Introduction
Radiation therapy is a major component of the
armamentarium for the treatment of various malignancies. Regardless
of the tumour cell type, radiation may have a central, adjuvant or
neoadjuvant role. In several gynaecological, urological and rectal
cancers, radiation treatment is limited to the pelvis, where a
maximum dose may be delivered to the target tissue while minimizing
inadvertent irradiation of other organs. Unfortunately, the
radiation fields required to treat these pelvic and, occasionally,
intra-abdominal or retroperitoneal tumours, frequently include the
small bowel and colon (1).
With the increased incidence of malignant tumours,
the higher long-term survival rate of cancer patients and the
increased popularity of radiotherapy for the treatment of pelvic
malignant tumours, the incidence of radiation enteritis (RE), a
common post-radiotherapy complication, has been continuously
increasing (2,3). Based on its clinical onset, RE may be
generally classified as acute or chronic (4). With regard to acute RE (ARE), most
cases are self-healing and require no special treatment. However,
chronic RE (CRE) is associated with more severe latent and
progressive manifestations, increased pain and reduced quality of
life, requiring surgical treatment in >30% of patients (5). Therefore, the diagnosis of CRE has been
attracting increasing clinical attention. As the most commonly
involved organs are the colon and rectum, pelvic RE is also
referred to as radiation colitis and rectitis (RC&R). Only few
studies have investigated the diagnosis of RC&R (6). Therefore, in the present study,
clinical, endoscopic and imaging data of 23 patients with CRC&R
were retrospectively analysed to explore the diagnostic value of
multi-slice spiral CT (MSCT).
Materials and methods
Patient selection and eligibility
The present study was a retrospective, observational
study. From a database of the internal medical records system, a
list of 151 patients with endoscopically proven RC&R who
underwent pelvic CT examination at Chongqing University Cancer
Hospital (Chongqing, China) over a 6-year period (from May 2013 to
May 2019) was obtained. Among those, 23 patients were finally
selected after reviewing their radiological as well as diagnostic
pathological and medical records. The diagnosis of RC&R in
these selected patients had been based on a history of radiotherapy
for malignant pelvic tumours, as well as pelvic CT prior to
radiotherapy displaying no abnormal changes in the pelvic
intestinal tract and follow-up examination after radiotherapy
included an enhanced pelvic CT scan. There was a latent period
(>3 months) with typical clinical manifestations of CRE after
radiotherapy (7). All diagnoses were
confirmed endoscopically. Patients without complete CT and
endoscopic data were excluded as were patients diagnosed with
primary and recurrent intestinal tumours, as well as primary
inflammatory and ischemic intestinal diseases. The patient
population comprised 4 males and 19 females, ranging in age from
41-74 years (mean, 53 years).
CT technique
CT was performed using a Philips 64-slice spiral CT
machine (Philips Medical Systems, Inc.). The scanning range was
from the inferior margin of the pubic symphysis to the superior
umbilicus or lower diaphragm. All patients were examined by plain
and enhanced CT. For the latter, 80-100 ml of a non-ionic iodinated
contrast agent was injected via the elbow vein with a high-pressure
injector at the flow rate of 2.5-3.0 ml/sec. Scanning was initiated
50-60 sec after the injection. The reformation slice thickness and
slice gap of the transverse section and sagittal section were 3 and
5 mm, respectively.
Statistical analysis
The CT findings were retrospectively reviewed by the
consensus of two experienced radiologists. The CT scans were
evaluated with an emphasis on the following: Site of involvement,
thickness of the intestinal wall, length of the lesion, presence or
absence of a layering pattern in the involved segment of the bowel
wall [known as the target sign, the target sign comprises three
rings of alternating attenuation. Two high attenuation value rings,
the mucosa (inner ring) and the muscularis propria (outer ring) are
separated by the low attenuation value submucosa (middle ring)],
resolution of the pericolic fat tissue, density of subcutaneous fat
and presence of pelvic abscesses or intestinal fistulas. The
clinical, colonoscopic and pathological findings were evaluated
simultaneously. All analyses were conducted using SPSS 16.0 (SPSS
Inc.). In order to determine whether the thickness of the
intestinal wall was associated with the presence of the target
sign, the cases were divided into ≤6.5 and >6.5-mm groups, and
the χ2 test or Fisher's exact probability method was
used. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
The characteristics of the 23 patients are
summarized in Tables I and SI.
 | Table ISummarized data of 23 patients with
chronic radiation colitis and rectitis. |
Table I
Summarized data of 23 patients with
chronic radiation colitis and rectitis.
