Introduction
Endometriosis (EM) is a disorder in which
endometrial tissue grows outside of the uterine cavity, such as
within the fallopian tubes or peritoneum (1). It has been suggested that EM affects
6-10% of women of reproductive age in the USA, whilst in the
clinic, EM is diagnosed in 4% of women undergoing tubal ligation
and 50% of teenagers with intractable dysmenorrhea (2). EM is generally considered as an
inflammatory, estrogen-related disease that can induce numerous
symptoms in patients, including pelvic pain or infertility;
however, the pathogenesis of EM remains relatively unclear. The
most current hypothesis is that EM occurs following retrograde
menstruation, which suggests that menstrual uterine contractions
stimulate the reflux of endometrial tissue into the fallopian tubes
and peritoneal cavity. However, retrograde menstruation is observed
in 76-90% of women during menstruation, whereas EM only affects
6-10% of women of reproductive age (3). Recent molecular cytogenetic studies
have provided novel evidence suggesting that acquired alterations
in specific genes or signaling pathways may induce EM (4,5). In
fact, in a previous study, through reanalyzing multiple genetic
datasets of EM, it was identified that numerous important genes may
contribute to the pathogenesis of EM, including angiotensin II
receptor type 1 (AGTR1), membrane metalloendopeptidase, myosin
heavy chain 11 and 15-hydroxyprostaglandin dehydrogenase (6). These genes regulate pathways involved
in the renin-angiotensin system (RAS), smooth muscle contraction,
lipoxin synthesis and the NF-κB signaling pathway (6). AGTR1 is a gene coding receptor for
angiotensin II (AngII) and is considered to mediate major cellular
events; for example, AGTR1 was reported to be expressed in human
epithelial ovarian carcinoma (7),
and it was found to be involved in the invasion, migration and
tumorigenesis of endometrial carcinoma (8). Notably, in a genome-wide association
study, Hsieh et al (9)
identified polymorphisms in the AGTR1 gene in EM. In addition,
AGTR1 activation has also been observed to induce inflammation
through the increased expression of leukocytic and endothelial
adhesion molecules and the increased production of pro-inflammatory
mediators (10). It is widely
accepted that inflammation serves an important role in the
pathogenesis and symptoms of EM.
NF-κB is a well-established pathway that
participates in the inflammatory response, and previous studies
have reported the activation of the NF-κB signaling pathway in EM.
For example, Wei and Shao (11)
suggested that the activation of the NF-κB signaling pathway
promoted the development of EM, while Taniguchi et al
(12) suggested that activated NF-κB
signaling may contribute to EM through inhibiting apoptosis in
endothelial cells.
Therefore, in the present study, AGTR1 expression
levels and the activity of NF-κB in human EM tissues were
investigated. AGTR1 expression levels were significantly increased
in human EM tissues, and in vitro, it was demonstrated that
the increased expression levels of AGTR1 were in response to the
inhibition of the estrogen receptor. AGTR1 was observed to activate
the NF-κB signaling pathway, and promote cell viability and
migration, whilst preventing apoptosis in EM cells, which may
contribute to EM development.
Materials and methods
Patient studies
Study protocols involving human subjects were
approved by the Institutional Ethics Committee of the Fourth
Hospital of Shijiazhuang City, and written informed consent was
obtained from all subjects. From June 2017 to December 2018, 34
women with EM were recruited to this study from the Fourth Hospital
of Shijiazhuang City; however, 2 cases withdrew, 6 cases were
confirmed to not have EM by laparoscopy and 9 cases were excluded
due to the quality control of the samples. In the control group, 32
patients were recruited in the Fourth Hospital of Shijiazhuang
City; however, 5 cases withdrew and 6 cases were excluded due to
the quality control of the samples. Altogether, 17 patients (age,
22-38 years old) with EM with ovarian endometriotic cysts and 21
controls (age, 23-36 years old) were enrolled in the present study.
The collected tissues were histologically confirmed by
pathologists. Data concerning the patients' menstrual cycle phases
were collected by ZZ, YY and LH, and were classified into the
proliferative phase (days 1-14) and the secretory phase (days
15-29). The exclusion criteria of this study included patients who
received hormonal treatment or any anti-inflammatory treatment for
≥6 months prior to surgery.
Cell culture and reagents
Human endometrial stromal cells (ESCs) (cat. no.
CRL-4003) were purchased from the ATCC. ESCs were maintained in
DMEM (Gibco; Thermo Fisher Scientific, Inc.), supplemented with 10%
FBS (Gibco; Thermo Fisher Scientific, Inc.) treated with
dextran-coated charcoal (final concentration, 0.25%; Sigma-Aldrich;
Merck KGaA), to remove any hormonal effects from the contents of
FBS; 100 U/ml penicillin (Invitrogen; Thermo Fisher Scientific,
Inc.); and 10 mg/ml streptomycin (Invitrogen; Thermo Fisher
Scientific Inc.), and maintained at 37˚C and 5% CO2.
Losartan potassium (AGTR1 antagonist) dissolved in
DMSO, AngII (AGTR1 activator) (13),
estrogen (17β-estradiol) and tamoxifen (the estrogen receptor
modulator) in DMSO (14,15) were purchased from Sigma-Aldrich.
Tamoxifen is an estrogen receptor modulator (16). Cells were treated with tamoxifen to
investigate the effect of estrogen on cells when its receptor was
regulated by tamoxifen. Cells were also treated with 17.9 nM TPCA-1
in DMSO (Selleck Chemicals) (17),
which is a selective inhibitor of IKKβ (IκB kinase β). ESCs were
divided into the various treatment groups as follows: i) 10 µM
AngII; ii) 10 µM AngII+10 µM losartan potassium; iii) 10 µM
losartan potassium; iv) 10 nM 17β-estradiol; v) 10 nM
17β-estradiol+10 µM tamoxifen; vi) 10 µM tamoxifen; vii) 10 µM
AngII+17.9 nM TPCA-1; viii) 17.9 nM TPCA-1; ix) DMSO
(controls).
Immunohistochemistry (IHC)
Endothelium tissue was fixed with 10% formalin for
24 h at room temperature, and embedded in paraffin.
Paraffin-embedded tissue samples were cut into 5-µm thick sections.
