Introduction
Idiopathic pulmonary fibrosis (IPF) is a lung
disease associated with a lower average survival time of ~2-5 years
after initial diagnosis (1). For
instance, Wolters et al (2)
reported that 50% of patients have a survival time of 3-5 years.
The characteristics of IPF include the proliferation of
myofibroblasts, excessive accumulation of extracellular matrix and
abnormal proliferation of alveolar epithelial cells (2). Moreover, fibroblasts in patients with
IPF may originate from the following three sources: i) Excessive
proliferation of resident fibroblasts in lung tissue; ii) the
epithelial-mesenchymal transition (EMT) of alveolar epithelial
cells into fibroblasts; and iii) excessive inflammation, which
attracts fibroblasts from other tissues and facilitates their
migration to the lungs (3). In
total, two drugs are currently available to slow the progression of
IPF, including prednisone and nintedanib (4). However, as the pathogenesis of IPF
remains unknown, these drugs can only alleviate the progression of
pulmonary fibrosis; thus, the disease cannot be fully treated
(4).
MicroRNAs (miRNAs/miRs) are non-coding RNA molecules
that have been revealed to interact with a variety of mRNAs and
affect the expression of target genes (5). Furthermore, miRNAs serve important
roles in the pathogenesis of IPF. For example, Rubio et al
(6) found that epigenetic gene
silencing mediated by the ribonucleoprotein complex multicomponent
RNA-protein complex results from reduced levels of miRNA lethal
7 d in IPF. Bodempudi et al (7) also reported that miR-210
promoted the proliferation of IPF fibroblasts to resist hypoxia.
Additionally, miR-101 attenuates IPF by inhibiting SMAD3,
thus reducing transforming growth factor (TGF)-β expression and
inhibiting fibroblast proliferation and activation (8). Other previous studies have shown that
the downregulation of miR-9 targets anoctamin-1, which
results in decreased expression levels of TGF-β and SMAD3, slowed
progression of IPF and reduced fibroblast proliferation in
bleomycin-induced mice (9,10).
Therefore, the aim of the present study was to
comprehensively identify and quantify miRNAs involved in the
occurrence and development of IPF using machine learning. The
results will improve the understanding of miRNAs in patients with
IPF, as well as demonstrate the differential expression of miRNAs
and the function of target genes during IPF. In addition, the
current results may provide miRNA expression profiles and potential
biomarkers for IPF, thus improving diagnosis and treatment
strategies.
Materials and methods
Data collection and patient
population
In the discovery phase, array data (GSE129126) from
the Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/geo/) were used as the
finding set. This dataset included 28 samples (eight healthy lung
tissues and 20 lung tissues from patients with IPF) from
individuals with forced vital capacities of >80, 50-80 or
<50%, respectively (8). GSE13316
array data were also included as the validation set with 20 samples
(ten healthy lung tissues and ten lung tissues from patients with
IPF) (11).
Paraffin-embedded tissue samples were collected from
4 men with IPF (age range, 56-73 years; median age, 63 years) or 3
age-matched men without fibrotic lung disease (controls) at
Shenyang Thoracic Hospital and Fushun Central Hospital of Liaoning
Province. All patients were enrolled between August 2001 and
December 2016 (Table SI). The
research was approved by the Ethics Committee of Shenyang Thoracic
Hospital and Fushun Central Hospital of Liaoning Province. All
selected patients or their families provided oral informed consent
for participation in the study.
Bioinformatics analysis Identification
of differentially expressed miRNAs in patients with IPF
The K-nearest neighbor and β-mixture quintile
dilation methods (12) were used to
perform imputation and normalization in the GSE129126 dataset.
Differences among the three groups were tested using single factor
ANOVA with post hoc Fisher's LSD. Results with P<0.05 were
considered significant. To further analyze the GEO data, glmnet
(version 4.0-2; http://cran.r-project.org/web/packages/glmnet/index.html)
(13) and e1071 (version 1.7-3;
https://cran.r-project.org/web/packages/e1071/index.html)
R packages were used to establish a least absolute shrinkage and
selector operation (LASSO) model and a support vector
machine-recursive feature elimination (SVM-RFE) model,
respectively. After primary filtration, a LASSO algorithm, with
penalty parameter tuning conducted using 5-fold cross-validation,
was constructed to select candidate miRNAs. In R 3.50 software
(14), the minimum absolute
contraction of glmnet and selection operator (LASSO) Cox regression
were used to determine the best candidate, ignoring miRNAs with a
regression coefficient of <0.1. Then, miRNAs from the LASSO and
SVM-RFE algorithms and ANOVA were combined; the obtained miRNAs
were considered potential markers.
Validation of the IPF signature
The validation set was used for internal validation.
The R package ‘pROC’ (version 1.16.2; https://CRAN.R-project.org/package=pROC) was used to
analyze the receiver operating characteristic curve (ROC) with area
under the curve (AUC) analysis. miRNAs with an AUC >0.7 were
considered ideal biomarkers (15).
Construction of the miRNA/mRNA
network
miRNA target sites were retrieved using miRDB
(version 6.0; www.mirdb.org) (16). mRNAs with a target score >90 were
selected as target genes and used to construct the miRNA/mRNA
network using Cytoscape software (version 3.8.0; https://cytoscape.org/).
Functional enrichment analysis
The Kyoto Encyclopedia of Genes and Genomes (KEGG;
https://www.genome.jp/kegg/) annotation
system and cumulative hypergeometric distribution were used to
determine the enrichment pathways of the targeted mRNAs.
ClusterProfiler (version 3.11) (17)
was used to annotate and analyze KEGG data. The screening
conditions were as follows: Enrichment score >2, and false
discovery rate <0.05.
Histological analysis
Formalin-fixed (formalin concentration, 10%; 12-24 h
at room temperature), paraffin-embedded lung sections (thickness,
2-5 µm) were deparaffinized, stained with hematoxylin (3-5 min) and
eosin (2-3 min) at room temperature and diagnosed by a lung
pathologist using a blinded method. Tissue sections were dehydrated
in xylene I (15 min), xylene II (5 min), 100% ethanol (twice for 5
min), 95% ethanol (2 min), 85% ethanol (2 min) and 75% ethanol (2
min) at room temperature. After soaking for 2 min in distilled
water, the slices were stained with hematoxylin (Beijing Solarbio
Science & Technology Co., Ltd.; cat. no. H8070) for 20 min and
soaked in tap water for 3 min at room temperature. The slices were
then re-differentiated in 1% hydrochloric acid-ethanol solution (5
sec) and washed with tap water for 10 min to return to a blue
color. Slices were then re-stained with eosin (Beijing Solarbio
Science & Technology Co., Ltd.; cat. no. G1100) for 1 min and
washed with distilled water for 30 sec to terminate the staining at
room temperature. Samples were then sealed with neutral resin glue
and observed under an optical microscope (Nikon Corporation;
magnification, x200).
