Introduction
Ovarian cancer is the eighth most common malignancy
among women worldwide, and is also the eighth leading cause of
cancer associated mortality in women (1). A previous study has indicated ~295,414
new patients with ovarian cancer patients were diagnosed and
~184,799 women die due to ovarian cancer every year (1). The high mortality that accompanies
ovarian cancer is due to lack of obvious symptoms and effective
methods for the early detection of the disease (2). The current treatments for ovarian
cancer are surgery and chemotherapy, but the prognosis of ovarian
cancer is poor and the 5-year survival rate is only ~44% (1). Therefore, the investigation into
mechanisms underlying the pathogenesis of ovarian cancer
tumorigenesis and the development of novel and effective methods
for the treatment of patients with ovarian cancer patients are
urgently required.
microRNAs (miRNAs) are a series of small, conserved
on-coding short RNAs, and miRNAs are formed by 18 to 25 nucleotides
in length (3). Previous studies have
indicated that miRNAs are dysregulated in a number of human
cancers, and regulated the target gene expression by binding to the
3' untranslated region (UTR) of target messenger RNAs (mRNAs).
Therefore, miRNAs have been suggested to regulate human cancer cell
growth and differentiation (4-8).
A previous study has also indicated that miRNAs can regulate human
tumorigenesis (9). In a variety of
human tumor types, distinct miRNA expression profiles have been
identified, which have been associated with tumor histological
subtypes (10). Therefore, miRNAs
can be used as biomarkers for the diagnosis and prognosis of a
number of cancer types. A growing body of evidence has suggested
that miR-193b is significantly down-regulated compared with
non-cancerous tissues in a number of human cancer types, including:
Liver cancer, lung cancer, pancreatic cancer, esophageal squamous
cell carcinoma, cervical cancer and ovarian cancer (11-15).
Studies have also demonstrated that miR-193b played as a
tumor-suppressive miRNA in human cancer by regulating cancer cell
proliferation, migration, invasion and metastasis (11-15).
Although these studies reported that the expression of miR-193b is
altered in a number of cancers, including in ovarian cancer, the
roles of miR-193b in ovarian cancer have not, to the best of our
knowledge, yet been fully determined.
The current study investigated the biological
effects of miR-193b in ovarian cancer cells, and studied the
underlying molecular mechanisms. The results demonstrated that
miR-193b was down-regulated in ovarian cancer cells and regulated
ovarian cancer cell proliferation and apoptosis by targeting the
stathmin 1 (STMN1) gene. These findings provide insights into the
development of potential diagnostic and therapeutic tools for use
in the treatment of ovarian cancer in the future.
Materials and methods
Cells culture
A2780cp, A2780s, SKOV3 and CAOV3 cells were
purchased from the American Type Culture Collection. Human ovarian
epithelium cell line HOEC was also purchased from ATCC. All cells
were cultured in Dulbecco's modified Eagle's medium (DMEM;
Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10%
fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific,
Inc.), 1% penicillin (Invitrogen; Thermo Fisher Scientific, Inc.)
and 1% streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.) in
a humidified incubator with 5% CO2 at
37̊C.
Transfection
miR-negative control (NC), miR-193b mimic, miR-193b
inhibitor and inhibitor control were purchased from Ambion; Thermo
Fisher Scientific, Inc. The miR-193b mimic or inhibitor were
diluted in Opti-MEM medium (Invitrogen; Thermo Fisher Scientific,
Inc.) at room temperature (RT) for 15 min. A2780cp, A2780s and
CAOV3 cells were transfected with miR-NC or miR-193b, while HOEC
cells were transfected with miR-193b inhibitor or inhibitor control
and cultured for 48 h. Transfection was performed using
Lipofectamine® 3000 (Thermo Fisher Scientific, Inc)
according to the manufacturer's recommendations.
Cell viability detection assay
The Cell Counting Kit-8 (CCK-8) assay (Beyotime
Institute of Biotechnology) was performed to measure cell viability
according to the manufacturer's recommendations. Mock or
transfected cells were seeded and cultured. Subsequently, 10 µl
CCK-8 was added into the cell culture medium and incubated at 37˚C
for an additional 4 h. Subsequently, absorbance was measured at 450
nm using a plate reader (Molecular Devices, Spectramax).
Cell apoptosis assay
Mock or transfected cells were seeded and cultured.