The tissue sections were subsequently deparaffinized with xylene at
55˚C and rehydrated with descending alcohol series, and then
subjected to antigen retrieval. Deparaffinized sections were
blocked with 5% goat serum (Thermo Fisher Scientific, Inc.) at room
temperature for 1 h. Tissue sections were incubated with a primary
rabbit monoclonal antibody targeting AGTR1 (1:200; Abcam; cat. no.
ab124734) overnight at 4˚C. Following the primary incubation,
sections were incubated with an anti-rabbit horseradish
peroxidase-conjugated secondary antibody (1:8,000; Abcam, cat. no.
ab99702) at room temperature for 1 h. The slides were subsequently
stained with 3,3'-diaminobenzidine, counterstained with hematoxylin
(0.5%) and visualized using a light microscope (Olympus
Corporation). Expression levels were semi-quantified according to
the regular IHC staining grade system (18).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from ESCs or tissues using a
RNeasy kit (Qiagen, Inc.) following the manufacturer's
instructions. Total RNA (500 ng) was reverse transcribed into cDNA
using SuperScript™ III Reverse Transcriptase (Invitrogen; Thermo
Fisher Scientific, Inc.). qPCR (SYBR™ Green; Thermo Fisher
Scientific, Inc.; cat. no. 4309155) was subsequently performed
using an ABI PRISM 7500 Real-Time PCR System (Applied Biosystems;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. Cycling conditions for the reaction were as follows: An
initial hold for 10 min at 95˚C; then 40 cycles of 15 sec at 95˚C
denaturation, 30 sec at an annealing temperature of 60˚C and a 30
sec extension at 72˚C. The following primer pairs were used for the
qPCR: β-actin forward, 5'-CATGTACGTTGCTATCCAGGC-3' and reverse,
5'-CTCCTTAATGTCACGCACGAT-3'; and AGTR1 forward,
5'-ATTTAGCACTGGCTGACTTATGC-3' and reverse,
5'-CAGCGGTATTCCATAGCTGTG-3'. Gene expression levels were quantified
using the 2-ΔΔCq method (19) and normalized to the internal
reference gene β-actin. Each experiment was performed in
triplicate.
Western blotting
Total protein was extracted from tissues or ESCs
using RIPA lysis buffer (Beyotime Institute of Biotechnology; cat.
no. P0013B) containing proteinase inhibitor (Beyotime Institute of
Biotechnology; cat. no. P1006). Total protein was quantified using
BCA assays, and 20 µg protein/lane was separated by 8-10% SDS-PAGE.
The separated proteins were subsequently electrotransferred onto
PVDF membranes (EMD Millipore) and blocked with 5% bovine serum
albumin for 1 h at room temperature. (Beyotime Institute of
Biotechnology; cat. no. ST023-50 g). The membranes were incubated
with the following primary antibodies (all 1:3,000) at 4˚C
overnight: Anti-AGTR1 (Abcam; cat. no. ab124734), anti-p65 (Santa
Cruz Biotechnology, Inc.; cat. no. sc-109), anti-phosphorylated
(pho)-p65 (Ser536; Santa Cruz Biotechnology, Inc.; cat. no.
sc-101752) and anti-β-actin (Santa Cruz Biotechnology, Inc.; cat.
no. sc-7210). Following the primary antibody incubation, membranes
were incubated with anti-rabbit horseradish peroxidase-conjugated
IgG secondary antibodies (1:3,000; Abcam; cat. no. ab99702) at room
temperature for 1 h. Protein bands were visualized using the
Western Bright ECL kit (Bio-Rad Laboratories, Inc.). β-actin was
used as the loading control. The protein expression levels were
quantified with ImageJ software (version 1.8.0; National Institutes
of Health).
Wound healing assay
To evaluate cell migration, ESCs were seeded into
12-well plates (0.1x106 cells per well) and cultured in
DMEM supplemented with 10% FBS until reaching 100% confluence at
37˚C. Next, DMEM was removed, and a P-200 pipette tip was used to
scratch a straight line into the cell monolayer. Cells were
subsequently washed with PBS to remove the cell debris. Cells were
then cultured with serum-free DMEM supplemented with 10 µM losartan
potassium, 10 µM AngII or 17.9 nM TPCA-1 depending on the treatment
group at 37˚C for 72 h. Cells were photographed at 0 and 72 h using
a light microscope (Olympus Corporation), magnification x100, and
the wound closure was quantitatively analyzed. The size of the
regions with and without cells were quantitatively evaluated with
ImageJ software (Version 1.8.0; National Institutes of Health) and
used calculating the percentage wound closure.
Cell transfection
A total of 105 ESCs/well were seeded into
12-well culture plates and transfected with 40 nM small interfering
RNA (siRNA) targeting AGTR1 (5'-CUGUAGAAUUGCAGAUAUU dTdT-3',
3'-dTdT GACAUCUUAACGUCUAUAA-5) or a scrambled control
(5'-UUCUCCGAACGUGUCACGUdTdT-3'; 3'-dTdT AAGAGGGUUGCACAGUGGA-5',
negative control; Santa Cruz Biotechnology, Inc.) using
Lipofectamine®2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Following 24 h of transfection, RT-qPCR and western blotting were
performed to prove the successful transfection, and then the ESCs
were used for subsequent experiments.
Flow cytometric analysis of
apoptosis
Flow cytometry was used to investigate the levels of
apoptosis in ESCs in vitro. Following treatment, ESCs were
treated with 0.25% trypsin and then gently centrifuged at 1,000 x g
for 5 min at room temperature. The collected ESCs were washed with
PBS. The cells were subsequently resuspended in 500 µl binding
buffer containing 5 µl Annexin V-FITC and 10 µl propidium iodide
using the Annexin V-FITC Apoptosis Detection kit (Bio-Rad
Laboratories, Inc.). Following incubation for 30 min in the dark at
room temperature, apoptotic cells were subsequently analyzed using
a BD FACScan™ flow cytometer (BD Biosciences). Data were analyzed
using FlowJo software (version 7.6.5; FlowJo, LLC).
Cell proliferation assay
A total of 5x103 cells/well were seeded
into 96-well plates and were cultured for 72 h at 37˚C. Cellular
proliferation was subsequently analyzed using a WST-1 assay (Roche
Diagnostics), according to the manufacturer's protocol. The
absorbance was measured at 440 nm using a multimode plate
reader.
ELISA
The hormone expression levels of estrogen (cat. no.
K4267-100; BioVision, Inc.), progesterone (cat. no. MBS494530;
MyBioSource, Inc.) and prolactin (cat. no. MBS580135; MyBioSource,
Inc.) in endometrial tissue (1 g) were determined using ELISA kits,
according to the manufacturers' protocols. Briefly, the frozen
tissues were homogenized using a sonicator (20 kHz/s) and were
subsequently centrifuged for 5 min at 5,000 x g at 37˚C to obtain
the supernatant, which was then suspended in PBS and added in the
plates (100 µl/well). A total of 0.1 ml biotinylated antibodies
(1:100) against the target hormones and the extracts from tissues
were then added to the plate. Standard reagents as the per the
ELISA kits were diluted and added to the wells to generate a
standard curve. Following incubation for 2 h at room temperature,
the absorbance was measured at 450 nm using a multimode plate
reader.
