Introduction
Neuropathic pain is one of the most common
categories of chronic pain and sensory dysfunction affecting a
considerable proportion of the global population, negatively
influencing their emotional health and overall quality of life
(1). The prevalence of trauma
related to the peripheral nervous system in the United States is
1.3-2.8% (2). Neuropathic pain is
characterized by a response to non-noxious stimuli (tactile
allodynia), spontaneous pain (exaggerated pain response to normal
painful stimulus), hyperalgesia and loss of sensation in local
areas (3,4). It can be triggered or initiated in the
peripheral or central nervous system (5). Owing to the complex etiology of
neuropathic pain, it is considered to be one of the most
challenging pathologies to treat in clinical practice (6). At present, most pain medications to
treat neuropathic pain are not satisfactory, and cause undesirable
side effects. Therefore, it is necessary to find an effective
therapy with minimal side effects.
It is well known that α2-adrenoceptor
(α2-AR) agonists have antinociceptive effects in the
spinal cord (7). α2-ARs
are located not only in the central nervous system, but also in the
peripheral nervous system (8). The
complex processes involved in α2-AR pain regulation have
been extensively researched. A previous study demonstrated that
α2-AR agonists could relieve nerve injury-induced pain
by binding to α2-AR in patients and animal models with
neuropathic pain (9). Blockade of
spinal 5-hydroxytryptamine (HT)3 receptors reduced
α2-AR-mediated anti-hypersensitivity by reducing total
GABA release (10). Furthermore, it
is well known that α2-AR agonists can enhance the
analgesic effects of morphine (11).
Recently, the α2-AR antagonists atipamezole and idazoxan
have been shown to block the induction of tolerance to morphine,
which was verified through intrathecal atipamezole in opioid naïve
and tolerant rats weakening the anti-nociceptive effect of morphine
(12).
Baicalin is a common flavonoid substance isolated
from the root of Scutellaria baicalensis Georgi. Previous
studies have demonstrated that baicalin possesses
anti-inflammatory, antioxidant, antitumor and antiallergy
properties (13-15).
Furthermore, Chou et al (16)
established the model of carrageenan-evoked thermal hyperalgesia in
rats and found that baicalin had a clear analgesic effect. A
previous study also demonstrated that baicalin helps relieve pain
in patients suffering from osteoarthritis of the knee (17).
In this study, it was determined whether baicalin
may reduce pain in a spinal nerve ligation rat model of neuropathic
pain, and the roles of the peripheral α2-AR subtypes in
the mechanism of action of baicalin were investigated.
Materials and methods
Animals
Male Sprague Dawley rats [Beijing Vital River
Laboratory Animal Technology Co., Ltd.; Charles River Laboratories,
Inc.; license no. SCXK9 (Beijing) 20160006; weight, 150-200 g; age,
3 months] were housed at 22-24˚C and 50-65% humidity on a 12 h
light/dark cycle and were provided with free access to food and
water. All experiments in this study were conducted in accordance
with the National Institute of Health Guide for the Care and Use of
Laboratory Animals. The procedures were all approved by the Animal
Ethics Committee of China Pharmaceutical University (Nanjing,
Jiangsu, China), where the study was carried out.
Neuropathic pain model
Animals were anesthetized with halothane, 1-3% in
oxygen, with spontaneous ventilation. A 3 cm paramedian incision
was made in the left L4-sacral area, and a bundle of paraspinal
muscles was removed to visualize the left L6 transverse process.
Using small scissors, the left L6 transverse process was removed
completely and the L4-L5 spinal nerves were exposed. After the L4
spinal nerve was separated, the L5 spinal nerve was cut and spread
laterally. The fascia and skin were closed using sutures, and the
animals were allowed to recover for 10 days prior to the epidural
catheterization. Paw withdrawal mechanical threshold (PWT) <4 g
after surgery was recognized as the standard of neuropathic pain
induction.
Drugs administration
A total of 70 rats were randomly divided into the
following groups: Control group (n=10); saline group (n=10);
baicalin group (n=10); baicalin combined α2-AR
antagonist groups (n=40). The α2-AR antagonist group was
subcategorized in to four groups based on the antagonist used,
which included the nonselective α2-AR antagonist
idazoxan (n=10); α2a-AR antagonist BRL 44408 (n=10);
α2b-AR antagonist ARC 239 (n=10); and α2c-AR
antagonist JP 1302 (n=10). The rats were treated with 20 mg/kg
baicalin by intrathecal injection. The drugs used in the study were
purchased from Tocris Bioscience. Idazoxan was dissolved in
distilled water, and the other drugs were dissolved in
physiological saline. All drugs were delivered in a volume of 2
µg/20 µl administered by intrathecal injection. In the control
group, the rats did not undergo any surgery. The rats in saline
group were injected with physiological saline (10 µl). Drugs were
administered once per day for 7 days.
Behavioral tests
The PWT was measured by the up and down method
(18). A series of von Frey
filaments (0.4, 0.7, 1.2, 2.0, 3.6, 5.5, 8.5 and 15 g) in a
perpendicular fashion were used to stimulate the surface of the
lateral paw. Each was applied until slightly bent and held for
approximately 5 sec. Reponses in the form of sharp withdrawal or
paw licking were regarded as a positive response. Only rats with
marked allodynia (withdrawal threshold <4 g) after spinal nerve
ligation were studied.