Ovarian cancer cell apoptosis was measured using the Apo-ONE
Homogeneous Caspase-3/7 Assay kit (Promega Corporation) according
to the manufacturer's instructions. CellTiter-Blue (Promega
Corporation) was used to measure cell number. The relative
Caspase-3/7 activity was calculated using the ratio of Apo-ONE and
CellTiter-Blue signals.
RNA extraction and reverse
transcription-quantitative (RT-q) PCR
Total RNA was isolated from mock or transfected
ovarian cancer cells using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
recommendations. miRNA complementary DNA (cDNA) was converted from
total RNA by using a PrimeScript miRNA cDNA Synthesis Kit (cat. no.
D350A; Takara Biotechnology Co., Ltd.). miR-193b quantification was
performed using specific primers and probes using TaqMan MicroRNA
Assays (cat. no. 4426961; Applied Biosystems, Thermo Fisher
Scientific, Inc.); RNA U6 was used as the internal control. The
primer sequences for U6 were: Forward:
5'-GTGCTCGCTTCGGCAGCACATATAC-3' and reverse:
5'-AAAAATATGGAACGCTCACGAATTTG-3'. Relative mRNA expression was
calculated by using the 2-ΔΔCq
method.
Bioinformatics analysis
To identify the potential target of miR-193b, genes
were predicted by searching targetscan (http://www.targetscan.org) and mirbase targets
(http://microrna.sanger.ac.uk/cgi-bin/targets/v5/search.pl).
STMN1 was predicted to be a target of miR-193b.
Luciferase reporter assay
A fragment from the 3'UTR of STMN1 gene containing
the predicted binding site of miR-193b was amplified using PCR from
genomic DNA. The amplified fragment was cloned into the UTR
downstream of the luciferase gene in the pMIR-reporter luciferase
vector (Ambion; Thermo Fisher Scientific, Inc.). A corresponding
mutant construct was used as the control. Ovarian cancer cells were
co-transfected with the testing firefly luciferase reporter plasmid
together with a Renilla luciferase plasmid. Dualluciferase
activities were measured by using a Dual-Glo Luciferase Assay
System (Promega Corporation). Results were presented as the ratio
between the treatment and the control group.
Western blot assay
Total protein was extracted by using RIPA lysis
buffer (Beyotime Institute of Biotechnology) according to the
manufacturer's recommendations. Extracted proteins were separated
on a 10% SDS-PAGE gel and transferred to a PVDF membrane (GE
Healthcare). The PVDF membrane was then blocked with 5% BSA for 2 h
at RT and incubated with antibodies against STMN1 (cat. no. 3352;
Cell Signaling Technology, Inc.) and GAPDH (cat. no. 2118; Cell
Signaling Technology, Inc.) at a 1:1,000 dilution for 2 h at RT.
The membrane was then washed and further incubated with the
secondary antibody (cat. no. 7074; Cell Signaling Technology, Inc.)
at a 1:1,000 dilution for 2 h at RT. The bands were then detected
by using the Novex ECL HRP chemiluminescent substrate reagent kit
(Invitrogen; Thermo Fisher Scientific, Inc.).
Statistical analysis
All data were analyzed using a one-way ANOVA and
Tukey's HSD test (post hoc test). Statistical analyses were
performed using SPSS Statistics v19. The data were expressed as the
mean ± standard deviation (SD). P<0.05 was considered to
indicate a statistically significant difference. All experiments
were performed in triplicate.
