Introduction
Helicobacter pylori (HP) infection is one of
the world's most common infections. HP, a Gram-negative bacteria,
is a human pathogen that is transmitted from human to human, and
causes chronic active gastritis in all colonized subjects. This
infection can lead to peptic ulcers, atrophic gastritis, gastric
adenocarcinoma, and mucosa-associated lymphoid tissue (MALT)
lymphoma. Therefore, HP is considered an infectious disease
regardless of clinical severity (1).
Routes of transmission are considered: direct
contact between subjects (2),
contaminated water sources or food (3-5),
zoonotic transmission and iatrogenic transmission during
endoscopies and dental care (6).
HP, is an intensely studied bacteria and plays a
very important role in the ethiopatogenesis of gastric cancer,
possibly carried by more than half of the world population
(7).
The latest data regarding HP infection in Europe are
from 2018 where it is shown that the lowest infection prevalence
was found in Northern Europe, while the highest was in Eastern and
Southern Europe, up to 84% in Portugal and Poland (8). Another systematic review and
meta-analysis published in 2016 showed that Africa had the highest
pooled prevalence of HP infection (70.1%; 95% CI, 62.6-77.7),
whereas Oceania had the lowest prevalence (24.4%; 95% CI,
18.5-30.4). Among individual countries, the prevalence of HP
infection varied from as low as 18.9% in Switzerland (95% CI,
13.1-24.7) to 87.7% in Nigeria (95% CI, 83.1-92.2) (9).
A review regarding the HP infection prevalence
trends in Europe over time from 1990 to 2014 was published in 2015.
The study reviewed the prevalence of HP across 35 European
countries using surveys of unselected population. It showed that
the prevalence of HP was much lower in northern and western regions
of Europe than in eastern and southern Europe. The review revealed
a significant reduction in the prevalence of HP over time with an
overall mean reduction of 3.1% per year. Statistics showed that HP
prevalence increased over younger age groups, often sharply, but
levelled off in many studies from ages of ~50 years onwards,
especially in areas of high prevalence.
Regarding gastric cancer incidence in Europe, in the
15-year period from 1993 to 2007, there was also a moderate to
large reductions over time in each of the 18 countries in which the
studies were done (10).
The first report on prevalence of HP infection in
Romania was published in 1990(11).
Helicobacter-like organisms were identified using
histological staining from antral gastric mucosa: 72.8% in gastric
ulcer, 69.6% in duodenal ulcer, 69.2% in bulbitis, 61% in chronic
gastritis, 50% in gastric cancer and 34.3% in healthy controls.
Currently there are various methods used for HP
detection in all categories of patients, but it is recommended to
use only those methods that have high specificity and sensitivity.
The IgG anti-HP antibodies have low sensitivity because they can
persist in blood plasma in high levels for many years even after
treatment. Studies show that levels of IgG anti-Hp antibodies in
the serum do not predict the presence of macroscopic gastroduodenal
diseases or the density of HP colonization in HP-infected dyspeptic
patients. In addition, there are some levels which do not allow a
precise determination of HP status (12-15).
Thus, the presence of anti-HP antibodies in the serum indicates
that the patient had contact with the bacteria, without being able
to determine definately whether the infection still exists.
The specific objectives of this study were: i) to
estimate the prevalence of HP exposure in dyspeptic patients who
presented to the hospital from this northwestern region of the
country; ii) to determine the association of the HP exposure with
potential risk factors such as age, sex and the area of residence;
iii) to analyze the epidemiological trends of HP infection
prevalence in a symptomatic population in this region of Romania by
comparing with previous published data.
Patients and methods
Study design and setting
A retrospective study was performed including 414
patients who attended a secondary center, ‘Salvosan Ciobanca’
Medical Center from Zalău, Salaj, Romania between 2014 and
2018.
Participants
Patients with dyspeptic symptoms presented either by
their own initiative or were referred by their general
practitioners for evaluation of the presence of IgG anti-HP
antibodies, were included in the study. There was no exclusion
criteria.
Variables
The main outcome measure was the serology test
result for HP antibodies. As predictors, we collected data on age,
sex, place of residence, and the year of serology test.
Serology analysis
Serology testing was made using
immunochromatographic Laboquiq and Intermedical test kit for IgG
anti-HP antibodies, and a result of >20 was considered positive.
