Introduction
Acute lung injury (ALI) is a type of a pulmonary
inflammatory response syndrome and is mainly characterized by
increased alveolar capillary permeability and alveolar epithelial
damage. In the process of ALI, acute, persistent alveolar-capillary
membrane damage results in large amounts of protein extravasations
and induces the formation of a transparent membrane in the alveolar
space (1). In addition, with an
imbalance in ventilated blood flow, lung compliance typically
declines, and resultant hypoxemia and dyspnea often occur in
patients with ALI. ALI is a commonly encountered critical type of
emergency in clinical practice, with a high mortality rate ranging
from 50 to 70% (2,3). While the pathogenesis of ALI is not
fully elucidated, previous research indicates that uncontrolled
systemic inflammatory responses (SIRS) caused by varied factors can
induce the onset and progression of ALI, especially as the lung is
the foremost organ most susceptible to such types of inflammatory
damage (4,5). Furthermore, it is certain that the
imbalance between pro-inflammatory responses and anti-inflammatory
responses is one of the main causes of the onset and progression of
ALI (6,7).
Polygonatum sibiricum polysaccharides (PSPs)
mainly composed of four chemically distinct monosaccharides, were
originally extracted from the dried rhizome of Polygonatum,
a genus of flowering plants. Recent modern pharmacological research
suggests that PSPs have many biological activities including
antioxidant, anti-aging, anti-fatigue, immune enhancement,
antibacterial, and anti-inflammatory activities (8,9). In
addition, previous research has indicated that PSPs can exert
antitumor effects by inhibiting the transmission of the Toll-like
receptor 4/mitogen-activated protein kinase/ nuclear factor-κB
(TLR4-MAPK/NF-κB) signaling pathway (10). The TLR4 signaling pathway has also
been found to be closely related to the dynamics underlying ALI
(11,12). TLR4 is expressed in immune cells such
as macrophages and neutrophils, and in non-immune cells such as
alveolar endothelial cells and epithelial cells. Infiltration of
alveolar inflammatory cells is the key to the inflammatory
response, and endothelial cell damage is the basis of structural
degradation of the alveolar basement membrane in ALI (13,14).
Moreover, previous research has suggested that TLR4-mediated
inflammation plays a key role in lipopolysaccharide (LPS)-induced
ALI (15), and plays a key role in
kidney injury (16).
TLR is a transmembrane transduction receptor for LPS
signaling from extracellular to intracellular space. Furthermore,
TLR4 can directly bind to LPS, and binds to
lipopolysaccharide-binding protein (LBP)-LPS-cluster of
differentiation 14 (CD14), thereby activating the NF-κB signaling
pathway through its impact upon dynamics underlying medullary
differentiation protein 88 (Myd88). This leads to the synthesis and
release of various inflammatory mediators, and finally initiates
and amplifies the inflammatory response (17,18).
However, whether or not PSPs are able to prevent LPS-induced ALI by
inhibiting inflammation via the TLR4/NF-κB pathway is unclear.
Therefore, in the present study, we sought to examine and answer
the above question.
Materials and methods
Animals and treatment
We used Wistar rats (5-6 weeks of age; weight,
130-180 g) to establish animal-based models for the present
research. In this study, total of 50 rats (male: female=1:1) were
all purchased from Shanghai SLAC Laboratory Animal Co., Ltd. Rats
were fed daily, housed at 22±2˚C, and we used a 12 h light/dark
cycle at our experimental center. Rats were randomly assigned to
five treatment groups, including the: Control group, LPS group,
L-PSP group, M-PSP group, and H-PSP group. The LPS group was
composed of the ALI rats model without PSP treatment. The ALI rat
model was composed of rats that were first intraperitoneally
injected with normal saline. After 30 min, we used 1 mg/kg
lipopolysaccharide (LPS, purity >95%, cat. no. L2880,
Sigma-Aldrich; Merck KGaA) which was intraperitoneally injected.
After 16 h, lipopolysaccharide (3 mg/kg) was instilled into the
trachea, and 1 h later, the rats were intraperitoneally injected
with normal saline. In total, 2 h prior to the establishment of the
ALI model, rats in the L-PSP group, the M-PSP group, and the H-PSP
group were injected intraperitoneally with PSPs (20 mg/kg for
L-PSP, 40 mg/kg for M-PSP, 80 mg/kg for H-PSP). PSP was purchased
from Ci Yuan Biotechnology Co., Ltd., and the purity was >98%.
Saline was used to dissolve the PSPs. In addition, all rats in this
study were anesthetized by intraperitoneal injection of 50 mg/kg
pentobarbital sodium before sacrifice.
All animal protocols were reviewed and approved by
The Animal Care and Use Committee of The First Affiliated Hospital
of Nanchang University (Nanchang, Jiangxi, China) and conformed to
standard guidelines from The National Institution of Health
(19).
Hematoxylin and eosin (H&E)
staining
Frozen slices of rat lung were slightly dried,
stained with hematoxylin for 3 min at room temperature, stained
with eosin for 10 sec at room temperature, dehydrated using an
ascending ethanol gradient, made transparent and then sealed with
neutral gum. Histopathological changes were observed under light
microscopy (magnification, x100; CKX41; Olympus Corporation) and
imaged for assessment.
Lung wet/dry (W/D) weight,
myeloperoxidase (MOP) and malondialdehyde levels
Rats were anesthetized by intraperitoneal injection
of 50 mg/kg pentobarbital sodium before euthanasia, before the rats
were euthanized by CO2 as previously described (20). Rats were sacrificed to obtain lung
tissues, which were drained and measured for wet weight.
Afterwards, lung tissues were incubated at 80˚C for 4 h and then
tissues were weighed to obtain measures of dry weight. Measures of
activity of MOP and the levels of malondialdehyde (MDA) were
measured as previously described (21,22).
