Introduction
The incidence of stroke is increasing on an annual
basis worldwide, with high disability and mortality rates (1). In excess of two-thirds of stroke cases
are of ischemic stroke, which has become the second leading cause
of mortality and disability worldwide (2). Stenosis that is caused by the formation
and development of atherosclerosis is one of the main risk factors
of cerebrovascular disease, especially ischemic cerebrovascular
disease, which accounts for 19-35% of all cases (3), and is closely associated with high
mortality rate due to acute cerebral infarction (4,5).
Therefore, the management of ischemic stroke to achieve the risk
stratification of patients is becoming increasingly important,
where not only the severity of carotid stenosis is evaluated, but
also the stability of carotid plaque. Previous studies have
indicated that the components of atherosclerotic plaque will also
affect its stability, leading to the emergence of vulnerable plaque
(6,7). The presence of components of vulnerable
plaque provide an indication that the probability of plaque rupture
is high, with plaque rupture subsequently leading to thrombus
events (8). In a large-scale study
of carotid plaque histology, it was demonstrated that the
histological characteristics of carotid plaque instability are
closely associated with the risk of stroke (9), and the presence and composition of
vulnerable plaque are also associated with the occurrence of
ipsilateral cerebrovascular events (10). Therefore, vulnerable plaque formation
fulfills an important role in the prevention and control of
cerebrovascular diseases.
With the development of imaging technology, it has
been previously demonstrated that high-resolution magnetic
resonance imaging (HR-MRI) exhibits high sensitivity and
specificity in terms of the detection of carotid vulnerable plaque
(11). Collected data has indicated
that the sensitivity and specificity of this technique can be as
high as 90-100% (12,13). HR-MRI is able to clearly discern the
structural characteristics of the vascular wall, the shape and size
of the arterial plaque and its specific components. However
currently, relatively few studies have been published on this
topic, and those on carotid plaque models with different degrees of
stenosis are scarce. Therefore, in the current study, the clinical
data of corresponding patients was collected for analysis to
explore the value of HR-MRI in the identification of vulnerable
carotid plaque.
Materials and methods
Patients
Between March 2018 and March 2019, 72 patients in
the Department of Neurology China-Japan Union Hospital of Jilin
University were selected, including 49 males and 23 females, aged
between 45 and 82 years. The present study was approved by the
Ethics Committee of China-Japan Union hospital of Jilin University
(approval no. 2020010812; Changchun, China). Patients who
participated in this research had complete clinical data. Signed
informed consents were obtained from the patients and/or the
guardians.
Inclusion and exclusion criteria
Patients were included in the present study if they
met the following criteria: i) Carotid plaque and different degrees
of carotid stenosis had been identified by carotid ultrasound; ii)
the diagnosis of acute cerebrovascular disease conformed to the
Chinese guidelines for the diagnosis and treatment of acute
ischemic stroke (2018 edition) (14); iii) the age of the patient fell
within the range 18-85 years; and iv) the patients and their
families were willing to sign informed consent forms.
The exclusion criteria were as follows: i) The
patient also suffered from serious heart disease and arrhythmia
(such as atrial fibrillation); ii) there were foreign bodies in the
body that could not be examined by MRI, such as metal prostheses or
pacemakers; iii) the patient was convulsing, and unable to
cooperate with the examination; iv) the patients had a severe high
fever; v) the patient experienced claustrophobia, drug or alcohol
abuse, or other mental disorders; and vi) the MRI quality score was
≤2 (as defined by the presence of motion and blood flow artifacts
in the image, such that the plaque components could not be clearly
identified).
Study groups
Patients who were admitted to the hospital between
March 2018 and March 2019 were divided into a symptomatic group
(group A) and an asymptomatic group (group B), according to whether
the patient was suffering from acute ischemic cerebrovascular
disease. After the degree of stenosis had been assessed by carotid
ultrasound, patients were further divided into mild stenosis
(stenosis degree <50%; groups A1 and B1), moderate stenosis
(stenosis degree 50-69%; groups A2 and B2) and severe stenosis
(stenosis degree ≥70%; groups A3 and B3) groups. A total of 12
patients were included in each group, and a total of 72 patients
were included in the study.
Collection of image data
Cervical vascular examination
The carotid ultrasound system utilizes a domestic
Mindray DC-8 color Doppler ultrasound imaging instrument (Mindray
Medical International Limited) with a probe frequency of 9 Hz. The
bilateral carotid arteries were examined separately, and the
morphology of carotid plaque was observed according to color
Doppler flow imaging. The echo characteristics were subsequently
analyzed. The plaque size, and peak and diastolic peaks were
measured at their narrowest dimensions, and the sizes of the distal
segments of the lumen were also measured. All carotid ultrasound
procedures were performed by an experienced sonographer.
Carotid HR-MRI examination
In order to perform the carotid HR-MRI examinations,
the Siemens Skyra 3.0T nuclear magnetic resonance scanning system
(Siemens Healthineers) was employed. The coil used was the Siemens
head-and-neck combined coil. The patient was instructed to lay down
on the examination bed, where the head was later tilted. Prior to
the scan, the examinee was subjected to a longer scan time, where
the examinees were instructed to allow their body to relax. After
having adjusted themselves to a comfortable position, the patients
were restricting from moving their bodies freely during the
scanning process in order to minimize swallowing, to reduce
artifacts during imaging and to improve image quality. In terms of
HR-MRI, carotid plaque three-dimensional time-of-flight (3D-TOF)
scans [time of repetition (TR), 29 msec; time of echo (TE), 4 msec;
field of view (FOV), 160/180 mesc], T1W1 black blood scans (TR, 800
msec; TE, 10 msec; FOV, 160/180 msec) and T2WI black blood nuclear
scans (TR, 3500 msec; TE, 60 msec; FOV, 160/180 msec) were
sequentially performed on each patient. Concerning the neck
positioning image scanning method, the following procedure was
followed: The sagittal position was centered at the position of the
3-4 level of the flat cervical vertebra, and in the coronal and
transverse positioning images, the target area was placed in the
center of the field-of-view (FOV) frame.
Image post-processing
The ultrasound reports were issued after evaluation
and analysis of the results obtained from the cervical vascular
ultrasound. These reports were produced by an ultrasound doctor
with a deputy chief physician, or by a physician with a higher
status. A multi-contrast MRI sequence image obtained after HR-MRI
by an experienced radiologist combined with a Magnetic
resonance-Vulnerable plaque diagnosis technology (MR-VPD)
examination was processed using the MRI-PlaqueView™ software (V2.1)
(V2.1; VPDiagnostics) to analyse and report on the data. The
evaluation included a determination of the degree of vascular
stenosis according to the following equation: Degree of vascular
stenosis=(B-A)/B x100%, where A is the narrowest diameter of the
stenosis, and B is the normal diameter of the stenosis telecentric
end. Diagnostic criteria of carotid stenosis were then consulted:
i) <50%, mild stenosis; >70%, severe stenosis (15).
The acoustic characteristics of the carotid
ultrasound were subsequently analyzed in order to provide the
accurate classification of stenosis, and to classify stenosis into
mild, moderate or severe stenosis, according to the standard
protocol (14). Before analyzing the
images using the MRI-PlaqueView™ software, the HR-MRI
multi-contrast images of the neck were evaluated for image quality.
According to the evaluation criteria (16), a quality score between 1 (for low
quality) and 4 (for high quality) was assigned. The American Heart
Association (AHA) plaque typing method was followed, which was
based on the modified plaque classification standard for nuclear
magnetics (17) in which plaque
types I-II, III, VII and VIII are classified as stable plaque, and
plaque types IV-V and VI are categorized as vulnerable plaque.
