Introduction
DVT is a type of vascular disease, and its
occurrence is ~0.1% globally (1).
DVT can lead to swelling, ulceration and post-thrombotic syndromes
in the legs, as well as pulmonary embolism, which results in ~15%
of mortality in the first three months post-diagnosis (1). Although anticoagulants are widely used
for the treatment of DVT, existing thrombus and pulmonary embolisms
still affect life quality of DVT patients (2). Therefore, it is urgent to develop of
effective targeted therapies against DVT, and studies are required
to elucidate the mechanisms underlying the development of this
disease.
EPCs are derived from bone marrow, are present in
the circulating blood and have the potential to differentiate into
mature endothelial cells during vascular injury repair (3). Additionally, they are considered to be
potential biomarkers and promising regenerative medicine for
cardiovascular diseases (3).
Previous studies have indicated that peripheral EPCs serve
essential roles in the resolution of thrombus (4-6).
These data also provided novel insights on the therapeutic
approaches of DVT treatment.
LncRNAs are novel non-protein-coding RNAs that are
>200 nucleotides in length (7).
Previous studies have suggested that lncRNAs are associated with
the progression of a variety of vascular diseases, where impaired
levels of lncRNAs were observed (8-13).
Among these non-coding RNAs, lncRNA metastasis associated lung
adenocarcinoma transcript 1 (MALAT1) has been indicated to be
involved in the pathogenesis of vascular diseases, as it is
downregulated in plaques and is associated with endothelial
phenotypic switch (14).
Furthermore, a previous study revealed that MALAT1 polymorphism
could result in vascular disease in the Chinese population
(15). However, the expression
profiles and potential functions of MALAT1 in DVT have not been
fully elucidated and require further investigation.
The Wnt pathway is an evolutionarily conserved
signaling pathway and is widely involved in a variety of biological
processes including cell proliferation and migration (16-19).
This axis is activated by the Wnt ligand and when activated,
β-catenin escapes serine and threonine phosphorylation by glycogen
synthase kinase-3β (GSK3β) at the N-terminus, which dictates the
stability of the β-catenin destruction complex (17). As a result, β-catenin accumulates in
the cytoplasm and translocates into the nucleus, further modulating
the expression of Wnt target genes (16). The Wnt pathway serves an essential
role in the progression of vascular diseases (16-19).
Furthermore, this pathway is also involved in the proliferation,
differentiation and apoptosis of EPCs (20,21). In
addition, MALAT1 could modulate the growth and migration of a
variety of cell types through Wnt signaling (22,23).
Taken together, the MALAT1/Wnt/β-catenin axis may also serve a role
in the proliferation and migration of EPCs, consequently
contributing to the progression of DVT.
In the present study, the effects of
MALAT1-regulated signaling on the growth and migration of EPCs were
investigated. The results revealed that MALAT1 is upregulated in
DVT tissues. Furthermore, MALAT1 was able to regulate the
biological behaviors of EPCs, such as proliferation, migration,
cell cycle arrest and apoptosis. In addition, the Wnt/β-catenin
signaling pathway is a promising downstream target of MALAT1 in
DVT. The changes of biological behaviors in EPCs caused by silenced
MALAT1 were reversed by inhibition of the Wnt/β-catenin signaling
pathway. In summary, the data indicated the roles of MALAT1 in the
pathogenesis of DVP, and the MALAT1/Wnt/β-catenin axis could be a
novel therapeutic target for the treatment of this disease.
Materials and methods
Clinical specimens
A total of 20 blood samples were obtained from
patients with DVT and healthy donors (age, 45-70 years; 10 males
and 10 females) at the First Affiliated Hospital of Jinzhou Medical
University (Jinzhou, China) between May 2015 and April 2017. None
of the patients received treatment prior to enrollment. The present
study was conducted according to the Declaration of Helsinki and
approved by the Review Committee of the First Affiliated Hospital
of Jinzhou Medical University (approval no. 20152874). Written
informed consent was signed by each patient. Samples were
snap-frozen using liquid nitrogen and immediately stored at -80˚C
until further use.
Cell culture
For the isolation of mononuclear cells (MNCs), ~100
ml of circulating blood was obtained from each patient with DVT or
healthy controls using BD Vacutainer EDTA tube (BD Biosciences) and
immediately stored in the dark. The samples were processed
following collection as follows: MNCs were extracted by density
gradient centrifugation using Biocoll (Biochrom, Ltd.) at 5,003 x g
at 4˚C for 20 min and washed three times using PBS. Isolated cells
were plated on culture dishes that were pre-coated with human
fibronectin (Sigma-Aldrich; Merck KGaA) and cultured in endothelial
cell growth medium (GE Healthcare Life Sciences) supplemented with
bovine brain extract (12 mg/ml), human epidermal growth factor (10
ng/ml), human insulin-like growth factor-1 (50 ng/ml),
hydrocortisone (1 mg/ml) and streptomycin (100 µg/ml) and
penicillin (100 U/ml; GE Healthcare Life Sciences) at 37˚C. Heparin
(10 U/ml; Tocris Bioscience) was used to avoid platelet coagulation
and cells were maintained in a humidified 5% CO2
atmosphere at 37˚C. After 3 days, floating cells were aspirated and
the culture medium was replaced. EPC colonies formed after 1-2
weeks of culture. Medium was replenished every day for the first 7
days and every other day for the following 7 days. The medium was
then replenished every 2-3 days. A total of two batches of cells
were used in the subsequent experiments.
Cell transfection
To establish a MALAT1 knockdown model, small
interfering RNA (siRNA) sequences targeting MALAT1 (si-MALAT1;
5'-GUACAUUCGUGGAGACUAGC-3' and 3'-ACCAUGUAAGCACCUCUGAU-5') and
negative control (si-NC; 5'-GCUACAUUCUGGAGACAUA-3' and
3'-CGAUGUAAGACCUCUGUAU-5') were purchased from GenePharma Co. Ltd.
Following annealing, siRNA were integrated into lentiviral
pU6-Luc-Puro vectors (GenePharma Co., Ltd.). Furthermore, to
establish a MALAT1 overexpression model, wild-type MALAT1
(LV-MALAT1) or mutant (LV-NC) fragments were amplified using PCR
and integrated into pcDNA3.1 vectors (Invitrogen; Thermo Fisher
Scientific, Inc.). Cells were transfected with corresponding
lentiviral vectors or controls. A total of 10 nM plasmids or the
aforementioned siRNA were used for transfection. Up- or
downregulation of MALAT1 was determined using reverse
transcription-quantitative PCR (RT-qPCR). The Wnt/β-catenin
signaling inhibitor XAV939 (10 nM; cat. no. ab120897; Abcam) was
used to treat si-MALAT1-transfected cells. All transfections were
performed using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). At 8 h post-transfection, endothelial
cell growth medium was replenished with fresh culture medium
containing 10% FBS. Cells were cultured for 24 h post-transfection
and subjected to further analysis.