Results
miR-193b is down-regulated in ovarian
cancer cells
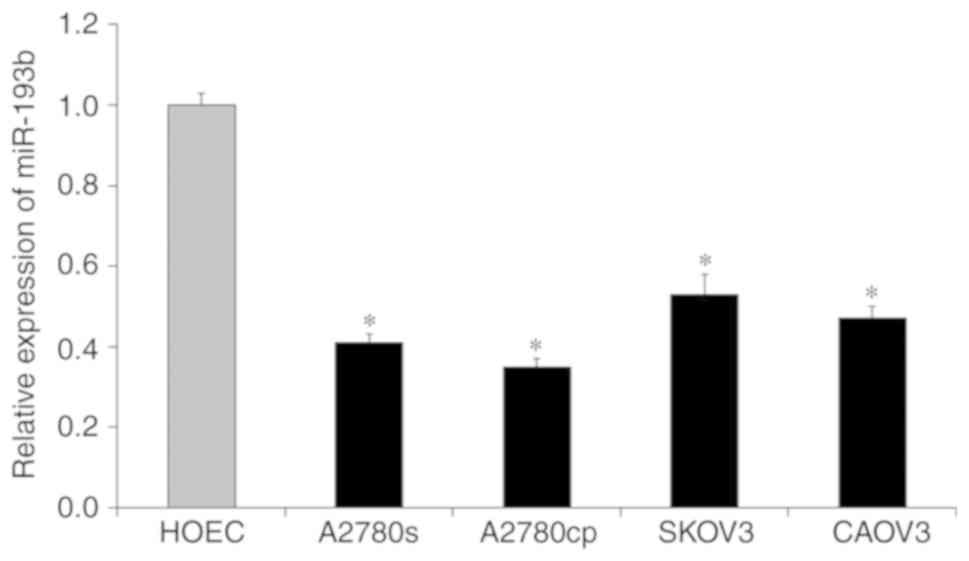
To investigate the biological roles of miR-193b in
ovarian cancer, the expression level of miR-193b in a number of
ovarian cancer cell lines was measured by using RT-qPCR assay. As
presented in Fig. 1, the expression
of miR-193b in ovarian cancer cells (A2780cp, A2780s, SKOV3 and
CAOV3) was significantly down-regulated compared with the
non-malignant HOEC cell line (all, P<0.05; Fig. 1). These results suggested that the
down-regulation of miR-193b may be associated with the development
of ovarian cancer.
Effects of miR-193b transfection on
ovarian cancer cell proliferation
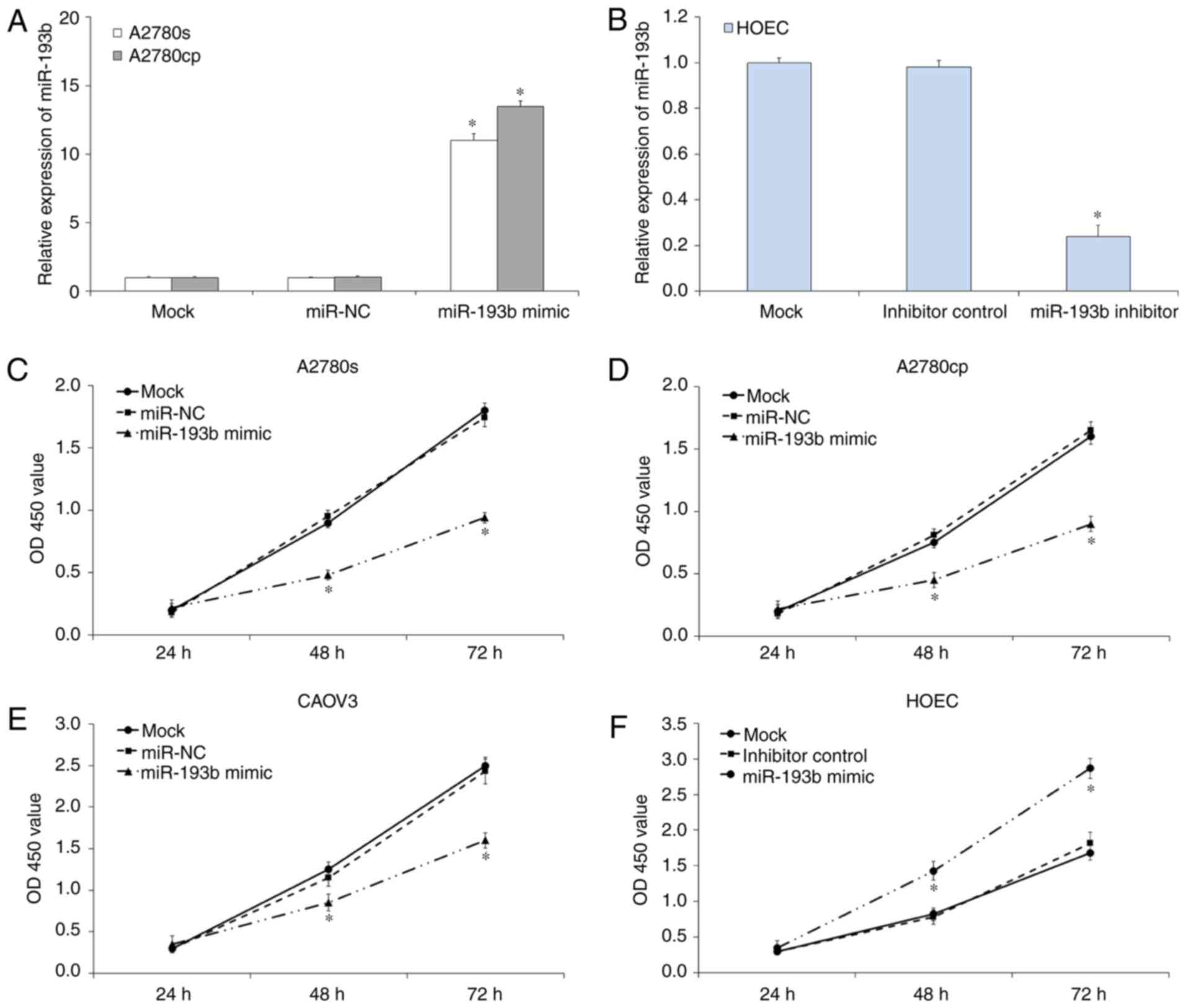
To investigate the effects of miR-193b on ovarian
cancer cell proliferation, a CCK-8 assay was performed. RT-qPCR
results indicated miR-193b mimic or inhibitor transfection
significantly regulated the expression of miR-193b in ovarian
cancer cells (P<0.05; Fig. 2A and
B). CCK-8 assay results demonstrated
that the up-regulation of miR-193b inhibited the proliferation of