Extraction of miRNAs and detection
using reverse transcription-quantitative PCR (RT-qPCR)
A miRNeasy FFPE kit (Qiagen GmbH; cat. no. 217504)
was used to extract miRNAs from paraffin-embedded samples from the
IPF and control groups. The extracted miRNAs were reverse
transcribed to cDNA using miScript II RT kits (Qiagen GmbH; cat.
no. 218160) at 42˚C for 60 min. cDNA was stored in a -20˚C
refrigerator until subsequent analysis using RT-qPCR, which was
performed according to a previously described method to amplify
miRNAs (18). Reactions were
conducted using SYBR Green PCR Master Mix (Thermo Fisher
Scientific, Inc.; cat. no. 4309155) following the manufacturer's
instructions for RT-qPCR. Triplicate reactions were performed in a
QuantStudio 7 system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) using the following thermocycling conditions: Initial
denaturation at 95˚C for 8 min, followed by 40 cycles of 95˚C for
15 sec, 60˚C for 15 sec, 72˚C for 15 sec, and a final extension
step at 55˚C for 15 sec. Using the 2-ΔΔCq method
(19), the relative expression
levels of hsa-miR-221 hsa-miR-524-5p, hsa-miR-194,
hsa-miRPlus-E1092, hsa-miR-17 and hsa-miR-133a
(Table SII) were normalized to U6
small nuclear RNA.
Statistical analysis
If the data were normally distributed, the
measurement data between the two groups were compared using the
independent sample t-test, and the measurement data of ≥3 groups
were compared using Fisher's and Welch's one-way ANOVA with post
hoc Fisher's LSD. If the results demonstrated that there was a
significant difference, then the non-parametric Mann-Whitney test
was used for comparison of two groups when the data were of skewed
distribution. P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using R version 3.5(14). In each
experiment, data are presented as the mean ± SEM. All experiments
were repeated ≥3 times.
Results
Identification of differentially
expressed miRNAs in patients with IPF using ANOVA
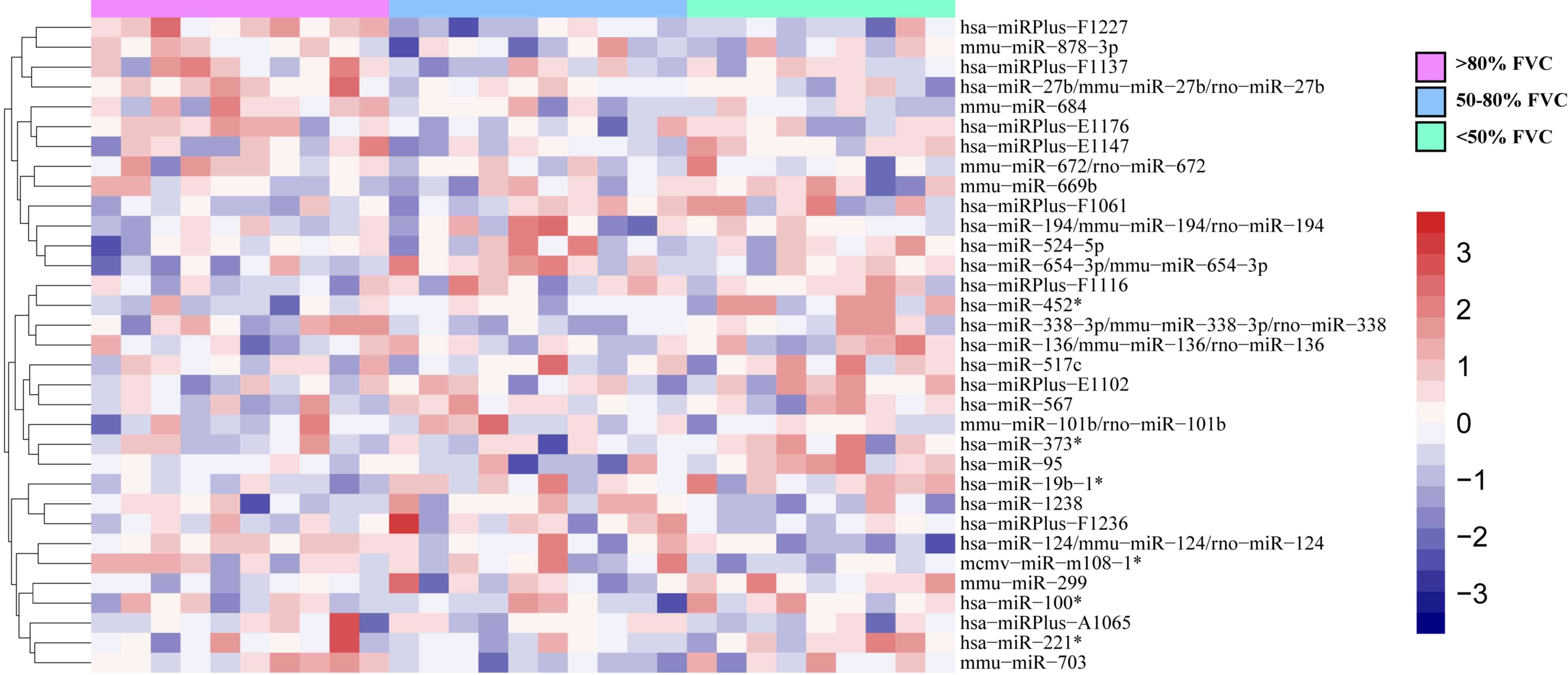
To identify IPF-specific genes and key biomarkers,
ANOVA was used to evaluate gene expression at specific disease
stages. Using the significance criteria (|Log2FC|≥1;
P<0.05) to filter the miRNAs, miRNAs as markers of IPF at
different stages determined according to the pre-bronchodilator
forced vital capacity % values, such as hsa-miR-124, hsa-miR-133a
and hsa-miR-524-5p, were obtained using one-way ANOVA. (Fig. 1).