Inflammatory cytokines and cell
counting assay
Rats were sacrificed and bronchoalveolar lavage
fluid (BALF) was collected. Neutrophils and total cell numbers in
BALF samples were counted as previously described (21,22). Rat
tumor necrosis factor (TNF)α (cat. no. ab100785, Abcam),
interleukin (IL)-1β (cat. no. ab100768, Abcam), IL-6 (cat. no.
ab100772, Abcam) and IL-8 (cat. no. SBJ-R0033, Nanjing Biological)
ELISA kits were used to detect the levels of inflammatory cytokines
in BALF samples.
Reverse transcription-quantitative
PCR
TNF-α, IL-1β, L-6, and IL-8 levels in samples from
rats were measured as previously described (23) by using fluorescence based real-time
quantitative PCR (RT-qPCR) and gene-specific TaqMan primer/probe
sets on a ABI Prism 7000 Sequence Detection System (Applied
Biosystems). GAPDH mRNA transcription was used for internal loading
control. PCR primer sets were composed as follows: TNF-α-F,
5'-CTGAACTTCGGGGTGATCGG-3' and TNF-α-R,
5'-GGCTTGTCACTCGAATTTTGAGA-3'; IL-1β-F,
5'-GAAATGCCACCTTTTGACAGTG-3' and IL-1β-R,
5'-TGGATGCTCTCATCAGGACAG-3'; IL-6-F, 5'-TCTATA CCACTTCACAAGTCGGA-3'
and IL-6-R, 5'-GAATTG CCATTGCACAACTCTTT-3'; IL-8-F, 5'-TCGAGACCA
TTTACTGCAACAG-3' and IL-8-R, 5'-CATTGCCGGTGG AAATTCCTT-3'.
Western blot analysis
Protein levels were analyzed by using western
blotting as previously described (23) and GAPDH protein transcription factor
was used for an internal loading control. All antibodies were
purchased from Abcam with the corresponding information as follows:
anti-TLR4 (cat. no. ab22048, dilution 1:2,000), anti-MyD88 (cat.
no. ab107585, dilution 1:3,000), anti-IκB-α (cat. no. ab32518,
dilution 1:500), anti-IκB-α (phospho) (cat. no. ab92700, dilution
1:500) anti-NF-kB p65 (acetyl K310) (cat. no. ab19870, dilution
1:300), or anti-p65 phospho S636 (cat. no. ab86299, dilution
1:5,000), or anti-GAPDH (cat. no. ab9484, dilution 1:3,000).
Flow cytometry detection of
apoptosis
Normal unafflicted human lung epithelial cells
(BEAS-2B) (CRL-9609; ATCC) were cultured using DMEM (cat. no.
12491-15, Thermo Fisher Scientific, Inc.) at a temperature of 37˚C
and in a constant atmosphere of 5% CO2. In the LPS
treatment group, only 24 h of LPS stimulation was permitted, but
for the PSP (1.5 g/l) treatment group, PSP was administered 1 h
before LPS stimulation in the PSP+LPS group. BEAS-2B cells were
collected 24 h post-LPS stimulation, and then the Annexin V FITC/PI
kit (Invitrogen; Thermo Fisher Scientific, Inc.) was used for flow
cytometry to detect apoptosis. Beckman CytoFLEX Flow cytometry
(Beckman Coulter, Inc.) was used to analyze levels of cell
apoptosis.
Statistical analysis
We used SPSS v20.0 software (IBM Corp.) to analyze
data. The Student's t-tests were used to compare differences
between two treatment groups. One-way ANOVA was used to compare
differences between multiple groups and the corresponding Tukey
post-hoc test was used. P<0.05 was considered to indicate a
statistically significant difference at which the null hypothesis
of no differences among treatment groups was rejected.
Results
PSPs attenuate LPS-induced lung injury
in ALI rats
We used H&E staining to detect pathological
changes in lungs. As exemplified in Fig.
1, the alveolar structure of the control group was intact, the
alveolar cavity was clearly visible, and there was no obvious
exudation (Fig. 1A). However, we
observed alveolar septal thickening, massive inflammatory cell
infiltration in the interstitial and alveolar spaces,
telangiectasia, hemorrhaging, alveolar insufficiency, or observed
alveolar fusion in some areas (Fig.
1B). Additionally, the results indicated that PSPs
significantly attenuated LPS-induced histopathological changes and
that this effect occurred in a dose-dependent manner (Fig. 1C-E). In addition, data related to
pathological changes in the lung tissues demonstrated that PSPs
reduced LPS-induced increases in lung tissue wet/dry weight ratio
(W/D) (Fig. 1F), reduced LPS-induced
increases in MOP activity (Fig. 1G)
and elevation in levels of MDA (Fig.
1H). Similarly, these functions of PSPs were found to have
occurred in a dose-dependent manner.