A2780s (P<0.05; Fig. 2C), A2780cp
(P<0.05; Fig. 2D) and CAOV3
(P<0.05; Fig. 2E) compared with
mock cells. In parallel, the miR-193b inhibitor or inhibitor
control was transfected into HOEC cells to investigate the effects
of miR-193b in normal ovary epithelial cell. As presented in
Fig. 2F, the down-regulation of
miR-193b increased HOEC cell proliferation (P<0.05). The miR-NC
and inhibitor control transfection was not indicated to affect
ovarian cancer cell proliferation. Therefore, miR-193b inhibited
the on ovarian cancer cell proliferation in vitro.
Effects of miR-193b transfection on
ovarian cancer cell apoptosis
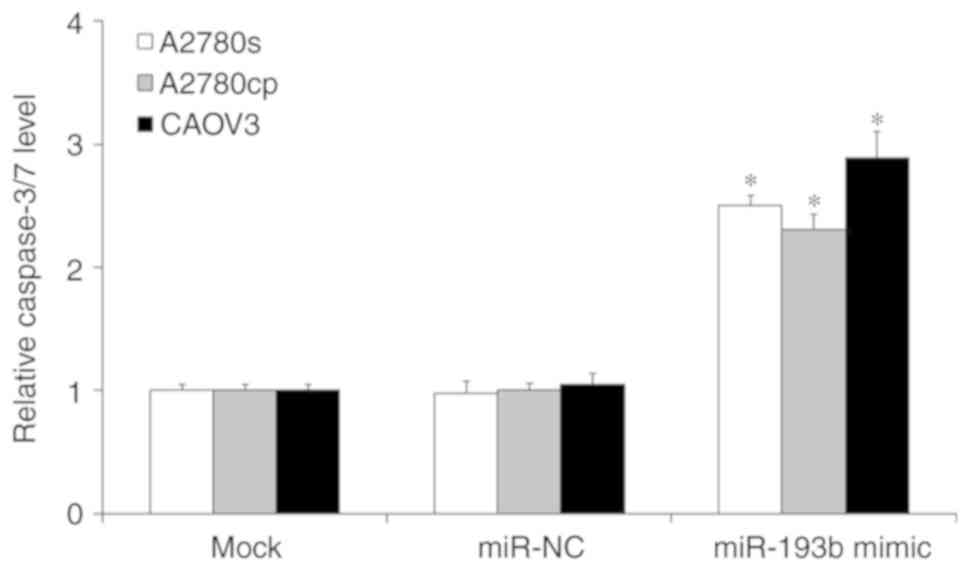
To investigate whether miR-193b regulated ovarian
cancer cell proliferation through the induction of cell apoptosis,
a cell apoptosis assay was performed. As presented in Fig. 3, the induction of miR-193b
significantly induced ovarian cancer cell apoptosis compared with
the mock ovarian cancer cells (P<0.05; Fig. 3), and the miR-NC transfection did not
affect ovarian cancer cell apoptosis (P>0.05; Fig. 3).
