Introduction
Worldwide, diabetes is one of the fastest growing
health challenges of the 21st century, with the number of adults
diagnosed with diabetes tripling in the last two decades (1-3).
By the year 2045, it is estimated that ~10% of the global
population will be diagnosed with diabetes or pre-diabetes
(1). In the United States, there
are ~27 million diabetic individuals, of which 11.7 million are
females (3,4). In addition to complications such as
nephropathy and cardiovascular disease, diabetic individuals may
also experience ocular complications (5,6). In
total, >50% of individuals diagnosed with diabetes exhibit at
least one ocular surface complication, such as dry eye disease,
delayed corneal epithelial healing or keratopathy, during their
lifetime, highlighting the unmet need for knowledge regarding the
onset and course of diabetic ocular complications (5). Understanding the molecular mechanisms
underlying the onset of these ocular defects may assist in
developing therapeutics to manage them.
The Opioid Growth Factor (OGF)-OGF receptor (OGFr)
pathway is active in corneal tissues, where it functions to
maintain epithelial homeostasis of the cornea (7-9).
Blockade of the OGF-OGFr pathway using naltrexone (NTX), an opioid
antagonist, reverses numerous ocular epithelial complications in
individuals with type 1 (T1D) or type 2 diabetes, including delayed
epithelial wound healing, abnormal corneal sensitivity and low tear
production (10-12).
OGF, which is chemically termed [Met5]-enkephalin, is an
endogenous pentapeptide that reduces cell replication, and its
levels are elevated in diabetic humans (13-15)
and animals (16). In addition, the
effects of differences in the sex of the patient, the pathology of
diabetes and the associated complications are reflected by the fact
that female subjects are more likely to develop certain
complications, whereas they are hormonally protected against
others. Studies have shown the importance of sex hormones in ocular
surface dysfunction, particularly in female subjects (17-19).
At present, to the best of our knowledge, there are no studies
using female animal models of T1D that document the timing and
degree of dysregulation of the OGF-OGFr pathway in the cornea, or
the effect of blockade of this pathway in preventing such
abnormalities. In the present study, female Sprague-Dawley rats
were treated with streptozotocin (STZ) to induce hyperglycemia to
assess whether sex influenced the relationship between the tissue
levels of OGF and OGFr in the corneal epithelium, and the onset and
magnitude of corneal surface complications related to diabetes. The
present study also investigated whether topical NTX treatment in
female T1D rats could block the OGF-OGFr axis and prevent or delay
diabetic complications of the ocular surface.
Materials and methods
Female T1D rat model
In the present study, 6-week old female Sprague
Dawley rats weighing 110-120 g (Charles River, Laboratories, Inc.)
were administered an intraperitoneal (i.p.) injection of 50 mg/kg
STZ (EMD Millipore) dissolved in sodium citrate buffer (pH 4.5),
once per a day for 2 consecutive days. Rats were fasted overnight
prior to injection of STZ. Blood glucose levels >350 mg/dl 48-72
h after induction were indicative of successful establishment of
the T1D rat model. A subset of T1D rats received insulin implants
(T1D-INS; LinShin). The implants (7 mm) were implanted
subcutaneously on the left upper quadrant of the abdomen and were
designed to release insulin for 45±2 days at a rate of 2 U/day.
Control rats received i.p. injections of citrate buffer.
Blood glucose and body weight
Female rats were weighed and blood glucose levels
were monitored on a weekly basis. The body weights, blood glucose
levels, as well as non-invasive corneal surface parameters of 12
rats from each group were evaluated at each time point over the
course of 8 weeks.
Evaluation of ocular surface
complications
Corneal sensitivity and tear production measurements
were assessed on unanesthetized rats, whereas corneal wounding was
assessed under anesthesia. All procedures were performed under
sterile conditions and in accordance with the ARVO Statement for
the Use of Animals in Ophthalmic and Vision Research (10,11).
Corneal sensitivity was evaluated by determining the
blink rate using the Cochet-Bonnet aesthesiometer (Boca Raton) as
described previously (11). The
test was performed by obtaining three readings/eye/time point. The
aesthesiometer pressure (g/mm2) was determined directly
using the conversion table from the protocol supplied by the
manufacturer.
Tear production was determined by inserting Schirmer
strips (Alcon) into the lower lid, proximal to the lateral canthus
for 1 min, to evaluate the wetting length to the nearest half mm,
using a scale provided by the manufacturer. The Schirmer strips
were cut into 1x17 mm strips in a sterile environment and inserted
into the cul-de-sac of unanesthetized rats, as described previously
(11).