| Case no./Age
(years)/sex | Lesion location | Intestinal wall
thickness (mm) | Length (cm) | Target sign | Increased mesenteric
density | Muscle
swelling/increased subcutaneous fat density | Primary tumour | Total radiation dose
(Gy) | After-loading
intracavitary therapy (Gy) |
|---|
| 1/46/F | Rectum, colon | 8.5 | 10.1 | Yes | Yes | No | Cervical cancer | 50 | 30 |
| 2/74/M | Rectum | 9.7 | 8.2 | Yes | Yes | No | Rectal cancer | 50 | - |
| 3/69/F | Rectum, colon | 6.3 | 10.3 | No | No | No | Cervical cancer | 50 | 30 |
| 4/41/F | Rectum | 8.4 | 3.8 | Yes | No | No | Bladder cancer | 50 | 54 |
| 5/55/F | Rectum | 9.6 | 3.8 | Yes | No | Yes | Cervical cancer | 45 | 33 |
| 6/53/F | Rectum | 13.5 | 6.2 | Yes | Yes | No | Cervical cancer | 50 | 30 |
| 7/63/F | Rectum, colon | 6.5 | 6.4 | No | Yes | No | Cervical cancer | 50 | 30 |
| 8/54/F | Rectum | 10.4 | 4.7 | Yes | Yes | Yes | Cervical cancer | 60 | 36 |
| 9/49/F | Rectum | 5.5 | 7.4 | No | Yes | No | Cervical cancer | 50 | 30 |
| 10/63/F | Rectum | 6.7 | 4.5 | Yes | No | No | Cervical cancer | 50 | 30 |
| 11/53/F | Rectum | 7.8 | 4.2 | Yes | No | No | Cervical cancer | 50 | 30 |
| 12/51/F | Rectum | 9.5 | 5.6 | Yes | Yes | No | Cervical cancer | 50 | 30 |
| 13/42/F | Rectum | 13.8 | 8.7 | Yes | Yes | Yes | Cervical cancer | 46 | 30 |
| 14/50/F | Rectum | 8.5 | 6.5 | Yes | Yes | No | Cervical cancer | 50 | 30 |
| 15/70/F | Rectum | 7.1 | 4.1 | Yes | Yes | No | Ovarian cancer | 50 | - |
| 16/59/F | Rectum, colon | 9.8 | 3.1 | Yes | No | No | Cervical
cancer | 50 | 30 |
| 17/62/F | Rectum, colon | 7.8 | 4.2 | No | Yes | No | Cervical
cancer | 50 | 36 |
| 18/53/F | Rectum | 10.3 | 3.3 | No | No | No | Cervical
cancer | 50 | 40 |
| 19/46/M | Colon | 6.2 | 3.4 | No | Yes | No | Rectal cancer | 50 | - |
| 20/61/M | Rectum | 5.8 | 5.6 | Yes | Yes | Yes | Rectal cancer | 50 | - |
| 21/50/M | Rectum | 8.9 | 4.8 | Yes | Yes | No | Rectal cancer | 50 | - |
| 22/51/F | Rectum, colon | 13.2 | 14.0 | Yes | Yes | No | Cervical
cancer | 46 | 30 |
| 23/59/F | Rectum | 7.6 | 3.6 | No | No | No | Cervical
cancer | 46 | 30 |
The primary tumours included cancer of the cervix
(n=17), rectum (n=4), ovaries (n=1) and bladder (n=1). The total
dose of radiation per patient was 46-60 Gy (mean, 49.7 Gy)
delivered over 5 weeks. Among the patients, those with cervical
cancer (n=17) and bladder cancer (n=1) were treated using
after-loading intracavitary therapy with a total dose of 30-54 Gy
(mean, 32.7 Gy). The major clinical symptoms included increased
frequency of defecation (n=16), abdominal pain (n=12) and
constipation and tenesmus (n=5), while there was a lack of obvious
symptoms in 3 cases (Table II).
Endoscopy and CT examination were performed from 5 to 29 months
after radiotherapy. Of the 23 patients, 22 exhibited involvement of
the rectum (96.7%), 7 of the sigmoid colon (30.5%) and 6 exhibited
both (26.1%). The length of the affected intestinal segment varied
from 3.1 to 14.0 cm.
 | Table IIMajor clinical symptoms. |
Table II
Major clinical symptoms.