RT-qPCR
RT-qPCR was performed to evaluate the levels of
MALAT1, proliferating cell nuclear antigen (PCNA), segment polarity
protein dishevelled homolog DVL-2 (DVL2), GSK3β, cyclin D1 and
β-catenin. Total RNA from clinical samples or cells was extracted
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
concentration of extracted RNA was measured using a
NanoDrop™ 1000 spectrophotometer (Thermo Fisher
Scientific, Inc.). The quality of RNA was determined with an
Agilent 2100 Bioanalyzer (Agilent Technologies, Inc.).
Subsequently, cDNA was synthesized using a PrimeScript™
RT Reagent kit (Takara Biotechnology Co., Ltd.), and qPCR was
performed using a SYBR Green PCR Master Mix (Takara Biotechnology
Co., Ltd.) according to the manufacturer's protocol. β-actin was
used as the internal reference gene. The temperature protocol of
reverse transcription was as follows: 42˚C for 45 min, 99˚C for 5
min and 5˚C for 5 min. The following primer pairs were used for
qPCR: MALAT1 forward, 5'-CTAAGGTCAAGAGAAGTGTCAG-3' and reverse,
5'-AAGACCTCGACACCATCGTTAC-3'; PCNA forward,
5'-CATGGTGAAACCCCGTCTCTACTA-3' and reverse,
5'-GAGCACTTAGGCAATTTTGGTGAT-3'; DVL2 forward,
5'-CATCCAGCCAATTGACCCTG-3' and reverse, 5'-AGGGATGGTGATCTTGAGCC-3';
GSK3β forward, 5'-GGAACTCCAACAAGGGAGCA-3' and reverse,
5'-TTCGGGGTCGGAAGACCTTA-3'; cyclin D1 forward,
5'-TGAACTACCTGGACCGCT-3' and reverse, 5'-GCCTCTGGCATTTTGGAG-3';
β-catenin forward, 5'-AGTTCCTTACCGTCCCCAAG-3' and reverse,
5'-CAGACACGCCTGTTTCGAAT-3' and β-actin forward,
5'-GCACCACACCTTCTACAATG-3' and reverse, 5'-TGCTTGCTGATCCACATCTG-3'.
The following thermocycling conditions were used for the PCR:
Initial denaturation at 95˚C for 5 min; 45 cycles of 95˚C for 15
sec, 60˚C for 20 sec and 72˚C for 10 sec, followed by 72˚C for 5
min. The relative expression of each gene was calculated using
2-∆∆Cq method (24).
Western blot analysis
Total protein was extracted from tissues or cells
using RIPA buffer (Beyotime Institute of Biotechnology). Protein
concentration was measured using a BCA assay (Beyotime Institute of
Biotechnology). Equal amounts (30 µg) of protein samples were
separated using 10% SDS-PAGE gel and then transferred onto a PVDF
membrane (EMD Millipore). Subsequently, the membranes were blocked
in TBS containing 5% skimmed milk at room temperature for 2 h and
incubated with the following primary antibodies: PCNA (1:2,000;
cat. no. 2586; Cell Signaling Technology, Inc.), DVL2 (1:1,000;
cat. no. 3216; Cell Signaling Technology, Inc.), GSK3β (1:1,000;
cat. no. 9832; Cell Signaling Technology, Inc.), cyclin D1
(1:1,000; cat. no. 2978; Cell Signaling Technology, Inc.),
β-catenin (1:1,000; cat. no. 2698; Cell Signaling Technology, Inc.)
and β-actin (1:1,000; cat. no. 3700; Cell Signaling Technology,
Inc.) overnight at 4˚C. Membranes were then incubated in
horseradish peroxidase-conjugated secondary antibodies (1:5,000;
cat. no. sc-2371 or 1:10,000; cat. no. sc-2004; Santa Cruz
Biotechnology Inc.) at room temperature for 1 h. Bands were
visualized using an ECL protein detection kit (Pierce; Thermo
Fisher Scientific, Inc). Protein blots were quantified by
densitometric analysis using ImageJ software (v1.48; National
Institutes of Health).
MTT assay
Following transfection for 24 h, cells were
harvested and a total of 2x104 cells were seeded onto a
96-well plate. Cell proliferation was examined using an MTT assay
(Sigma-Aldrich; Merck KGaA) at days 1, 2, 3 and 4 post-inoculation.
Briefly, 20 µl of MTT solution was added into each well followed by
incubation at 37˚C for 4 h. Dimethyl sulfoxide was subsequently
used to dissolve formazan. The absorbance was read at a wavelength
of 450 nm and measured using a microplate reader (Bio-Rad
Laboratories, Inc.).
Transwell assay
The migratory abilities of cells were examined using
a Transwell assay. For the migration assay, a total of
1x105 cells in serum-free endothelial cell growth medium
were seeded into the upper chamber of Transwell plates (BD
Biosciences) with 8-µm pore size. Subsequently, 500 µl of culture
medium supplemented with 10% FBS was added into the lower chamber.
Following overnight incubation at 37˚C, non-migratory cells were
removed using a cotton swab, whereas the migrated cells in the
lower chamber were fixed using 4% paraformaldehyde at room
temperature for 30 min and stained with 0.5% crystal violet at room
temperature for 10 min. The numbers of migratory cells were counted
in five randomly selected fields using an inverted light microscope
(magnification, x200; Olympus Corporation).
Cell cycle and apoptosis analysis
Cells were plated onto a six-well plate at a density
of 3x105 cells/well. Cells were then collected using
low-speed centrifugation (1,000 x g at 4˚C for 5 min. Cell pellets
were rinsed and resuspended in PBS, fixed with 70% pre-chilled
ethanol at room temperature for 15 min and stored at 4˚C for two
days. Cells were lysed prior to flow cytometry, centrifuged at
10,000 x g at room temperature for 5 min and then resuspended using
propidium iodide (PI; Sigma-Aldrich; Merck KGaA) staining buffer
containing 50 µl/ml of PI with 250 µl/ml RNase A. To evaluate cell
apoptosis, suspended cells were incubated in the dark at 4˚C for 30
mins and stained with 5 µl Annexin V-FITC (JingMei Biotech Co.,
Ltd.). Both cell cycle distribution and the number of apoptopic
cells were analysed using a flow cytometer (BD Biosciences) and
FlowJo software (version 7.6; FlowJo LLC).
Statistical analysis
Data were presented as the mean ± standard deviation
and analysed using SPSS 17.0 software (SPSS, Inc.). Any significant
difference between groups was analysed using Student's t-test or
one-way ANOVA followed by Student-Newman-Keuls test. P<0.05 was
considered to indicate a statistically significant difference.