Corneal epithelial wound healing was determined
following corneal abrasion (12).
Surgeries were performed between 8 and 9 AM to prevent disparities
in diurnal rhythm. Rats were anesthetized with a 0.1 ml cocktail of
ketamine (100 mg/kg), xylazine (10 mg/kg) and acepromazine (1
mg/kg) delivered by i.p. injection. A topical anesthetic,
proparacaine-HCl (0.5%; Bausch and Lomb), was applied to the cornea
prior to creating a 5-mm diameter abrasion in the right eye using a
dermal trephine (Acuderm). The area of epithelium was abraded
without penetrating below the level of the epithelium. The outlined
epithelium was removed without damaging the underlying corneal
tissue. Beginning at 16 h, rats were anesthetized with 3%
isoflurane (VEDCO) regulated with oxygen flow from a certified
vaporizer, and the eyes were stained with fluorescein strips and
imaged using cobalt blue filtered light to assess wound closure.
Rats were not imaged prior to this 16 h period in order to ensure
proper initiation of the epithelial wound healing process. Baseline
images were taken at time 0, 16, 24, 32, 40 and 48 h following
wounding, and wound closure was documented as a percentage of the
original wound area. After surgery, and as required, rats received
buprenorphine (0.025 mg/kg; i.p.) every 8-12 h to offset any signs
of pain (12).
Levels of OGF and OGFr in ocular
tissue
Rats were humanely euthanized using an overdose of
sodium pentobarbital (>150 mg/kg). The eyes were removed from
the orbital cavity, processed and immunohistochemically stained
with OGF (Penn State University, 1:200) and OGFr (MyBioSource
MBS2124207, 1:150) antibodies as described previously (16). Frozen corneal sections (6-8 µm) from
each group were stained following previously published protocols
(7,11,12,16);
control sections were stained with the secondary antibody only
(Alexa Flour568; Invitrogen; Thermo Fisher Scientific, Inc.).
Stained sections were imaged using a Keyence Scope (KEYENCE, Ltd.)
and further quantified using ImageJ 1.8.0_172 (National Institutes
of Health) to quantify optical density (OD).
Serum levels of OGF, OGFr and the enkephalinase CD10
(also known as neprilysin) were measured in female rats using ELISA
kits (MyBioSource) for CD10 (cat. no. MBS764927), OGF (cat. no.
MBS756126) and OGFr (cat. no. MBS109224) (16). ELISA kits were specifically designed
for rat serum, and experiments were performed according to the
manufacturer's protocol. In order to ensure rigor and
reproducibility, serum samples were utilized from multiple
experiments and samples were replicated from other assays as
additional positive controls. A minimum of three repeats of ELISA
were performed with replicate samples from 6-8 different female
rats at each time point.
Blockade of the OGF-OGFr pathway:
Mechanistic approach to therapy
In order to evaluate the mechanisms underlying the
interactions between ocular surface complications and the OGF-OGFr
pathway, experiments were designed to block this pathway using NTX.
Whether continuous opioid receptor blockade using topical NTX would
prevent or substantially reduce the magnitude of ocular surface
complications in female diabetic rats was assessed. A cohort of T1D
female rats received one drop (0.05 ml) of 5x10-5 M NTX
dissolved in saline (pH 7.0; T1DTopNTX-2Drop group)
twice daily, as described previously (11,12,16).
Each drop was administered between 8 and 9 AM as well as between 3
and 4 PM for 4 weeks. For comparison, female rats in the T1D,
T1D-INS and non-diabetic control groups not receiving NTX were
included. The dose of NTX was based on previous studies which are
reviewed elsewhere (8), and was
considered an effective dosage to ensure prolonged blockade of the
OGF-OGFr axis.
Statistical analysis
A total of five independent experiments were
performed. Multiple experiments were performed with a smaller
number of rats per group in order to ensure rigor and
reproducibility. For all parameters assessed, 12-15 rats per
treatment condition were evaluated. Data were analyzed using a one-
or two-way ANOVA. If interactions were significant, a Tukey
post-hoc test was used for multiple comparisons. Analysis was
performed using GraphPad Prism version 8.0 (GraphPad Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Female T1D rat model phenotype
Female STZ-treated rats presented with a blood
glucose level >300 mg/dl within 48-72 h post-STZ injection.