All tests were processed according to the manufacturer's
recommendations. The data were collected from the hospital
archives.
Statistical analysis
Qualitative data are presented by counts and
percentages, and normally distributed continuous data as means and
standard deviation. Associations between qualitative variables were
checked with Chi-square test. Comparisons between two groups
regarding normally distributed continuous data were performed with
independent samples t-test. Tests were presented as two-tailed
P-value, of 0.05 level of confidence. The statistical analysis was
made using the program R Environment for statistical computing and
graphics (R Foundation for Statistical Computing, Vienna, Austria)
version 3.2.1.
Results
Demographic characteristics of the
study population
Of the 414 patients, 42.2% (175 patients) were from
rural areas and 57.7% (239 patients) from urban places. Regarding
sex distribution 63.8% were females (264 persons) and 36.2% males
(150 persons). The mean age of the group was 45.89, ranging from 6
years to 97 years. The patients characteristics and the prevalence
of infection are shown in Table
I.
 | Table IPrevalence of infection and patients
characteristics. |
Table I
Prevalence of infection and patients
characteristics.
| Test | All (n=414) | Positive (n=169) | Negative (n=245) | P-value |
|---|
| Age (years), mean
(SD) | 45.89 (17.24) | 47.22 (15.27) | 44.97 (18.45) | 0.176 |
| Age groups (years), n
(%) | | | | 0.015 |
|
<18 | 16 (3.86) | 3 (1.78) | 13 (5.31) | |
|
18-29 | 59 (14.25) | 18 (10.65) | 41 (16.73) | |
|
30-39 | 85 (20.53) | 35 (20.71) | 50 (20.41) | |
|
40-49 | 92 (22.22) | 41 (24.26) | 51 (20.82) | |
|
50-59 | 71 (17.15) | 40 (23.67) | 31 (12.65) | |
|