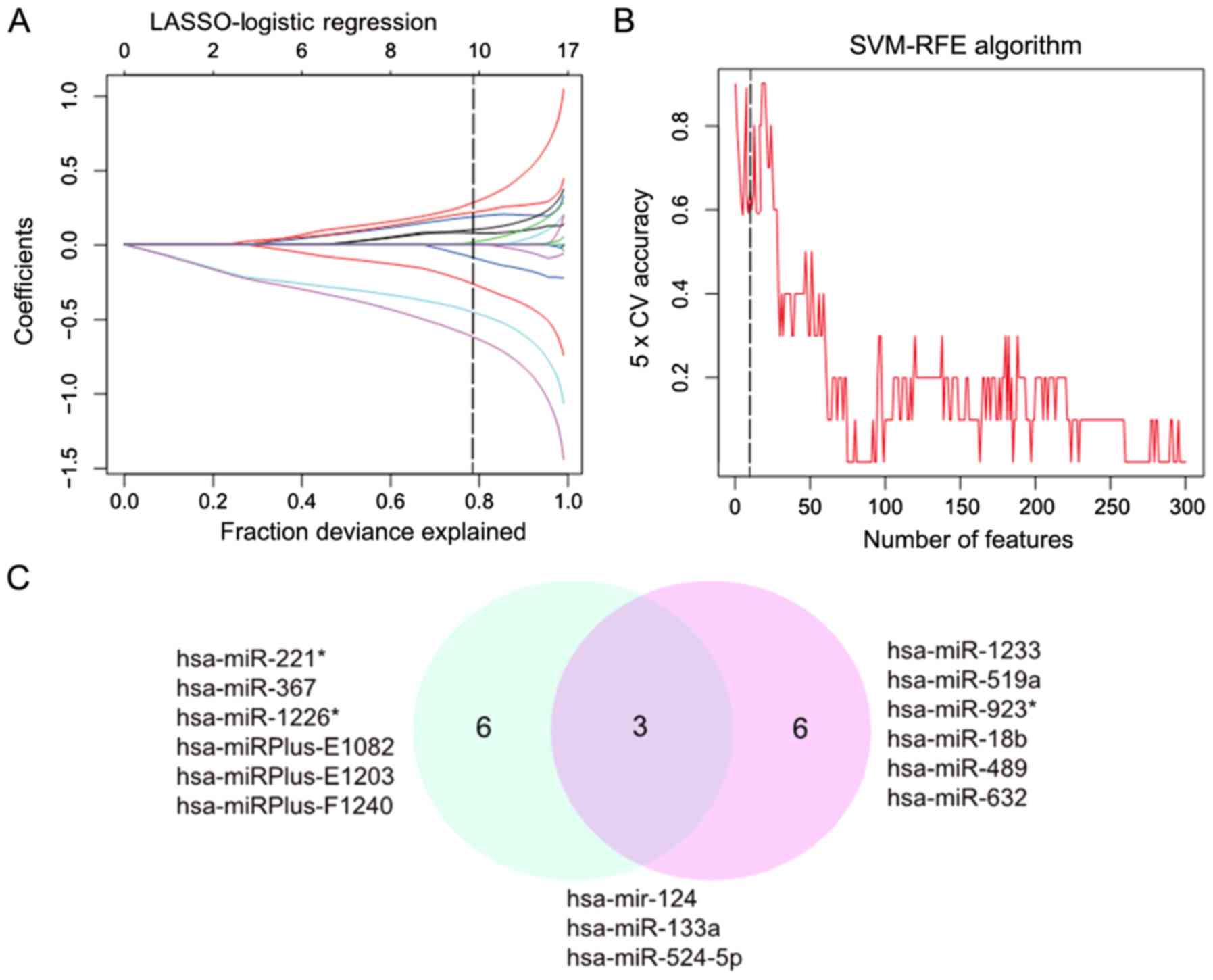
Feature selection of IPF using LASSO
and SVM-RFE
The LASSO algorithm was used to distinguish between
healthy lung tissues and mild IPF. In total, nine eigenvalues were
obtained (Fig. 2A). Of the 300
differentially expressed miRNAs, nine demonstrated features with
non-zero coefficients in the LASSO logistic regression model,
including four upregulated and five downregulated miRNAs, and were
selected on the basis of the training set for mild IPF (Fig. 2B). From the two algorithms,
hsa-miR-124, hsa-miR-133a and hsa-miR-524-5p
were identified (Fig. 2C). Among
these miRNAs, hsa-miR-124 appeared in the three algorithms,
demonstrating the reliability of the association between mild IPF
and miR-124.
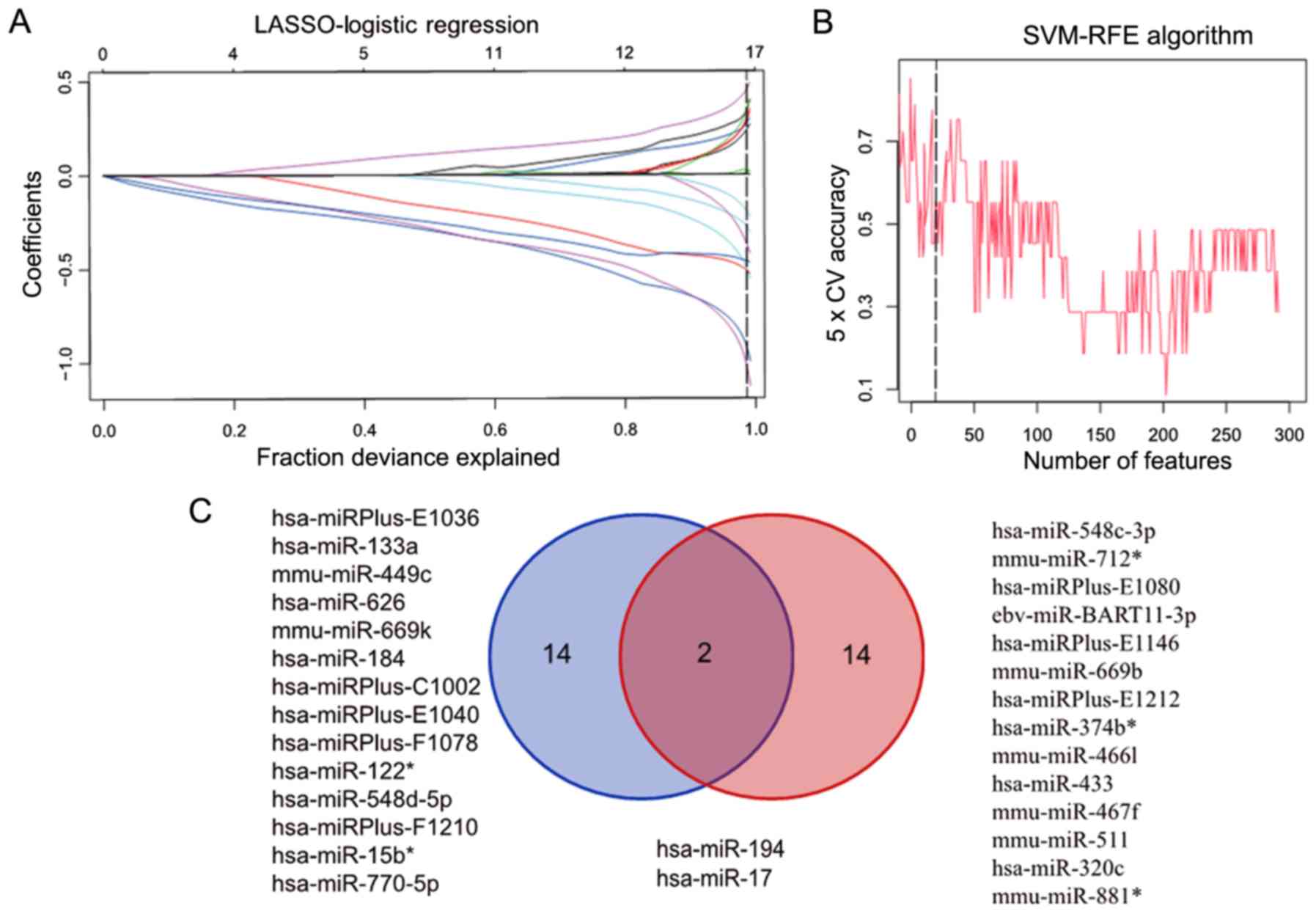