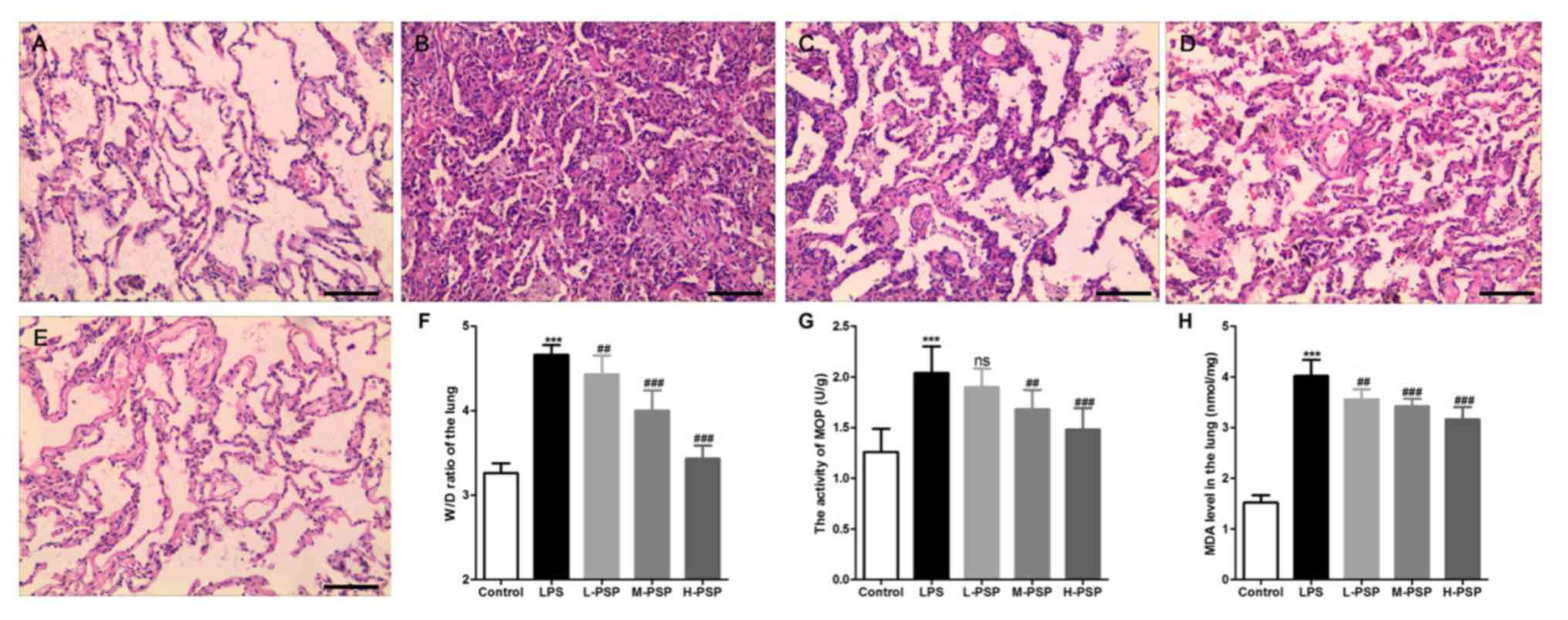 | Figure 1Effect of PSPs on LPS-induced
pathological changes in the lungs in ALI rats. (A-E) Hematoxylin
and eosin (H&E) staining was used to observe pathological
changes in the lungs of (A) control group, (B) LPS group, (C) L-PSP
group, (D) M-PSP group and (E) H-PSP group of rats. (F) The value
of the W/D in lung tissues of the different groups. (G) MOP
activity in lung tissues of the different groups. (H) The MDA level
in lung tissues of the different groups. A total of 10 rats in each
group, each indicator for each rat was tested at least 3 times
independently; ***P<0.001 vs. the control group; NS,
not significant at P>0.05; ##P<0.01 and
###P<0.001 vs. the LPS group. Scale bar, 100 µm.
PSPs, Polygonatum sibiricum polysaccharides; ALI, acute lung
injury; LPS, lipopolysaccharide; MOP, myeloperoxidase; MDA,
malondialdehyde; W/D, wet/dry weight ratio. Groups: L-PSP, 20
mg/kg; M-PSP, 40 mg/kg; H-PSP, 80 mg/kg PSPs. |
PSPs attenuate LPS-induced pulmonary
inflammation in ALI rats
Excessive or uncontrolled inflammation is an
underlying cause of ALI, and neutrophils play an important role in
associated dynamics. Thus, we used BALF tests to assess measures of
lung inflammation in the different treatment groups of rats, and
found that PSPs were able to attenuate LPS-induced increases in
neutrophil ratios (Fig. 2A) and
attenuated levels of inflammatory factors in BALF (Fig. 2B-E). Moreover, PSPs were able to
reduce LPS-induced increases in mRNA expression of inflammatory
factors in lung tissues of the ALI-afflicted rats (Fig. 2F).
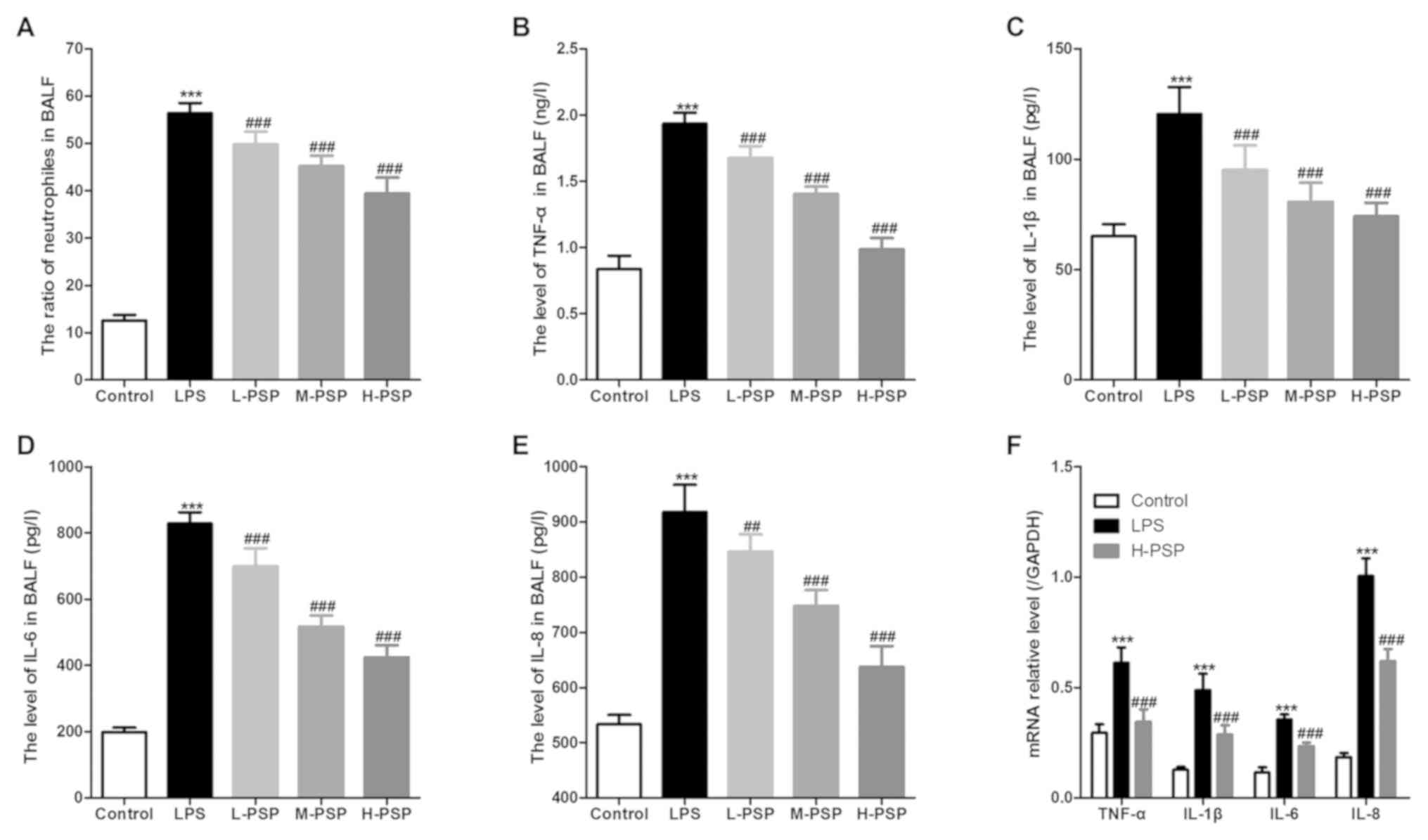 | Figure 2Effect of PSPs on neutrophil ratio
and inflammatory factor content in BALF of the ALI rats. (A)
Proportion of neutrophils in BALF of the different groups of rats.