Results
MALAT1 is upregulated in DVT
samples
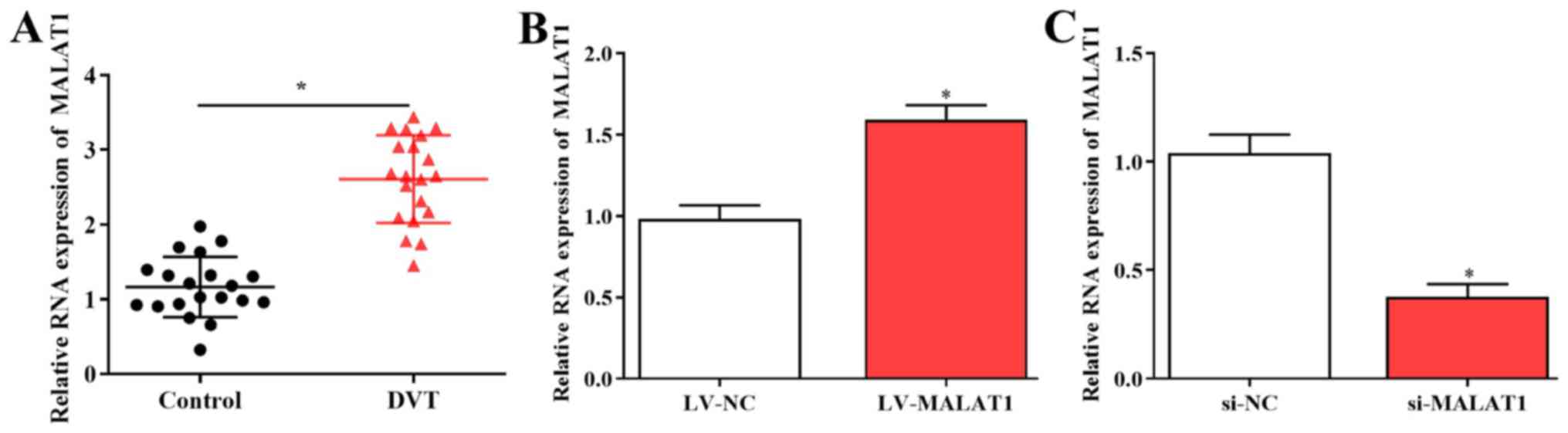
The expression of MALAT1 was evaluated in 20 DVT
samples and healthy controls using RT-qPCR. The results revealed
that expression of MALAT1 was significantly increased in DVT
tissues compared with controls (Fig.
1A). To study the role of MALAT1 in DVT, EPCs isolated from
patients were transfected with LV-MALAT1, si-MALAT1 or control
vectors, and transfection efficiencies were measured using RT-qPCR
(Fig. 1B and C).
MALAT1 regulates the biological
behaviors of EPCs
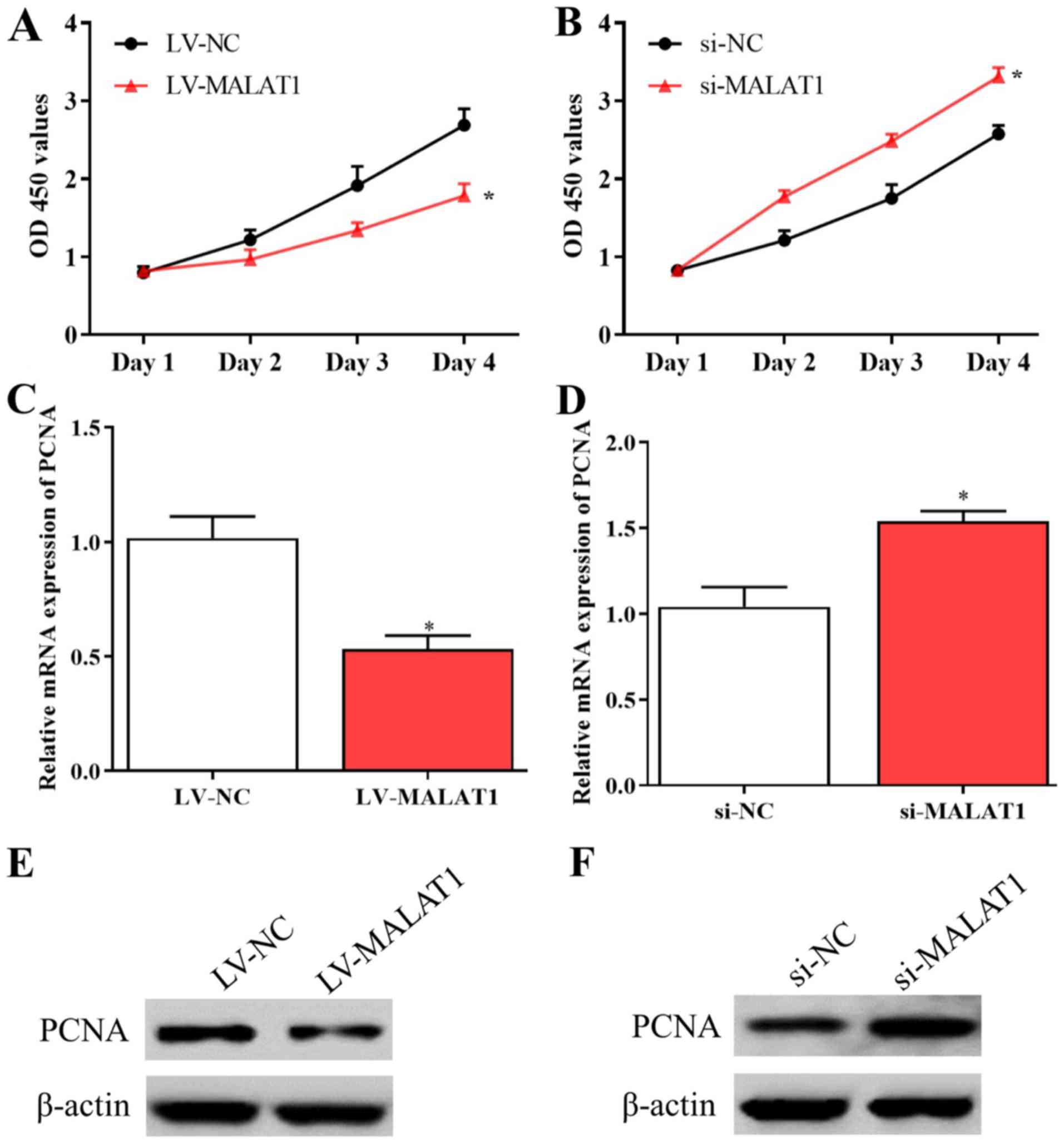
Further experiments were conducted to explore the
effects of MALAT1 on the proliferation and migration of EPCs. The
results of the MTT assay suggested that the proliferative activity
of EPCs was inhibited by overexpressed MALAT1 and enhanced by
silenced MALAT1 compared with negative controls (Fig. 2A and B). Consistent with these data, the levels
of PCNA significantly decreased in EPCs overexpressing MALAT1 and
significantly increased in cells with silenced MALAT1 compared with
corresponding negative controls (Fig.