60-69 | 47 (11.35) | 14 (8.28) | 33 (13.47) | |
|
≥70 | 44 (10.63) | 18 (10.65) | 26 (10.61) | |
| Sex, n (%) | 18-29: 59/414
(14.25) | | | 0.873 |
|
Female | 264 (63.77) | 107 (63.31) | 157 (64.08) | |
|
Male | 150 (36.23) | 62 (36.69) | 88 (35.92) | |
| Place of residence, n
(%) | | | | 0.604 |
|
Urban | 239 (57.73) | 95 (56.21) | 144 (58.78) | |
|
Rural | 175 (42.27) | 74 (43.79) | 101 (41.22) | |
Prevalence of HP infection
Of the 414 patients, 40.8% (n=169) had positive IgG
anti-HP and 59.2% (n=245) had negative IgG anti-HP.
HP prevalence by sex, age group and
place of residence
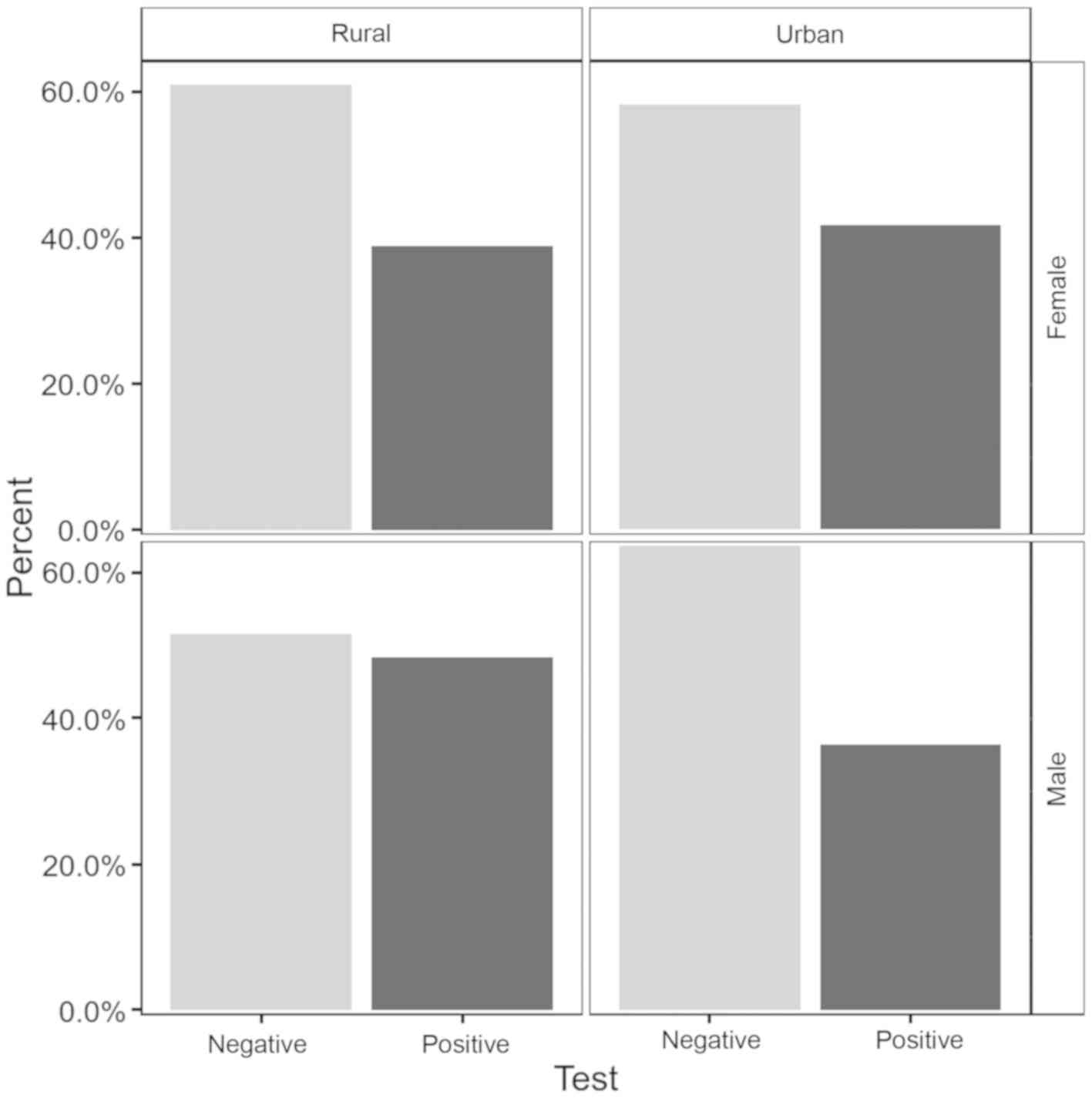
In terms of the positivity of the tests, the
percentages were about the same with a slightly higher values among
males 41.33% versus females 40.53%. The differences were not
statistically significant (P=0.87). There was a higher prevalence
of the positivity in the rural area (42.29%) versus (39.75%) in the
urban area, but with no statistically significant differences
(P=0.6). The prevalence of infection according to sex and place of
residence is displayed in Fig.
1.
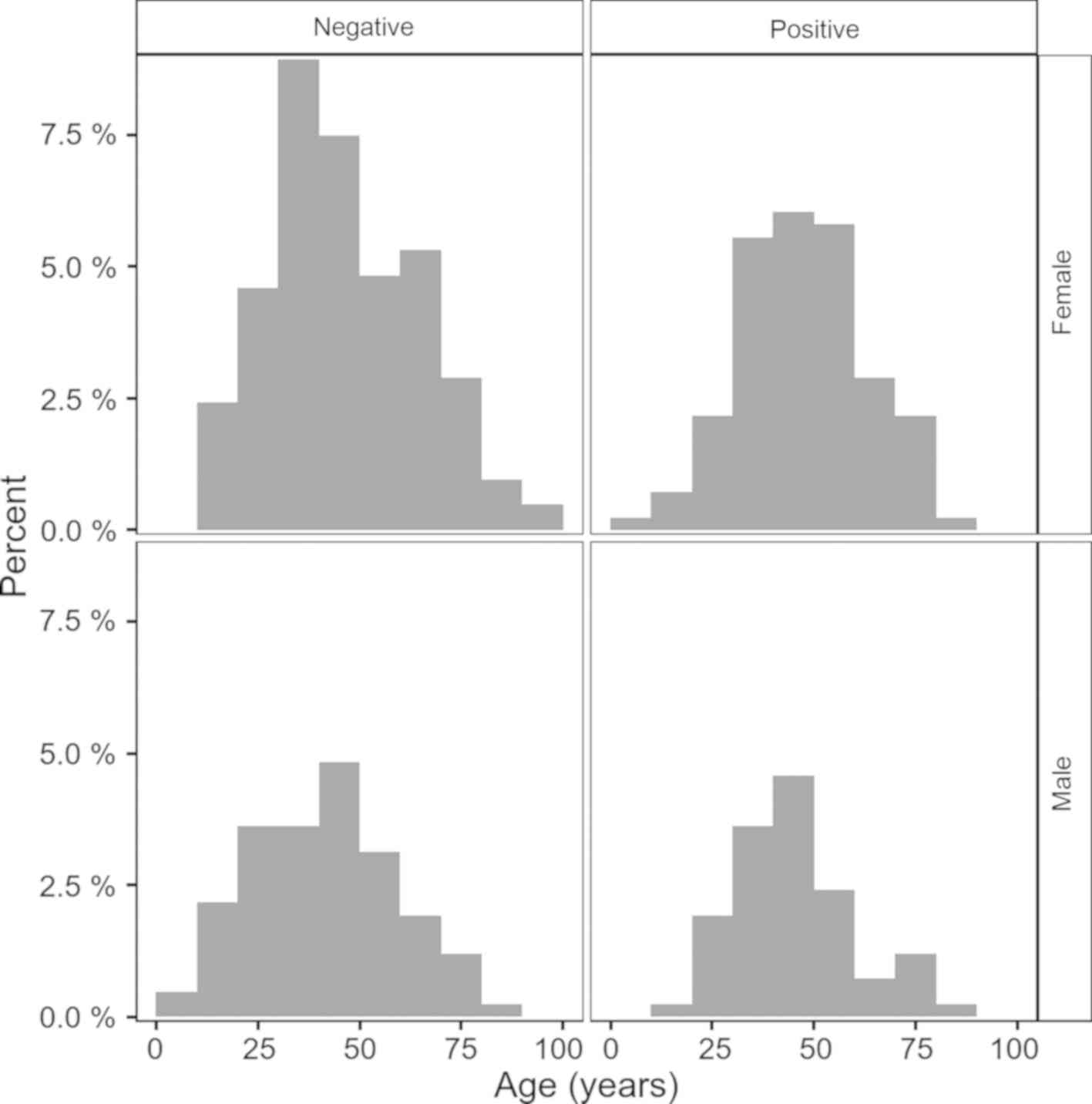
The group with negative tests had younger mean age
with 2.26 (95% CI, 1.01-5.53) years, than the group with positive
tests, a difference that did not reach the level of significance
(P=0.176). Fig. 2 shows the test
result distribution according to age and sex.
Discussion
The present study shows that >40% of dyspeptic
patients had or have HP infection. Comparing these values with
those of previous studies on the prevalence of HP infection from
the same region, it seems that they are declining. In medical
literature there is no clear country wide recent data on this
topic.
There are some interesting studies regarding the