| Clinical
symptoms | N (%) |
|---|
| Increased frequency
of defecation | 16 (69.6) |
| Abdominal pain | 12 (52.2) |
| Constipation and
tenesmus | 5 (21.7) |
CT manifestations
The patients exhibited different degrees of
increased thickness of the intestinal wall (n=20, 87.0%), with a
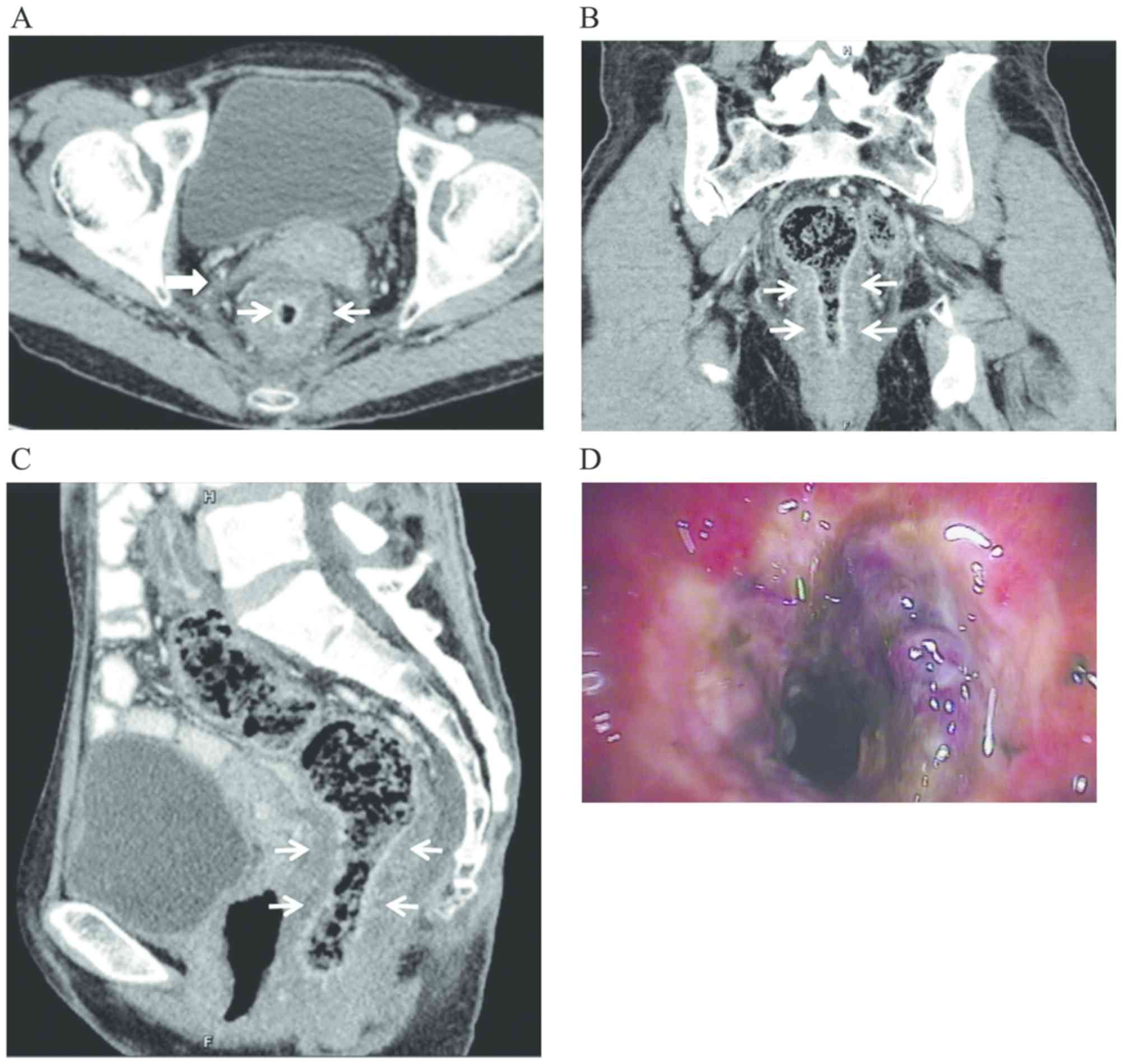
maximum of 16.6 mm, as exemplified in the representative case in
Fig. 1. The intestinal wall was
indicated to be thickened, with smooth contours and no obvious
protrusions or masses. The target sign (n=16, 69.9%) on enhanced CT
was observed as a significant enhancement of the mucosal and/or
serosal layers, presenting a stratified change. The target sign was
