Introduction
Idiopathic necrosis of the femoral head (INFH) is
the osteonecrosis of the femoral head with unknown causes. It is a
common disease and the number of cases is estimated at 10,000 to
20,000 cases per year in the USA (1,2). This
number increases every year. A recent study from Japan reported an
annual incidence of 2.51 cases per 100,000 person per year
(3). Early manifestations of INFH
are atypical. With the progression of the disease, patients may
develop collapsed femoral head, pain, shortened limbs and
arthritis, eventually surgical treatments are needed. One of the
available early treatment is core compression (4). It can reduce pain in patients with
early stage of the disease (5).
However, a significant number of patients are dissatisfied with the
repair of the femoral head necrotic area (5). The other promising treatment is stem
cell therapy. The necrosis of femoral head involves the local stem
cell activity (6).
Human bone marrow mesenchymal stem cells (hBMSCs)
are multipotent stem cells isolated from the bone marrow that are
important for making and repairing skeletal tissues. It has strong
self-renewal abilities and can undergo chondrogenic, osteogenic and
adipogenic differentiation. It also maintains the balance of bone
metabolism in the human skeletal system (7). Recently, the decrease in hBMSC and the
change in cell behavior have been linked to the occurrence and
development of osteonecrosis of the femoral head (8). This suggests that BMSC maybe a great
target for INFH treatment. Wnt signaling has been implicated in
controlling BMSC fate, but it has not been well investigated in
hBMSC from INFH patients.
Recently, Wang et al conducted a microRNA
expression profiling of BMSCs in patients with femoral head
osteonecrosis compared to femoral neck fracture. They found a group
of differentially expressed microRNAs and predicted their target
genes. Among these, the expression of Leucine-rich
repeat-containing 17 (LRRC17) was significantly lower in hBMSCs
obtained from patients with femoral head osteonecrosis compared to
femoral neck fracture (9). LRRC17
is a secretory protein containing five leucine-rich repeat domains.
It was first characterized in bone metabolism as an inhibitor of
receptor activator of nuclear factor-κB ligand (RANKL)-induced
osteoclast differentiation (10).
RANKL is the ligand of the NF-κB receptor activator, which is an
indispensable biomolecule in osteoclast activation and
proliferation (10). LRRC17
negatively regulates RANKL to inhibit bone degradation.
Later, Kim et al demonstrated that postmenopausal women with
lower LRRC17 level had a 3.32-fold higher odds ratio for
osteoporotic fracture and associated with a 46% higher risk of
osteoporotic fracture than the group with higher LRRC17 levels,
suggesting LRRC17's potential as a marker for osteoporotic fracture
(11). However, the role of LRRC17
in the INFH has never been fully investigated.
In this study, we aimed to investigate the role of
LRRC17 in hBMSCs derived from INFH patients. By manipulating the
expression of LRRC17, we explored the potential mechanisms with a
focus on the Wnt signaling pathways. This study may help us
understand the effect of LRRC17 on the pathogenesis of INFH and
identify a potential treatment target for INFH patients.
Materials and methods
Clinical samples
The present study was approved by the Ethics
Committee of Liaocheng People's Hospital, and all patients who
participated in this study provided a signed written informed
consent.
Hematoxylin-eosin staining
The surgically resected femoral head was
semi-dissected from the coronal face, and the appearance of the
specimen and the pathology of the section were observed. A bone
grain of ~10x10x5 mm was taken from the necrotic area
(INFH-derived) and normal area (FNF-derived) under naked eye
observation. All the bone blocks were fixed in 10% formalin
solution at 4˚C for 3 days, decalcified with 10% EDTA Tris-HCl (pH
7.4) buffer and changed every day at 4˚C. When acupuncturing
specimen had no resistance, this indicated that the bone tissue had
been decalcified completely. The specimen was embedded in paraffin
and cut into sections with a thickness of 4-5 µm. Sections were
dewaxed using xylene for three times with 5 min each, rehydrated
using gradient ethanol (100, 95, 85 and 75%), and stained with
hematoxylin solution at 25˚C for 10 min. After that, the sections
were dipped in 1% hydrochloric acid for 2-5 sec, 1% ammonia for 1
min, and then 0.5% eosin for 5-10 sec at 25˚C. Finally, sections
were dehydrated with 95 and 100% ethanol and mounted with
xylene-based glue. Blocking was not performed. Sections were
observed under a light microscope at a magnification of x200 and
images were captured.
Bone marrow were harvested from five patients with
INFH (three males and two females; average age, 61.33±7.2 years
old) and five patients with femoral neck fracture (FNF; two males
and three females; average age, 67.33±8.6 years old) between
February 2017 and February 2018, who received femoral head
replacement in the hospital. According to the Ficat staging system
(12), all patients with INFH were
at stages III or IV. The exclusion criteria were: i) Medical
history of cancer; ii) other metabolic bone disease; iii) previous
hip surgery or infectious disease; and iv) long-term use of
hormones and alcohol. hBMSCs harvested from five patients with INFH
or FNF (INFH-hBMSC or FNF-hBMSC) were pooled together for following
experiments.
All experimental procedures were conducted in strict
conformity with the work described, and carried out in accordance
with the Code of Ethics of the World Medical Association
(Declaration of Helsinki) for experiments involving humans.
Isolation and culture of hBMSCs
The hBMSCs were isolated by subjecting to Ficoll
(1.077 g/ml; Tianjin Haoyang Biological Products Technology Co.,
Ltd.) density gradient separation. The mononuclear fraction was
collected and plated in T-25 flasks (Corning, Inc.) using complete
DMEM/F-12 containing 10% fetal bovine serum and 1% penicillin and
streptomycin (Gibco; Thermo Fisher Scientific, Inc.) medium at 37˚C
with 5% CO2. After three days, the medium was changed to
remove non-adherent cells. Then, the media was changed every three
days. When the primary cells reached 70-80% confluence, the cells
were passaged by trypsinization (Hyclone; Cyvita), and cultured
based on the aforementioned method.
Flow cytometry
The cell-specific surface markers were examined by
flow cytometry. The hBMSCs were dissociated and fixed in 70%
ethanol at 4˚C for 24 h. Anti-CD34-FITC (cat. no. 560942),
anti-CD44-FITC (cat. no. 560977), anti-CD45-FITC (cat. no. 560976)
and anti-CD90-FITC (cat. no. 561969) (all ready to use; BD
Biosciences PharMingen) antibodies were used and incubated at 25˚C
for 20 min. The data analysis was conducted using the BD FACSDiva
software (version. 8.0.1; BD Biosciences).
Osteogenic induction and
evaluation
To induce osteogenic differentiation, hBMSCs were
incubated in DMEM supplemented with dexamethasone (100 nM),
β-glycerophosphate (10 mM) and ascorbic acid 2-phosphate (200 µM)
(all from Sigma-Aldrich; Merck KGaA), and 10% fetal bovine serum
(Hyclone; Cyvita). The medium was changed every three days. After
osteogenic induction for 14 days, cells were stained with 2%
alizarin red S (Sigma-Aldrich; Merck KGaA) at 25˚C for 15 min to
evaluate the calcium deposition. Matrix mineral-bound staining was
detected as a bright orange-red color under a light microscope
(magnification, x200).