STMN1 is a direct target of
miR-193b
The results of the current study indicated that
miR-193b regulated ovarian cancer cell proliferation and apoptosis,
therefore, the present study aimed to determine the downstream
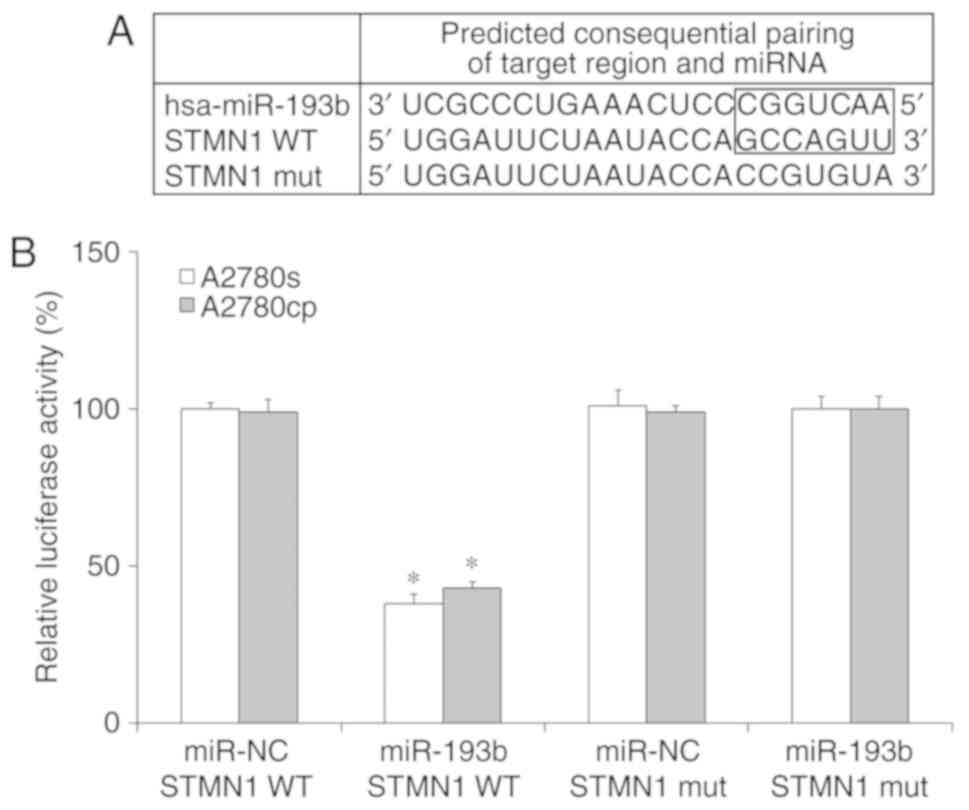
target of miR-193b in ovarian cancer cells. By using prediction
programs, STMN1 was indicated as a potential target of miR-193b
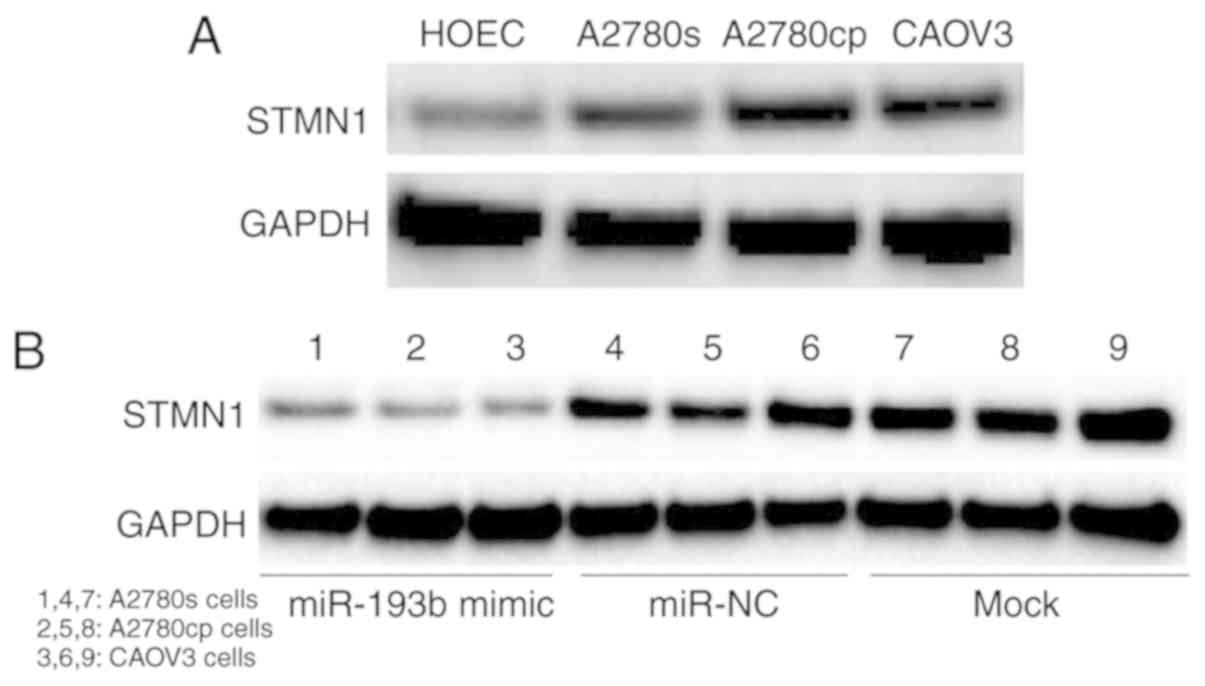
(Fig. 4A). Western blot results
showed that the expression of STMN1 was significantly increased in
ovarian cancer cells compared with the non-malignant HOEC cell line
(Fig. 5A). To confirm whether STMN1
is a direct target of miR-193b in ovarian cancer cells, miR-193b
and luciferase reporter plasmids were co-transfected with wild type
(WT) or mutant type (Mut) 3'-UTRs of STMN1, into ovarian cancer
cells, and the luciferase value was measured. As presented in
Fig. 4B, miR-193b remarkably
decreased the luciferase activity in the WT group but not in the
Mut group in ovarian cancer cells. The results suggested that STMN1
is a direct target of miR-193b in ovarian cancer cells.
miR-193b modulated ovarian cancer cell
growth through targeting STMN1
To investigate whether miR-193b regulated ovarian
cancer cell growth through targeting STMN1, a western blot assay
was performed. Ovarian cancer cells were transfected with miR-193b
mimic or miR-NC and cultured for 48 h, and the expression of STMN1
was determined. The results indicated that the up-regulation of
miR-193b decreased the expression of STMN1 in ovarian cancer cells,
while miR-NC transfection did not affect the expression of STMN1 in
ovarian cancer cells (Fig. 5B).
These results suggested that miR-193b modulated ovarian cancer cell
growth through targeting STMN1.
Discussion
Ovarian cancer exhibits the eighth highest mortality