When comparing advanced fibrosis with healthy lung
tissue, the LASSO algorithm calculated a total of 16 features
(Fig. 3A), and SVM-RFE selected the
first 16 features (Fig. 3B). Further
analysis identified that hsa-miR-194 and hsa-miR-17
were found in both algorithms (Fig.
3C).
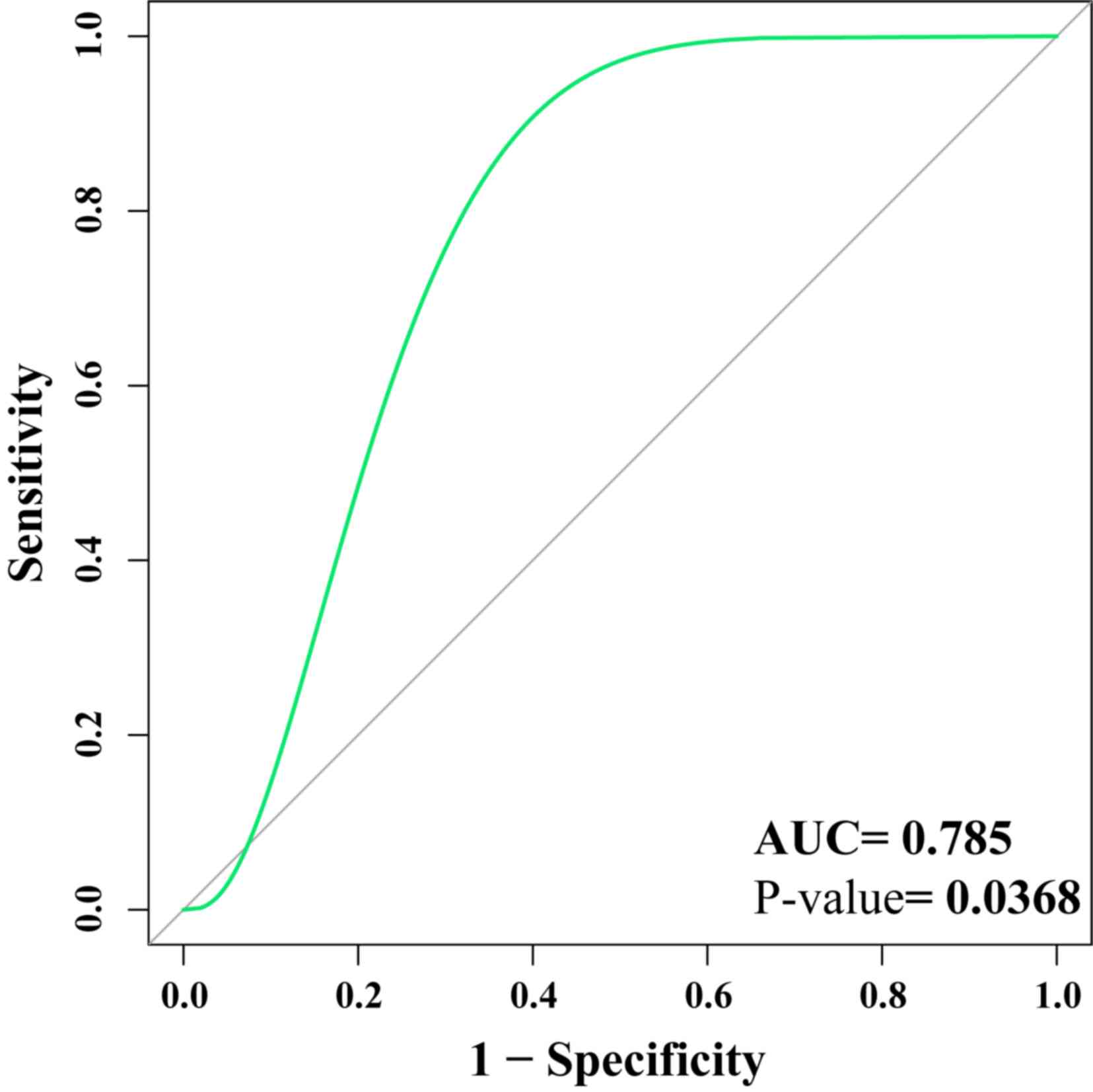
Verification of biomarkers using ROC
curves
GSE13316 was used as the verification set to assess
the potential biomarkers. The results demonstrated that
hsa-miR-124, hsa-miR-194 and hsa-miR-524-5p
could distinguish between healthy tissue and pulmonary fibrosis
tissue (AUC=0.785; P=0.0368), indicating that the biomarkers may be
clinically useful (Fig. 4). The ROC
curve was above the diagonal, indicating a good sensitivity
(96.52%) and specificity (96.32%).