Statistical analysis
All the data were analyzed and graphics produced
using R (version 3.6.2). Data are presented as the mean ± SEM of
three independent experimental repeats. Statistical differences
between >2 groups were determined using one-way ANOVA with
Tukey's post hoc test, whereas differences between 2 groups were
determined using Student's t-test. Correlation analysis was
performed using Pearson's correlation test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Increased AGTR1 and NF-κB expression
levels are present in human EM tissues
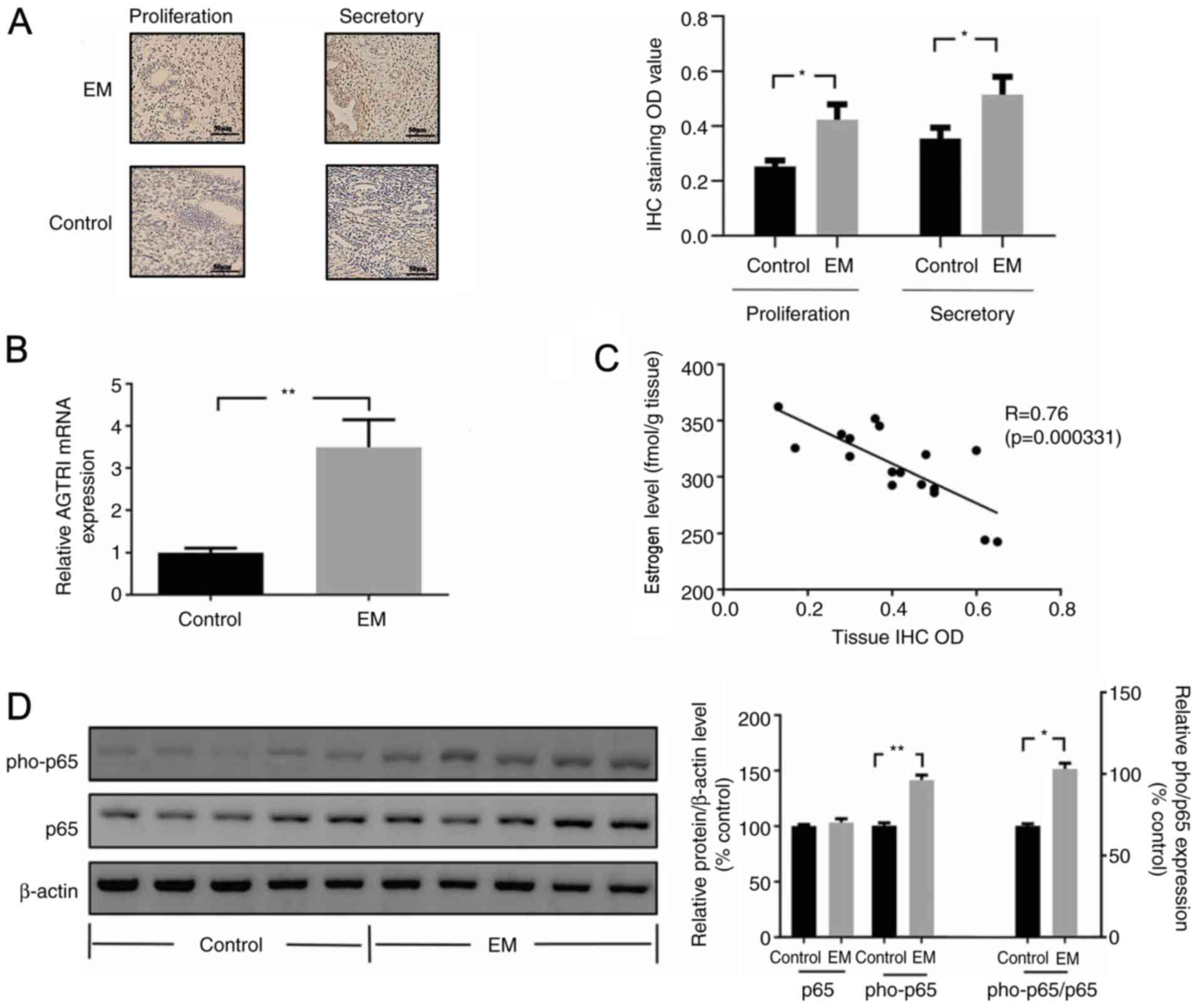
AGTR1 expression levels in EM tissues were
determined using IHC. Compared with that of normal endometrial
tissues (control), Immunohistochemical staining showed that there
were numerous brown red immunoreactive particles of AGTR1
distributed densely in stromal cells and glandular epithelial cells
in the endometrium. The expression levels of AGTR1 in EM tissues
were significantly increased compared with those of the control
tissues (Fig. 1A). Subsequently, the
mRNA expression levels of AGTR1 in EM tissues were evaluated using
RT-qPCR, and it was demonstrated that AGTR1 expression levels were
significantly increased in EM tissue compared with those in control
tissue (Fig. 1B).
Previously, multiple studies have reported that
estrogen serves a critical role in the development of EM (20). In the present study, the levels of
estrogen, progesterone and prolactin in both EM and normal tissues
were investigated, and it was observed that the levels of estrogen
in EM tissues were significantly decreased compared with those in
control tissues (Fig. S1).
Moreover, the levels of estrogen in EM were negatively correlated
with the expression levels of AGTR1 (Fig. 1C). However, the results also
demonstrated that there was no significant difference between the
levels of progesterone or prolactin found in EM tissues and those
found in control tissues (Fig. S1).
Notably, the ratio of estrogen/progesterone was significantly
decreased in EM tissues compared with that of control tissues
(Fig. S1).
In addition, NF-κB activity in EM was investigated
using western blotting, and the data revealed that pho-p65
expression levels (the ratio of pho-p65:p65) were significantly
increased in EM tissues compared with those in control tissues
(Fig. 1D).
AGTR1 promotes the activity of
NF-κB
The effect of AGTR1 on the NF-κB signaling pathway
in ESCs was subsequently investigated. In vitro, losartan
treatment significantly decreased the expression levels of pho-p65
(the ratio of pho-p65:p65) in ESCs compared with those of untreated
control cells, which suggested that AGTR1 antagonist may exert an
inhibitory effect on the NF-κB signaling pathway (Fig. 2A). By contrast, ESCs treated with
AngII, an activator of AGTR1(21),
exhibited significantly increased pho-p65 expression levels (the
ratio of pho-p65:p65) compared with those of control cells, and the
upregulation effect of AngII on pho-p65 was attenuated by losartan
(Fig. 2A) when cells were treated
with AngII in combination with losartan. In addition, following the
genetic knockdown of AGTR1 expression in ESCs using siRNA (Fig. 2B and C), the expression levels of pho-p65 (the
ratio of pho-p65:p65)were observed to be significantly decreased
compared with those of scramble-treated cells (Fig. 2C).