Insulin implants were introduced into 13 rats. Of the 64 rats
injected with STZ, five rats did not become hyperglycemic and four
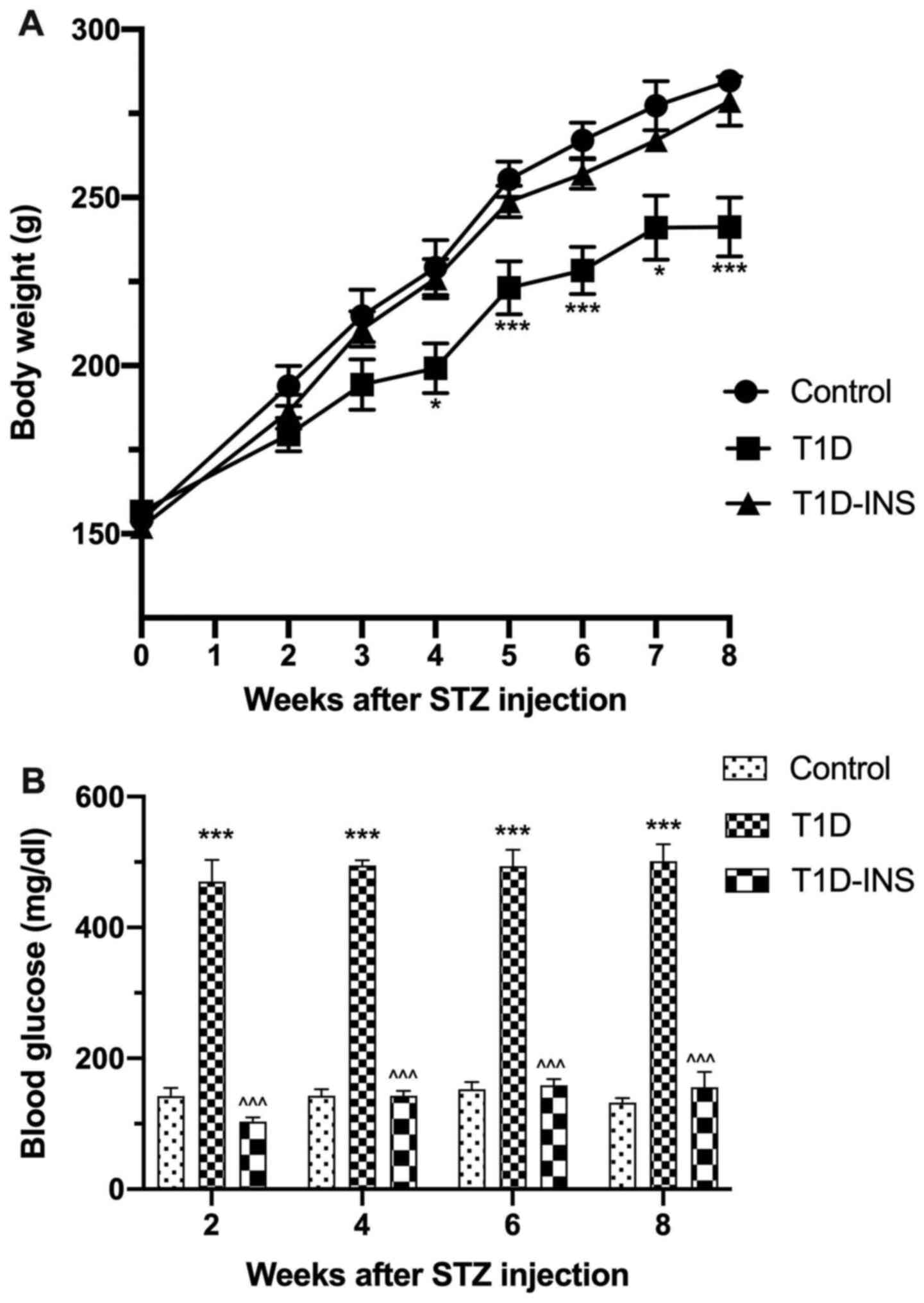
rats died within the first 2 weeks of STZ exposure. Fig. 1A and B present the body weights and blood
glucose levels of the rats, respectively, showing that female T1D
rats gained less weight over the 8-week period relative to the
control and T1D-INS female rats. The body weights of the control
female rats increased by a mean of 154 g from baseline to 284 g by
week 8, whereas the T1D rats only exhibited an increase to 241 g by
week 8. T1D-INS rats weighed ~278 g by week 8. Blood glucose levels
of the control rats ranged between 130-150 mg/dl, whereas glucose
levels were measured in the T1D between 456-470 mg/dl. Glucose
levels in the T1D-INS rats ranged between 103-155 mg/dl.