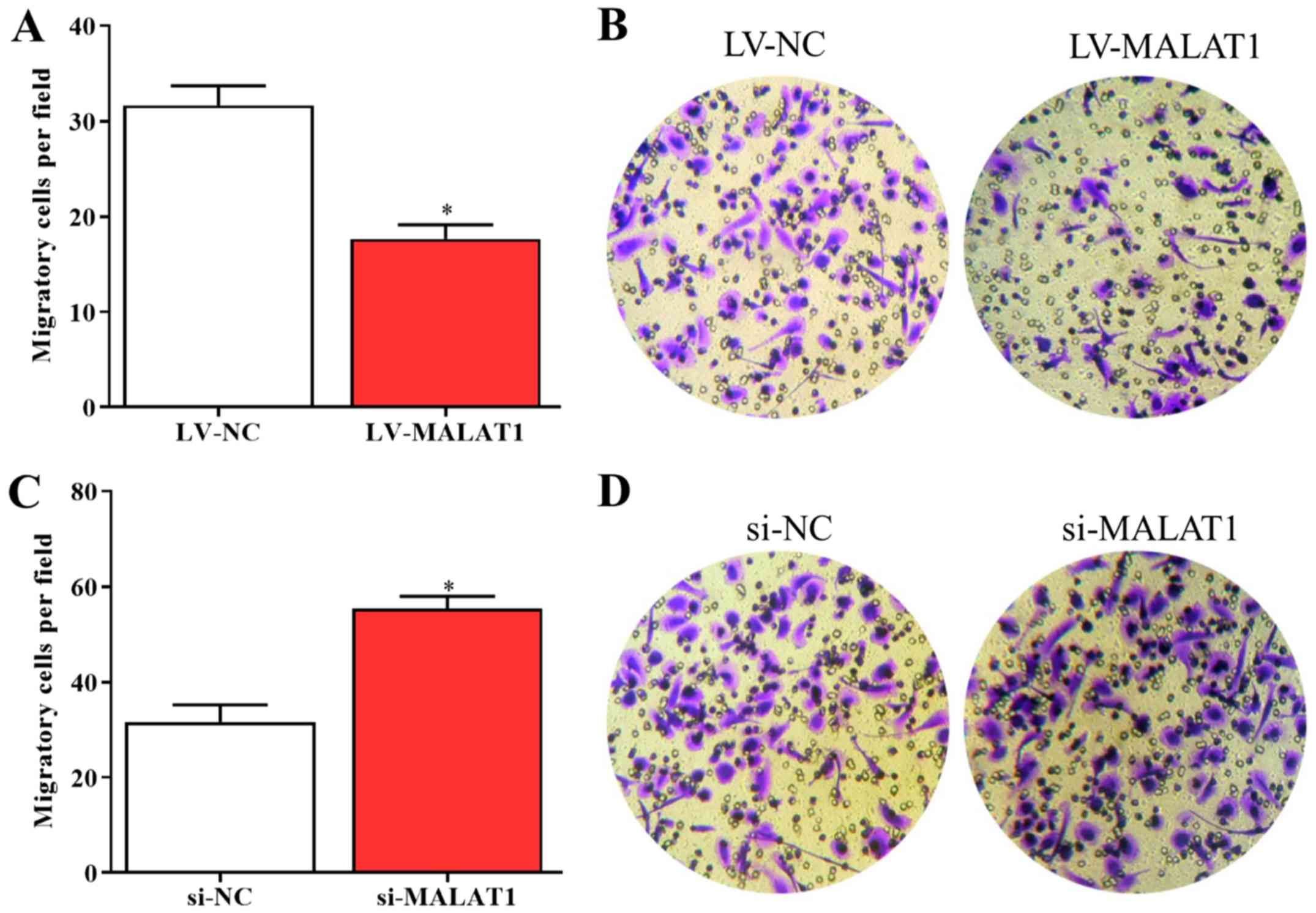
2C-F). In addition, the results of the Transwell assay
indicated that the migration of LV-MALAT1-transfected EPCs
significantly decreased whereas the migratory abilities of EPCs
significantly increased by silenced MALAT1 compared with negative
controls (Fig. 3A-D). The results
revealed that the growth and migration of EPCs could be inhibited
by upregulated MALAT1 and enhanced by downregulated MALAT1.
MALAT1 affects cell cycle arrest and
apoptosis in EPCs
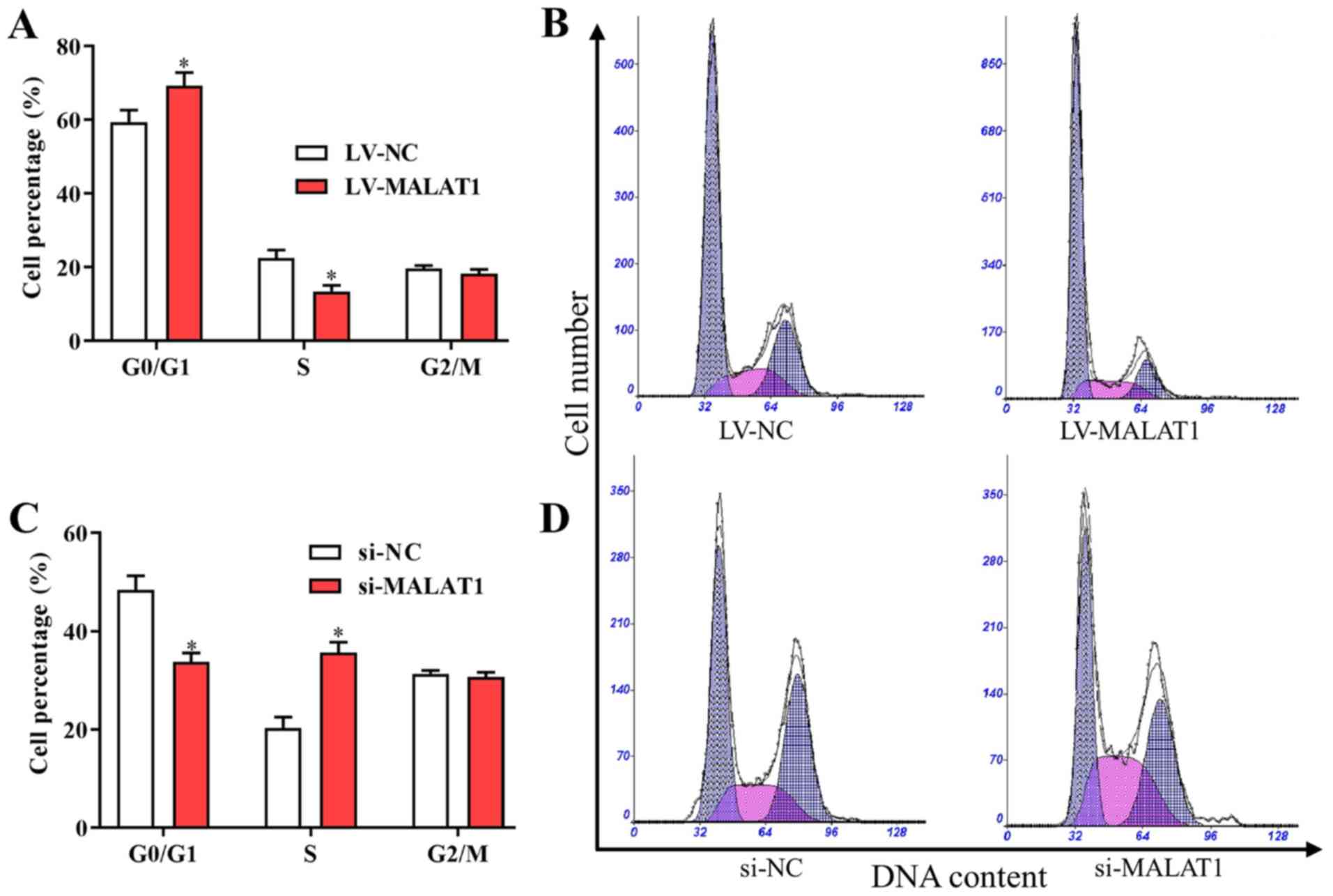
Based on the aforementioned findings, MALAT1 was
associated with the proliferation and metastasis of EPCs in
vitro. Furthermore, to investigate the potential effects of
MALAT1 on the cell cycle and apoptosis, the cell cycle distribution
and apoptosis of EPCs transfected with LV- MALAT1 or si-MALAT1 were