Identification of differential miRNA
expression using RT-qPCR
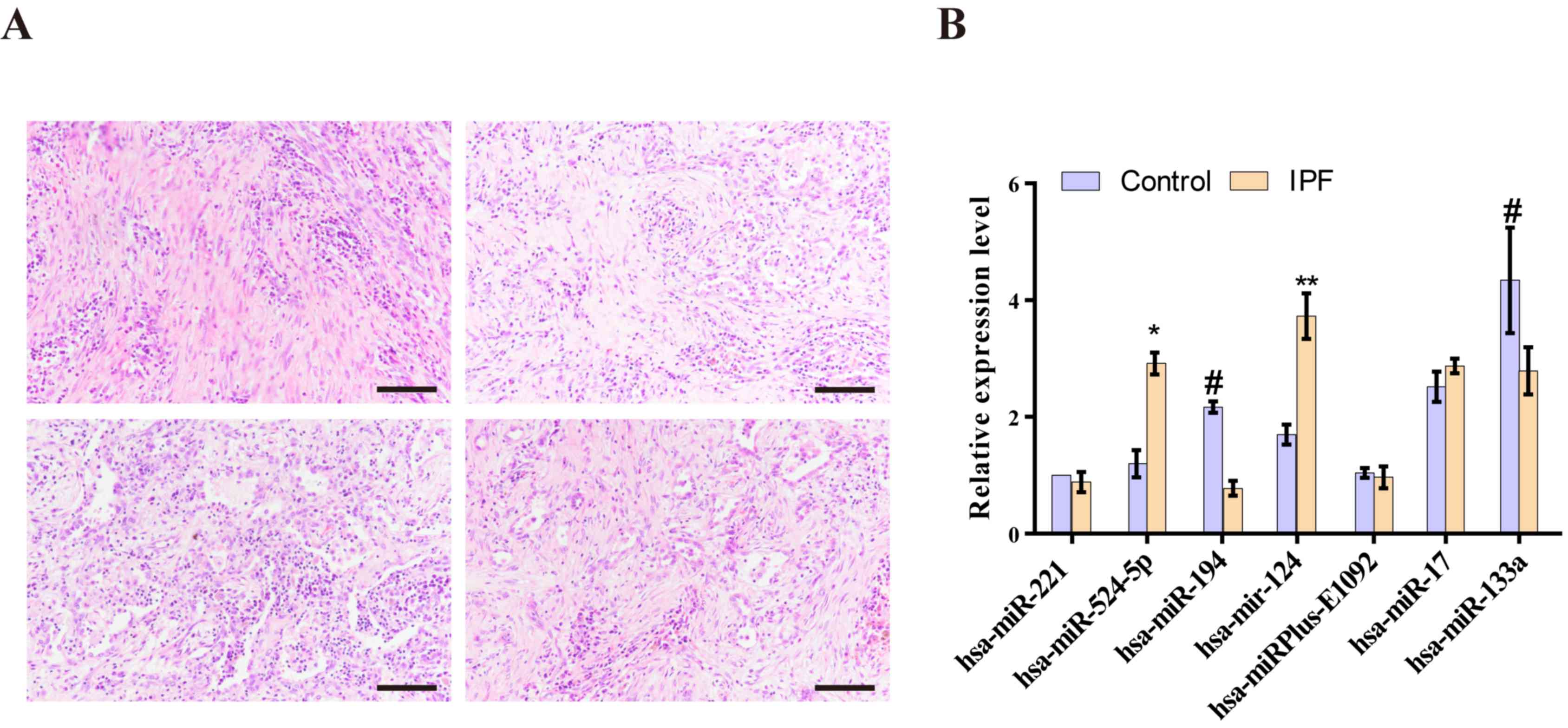
The number of interstitial cells increased and
interstitial sclerosis appeared in the lungs of the patients with
IPF. Furthermore, inflammatory cell infiltration was found around
the interstitial lesion, and an inflammatory reaction was
identified in the lung tissue around the lesion area in the
patients with IPF (Fig. 5A).
It was demonstrated that miR-124 (P=0.0086)
and hsa-miR-524-5p (P=0.0362) were highly expressed in the
IPF group compared with the control (Fig. 5B). These results suggested that these
miRNAs may contribute to IPF by reducing the expression of target
genes. Moreover, hsa-miR-194 (P=0.0399) and
hsa-miR-133a (P=0.0489) were expressed at lower levels in
patients with IPF compared with the control group. Therefore, the
results indicated that these miRNAs may also contribute to IPF by
increasing the expression of target genes. Other differentially
expressed miRNAs were detected in the lungs of patients with IPF
and age-matched men without fibrotic lung disease; however, the
differences were not statistically significant (Fig. 5B).
Functional enrichment analysis
miRDB was used to predict the mRNAs targeted by
miR-124, hsa-miR-194 and hsa-miR-524-5p, and
selected genes with a target score >90 as candidate genes. In
total, 329 genes targeted by miR-124 were enriched in
‘PI3K/AKT’, ‘cAMP’ and ‘mitogen-activated protein kinase’ (MAPK)
signaling pathways. In addition, 63 genes targeted by
miR-194 were enriched in the regulation of signaling
pathways, such as ‘stem cell pluripotency’, ‘epithelial cell signal
transduction’ and ‘lysine degradation’. A total of 401 genes
targeted by hsa-miR-524-5p were enriched in the ‘MAPK’,
‘amphetamine addiction’ and ‘cAMP’ signaling pathways. The top six
input pathways with P<0.05 enriched by the three miRNAs are
presented in Table SIII. Most of
these signaling pathways were cascade signaling pathways that
regulate ‘growth’, ‘differentiation’ and the ‘EMT’ in
fibroblasts.
Discussion
IPF is a progressive disease; however, as current
treatments can only delay the disease progression, there is an
urgent need for improved methods to treat IPF (20). miRNAs are important regulators of
cell function during disease and can serve roles in other cells via
exocrine secretion (21,22). The present study analyzed miRNA
expression profiles at different stages of IPF to identify miRNAs
involved in disease progression. To further reduce the number of
differentially expressed miRNAs, LASSO and SVM-RFE were used to
calculate the differential gene set. In total, four miRNA were
obtained by intersecting the results with ANOVA. At present, to the
best of our knowledge, no in-depth studies regarding the expression
levels of these three miRNAs in tissue samples of patients with IPF
have been published. However, in other diseases, these three miRNAs
have been reported to be associated with the extracellular matrix
and TGF-β signaling, supporting the relevance of the present
analysis in IPF.