Expression of α2a-AR,
α2b-AR, α2c-AR mRNA in the spinal cord
When the final test was completed, three rats from
each experimental group were sacrificed by cervical dislocation
whilst anesthetized to obtain the L4-L5 dorsal spinal cord. Tissue
samples were frozen immediately at -80˚C. Total RNA in spinal cord
tissues was extracted using an RNeasy kit (cat. no. 74104; Qiagen
GmbH) following the manufacturer's instructions. RNA quality and
quantity were measured using a Nanodrop Spectrophotometer (UL-2000;
Macylab Instruments, Inc.), while RNA integrity was assessed by gel
electrophoresis. A total of 500 ng RNA was used to generate cDNA
with a reverse transcription (RT) kit (Takara Biotechnology Co.,
Ltd.). The temperature conditions for the RT procedure were 65˚C
for 10 min, 37˚C for 10 min, 75˚C for 15 min and 37˚C for 20 min. A
Mastercycler® nexus X2 (Eppendorf) was used for
RT-quantitative (q) PCR (Power SYBR™ Green
RNA-to-CT™ 1-step kit, cat. no. 4389986;
Thermo Fisher Scientific, Inc.). The thermocycling conditions were
95˚C for 15 sec followed by 35 cycles of 95˚C for 15 sec and 60˚C
for 1 min. The relative levels of target mRNAs were standardized to
the reference gene β-actin gene. The results were quantified using
the 2-ΔΔCq method (19). Primers for RT-qPCR in this study were
as follows: α2a forward 5'-GCGCCCCAGAACCTCTTCCTGGTG-3',
reverse 5'-CCAGCGCCCTTCTTCTCTATGGAG-3'; α2b forward
5'-AAACGCAGCCACTGCAGAGGTCTC-3', reverse
5'-ACTGGCAACTCCCACATTCTTGCC-3'; α2c forward
5'-CTGGCAGCCGTGGTGGGTTTCCTC-3'; reverse
5'-GTCGGGCCGGCGGTAGAAAGAGAC-3'; and β-actin forward
5'-CGGGAAATCGTGCGTGACAT-3', reverse 5'-GAAGGAAGGCTGGAAGAGTG-3'.
Quantification of inflammatory
mediators in serum
Blood was collected via the tail vein at 72 h after
drug injection. Serum inflammatory mediators, including tumor
necrosis factor (TNF)-α (cat. no. DY510), interleukin (IL)-6, (cat.
no. DY506), IL-17 (cat. no. DY4437) and IL-1β (cat. no. DY401) were
tested with ELISA kits supplied by R&D Systems China Co., Ltd.
according to the manufacturers' instructions.
Histological evaluation
Spinal cord tissues were fixed with 4%
paraformaldehyde (Beijing Solarbio Science & Technology Co.,
Ltd.) at 37˚C for 24 h, dehydrated and then embedded in paraffin
wax. The tissues were then cut into 5-µm-thick sections, and
stained with hematoxylin and eosin solution (Fuzhou Maixin Biotech
Co., Ltd.) at 37˚C for 5 min. Pathological changes were observed
under a light microscope (magnification, x400; Nikon
Corporation).
Flow cytometry analyses
The peripheral blood mononuclear cells (PBMC) were
separated from blood following the Ficoll 400 and uropolinum
density centrifugation method (20).
The T-lymphocyte subset CD4+ was identified by flow
cytometry analysis of cells isolated from the PBMCs. PBMCs adjusted
to a density of 1x106 cells/ml in complete medium were
used for analysis. Phytohemagglutinin solution (25 µl; Cylex, Inc.)
was added to block non-specific binding and the cells were
incubated for 15-18 h at 37˚C and 5% CO2. The frequency
of the T-lymphocyte subset was evaluated after staining with the
FITC-conjugated mouse anti-rat CD4 (1:100; cat. no. FAB554F, BD
Biosciences) at 4˚C for 25 min. After washing, cells were incubated
with the pacific blue-A fluorochrome-conjugated isotype control
(1:100; cat. no. A10478, Thermo Fisher Scientific, Inc.), to gate
nonspecific fluorescence signals, at 4˚C for 25 min. Data were
analyzed using FlowJo software (version 7.2.5; FlowJo LLC).
Statistical analysis
Statistical analysis was implemented using SPSS 20.0
(IBM Corp.). Statistical comparisons between groups were analyzed
using one-way ANOVAs followed by Duncan multiple range post hoc
tests. All results are reported as the mean ± SD. P<0.05 was
considered to indicate a statistically significant difference. Each
test was repeated 3 times.
