR stimulation. Thus, activation of TLRs plays an important role
in the immune process.
In the present study, the MTT assay results revealed
that knockdown of the TLR4 gene had a certain inhibitory effect on
the proliferation of A549 cells, whereas overexpression could
improve proliferation. In the subsequent ELISA experiment,
overexpression of TLR4 gene increased the concentration of TLR4,
MyD88, NF-κB p65, TNF-α and GR, indicating that the expression of
TLR4 could promote cell survival. RT-qPCR and western blot analysis
results revealed that the levels of TLR4, MyD88 and NF-κB p65 in
RSV and RSV+MP groups were significantly higher than those in the
control group; however, the increase in the RSV+MP group was
significantly lower than that in the RSV group. In the RSV group,
the level of GR was significantly lower than that in the control
group; however, the level of GR in the RSV+MP group was not
significantly different from that in the control group. After
pretreatment with GR activator MP, RSV had a certain reversal
effect on the inhibition of A549 cells, indicating that activation
of GR could alleviate the damage of RSV on A549 cells. It was
speculated that in RSV-infected AEC Ⅱ cells, TLR4 might be the
starting point for initiating antiviral immunity, regulating the
immune response of the body by regulating TLR4/MyD88/NF-κB p65
signaling pathway.
GR is a member of the conserved nuclear receptor
superfamily and a transcription factor which is present in the
cytoplasm of various cells of the body, including α and β
receptors. Among them, α receptor plays a major role (27). Under normal physiological conditions,
GR forms a complex with other proteins, such as heat shock protein
90, preventing the GR receptor from entering the nucleus and
interacting with DNA (28). However,
when GR is combined with GC, the conformation of heat shock protein
90 changes, causing the GR receptor to separate from the complex
and enter the nucleus, resulting in enhanced transcriptional
activity of the target gene and production of corresponding
proteins, such as adhesion molecules. GR can also block the
transcription of related transcription factors and inhibits the
production of related proteins, such as IL-1 and TNF-α. At the same
time, the activation of GR can interact with NF-κB to inhibit the
secretion of inflammatory factors, such as IL-1 and TNF-α in cells
(29). A study by Kamiyama et
al (30) has revealed that GR
also plays an important regulatory part in the inflammatory
response. GR can inhibit the activation of p38 MAPK and prevent the
cascade of downstream inflammatory factors (31,32). GR
can also destroy the downstream product of TLR4, the IRF3, thereby
inhibiting the expression of related inflammatory genes. Therefore,
GR was highly likely to play an important role in the immune
response. In the present study, the experimental results on
concentration and expression levels, suggest that insufficient or
low activity of GR levels could cause a large number of release of
inflammation factors, aggravating the human AEC Ⅱ injury. However,
in the use of preincubation of GR activator MP, the cell damage was
relieved, suggesting that GR was involved in immune response and
could reduce cell damage.
In the present study, the results revealed that RSV
infection of human AEC Ⅱ A549 pretreated with the GR activator MP
could reduce the activation of TLR4 and downregulate the expression
of downstream MyD88, NF-κB p65, and TNF-α, indicating that
upregulation of GR expression is involved in antiviral protection.
TLR4 and GR, as key parts of the overall pathway, can provide a
theoretical basis for the clinical viral infections in the
lungs.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
DW and JW conceived and designed the study,
collected, analyzed and interpreted the experimental data, drafted
the manuscript, and revised it critically for important
intellectual content. Both authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Xuzhou Children's Hospital Affiliated to Xuzhou Medical University
(Xuzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hofer CC, Woods PS and Davis IC: Infection
of mice with influenza A/WSN/33 (H1N1) virus alters alveolar type Ⅱ
cell phenotype. Am J Physiol Lung Cell Mol Physiol. 308:L628–L638.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Liu Y, Kumar VS, Zhang W, Rehman J and
Malik AB: Activation of type Ⅱ cells into regenerative stem cell
antigen-1(+) cells during alveolar repair. Am J Respir Cell Mol
Biol. 53:113–124. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Schmiedl A, Kerber-Momot T, Munder A,
Pabst R and Tschernig T: Bacterial distribution in lung parenchyma
early after pulmonary infection with Pseudomonas aeruginosa.
Cell Tissue Res. 342:67–73. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tumurkhuu G, Dagvadorj J, Jones HD, Chen
S, Shimada K, Crother TR and Arditi M: Alternatively spliced
myeloid differentiation protein-2 inhibits TLR4-mediated lung
inflammation. J Immunol. 194:1686–1694. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hartshorn KL: Role of surfactant protein A
and D (SP-A and SP-D) in human antiviral host defense. Front Biosci
(Schol Ed). 2:527–546. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Ariki S, Nishitani C and Kuroki Y: Diverse
functions of pulmonary collectins in host defense of the lung. J
Biomed Biotechnol. 2012(532071)2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Beutler B: Inferences, questions and
possibilities in Toll-like receptor signalling. Nature.
430:257–263. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Takeuchi O, Hoshino K, Kawai T, Sanjo H,
Takada H, Ogawa T, Takeda K and Akira S: Differential roles of TLR2
and TLR4 in recognition of gram-negative and gram-positive
bacterial cell wall components. Immunity. 11:443–451.
1999.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hoshino K, Tsutsui H, Kawai T, Takeda K,
Nakanishi K, Takeda Y and Akira S: Cutting edge: generation of
IL-18 receptor-deficient mice: evidence for IL-1 receptor-related
protein as an essential IL-18 binding receptor. J Immunol.