rate in women worldwide (1). Despite
the low prevalence rate, the majority of patients exhibit cancer
recurrence and cancer-associated mortality (16). Therefore, the investigation into the
mechanisms underlying the pathogenesis of ovarian cancer is
urgently required. A number of studies have indicated that miRNAs
are associated in the pathogenesis of human cancers, indicating
that they contributed to the cancer cell proliferation,
differentiation, cell cycle and apoptosis and also serve as
oncogenes and tumor suppressors (17-19).
Recent studies have indicated that miR-193b is dysregulated and
serves as a tumor suppressor in patients exhibiting a variety of
primary tumors, including breast cancer, gastric cancer, cervical
cancer, prostate cancer and ovarian cancer (11-15,20-23).
Wu et al reported that the expression of miR-193b was
significantly down-regulated in endometrioid adenocarcinoma
(24). Rauhala et al reported
that miR-193b is a tumor suppressor in prostate cancer (25). Chen et al revealed that
miR-193b was significantly down-regulated in melanoma tissues, and
the up-regulation of miR-193b decreased cell proliferation by
regulating cyclin D1(26). Li et
al indicated that miR-193b was a novel biomarker for patients
with ovarian cancer (27). However,
the role of miR-193b in ovarian cancer is yet to be determined.
In the present study, miR-193b was demonstrated to
be down-regulated in human ovarian cancer cells compared with
non-malignant cells. Therefore, it was hypothesized that miR-193b
may act as a tumor suppressor in the development of ovarian cancer.
The current study indicated that the up-regulation of miR-193b
inhibited ovarian cancer proliferation and induced ovarian cancer
apoptosis. The results also revealed that the down-regulation of
miR-193b increased the non-malignant HOEC cell proliferation.