(B) TNF-α, (C) IL-1β, (D) IL-6 and (E) IL-8 levels in BALF of the
different groups of rats. (F) mRNA expression of TNF-α, IL-1β, IL-6
and IL-8 in lung tissues of the different groups of rats. A total
of 10 rats in each group, each indicator for each rat was tested at
least 3 times independently; ***P<0.001 vs. the
control group; ##P<0.01 and ###P<0.001
vs. the LPS group. PSPs, Polygonatum sibiricum
polysaccharides; LPS, lipopolysaccharide; BALF, bronchoalveolar
lavage fluid; ALI, acute lung injury; TNF-α, tumor necrosis
factor-α; IL, interleukin. Groups: L-PSP, 20 mg/kg; M-PSP, 40
mg/kg; H-PSP, 80 mg/kg PSPs. |
PSPs inhibit activation of the
TLR4/NF-κB pathway in lung tissue of ALI rats
The TLR4/NF-κB pathway is known to be closely
related to the dynamics of LPS-induced inflammation. Thus, we
measured the expression levels of key proteins in the TLR4/NF-κB
pathway by using western blotting. As shown in Fig. 3, LPS was able to induce elevated
levels of TLR4, Myd88, p-IKB-α/IKB-α, and p-p65/p65 proteins in
lung tissue of ALI afflicted rats. In contrast, the expression
levels of TLR4, Myd88, p-IKB-α/IKB-α, and p-p65/p65 proteins in
lung tissue of the H-PSP rat treatment group were significantly
decreased when compared with the levels in the LPS treatment group
(Fig. 3).
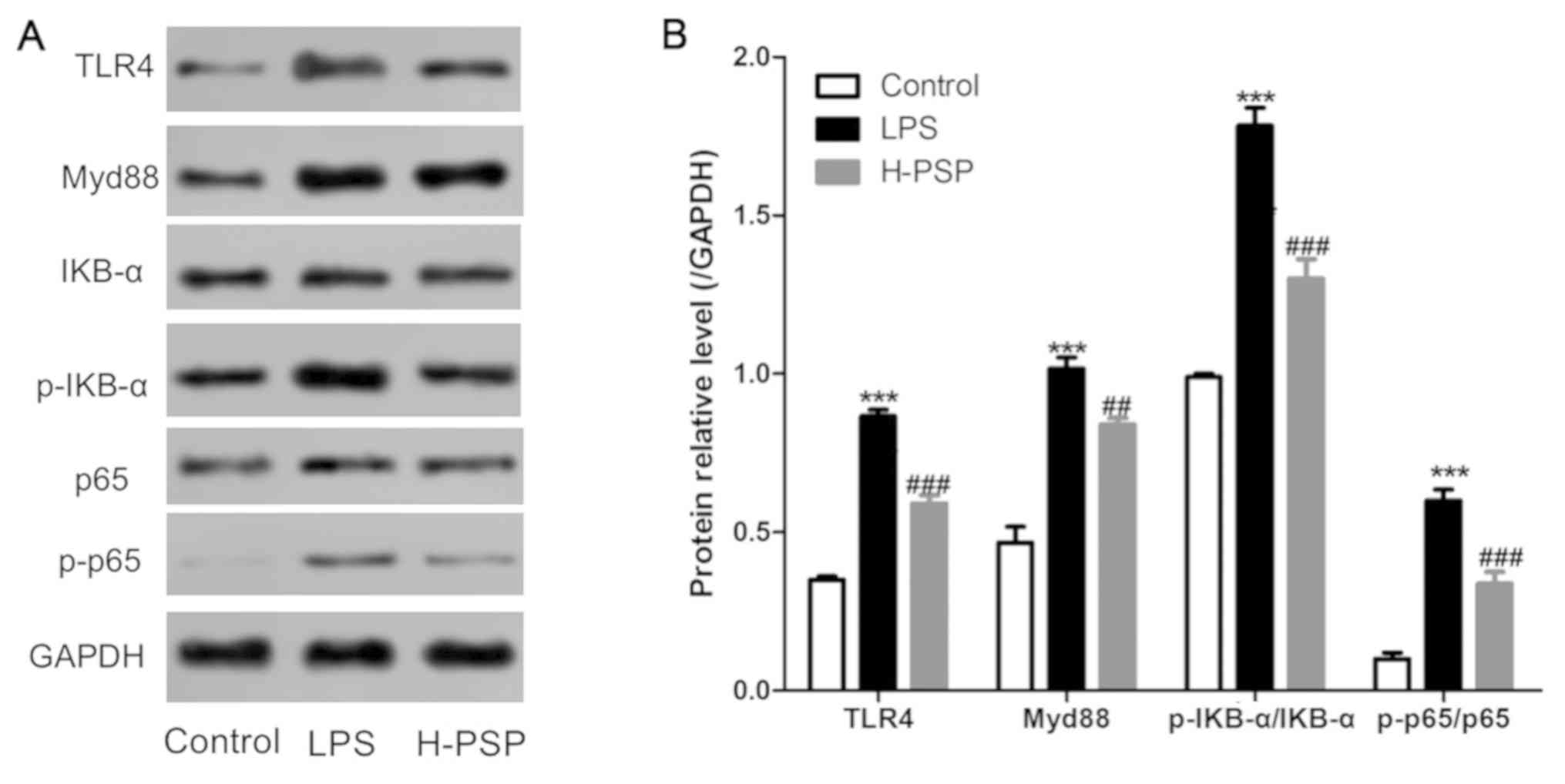 | Figure 3Effect of PSPs on the TLR4/NF-κB
pathway in lung tissue of ALI rats. (A) Western blot analysis was
used to detect the expression of proteins in lung tissues of the
different group of rats: control group, LPS group and H-PSP group.