Specifically, the descriptions of the plaque types are as follows:
Type I-II, near-normal wall thickening without calcification; type
III, diffuse thickening or small eccentric non-calcified plaque
under the endometrium; type IV-V, a lipid or necrotic core exists
in the plaque, which is surrounded by fibrous tissue and may have
calcification; type VI, complex plaque, which may have surface
defects, bleeding, or thrombosis; type VII, calcified plaques; and
type VIII, fibrous plaque without a lipid core that may have small
calcifications.
Statistical analysis
The obtained research data were statistically
analyzed using SPSS software version 19.0 (IBM Corp.), and
comparisons between the data groups were performed by using the
χ2 test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Comparison of the number of cases with
vulnerable plaque in the symptomatic and asymptomatic groups
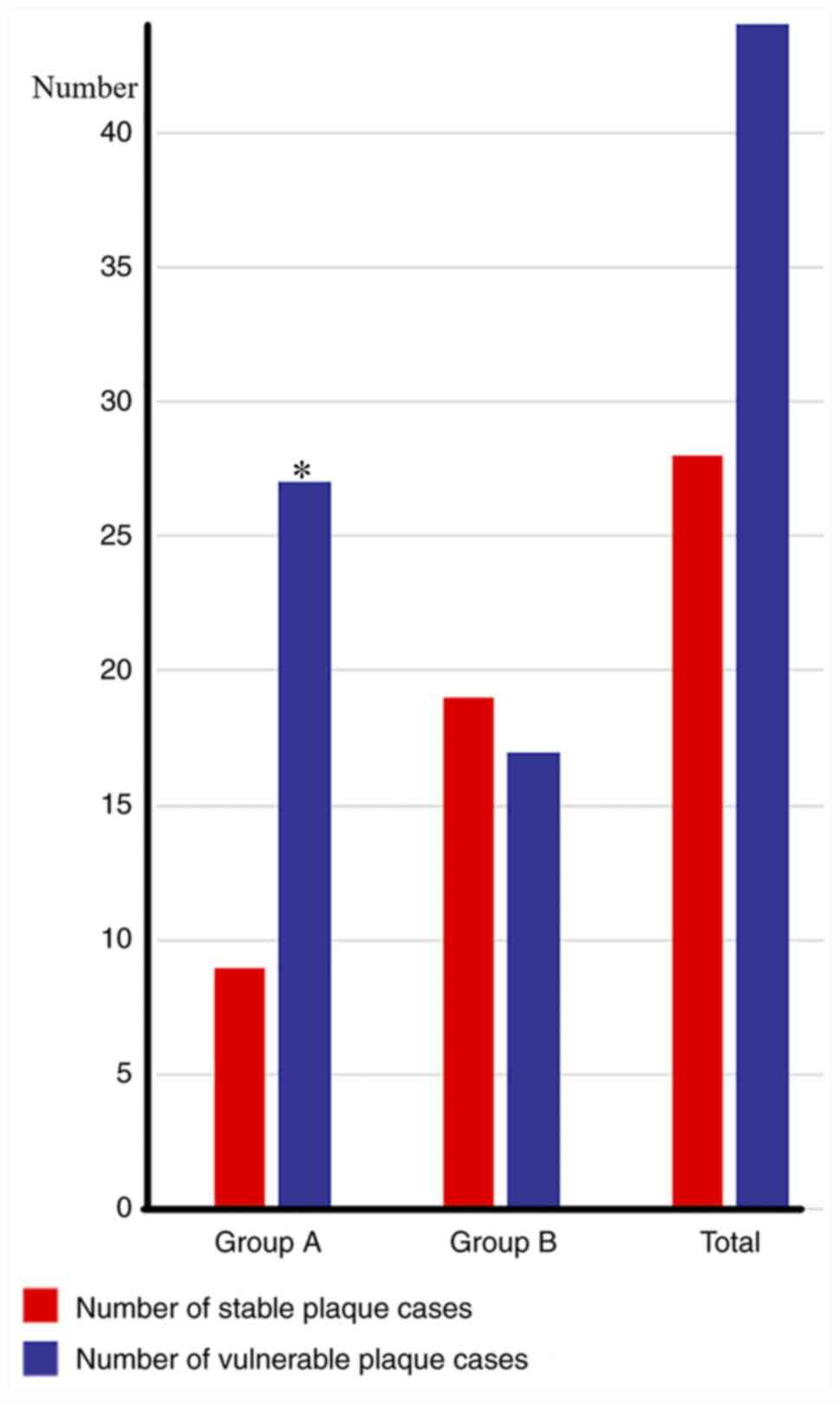
The number of cases with vulnerable plaque in groups
A and group B were statistically analyzed. A total of 27 cases with
vulnerable plaque were identified in group A (27/36; 75.0%), and
the number of cases with vulnerable plaque in group B was indicated
to be 17 cases (17/36; 47.2%); therefore, the proportion of
patients with vulnerable plaque in group A was greater compared
with that in group B. The number of unstable plaques in group A was
significantly higher compared with that in group B (P<0.05;
Fig. 1).
Comparison of the number of vulnerable
plaque in different stenosis groups
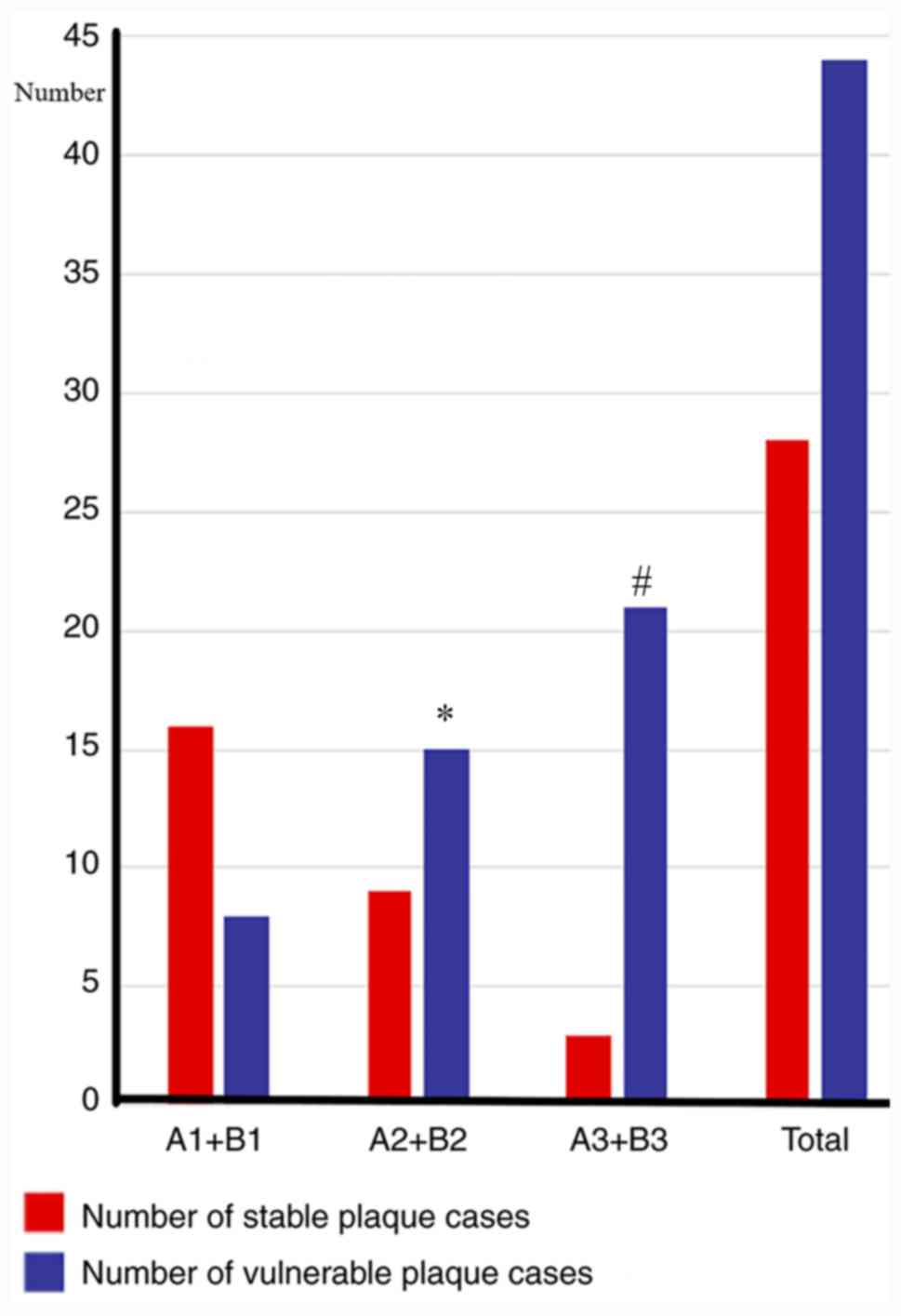
The number of cases containing vulnerable plaque in
the A1+B1, A2+B2 and A3+B3 groups were counted, and the number of
cases containing vulnerable plaque in the A1+B1 group was indicated
to be 8 cases (8/24; 33.3%). The number of cases containing
vulnerable plaque in the A2+B2 group was 15 (15/24; 62.5%), whereas
the number of cases with vulnerable plaque in the A3+B3 group was
21 (21/24; 87.5%). The proportions of vulnerable plaque in the
three groups with different degrees of stenosis were therefore
significantly different (P<0.05), and a comparison of the groups
revealed that, the more severe the stenosis, the higher the
proportion of vulnerable plaque identified (P<0.05; Fig. 2).