also examined. The results indicated that the number of cells in
G0/G1 was increased and the number of cells in S phase was
decreased following transfection with LV-MALAT1 (Fig. 4A and B). In comparison, the percentage of cells
in the G0/G1 phase significantly decreased, while cells in the S
phase significantly increased in cells with silenced MALAT1
compared with negative controls (Fig.
4C and D). Additionally, flow
cytometry data indicated that overexpressed MALAT1 induced
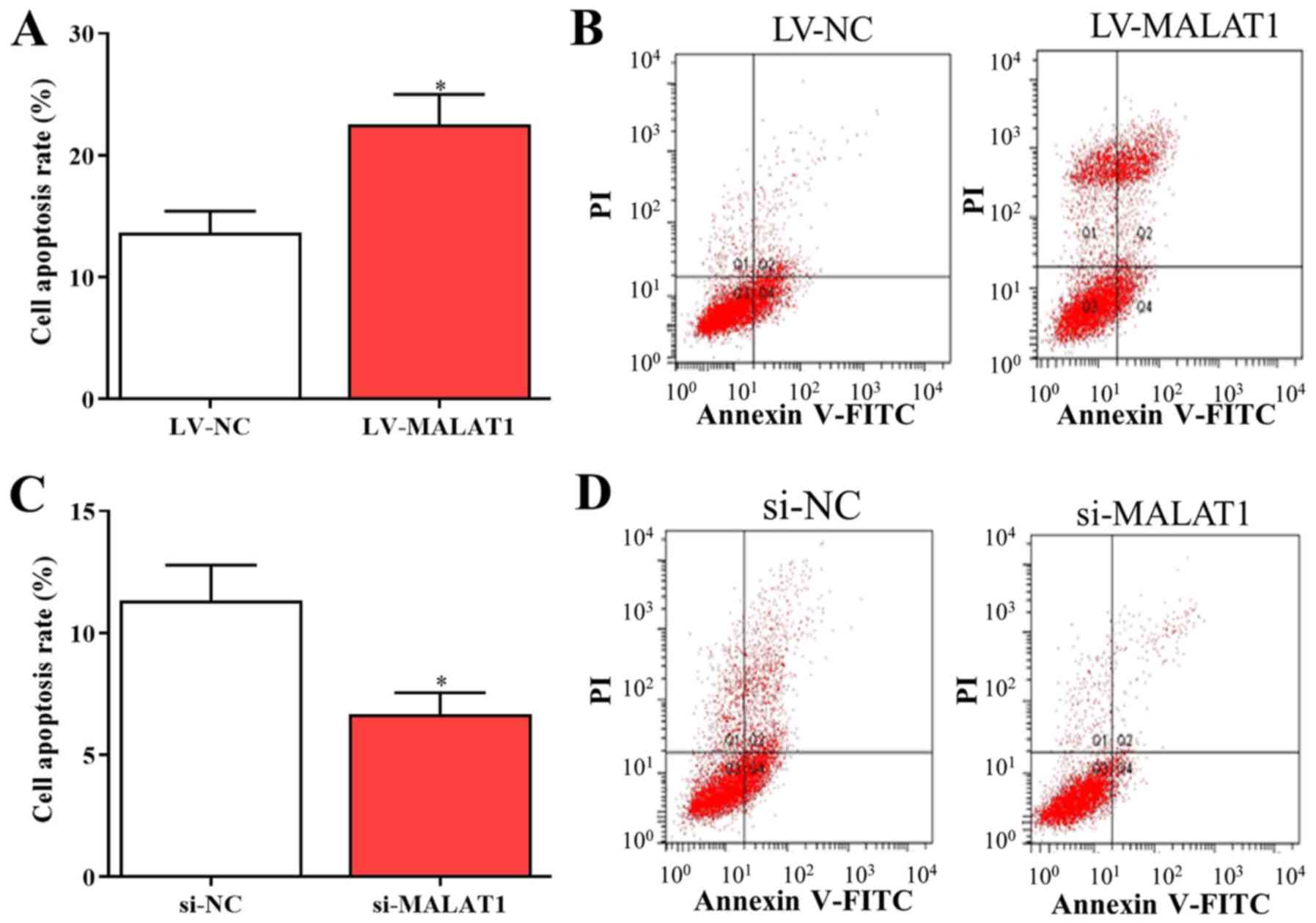
apoptosis of EPCs (Fig. 5A and
B), while cell apoptosis was
significantly reduced by silenced MALAT1 compared with negative
controls (Fig. 5C and D). These findings indicated that the
upregulation of MALAT1 was able to arrest the cell cycle in the
G0/G1 phase to promote cell apoptosis, and vice versa.