more commonly observed when the thickness of the intestinal wall
was >6.5 mm and the difference was statistically significant
(χ2=4.72, P<0.05; Table
III). Other findings included oedema and increased density of
the mesentery (n=15, 65.2%); increased density of subcutaneous fat
and blurred and swollen pelvic wall muscles (n=4, 17.4%), with the
obturator internus and levator ani muscles being most commonly
affected; narrowed intestinal lumen (n=3, 13.0%); and a small
amount of ascitic fluid (n=2, 8.7%) located in the paracolic sulci
and bladder or Douglas pouch. The thickness of the bladder wall was
increased evenly (n=5, 21.7%); patients with urocystitis (n=2) were
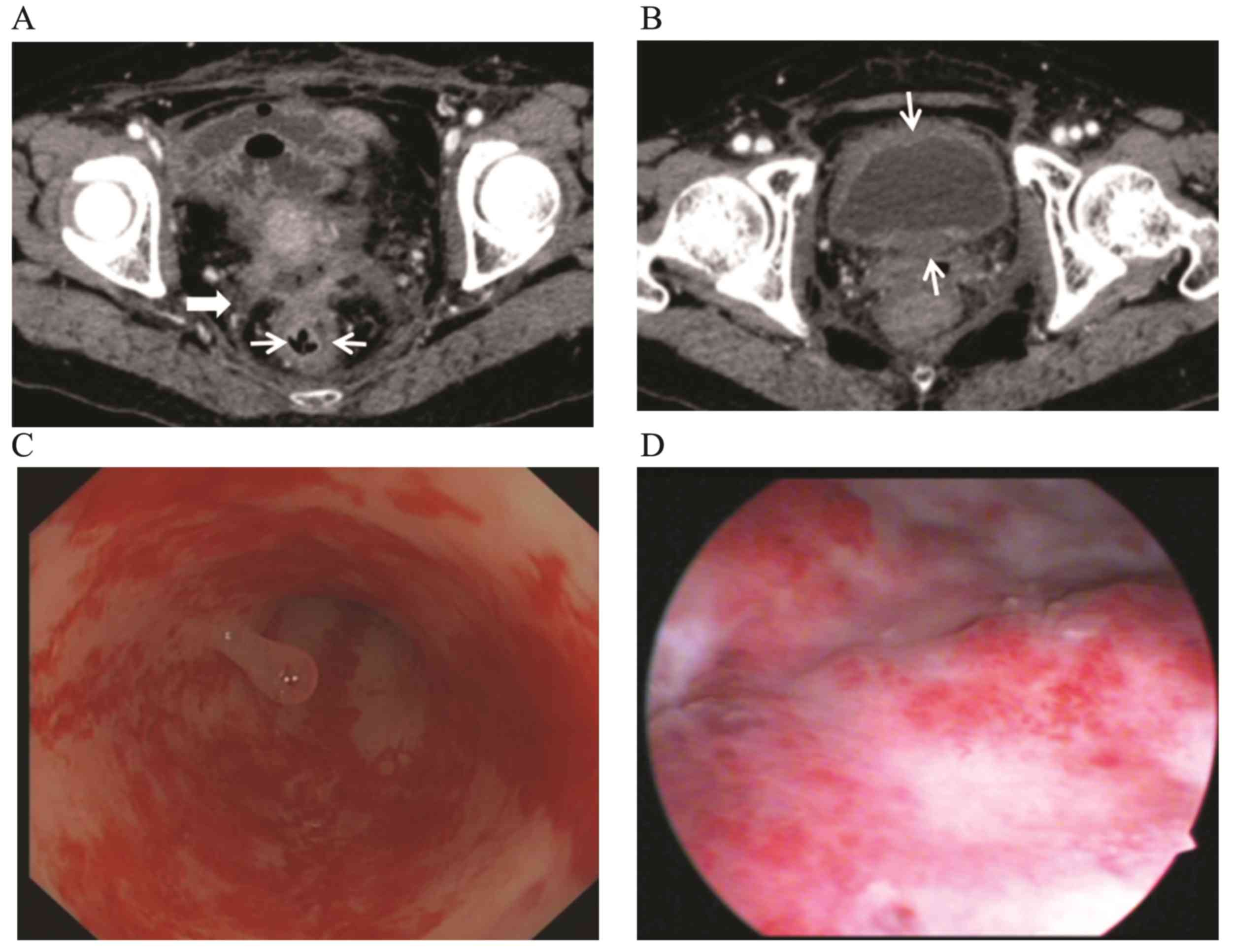
diagnosed using a cystoscope (Fig.