pediatric population and HP infection from Romania. In 2002, an
epidemiological study was performed on dyspeptic children from
northwestern region of Romania. The authors included 267 subjects,
aged between 5-18 years, and the presence of infection was
considered if both urease test and histological staining revealed
the infection. The results showed a high prevalence of infection of
40% (16). In our study, from 16
patients <18 years, 3 of them (1.7%) had a positive HP infection
test.
One year later, in 2003, another study by Miller
et al was published regarding the seroprevalence of
antibodies to HP in Romanian adopted children, with age between 4
months and 16 years. A high prevalence was found for Romanian
children of 20% (17).
A similar study published in 2018 also reported a
high prevalence of HP infection among pediatric population (almost
25%) from 7,100 children studied from Cluj-Napoca, Romania
(18).
An epidemiological study on asymptomatic subjects,
from the western part of the county, Timisoara, using a serological
test, found high rates of infection in the adult population in
2003. The study population consisted of 960 employees, and it was
standardized for age and sex distribution of the western region
population. The prevalence of HP infection in the adult population
was 68.5%. The prevalence by age group were: 18-30 years, 65.3%; in
group 31-40 years, 71.6%; in group 41-50 years, 75%; and in group
51-60 years, 88.7% (19). Even
though the study was done on a smaller population (416 vs. 960),
comparing the data, to our study the prevalence of HP infection was
lower, as follows: in the group age 18-30 years, 10.6%; in group
30-40 years, 20.7%; in group 40-50 years, 24.2%; and in group 50-60
years, 23.6%.