The Wnt/β-catenin signaling pathway is
a potential downstream target of MALAT1 in DVT
Further experiments were performed to investigate
whether MALAT1 modulates the proliferation and migration of EPCs
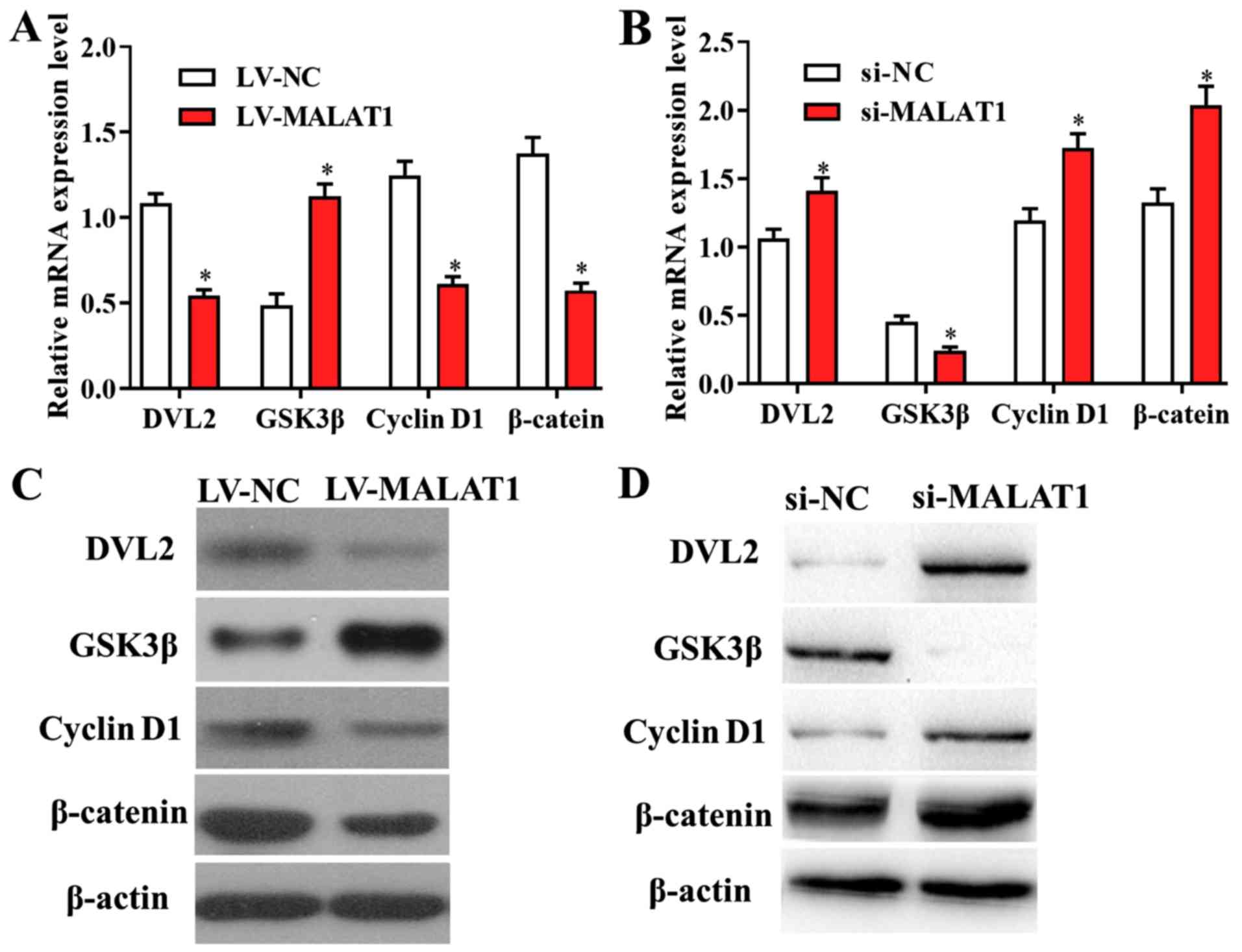
though targeting its downstream signaling pathways. RT-qPCR results
revealed that the levels of genes involved in Wnt/β-catenin
signalling were altered in cells transfected with LV-MALAT1
compared with negative controls, suggesting that overexpressed
MALAT1 significantly suppressed the activity of the Wnt/β-catenin
pathway, and vice versa (Fig. 6A and
B). In addition, these findings were
confirmed by western blot analysis, as the protein levels of
Wnt/β-catenin-associated genes were also affected by overexpressed
or silenced MALAT1 in EPCs (Fig. 6C
and D). The data suggested that the
Wnt/β-catenin signalling pathway is a potential downstream target
of MALAT1 in DVT.
Wnt/β-catenin signaling is involved in
the growth and migration of EPCs
Since the Wnt/β-catenin pathway may be a novel
target of MALAT1 in DVT, its effects on the growth and migration of
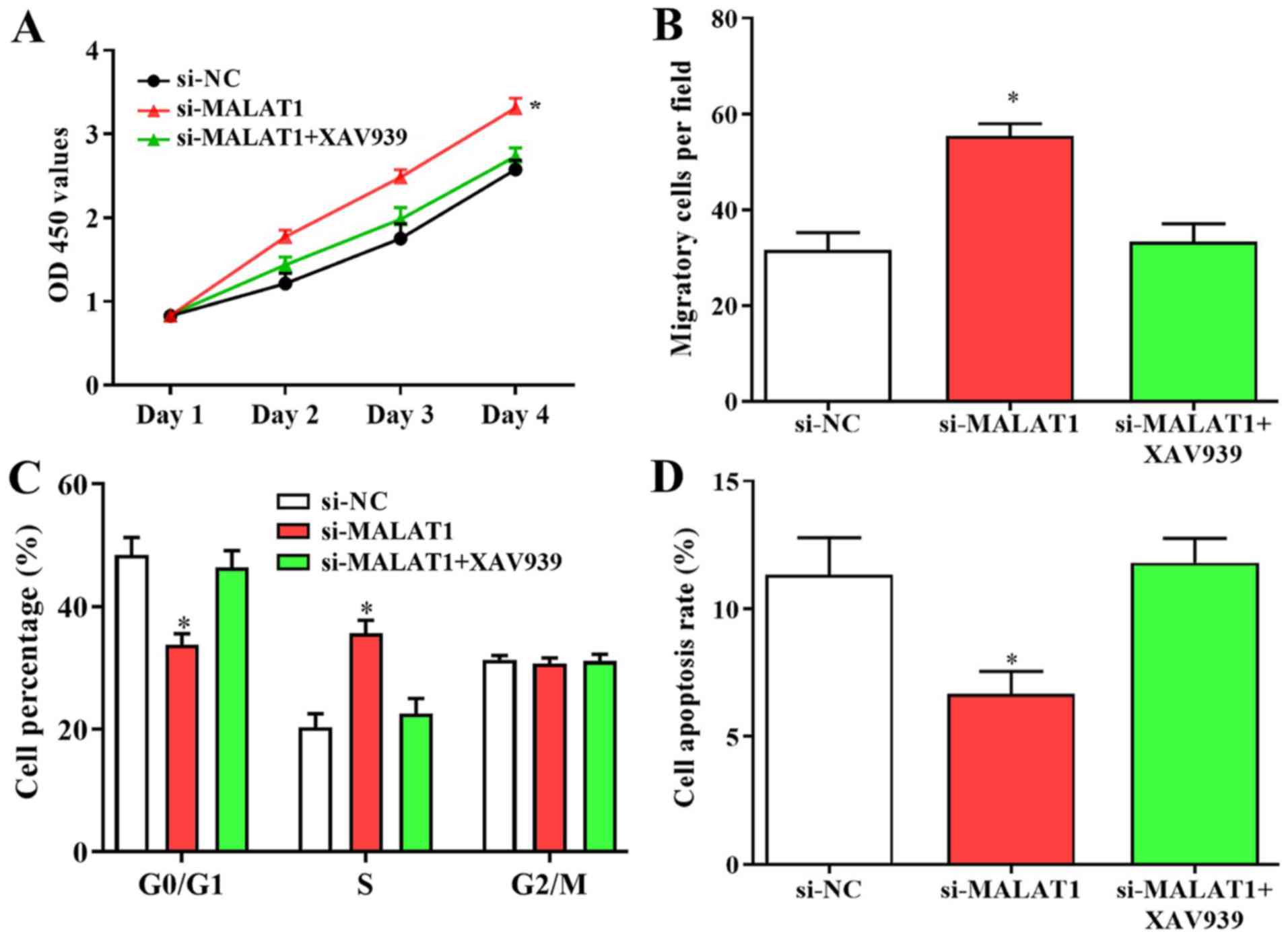
EPCs were also investigated. Enhanced cell proliferation and
migration caused by downregulated MALAT1 was suppressed following
treatment with the Wnt/β-catenin inhibitor XAV939 (Fig. 7A and B). Furthermore, the number of cells in
G0/G1 phase was increased and the number of cells in S phase was
decreased following XAV939 treatment, and suppressed apoptosis in
cells transfected with si-MALAT1 were reversed by Wnt/β-catenin
inhibitor transfection (Fig. 7C and
D). The results revealed that
changes in biological behaviors of EPCs caused by silenced MALAT1
were suppressed by the inhibition of Wnt/β-catenin signalling,
suggesting that the Wnt/β-catenin signalling pathway may serve a
role in MALAT1-modulated growth and the migration of EPCs.
Discussion
In the present study, the detailed expression
profile and potential functions of MALAT1 in DVT were elucidated.
The results indicated that MALAT1 was upregulated in DVT samples
compared with healthy controls. Additionally, MALAT1 was able to
regulate the biological behaviors of EPCs, including proliferation,
migration, cell cycle arrest and apoptosis. The growth and
migration of EPCs was inhibited by upregulated MALAT1 and enhanced
by downregulated MALAT1. Furthermore, upregulation of MALAT1 was
able to arrest cell cycle in the G0/G1 phase to promote cell
apoptosis, and vice versa. Consistent with the present findings,
MALAT1 is involved in the growth and migration of various types of
cells in numerous diseases, including esophageal squamous cell
carcinoma, pancreatic cancer and colorectal cancer (25-27).
The growth and migration of EPCs involves a number
of different pathways, and among these, the Wnt/β-catenin axis has
been widely investigated. It serves essential roles in the
development of various vascular diseases such as hypertensive heart
disease (16-19).
Furthermore, it is associated with the proliferation,
differentiation and apoptosis of EPCs by regulating its downstream
pathways including those of MYC and CDKN1A (20,21).
Additionally, MALAT1 could regulate the growth and migration of
numerous types of cells including neural stem cells and ovarian
cancer cells, via the Wnt signaling pathway by affecting the levels
of DVL2, GSK3β, β-catenin and cyclin D1 (22,23). In
the present study, the data suggested that Wnt/β-catenin signaling