(B) Statistical analysis of the western blotting. A total of 10
rats in each group, each indicator for each rat was tested at least
3 times independently; ***P<0.001 vs. the control
group; ##P<0.01 and ###P<0.001 vs. the
LPS group. PSPs, Polygonatum sibiricum polysaccharides;
TLR4, Toll-like receptor 4; NF-κB, nuclear factor-κB; ALI, acute
lung injury; LPS, lipopolysaccharide. Groups: L-PSP, 20 mg/kg;
M-PSP, 40 mg/kg; H-PSP, 80 mg/kg PSPs. |
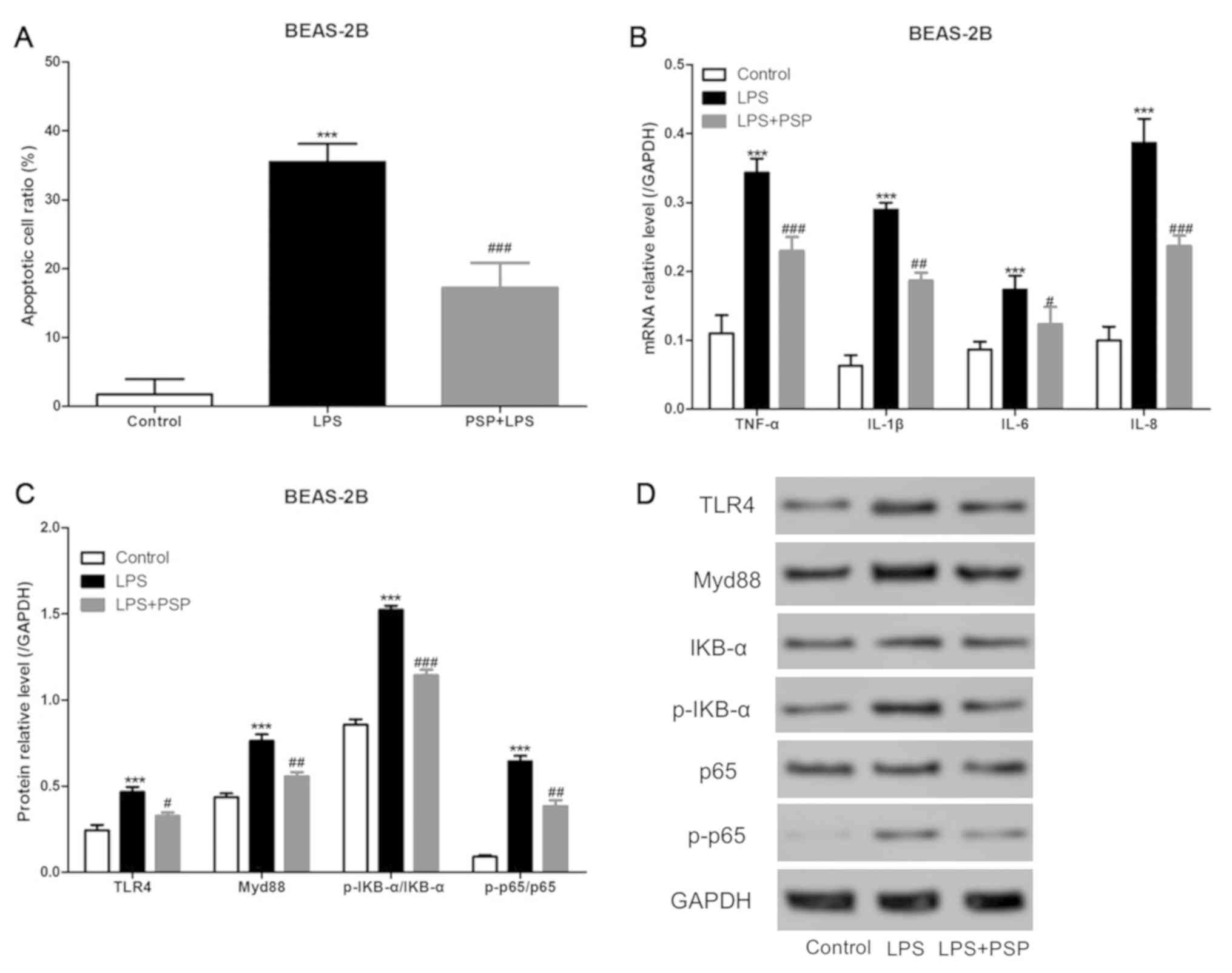
PSPs reduce LPS-induced apoptosis in
human normal lung epithelial cells in vitro
We cultured normal unafflicted human lung epithelial
cells (BEAS-2B cells) in vitro, added LPS to the culture
medium, and detected apoptosis by using flow cytometry. As shown in
Fig. 4A, LPS was able to
significantly increase the proportion of cells that underwent
apoptosis in the BEAS-2B cell treatment group, and PSPs
significantly reduced the proportion of apoptosis induced by LPS in
the BEAS-2B cells. The mRNA expression levels of inflammatory
factors were assessed using RT-qPCR, and the results indicated that
PSPs could also reduce LPS-induced increases in mRNA expression
levels of inflammatory factors in BEAS-2B cells in vitro
(Fig. 4B). Furthermore, we measured
the levels of expression of key proteins in the TLR4/NF-κB pathway
by using western blotting and found that PSPs were also able to
reduce LPS-induced increases in the expression levels of TLR4,
Myd88, p-IKB-α/IKB-α, and p-p65/p65 proteins in BEAS-2B cells
cultured in vitro (Fig. 4C
and D).