Carotid ultrasound and HR-MRI plaque
properties and image comparison
Comparison of plaque properties in
carotid ultrasound and HR-MRI in each group
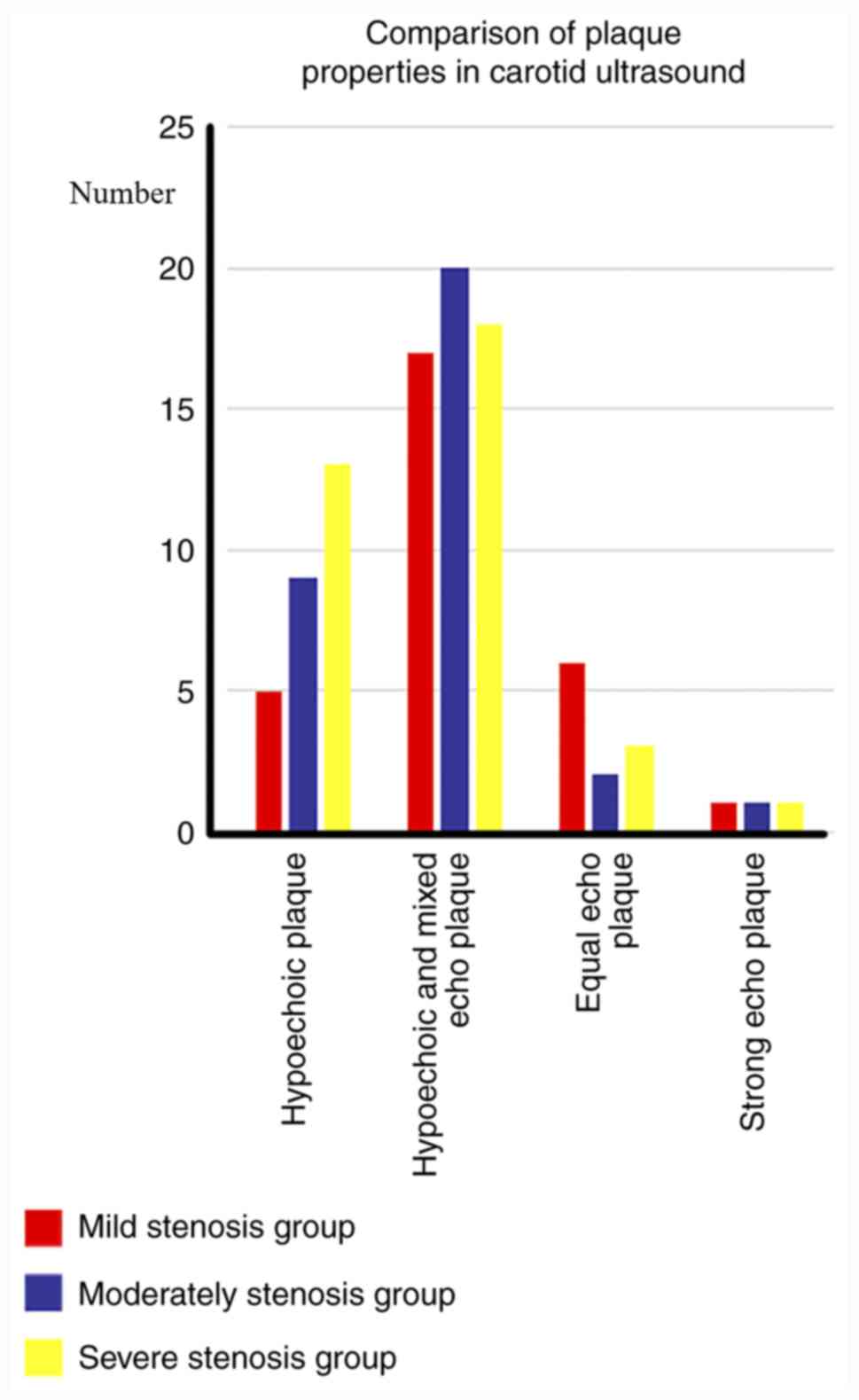
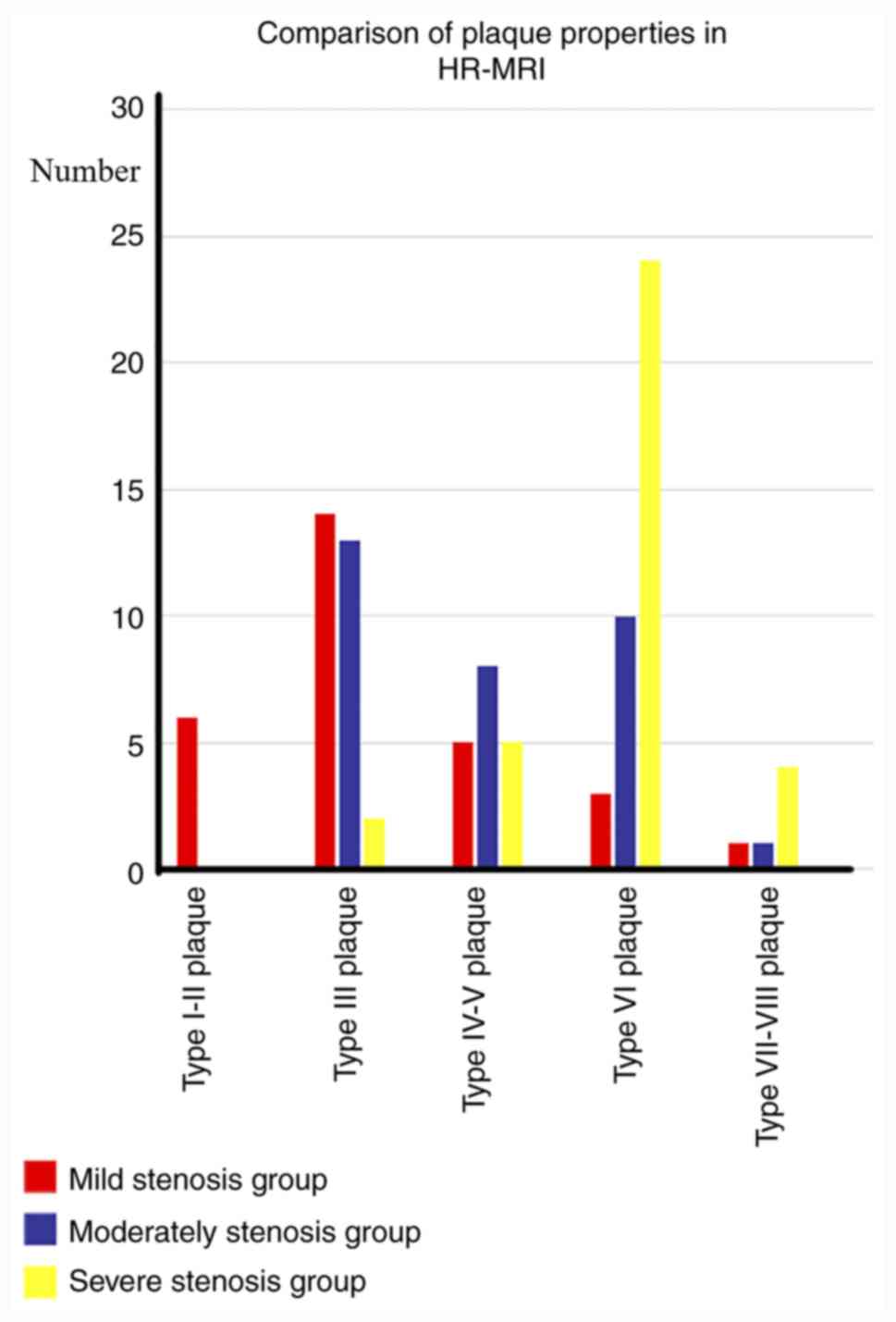
In the mild stenosis group (n=24), a total of 5
hypoechoic plaques, 17 hypoechoic and mixed echo plaques, 6 equal
echo plaques and 1 strong echo plaque were screened using carotid
ultrasound. A total of 6 type I-II plaques, 14 type III plaques, 5
IV-V plaques, 3 type VI plaques, and 1 type VII plaque were
detected by HR-MRI. In the moderate stenosis group (n=24), 9
hypoechoic plaques, 20 hypoechoic and mixed echo plaques, 2 equal
echo plaques and 1 strong echo plaque were screened by carotid
ultrasound. A total of 13 type III plaques, 8 IV-V plaques, 10 type
VI plaques, and 1 VII type plaque were detected using HR-MRI.
Finally, in the severe stenosis group (n=24), 13 hypoechoic
plaques, 18 hypoechoic and mixed echo plaques, 3 equal echo plaques
and 1 strong echo plaque were screened using carotid ultrasound.
And in the severe stenosis group, 2 type III plaques, 5 IV-V
plaques, 24 type VI plaques, 2 type VII plaques and 2 type VIII
plaques were detected using HR-MRI (Figs. 3 and 4).
Comparison of image characteristics of
each group in carotid ultrasound and HR-MRI
Carotid ultrasound and HR-MRI were consistent in the
assessment of plaque vulnerability for the three established
groups: mild, moderate and severe stenosis. Carotid artery
ultrasound revealed low echo and mixed echo plaques, multiple
hypoechoic and mixed echo plaques and hypoechoic and mixed echo
plaques on the wall of the carotid artery (Figs.
5-7). In comparing the results, carotid ultrasound was
demonstrated to have the ability to identify plaque, and the plaque
position, size and shape were all displayed. The characteristics of
plaque were assessed according to the plaque echo intensity. HR-MRI
was able to identify different components inside the plaque. After
subsequent processing with MRI-PlaqueView™ software, it was found
that the size of the plaque was 1.36x0.26 cm, where the lumen of
the internal carotid artery was narrow and the inner diameter of
the narrower section was 0.39 cm (Fig.
5). The diameter stenosis rate was calculated to be 47% in the