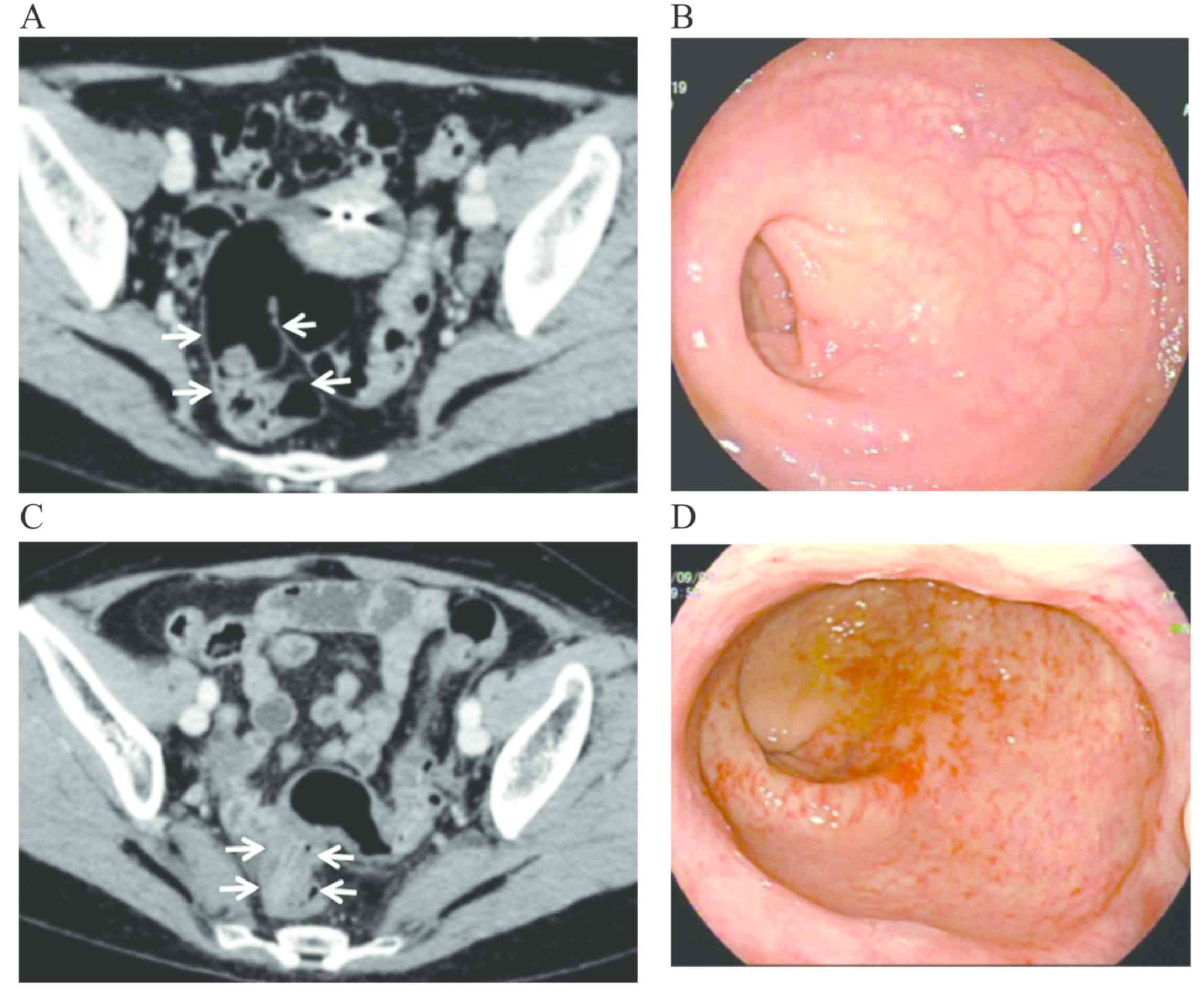
2). One patient underwent CT and colonoscopic examination prior
to and after radiotherapy (Fig. 3).
Through comparing the findings, the CT manifestations and
colonoscopic changes of CRC&R were evaluated. A rectovaginal
fistula was found in 1 patient and pelvic abscess was identified in
1 patient by CT.
 | Table IIIAssociation between intestinal wall
thickness and ’target signs’. |
Table III
Association between intestinal wall
thickness and ’target signs’.
| Intestinal wall
thickness (mm) | ‘Target sign’ | No ‘target
sign’ | Total | Statistics |
|---|
| ≤6.5 | 1 | 4 | 5 | χ2=4.72
(P<0.05) |
| >6.5 | 15 | 3 | 18 | |
| Total | 16 | 7 | 23 | |
Colonoscopy
The 23 patients underwent colonoscopy and were
diagnosed with RC&R. The diagnoses included telangiectasia and
mucosal hyperaemia (n=21, 91.3%), rectal ulcers (n=3, 13.0%) and
narrowed intestinal lumen, which made it difficult for the
colonoscope to pass through (n=3, 13.0%). A total of 2 patients
were subjected to biopsy and chronic mucosal inflammation was
confirmed.
Discussion
RE is a common complication of radiotherapy for
pelvic malignant tumours and is reported in 5-15% of such cases
(8). It is generally thought that
the occurrence of RE is more likely when the pelvic external
radiation dose exceeds 50 Gy delivered within 5 weeks (9).
According to the clinical onset time and symptoms,
RE may be classified as ARE or CRE. The symptoms of ARE mostly
occur within 1-2 weeks after radiotherapy and last for several
weeks, and usually subside with conservative treatment. By
contrast, CRE is a slow process and may occur 3 months or as long
as 30 years after the completion of treatment. In addition to acute
cytotoxicity, radiation can cause progressive occlusive arteritis
and submucosal fibrosis. Transmucosal damage to the intestinal wall
may lead to progressive vasculitis, thrombosis, and eventually to
varying degrees of ischemia and necrosis. This process may lead to
narrowing of the intestinal lumen and eventual blockage. The
chronic effects of radiation are mainly related to the total dose
of radiation received and the total volume of the irradiated tissue
(4).