Discussion
The main causes of acute lung injury (ALI) include
infection, trauma, shock, and inhalation of toxic gases among
others, and approximately 30 to 50% of cases are caused by serious
infections. Endotoxin is the main pathogenic substance of bacteria,
and the active ingredient of endotoxin is lipopolysaccharide (LPS),
which is an important pathogenic factor of ALI (24,25).
Therefore, in the present study, we sought to establish a rat model
for assessment of factors related to ALI by using intraperitoneal
injections of LPS in combination with tracheal instillation of LPS,
and by treatments with pre-intraperitoneal injection of PSPs. The
results indicated that PSPs attenuated LPS-induced lung
pathological changes in ALI rats, and decreased LPS-induced
increases in MOP activity as well as the elevated levels of MDA in
lung tissue. The occurrence of endotoxin-induced ALI involves a
variety of pathophysiological mechanisms, which can be divided into
classifications of direct and indirect LPS injury (such as
inflammatory theory, oxidative stress and apoptosis), among which
inflammatory reactions have been the most extensively studied
(26). Previous research has
indicated that excessive, uncontrolled, inflammatory responses are
an underlying cause of ALI, and has shown that most are
characterized by neutrophil dependence. Accordingly, aggregation
and activation of neutrophils are one of the main causes of
capillary and alveolar damage in lungs (27,28).
In the present study, we found that PSPs
significantly decreased the LPS-induced increase in neutrophil
ratio and reduced levels of inflammatory factors in the
bronchoalveolar lavage fluid (BALF) of ALI rats. The activation and
accumulation of neutrophils in lung tissues were found to have
increased levels of pro-inflammatory cytokines, and to have caused
pulmonary vascular endothelium and parenchymal cell damage by
releasing toxic substances such as oxygen-free radicals,
proteolytic enzymes, and arachidonic acid metabolites. Generally,
ALI caused by neutrophils can be divided into several stages,
including activation, accumulation, adhesion, migration, final
release of inflammatory mediators, and oxygen-free radicals, and
lung tissue can be protected if the drug can block any of these
stages (27,28). Previous research has confirmed that
PSPs have antibacterial and anti-inflammatory effects (8,9). An
experiment using the filter paper and cup method indicated that
PSPs displayed antibacterial effects against Micrococcus
luteus (29), Saccharomyces
cerevisiae (30) and other
similar bacterial strains. Previous research also indicated that
PSPs were able to control inflammation caused by bacterial
infections, and that the mechanism of these effects may involve
reducing the level of inflammatory mediators in the serum; however,
the details of any such mechanisms remain undescribed (8,9).
In theory, regarding the underlying dynamics of
inflammatory effects upon ALI, LPS enters the body and is
recognized by lipopolysaccharide-binding protein (LBP), and then
binds to cluster of differentiation 14 (CD14) to form the
LPS/LBP/CD14 complex, which can induce inflammation through
multiple signal transduction pathways to ultimately release a large
amount of inflammation factors. This release causes lung damage, of
which TLR4/Myd88/NF-κB is one of the most critical pathways
(31,32). Our results also indicated that PSPs
decreased LPS-induced increases in mRNA expression of inflammatory
factors, and of TLR4, Myd88, p-IKB-α/IKB-α and p-p65/p65 proteins
in lung tissue. In vitro, we found that PSPs could reduce
apoptosis induced by LPS in BEAS-2B cells by inhibiting
inflammation via limiting the TLR4/Myd88/NF-κB pathway.
Toll-like receptor 4 (TLR4) is the first TLR member
to have been discovered, and it is now widely accepted that TLR4 is
a transmembrane transduction receptor for LPS signaling from
extracellular to intracellular space (33,34).
TLR4 directly binds to LPS and binds to LBP-LPS-CD14, which signals
into the cell and initiates an inflammatory response that activates
a series of downstream molecules whereby amplification of the
inflammatory response occurs (34).
The TLR4/NF-κB pathway is divided into a MyD88 (medullary
differentiation protein 88)-independent pathway and a
MyD88-dependent pathway. The MyD88-independent pathway is
controlled by the MyD88 linker protein MAL (MyD88 linker protein),
which interacts with MAL to induce activation of NF-κB (35,36). The
MyD88-dependent pathway involves the interaction of TLR4 with
MyD88, whereby MyD88 is activated and phosphorylates TLR4 with an
IL-1-related enzyme (IRAK), thereby activating NF-κB (37). NF-κB is an important transcription
factor that binds to the binding site of the target gene, initiates
and regulates the expression of a series of inflammatory cytokines
involved in the inflammatory response, participates in the
inflammatory process of ALI, and mediates organ damage such as in
the lungs (38). Previous research
has shown that the IKK/IκB /NF-κB signaling pathway is an important
pathway for the activation of NF-κB (39), in which it initiates signal
transduction after LPS stimulation and activates NF-κB. Sustained
activation of NF-κB promotes massive cell synthesis and release of
cytokines including such as TNF-α, IL-1β, IL-6, IL-8, IL-10, and
IL-12 (40,41). These cytokines directly lead to lung
injury, and can also activate other signaling pathways, as well as
promote expression of inflammatory factors, ultimately leading to
lung injury (42,43).