Results
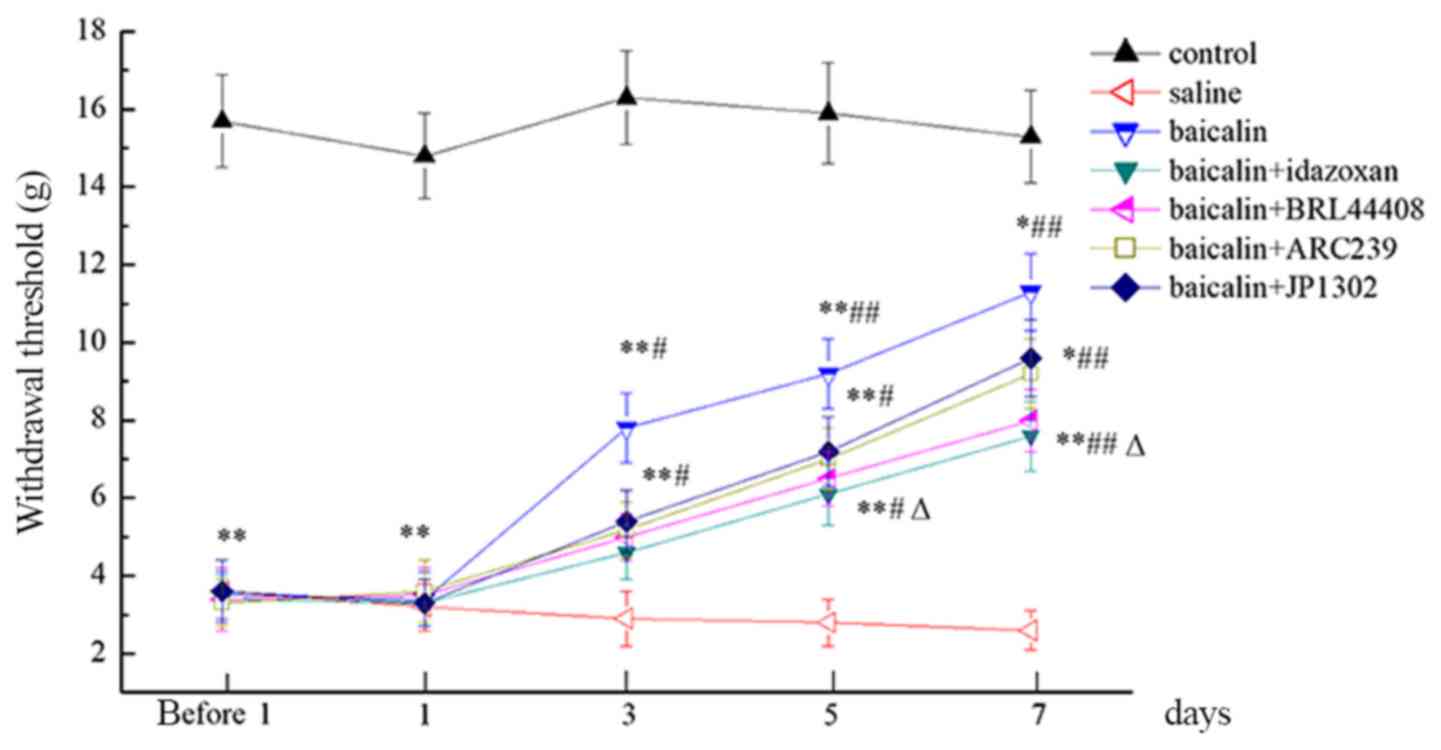
Baicalin increases the PWT
Compared with the control group, a significant
decrease in PWT was observed in all treatment groups (P<0.05;
Fig. 1). After baicalin treatment,
PWT increased, but the antiallodynic effect of baicalin was
antagonized by intrathecal injection of α2-AR
antagonists. PWT was reduced after 5 days of idazoxan
administration when compared to baicalin group (P<0.05).
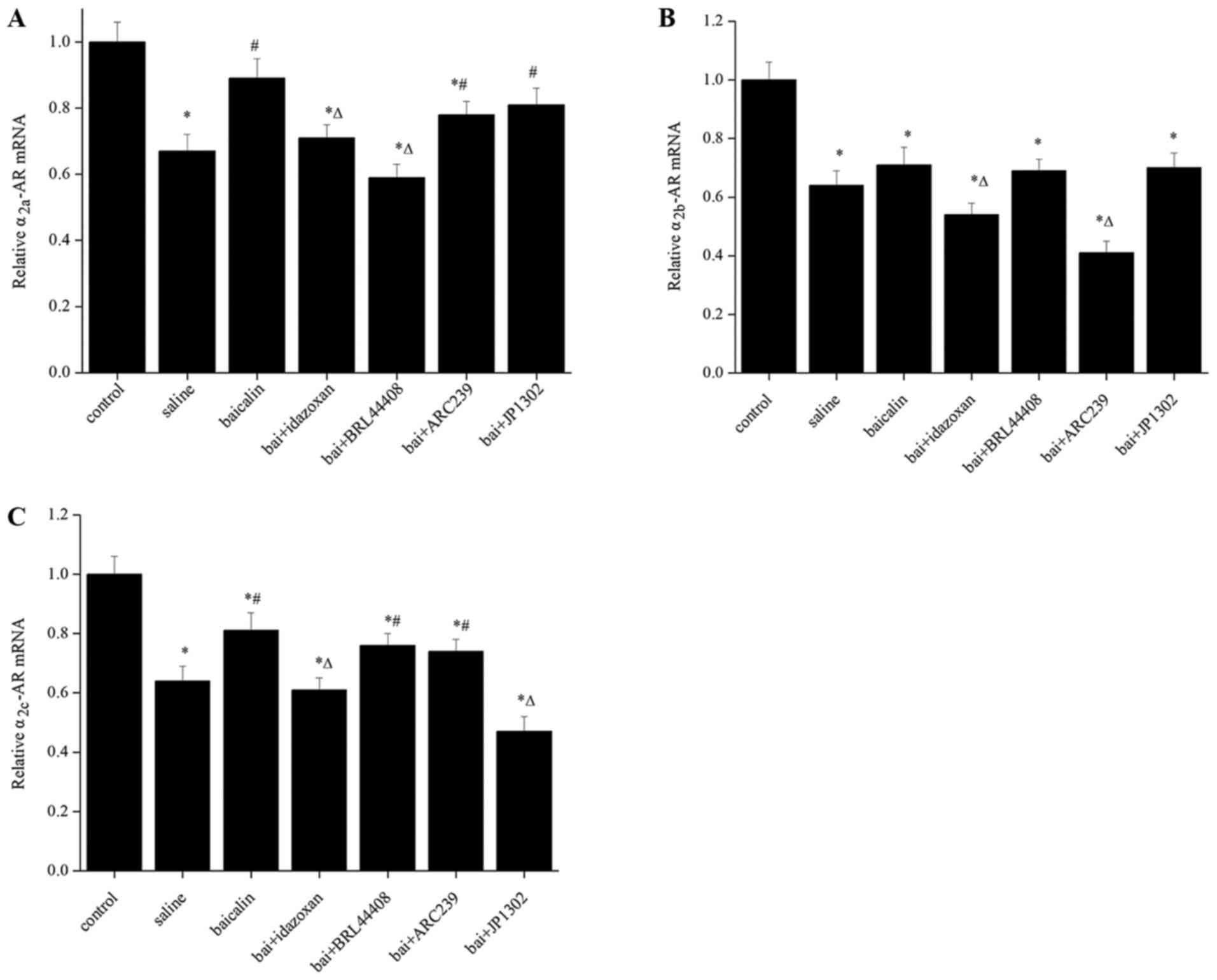
Baicalin contributes to the increase
in α2-AR mRNA
The expression of α2a-AR,
α2b-AR and α2c-AR mRNA were significantly
downregulated in the saline group, compared with the control group
(P<0.05; Fig. 2). Intrathecal
administration of baicalin upregulated the levels of
α2-AR mRNA, especially the levels of α2a-AR
and α2c-AR mRNA (P<0.05; Fig. 2A and C). Compared with baicalin group, the levels
of α2a-AR, α2b-AR and α2c-AR mRNA