Adipogenic induction and
evaluation
To induce adipogenic differentiation, lipid
induction A solution was prepared by supplementing DMEM with
dexamethasone (1 µM), 3-isobutyl-1-methylxanthine (0.5 mM), insulin
(10 µg/ml), rosiglitazone (0.5 µM) and indomethacin (100 µM) (all
from Sigma-Aldrich; Merck KGaA) and 10% fetal bovine serum
(Hyclone; Cyvita). Then, B solution was prepared by adding insulin
(20 µg/ml) to the DMEM and 10% fetal bovine serum. Subsequently,
2x105/ml hBMSCs were incubated with the A solution for
three days, and this was switched to the B solution for one day.
This was repeated for four times. In order to evaluate the
adipogenesis, oil red O (Sigma-Aldrich; Merck KGaA) staining was
performed at 25˚C for 15 min and observed under a light microscope
at a magnification of x200.
Cell transfection
The INFH-hBMSCs were randomly divided into four
groups: LRRC17 overexpression group and its vector control group,
and LRRC17 knockout group and its negative control group. For the
overexpression or knockout, INFH-hBMSCs were transfected with
lentiviral particles of GV492-LRRC17 or GV493-LRRC17 short hairpin
(sh)RNA, and the respective control vectors. The quantity of
lentiviral plasmid used for transfection was 9 µg. The ratio of the
lentiviral plasmid: Packaging vector: Envelope was 9:13:4. The
duration of transfection into cells was 3 days and the multiplicity
of infection (MOI) was 70%.
The lentiviral supernatants that contained
lentiviral vectors for LRRC17 overexpression or knockout,
and the respective controls were purchased from Shanghai GeneChem
Co., Ltd.
Positive clones were selected with 200 ng/ml of
puromycin for three days. After five days, the infection efficiency
was observed under a fluorescence microscope (cat. no. CKX71;
Olympus Corporation). When the transduction was over, the
subsequent experiments were performed immediately.
Dickkopf-related protein 1 (DKK1) and
Wnt inhibition treatment
INFH-hBMSCs were seeded at 1x103 in a
6-well plate, and randomly divided into four groups: LRRC17
overexpression treatment with or without 100 ng/ml DKK1
(MedChemExpress) treatment, and LRRC17-knockout cells with
or without 10 µM SP600125 (Beyotime Institute of Biotechnology).
Then, cells were transfected for the LRRC17 overexpression or
knockout cells at 24 h after plating, followed by DKK1 or SP600125
treatment after 48 h. Cells were harvested after 24 h for
downstream analysis.
In vitro cell proliferation assay
In order to generate the standard curve, hBMSCs were
trypsinized and seeded at 2x103, 4x103,
8x103, 1.0x104, 1.2x104,
1.6x104, 2.0x104 and 2.4x104 cells
per well in a 96-well plate. Each well was repeated for three
times. After 2 h, cells were incubated with Cell Counting Kit-8
(CCK-8) (Dojindo Molecular Technologies, Inc.) for another 2 h at
25˚C and detected using an enzyme-labeling instrument.
The INFH-hBMSCs or FNF-hBMSCs were trypsinized, and
5x103 cells per well were plated in a 96-well plate. For
each of the following six days, cells were incubated with CCK-8 for
2 h at 37˚C and detected using an enzyme labeling instrument.
Cell apoptosis assay
Annexin V-FITC (BD Biosciences) was used to evaluate
the apoptosis in INFH-hBMSCs or FNF-hBMSCs. Then, 1x106
cells from each group were resuspended with 1X binding buffer, and
5 µl of Annexin V-FITC was added and incubated for 15 min at room
temperature in the dark. Propidium iodide (PI) staining with a
volume of 5 µl was added at 5 min before detection. Then, cells
were analyzed by flow cytometry (Becton, Dickinson and
Company).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRIzol RNA isolation reagents (Thermo Fisher
Scientific, Inc.) was used to extract the total RNA. A NanoDrop
2000 Spectrophotometer (Thermo Fisher Scientific, Inc.) was used to
measure the concentration and purity of the total RNA. Then, 1 µg
RNA was converted to cDNA using a PrimeScript™ RT reagent kit
(Takara Bio, Inc.) with a gDNA Eraser at 37˚C for 15 min, then 85˚C
for 5 sec and 4˚C continuously, and amplified in the ABI 7500
Sequence Detection System (Applied Biosystems; Thermo Fisher
Scientific, Inc.) using a SYBR® Premix Ex Taq™ II kit
(all obtained from Takara Biotechnology Co., Ltd.). The thermal
profile of the RT-qPCR was: Pre-incubation at 95˚C for 30 sec for
one cycle, followed by 40 cycles incubated at 95˚C for 5 sec, and
60˚C for 34 sec. The polymerase chain reaction (PCR) primers were
synthesized by Sangon Biotech Co., Ltd. The primer sequences are
summarized in Table I. The
housekeeping gene GAPDH was used as an internal control. The
relative expression levels of each gene were calculated using
2-ΔΔCq (13). Each
experiment was evaluated by the same PCR reactions for three
times.
 | Table ISequences of primers. |
Table I
Sequences of primers.
| Gene | Refseq accession
number | Direction | Sequence, 5' to
3' |
|---|
| LRRC17 | NM_001031692 | Forward |
AAAGTGCCAAACAACATCCCT |
| | | Reverse |
TGGGTCGAAGTTGGTTGATTTT |
| OPG | NM_002546.4 | Forward |
GCCCCTTGCCCTGACCACTAC |
| | | Reverse |
TGCGATTGCACTCCTGCTTGAC |
| BMP2 | NM_001200.4 | Forward |
GTCCTGAGCGAGTTCGAGTTGC |
| | | Reverse |
GTGGTCTGGGGCGGGTGAG |
| CEBPα | NM_004364.4 | Forward |
TCGGTGGACAAGAACAGCAACG |
| | | Reverse |
GGCGGTCATTGTCACTGGTCAG |
| PPARγ | NM_015869.4 | Forward |
GCTGAATCCAGAGTCCGCTGAC |
| | | Reverse |
ATCGCCCTCGCCTTTGCTTTG |
| Wnt3a | NM_033131.4 | Forward |
TCGGAGATGGTGGTGGAGAAGC |
| | | Reverse |
GGGTTGGGCTCGCAGAAGTTG |
| Wnt5a | NM_003392.5 | Forward |
GACTTCCGCAAGGTGGGTGATG |
| | | Reverse |
GTCTTGTGTGGTGGGCGAGTTG |
| β-catenin | NM_001904.4 | Forward |
TACTGTCCTTCGGGCTGGTGAC |
| | | Reverse |
GCTTCTTGGTGTCGGCTGGTC |
| Rankl | NM_003701.4 | Forward |
CAGCATCGCTCTGTTCCTGTA |
| | | Reverse |
CTGCGTTTTCATGGAGTCTCA |
| GAPDH | NM_002046.7 | Forward |
CGGAGTCAACGGATTTGGTCGTAT |
| | | Reverse |
AGCCTTCTCCATGGTGGTGAAGAC |
Western blotting
The protein expression was measured after
transfecting hBMSCs with lentivirus that contained the LRRC17 gene.