Further study indicated that STMN1 is a direct target of miR-193b
in human ovarian cancer cell. STMN1, which is also known as
metablastin and oncoprotein 18, is a protein that is encoded by the
STMN1 gene. STMN1 is highly conserved and is important for the
regulation of the cell cytoskeleton (28). STMN1 encode for s a cytosolic
phosphoprotein, and this protein serves an important role in the
function of the mitotic spindle (29). Therefore, it can be suggested that
STMN1 serves an important role in the regulation of cell growth
(30). Previous studies have
indicated that STMN1 is over-expressed in many types of human
cancer types, including in hepatocellular cancer, gastric cancer,
breast cancer and prostate cancer (31-34).
STMN1 has also been suggested to be an an oncogene in human cancer.
In the current study, the expression of STMN1 was indicated to be
up-regulated in human ovarian cancer cells, and the up-regulation
of miR-193b significantly decreased the expression of STMN1 in
human ovarian cancer cells. Based on these findings, it can be
suggested that the up-regulation of miR-193b effectively regulated
ovarian cancer cell growth, at least in part, through the
inhibition of STMN1 expression. However, ovarian tumorigenesis is a
complicated process and a lot of other factors are involved within
this, and whether STMN1 is the key targeting gene of miR-193b
requires further investigation.
In conclusion, our study revealed that miR-193b was
down-regulated in human ovarian cancer cells and the regulation of
miR-193b regulated human ovarian cancer cell growth through
targeting STMN1. These results provide novel information into the
molecular mechanism underlying the effects of miR-193b on human
ovarian cancer cells, and suggest that the regulation of miR-193b
may benefit ovarian cancer treatment in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL designed the study; HL, YX and DZ performed the
experiments, analyzed the data and prepared the manuscript. HL
reviewed the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Vaughan S, Coward JI, Bast RC Jr, Berchuck
A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R,
Etemadmoghadam D, et al: Rethinking ovarian cancer: Recommendations
for improving outcomes. Nat Rev Cancer. 11:719–725. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Weidle UH, Dickopf S, Hintermair C,
Kollmorgen G, Birzele F and Brinkmann U: The role of micro RNAs in
breast cancer metastasis: Preclinical validation and potential
therapeutic targets. Cancer Genomics Proteomics. 15:17–39.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jiang YW and Chen LA: microRNAs as tumor
inhibitors, oncogenes, biomarkers for drug efficacy and outcome
predictors in lung cancer (review). Mol Med Rep. 5:890–894.
2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kelly BD, Miller N, Healy NA, Walsh K and
Kerin MJ: A review of expression profiling of circulating microRNAs
in men with prostate cancer. BJU Int. 111:3–4. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nair VS, Maeda LS and Ioannidis JP:
Clinical outcome prediction by microRNAs in human cancer: A
systematic review. J Natl Cancer Inst. 104:528–540. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Iorio MV and Croce CM: MicroRNAs in
cancer: Small molecules with a huge impact. J Clin Oncol.
27:5848–5856. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Glud M, Rossing M, Hother C, Holst L,
Hastrup N, Nielsen FC, Gniadecki R and Drzewiecki KT:
Downregulation of miR-125b in metastatic cutaneous malignant
melanoma. Melanoma Res. 20:479–484. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bushati N and Cohen SM: microRNA
functions. Annu Rev Cell Dev Biol. 23:175–205. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714.
2009.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Yin W, Nie Y, Chen L, Wang Q, Liu S, He X
and Wang W: Deregulation of microRNA-193b affects the proliferation
of liver cancer via myeloid cell leukemia-1. Oncol Lett.
15:2781–2788. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wu H and Zhou C: Long non-coding RNA UCA1
promotes lung cancer cell proliferation and migration via
microRNA-193a/HMGB1 axis. Biochem Biophys Res Commun. 496:738–745.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fang C, Dai CY, Mei Z, Jiang MJ, Gu DN,
Huang Q and Tian L: microRNA-193a stimulates pancreatic cancer cell
repopulation and metastasis through modulating TGF-β2/TGF-βRIII
signalings. J Exp Clin Cancer Res. 37(25)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chan CM, Lai KKY, Ng EKO, Kiang MN, Kwok
TWH, Wang HK, Chan KW, Law TT, Tong DK, Chan KT, et al: Serum
microRNA-193b as a promising biomarker for prediction of
chemoradiation sensitivity in esophageal squamous cell carcinoma
patients. Oncol Lett. 15:3273–3280. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Han D, Wang J and Cheng G: LncRNA NEAT1
enhances the radio-resistance of cervical cancer via
miR-193b-3p/CCND1 axis. Oncotarget. 9:2395–2409. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chan JK, Cheung MK, Husain A, Teng NN,
West D, Whittemore AS, Berek JS and Osann K: Patterns and progress
in ovarian cancer over 14 years. Obstet Gynecol. 108:521–528.