mild stenosis group (Fig. 5). By
contrast, the larger plaque was revealed to be located at the
bifurcation of the carotid artery, where the size was 1.43x0.43 cm
and the lumen of the internal carotid artery was narrow, with a
stenosis rate of 50-69% in the moderate stenosis group (Fig. 6). In the severe stenosis group, the
larger plaque on the left side is located at the bifurcation of the
carotid artery, where the size of the plaque is 2.08x0.63 cm
(Fig. 7).
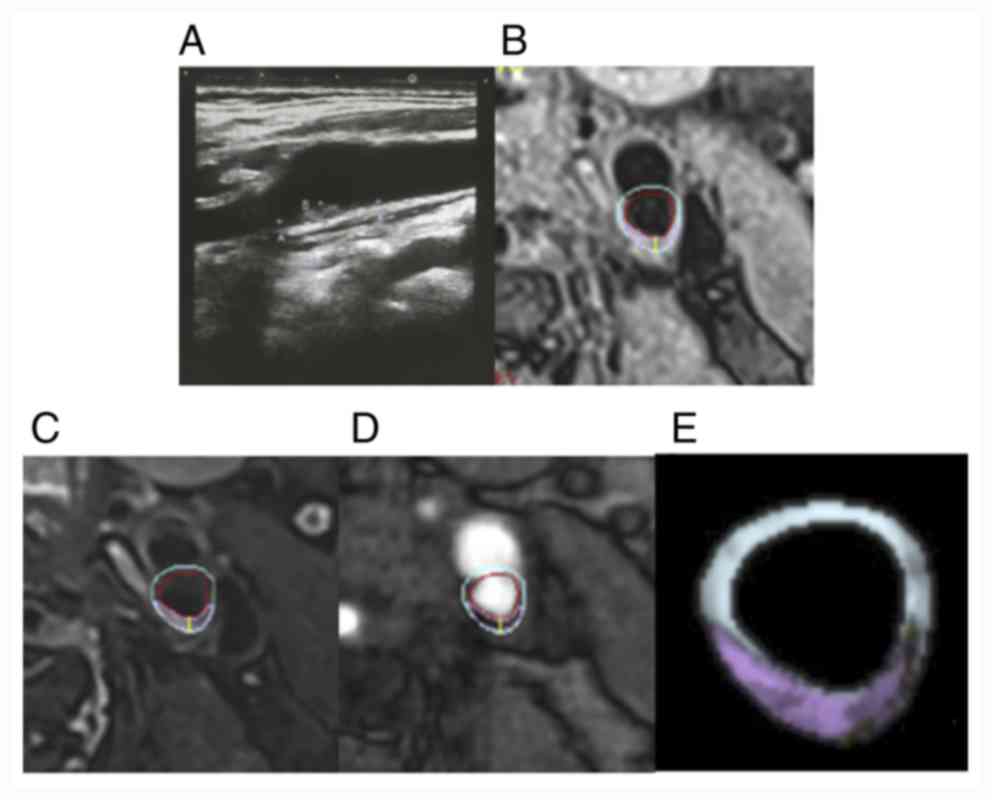 | Figure 5Comparison of carotid ultrasound and
cervical HR-MRI images in the mild stenosis group. (A)
Representative carotid artery ultrasound images, (B) T1, (C) T2,
(D) TOF and (E) vessel examinations. Green, fiber; white,
calcification; yellow, lipid-rich core; pink, loose substrate area;
red, lumen or hemorrhage; blue, tube wall. HR-MRI, High resolution
magnetic resonance imaging; TOF, time-of-flight. |
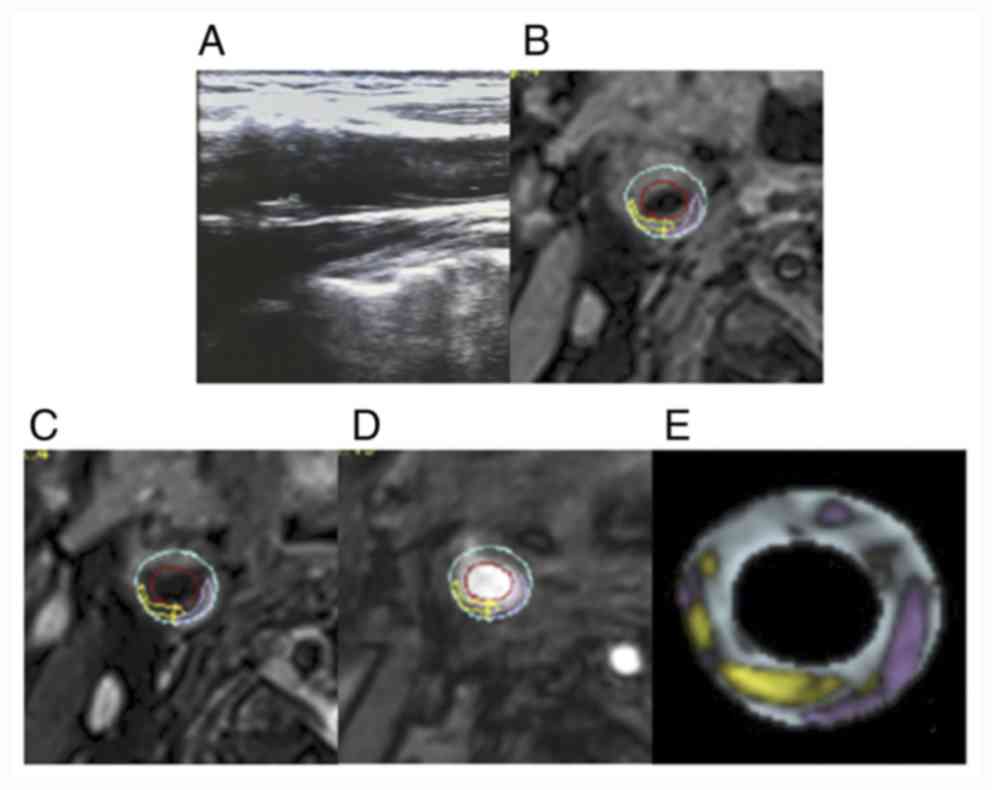 | Figure 6Comparison of carotid ultrasound and
cervical HR-MRI images in the moderate stenosis group. (A)
Representative carotid artery ultrasound images. (B) T1, (C) T2,
(D) TOF and (E) vessel examinations. Green, fiber; white,
calcification; yellow, lipid-rich core; pink, loose substrate area;
red, lumen or hemorrhage; blue, tube wall. HR-MRI, high-resolution
magnetic resonance imaging; TOF, time-of-flight. |
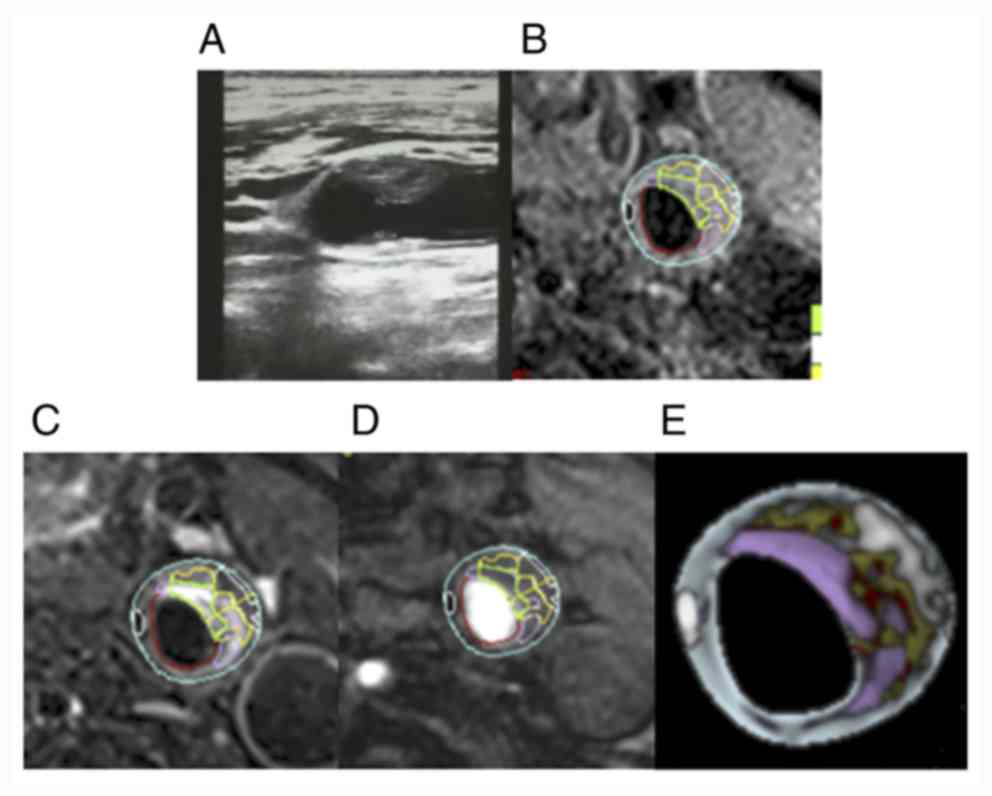 | Figure 7Comparison of carotid ultrasound and
cervical HR-MRI images in the severe stenosis group. (A)
Representative carotid artery ultrasound images. (B) T1, (C) T2,
(D) TOF and (E) vessel examinations. Green, fiber; white,
calcification; yellow, lipid-rich core; pink, loose substrate area;
red, lumen or hemorrhage; blue, tube wall. HR-MRI, high-resolution
magnetic resonance imaging; TOF, time-of-flight. |
The MRI-PlaqueView™ software made it possible to
quantify the different components inside the plaque through the
multi-dimensional color display. The MRI-PlaqueView™ is more
effective for the identification of vulnerable plaques, and there
are a number of clear advantages compared with ultrasonography
(Figs.
5-7).
Discussion
Atherosclerosis is an inflammatory disease involving
the blood vessels of the entire body (18). Epidemiological studies have revealed