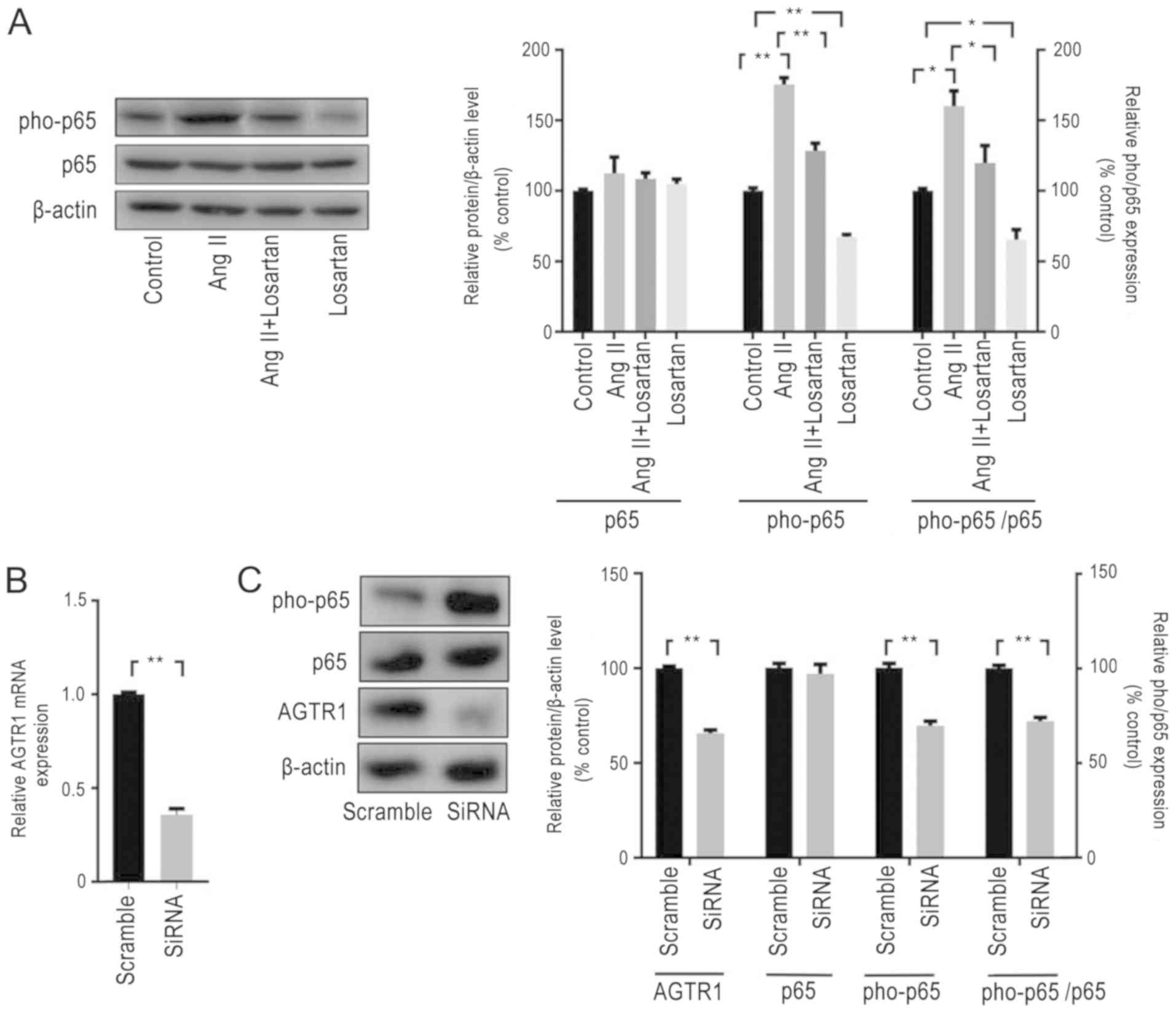 | Figure 2AGTR1 expression promotes the
activity of NF-κB in ESCs. (A) ESCs were exposed to AngII, losartan
or DMSO for 72 h, and expression levels of pho-p65 were analyzed
using western blotting. Activation of AGTR1 by AngII increased the
expression levels of pho-p65, which was inhibited by losartan
treatment. (B) AGTR1 expression was knocked down using siRNA. The
expression levels of AGTR1 were analyzed using reverse
transcription-quantitative PCR; siRNA significantly decreased AGTR1
expression. (C) The expression levels of ATGR1 and pho-p65 were
determined using western blotting. siRNA decreased the
phosphorylation of p65 and the pho-p65/p65 ratio, as well as the
expression levels of ATGR1. All experiments were performed in
triplicate, and data are presented as the mean ± SEM.
*P<0.05, **P<0.01. AGTR1, angiotensin
II receptor type 1; pho, phosphorylated; siRNA, small interfering
RNA; ESCs, endometrial stromal cells; AngII, angiotensin II. |
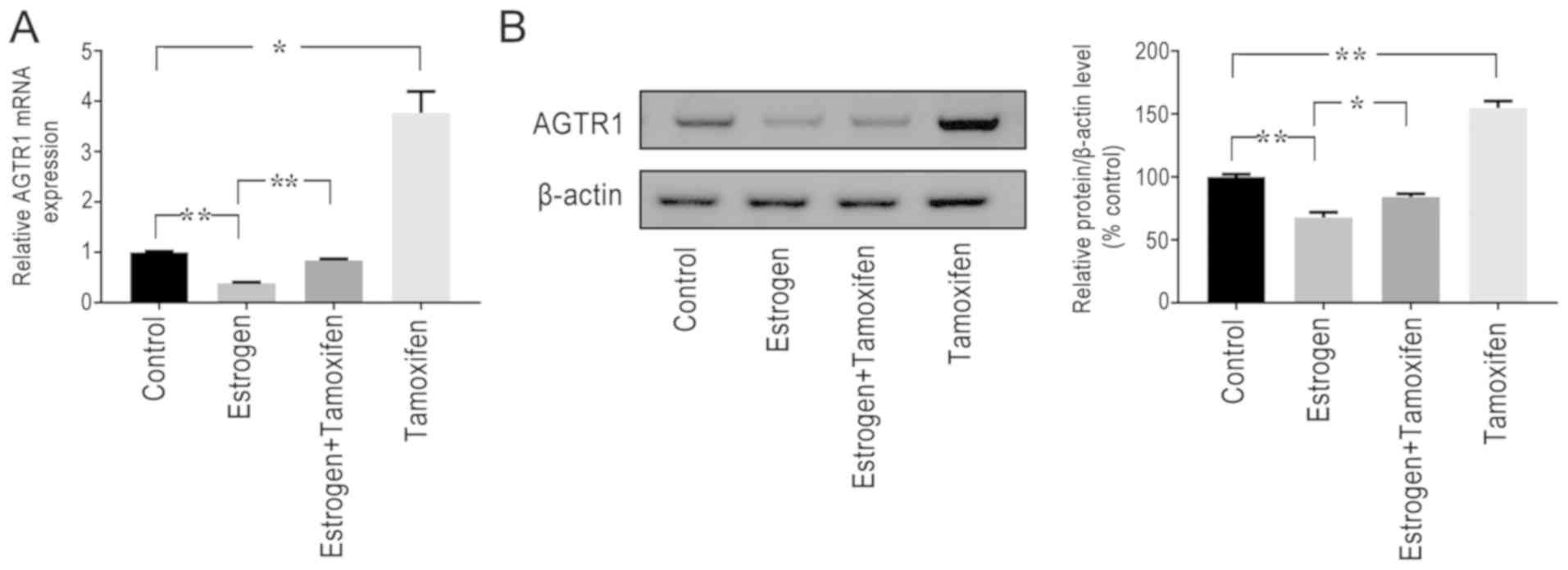
Estrogen inhibits the expression
levels of AGTR1
This study found that estrogen and AGTR1 levels were
negatively correlated in EM. It was subsequently identified that
the loss of estrogen expression could significantly increase AGTR1
expression levels; upon culturing ESCs and subsequently treating
them with estrogen in vitro, it was discovered that
estradiol treatment significantly decreased the expression levels
of AGTR1 compared with those of the control, whereas the inhibition
of AGTR1 expression by estrogen could be alleviated using the
estrogen receptor modulator tamoxifen (Fig. 3), when cells were treated with
estrogen combination with tamoxifen. Tamoxifen also increased the
expression levels of AGTR1 compared with those of the control.