is a novel downstream target of MALAT1 in DVT. The changes in
biological behaviors in EPCs caused by silenced MALAT1 were
reversed by the treatment with Wnt/β-catenin inhibitors. Enhanced
cell proliferation and migration caused by downregulated MALAT1 was
suppressed following treatment with Wnt/β-catenin inhibitor XAV939.
Similarly, Wnt signaling can be regulated by other lncRNAs such as
HOTAIR and LINC01197, which can affect cartilage damage and
pancreatic adenocarcinoma cell proliferation (28,29).
Consistent with the present findings, Wnt signaling blockage using
XAV939 affected the growth and migration of numerous types of
cells, such as lung adenocarcinoma A549 and breast cancer cells
(30-32).
Furthermore, the shift of cells from G0/G1 to S stage and
suppressed apoptosis in cells transfected with si-MALAT1 were
suppressed by Wnt/β-catenin inhibitors. However, there are some
limitations to the present study. For example, a time-dependent
model was observed for some indexes, but longer periods could be
used till the maximum index value is reached. Additionally, the
subcellular distribution of PCNA and β-catenin could be examined by
immunostaining to investigate the nuclear expression of these
genes. Furthermore, in vivo xenograft experiments should be
performed to confirm the existing findings. Taken together, the
MALAT1/Wnt/β-catenin axis could serve a role in the proliferation
and migration of EPCs, contributing to the progression of DVT. This
novel signaling pathway could be a potential therapeutic target for
the treatment of patients with DVT.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BD and YD designed the study. BD, JW, SZ and XM
performed the experiments and analyzed the data. BD and YD drafted
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The First Affiliated Hospital of Jinzhou Medical University
(Jinzhou, China). Written informed consent was obtained from all
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sáez-Giménez B, Berastegui C, Loor K,
López-Meseguer M, Monforte V, Bravo C, Santamaría A and Roman A:
Deep vein thrombosis and pulmonary embolism after solid organ
transplantation: An unresolved problem. Transplant Rev (Orlando).
29:85–92. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Weitz JI, Eikelboom JW and Samama MM: New
antithrombotic drugs: Antithrombotic therapy and prevention of
thrombosis, 9th ed: American college of chest physicians
evidence-based clinical practice guidelines. Chest. 141 (Suppl
2):e120S–e151S. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bakogiannis C, Tousoulis D, Androulakis E,
Briasoulis A, Papageorgiou N, Vogiatzi G, Kampoli AM, Charakida M,
Siasos G, Latsios G, et al: Circulating endothelial progenitor
cells as biomarkers for prediction of cardiovascular outcomes. Curr
Med Chem. 19:2597–2604. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Li W and Li X: Endothelial progenitor
cells accelerate the resolution of deep vein thrombosis. Vascul
Pharmacol. 83:10–16. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Modarai B, Burnand KG, Sawyer B and Smith
A: Endothelial progenitor cells are recruited into resolving venous
thrombi. Circulation. 111:2645–2653. 2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang W, Li C, Li W, Kong L, Qian A, Hu N,
Meng Q and Li X: MiR-150 enhances the motility of EPCs in vitro and
promotes EPCs homing and thrombus resolving in vivo. Thromb Res.