Ocular surface defects in the T1D
female rats
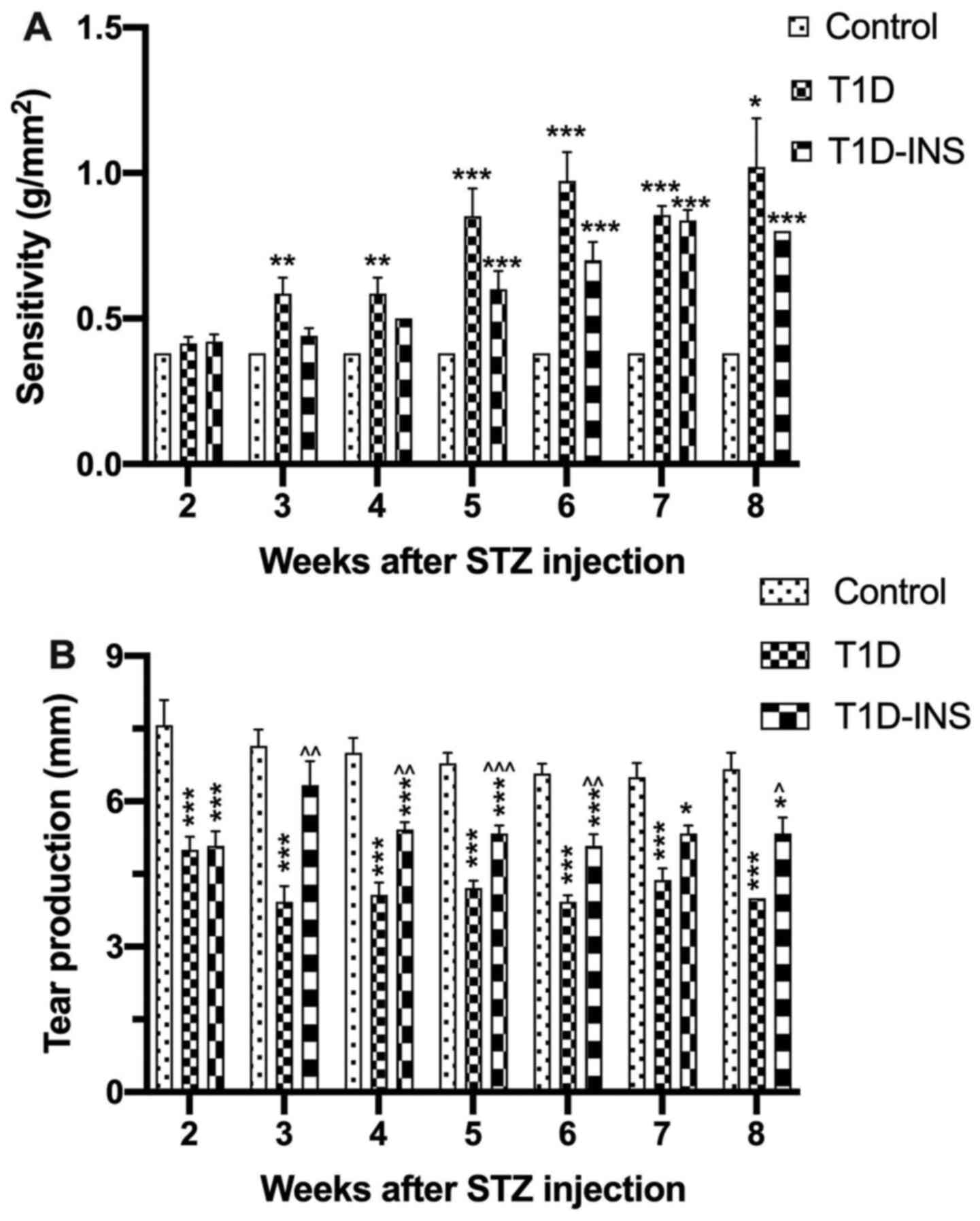
Beginning at week 2 post-STZ injection, corneal
surface sensitivity (Fig. 2A) and
tear production (Fig. 2B) were
evaluated weekly. On week 4, the results showed that the onset of
significant changes in esthesiometry in T1D rats significantly
decreased compared with the control group. Both the uncontrolled
and controlled diabetic groups had reduced corneal surface
sensitivity on weeks 5-8, requiring greater pressure to induce a
positive blink response. Female T1D rats required ~0.58
g/mm2 pressure on week 4 and 1.02 g/mm2
pressure by week 8 to elicit a blink response. The pressure
required for the T1D-INS rats was ~0.50 g/mm2 at week 4,
but this increased to 0.8 g/mm2 by week 8, whereas the
control rats required a constant pressure of 0.38 g/mm2
throughout the entirety of the study (Fig. 2A). Tear production was used to
assess dry eye conditions, and was measured as the length of
wetting on the Schirmer strips. Tear production for the control
rats ranged between 6.5-7.6 mm wetting distance, whereas both T1D
and T1D-INS had decreased tear production 2 weeks after STZ
exposure. T1D rats exhibited a steady decline in tear production
from week 2 starting at an average of 5 mm and dropping to 4 mm on
week 8, whereas the T1D-INS rats exhibited wetting distances
between 5.5-6.5 mm over the 8 weeks.