AGTR1 promotes cell migration and
proliferation, and inhibits apoptosis in ESCs through the NF-κB
signaling pathway
The effects of AGTR1 on the migration of ESCs were
investigated using wound healing assays. Pre-treatment with AngII
for 72 h resulted in a significant increase in cell migratory
ability compared with that of the control group; however, this
effect was significantly prevented following treatment with TPCA-1,
an antagonist of the signaling NF-κB pathway (Figs. 4A and S2A). In addition, losartan treatment also
significantly decreased the migratory ability of ESCs compared with
that of the control group (Fig.
4A).
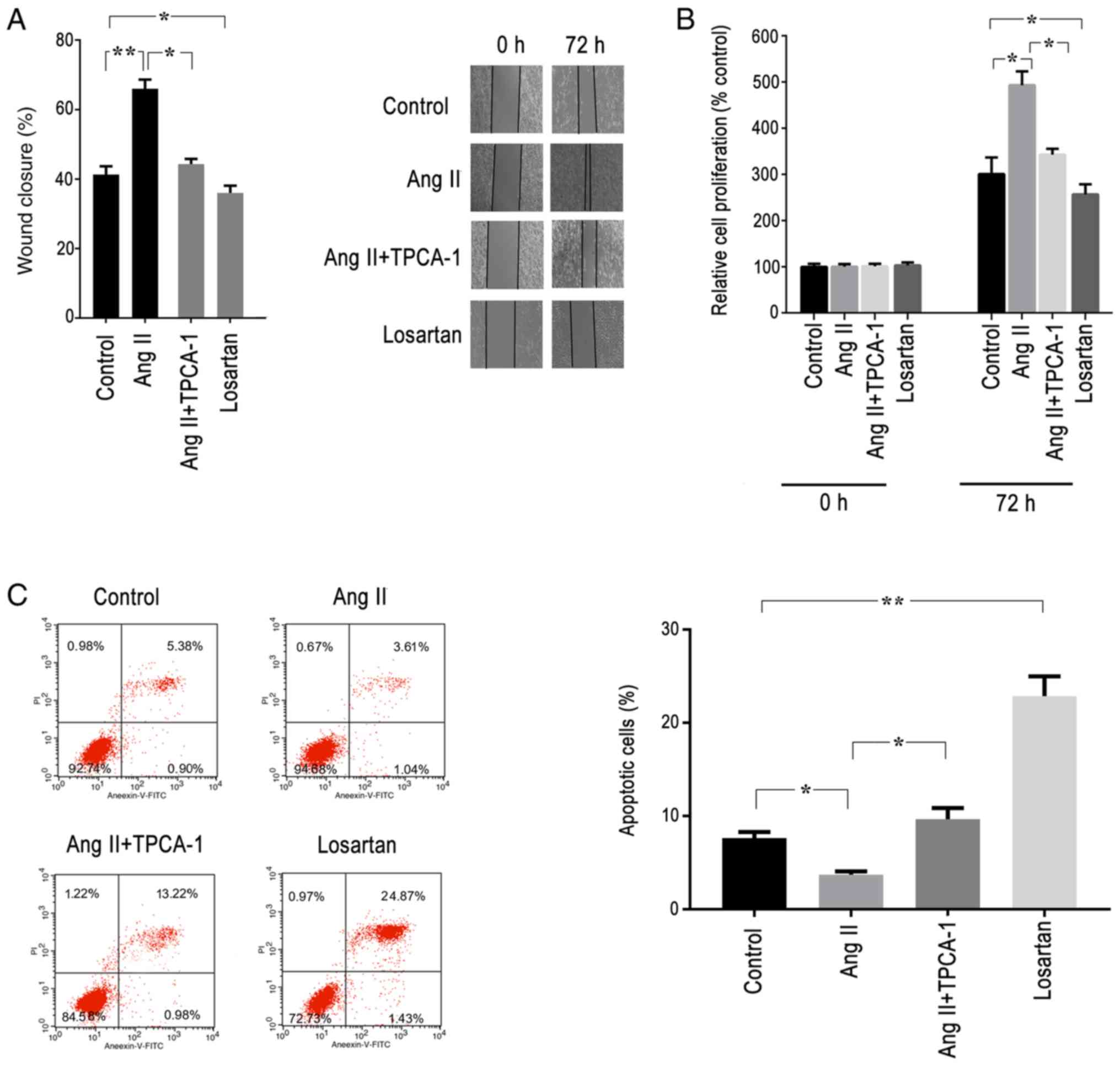 | Figure 4Activation of AGTR1 promotes cell
proliferation and migration, and prevents apoptosis in ESCs in
vitro via the NF-κB signaling pathway. ESCs were treated with
AngII (AGTR1 activator), TPCA-1 (selective inhibitor of IκB kinase
β), losartan (AGTR1 antagonist) or DMSO (control) for 72 h in
vitro, and cell proliferation, apoptosis and migration were
analyzed. (A) Wound healing assay was performed, and images were
acquired using light microscopy (magnification, 100x). The
activation of AGTR1 promoted wound closure, which was blocked by
TCPA-1. Losartan significantly inhibited cell migration compared
with that of the control. (B) Cell proliferation assay. The
activation of AGTR1 by AngII increased cell proliferation compared
with that of the control, whereas TPCA-1 inhibited the cell
proliferation induced by AngII. Losartan also inhibited the cell
proliferation. (C) Cell apoptosis assay. Following treatment for 72
h in vitro, ESCs were collected, stained with Annexin-V and
PI, and analyzed by flow cytometry. The number of apoptotic cells
(Annexin V-positive cells) was determined as the percentage of
gated cells at upper-right and lower-right quadrants.