were significantly reduced after the administration of idazoxan
(P<0.05). The antagonist BRL 44408 markedly reduced
α2a-AR mRNA expression when compared with the baicalin
group (P<0.05; Fig. 2A). The
antagonist ARC239 markedly reduced α2b-AR mRNA when
compared with the baicalin group (P<0.05; Fig. 2B). The antagonist JP1302 markedly
reduced α2c-AR mRNA expression compared with the
baicalin group (P<0.05; Fig.
2C).
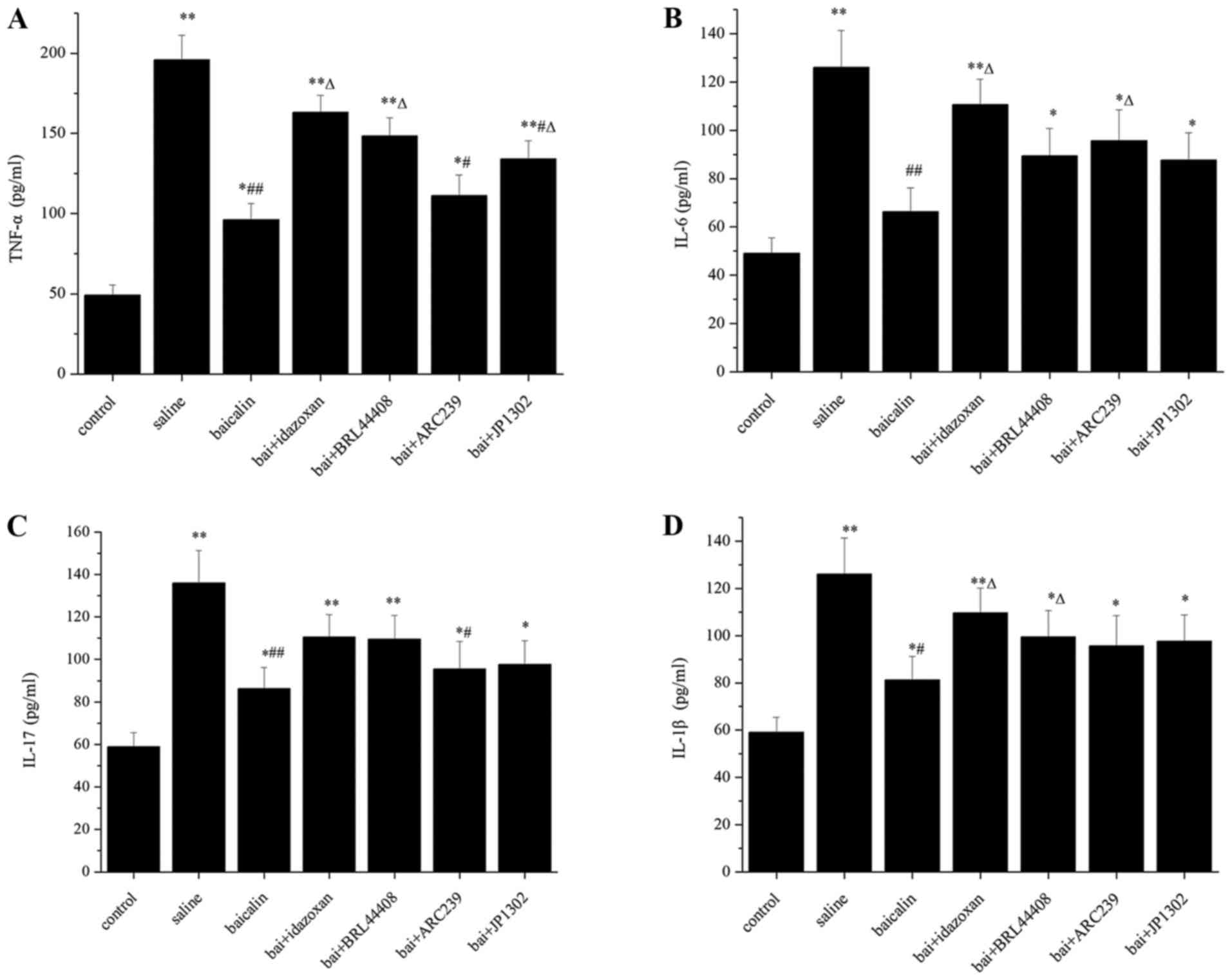
Baicalin decreases the levels of
TNF-α, IL-6, IL-17 and IL-1β in the serum
As shown in Fig. 3,
the levels of TNF-α, IL-6, IL-17 and IL-1β in the saline group were
all markedly higher than those in the control group (P<0.05).
Intrathecal administration of baicalin significantly reduced the
levels of TNF-α, IL-6, IL-17 and IL-1β when compared to the saline
group (P<0.05). TNF-α, IL-6 and IL-1β release was significantly