162:5041–5044. 1999.PubMed/NCBI
|
|
10
|
Hayashi F, Smith KD, Ozinsky A, Hawn TR,
Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM and Aderem A: The
innate immune response to bacterial flagellin is mediated by
Toll-like receptor 5. Nature. 410:1099–1103. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Heil F, Hemmi H, Hochrein H, Ampenberger
F, Kirschning C, Akira S, Lipford G, Wagner H and Bauer S:
Species-specific recognition of single-stranded RNA via toll-like
receptor 7 and 8. Science. 303:1526–1529. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ricard JD, Dreyfuss D and Saumon G:
Production of inflammatory cytokines in ventilator-induced lung
injury: A reappraisal. Am J Respir Crit Care Med. 163:1176–1180.
2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kawai T, Takeuchi O, Fujita T, Inoue J,
Mühlradt PF, Sato S, Hoshino K and Akira S: Lipopolysaccharide
stimulates the MyD88-independent pathway and results in activation
of IFN-regulatory factor 3 and the expression of a subset of
lipopolysaccharide-inducible genes. J Immunol. 167:5887–5894.
2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Barton GM and Medzhitov R: Toll-like
receptor signaling pathways. Science. 300:1524–1525.
2003.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cyr SL, Angers I, Guillot L,
Stoica-Popescu I, Lussier M, Qureshi S, Burt DS and Ward BJ: TLR4
and MyD88 control protection and pulmonary granulocytic recruitment
in a murine intranasal RSV immunization and challenge model.
Vaccine. 27:421–430. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhou B, Zhou H, Ling S, Guo D, Yan Y, Zhou
F and Wu Y: Activation of PAR2 or/and TLR4 promotes SW620 cell
proliferation and migration via phosphorylation of ERK1/2. Oncol
Rep. 25:503–511. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yan ZQ: Regulation of TLR4 expression is a
tale about tail. Arterioscler Thromb Vasc Biol. 26:2582–2584.
2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Akira S and Takeda K: Toll-like receptor
signalling. Nat Rev Immunol. 4:499–511. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Gui J, Yue Y, Chen R, Xu W and Xiong S:
A20 (TNFAIP3) alleviates CVB3-induced myocarditis via inhibiting
NF-κB signaling. PLoS One. 7(e46515)2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Janssen R, Pennings J, Hodemaekers H,
Buisman A, van Oosten M, de Rond L, Oztürk K, Dormans J, Kimman T
and Hoebee B: Host transcription profiles upon primary respiratory
syncytial virus infection. J Virol. 81:5958–5967. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lizundia R, Sauter KS, Taylor G and
Werling D: Host species- specific usage of the TLR4-LPS receptor
complex. Innate Immun. 14:223–231. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Baschant U and Tuckermann J: The role of
the glucocorticoid receptor in inflammation and immunity. J Steroid
Biochem Mol Biol. 120:69–75. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wei SD, Li JZ, Liu ZJ, Chen Q, Chen Y,
Chen M and Gong JP: Dexamethasone attenuates
lipopolysaccharide-induced liver injury by downregulating
glucocorticoid-induced tumor necrosis factor receptor ligand in
Kupffer cells. Hepatol Res. 41:989–999. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Pizzi M: Sampling variation of the fifty
percent end-point, determined by the Reed-Muench (Behrens) method.
Hum Biol. 22:151–190. 1950.PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Delgado MF, Coviello S, Monsalvo AC,
Melendi GA, Hernandez JZ, Batalle JP, Diaz L, Trento A, Chang HY,
Mitzner W, et al: Lack of antibody affinity maturation due to poor
Toll-like receptor stimulation leads to enhanced respiratory
syncytial virus disease. Nat Med. 15:34–41. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Caratti G, Matthews L, Poolman T and
Kershaw S: Baxter MA and Ray D: Glucocorticoid receptor function in
health and disease. Clin Endocrinol (Oxf). 4:185–193. 1997.
|
|
28
|
Oakley RH, Jewell CM, Yudt MR, Bofetiado
DM and Cidlowski JA: The dominant negative activity of the human
glucocorticoid receptor beta isoform. Specificity and mechanisms of
action. J Biol Chem. 274:27857–27866. 1999.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zen M, Canova M, Campana C, Bettio S,
Nalotto L, Rampudda M, Ramonda R, Iaccarino L and Doria A: The
kaleidoscope of glucorticoid effects on immune system. Autoimmun
Rev. 10:305–310. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kamiyama K, Matsuda N, Yamamoto S, Takano
K, Takano Y, Yamazaki H, Kageyama S, Yokoo H, Nagata T, Hatakeyama
N, et al: Modulation of glucocorticoid receptor expression,
inflammation, and cell apoptosis in septic guinea pig lungs using
methylprednisolone. Am J Physiol Lung Cell Mol Physiol.
295:L998–L1006. 2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Bhattacharyya S, Brown DE, Brewer JA, Vogt
SK and Muglia LJ: Macrophage glucocorticoid receptors regulate
Toll-like receptor 4-mediated inflammatory responses by selective
inhibition of p38 MAP kinase. Blood. 109:4313–4319. 2007.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Vollmer TR, Stockhausen A and Zhang JZ:
Anti-inflammatory effects of mapracorat, a novel selective
glucocorticoid receptor agonist, is partially mediated by MAP
kinase phosphatase-1 (MKP-1). J Biol Chem. 287:35212–35221.
2012.PubMed/NCBI View Article : Google Scholar
|