Lu et al (23)
demonstrated that miR-124 responded to TGF-β1-induced
fibrogenic differentiation by regulating Axin-1 expression and
activating the Wnt signaling pathway. Panganiban et al
(24) revealed significant changes
in the serum levels of miR-124-26a, let-7a and
let-7d in patients with asthma compared with healthy
controls, which suggested that miR-124 may be involved in
the development of lung asthma. Moreover, Chen et al
(25) demonstrated the inhibitory
effects of miR-124 on the DNA repair enzyme poly(ADP) ribose
polymerase 1. In addition, overexpression of miR-124 reduces
DNA repair ability and leads to a decrease in the drug sensitivity
of cells (25). Liang et al
(26) also reported that
miR-124 may regulate the EMT process by targeting snail
family transcriptional repressor 2 to promote breast cancer
metastasis, while Cui et al (27) suggested that miR-124 may
induce hepatocellular carcinoma metastasis by targeting Slug.
Additionally, homeoboxA11 has been shown to induce the
formation of type I collagen in scars via the activation of
miR-124-3p and the SMAD signaling pathway in the cavernous
body (28). Thus, miR-124
contributes to the formation of fibrotic tissue in a variety of
diseases by responding to the TGF-β and EMT signaling pathways.
miR-524-5p is a member of the
primate-specific chromosome 19 miRNA cluster (C19MC), which is
highly homologous to reprogrammed miR-520d-5p. Nguyen et
al (29) demonstrated that
miR-524-5p regulated stem cell programming by targeting
tumor protein p53 and EMT-related genes. Similarly, Liu et
al (27) reported that
miR-524-5p can positively regulate the expression of distal
homeobox 1 and modulate the TGF-β signaling pathway by competing
with taurine upregulated-1. It has also been shown that low
expression of miR-524-5p in thyroid papillary carcinoma
increases the expression levels of Forkhead box protein E1 and
integrin subunit α3 in thyroid papillary carcinoma, thus inhibiting
cell migration and proliferation and promoting apoptosis (27). Eftekharian et al (30) demonstrated that the expression of
miR-524-5p was significantly lower in the peripheral blood
of patients with multiple sclerosis compared with healthy controls.
Moreover, miR-524-5p can be used as a biomarker of the
response to fengomod in patients with multiple sclerosis (30). Zhao et al (31) also revealed that high expression
levels of miR-524-3p and miR-524-5p inhibit the
TGF-β, Notch and Hippo pathways by targeting SMAD2, hairy and
enhancer of split-1 and TEA domain transcription factor 1,
respectively. It has also been reported that knockout of H19
imprinted maternally expressed transcript inhibits the activation
of the TGF-β/SMAD3 pathway by regulating miR-140. The
aforementioned results suggested that miR-524-5p, a member
of the C19MC, may serve the same role as miR-140 (32). Furthermore, the current findings
indicated that miR-524-5p was involved in signaling pathways
and target genes associated with IPF. For instance,
miR-524-5p was shown to be downregulated in patients with
moderate IPF compared with that in other groups, and was
upregulated in healthy lung tissues. Therefore, it was speculated
that this miRNA may be involved in the EMT process during the
development of IPF.
Xu et al (33)
reported that high expression of miR-194-3p inhibits the
proliferation and migration of fibroblasts by directly blocking the
expression of genes encoding cyclin-dependent kinase 4 and matrix
metalloproteinase 2, as well as interacting with RUNX family
transcription factor 2 in keloids. Furthermore, Hu et al
(34) showed that miR-194 can
be used as a biomarker of drug resistance in non-small lung cancer.
Downregulation of miR-194 reduces nuclear accumulation of
β-catenin and inhibits the Wnt signaling pathway in gastric cancer
(35). In addition, the
proliferation and infiltration of breast cancer cells are inhibited
by knockout of miR-194, which regulates the Wnt/β-catenin
signaling pathway (36). Miao et
al (37) also revealed that
miR-194 targets cadherin 2 (CDH2) to inhibit cell expansion
and promote apoptosis in osteosarcoma cells. Thus, miR-194
is involved in the EMT and cell adhesion in a variety of diseases
by regulating various genes, such as Wnt and CDH2. Of
the three significant miRNAs identified in the present study,
including miR-124, hsa-miR-524-5p and hsa-miR-194, two may be used
as biomarkers in the diagnosis of IPF. All three miRNAs are related
to the EMT process, and the EMT is a key process involved in the
pathogenesis of IPF (38-40).
Therefore, the present results suggested that the three miRNAs were
specific to, and significant for, IPF.
Various changes in miRNA expression have been
identified in IPF (41). For
example, miR-21 positively regulates IPF by targeting the
TGF-β inhibitor SMAD7 and reducing the phosphorylation level of the
SMAD2 complex (42). In addition,
significant upregulation of serum EVmiR-21-5p is observed in
acute and chronic/late fibrosis in a mouse bleomycin-induced lung
fibrosis model (43). The upstream
region of the miR-154 promoter contains the binding site for
the transcription factor SMAD3. In the pathogenesis of IPF, the
TGF-β signaling pathway enhances the phosphorylation of SMAD3 and
promotes the transcription of miR-154 (44). Subsequently, upregulation of
miR-154 protects myofibroblasts from apoptosis by regulating
the activity of cyclin (45).
The current results indicated that there were
significant differences in the expression levels of miR-124,
hsa-miR-524-5p and hsa-miR-194 between patients with
IPF and age-matched men without fibrotic lung disease. These
findings further support the reliability of the present analysis
results. However, due to the small number of samples, additional
studies are required to assess the present findings. In addition to
verifying the aforementioned three miRNAs, other differences in
miRNA expression levels were demonstrated using two machine
learning models. For example, hsa-miR-133a expression was
found to be significantly different between the experimental and
control groups, although the ROC curve AUC was <0.5. Thus, it
was suggested that hsa-miR-133a could not be used as a
potential biomarker to distinguish pulmonary fibrosis tissue from
healthy tissue. Therefore, due to limitations of this study,
prospective studies with larger sample sizes are required to
confirm the current results. Moreover, additional RT-qPCR
experiments should be performed using known IPF biomarkers as
reference miRNAs, such as miR-29b and miR-let-7d.
In conclusion, the present study demonstrated the
expression patterns of miRNAs in different stages of IPF using
ANOVA and identified candidate biomarkers of IPF using two machine
learning models. The reliability of these candidate markers was
assessed using a validation dataset. The current study successfully
identified nine specific miRNAs and obtained a collection of
biomarkers including three miRNAs (AUC=0.785). The miRNA expression
levels in patients with IPF and age-matched men without fibrotic
lung disease were compared using RT-qPCR, and the differences in
the expression levels of the four miRNAs obtained were further
evaluated using bioinformatics methods.