increased with idazoxan treatment compared with baicalin group
(P<0.05; Fig. 3A, B and D).
TNF-α release was also significantly increased after treatment with
BRL44408 and JP1302 (P<0.05; Fig.
3A). Treatment with ARC239 also significantly increased IL-6
release (P<0.05; Fig. 3B).
Treatment with BRL44408 also increased IL-1β release (P<0.05;
Fig. 3C).
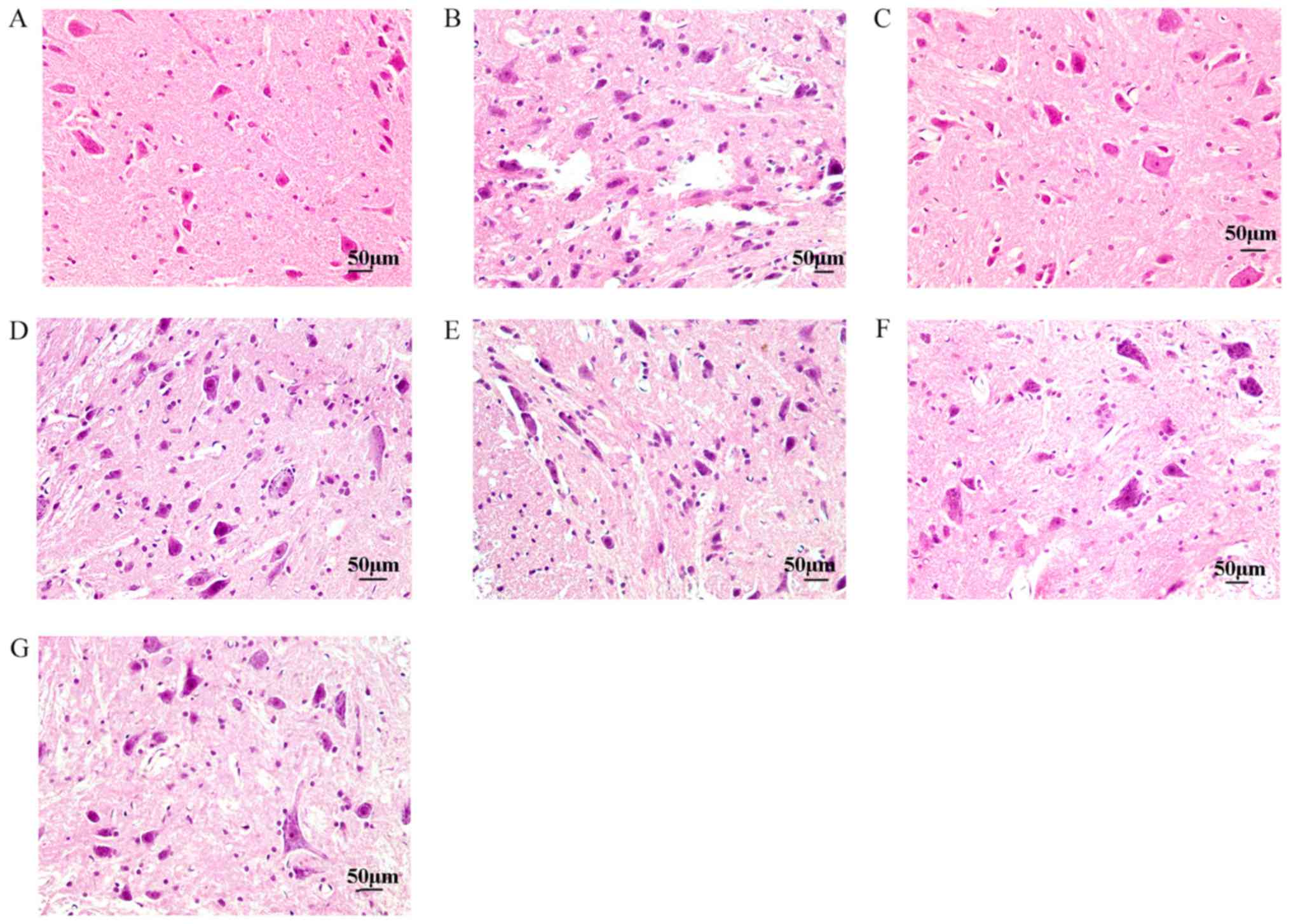
Histopathological changes in spinal
cord tissue
As shown in Fig. 4A,
the distribution of spinal cord neurons was orderly, and the nuclei
were clearly visible in the control group. However, the number of
neurons decreased and the distribution of neurons was uneven in the
saline group (Fig. 4B). Compared
with the saline group, baicalin treatment decreased neuronal
apoptosis and reversed the pathomorphology (Fig. 4C). Intrathecal administration of
different α2-AR antagonists reversed the effects of the
baicalin treatment (Fig. 4D-G).