In conclusion, we found that PSPs were able to
reduce measures of inflammation of lung tissue in ALI afflicted
rats through its effects upon the TLR4/Myd88/NF-κB pathway. Thus,
PSPs are a promising potential drug that can be used for the
treatment of LPS-induced ALI.
Acknowledgements
Not applicable.
Funding
No funding was recieved.
Availability of data and material
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
TYL and LLZ designed and performed this study, and
wrote the article. TYL, SBC, BCH, JH, XH, LQ, YF and ZT carried out
the data collection and analysis. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The Ethics Committee of The First Affiliated
Hospital of Nanchang University (Nanchang, China) has reviewed and
approved this study. All the experimental procedures were carried
out according to the National Institutes of Health guidelines for
the use of experimental animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Butt Y, Kurdowska A and Allen TC: Acute
lung injury: A clinical and molecular review. Arch Pathol Lab Med.
140:345–350. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rubenfeld GD and Herridge MS: Epidemiology
and outcomes of acute lung injury. Chest. 131:554–562.
2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bastarache JA, Ware LB and Bernard GR: The
role of the coagulation cascade in the continuum of sepsis and
acute lung injury and acute respiratory distress syndrome. Semin
Respir Crit Care Med. 27:365–376. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fujishima S, Morisaki H, Ishizaka A,
Kotake Y, Miyaki M, Yoh K, Sekine K, Sasaki J, Tasaka S, Hasegawa
N, et al: Neutrophil elastase and systemic inflammatory response
syndrome in the initiation and development of acute lung injury
among critically ill patients. Biomed Pharmacother. 62:333–338.
2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Nie L, Wu W, Lu Z, Zhu G and Liu J: CXCR3
may help regulate the inflammatory response in acute lung injury
via a pathway modulated by IL-10 secreted by CD8+ CD122+ regulatory
T cells. Inflammation. 39:526–533. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Goodman RB, Pugin J, Lee JS and Matthay
MA: Cytokine-mediated inflammation in acute lung injury. Cytokine
Growth Factor Rev. 14:523–535. 2003.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lin SH, Fu J, Wang CJ, Gao F, Feng XY, Liu
Q, Cao J and Xu F: Inflammation elevated IL-33 originating from the
lung mediates inflammation in acute lung injury. Clin Immunol.
173:32–43. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cui X, Wang S, Cao H, Guo H, Li Y, Xu F,
Zheng M, Xi X and Han C: A Review: The bioactivities and
pharmacological applications of Polygonatum sibiricum
polysaccharides. Molecules. 23(1170)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li L, Thakur K, Liao BY, Zhang JG and Wei
ZJ: Antioxidant and antimicrobial potential of polysaccharides
sequentially extracted from Polygonatum cyrtonema Hua. Int J Biol
Macromol. 114:317–323. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Long T, Liu Z, Shang J, Zhou X, Yu S, Tian
H and Bao Y: Polygonatum sibiricum polysaccharides play anti-cancer
effect through TLR4-MAPK/NF-κB signaling pathways. Int J Biol
Macromol. 111:813–821. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yang HZ, Wang JP, Mi S, Liu HZ, Cui B, Yan
HM, Yan J, Li Z, Liu H, Hua F, et al: TLR4 activity is required in
the resolution of pulmonary inflammation and fibrosis after acute
and chronic lung injury. Am J Pathol. 180:275–292. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tao H, Li N, Zhang Z, Mu H, Meng C, Xia H,
Fu L, Xu Y and Zhang S: Erlotinib protects LPS-induced acute lung
injury in mice by inhibiting EGFR/TLR4 signaling pathway. Shock.
51:131–138. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Becker CE and O'Neill LAJ: Inflammasomes
in inflammatory disorders: The role of TLRs and their interactions
with NLRs. Semin Immunopathol. 29:239–248. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lin Q, Li M, Fang D, Fang J and Su SB: The
essential roles of Toll-like receptor signaling pathways in sterile
inflammatory diseases. Int Immunopharmacol. 11:1422–1432.
2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wu H, Yang Y, Guo S, Yang J, Jiang K, Zhao
G, Qiu C and Deng G: Nuciferine ameliorates inflammatory responses
by inhibiting the TLR4-mediated pathway in
lipopolysaccharide-induced acute lung injury. Front Pharmacol.
8(939)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Qi M, Yin L, Xu L, Tao X, Qi Y, Han X,
Wang C, Xu Y, Sun H, Liu K, et al: Dioscin alleviates
lipopolysaccharide-induced inflammatory kidney injury via the
microRNA let-7i/TLR4/MyD88 signaling pathway. Pharmacol Res.
111:509–522. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Verstak B, Nagpal K, Bottomley SP,
Golenbock DT, Hertzog PJ and Mansell A: MyD88 adapter-like
(Mal)/TIRAP interaction with TRAF6 is critical for TLR2- and
TLR4-mediated NF-kappaB proinflammatory responses. J Biol Chem.
284:24192–24203. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
He W, Qu T, Yu Q, Wang Z, Lv H, Zhang J,
Zhao X and Wang P: LPS induces IL-8 expression through TLR4, MyD88,
NF-kappaB and MAPK pathways in human dental pulp stem cells. Int
Endod J. 46:128–136. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals. Guide for the Care and Use of Laboratory Animals.
Publication. 327:963–965. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Hawkins P, Prescott MJ, Carbone L,
Dennison N, Johnson C, Makowska IJ, Marquardt N, Readman G, Weary
DM and Golledge HD: A good death? Report of the second Newcastle
Meeting on Laboratory Animal Euthanasia. Animals (Basel).