In 2008 a study targeting the prevalence of HP
infection was done in Cluj-Napoca including 955 patients. The
global prevalence was 29.7%. The highest prevalence was seen in the
age group >80 years (42.8%), followed by the group 21-30 years
(38.8%) and the group 41-50 years (35.9%). Also, the prevalence of
HP infection was significantly higher in rural areas (20).
In a study published in 2014 by Ciobanu et al
(23), a comparison regarding the
data on HP epidemics from Romania was made to see if the tendency
of the prevalence was decreasing. The first comparison was made in
the same category of age, in different periods of time. The second
comparison followed the prevalence trends over 15 years in the same
population. In the group of children, a 10% decreased prevalence
was noted from 1994 to 2003. Also, in young adults (20-30 years)
from 1994 until 2009, the prevalence dropped significantly from
78.1% (21) to 51.7% (22). A similar prevalence was observed in
the youngest group, followed over time: 50% in 1994, without a
significant increase in 2009: 51.71%. For this age group, the rate
of infection in adult life is very small. These data may reflect
the first epidemiological trends of decreasing prevalence of
infection in Romania.
Our data show a small difference between patients
with HP infection from urban areas compared with rural areas. In
urban areas people are more likely to have a higher education and
easier access to medical care.
In a study from 2017 made in Craiova on 1,525
dyspeptic patients, in which the prevalence of HP infection was
63.67%, there were no significant percentage differences of the HP
infection between the patients from urban area and those from rural
area (24). In the study the
prevalence of HP infection increased with age, the percentages by
age groups were: age <20 years, 2.16%; group 20-29 years,
12.36%; group 30-39 years, 16.7%; group 40-49 years, 17.1%; group
50-59 years, 20.7%; and in group 60-60 years, 17.9%. Comparing the
data with those of the present study, the global prevalence (63.67
vs. 40.8%) and the prevalence in all age groups were lower. The
epidemiological trends of HP prevalence in Romania is presented in
Table II.
 | Table IIEpidemiological trends of HP
prevalence among symptomatic patients in Nortwestern Romania. |
Table II
Epidemiological trends of HP
prevalence among symptomatic patients in Nortwestern Romania.
| Population age
(years) | Prevalence (%) | Reported year | Refs. | Methodology |
|---|
| <20 | 50 | 1994 | (21) | Urease test |
| 21-30 | 78.1 | 1994 | (21) | Urease test |
| 31-40 | 85.9 | 1994 | (21) | Urease test |
| 41-50 | 80.3 | 1994 | (21) | Urease test |
| 71-80 | 61.5 | 1994 | (21) | Urease test |
| 5-18 | 40 | 2003 | (19) | Urease test and
histology |
| 18-30 | 65.3 | 2003 | (19) | Urease test and
histology |
| 31-40 | 71.6 | 2003 | (19) | Urease test and
histology |
| 41-50 | 75 | 2003 | (19) | Urease test and
histology |
| 21-30 | 51.71 | 2009 | (22) | C14-Urease breath
test |
| <20 | 63.7 | 2017 | (24) | Urease test and IgG
anti-HP |
| 20-29 | 66.22 | 2017 | (24) | Urease test and IgG
anti-HP |
| 50-69 | 61.78 | 2017 | (24) | Urease test and IgG
anti-HP |
| 21-30 | 38.8 | 2018 | (20) | Urease test |
| 41-50 | 35.9 | 2018 | (20) | Urease test |
| >80 | 42.9 | 2018 | (20) | Urease test |
| <18 | 1.78 | 2019 | Our study | IgG-anti-HP |
| 18-30 | 10.65 | 2019 | Our study | IgG-anti-HP |
| 31-40 | 20.7 | 2019 | Our study | IgG-anti-HP |
| 41-50 | 24.26 | 2019 | Our study | IgG-anti-HP |
| >70 | 10.65 | 2019 | Our study | IgG-anti-HP |
We argue that this decrease in the prevalence of HP
infection over time could be attributed to better socio-economic
conditions. The fact that patients have easier access to medical
services, that more investigation are made when they show various
dyspeptic symptoms and due to the existence of non-invasive methods
of diagnosing this infection (serology, stool samples, respiratory
test), with a more rapid treatment could explain the decreasing
trends.