Supplementary Material
Patient characteristics.
miRNA primers.
Enrichment results of target gene KEGG
with differentially expressed miRNAs.
Acknowledgements
Not applicable.
Funding
This work was supported by the ‘Financial Support
for Selected Researchers Back from Abroad (2012)’ from Liaoning
Province (grant no. 88030312004).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QL wrote the article and analyzed the bioinformatics
data. ML performed the mathematical modeling and ROC calculations.
KZ, HL and HY performed sectioning and analysis of qPCR and IPF
data. SM and MZ designed the study and wrote the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The research was approved by the Ethics Committee of
Shenyang Thoracic Hospital and Fushun Central Hospital of Liaoning
Province. All selected patients or their families provided informed
consent for participation in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Raghu G, Freudenberger TD, Yang S, Curtis
JR, Spada C, Hayes J, Sillery JK, Pope CE II and Pellegrini CA:
High prevalence of abnormal acid gastro-oesophageal reflux in
idiopathic pulmonary fibrosis. Eur Respir J. 27:136–142.
2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wolters PJ, Blackwell TS, Eickelberg O,
Loyd JE, Kaminski N, Jenkins G, Maher TM, Molina-Molina M, Noble
PW, Raghu G, et al: Time for a change: Is idiopathic pulmonary
fibrosis still idiopathic and only fibrotic? Lancet Respir Med.
6:154–160. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Selman M and Pardo A: Revealing the
pathogenic and aging-related mechanisms of the enigmatic idiopathic
pulmonary fibrosis An integral model. Am J Respir Crit Care Med.
189:1161–1172. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Antoniou K, Markopoulou K, Tzouvelekis A,
Trachalaki A, Vasarmidi E, Organtzis J, Tzilas V, Bouros E, Kounti
G, Rampiadou C, et al: Efficacy and safety of nintedanib in a Greek
multicentre idiopathic pulmonary fibrosis registry: A
retrospective, observational, cohort study. ERJ Open Res.
6:00172–2019. 2020.
|
|
5
|
Kooshapur H, Choudhury NR, Simon B,
Mühlbauer M, Jussupow A, Fernandez N, Jones AN, Dallmann A, Gabel
F, Camilloni C, et al: Structural basis for terminal loop
recognition and stimulation of pri-miRNA-18a processing by hnRNP
A1. Nat Commun. 9(2479)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rubio K, Singh I, Dobersch S, Sarvari P,
Günther S, Cordero J, Mehta A, Wujak L, Cabrera-Fuentes H, Chao CM,
et al: Inactivation of nuclear histone deacetylases by EP300
disrupts the MiCEE complex in idiopathic pulmonary fibrosis. Nat
Commun. 10(2229)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bodempudi V, Hergert P, Smith K, Xia H,
Herrera J, Peterson M, Khalil W, Kahm J, Bitterman PB and Henke CA:
MiR-210 promotes IPF fibroblast proliferation in response to
hypoxia. Am J Physiol Lung Cell Mol Physiol. 307:L283–L294.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Huang C, Xiao X, Yang Y, Mishra A, Liang
Y, Zeng X, Yang X, Xu D, Blackburn MR, Henke CA and Liu L:
MicroRNA-101 attenuates pulmonary fibrosis by inhibiting fibroblast
proliferation and activation. J Biol Chem. 292:16420–16439.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dai WJ, Qiu J, Sun J, Ma CL, Huang N,
Jiang Y, Zeng J, Ren BC, Li WC and Li YH: Downregulation of
microRNA-9 reduces inflammatory response and fibroblast
proliferation in mice with idiopathic pulmonary fibrosis through
the ANO1-mediated TGF-β-Smad3 pathway. J Cell Physiol.
234:2552–2565. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kang H: Role of MicroRNAs in TGF-β
signaling pathway-mediated pulmonary fibrosis. Int J Mol Sci.
18(2527)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pandit KV, Corcoran D, Yousef H,
Yarlagadda M, Tzouvelekis A, Gibson KF, Konishi K, Yousem SA, Singh
M, Handley D, et al: Inhibition and role of let-7d in idiopathic
pulmonary fibrosis. Am J Respir Crit Care Med. 182:220–229.
2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sung J, Loughin C, Marino D, Leyva F,
Dewey C, Umbaugh S and Lesser M: Medical infrared thermal imaging
of canine appendicular bone neoplasia. BMC Vet Res.
15(430)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Friedman J, Hastie T and Tibshirani R:
Regularization paths for generalized linear models via coordinate
descent. J Stat Softw. 33:1–22. 2010.PubMed/NCBI
|
|
14
|
Team RC: R: A language and environment for
statistical computing, 2013.
|
|
15
|
Xia J, Broadhurst DI, Wilson M and Wishart
DS: Translational biomarker discovery in clinical metabolomics: An
introductory tutorial. Metabolomics. 9:280–299. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liu W and Wang X: Prediction of functional
microRNA targets by integrative modeling of microRNA binding and
target expression data. Genome Biol. 20(18)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yu G, Wang LG, Han Y and He QY:
ClusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Buglyó G, Magyar Z, Romicsné Görbe É,
Bánusz R, Csóka M, Micsik T, Berki Z, Varga P, Sápi Z and Nagy B:
Quantitative RT-PCR-based miRNA profiling of blastemal Wilms'
tumors from formalin-fixed paraffin-embedded samples. J Biotechnol.
298:11–15. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Akiyama N, Hozumi H, Isayama T, Okada J,
Sugiura K, Yasui H, Suzuki Y, Kono M, Karayama M, Furuhashi K, et
al: Clinical significance of serum S100 calcium-binding protein A4
in idiopathic pulmonary fibrosis. Respirology. 25:743–749.
2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Carthew RW and Sontheimer EJ: Origins and
mechanisms of miRNAs and siRNAs. Cell. 136:642–655. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Thum T and Condorelli G: Long noncoding
RNAs and microRNAs in cardiovascular pathophysiology. Circ Res.
116:751–762. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lu Y, Zhang T, Shan S, Wang S, Bian W, Ren
T and Yang D: miR-124 regulates transforming growth factor-β1
induced differentiation of lung resident mesenchymal stem cells to
myofibroblast by repressing Wnt/β-catenin signaling. Dev Biol.