2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fabbri M, Ivan M, Cimmino A, Negrini M and
Calin GA: Regulatory mechanisms of microRNAs involvement in cancer.
Expert Opin Biol Ther. 7:1009–1019. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Croce CM and Calin GA: miRNAs, cancer, and
stem cell division. Cell. 122:6–7. 2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Brown JR and Sanseau P: A computational
view of microRNAs and their targets. Drug Discov Today. 10:595–601.
2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tahiri A, Leivonen SK, Lüders T, Steinfeld
I, Ragle Aure M, Geisler J, Mäkelä R, Nord S, Riis ML, Yakhini Z,
et al: Deregulation of cancer-related miRNAs is a common event in
both benign and malignant human breast tumors. Carcinogenesis.
35:76–85. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhou H, Wang K, Hu Z and Wen J: TGF-β1
alters microRNA profile in human gastric cancer cells. Chin J
Cancer Res. 25:102–111. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cheung TH, Man KN, Yu MY, Yim SF, Siu NS,
Lo KW, Doran G, Wong RR, Wang VW, Smith DI, et al: Dysregulated
microRNAs in the pathogenesis and progression of cervical neoplasm.
Cell Cycle. 11:2876–2884. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Xie C, Jiang XH, Zhang JT, Sun TT, Dong
JD, Sanders AJ, Diao RY, Wang Y, Fok KL, Tsang LL, et al: CFTR
suppresses tumor progression through miR-193b targeting urokinase
plasminogen activator (uPA) in prostate cancer. Oncogene.
32:2282–2291. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wu W, Lin Z, Zhuang Z and Liang X:
Expression profile of mammalian microRNAs in endometrioid
adenocarcinoma. Eur J Cancer Prev. 18:50–55. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Rauhala HE, Jalava SE, Isotalo J, Bracken
H, Lehmusvaara S, Tammela TL, Oja H and Visakorpi T: miR-193b is an
epigenetically regulated putative tumor suppressor in prostate
cancer. Int J Cancer. 127:1363–1372. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chen J, Feilotter HE, Paré GC, Zhang X,
Pemberton JG, Garady C, Lai D, Yang X and Tron VA: MicroRNA-193b
represses cell proliferation and regulates cyclin D1 in melanoma.
Am J Pathol. 176:2520–2529. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li H, Xu Y, Qiu W, Zhao D and Zhang Y:
Tissue miR-193b as a novel biomarker for patients with ovarian
cancer. Med Sci Monit. 21:3929–3934. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kueh HY and Mitchison TJ: Structural
plasticity in actin and tubulin polymer dynamics. Science.
325:960–963. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rana S, Maples PB, Senzer N and Nemunaitis
J: Stathmin 1: A novel therapeutic target for anticancer activity.
Expert Rev Anticancer Ther. 8:1461–1470. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rubin CI and Atweh GF: The role of
stathmin in the regulation of the cell cycle. J Cell Biochem.
93:242–250. 2004.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Belletti B and Baldassarre G: Stathmin: A
protein with many tasks. New biomarker and potential target in
cancer. Expert Opin Ther Targets. 15:1249–1266. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mistry SJ and Atweh GF: Role of stathmin
in the regulation of the mitotic spindle: Potential applications in
cancer therapy. Mt Sinai J Med. 69:299–304. 2002.PubMed/NCBI
|
|
33
|
Ke B, Wu LL, Liu N, Zhang RP, Wang CL and
Liang H: Overexpression of stathmin 1 is associated with poor
prognosis of patients with gastric cancer. Tumour Biol.
34:3137–3145. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tamura K, Yoshie M, Miyajima E, Kano M and
Tachikawa E: Stathmin regulates hypoxia-inducible factor-1α
expression through the mammalian target of rapamycin pathway in
ovarian clear cell adenocarcinoma. ISRN Pharmacol.
2013(279593)2013.PubMed/NCBI View Article : Google Scholar
|