CRC&R is a common complication of radiotherapy
for pelvic malignant tumours and is associated with a variable
latency period. The time interval from the beginning of
radiotherapy to diagnosis and CT and endoscopy examination in the
present study varied between 4 and 28 months, and all patients
exhibited late RE. Previously reported evidence suggests that
chronic radiation proctitis is more likely to occur in those
initially experiencing severe acute proctitis (10), which is referred to as ‘the
consequential late effect’ (11).
However, not all CRC&R cases develop following an acute phase.
Additional factors that may increase the incidence of CRC&R
were also identified. Mounting evidence has demonstrated that
previous surgeries may increase the incidence rate of CRC&R,
which may be attributed to partly fixed intestinal loops by
postoperative adhesions with excessive radiation exposure (12,13). A
correlation also exists between the selection of various surgical
methods for different diseases and RE caused by postoperative
adhesions (12). In addition, pelvic
inflammatory disease, radiation dose, diabetes, cardiovascular
diseases and smoking may serve as facilitators of CRC&R
(14).
Various symptoms and signs may occur in patients
with CRC&R, including chronic abdominal pain, constipation,
ileus and haematochezia (15). In
the present study, the majority of the patients suffered from
abdominal pain (n=15, 52.2%) with increased frequency of defecation
and haematochezia (n=16, 69.6%). For any patient with a history of
abdominal or pelvic irradiation, if gastrointestinal discomfort
occurs, radiation damage should be considered, unless there is a
diagnosis of other gastrointestinal diseases. For those patients
with CRC&R, preoperative imaging may help the surgeons plan the
surgical strategy (16). At present,
the radiological assessment of CRC&R mostly involves barium
enema. A series of studies have verified that the major
radiological findings varied from normal (15% of the initial
examinations) to decreased distensibility of the bowel wall,
intestinal fixation, mucosal and contour abnormalities, ulceration,
stenoses and fistula formation (17-19).
The ‘omega sign’, caused by bilateral retraction at the base of a
narrowed sigmoid loop was detected in ~60% of patients with
radiation colitis. In addition, due to the more accurate assessment
of mucosal abnormalities and ulcers, colonoscopy is frequently
applied in the evaluation of CRC&R. The symptoms of CRC&R
include a thickened and hardened mucosa, as well as the expansion,
ulceration, stenosis and necrosis of capillaries, among which
expansion is the most common finding. Colonoscopy is able to
readily identify intestinal mucosal lesions or may be used for
biopsy and pathological evaluation in cases with suspected
malignancy. Without colonoscopy and biopsy, it is difficult to
distinguish CRC&R from intestinal tumours or other inflammatory
lesions. Due to the risk of fistula formation, the decision to
perform a biopsy should be made with caution. If possible, the
anterior rectal wall, which is usually exposed to high radiation
doses, should be avoided (20). In
the present study, following the diagnosis of RE via colonoscopy,
the risk of fistulas was also considered; only 2 patients, in whom
large ulcers were identified on colonoscopy, underwent biopsy to
eliminate malignant tumours, and were ultimately diagnosed with
chronic mucosal inflammation. As an invasive examination, in
addition to not being suitable for high-risk patients, colonoscopy
is also associated with pain and the risk of perforation; by
contrast, CT is a non-invasive, safe and efficient method for
almost all patients, including those considered as high-risk
(21).
At present, only few MSCT studies on CRC&R are
available (21,22). In the present study, based on the
abovementioned data, the MSCT characteristics of CRC&R may be
summarized as follows: i) The highest incidence (96.7%) was in
patients with rectal lesions, followed by 30.5% in those with
sigmoid colon lesions and 26.1% in those with both rectal and
sigmoid colon lesions. It has been demonstrated that the intestinal
sensitivity to radiation varies among different sites, and the
order from highest to lowest is as follows: Rectum, sigmoid colon,
transverse colon, ileum, jejunum and duodenum, consistently with
data reported by relevant studies (23,24). ii)
Intestinal changes included thickening of the involved intestinal
walls and most cases exhibited a thickening of no more than 10 mm,
and even thickening of the smooth muscle layer and entire
intestinal wall. When an obvious submucosal oedema occurred, the
‘target sign’ was observed on the enhanced scans (6), In the present study, the majority of
patients (87%) exhibited even thickening of the intestinal walls,
among whom 69.6% displayed the target sign. In addition, when the
thickness of the intestinal wall was >6.5 mm, the target sign
was more commonly observed (P<0.05), but further confirmation in
a larger sample is required. Ileal ulcers, mostly of the incomplete
type, were occasionally observed, which was likely the result of
the narrowed intestinal lumen caused by fibrosis and chronic
inflammation-associated adhesions to the surrounding tissues. iii)
Changes in the mesentery, peritoneum and pelvic wall: Following
radiotherapy, widespread oedema occurred in the subcutaneous fat,
mesentery and pelvic wall soft tissues, with interstitial and
fibrous tissue proliferation in the late stages. On CT, an
increased density but blurred appearance of the subcutaneous fat