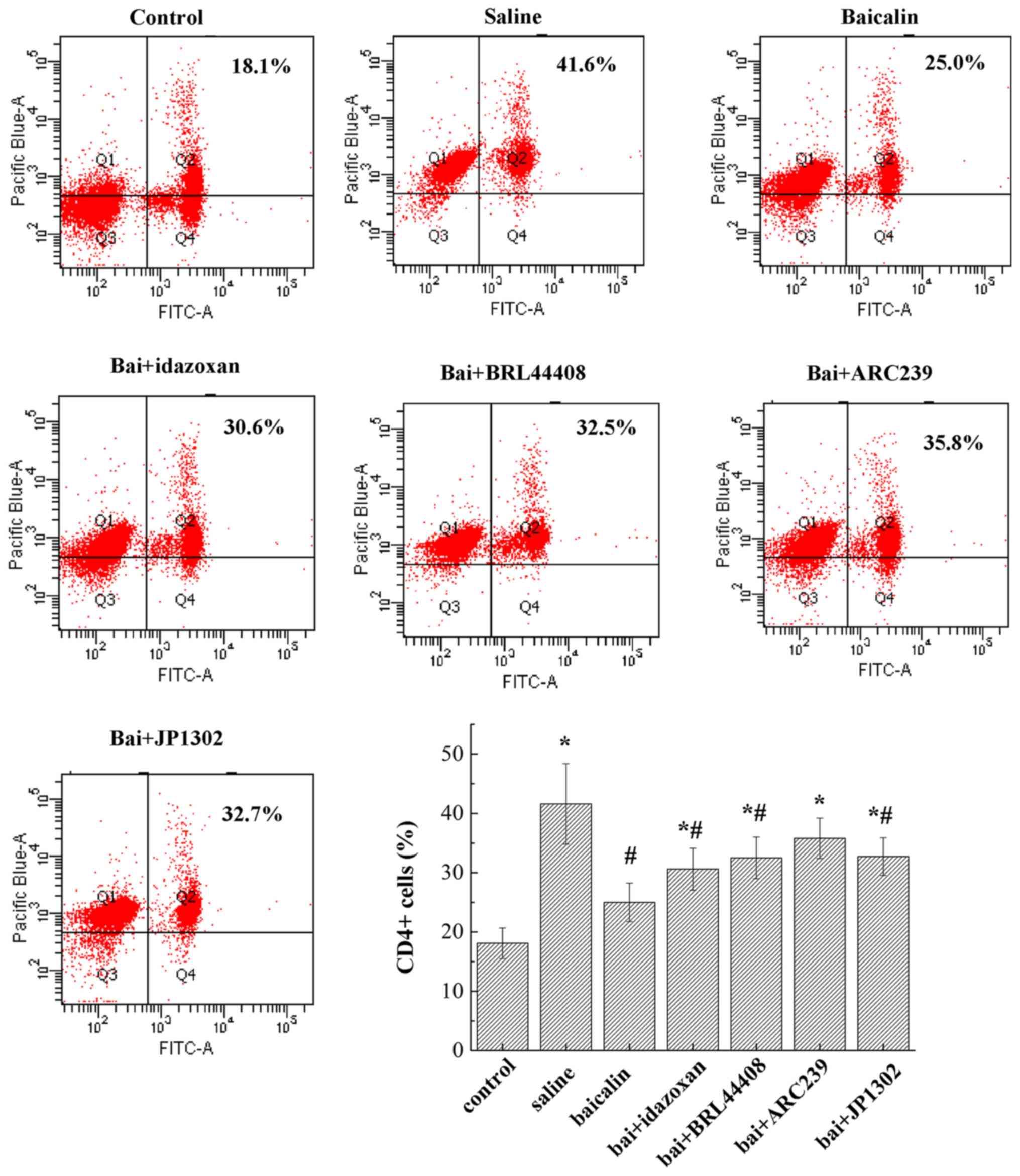
Baicalin decreases the expression of
CD4+ cells
To analyze the effects of α2-AR
expression on CD4+ T cells, the percentage of
CD4+ PBMCs was measured by flow cytometric analysis
(Fig. 5). The results showed that
the frequency of CD4+ cells was significantly increased
in rats following spinal nerve injury (P<0.05). Compared with
the saline group, baicalin treatment suppressed the frequency of
CD4+ cells (P<0.05). The administration of different
α2-AR antagonists appeared to increase the number of
CD4+ cells compared with that in baicalin group but this
difference was not significant.