This decreasing prevalence of HP infection is
correlated with the evolution of gastric cancer. An epidemiological
study published in 2018, regarding the gastric cancer mortality in
Romania, in period 1955-2012 shows a decreasing trend in both
sexes; from 17.8% in 1990 to 15.2% in 2008 and 13% in 2012.
Regarding gastric cancer incidence in period 2008-2012 it seems to
be increased in males from 15.9% in 2008 to 16.3% in 2012 and
appears to be stabilized in females; 5.8% in 2008 with the same
value in 2012(25).
Our study has several limitations. First, we would
have preferred a larger sample, but routine HP screening is not
usual in this area. Secondly, as data were collected from HP
testing registry, we were not able to record detailed symptoms, the
history of HP infection, the followed treatment, and the endoscopic
findings. Therefore, further studies should be undertaken. A more
detailed analysis on epidemiological trends in Romania in this
region, over the last 30 years will be of great interest. However,
the present results bring novel information on HP infection
prevalence in this area, which was the main objective of our
study.
This study shows that, among dyspeptic patients from
Northwestern part of Romania, >40% have or had a positive HP
infection. Comparing our results with those of previous studies on
the prevalence of HP infection from the same region, we were able
to signal a decline in prevalence in HP infection over a 30-year
interval.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ALC designed the study, acquired and analyzed the
data, and drafted the manuscript. DLD conceived the study and
contributed to the analysis of the data and the writing of the
manuscript. PC participated in the acquisition and analysis of the
data, and reviewed the manuscript. DCL performed the statistical
analysis and reviewed the manuscript. All authors had major
intellectual contribution to deserve authorship. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Malfertheiner P, Megraud F, O'Morain CA,
Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton
J, Graham DY, et al: European Helicobacter and Microbiota
Study Group and Consensus panel: Management of Helicobacter
pylori infection - the Maastricht V/Florence Consensus Report.
Gut. 66:6–30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cheng H, Hu F, Zhang L, Yang G, Ma J, Hu
J, Wang W, Gao W and Dong X: Prevalence of Helicobacter
pylori infection and identification of risk factors in rural
and urban Beijing, China. Helicobacter. 14:128–133. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Klein PD, Graham DY, Gaillour A, Opekun AR
and Smith EO: Gastrointestinal Physiology Working Group. Water
source as risk factor for Helicobacter pylori infection in
Peruvian children. Lancet. 337:1503–1506. 1991.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hopkins RJ, Vial PA, Ferreccio C, Ovalle
J, Prado P, Sotomayor V, Russell RG, Wasserman SS and Morris JG Jr:
Seroprevalence of Helicobacter pylori in Chile: Vegetables
may serve as one route of transmission. J Infect Dis. 168:222–226.
1993.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Goodman KJ, Correa P, Tenganá Aux HJ,
Ramírez H, DeLany JP, Guerrero Pepinosa O, López Quiñones M and
Collazos Parra T: Helicobacter pylori infection in the
Colombian Andes: A population-based study of transmission pathways.
Am J Epidemiol. 144:290–299. 1996.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Brown LM: Helicobacter pylori:
Epidemiology and routes of transmission. Epidemiol Rev. 22:283–297.
2000.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hu Y, Wan JH, Li XY, Zhu Y, Graham DY and
Lu NH: Systematic review with meta-analysis: The global recurrence
rate of Helicobacter pylori. Aliment Pharmacol Ther.
46:773–779. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Venneman K, Huybrechts I, Gunter MJ,
Vandendaele L, Herrero R and Van Herck K: The epidemiology of
Helicobacter pylori infection in Europe and the impact of
lifestyle on its natural evolution toward stomach cancer after
infection: A systematic review. Helicobacter.
23(e12483)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hooi JKY, Lai WY, Ng WK, Suen MMY,
Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu
JC, et al: Global prevalence of Helicobacter pylori
infection: Systematic review and meta-analysis. Gastroenterology.