was observed, with common cords, mesh intervals, thickened
mesenteric vessels, blurred edges, swollen pelvic wall muscles and
blurred muscle intervals; the obturator internus and externus and
levator ani muscles were the ones most commonly affected. The
peritoneum and pelvic fascia were observed to be thickened,
particularly the rectal fascia. iv) Abdominal cavity changes: When
the intestinal wall ischemia progresses further, the ulcers may
perforate the intestinal wall and cause peritonitis and formation
of a pelvic abscess or an intestinal fistula. The pelvic abscess
may be confined to the vicinity of the lesions and the low-lying
area of the pelvic cavity away from the crevasse. The abscess
borders may be better displayed on enhanced CT scans. Sinus tracts
may be frequently difficult to image directly using CT, but their
presence may be indicated by luminal fluid and gases. Due to the
weakened tissue repair capacity caused by radiotherapy and constant
leakage of intestinal contents, it is difficult for the sinus tract
to close spontaneously and surgical treatment is frequently
required (25). A small amount of
ascitic fluid is rarely observed. v) Other changes: Radiation may
also damage other organs in the pelvic cavity, particularly the
urinary system, manifesting as radiation-induced urocystitis and
inflammatory stenosis of the lower ureter. In the present study,
21.7% of patients had a thickened bladder wall and 2 patients had
confirmed radiation-induced cystitis. In addition, MSCT has a
variety of image post-processing modes, and the multiplanar
reconstruction (MPR) is able to display the characteristics of
thickened intestinal walls and surrounding tissues, allowing for a
comprehensive evaluation of the intestinal walls.
The appearance of CRE on imaging lacks specificity
and it must be clinically distinguished from intestinal tumours,
inflammatory bowel disease and ischemic bowel disease. Intestinal
tumours frequently invade the intestinal wall and form a mass,
which may be easily distinguished from the intestinal wall
thickening observed in CRE. Inflammatory lesions are more common in
ulcerative colitis and Crohn's disease, both of which have
characteristic predominantly affected sites. Crohn's disease is
characterized by multiple segment involvement. Both may display
irregular thickening of the intestinal wall, stenosis, intestinal
ulcers, even formation of fistulas or pelvic abscesses. However,
the changes in the mesentery and pelvic wall are minor and
consideration of the patient's history may help distinguish it from
CRE (26). Acute ischemic bowel
disease frequently manifests as oedema and thickening of the
intestinal wall, oedema and blurred appearance of the mesentery,
and it is difficult to distinguish from CRE. Ischemic bowel disease
is mostly caused by vasculitis, trauma, intestinal obstruction,
intestinal torsion or vascular sclerosis. There is no evidence
suggesting that the development of chemic bowel disease is related
to radiotherapy. Intestinal wall gas is a characteristic
manifestation of intestinal ischemic necrosis (27).
In summary, the CT manifestations of CRC&R are
variable and non-specific but are easily diagnosed when combined
with a history of radiotherapy and clinical manifestations. The
MSCT of CRC&R exhibits certain distinct characteristics,
including an evenly thickened intestinal wall, particularly in the
rectum, mostly increase to <10 mm. When submucosal oedema
develops, particularly in cases with wall thickening to >6.5 mm,
the ‘target sign’ may be observed through enhanced CT scans. An
increased density but blurred appearance of the subcutaneous fat
may be observed, with common cords, mesh intervals, thickened
mesenteric vessels, blurred edges, swollen pelvic wall muscles and
blurred muscle borders. In addition to demonstrating the intestinal
lesions, CT also reveals changes outside the intestine,
particularly mesenteric oedema and pelvic abscesses, which may
prompt early clinical treatment measures. MPR is able to display
the characteristics of a thickened intestinal wall and surrounding
tissues, allowing for a comprehensive evaluation of the intestinal
walls.
Supplementary Material
Table SI. Summarized data of 23
patients with chronic radiation colitis and rectitis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JX and QDL designed the experiments, analyzed the
data and wrote the manuscript. Both authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Chongqing University Cancer Hospital (approval no.
2020060) and written informed consent was obtained from all
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kennedy GD and Heise CP: Radiation colitis
and proctitis. Clin Colon Rectal Surg. 20:64–72. 2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shadad AK, Sullivan FJ, Martin JD and Egan
LJ: Gastrointestinal radiation injury: Prevention and treatment.
World J Gastroenterol. 19:199–208. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li YS: Radiation pelvicopathy: A
comprehensive and interdisciplinary approach. J Med Postgrad.
29:449–452. 2016.
|
|
4
|
Regimbeau JM, Panis Y, Gouzi JL and
Fagniez PL: French University Association for Surgical Research:
Operative and long term results after surgery for chronic radiation
enteritis. Am J Surg. 182:237–242. 2001.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang XH, Gao MY, Zhou XH, et al: MRI
diagnosis of radiation enteritis in patients with gynecological
pelvic malignancies after radiotherapy. Chin J Med Imaging Technol.