Representative images and relative quantifications are shown. The
results indicated that the activation of AGTR1 by AngII inhibited
cell apoptosis, whereas the anti-apoptotic effect was inhibited by
TPCA-1. Losartan promoted the apoptosis of cells. All experiments
were performed in triplicate, and the data are presented as the
mean ± SEM. *P<0.05, **P<0.01. ESCs,
endometrial stromal cells; AGTR1, angiotensin II receptor type 1;
AngII, angiotensin II; PI, propidium iodide. |
To investigate the role of AGTR1 on the
proliferative activity of ESCs, cell proliferation and apoptosis
assays were conducted. The cells were cultured for 72 h in the
presence of AngII, TPCA-1 or losartan. ESCs treated with AngII
exhibited a significantly increased proliferative rate compared
with that of the control group, alongside a significantly decreased
apoptotic rate (Fig. 4B and C). By contrast, TPCA-1 treatment
significantly blocked the effects of AngII on ESCs (Figs. 4B, C,
S2B and C). ESCs treated with losartan or TPCA-1
displayed significantly decreased proliferation rates and increased
apoptotic rates compared with those of the control group (Fig. 4B and C).
Discussion
AGTR1 is a component of the RAS, and has been
reported to be upregulated in EM (6,21). In
the present study, decreased levels of estrogen in EM tissues were
found to be associated with increased expression levels of AGTR1,
which in turn promoted cell proliferation and prevented cell
apoptosis through the activation of the NF-κB signaling pathway. In
the RAS, bioactive effector molecules, AngI and AngII serve
antagonistic roles by binding to different receptors, namely AGTR1,
AGTR2, respectively (22), which
subsequently exert vasoactive or proliferative roles. In recent
years, increasing attention has been paid to the local activity of
the RAS in ovarian and endometrial tissues, which may subsequently
contribute to physiological and pathological processes such as
follicle maturation, regulation of reproduction, angiogenesis and
tumor cell proliferation (23-25). Notably, the
dysregulation of the RAS has been observed in EM; for example,
Abraham et al (26) reported
that, in rat endometrial stromal cells, AngII induced
Cyclooxygenase-2 gene expression by activating the calcineurin/NFAT
signaling pathway. In another study, Kowalczyńska et al
(27) investigated the polymorphisms
in the angiotensin I converting enzyme (ACE) gene and AGTR1 in
women with EM, and their results indicated that the A2350G
polymorphism in the ACE gene was associated with the development of
EM. Nakao et al (28)
discovered that the expression of AGTR1 was largely located in
endometrial glandular epithelium and stromal cells, and AGTR1
expression levels were markedly increased in EM compared with those
of normal tissue. Therefore, these findings suggested that the
increased expression levels of AGTR1 may increase RAS sensitivity
in endothelium tissue, and the subsequent activation of the
RAS-AGTR1 system may promote the pathogenesis of EM.
The NF-κB family represents a family of
transcription factors that serve vital roles in various processes
such as cellular survival, proliferation and differentiation.
Furthermore, the NF-κB signaling pathway has been found to regulate
menstruation (20). King et
al (29) reported that IκB
kinase α and TANK Binding Kinase 1 mRNA expression levels were
increased in the human endometrium during the perimenstrual phase
of the menstrual cycle in response to premenstrual progesterone
withdrawal. In addition, a previous study have demonstrated the
constitutive activation of NF-κB in endometriotic lesions (30). The activation of the NF-κB signaling
pathway induces the production of pro-inflammatory cytokines such
as IL-8 and matrix metalloproteinases, which subsequently induce
tissue breakdown (31). Nie et
al (32) reported the expression
of NF-κB in the eutopic endometrium of patients with adenomyosis,
which reportedly increased the expression of nuclear p65 and p52,
whilst decreasing the expression of progesterone receptor B and
cytoplasmic IκBα. Park et al (33) reported that the expression levels of
NF-κB p65 were increased in the eutopic endometrium and adenomyosis
nodules of women with adenomyosis, which strongly suggested that
NF-κB served a critical role in the pathogenesis and
pathophysiology of adenomyosis. Finally, Wei and Shao (11) observed that blocking NF-κB activity
with nobiletin reduced the lesion size and pain in a mouse model of
EM. In the present study, the activation of AGTR1 in EM increased
the activity of NF-κB, and subsequently promoted cell proliferation
and migration. The interaction between AGTR1 and NF-κB has been
reported in several studies; Li et al (34) found that the treatment of hepatic
stellate cells with AngII activated its receptor AGTR1, which
subsequently increased the activity of NF-κB; Du et al
(35) reported that an AGTR1
antagonist inhibited the expression of NF-κB, which then decreased
the proliferation of breast cancer cells; and Ekambaram et
al (36) suggested that AGTR1
overexpression may activate the NF-κB signaling pathway, and may
promote cell proliferation, migration and invasion, as well as
angiogenesis. In addition, AngII/AGTR1 exacerbated vascular
calcification following the activation of NF-κB, which induced the
inflammatory response in human vascular smooth muscle cells
(37). Therefore, it was
hypothesized that, in EM, the upregulation of AGTR1 expression may
activate the NF-κB signaling pathway, which may promote cell
migration and proliferation to contribute to EM development, as
well as the inflammatory response to induce symptoms of EM.
The present study also discovered that the
expression of AGTR1 was regulated by estrogen. EM is known to be an
estrogen-dependent disease; Galvankar et al (38) reported that estrogen was essential
for the induction of EM, whereas Wang et al (39) suggested that estradiol may promote
inflammation, and increasing expression of both C-X-C motif
chemokine 12(CXCL12) and C-X-C Motif Chemokine Receptor 4 in human
endometrial stromal cells, which contributes to EM pathogenesis.
CXCL12 is a chemokine and plays a crucial role inflammatory
reaction and cell migration (40).
Moreover, low expression levels of estrogen and progesterone have
been found in the serum and urine of women with EM (41,42). In
the present study, it was suggested that the estrogen/AGTR1
signaling pathway may be involved in EM pathogenesis. In stromal
cells derived from human endometrial tissue, estrogen treatment
decreased the expression levels of ATGR1, which subsequently
inhibited NF-κB activity, whereas the estrogen receptor modulator
tamoxifen increased the expression levels of components of the
ATGR1/NF-κB signaling pathway. The regulatory effect of estrogen on
AGTR1 expression has been found in other studies; Kooptiwut et
al (43) suggested that, under
high-glucose conditions, estradiol could decrease the mRNA
expression levels of AGTR1 in pancreatic β-cells, while Gao et
al (44) reported that estradiol
could decrease the expression levels of AGTR1 in the uterine
artery. Nickenig et al (45)
also found that the AGTR1 protein density in rat aortic tissue was
increased during estrogen deficiency. Therefore, during
menstruation, the low levels of estrogen may induce an increase in
AGTR1 expression, and then activate its downstream signaling
pathway. However, in a study on ischemic injury of the heart in
rats, Xue et al (46) argued
that treatment with estradiol increased the expression levels of
AGTR1in the heart. This paradoxical result suggests that these
effects may be organ specific, and further research is required to
understand the role of AGTR1 in EM. In addition, the present study
was limited by the fact that it did not involve in vivo
studies to verify the effect of AGTR1; thus, further investigations
are required to understand the pathogenesis of EM.