133:590–598. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hsiao J, Yuan TY, Tsai MS, Lu CY, Lin YC,
Lee ML, Lin SW, Chang FC, Liu Pimentel H, Olive C, et al:
Upregulation of haploinsufficient gene expression in the brain by
targeting a long non-coding RNA improves seizure phenotype in a
model of Dravet syndrome. EBioMedicine. 9:257–277. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Holdt LM, Sass K, Gabel G, Bergert H,
Thiery J and Teupser D: Expression of Chr9p21 genes CDKN2B
(p15(INK4b)), CDKN2A (p16(INK4a), p14(ARF)) and MTAP in human
atherosclerotic plaque. Atherosclerosis. 214:264–270.
2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ballantyne M, Pinel K, Dakin R, Vesey A,
Diver L, Mackenzie R, Garcia R, Welsh P, Sattar N, Hamilton G, et
al: Smooth muscle enriched long noncoding RNA (SMILR) regulates
cell proliferation. Circulation. 133:2050–2065. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sallam T, Jones M, Thomas BJ, Wu X,
Gilliland T, Qian K, Eskin A, Casero D, Zhang Z, Sandhu J, et al:
Transcriptional regulation of macrophage cholesterol efflux and
atherogenesis by a long noncoding RNA. Nat Med. 24:304–312.
2018.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Boulberdaa M, Scott E, Ballantyne M,
Garcia R, Descamps B, Angelini GD, Brittan M, Hunter A, McBride M,
McClure J, et al: A role for the long noncoding RNA SENCR in
commitment and function of endothelial cells. Mol Ther. 24:978–990.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ishii N, Ozaki K, Sato H, Mizuno H, Saito
S, Takahashi A, Miyamoto Y, Ikegawa S, Kamatani N, Hori M, et al:
Identification of a novel non-coding RNA, MIAT, that confers risk
of myocardial infarction. J Hum Genet. 51:1087–1099.
2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vausort M, Wagner DR and Devaux Y: Long
noncoding RNAs in patients with acute myocardial infarction. Circ
Res. 115:668–677. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Arslan S, Berkan Ö, Lalem T, Özbilüm N,
Göksel S, Korkmaz Ö, Çetin N and Devaux Y: Cardiolinc™
network. Long non-coding RNAs in the atherosclerotic plaque.
Atherosclerosis. 266:176–181. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhuo Y, Zeng Q, Zhang P, Li G, Xie Q and
Cheng Y: Functional polymorphism of lncRNA MALAT1 contributes to
pulmonary arterial hypertension susceptibility in Chinese people.
Clin Chem Lab Med. 55:38–46. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hermans KC and Blankesteijn WM: Wnt
signaling in cardiac disease. Compr Physiol. 5:1183–1209.
2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhao Y, Wang C, Wang C, Hong X, Miao J,
Liao Y, Zhou L and Liu Y: An essential role for Wnt/β-catenin
signaling in mediating hypertensive heart disease. Sci Rep.
8(8996)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cohen ED, Tian Y and Morrisey EE: Wnt
signaling: An essential regulator of cardiovascular
differentiation, morphogenesis and progenitor self-renewal.
Development. 135:789–798. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gay A and Towler DA: Wnt signaling in
cardiovascular disease: Opportunities and challenges. Curr Opin
Lipidol. 28:387–396. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nayak G, Odaka Y, Prasad V, Solano AF, Yeo
EJ, Vemaraju S, Molkentin JD, Trumpp A, Williams B, Rao S and Lang
RN: Developmental vascular regression is regulated by a
Wnt/β-catenin, MYC and CDKN1A pathway that controls cell
proliferation and cell death. Development. 145:
pii(dev154898)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Du Y, Zhang S, Yu T, Du G, Zhang H and Yin
Z: Wnt3a is critical for endothelial progenitor cell-mediated
neural stem cell proliferation and differentiation. Mol Med Rep.
14:2473–2482. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Guo C and Wang X, Chen LP, Li M, Li M, Hu
YH, Ding WH and Wang X: Long non-coding RNA MALAT1 regulates
ovarian cancer cell proliferation, migration and apoptosis through
Wnt/β-catenin signaling pathway. Eur Rev Med Pharmacol Sci.
22:3703–3712. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li GQ, Fang YX, Liu Y, Meng FR, Wu X,
Zhang CW, Zhang Y, Liu D and Gao B: MALAT1-driven inhibition of Wnt
signal impedes proliferation and inflammation in fibroblast-like
synoviocytes through CTNNB1 promoter methylation in rheumatoid
arthritis. Hum Gene Ther. 30:1008–1022. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hu L, Wu Y, Tan D, Meng H, Wang K, Bai Y
and Yang K: Up-regulation of long noncoding RNA MALAT1 contributes
to proliferation and metastasis in esophageal squamous cell
carcinoma. J Exp Clin Cancer Res. 34(7)2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cheng Y, Imanirad P, Jutooru I, Hedrick E,
Jin UH, Rodrigues Hoffman A, Leal de Araujo J, Morpurgo B, Golovko
A and Safe S: Role of metastasis-associated lung adenocarcinoma
transcript-1 (MALAT-1) in pancreatic cancer. PLoS One.
13(e0192264)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xu Y, Zhang X, Hu X, Zhou W, Zhang P,
Zhang J, Yang S and Liu Y: The effects of lncRNA MALAT1 on
proliferation, invasion and migration in colorectal cancer through
regulating SOX9. Mol Med. 24(52)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ling J, Wang F, Liu C, Dong X, Xue Y, Jia
X, Song W and Li Q: FOXO1-regulated lncRNA LINC01197 inhibits
pancreatic adenocarcinoma cell proliferation by restraining
Wnt/β-catenin signaling. J Exp Clin Cancer Res.
38(179)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhou W, He X, Chen Z, Fan D, Wang Y, Feng
H, Zhang G, Lu A and Xiao L: LncRNA HOTAIR-mediated Wnt/β-catenin
network modeling to predict and validate therapeutic targets for
cartilage damage. BMC Bioinformatics. 20(412)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li C, Zheng X, Han Y, Lv Y, Lan F and Zhao
J: XAV939 inhibits the proliferation and migration of lung
adenocarcinoma A549 cells through the WNT pathway. Oncol Lett.
15:8973–8982. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Bilir B, Kucuk O and Moreno C: Wnt
signaling blockage inhibits cell proliferation and migration, and
induces apoptosis in triple-negative breast cancer cells. J Transl
Med. 11(280)2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bao R, Christova T, Song S, Angers S, Yan
X and Attisano L: Inhibition of tankyrases induces Axin
stabilization and blocks Wnt signalling in breast cancer cells.
PLoS One. 7(e48670)2012.PubMed/NCBI View Article : Google Scholar
|