The expression levels of osteoprotegerin (OPG), bone morphogenetic
protein 2 (BMP2), peroxisome proliferator-activated receptor γ
(PPARγ), CCAAT/enhancer-binding protein α (CEBPα), Wnt3a,
β-catenin, Wnt5a and Rankl proteins were measured for all groups of
hBMSCs.
PMSF was used to lyse the cell precipitation. The
cell lysates were centrifuged at 10,000 x g for 4 min at 4˚C, and
the BCA protein assay kit was used to measure the protein
concentrations (all were obtained from Beyotime Institute of
Biotechnology). The same amount of protein (10 µg) and Prestained
Protein Ladder (4 µl) were injected into the 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis lanes, and
electrophoresis was performed for 1 h at 100 V. Then, the proteins
were transferred onto polyvinylidene fluoride membranes (EMD
Millipore) for 1.5 h at 200 mA. Next, 5% skimmed milk (dissolved in
TBST) was used to block the non-specific binding membranes at room
temperature for 1 h. Then, the membranes were incubated with the
appropriate primary antibodies overnight at 4˚C: Anti-LRRC17
(1:600), anti-CEBPα (1:750), anti-β-catenin (1:30,000) (all
obtained from Proteintech Group, Inc.); anti-OPG (1:1,000; ABclonal
Biotech Co., Ltd.); anti-Wnt5a (1:1,000), anti-BMP2 (1:1,000),
anti-PPARγ (1:1,000), anti-RANKL (1:700), anti-Wnt3a (1:1,000),
mouse monoclonal anti-β-actin antibody (1:10,000) (all obtained
from Beyotime Institute of Biotechnology). TBST was used to wash
the membranes three times, for 10 min each. The membranes were
incubated with species-specific horseradish peroxidase coupled with
the secondary antibodies (goat anti-mouse or anti-rabbit IgG; cat.
nos. SA00001-1 and SA00001-2, respectively) at 1:5,000 for 1 h at
room temperature. After washing for 30 min, an ECL western blotting
kit (Beyotime Institute of Biotechnology) was used to develop the
blots. The quantification of the protein bands was visualized using
the AlphaView analysis system (ProteinSimple). The β-actin antibody
was used as the protein loading control. The LRRC17, CEBPα,
β-catenin, OPG, Wnt5a, PPARγ, BMP2, RANKL and Wnt3a protein
expression was normalized against β-actin.
Statistical analysis
The data are presented as mean ± standard deviation
(SD), and at least three independent experiments were performed.
The quantitative data was collected and statistically analyzed
using the SPSS 20.0 software (IBM Corp.) by one-way ANOVA between
two groups followed by SNK-q and LSD test. The level of statistical
significance was set at P<0.05.
Results
Isolation and culture of hBMSCs from
patients with FNF or INFH
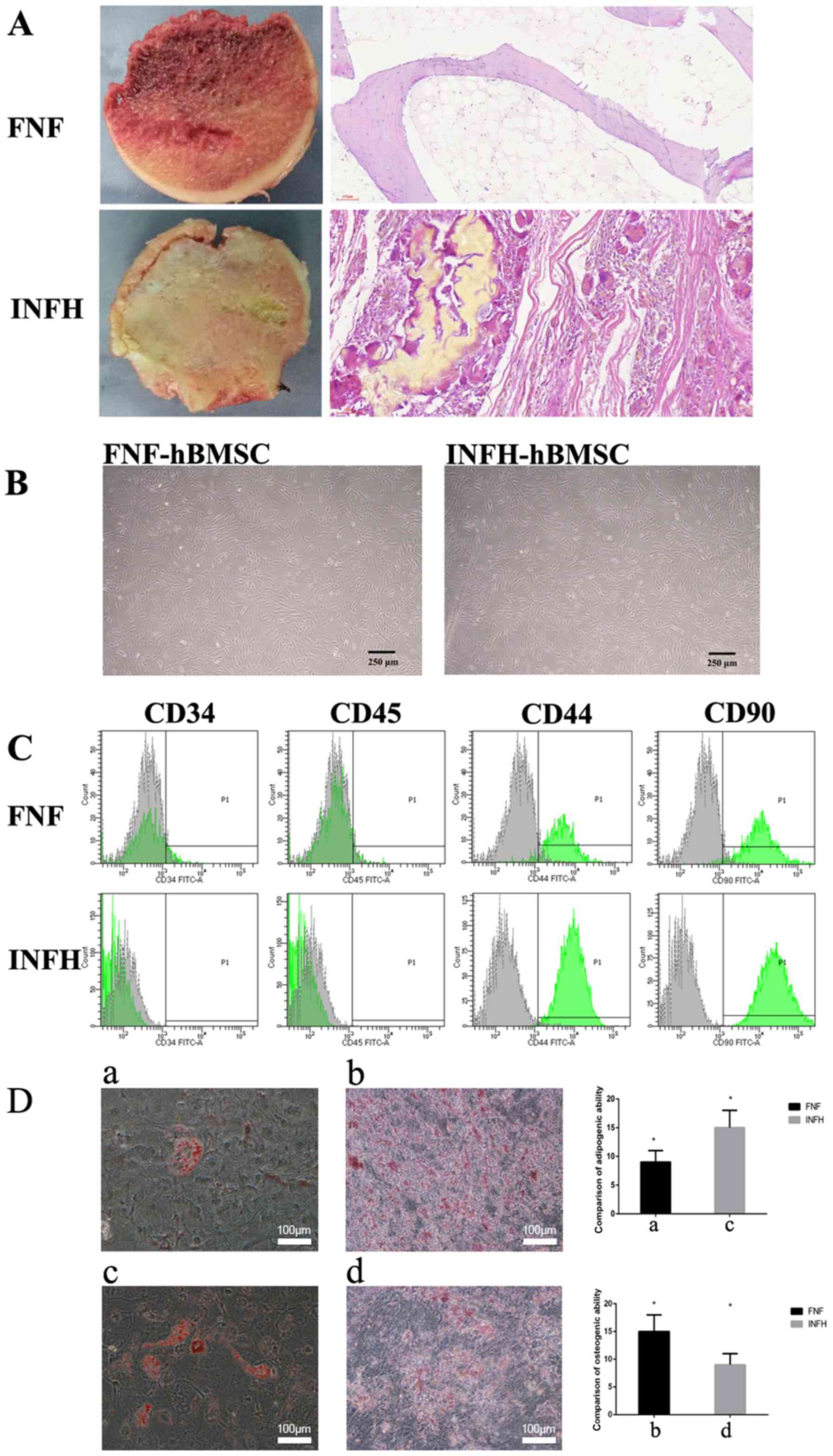
Femoral head tissues were harvested from five
patients with FNF and five patients with INFH (representatively
shown in Fig. 1A). The hematoxylin
and eosin (H&E) staining of femoral head tissues suggested that
the destruction of the bone structure in INFH patients was more
severe compared with that of patients with FNF.