153:420–429. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Roberts SE, Morrison-Rees S, Samuel DG,
Thorne K, Akbari A and Williams JG: The prevalence of
Helicobacter pylori and the incidence of gastric cancer
across Europe (Review). Aliment Pharmacol Ther. 43:334–345.
2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Andreica V, Dumitraşcu D, Sască N, Toganel
E, Suciu A, Drăghici A, Pascu O, Sască C, Suciu M, Andreica M, et
al: Helicobacter-like organisms in gastroduodenal diseases.
Gastroenterol Clin Biol. 14:437–441. 1990.PubMed/NCBI
|
|
12
|
Chen TS, Li FY, Chang FY and Lee SD:
Immunoglobulin G antibody against Helicobacter pylori:
Clinical implications of levels found in serum. Clin Diagn Lab
Immunol. 9:1044–1048. 2002.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lahner E, Bordi C, Di Giulio E, Caruana P,
D'Ambra G, Milione M, Grossi C, Delle Fave G and Annibale B: Role
of Helicobacter pylori serology in atrophic body gastritis
after eradication treatment. Aliment Pharmacol Ther. 16:507–514.
2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pérez-Pérez GI, Cutler AF and Blaser MJ:
Value of serology as a noninvasive method for evaluating the
efficacy of treatment of Helicobacter pylori infection. Clin
Infect Dis. 25:1038–1043. 1997.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Cutler A, Schubert A and Schubert T: Role
of Helicobacter pylori serology in evaluating treatment
success. Dig Dis Sci. 38:2262–2266. 1993.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Serban R, Grigorescu-Sido P, Gheban D and
Kiss E: Helicobacter pylori gastritis in children:
Endoscopical and histological aspects. Rom J Gastroenterol.
11:297–301. 2002.PubMed/NCBI
|
|
17
|
Miller LC, Kelly N, Tannemaat M and Grand
RJ: Serologic prevalence of antibodies to Helicobacter
pylori in internationally adopted children. Helicobacter.
8:173–178. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Domșa T, Gheban D, Rădulescu A and Borzan
C: Preliminary research on Helicobacter pylori infection in
hospitalized children from northwestern Romania. J Mol Biol.
1:119–120. 2018.
|
|
19
|
Sporea I, Popescu A, van Blankenstein M,
Sirli R, Focşea M and Dănilă M: The prevalence of Helicobacter
pylori infection in western Romania. Rom J Gastroenterol.
12:15–18. 2003.PubMed/NCBI
|
|
20
|
Prunduș C, Ciobanu L, Bolboacă S, Tanțău
M, Matei D, Cruciat C, Pojoga C and Andreica V: The evolution over
time of the prevalence of Helicobacter pylori infection
among patients with dyspeptic syndrome - the experience of a
tertiary center in Romania. Med Connect. 13:23–27. 2018.
|
|
21
|
Andreica V and Andreica M: Helicobacter
pylori infection in stomach and duodenal diseases. Hipocrate,
Sibiu, pp10-25, 1994.
|
|
22
|
Ciobanu L, Prundus R and Diaconu B:
Epidemiological trends of Helicobacter pylori prevalence in
central-western part of Romania. J Gastrointest Liv Dis.
18(17)2009.
|
|
23
|
Ciobanu L, Taulescu M and Dumitrascu DL:
Helicobacter pylori in Romania: Epidemiology, diagnosis and
treatment. In: Helicobacter pylori: A Worldwide Perspective,
2014. Buzas GM (ed). Vol 1. Bentham Science Publishers, Oak Park,
IL, pp183-201, 2014.
|
|
24
|
Olar L, Mitrut P, Florou C, Mălăescu GD,
Predescu OI, Rogozea LM, Mogoantă L, Ionovici N and Pirici I:
Evaluation of Helicobacter pylori infection in patients with
eso-gastro-duodenal pathology. Rom J Morphol Embryol. 58:809–815.
2017.PubMed/NCBI
|
|
25
|
Valean S, Chira R and Dumitrascu D:
Epidemiological trends in digestive cancers in Romania, 1955-2012,
compared to alcohol consumption Correlation or coincidence? Clujul
Med. 91:376–386. 2018.PubMed/NCBI View Article : Google Scholar
|