28:1695–1698. 2012.
|
|
6
|
Chen S, Harisinghani MG and Wittenberg J:
Small bowel CT fat density target sign in chronic radiation
enteritis. Australas Radiol. 47:450–452. 2003.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Harb AH, Abou Fadel C and Sharara AI:
Radiation enteritis. Curr Gastroenterol Rep. 16(383)2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zimmerer T, Böcker U, Wenz F and Singer
MV: Medical prevention and treatment of acute and chronic radiation
induced enteritis-is there any proven therapy? A short review. Z
Gastroenterol. 46:441–448. 2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
DeVita VT, Hellman S and Rosenberg SA
(eds): Cancer: Rrinciples and practice of oncology. Philadelphia,
New York, Lippincott Williams and Wilkins Press. pp1527–1528.
2008.
|
|
10
|
Denham JW, O'Brien PC, Dunstan RH,
Johansen J, See A, Hamilton CS, Bydder S and Wright S: Is there
more than one late radiation proctitis syndrome? Radiother Oncol.
51:43–53. 1999.
|
|
11
|
Dorr W and Hendry JH: Consequential late
effects in normal tissues. Radiother Oncol. 61:223–231.
2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Huang Y, Guo F, Yao D, Li Y and Li J:
Surgery for chronic radiation enteritis: Outcome and risk factors.
J Surg Res. 204:335–343. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Theis VS, Sripadam R, Ramani V and Lal S:
Chronic radiation enteritis. Clin Oncol (R Coll Radiol). 22:70–83.
2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Iraha S, Ogawa K, Moromizato H, Shiraishi
M, Nagai Y, Samura H, Toita T, Kakinohana Y, Adachi G, Tamaki W, et
al: Radiation enterocolitis requiring surgery in patients with
gynecological malignancies. Int J Radiat Oncol Biol Phys.
68:1088–1093. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Krol R, Smeenk RJ, van Lin EN and Hopman
WP: Impact of late anorectal dysfunction on quality of life after
pelvic radiotherapy. Int J Colorectal Dis. 28:519–526.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Haddad MC, Khouzami RA, Saad HA and Azzi
MC: Imaging fifindings of radiation enteritis. J Med Liban.
52:55–57. 2004.PubMed/NCBI
|
|
17
|
Smith DH and DeCosse JJ: Reliation damage
to the small intestine. World J Surg. 10:189–194. 1986.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chater C, Saudemont A and Zerbib P:
Chronic radiation enteritis. J Visc Surg. 156:175–176.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang SY and LI YS: Diagnosis of chronic
radiation enteritis. J Med Postgrad. 25:654–657. 2012.
|
|
20
|
Wu XR, Liu XL, Katz S and Shen B:
Pathogenesis, diagnosis, and management of ulcerative proctitis,
chronic radiation proctopathy, and diversion proctitis. Inflamm
Bowel Dis. 21:703–715. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Raman SP, Horton KM and Fishman EK: MDCT
and CT angiography evaluation of rectal bleeding: The role of
volume visualization. AJR Am J Roentgenol. 201:589–597.
2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen K, Yin F and Chen HT: Evaluation of
clinical use of CT virtual colonoscopy. China Prac Med. 4:115–116.
2009.
|
|
23
|
Kalaoselvan R, Theis VS, Dibb M, Teubner
A, Anderson ID, Shaffer JL, Carlson GL and Lal S: Radiation
enteritis leading to intestinal failure: 1994 patient-years of
experience in a national referral centre. Eur J Clin Nutr.
68:166–170. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kasuya G, Ogawa K, Iraha S, Nagai Y,
Shiraishi M, Hirakawa M, Samura H, Toita T, Kakinohana Y, Kudaka W,
et al: Severe late complications in patients with uterine cancer
treated with postoperative radiotherapy. Anticancer Res.
31:3527–3533. 2011.PubMed/NCBI
|
|
25
|
Chen MC, Chiang FF, Hsu TW, Chen JB, Chao
TH, Ma HF and Wang HM: Clinical experience in 89 consecutive cases
of chronic radiation enterocolitis. J Chin Med Assoc. 74:69–74.
2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Seastedt KP, Trencheva K, Michelassi F,
Alsaleh D, Milsom JW, Sonoda T, Lee SW and Nandakumar G: Accuracy
of CT enterography and magnetic resonance enterography imaging to
detect lesions preoperatively in patients undergoing surgery for
Crohn's disease. Dis Colon Rectum. 57:1364–1370. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cox VL, Tahvildari AM, Johnson B, Wei W
and Jeffrey RB: Bowel obstruction complicated by ischemia: Analysis
of CT findings. Abdom Radiol (NY). 43:3227–3232. 2018.PubMed/NCBI View Article : Google Scholar
|