that ~15% of all stroke cases occur secondary to carotid
atherosclerosis (19). Stenosis
caused by atherosclerosis results in 10-20% of all cases of
cerebral infarction worldwide, or transient ischemic attack
(20). Over the course of the last
20 years, numerous clinical trials (21-23)
have used carotid stenosis as a means of risk stratification.
Clinical studies have convincingly demonstrated that certain plaque
components, that is, intraplaque hemorrhage and lipid core, are
associated with plaque instability and plaque rupture, and also
with an increased risk of stroke (24). In the present clinical study, the
proportion of patients with vulnerable plaque in the symptomatic
group was indicated to be higher compared with that in the
asymptomatic group, a finding that was similar to a previous study
(25). The results of the
aforementioned study (25) revealed
that the main causes of ipsilateral cerebrovascular disease were as
follows: i) Ruptured vulnerable plaque is able to cause cerebral
embolism with blood flow; and ii) the surface of vulnerable plaque
is not smooth, thereby facilitating the process of adsorption of
platelets and coagulation factors. The plaque composition has also
been studied in a postmortem series, which demonstrated that
intracranial plaques associated with infarct had i) a higher area
of lipid core, ii) a higher frequency of neovasculature and
thrombus, iii) a trend toward a higher frequency of intraplaque
hemorrhage (25), meaning the more
unstable the intracranial plaque, the higher the probability of
cerebral infarction. This may lead to thrombosis on the plaque
surface due to the coagulation pathway (26). On the one hand, thrombosis on the
plaque surface can cause the continuous formation and expansion of
plaque, with the blockage of blood vessels occurring in serious
cases, resulting in insufficient blood perfusion; and on the other
hand, it can result in the rupture of thrombus and cerebral
embolism (27,28). At present, relatively few studies
have been published on this topic, and in assessing different
degrees of stenosis according to the carotid plaque model, the
nature of the plaque has been infrequently studied. Atherosclerosis
provides the underlying pathological basis of vascular diseases.