In conclusion, the present study suggested that
AGTR1 may contribute to the development of EM through the NF-κB
signaling pathway, and the increased expression levels of AGTR1
observed in EM tissue may be due to the low levels of estrogen
during menstruation.
Supplementary Material
Figure S1. Expression levels of
estrogen, progesterone and prolactin in EM and normal tissues. Both
the estrogen level and the ratio of estrogen/progesterone were
significantly decreased in EM tissues compared with those in
control tissues, whereas there was no significant difference in
progesterone or prolactin between EM and control tissues. Data are
presented as the mean ± SEM. *P<0.05. EM, endometriosis.
Figure S2. TPCA‑1 inhibits cell
viability and migration, and induces apoptosis in ESCs in
vitro. ESCs were treated with TPCA‑1 (selective inhibitor of
IκB kinase β) or DMSO (control) for 72 h in vitro, and cell
migration, viability and apoptosis were analyzed. (A) Wound healing
assay was performed, and images were acquired using light
microscopy (magnification, 100x). TCPA‑1 significantly inhibited
cell migration compared with that of the control. (B) Cell
proliferation assay, TPCA‑1 inhibited cell proliferation compared
with that of the control. (C) Cell apoptosis assay, following
treatment for 72 h in vitro, ESCs were collected, stained
with annexin‑V and PI, and analyzed by flow cytometry. The number
of apoptotic cells (annexin V‑positive cells) was determined as the
percentage of gated cells at upper‑right and lower‑right quadrants.
Representative images and relative quantifications are shown. The
data demonstrated that TPCA‑1 reduced the viability and migration
of cells, whilst increasing the apoptotic index of cells compared
with the values exhibited by the control. All experiments were
performed in triplicate and data are presented as the mean ± SEM.
*P<0.05, **P<0.01. ESCs, endometrial stromal cells; PI,
propidium iodide.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Fund of China (grant no. 81503608) and the Natural Science
Fund of Shijiazhuang city (grant no. 121461783).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZZ and YY performed the experiments. ZZ, YY, XY and
LH collected and analyzed the data. ZZ and JC designed the study
and analyzed the data, drafted and reviewed the manuscript and
supervised the entire study. YY designed the study, revised the
manuscript and provided material support. All the authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
Study protocols involving human subjects were
approved by the Institutional Ethics Committee of The Fourth
Hospital of Shijiazhuang City, and written informed consent was
obtained from all subjects.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Czyzyk A, Podfigurna A, Szeliga A and
Meczekalski B: Update on endometriosis pathogenesis. Minerva
Ginecol. 69:447–461. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cramer DW and Missmer SA: The epidemiology
of endometriosis. Ann N Y Acad Sci. 955:11–22, Discussion 34-16,
396-406. 2002.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Garcia-Velasco JA and Somigliana E:
Management of endometriomas in women requiring IVF: To touch or not
to touch. Hum Reprod. 24:496–501. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Diao R, Wei W, Zhao J, Tian F, Cai X and
Duan YG: CCL19/CCR7 contributes to the pathogenesis of
endometriosis via PI3K/Akt pathway by regulating the proliferation
and invasion of ESCs. Am J Reprod Immunol. 7(e12744)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pazhohan A, Amidi F, Akbari-Asbagh F,
Seyedrezazadeh E, Farzadi L, Khodarahmin M, Mehdinejadiani S and
Sobhani A: The Wnt/β-catenin signaling in endometriosis, the
expression of total and active forms of β-catenin, total and
inactive forms of glycogen synthase kinase-3β, WNT7a and
DICKKOPF-1. Eur J Obstet Gynecol Reprod Biol. 220:1–5.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang Z, Ruan L, Lu M and Yao X: Analysis
of key candidate genes and pathways of endometriosis
pathophysiology by a genomics-bioinformatics approach. Gynecol
Endocrinol. 35:576–581. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Park YA, Choi CH, Do IG, Song SY, Lee JK,
Cho YJ, Choi JJ, Jeon HK, Ryu JY, Lee YY, et al: Dual targeting of
angiotensin receptors (AGTR1 and AGTR2) in epithelial ovarian
carcinoma. Gynecol Oncol. 135:108–117. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Watanabe Y, Shibata K, Kikkawa F, Kajiyama
H, Ino K, Hattori A, Tsujimoto M and Mizutani S: Adipocyte-derived
leucine aminopeptidase suppresses angiogenesis in human endometrial
carcinoma via renin-angiotensin system. Clin Cancer Res.
9:6497–6503. 2003.PubMed/NCBI
|
|
9
|
Hsieh YY, Chang CC, Chen SY, Chen CP, Lin
WH and Tsai FJ: XRCC1 399 Arg-related genotype and allele, but not
XRCC1 His107Arg, XRCC1 Trp194Arg, KCNQ2, AT1R, and hOGG1
polymorphisms, are associated with higher susceptibility of
endometriosis. Gynecol Endocrinol. 28:305–309. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
MacKenzie A: Endothelium-derived
vasoactive agents, AT1 receptors and inflammation. Pharmacol Ther.
131:187–203. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wei X and Shao X: Nobiletin alleviates
endometriosis via down-regulating NF-κB activity in endometriosis
mouse model. Biosci Rep. 38(BSR20180470)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Taniguchi F, Uegaki T, Nakamura K, Mon KY,
Harada T and Ohbayashi T: Inhibition of IAP (inhibitor of
apoptosis) proteins represses inflammatory status via nuclear
factor-kappa B pathway in murine endometriosis lesions. Am J Reprod
Immunol. 79:2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Diop-Frimpong B, Chauhan VP, Krane S,
Boucher Y and Jain RK: Losartan inhibits collagen I synthesis and
improves the distribution and efficacy of nanotherapeutics in
tumors. Proc Natl Acad Sci USA. 108:2909–2914. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Block M, Fister S, Emons G, Seeber S,
Gründker C and Günthert AR: Antiproliferative effects of
antiestrogens and inhibitors of growth factor receptor signaling on
endometrial cancer cells. Anticancer Res. 30:2025–2031.
2010.PubMed/NCBI
|
|
15
|
Slopien R and Meczekalski B: Aromatase
inhibitors in the treatment of endometriosis. Prz Menopauzalny.
15:43–47. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Polin SA and Ascher SM: The effect of
tamoxifen on the genital tract. Cancer Imaging. 8:135–145.
2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Podolin PL, Callahan JF, Bolognese BJ, Li
YH, Carlson K, Davis TG, Mellor GW, Evans C and Roshak AK:
Attenuation of murine collagen-induced arthritis by a novel,
potent, selective small molecule inhibitor of IkappaB Kinase 2,
TPCA-1
(2-[(aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide),
occurs via reduction of proinflammatory cytokines and
antigen-induced T cell Proliferation. J Pharmacol Exp Ther.