Discussion
Previous studies have shown that peripheral
administration of an α2-AR agonist attenuates
nociceptive responses in both control animals and hypersensitive
animals under neuropathic conditions (20,21). The
results of this present study showed that intrathecal injection of
baicalin attenuated neuropathic pain induced by spinal cord
ligation, and the antiallodynic effects of baicalin were attenuated
by α2-AR antagonists. This present study revealed that
baicalin relieved pain by reducing inflammation, and this
beneficial effect may be associated with the expression of
α2-AR in spinal cord.
A previous study reported that norepinephrine and
other α2a-AR agonists decreased the release of glutamate
in healthy rat dorsal horn synaptosomes, and had analgesic as well
as anti-sympathetic effects (22).
α2c-AR was recognized to contribute to spinal cord
analgesia induced by α2 adrenoreceptor agonists
(23). Blockade of spinal 5-HT3
receptors reduced α2-adrenoceptor-mediated
anti-hypersensitivity via reducing total GABA release (24). α2-AR stimulation induces
Gs-mediated acetylcholine release in the dorsal horn after
peripheral nerve injury (25). In
this present study, the expression levels of α2-ARs were
changed in spinal nerve injury rats, compared to untreated rats.
Notably, intrathecal administration baicalin increased
α2a and α2c-AR mRNA. These results indicated
that baicalin relieved the pain by regulating α2-AR mRNA
levels.
There is increasing evidence demonstrating that
neuro- inflammation is one of the pivotal contributors to the
development of neuropathic pain. Some pro-inflammatory cytokines
produced by microglia in the spinal cord, such as IL-6, IL-17 and
IL-1β, play an important role in inflammatory processes (26). TNF-α is also a biomarker of acute
neuro-inflammatory responses (27).
The present study showed that the serum levels of TNF-α, IL-6,
IL-17 and IL-1β increased after spinal nerve ligation; however, the
release of TNF-α, IL-6 IL-17 and IL-1β was reduced by intrathecal
administration of baicalin. Furthermore, baicalin treatment
appeared to improve the order of nerve fibers and reduced the
percentage of CD4+ PBMCs. These data suggested that the
baicalin was capable of reducing neuropathic pain by regulating the
inflammatory response.
In conclusion, this present study indicated that
intrathecal administration of baicalin relieved neuropathic pain
following spinal nerve ligation in rats. The mechanisms of action
may be through upregulating the expression of α2-ARs in
the spinal cord. This may suggest that baicalin has therapeutic
potential for neuropathic pain.
Acknowledgements
Not applicable.
Funding
This work was supported by Nature Science Foundation
of Shandong Province (grant no. ZR2015HL029) and Science and
Technology Planning Project of Yantai Urban (grant nos. 2014WS046
and 2015WS070).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LJH and SSJ participated in the design of the study
and XHS, XYL, FFW, WL and QSJ carried out the study and performed
statistical analysis. LJH, and SSJ drafted the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments in this study were conducted in
accordance with the National Institute of Health Guide for the Care
and Use of Laboratory Animals. The procedures were all approved by
animal Ethics Committee of China Pharmaceutical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhao Y, Xin Y and Chu H: MC4R is involved
in neuropathic pain by regulating JNK signaling pathway after
chronic constriction injury. Front Neurosci. 13(919)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Matias Júnior I, Medeiros P, de Freita RL,
Vicente-César H, Ferreira Junior JR, Machado HR and Menezes-Reis R:
Effective parameters for gait analysis in experimental models for
evaluating peripheral nerve injuries in rats. Neurospine.
16:305–316. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yao C, Zhou X, Zhao B, Sun C, Poonit K and
Yan H: Treatments of traumatic neuropathic pain: A systematic
review. Oncotarget. 8:57670–57679. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Taheri A, Lajevardi M, Emami S, Shabani S
and Sharifi H: Commentary: Non-invasive brain stimulation, a tool
to revert maladaptive plasticity in neuropathic pain. Front Hum
Neurosci. 11(172)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mankowski C, Poole CD, Ernault E, Thomas
R, Berni E, Currie CJ, Treadwell C, Calvo JI, Plastira C,
Zafeiropoulou E and Odeyemi I: Effectiveness of the capsaicin 8%
patch in the management of peripheral neuropathic pain in european
clinical practice: The ASCEND study. BMC Neurol.
17(80)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
D'Arcy Y, McCarberg B, Parsons B, Behar R,
Thorpe A and Alexander A: Pregabalin for the treatment of
neuropathic pain: A narrative review for primary care providers.