The hBMSCs were isolated from the bone marrow of
patients with FNF and INFH and pooled together respectively for
downstream analysis. INFH-hBMSCs or FNF-hBMSCs were characterized
with surface markers. The cell morphology revealed that the hBMSCs
from both groups were as follows: Spindle-shaped, had a long
fusiform, uniform in size and easily adherent to the plate,
arranged in a certain direction, and rich in cytoplasm with a
strong refraction (Fig. 1B).
Characterization of hBMSCs by surface
marker expression and multi-lineage differentiation
The expression levels of MSC surface markers (CD44
and CD90) and hematopoietic markers (CD45 and CD34) were analyzed
in hBMSCs from both groups by flow cytometry (14). The results showed that hBMSCs from
both INFH and FNF groups consistently expressed CD44 and CD90 and
were negative for CD45 and CD34 (Fig.
1C).
In order to evaluate the adipogenic differentiation,
the lipid droplet accumulation was assessed by oil red O staining.
The present quantification data revealed that the FNF-hBMSC-induced
lipid droplets were smaller and fewer in number compared with those
from INFH-hBMSCs (P<0.05; Fig.
1D).
After incubation in the osteogenic induction medium
for 14 days, calcium nodules were observed through alizarin red
staining, and these were further quantified. The present data show
that calcium deposition was significantly higher in the FNF-hBMSCs
compared with the INFH-hBMSCs (P<0.05; Fig. 1D). These data suggested that
INFH-hBMSCs had a diminished ability for osteogenic differentiation
and increased potential for adipogenic differentiation.
Cell proliferation and apoptosis
analysis of hBMSCs from patients with INFH or FNF
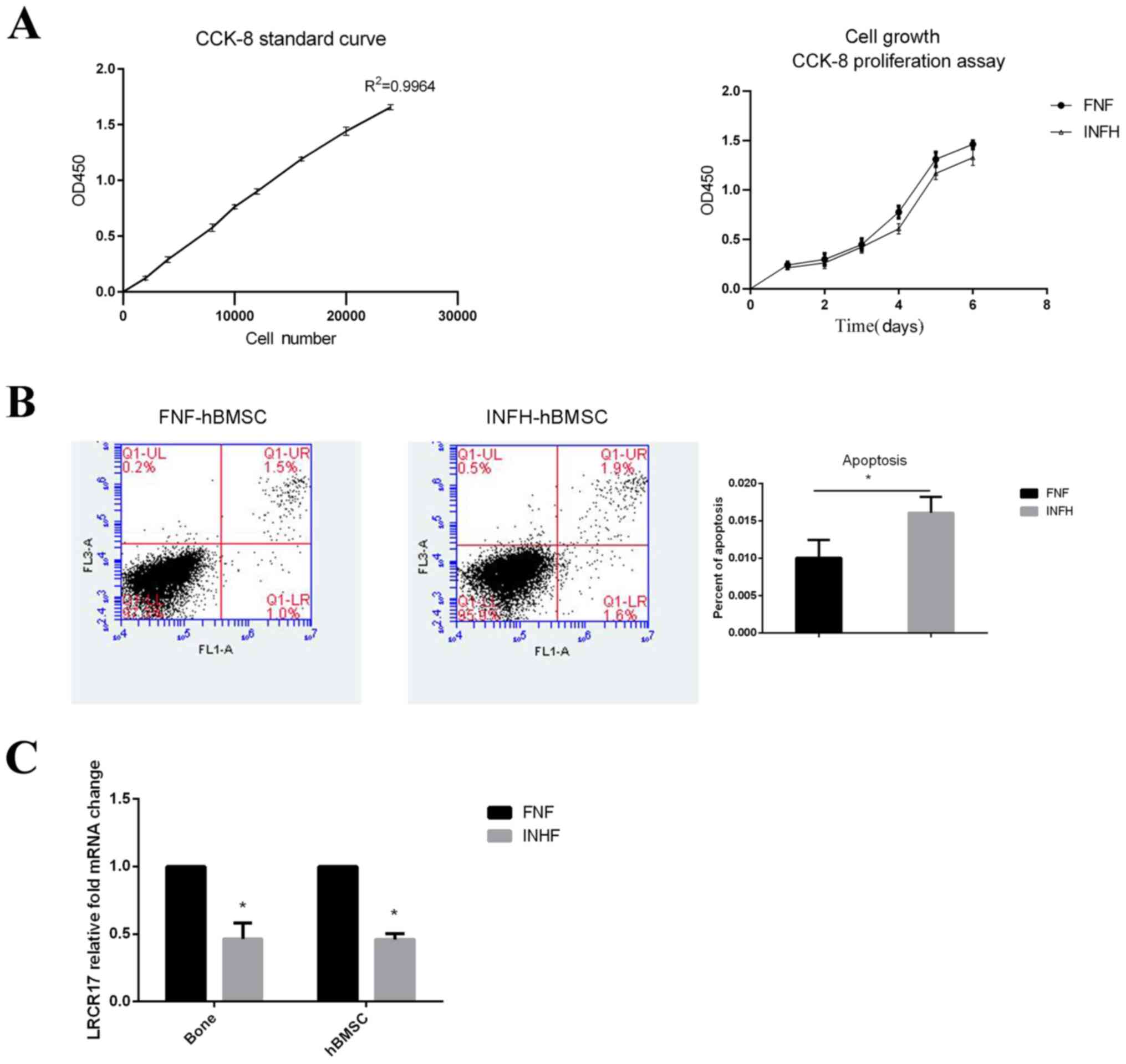
Cell proliferation was further analyzed using the
classic CCK-8 assay. Both groups of cells were seeded at the same
density on day 0 and cultured for 6 days. After generating a
standard curve with R2=0.9964, the CCK-8 levels of each
sample was measured. The data showed that hBMSCs from both groups
continued to proliferate during the culture. The cell number of
FNF-hBMSCs was comparable to INFH-hBMSCs for the first 3 days, then
became higher from day 4 with a statistically significant
difference observed on day 4 (P<0.05; Fig. 2A).
hBMSCs from both INFH and FNF groups did not have
high level of cell apoptosis, despite that the number of cells
undergoing apoptosis in INFH-hBMSCs was higher compared with those
from FNF-hBMSCs (FNF, 1.0%; INFH, 1.6%; P<0.05; Fig. 2B). These data suggest that
INFH-hBMSCs have decreased proliferative ability and tendency to
undergo apoptosis.