312:373–381. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chatterjee S, Malhotra R, Varghese F,
Bukhari AB, Patil A, Budrukkar A, Parmar V, Gupta S and De A:
Quantitative immunohistochemical analysis reveals association
between sodium iodide symporter and estrogen receptor expression in
breast cancer. PLoS One. 8(e54055)2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Reis FM, Petraglia F and Taylor RN:
Endometriosis: Hormone regulation and clinical consequences of
chemotaxis and apoptosis. Hum Reprod Update. 19:406–418.
2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nehme A, Zouein FA, Zayeri ZD and Zibara
K: An update on the tissue renin angiotensin system and its role in
physiology and pathology. J Cardiovasc Dev Dis.
6(14)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Herr D, Bekes I and Wulff C: Local
renin-angiotensin system in the reproductive system. Front
Endocrinol (Lausanne). 4(150)2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Vinson GP, Teja R, Ho MM, Hinson JP and
Puddefoot JR: The role of the tissue renin-angiotensin system in
the response of the rat adrenal to exogenous angiotensin II. J
Endocrinol. 158:153–159. 1998.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yoshimura Y: The ovarian renin-angiotensin
system in reproductive physiology. Front Neuroendocrinol.
18:247–291. 1997.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Brunswig-Spickenheier B and Mukhopadhyay
AK: Local regulatory factors in regulation of ovarian function:
Role of prorenin-renin-angiotensin-system. Indian J Exp Biol.
41:669–681. 2003.PubMed/NCBI
|
|
26
|
Abraham F, Sacerdoti F, De Leon R, Gentile
T and Canellada A: Angiotensin II activates the calcineurin/NFAT
signaling pathway and induces cyclooxygenase-2 expression in rat
endometrial stromal cells. PLoS One. 7(e37750)2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kowalczyńska LJ, Ferenc T, Wojciechowski
M, Mordalska A, Pogoda K and Malinowski A: Endometriosis and RAS
system gene polymorphisms: The association of ACE A2350G
polymorphism with endometriosis in Polish individuals. DNA Cell
Biol. 33:328–335. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nakao T, Chishima F, Sugitani M, Tsujimura
R, Hayashi C and Yamamoto T: Expression of angiotensin II types 1
and 2 receptors in endometriotic lesions. Gynecol Obstet Invest.
82:294–302. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
King AE, Critchley HO and Kelly RW: The
NF-kappaB pathway in human endometrium and first trimester decidua.
Mol Hum Reprod. 7:175–183. 2001.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kaponis A, Iwabe T, Taniguchi F, Ito M,
Deura I, Decavalas G, Terakawa N and Harada T: The role of
NF-kappaB in endometriosis. Front Biosci (Schol Ed). 4:1213–1234.
2012.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Luca M, Huang S, Gershenwald JE, Singh RK,
Reich R and Bar-Eli M: Expression of interleukin-8 by human
melanoma cells up-regulates MMP-2 activity and increases tumor
growth and metastasis. Am J Pathol. 151:1105–1113. 1997.PubMed/NCBI
|
|
32
|
Nie J, Lu Y, Liu X and Guo SW:
Immunoreactivity of progesterone receptor isoform B, nuclear factor
kappaB, and IkappaBalpha in adenomyosis. Fertil Steril. 92:886–889.
2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Park H, Kim SH, Cho YM, Ihm HJ, Oh YS,
Hong SH, Chae HD, Kim CH and Kang BM: Increased expression of
nuclear factor kappa-B p65 subunit in adenomyosis. Obstet Gynecol
Sci. 59:123–129. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li X, Zhang YJ, Meng Y, Zhou GS and Zhang
ZS: Effect of angiotensin II type 1 receptor and
angiotensin-converting enzyme gene silencing on nuclear
factor-kappaB activity in hepatic stellate cells. Nan Fang Yi Ke Da
Xue Xue Bao. 29:402–404. 2009.(In Chinese). PubMed/NCBI
|
|
35
|
Du N, Feng J, Hu LJ, Sun X, Sun HB, Zhao
Y, Yang YP and Ren H: Angiotensin II receptor type 1 blockers
suppress the cell proliferation effects of angiotensin II in breast
cancer cells by inhibiting AT1R signaling. Oncol Rep. 27:1893–1903.
2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ekambaram P, Lee JL, Hubel NE, Hu D,
Yerneni S, Campbell PG, Pollock N, Klei LR, Concel VJ, Delekta PC,
et al: The CARMA3-Bcl10-MALT1 signalosome drives NFκB activation
and promotes aggressiveness in angiotensin II receptor-positive
breast cancer. Cancer Res. 78:1225–1240. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Jia G, Stormont RM, Gangahar DM and
Agrawal DK: Role of matrix Gla protein in angiotensin II-induced
exacerbation of vascular calcification. Am J Physiol Heart Circ
Physiol. 303:H523–H532. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Galvankar M, Singh N and Modi D: Estrogen
is essential but not sufficient to induce endometriosis. J Biosci.
42:251–263. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang X, Mamillapalli R, Mutlu L, Du H and
Taylor HS: Chemoattraction of bone marrow-derived stem cells
towards human endometrial stromal cells is mediated by estradiol
regulated CXCL12 and CXCR4 expression. Stem Cell Res. 15:14–22.
2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Dotan I, Werner L, Vigodman S, Weiss S,
Brazowski E, Maharshak N, Chen O, Tulchinsky H, Halpern Z and
Guzner-Gur H: CXCL12 is a constitutive and inflammatory chemokine
in the intestinal immune system. Inflamm Bowel Dis. 16:583–592.
2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Cunha-Filho JS, Gross JL, Bastos de Souza
CA, Lemos NA, Giugliani C, Freitas F and Passos EP:
Physiopathological aspects of corpus luteum defect in infertile
patients with mild/minimal endometriosis. J Assist Reprod Genet.
20:117–121. 2003.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Smith MP, Keay SD, Margo FC, Harlow CR,
Wood PJ, Cahill DJ and Hull MG: Total cortisol levels are reduced
in the periovulatory follicle of infertile women with minimal-mild
endometriosis. Am J Reprod Immunol. 47:52–56. 2002.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kooptiwut S, Wanchai K, Semprasert N,
Srisawat C and Yenchitsomanus PT: Estrogen attenuates AGTR1
expression to reduce pancreatic β-cell death from high glucose. Sci
Rep. 7(16639)2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Gao H, Yallampalli U and Yallampalli C:
Protein restriction to pregnant rats increases the plasma levels of
angiotensin II and expression of angiotensin II receptors in
uterine arteries. Biol Reprod. 86(68)2012.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Nickenig G, Baumer AT, Grohe C, Bäumer AT,
Grohè C, Kahlert S, Strehlow K, Rosenkranz S, Stäblein A, Beckers
F, et al: Estrogen modulates AT1 receptor gene expression in vitro
and in vivo. Circulation. 97:2197–2201. 1998.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Xue Q, Xiao D and Zhang L: Estrogen
regulates angiotensin II receptor expression patterns and protects
the heart from ischemic injury in female rats. Biol Reprod.
93(6)2015.PubMed/NCBI View Article : Google Scholar
|