6(50)2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Huang CH, Yang ML, Tsai CH, Li YC, Lin YJ
and Kuan YH: Ginkgo biloba leaves extract (EGb 761) attenuates
lipopolysaccharide-induced acute lung injury via inhibition of
oxidative stress and NF-κB-dependent matrix metalloproteinase-9
pathway. Phytomedicine. 20:303–309. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Leung WS, Yang ML, Lee SS, Kuo CW, Ho YC,
Huang-Liu R, Lin HW and Kuan YH: Protective effect of zerumbone
reduces lipopolysaccharide-induced acute lung injury via
antioxidative enzymes and Nrf2/HO-1 pathway. Int Immunopharmacol.
46:194–200. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tao J, Zhang J, Ling Y, McCall CE and Liu
TF: Mitochondrial sirtuin 4 resolves immune tolerance in monocytes
by rebalancing glycolysis and glucose oxidation homeostasis. Front
Immunol. 9(419)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang B, Liu ZY, Li YY, Luo Y, Liu ML,
Dong HY, Wang YX, Liu Y, Zhao PT, Jin FG, et al: Antiinflammatory
effects of matrine in LPS-induced acute lung injury in mice. Eur J
Pharm Sci. 44:573–579. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jiang W, Luo F, Lu Q, Liu J, Li P, Wang X,
Fu Y, Hao K, Yan T and Ding X: The protective effect of Trillin
LPS-induced acute lung injury by the regulations of inflammation
and oxidative state. Chem Biol Interact. 243:127–134.
2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wu J, Zhang YY, Guo L, Li H and Chen DF:
Bupleurum polysaccharides attenuates lipopolysaccharide-induced
inflammation via modulating Toll-like receptor 4 signaling. PLoS
One. 8(e78051)2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Caudrillier A, Kessenbrock K, Gilliss BM,
Nguyen JX, Marques MB, Monestier M, Toy P, Werb Z and Looney MR:
Platelets induce neutrophil extracellular traps in
transfusion-related acute lung injury. J Clin Invest.
122:2661–2671. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Narasaraju T, Yang E, Samy RP, Ng HH, Poh
WP, Liew AA, Phoon MC, van Rooijen N and Chow VT: Excessive
neutrophils and neutrophil extracellular traps contribute to acute
lung injury of influenza pneumonitis. Am J Pathol. 179:199–210.
2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhi-Tao LI, Sun JX, Zhu HX and Chu ZF:
Extracting of Polygonatum polysaccharides and its antimicrobial
activity. Food Res Dev. 38:36–37. 2017.
|
|
30
|
Zheng CY, Wang HF and Zhang TT: Studies on
the anti-micromial and anti-inflammatory activities of Polygonatum
cyrtonema Hua. polysaccharides. J Anhui Norm Univsity. 33:272–276.
2010.(In Chinese).
|
|
31
|
Jiang Q, Yi M, Guo Q, Wang C, Wang H, Meng
S, Liu C, Fu Y, Ji H and Chen T: Protective effects of polydatin on
lipopolysaccharide-induced acute lung injury through
TLR4-MyD88-NF-κB pathway. Int Immunopharmacol. 29:370–376.
2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Akhter N, Hasan A, Shenouda S, Wilson A,
Kochumon S, Ali S, Tuomilehto J, Sindhu S and Ahmad R: TLR4/MyD88
-mediated CCL2 production by lipopolysaccharide (endotoxin):
Implications for metabolic inflammation. J Diabetes Metab Disord.
17:77–84. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Rocha DM, Caldas AP, Oliveira LL, Bressan
J and Hermsdorff HH: Saturated fatty acids trigger TLR4-mediated
inflammatory response. Atherosclerosis. 244:211–215.
2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lu YC, Yeh WC and Ohashi PS: LPS/TLR4
signal transduction pathway. Cytokine. 42:145–151. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Doyle SL and O'Neill LAJ: Toll-like
receptors: From the discovery of NFkappaB to new insights into
transcriptional regulations in innate immunity. Biochem Pharmacol.
72:1102–1113. 2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
O'Neill LAJ and Bowie AG: The family of
five: TIR-domain-containing adaptors in Toll-like receptor
signalling. Nat Rev Immunol. 7:353–364. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
37
|
Jiang Z, Ninomiya-Tsuji J, Qian Y,
Matsumoto K and Li X: Interleukin-1 (IL-1) receptor-associated
kinase-dependent IL-1-induced signaling complexes phosphorylate
TAK1 and TAB2 at the plasma membrane and activate TAK1 in the
cytosol. Mol Cell Biol. 22:7158–7167. 2002.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Monick MM and Hunninghake GW: Activation
of second messenger pathways in alveolar macrophages by endotoxin.
Eur Respir J. 20:210–222. 2002.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Perkins ND: Integrating cell-signalling
pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol.
8:49–62. 2007.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Dolinay T, Kim YS, Howrylak J, Hunninghake
GM, An CH, Fredenburgh L, Massaro AF, Rogers A, Gazourian L,
Nakahira K, et al: Inflammasome-regulated cytokines are critical
mediators of acute lung injury. Am J Respir Crit Care Med.
185:1225–1234. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Montravers P, Chollet-Martin S, Marmuse
JP, Gougerot-Pocidalo MA and Desmonts JM: Lymphatic release of
cytokines during acute lung injury complicating severe
pancreatitis. Am J Respir Crit Care Med. 152:1527–1533.
1995.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Barreto TR, Costola-de-Souza C, Margatho
RO, Queiroz-Hazarbassanov N, Rodrigues SC, Felício LF, Palermo-Neto
J and Zager A: Repeated Domperidone treatment modulates pulmonary
cytokines in LPS-induced acute lung injury in mice. Int
Immunopharmacol. 56:43–50. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lin S, Wu H, Wang C, Xiao Z and Xu F:
Regulatory T cells and acute lung injury: Cytokines, uncontrolled
inflammation, and therapeutic implications. Front Immunol.
9(1545)2018.PubMed/NCBI View Article : Google Scholar
|