Curr Med Res Opin. 33:1353–1359. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang YX, Mao XF, Li TF, Gong N and Zhang
MZ: Dezocine exhibits antihypersensitivity activities in neuropathy
through spinal µ-opioid receptor activation and norepinephrine
reuptake inhibition. Sci Rep. 7(43137)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Di Cesare Mannelli L, Micheli L, Crocetti
L, Giovannoni MP, Vergelli C and Ghelardini C: α2 adrenoceptor: A
target for neuropathic pain treatment. Mini Rev Med Chem.
17:95–107. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li C, Ji BU, Kim Y, Lee JE, Kim NK, Kim ST
and Koo S: Electroacupuncture enhances the antiallodynic and
antihyperalgesic effects of milnacipran in neuropathic rats. Anesth
Analg. 122:1654–1662. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Romero-Sandoval EA, McCall C and Eisenach
JC: Alpha2-adrenoceptor stimulation transforms immune responses in
neuritis and blocks neuritis-induced pain. J Neurosci.
25:8988–8994. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chabot-Doré AJ, Millecamps M, Naso L,
Devost D, Trieu P, Piltonen M, Diatchenko L, Fairbanks CA, Wilcox
GL, Hébert TE and Stone LS: Dual allosteric modulation of opioid
antinociceptive potency by α2A-adrenoceptors. Neuropharmacology.
99:285–300. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hughes S, Hickey L, Donaldson LF, Lumb BM
and Pickering AE: Intrathecal reboxetine suppresses evoked and
ongoingneuropathic pain behaviours by restoring spinal
noradrenergic inhibitory tone. Pain. 156:328–334. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lin CC and Shieh DE: The anti-inflammatory
activity of scutellaria rivularis extracts and its active
components, baicalin, baicalein and wogonin. Am J Chin Med.
24:31–36. 1996.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Boadas-Vaello P, Vela JM and Verdu E: New
pharmacological approaches using polyphenols on the physiopathology
of neuropathic pain. Curr Drug Targets. 18:160–173. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cherng CH, Lee KC, Chien CC, Chou KY,
Cheng YC, Hsin ST, Lee SO, Shen CH, Tsai RY and Wong CS: Baicalin
ameliorates neuropathic pain by suppressing HDAC1 expression in the
spinal cord of spinal nerve ligation rats. J Formos Med Assoc.
113:513–520. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chou TC, Chang LP, Li CY, Wong CS and Yang
SP: The antiinflammatory and analgesic effects of baicalin in
carrageenan-evoked thermal hyperalgesia. Anesth Analg.
97:1724–1729. 2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Levy RM, Saikovsky R, Shmidt E, Khokhlov A
and Burnett BP: Flavocoxid is as effective as naproxen for managing
the signs and symptoms of osteoarthritis of the knee in humans: A
short-term randomized, double-blind pilot study. Nutr Res.
29:298–304. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chaplan SR, Bach FW, Pogrel JW, Chung JM
and Yaksh TL: Quantitative assessment of tactile allodynia in the
rat paw. J Neurosci Methods. 53:55–63. 1994.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C (T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Listowska M, Glac W, Grembecka B,
Grzybowska M and Wrona D: Changes in blood CD4+T and
CD8+T lymphocytes in stressed rats pretreated
chronically with desipramine are more pronounced after chronic open
field stress challenge. J Neuroimmunol. 282:54–62. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xue ZJ, Shen L, Wang ZY, Hui SY, Huang YG
and Ma C: STAT3 inhibitor WP1066 as a novel therapeutic agent for
bCCI neuropathic pain rats. Brain Res. 1583:79–88. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li X and Eisenach JC: alpha2A-adrenoceptor
stimulation reduces capsaicin-induced glutamate release from spinal
cord synaptosomes. J Pharmacol Exp Ther. 299:939–944.
2001.PubMed/NCBI
|
|
23
|
Donello JE, Guan Y, Tian M, Cheevers CV,
Alcantara M, Cabrera S, Raja SN and Gil DW: A peripheral
adrenoceptor-mediated sympathetic mechanism can transform
stress-induced analgesia into hyperalgesia. Anesthesiology.
114:1403–1416. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hayashida K, Kimura M, Yoshizumi M, Hobo
S, Obata H and Eisenach JC: Ondansetron reverses
anti-hypersensitivity from clonidine in rats following peripheral
nerver injury: Role of γ-amino butyric acid in α2-adrenoceptor and
5-HT3 serotonin receptor analgesia. Anesthesiolgy. 117:389–398.
2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hayashida K and Eisenach JC: Spinal alpha
2-adrenoceptor- mediated analgesia in neuropathic pain reflects
brain-derived nerve growth factor and changes in spinal cholinergic
neuronal function. Anesthesiology. 113:406–412. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kiasalari Z, Rahmani T, Mahmoudi N,
Baluchnejadmojarad T and Roghani M: Diosgenin ameliorates
development of neuropathic pain in diabetic rats: Involvement of
oxidative stress and inflammation. Biomed Pharmacother. 86:654–661.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ghasemzadeh RM, Amin B, Mehri S,
Mirnajafi-Zadeh SJ and Hosseinzadeh H: Anti-inflammatory effects of
ethanolic extract of rosmarinus officinalis L. and rosmarinic acid
in a rat model of neuropathic pain. Biomed Pharmacother.
86:441–449. 2017.PubMed/NCBI View Article : Google Scholar
|