LRRC17 expression in the bone and
hBMSCs of patients with INFH or FNF
Next, the expression of LRRC17 was examined
in the bone tissues and hBMSCs in each patient. It was found that
the expression of LRRC17 was 2.17-fold higher on average in
the bone tissue of patients with FNF compared with that from
patients with INFH. Similarly, the mRNA expression of LRRC17
in the FNF-hBMSCs was 2.38-fold higher compared with that of the
INFH-hBMSCs (Fig. 2C). The
aforementioned data confirmed the finding presented by Wang et
al (9).
LRRC17 overexpression inhibits
adipogenic differentiation and promotes the osteogenic
differentiation of INFH-hBMSCs
To investigate the role of LRRC17 in the
pathogenesis of INFH, LRRC17 was first overexpressed in the
INFH-hBMSCs, and the adipogenic and osteogenic differentiation
abilities were assessed. The results showed that the mRNA levels of
LRRC17 increased 5.4-fold in the LRRC17-overexpressed
group, as well as the osteogenic differentiation marker genes
OPG and BMP2, which increased by 6.1- and 6.6-fold
respectively, compared with its vector control group. The
aforementioned changes were also confirmed at the protein level
using western blotting. The protein expression of LRRC17, BMP2 and
OPG was increased by 1.8-, 1.6- and 2.7-fold, respectively.
Meanwhile, the mRNA expression of adipogenic markers PPARG
and CEBPα decreased by 6.7- and 10.0-fold, respectively, in
the LRRC17 overexpression group compared with the control
group; similarly, their protein expression levels were decreased by
1.5- and 1.8-fold, respectively (Fig.
3A).
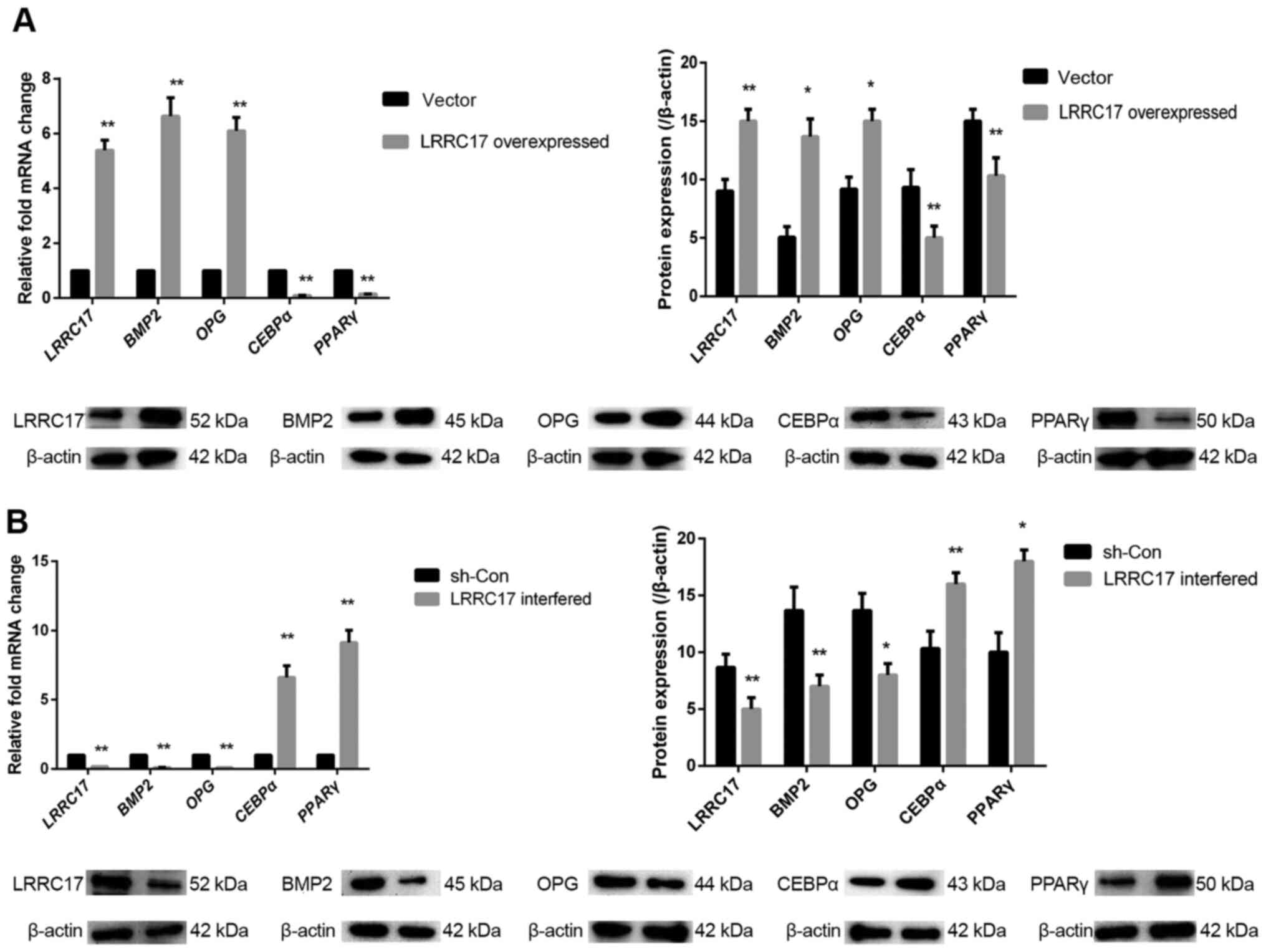 | Figure 3Effect of LRRC17 expression on
osteogenic and adipogenic differentiation of INFH-hBMSCs. (A and B)
Changes in LRRC17, BMP2, OPG, CEBPα, PPARγ mRNA and protein
expression after the overexpression and interference with LRRC17.
*P<0.05, **P<0.01 vs. respective
control. hBMSCs, human bone marrow mesenchymal stem cells; INFH,
idiopathic necrosis of the femoral head; LRRC17, leucine-rich
repeat-containing 17; OPG, osteoprotegerin; BMP2, bone
morphogenetic protein 2; PPARγ, peroxisome proliferator-activated
receptor γ; CEBPα, CCAAT/enhancer-binding protein α; sh-, short
hairpin RNA; con-, control. |
The knockout of LRRC17 gene expression in hBMSCs was
performed using shRNA. The data showed that the LRRC17 mRNA
expression was decreased by 5.5-fold with LRRC17 knockout.
OPG and BMP2 expression levels decreased by 10.0- and
10.0-fold, respectively, in the knockout group, compared with the
control; consistently, the protein expression levels were also
decreased by 1.7-, 1.7- and 1.9-fold, respectively. Moreover, the
mRNA expression of PPARG and CEBPα was increased by
9.1- and 6.6-fold, respectively, in the knockout group compared
with its negative control group, and the protein expression was
increased by 1.8- and 1.6-fold, respectively (Fig. 3B). This suggests that LRRC17
promotes osteogenesis and inhibits adipogenesis in INFH-hBMSCs.