The plaque or stenosis resulting from atherosclerosis is the key
factor for the progression and recurrence of cerebrovascular
diseases (29). Only a narrow
percentage difference in the extent of stenosis caused by the
blockage of the lumen determines whether carotid endarterectomy and
carotid stent placement will be necessary for the patient (30). Dong et al (31) found that the proportion of vulnerable
plaque was also high in patients without any degree of vascular
stenosis. Therefore, taking only carotid stenosis as the clinical
intervention standard, the risk assessment will lead to a missed
diagnosis or misdiagnosis of some patients. In addition, a previous
study (32) have demonstrated that
compared with vascular stenosis, vulnerable plaque is also
associated with a great risk of vascular disease. With an increase
in the degree of stenosis, the prevalence of the lipid-rich
necrotic core (LRNC) also increases significantly. A previous study
reported that, for patients identified as having an 80-99% extent
of carotid artery stenosis, 91.8% of the patients also had LRNC
lesions (33). In patients with mild
stenosis of carotid artery, the proportion of intraplaque
hemorrhage (IPH) was indicated to be 7% (34). Therefore, attention must be paid not
only to the degree of stenosis, but also to the stability of plaque
associated with the different degrees of stenosis. In the present
clinical study, it was demonstrated that the proportion of patients
with vulnerable plaque was 33.3% in the mild stenosis group, 62.5%
in the moderate stenosis group and 87.5% in the severe stenosis
group. The proportions of vulnerable plaque in the three groups
with differing degrees of stenosis were therefore different, and
the more severe the stenosis, the higher the proportion of
vulnerable plaque that was identified. However, vulnerable plaques
were also identified in patients with only mild stenosis, which is
a finding consistent with that of previous studies (35-37).
This also revealed the danger that, if the degree of vascular
stenosis is taken into consideration as the sole determining factor
of clinical surgical intervention, the risk assessment is impaired
and will result in some patients not receiving effective
treatment.
At present, in all the common non-invasive clinical
imaging methods used to evaluate carotid artery plaque, carotid
artery ultrasound is widely used in clinical practice as it is
simple, convenient and non-invasive and is associated with low
costs. However, due to the limitations of space, time and
resolution, the accuracy of ultrasound in the evaluation of carotid
plaque properties is reduced (38).
Therefore, carotid ultrasound is limited in its ability to identify
vulnerable plaque. According to the degree of stenosis suggested by
vascular ultrasound, mild stenosis, moderate stenosis and severe
stenosis were classified. There is contrast in the analysis of
plaque properties between ultrasound and HR-MRI in different
degrees of stenosis when evaluating the vulnerability of plaque,
and the current study suggested that HR-MRI is more specific, as
the higher the stenosis rate, the heavier the vulnerable plaque
rate. Compared with ultrasound, HR-MRI is an ideal method for the
continuous examination of the artery wall, since it has a better
ability to distinguish tissue characteristics (39-41).
The comparative study of MRI and histopathology has revealed that
MRI can accurately differentiate between the plaque fiber cap, IPH,
LRNC and calcification (42), since
different components have different signal characteristics in
multi-contrast sequence images. A large number of comparative
studies on imaging and pathology have demonstrated that HR-MRI
produces results that are highly consistent with those of
histopathology in identifying and quantitatively analyzing carotid
atherosclerotic plaque (13,17,43), and
its levels of sensitivity and specificity have reached 90-100%
accuracy (12,13). The calcification components in
atherosclerosis exhibit a low signal on T1WI, PDWI and T2 images
(44). Saam et al (13) identified that HR-MRI has both high
sensitivity and specificity in identifying calcified components (86
and 76%, respectively). In terms of the imaging of plaque with or
without a fiber cap, on the 3D-TOF sequence images, the complete
thick fiber cap was identified to be a continuous low signal close
to the vascular lumen in the image, and the signal was uniform
(45). However, on the T1WI, PDWI
and T2 sequence images, the signal was high. On the 3D-TOF
sequence, the broken fiber cap was revealed as an interrupted low
signal, or no signal, close to the vascular lumen in the image, and
the lumen was found to be irregular on each sequence image
(45). A subsequent study revealed
that an important feature of vulnerable plaque is IPH, which is
crucial for the stable development of vulnerable plaque (46). Different periods of IPH have
different signal characteristics, as revealed by multi-contrast MRI
sequences (47). Fresh IPH, with a
bleeding time of <1 week, is identified by a high signal on T1WI
and TOF sequence images, whereas it appears as an isointense or low
signal on T2WI and precision diagnostic imaging (PDI) sequence
images (47). Recent IPH with a
bleeding time >1 and <6 weeks is revealed on T1WI, T2W and
TOF sequence images, the images and PDI sequence images often
exhibit a clear high signal and the old bleeding with bleeding time
>6 weeks is revealed as a low signal on T1WI sequence images,
T2WI sequence images and TOF sequence images (47). These results confirm that HR-MRI can
distinguish between the different stages of bleeding in advanced
atherosclerotic carotid atherosclerotic plaques with high
sensitivity and specificity (48).
The presence and size of the LRNC is considered to be an important
indicator of atherosclerotic plaque vulnerability (49-51).
In the present study, HR-MRI based on multi-contrast MRI
acquisition parameters combined with MRI-PlaqueView™ software could
correctly identify fiber cap, IPH, LRNC and calcification, and
distinguish these features from each other. Additionally, it was
possible to quantify each component in the plaque by utilizing the
differences in the different colors, based on the multi-dimensional
color display of the plaque components. By comparing the types and
image characteristics of plaque in carotid ultrasound, HR-MRI was
indicated to have the ability to identify vulnerable plaque, and it
is a more intuitive method with several clear advantages compared
with ultrasonography. HR-MRI can qualitatively and quantitatively
evaluate differences in the different shapes and components of
carotid stable and vulnerable plaque in vivo, which will
help patients with carotid disease and ischemic stroke to achieve
better results in terms of risk stratification and management
guidance (52,53).
However, there are several limitations associated
with the method that has been implemented in the present clinical
study. Firstly, after the examination, the patients did not undergo
a follow up examination at a later stage. Therefore, it was not
clear whether the prognosis is worse if the blood vessel stenosis
is more severe, or whether the prognosis is worse when more
vulnerable plaques are present. Also, there is the possibility that
some asymptomatic neck plaques can undergo a further stroke.
Secondly, the sample size of the present case study was small, and
there may be a certain bias associated with some of the
conclusions. Thirdly, the patients included in the current study
did not undergo endometrial exfoliation treatment, so plaque
pathology examination was not possible. Lack of a pathological gold
standard as a validation tool is a limitation to the current study.
However, HR-MRI has been demonstrated to be the most sensitive and
specific diagnostic tool for in vivo morphological
characterization of atheromatous carotid artery plaques, which is
strongly in agreement with histological findings (54). In future studies, it should be
possible to further analyze a comparison of the plaque HR-MRI
performance and pathology. Taken together, the results of the
present study have clearly shown, however, that HR-MRI may identify
plaque properties and quantitatively analyze them, and that this
method has unique advantages in terms of the hierarchical
management of ischemic stroke.