449:115–121. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Panganiban RP, Pinkerton MH, Maru SY,
Jefferson SJ, Roff AN and Ishmael FT: Differential microRNA
epression in asthma and the role of miR-1248 in regulation of IL-5.
Am J Clin Exp Immunol. 1:154–165. 2012.PubMed/NCBI
|
|
25
|
Chen SM, Chou WC, Hu LY, Hsiung CN, Chu
HW, Huang YL, Hsu HM, Yu JC and Shen CY: The effect of microRNA-124
overexpression on anti-tumor drug sensitivity. PLoS One.
10(e0128472)2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liang YJ, Wang QY, Zhou CX, Yin QQ, He M,
Yu XT, Cao DX, Chen GQ, He JR and Zhao Q: MiR-124 targets Slug to
regulate epithelial-mesenchymal transition and metastasis of breast
cancer. Carcinogenesis. 34:713–722. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liang YJ, Wang QY, Zhou CX, Yin QQ, He M,
Yu XT, Cao DX, Chen GQ, He JR and Zhao Q: miR-124 targets Slug to
regulate epithelial-mesenchymal transition and metastasis of breast
cancer. Carcinogenesis. 34:713–722. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jin J, Zhai HF, Jia ZH and Luo XH: Long
non-coding RNA HOXA11-AS induces type I collagen synthesis to
stimulate keloid formation via sponging miR-124-3p and activation
of Smad5 signaling. Am J Physiol Cell Physiol. 317:C1001–C1010.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Nguyen PNN, Choo KB, Huang CJ, Sugii S,
Cheong SK and Kamarul T: MiR-524-5p of the primate-specific C19MC
miRNA cluster targets TP53IPN1-and EMT-associated genes to regulate
cellular reprogramming. Stem Cell Res Ther. 8(214)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Eftekharian MM, Komaki A, Mazdeh M,
Arsang-Jang S, Taheri M and Ghafouri-Fard S: Expression profile of
selected microRNAs in the peripheral blood of Multiple Sclerosis
patients: A multivariate statistical analysis with ROC curve to
find new biomarkers for fingolimod. J Mol Neurosci. 68:153–161.
2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhao K, Wang Q, Wang Y, Huang K, Yang C,
Li Y, Yi K and Kang C: EGFR/c-myc axis regulates TGFβ/Hippo/Notch
pathway via epigenetic silencing miR-524 in gliomas. Cancer Lett.
406:12–21. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang X, Cheng Z, Dai L, Jiang T, Jia L,
Jing X, An L, Wang H and Liu M: Knockdown of long noncoding RNA H19
represses the progress of pulmonary fibrosis through the
transforming growth factor β/Smad3 pathway by regulating microRNA
140. Mol Cell Biol. 39:e00143–00119. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xu Z, Guo B, Chang P, Hui Q, Li W and Tao
K: The differential expression of miRNAs and a preliminary study on
the mechanism of miR-194-3p in Keloids. Biomed Res Int.
2019(8214923)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hu S, Yuan Y, Song Z, Yan D and Kong X:
Expression profiles of microRNAs in drug-resistant non-small cell
lung cancer cell lines using microRNA sequencing. Cell Physiol
Biochem. 51:2509–2522. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Peng Y, Zhang X, Lin H, Deng S, Huang Y,
Qin Y, Feng X, Yan R, Zhao Y, Cheng Y, et al: Inhibition of miR-194
suppresses the Wnt/β-catenin signalling pathway in gastric cancer.
Oncol Rep. 40:3323–3334. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yang F, Xiao Z and Zhang S: Knockdown of
miR-194-5p inhibits cell proliferation, migration and invasion in
breast cancer by regulating the Wnt/β-catenin signaling pathway.
Int J Mol Med. 42:3355–3363. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Miao J, Wang W, Wu S, Zang X, Li Y, Wang
J, Zhan R, Gao M, Hu M, Li J and Chen S: MiR-194 suppresses
proliferation and migration and promotes apoptosis of osteosarcoma
cells by targeting CDH2. Cell Physiol Biochem. 45:1966–1974.
2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hill C, Li J, Liu D, Conforti F, Brereton
CJ, Yao L, Zhou Y, Alzetani A, Chee SJ, Marshall BG, et al:
Autophagy inhibition-mediated epithelial-mesenchymal transition
augments local myofibroblast differentiation in pulmonary fibrosis.
Cell Death Dis. 10(591)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Milara J, Navarro R, Juan G, Peiró T,
Serrano A, Ramón M, Morcillo E and Cortijo J:
Sphingosine-1-phosphate is increased in patients with idiopathic
pulmonary fibrosis and mediates epithelial to mesenchymal
transition. Thorax. 67:147–156. 2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Senoo T, Hattori N, Tanimoto T, Furonaka
M, Ishikawa N, Fujitaka K, Haruta Y, Murai H, Yokoyama A and Kohno
N: Suppression of plasminogen activator inhibitor-1 by RNA
interference attenuates pulmonary fibrosis. Thorax. 65:334–340.
2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Parker MW, Rossi D, Peterson M, Smith K,
Sikström K, White ES, Connett JE, Henke CA, Larsson O and Bitterman
PB: Fibrotic extracellular matrix activates a profibrotic positive
feedback loop. J Clin Invest. 124:1622–1635. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Liu G, Friggeri A, Yang Y, Milosevic J,
Ding Q, Thannickal VJ, Kaminski N and Abraham E: MiR-21 mediates
fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J
Exp Med. 207:1589–1597. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Makiguchi T, Yamada M, Yoshioka Y, Sugiura
H, Koarai A, Chiba S, Fujino N, Tojo Y, Ota C, Kubo H, et al: Serum
extracellular vesicular miR-21-5p is a predictor of the prognosis
in idiopathic pulmonary fibrosis. Respir Res.
17(110)2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Chao CM, Carraro G, Rako Z, Kolck J, Morty
RE, Rottier RJ and Bellusci S: MiR-154 controls branching
morphogenesis and alveologenesis in lung development involving
Tgf-β signaling. Eur Respir J. 50 (Suppl 61)(OA3229)2017.
|
|
45
|
Milosevic J, Pandit K, Magister M,
Rabinovich E, Ellwanger DC, Yu G, Vuga LJ, Weksler B, Benos PV,
Gibson KF, et al: Profibrotic role of miR-154 in pulmonary
fibrosis. Am J Respir Cell Mol Biol. 47:879–887. 2012.PubMed/NCBI View Article : Google Scholar
|