LRRC17 regulates BMSC through Wnt
signaling pathways
Since both canonical and non-canonical Wnt pathways
have been shown to regulate osteogenesis and adipogenesis in BMSCs,
the involvement of these pathways were further investigated to
dissect the mechanisms of how LRRC17 regulates the multilineage
differentiation in INFH-hBMSCs. Upon the overexpression of LRRC17,
the mRNA levels of WNT3A and CTNNB1 increased by 3.3-
and 8.8-fold, respectively; their protein expression levels were
also increased by 2.5- and 2.3-fold. On the contrary, the mRNA
levels of WNT5A and RANKL decreased by 4.0- and
2.9-fold, respectively, due to LRRC17 overexpression, and
their protein expression decreased by 2.0- and 1.9-fold,
respectively (Fig. 4A).
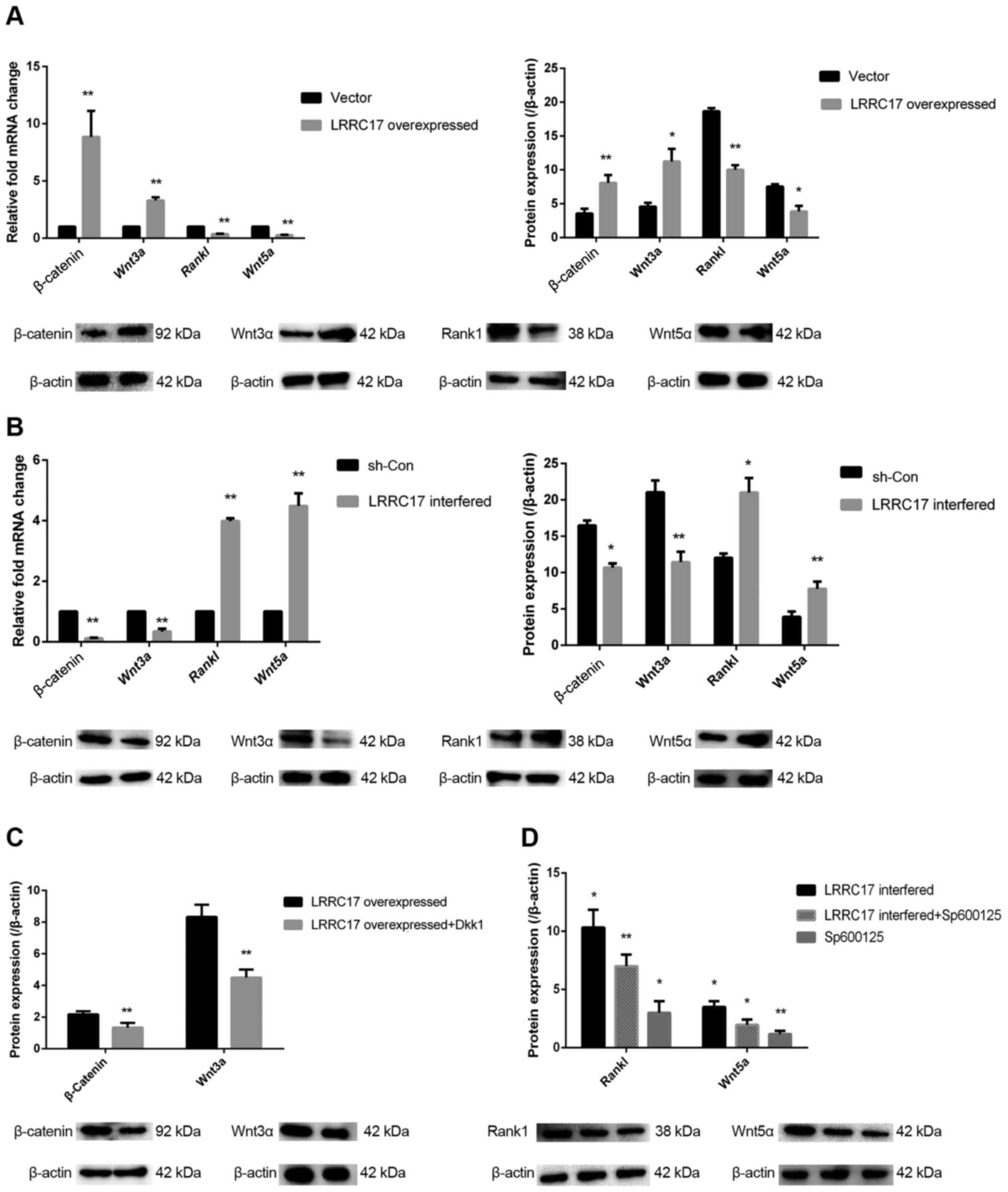 | Figure 4Effect of LRRC17 on Wnt signaling in
the INFH-hBMSCs. (A and B) Changes in β-catenin, Wnt3a, Rankl and
Wnt5a mRNA and protein expression after the overexpression and
interference with LRRC17. (C) Changes in β-catenin and Wnt3a
protein expression after the overexpression of LRRC17 and
the addition of DKK1. (D) Changes in Rankl and Wnt5a protein
expression after the interference of LRRC17 and the addition
of Sp600125. *P<0.05, **P<0.01 vs. the
control groups. hBMSCs, human bone marrow mesenchymal stem cells;
INFH, idiopathic necrosis of the femoral head; LRRC17, leucine-rich
repeat-containing 17; Rankl, receptor activator of nuclear factor
κ-B ligand; DKK1, Dickkopf-related protein 1; sh-, short hairpin
RNA; con-, control. |
Meanwhile, the mRNA expression of WNT3A and
CTNNB1 was decreased by 2.9- and 9.9-fold, respectively,
with LRRC17 knockout; their protein expression levels
decreased by 1.9- and 1.6-fold, respectively. The mRNA expression
levels of WNT5A and RANKL were increased by 4.5- and
4.0-fold, respectively, due to LRRC17 knockout.
Correspondingly, the protein expression levels were increased by
2.0- and 1.7-fold, respectively (Fig.
4B). This suggests that both Wnt3a- and Wnt5a-mediated
signaling pathways are involved in the regulation of hBMSC
differentiation by LRRC17.
To further confirm the role of Wnt signaling
pathways in INFH-hBMSCs, the canonical Wnt pathway inhibitor Dkk1
was incubated with LRRC17-overexpressed cells and the protein
levels of Wnt3a and β-catenin were evaluated. The results confirmed
that the expression of Wnt3a and β-catenin was decreased by 1.8-
and 1.7-fold, respectively, with the addition of Dkk1 (Fig. 4C). On the other hand, the protein
expression levels of Wnt5a and Rankl in the LRRC17-knockout
group with SP600125 treatment was decreased by 1.7- and 1.5-fold,
respectively. These data further confirm the involvement of Wnt
signaling in LRRC17 in regulating bone metabolism, and
provides the target of promoting osteogenesis in hBMSCs from
patients with INFH (Fig. 4D).