Acknowledgements
Not applicable.
Funding
Funding was received from the Jilin Province Science
and Technology Project (grant no. 20190303163SF).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YS interpreted and analyzed the data. LX wrote the
manuscript, analyzed the data and designed the study. YJ and MM
performed statistical analysis and data analysis. XW collected the
ultrasound and MRI data of the patients, and acquired and
interpreted data. YX reviewed the article and designed the study.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of China-Japan Union hospital of Jilin University
(approval no. 2020010812). Patients who participated in this
research had complete clinical data. Signed informed consents were
obtained from the patients and/or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
GBD 2016 Stroke Collaborators: Global,
regional, and national burden of stroke, 1990-2016: A systematic
analysis for the Global Burden of Disease Study 2016. Lancet Neurol
18: 439-458, 2019.
|
|
2
|
Habibi-Koolaee M, Shahmoradi L, Niakan
Kalhori SR, Ghannadan H and Younesi E: Prevalence of stroke risk
factors and their distribution based on stroke subtypes in Gorgan:
A retrospective hospital-based study-2015-2016. Neurol Res Int.
2018(2709654)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mead GE, Murray H, Farrell A, O'Neill PA
and McCollum CN: Pilot study of carotid surgery for acute stroke.
Br J Surg. 84:990–992. 1997.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Polak JF, Pencina Mj, O'Leary DH and
D'Agostino RB: Common carotid artery intima-media thickness
progression as a predictor of stroke in multi-ethnic study of
atherosclerosis. Stroke. 42:3017–3021. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li GW, Zheng Gy, Li JG and Sun XD:
Relationship between carotid atherosclerosis and cerebral
infarction. Chin Med Sci J. 25:32–37. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fisher M, Paganini-Hill A, Martin A,
Cosgrove M, Toole JF, Barnett HJ and Norris J: Carotid plaque
pathology: Thrombosis, ulceration, and stroke pathogenesis. Stroke.
36:253–257. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Seeger JM, Barratt E, Lawson GA and
Klingman N: The relationship between carotid plaque composition,
plaque morphology, and neurologic symptoms. J Surg Res. 58:330–336.
1995.PubMed/NCBI View Article : Google Scholar
|
|
8
|
van Lammeren GW, Reichmann BL, Moll FL,
Bots ML, de Kleijn DP, de Vries JP, Pasterkamp G and de Borst GJ:
Atherosclerotic plaque vulnerability as an explanation for the
increased risk of stroke in elderly undergoing carotid artery
stenting. Stroke. 42:2550–2555. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Howard DP, van Lammeren GW, Rothwell PM,
Redgrave JN, Moll FL, de Vries JP, de Kleijn DP, den Ruijter HM, de
Borst GJ and Pasterkamp G: Symptomatic carotid atherosclerotic
disease: Correlations between plaque composition and ipsilateral
stroke risk. Stroke. 46:182–189. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Brinjikji W, Huston J III, Rabinstein AA,
Kim GM, Lerman A and Lanzino G: Contemporary carotid imaging: From
degree of stenosis to plaque vulnerability. J Neurosurg. 124:27–42.
2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sadat U, Teng Z, Young VE, Graves MJ,
Gaunt ME and Gillard JH: High-resolution magnetic resonance
imaging-based biomechanical stress analysis of carotid atheroma: A
comparison of single transient ischaemic attack, recurrent
transient ischaemic attacks, non-disabling stroke and asymptomatic
patient groups. Eur J Vasc Endovasc Surg. 41:83–90. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kang X, Polissar NL, Han C, Lin E and Yuan
C: Analysis of the measurement precision of arterial lumen and wall
areas using high-resolution MRI. Magn Reson Med. 44:968–972.
2000.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Saam T, Ferguson MS, Yarnykh VL, Takaya N,
Xu D, Polissar NL, Hatsukami TS and Yuan C: Quantitative evaluation
of carotid plaque composition by in vivo MRI. Arterioscler Thromb
Vasc Biol. 25:234–239. 2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Peng B and Wu B: Guidelines for the
diagnosis and treatment of acute ischemic stroke in China 2018.
Chinese J Neurol. 9:666–682. 2018.
|
|
15
|
Grant EG, Benson CB, Moneta GL, Alexandrov
AV, Baker JD, Bluth EI, Carroll BA, Eliasziw M, Gocke J, Hertzberg
BS, et al: Carotid artery stenosis: Grayscale and Doppler
ultrasound diagnosis-society of radiologists in ultrasound
consensus conference. Ultrasound Q. 19:190–198. 2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xu D, Hippe DS, Underhill HR,
Oikawa-Wakayama M, Dong L, Yamada K, Yuan C and Hatsukami TS:
Prediction of high-risk plaque development and plaque progression
with the carotid atherosclerosis score. JACC Cardiovasc Imaging.
7:366–373. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cai JM, Hatsukami TS, Ferguson MS, Small
R, Polissar NL and Yuan C: Classification of human carotid
atherosclerotic lesions with in vivo multicontrast magnetic
resonance imaging. Circulation. 106:1368–1373. 2002.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Androulakis E, Norrington K, Bakogiannis
C, Lioudaki E, Siasos G and Tousoulis D: The impact of antiplatelet
treatment on endothelial function. Curr Pharm Des. 22:4512–4518.
2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chaturvedi S, Bruno A, Feasby T, Holloway
R, Benavente O, Cohen SN, Cote R, Hess D, Saver J, Spence JD, et
al: Carotid endarterectomy-an evidence-based review: Report of the
therapeutics and technology assessment subcommittee of the American
Academy of Neurology. Neurology. 65:794–801. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fairhead JF and Rothwell PM: The need for
urgency in identification and treatment of symptomatic carotid
stenosis is already established. Cerebrovasc Dis. 19:355–358.
2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Topakian R, King A, Kwon SU, Schaafsma A,
Shipley M and Markus HS: ACES Investigators: Ultrasonic plaque
echolucency and emboli signals predict stroke in asymptomatic
carotid stenosis. Neurology. 77:751–758. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Saba L, Biswas M, Suri HS, Viskovic K,
Laird JR, Cuadrado-Godia E, Nicolaides A, Khanna NN, Viswanathan V
and Suri JS: Ultrasound-based carotid stenosis measurement and risk
stratification in diabetic cohort: A deep learning paradigm.
Cardiovasc Diagn Ther. 9:439–461. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Basic J, Assadian A, Strassegger J,
Senekowitsch C, Wickenhauser G, Koulas S, Waldhör T and Duschek N:
Degree of contralateral carotid stenosis improves preoperative risk
stratification of patients with asymptomatic ipsilateral carotid
stenosis. J Vasc Surg. 63:82–88.e2. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
van den Bouwhuijsen QJ, Bos D, Ikram MA,
Hofman A, Krestin GP, Franco OH, van der Lugt A and Vernooij MW:
Coexistence of calcification, intraplaque hemorrhage and lipid core
within the asymptomatic atherosclerotic carotid plaque: The
Rotterdam Study. Cerebrovasc Dis. 39:319–324. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen XY, Wong KS, Lam WW, Zhao HL and Ng
HK: Middle cerebral artery atherosclerosis: Histological comparison
between plaques associated with and not associated with infarct in
a postmortem study. Cerebrovasc Dis. 25:74–80. 2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ananyeva NM, Kouiavskaia DV, Shima M and
Saenko EL: Intrinsic pathway of blood coagulation contributes to
thrombogenicity of atherosclerotic plaque. Blood. 99:4475–4485.