Rate of corneal surface healing in the
female T1D model
Corneal surface wound healing was assessed at week 6
following the creation of a 5-mm central corneal epithelial wound
(Fig. 3A). At 16 h post wounding,
the T1D rats exhibited significantly delayed wound healing compared
with the control rats. The T1D rats had 80% of their original wound
still present, whereas the control rats had only 34% of their
original wound area present. After 40 h, the wounds in the control
rats were completely healed (<1% residual wound), whereas in the
T1D group, 40% of the wound area remained, and in the T1D-INS
group, 10% remained (Fig. 3B).
Wound closure did not differ between the T1D-INS rats and the
control group, suggesting that systemic administration of insulin
may improve re-epithelialization of the cornea.
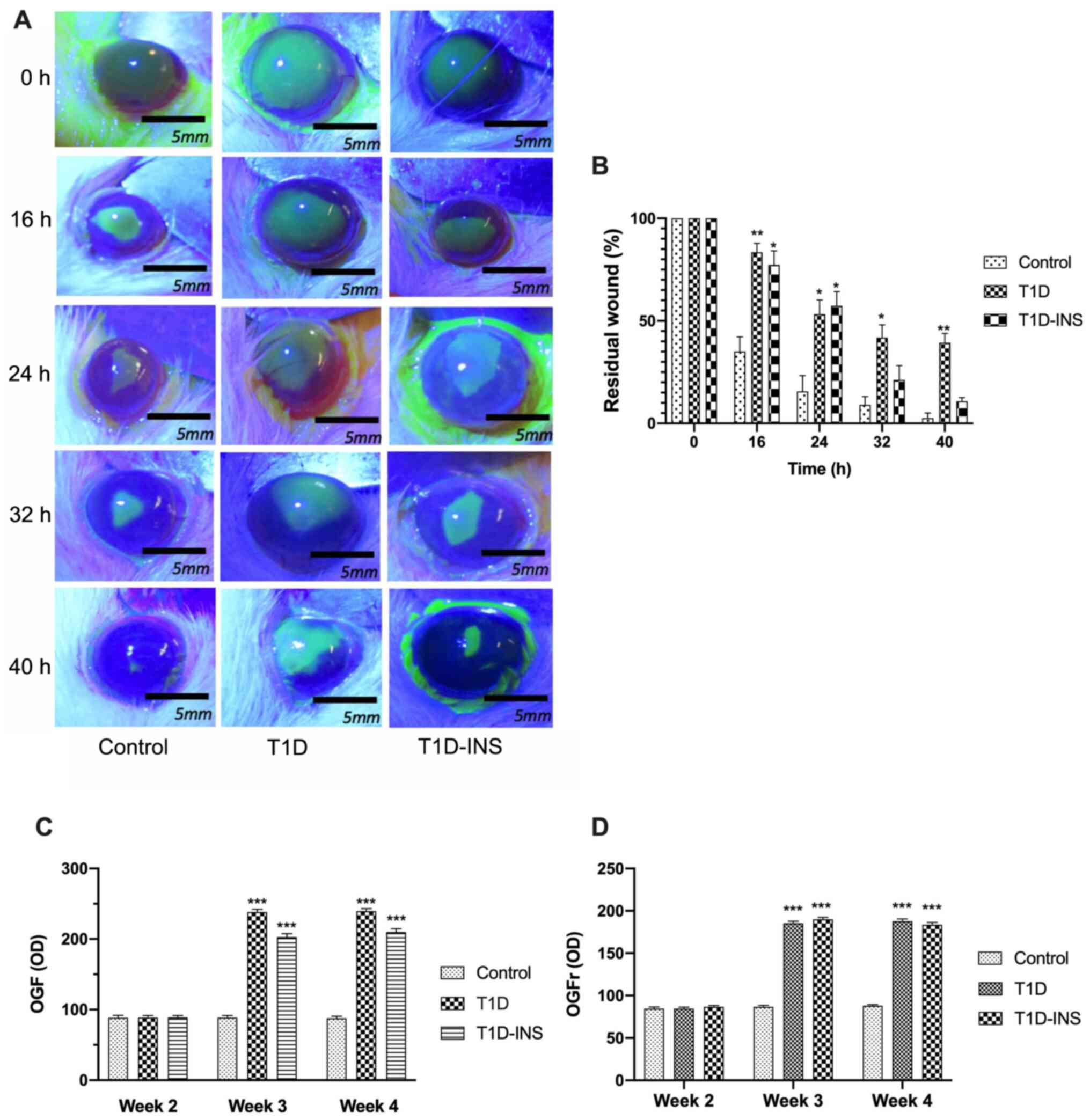 | Figure 3Photographs and percent residual
corneal defects in T1D, T1D-INS and control rats. (A) Photographs
(scale bar, 5 mm) and (B) percent residual corneal defects in T1D,
T1D-INS and control rats. Eyes were stained with fluorescein and
photographed prior to wounding (0 h) and at 16, 24, 32 and 40 h
after abrasion. Images with residual defects underwent areal
analyses and are presented as the percentage of the original wound
for the same rat until healed. (C) Expression patterns of OGF and
OGFr. Immunohistochemically stained sections of corneal epithelium
at 2, 3 and 4 weeks following hyperglycemia in control, T1D and
T1D-INS rats. Images were scanned, and (C) OGF and (D) OGFr
expression was determined using ImageJ calibrated to measure OD.
Values represent the mean ± SE for 20 readings from 3 slides from
each of 3 tissue specimens. *P<0.05,
**P<0.01 and ***P<0.001 vs. control.
OD, optical density; T1D, type 1 diabetes; INS, insulin; OGF,
opioid growth factor; OGFr, OGF receptor. |
Ocular surface tissue and serum OGF
and OGFr expression levels
The expression levels of OGF and OGFr in corneal
epithelial tissues were semi-quantitatively measured after 2, 3 and