Discussion
The present study characterized the morphology,
surface marker expression, cell proliferation and multi-lineage
differentiation abilities of hBMSCs from patient with INFH, and
demonstrated that LRRC17 may be a pivotal regulator of bone
metabolism in the pathogenesis of INFH. The present study data
suggested that INFH-hBMSCs were similar in cell morphology, surface
marker expressions, but with enhanced adipogenic differentiation
and decreased osteogenic differentiation abilities compared with
FNF-hBMSCs. INFH-hBMSCs also expressed lower levels of
LRRC17 and were highly apoptotic compared with FNF-hBMSCs.
The overexpression of LRRC17 promoted osteogenesis, and
inhibited adipogenesis, while the knockout of LRRC17
reversed these changes in the INFH-hBMSCs; this may be mediated
through both canonical and non-canonical Wnt signaling pathways.
The results suggested that LRRC17 may be a potential target
for the stem cell therapeutic treatment of INFH.
The present study data demonstrated that
LRRC17 has an important role in the pathogenesis of INFH.
Abnormal bone metabolism is part of the pathogenesis of femoral
head osteonecrosis (15). During
INFH, the proliferative and osteogenic differentiation abilities of
hBMSCs decrease. This diminishes the bone-repair ability of the
body, further leading to subchondral bone weakening, stress
fracture, and even articular surface collapse. Consistently, it was
also found that the osteogenic differentiation of INFH-hBMSCs was
significantly decreased and adipogenic differentiation ability was
increased compared with FNF-hBMSCs. LRRC17 expression was
significantly lower in patients with INFH. Overexpression of
LRRC17 promoted osteogenesis, and inhibited the adipogenesis
of INFH-hBMSCs. Therefore, targeting LRRC17 provides a
promising therapeutic target for stem cell treatment of patients
with INFH.
Previous studies have confirmed that Wnt signaling
pathways participate in regulating MSC self-renewal and osteogenic
differentiation, and maintaining the balance between osteoblast and
osteoclast differentiation (16,17).
This has important clinical significance for bone injury repair,
tissue renewal, and intra-articular homeostasis (18). Wnt signal pathways are
evolutionarily conserved, and can be mainly classified as follows:
Canonical- or Wnt/β-catenin-dependent pathway, and the
non-canonical or β-catenin-independent pathway. These two pathways
intersect with each other, and their association is very
complicated, and has not been fully elaborated currently (19). The members of the Wnt family are
involved in bone regeneration, including the proliferation and
differentiation of osteoblasts, and their progenitors (20). The Wnt/β-catenin signaling
stimulates the generation of osteoblasts by promoting the
osteogenic differentiation of MSCs, while suppressing the other two
lineages differentiation (21). It
has been shown that MSC osteogenesis can be induced by increasing
the transcription of β-catenin (22). The overexpression of the
Wnt/β-catenin signal in periosteal cells can increase
intramembranous ossification and endochondral ossification
(23,24).
Wnt3a can regulate the proliferation of
differentiated osteoblasts and their progenitors, and promote the
differentiation of MSCs into osteoblasts under appropriate
conditions (25). In addition,
Wnt3a binds to the Frizzled and LRP5 or LRP6 receptor complex,
inhibits GSK-3β, and promotes the accumulation of β-catenin in
osteoblasts (26,27). The accumulated β-catenin
translocates into the nucleus, and together with TCF/LEF, induces
the expression of OPG (28).
Furthermore, Wnt3a exhibits an inhibitory effect on osteoclast
formation, which is mediated through the canonical pathway
(29). The present results also
revealed that LRRC17 may promote osteogenesis by increasing
the Wnt3a levels in patients with INFH.
Wnt5a stimulates non-canonical Wnt signals in
osteoclast precursors, and promotes RANKL-induced osteoclast
precursor differentiation and JNK phosphorylation in osteoclasts.
Furthermore, JNK appears to be involved in the crosstalk between
RANK and Ror2-mediated signals (30,31).
These results suggest that Wnt5a is produced by osteoblasts, and
that this promotes osteoclast differentiation through the
non-canonical Wnt pathway in osteoclast precursors. Together with
the present findings, LRRC17 overexpression may inhibit
RANKL-induced osteoclast differentiation by inhibiting Wnt5a, while
LRRC17 interference may enhance RANKL-induced osteoclast
differentiation by promoting Wnt5a.
The potential mechanism on how LRRC17 regulates Wnt
signaling was investigated. Proteins that contain LRR domains
control a variety of physiological processes, including bone
metabolism. For example, glycan and decorin are highly expressed in
the extracellular bone matrix, affecting the differentiation and
proliferation of osteocytes. Mice with leucine proteoglycan
deficiency develop osteoporosis, which is characterized by failure
to reach the peak bone mass due to decreased bone formation
(8).
One of the limitations of the present study was the
small number of patient samples. In future studies, the present
findings require further validation in hBMSCs derived from a larger
number of patients with INFH or FNF and specimens with different
causes of avascular necrosis.
Another limitations of the present study is the lack
of experiments in the FNF-hBMSCs; the hBMSCs from patients with FNF
should be included in the LRRC17 overexpression or knockout
experiments as a control. Based on the present findings that LRRC17
was decreased in INFH-hBMSCs, it can be anticipated that a similar
trend of changes could be observed in the FNF-hBMSCs, with the
manipulation of LRRC17 as in the INFH-hBMSCs.
In conclusion, the present study is the first to
expound the pathogenesis of INFH from the perspective of bone
metabolism. The modulation of the LRRC17 gene may delay or
even change the process of INFH, providing a new target for the
treatment of osteonecrosis of the femoral head.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
DWW and DS designed and performed most of the
investigation, data analysis, confirm the authenticity of all the
raw data and wrote the manuscript; ZSW and QX provided pathological
assistance; KW, MTX, CZH and CZ contributed to the interpretation
of the data and analyses. All of the authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Liaocheng People's Hospital (approval no. 2018010).
All procedures performed in studies involving human participants
were in accordance with the ethical standards of the institutional
and/or national research committee and with the 1964 Helsinki
declaration and its later amendments or comparable ethical
standards. All patients who participated in this study provided a
signed written informed consent.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Mankin HJ: Nontraumatic necrosis of bone
(osteonecrosis). N Engl J Med. 326:1473–1479. 1992.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Maillefert JF, Tavernier C, Toubeau M and
Brunotte F: Non-traumatic avascular necrosis of the femoral head. J
Bone Joint Surg Am. 78:473–474. 1995.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yamaguchi R, Yamamoto T, Motomura G,
Ikemura S and Iwamoto Y: Incidence of nontraumatic osteonecrosis of
the femoral head in the Japanese population. Arthritis Rheum.