2002.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wong KS, Gao S, Chan YL, Hansberg T, Lam
WW, Droste DW, Kay R and Ringelstein EB: Mechanisms of acute
cerebral infarctions in patients with middle cerebral artery
stenosis: A diffusion-weighted imaging and microemboli monitoring
study. Ann Neurol. 52:74–81. 2002.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bogousslavsky J, Barnett HJ, Fox AJ,
Hachinski VC and Taylor W: Atherosclerotic disease of the middle
cerebral artery. Stroke. 17:1112–1120. 1986.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lu M, Peng P, Cui Y, Qiao H, Li D, Cai J
and Zhao X: Association of progression of carotid artery wall
volume and recurrent transient ischemic attack or stroke: A
magnetic resonance imaging study. Stroke. 49:614–620.
2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kerwin WS: Carotid artery disease and
stroke: Assessing risk with vessel wall MRI. ISRN Cardiol.
2012(180710)2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Dong L, Underhill HR, Yu W, Ota H,
Hatsukami TS, Gao TL, Zhang Z, Oikawa M, Zhao X and Yuan C:
Geometric and compositional appearance of atheroma in an
angiographically normal carotid artery in patients with
atherosclerosis. AJNR Am J Neuroradiol. 31:311–316. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yoshida K, Yang T, Yamamoto Y, Kurosaki Y,
Funaki T, Kikuchi T, Ishii A, Kataoka H and Miyamoto S: Expansive
carotid artery remodeling: Possible marker of vulnerable plaque. J
Neurosurg. 1–6. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Saam T, Underhill HR, Chu B, Takaya N, Cai
J, Polissar NL, Yuan C and Hatsukami TS: Prevalence of American
Heart Association type VI carotid atherosclerotic lesions
identified by magnetic resonance imaging for different levels of
stenosis as measured by duplex ultrasound. J Am Coll Cardiol.
51:1014–1021. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cheung HM, Moody AR, Singh N, Bitar R,
Zhan J and Leung G: Late stage complicated atheroma in low-grade
stenotic carotid disease: MR imaging depiction-prevalence and risk
factors. Radiology. 260:841–847. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Xu L, Wang R, Liu H, Wang J, Liang W, Mang
J and Xu Z: Comparison of the diagnostic performances of
ultrasound, high-resolution magnetic resonance imaging, and
positron emission tomography/computed tomography in a rabbit
carotid vulnerable plaque atherosclerosis model. J Ultrasound Med:
May 12, 2020 (Epub ahead of print).
|
|
36
|
Takai H, Uemura J, Yagita Y, Ogawa Y,
Kinoshita K, Hirai S, Ishihara M, Hara K, Toi H, Matsubara S,
Nishimura H and Uno M: Plaque characteristics of patients with
symptomatic mild carotid artery stenosis. J Stroke Cerebrovasc Dis.
27:1930–1936. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kashiwazaki D, Shiraishi K, Yamamoto S,
Kamo T, Uchino H, Saito H, Akioka N, Kuwayama N, Noguchi K and
Kuroda S: Efficacy of carotid endarterectomy for mild (<50%)
symptomatic carotid stenosis with unstable plaque. World Neurosurg.
121:e60–e69. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Anzidei M, Napoli A, Zaccagna F, Di Paolo
P, Saba L, Cavallo Marincola B, Zini C, Cartocci G, Di Mare L,
Catalano C and Passariello R: Diagnostic accuracy of colour Doppler
ultrasonography, CT angiography and blood-pool-enhanced MR
angiography in assessing carotid stenosis: A comparative study with
DSA in 170 patients. Radiol Med. 117:54–71. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Toussaint JF, LaMuraglia GM, Southern JF,
Fuster V and Kantor HL: Magnetic resonance images lipid, fibrous,
calcified, hemorrhagic, and thrombotic components of human
atherosclerosis in vivo. Circulation. 94:932–938. 1996.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yuan C, Hatsukami TS, Beach KW, Hayes CE,
Nelson JA, Ferguson MS, Clowes AW and Strandness E Jr: In vivo MR
evaluation of atherosclerosis in human carotid artery with use of
phased-array coils. J Vasc Interv Radiol. 7:46–48. 1996.
|
|
41
|
Toussaint JF, Southern JF, Fuster V and
Kantor HL: T2-weighted contrast for NMR characterization of human
atherosclerosis. Arterioscler Thromb Vasc Biol. 15:1533–1542.
1995.PubMed/NCBI
|
|
42
|
den Hartog AG, Bovens SM, Koning W,
Hendrikse J, Luijten PR, Moll FL, Pasterkamp G and de Borst GJ:
Current status of clinical magnetic resonance imaging for plaque
characterisation in patients with carotid artery stenosis. Eur J
Vasc Endovasc Surg. 45:7–21. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Hatsukami TS, Ross R, Polissar NL and Yuan
C: Visualization of fibrous cap thickness and rupture in human
atherosclerotic carotid plaque in vivo with high-resolution
magnetic resonance imaging. Circulation. 102:959–964.
2000.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Cappendijk VC, Cleutjens KB, Kessels AG,
Heeneman S, Schurink GW, Welten RJ, Mess WH, Daemen MJ, van
Engelshoven JM and Kooi ME: Assessment of human atherosclerotic
carotid plaque components with multisequence MR imaging: Initial
experience. Radiology. 234:487–492. 2005.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Mitsumori LM, Hatsukami TS, Ferguson MS,
Kerwin WS, Cai J and Yuan C: In vivo accuracy of multisequence MR
imaging for identifying unstable fibrous caps in advanced human
carotid plaques. J Magn Reson Imaging. 17:410–420. 2003.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kolodgie FD, Gold HK, Burke AP, Fowler DR,
Kruth HS, Weber DK, Farb A, Guerrero LJ, Hayase M, Kutys R, et al:
Intraplaque hemorrhage and progression of coronary atheroma. N Engl
J Med. 349:2316–2325. 2003.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Yang L, Li D and Wei Y: The clinical
application and technology progress of high resolution MRI in
carotid intraplaque hemorrhage. Chinese J Magn Reson Imaging.
6:711–715. 2015.
|
|
48
|
Chu B, Kampschulte A, Ferguson MS, Kerwin
WS, Yarnykh VL, O'Brien KD, Polissar NL, Hatsukami TS and Yuan C:
Hemorrhage in the atherosclerotic carotid plaque: A high-resolution
MRI study. Stroke. 35:1079–1084. 2004.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Gutstein DE and Fuster V: Pathophysiology
and clinical significance of atherosclerotic plaque rupture.
Cardiovasc Res. 41:323–333. 1999.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Naghavi M, Libby P, Falk E, Casscells SW,
Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P,
Pasterkamp G, et al: From vulnerable plaque to vulnerable patient:
A call for new definitions and risk assessment strategies: Part I.
Circulation. 108:1664–1672. 2003.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Falk E: Stable versus unstable
atherosclerosis: Clinical aspects. Am Heart J. 138:S421–S425.
1999.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Li ZY, Tang T, U-King-Im J, Graves M,
Sutcliffe M and Gillard JH: Assessment of carotid plaque
vulnerability using structural and geometrical determinants. Circ
J. 72:1092–1099. 2008.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Xia Z, Yang H, Yuan X, Wang J, Zhang S,
Zhang L, Qu Y, Chen J, Jiao L, Wang LX and Du Y: High-resolution
magnetic resonance imaging of carotid atherosclerotic plaques-a
correlation study with histopathology. Vasa. 46:283–290.
2017.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Chiocchi M, Chiaravalloti A, Morosetti D,
Loreni G, Gandini R, Mancino S, Fabiano S and Simonetti G: Virtual
histology-intravascular ultrasound as a diagnostic alternative for
morphological characterization of carotid plaque: Comparison with
histology and high-resolution magnetic resonance findings. J
Cardiovasc Med (Hagerstown). 20:335–342. 2019.PubMed/NCBI View Article : Google Scholar
|