63:3169–3173. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang W, Sun QM, Zhang FQ, Zhang QL, Wang
LG and Wang WJ: Core decompression combined with autologous bone
marrow stem cells versus core decompression alone for patients with
osteonecrosis of the femoral head: A meta-analysis. Int J Surg.
69:23–31. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Andriolo L, Merli G, Tobar C, Altamura SA,
Kon E and Filardo G: Regenerative therapies increase survivorship
of avascular necrosis of the femoral head: A systematic review and
meta-analysis. Int Orthop. 42:1689–1704. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mutijima E, Maertelaer VD, Deprez M,
Malaise M and Hauzeur JP: The apoptosis of osteoblasts and
osteocytes in femoral head osteonecrosis: Its specificity and its
distribution. Clin Rheumatol. 33:1791–1795. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Boregowda SV, Krishnappa V, Strivelli J,
Haga CL, Booker CN and Phinney DG: Basal p53 expression is
indispensable for mesenchymal stem cell integrity. Cell Death
Differ. 25:679–692. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kim T, Kim K, Lee SH, So HS, Lee J, Kim N
and Choi Y: Identification of LRRc17 as a negative regulator of
receptor activator of NF-kappaB ligand (RANKL)-induced osteoclast
differentiation. J Biol Chem. 284:15308–15316. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang A, Ren M, Song Y, Wang X, Wang Q,
Yang Q, Liu H, Du Z, Zhang G and Wang J: MicroRNA expression
profiling of bone marrow mesenchymal stem cells in steroid-induced
osteonecrosis of the femoral head associated with osteogenesis. Med
Sci Monit. 24:1813–1825. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hong N, Kim BJ, Kim CH, Baek KH, Min YK,
Kim DY, Lee SH, Koh JM, Kang MI and Rhee Y: Low plasma level of
leucine-rich repeat-containing 17 (LRRc17) is an independent and
additive risk factor for osteoporotic fractures in postmenopausal
women. J Bone Miner Res. 31:2106–2114. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kim BJ, Lee SH and Koh JM: Potential
biomarkers to improve the prediction of osteoporotic fractures.
Endocrinol Metab (Seoul). 35:55–63. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ficat RP: Idiopathic bone necrosis of the
femoral head. Early diagnosis and treatment. J Bone Joint Surg Br.
67:3–9. 1985.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tan SL, Ahmad TS, Selvaratnam L and
Kamarul T: Isolation, characterization and the multi-lineage
differentiation potential of rabbit bone marrow-derived mesenchymal
stem cells. J Anat. 222:437–450. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ko JY, Wang FS, Wang CJ, Wong T, Chou WY
and Tseng SL: Increased dickkopf-1 expression accelerates bone cell
apoptosis in femoral head osteonecrosis. Bone. 46:584–591.
2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Phetfong J, Sanvoranart T, Nartprayut K,
Nimsanor N, Seenprachawong K, Prachayasittikul V and Supokawej A:
Osteoporosis: The current status of mesenchymal stem cell-based
therapy. Cell Mol Biol Lett. 21(12)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Albers J, Keller J, Baranowsky A, Beil FT,
Catala-Lehnen P, Schulze J, Amling M and Schinke T: Canonical Wnt
signaling inhibits osteoclastogenesis independent of
osteoprotegerin. J Cell Biol. 200:537–549. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tornero-Esteban P, Peralta-Sastre A,
Herranz E, Rodriguez-Rodriguez L, Mucientes A, Abasolo L, Marco F,
Fernandez-Gutierrez B and Lamas JR: Altered expression of wnt
signaling pathway components in osteogenesis of mesenchymal stem
cells in osteoarthritis patients. PLoS One.
10(e0137170)2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Duchartre Y, Kim YM and Kahn M: The Wnt
signaling pathway in cancer. Crit Rev Oncol Hematol. 99:141–149.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen J, Tu X, Esen E, Joeng KS, Lin C,
Arbeit JM, Rüegg MA, Hall MN, Ma L and Long F: WNT7B promotes bone
formation in part through mTORC1. PLoS Genet.
10(e1004145)2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rodda SJ and McMahon AP: Distinct roles
for Hedgehog and canonical Wnt signaling in specification,
differentiation and maintenance of osteoblast progenitors.
Development. 133:3231–3244. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Krause U, Harris S, Green A, Ylostalo J,
Zeitouni S, Lee N and Gregory CA: Pharmaceutical modulation of
canonical Wnt signaling in multipotent stromal cells for improved
osteoinductive therapy. Proc Natl Acad Sci USA. 107:4147–4152.
2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Nagayama M, Iwamoto M, Hargett A, Kamiya
N, Tamamura Y, Young B, Morrison T, Takeuchi H, Pacifici M,
Enomoto-Iwamoto M and Koyama E: Wnt/beta-catenin signaling
regulates cranial base development and growth. J Dent Res.
87:244–249. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tamamura Y, Otani T, Kanatani N, Koyama E,
Kitagaki J, Komori T, Yamada Y, Costantini F, Wakisaka S, Pacifici
M, et al: Developmental regulation of Wnt/beta-catenin signals is
required for growth plate assembly, cartilage integrity, and
endochondral ossification. J Biol Chem. 280:19185–19195.
2005.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cho HH, Kim YJ, Kim SJ, Kim JH, Bae YC, Ba
B and Jung JS: Endogenous Wnt signaling promotes proliferation and
suppresses osteogenic differentiation in human adipose derived
stromal cells. Tissue Eng. 12:111–121. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ring L, Neth P, Weber C, Steffens S and
Faussner A: β-catenin-dependent pathway activation by both
promiscuous ‘canonical’ WNT3a-, and specific ‘noncanonical’ WNT4-
and WNT5a-FZD receptor combinations with strong differences in LRP5
and LRP6 dependency. Cell Signal. 26:260–267. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang S, Chen X, Hu Y, Wu J, Cao Q, Chen S
and Gao Y: All-trans retinoic acid modulates Wnt3A-induced
osteogenic differentiation of mesenchymal stem cells via activating
the PI3K/AKT/GSK3β signalling pathway. Mol Cell Endocrinol.
422:243–253. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yamane T, Kunisada T, Tsukamoto H,
Yamazaki H, Niwa H, Takada S and Hayashi SI: Wnt signaling
regulates hemopoiesis through stromal cells. J Immunol.
167:765–772. 2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Takahashi N, Maeda K, Ishihara A, Uehara S
and Kobayashi Y: Regulatory mechanism of osteoclastogenesis by
RANKL and Wnt signals. Front Biosci (Landmark Ed). 16:21–30.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Oishi I, Suzuki H, Onishi N, Takada R,
Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, et
al: The receptor tyrosine kinase Ror2 is involved in non-canonical
Wnt5a/JNK signalling pathway. Genes Cells. 8:645–654.
2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sato A, Yamamoto H, Sakane H, Koyama H and
Kikuchi A: Wnt5a regulates distinct signalling pathways by binding
to Frizzled2. EMBO J. 29:41–54. 2010.PubMed/NCBI View Article : Google Scholar
|