4 weeks (Fig 3C and D). OGF expression was ~88 OD units in all
groups at week 2. OGF expression levels were significantly elevated
in both diabetic groups at week 3 and 4; 239 OD units in the T1D
rats and 203 OD units in the T1D-INS rats. Similarly, OGFr
expression levels in the corneal epithelium were comparable between
all groups at week 2. OGFr expression at week 3 post-STZ in the
control rats was ~86 OD units, and was elevated in both diabetic
groups. Expression of OGFr in corneal epithelial tissue increased
to >95 OD units by week 8 in both T1D and T1D-INS groups.
Insulin treatment did not reduce the OGF or OGFr levels in the
diabetic rats.
Serum OGF levels of the control, T1D and T1D-INS
groups were comparable to each other 2 weeks after STZ injection
(Fig. 4A). At week 3, T1D and
T1D-INS OGF levels increased, and by week 4 they were 3-fold
greater than the OGF values at baseline or in the non-diabetic
group. Serum OGF levels in the T1D rats remained elevated
throughout the study. T1D-INS rats had elevated OGF serum levels
compared with the control group, with significantly higher values
at weeks 4 and 8. Relative to OGF values, OGFr serum levels were
~1,000-fold higher in female rats (Fig.
4B). Mean OGFr levels for the control female rats ranged from
2.4-3.5 ng/ml over an 8-week period. A two-way ANOVA did not reveal
a significant difference between time point and treatment. OGFr
values were lower in the T1D group compared with the control group
at all time points, and this decrease was significant at weeks 2,
3, 4 and 8. T1D-INS rats had comparable levels to the control rats,
except at week 4 when there was a significant decrease in OGFr
levels detected in the serum of the T1D-INS rats. CD10 is an
enkephalinase that metabolizes OGF. At weeks 4 and 8 post-STZ
injection, controlled and uncontrolled diabetic rats had a
significantly reduced CD10 concentration relative to the control
level of ~16 ng/µl, with the levels in the diabetic rats being
~2-fold less (Fig. 4C).
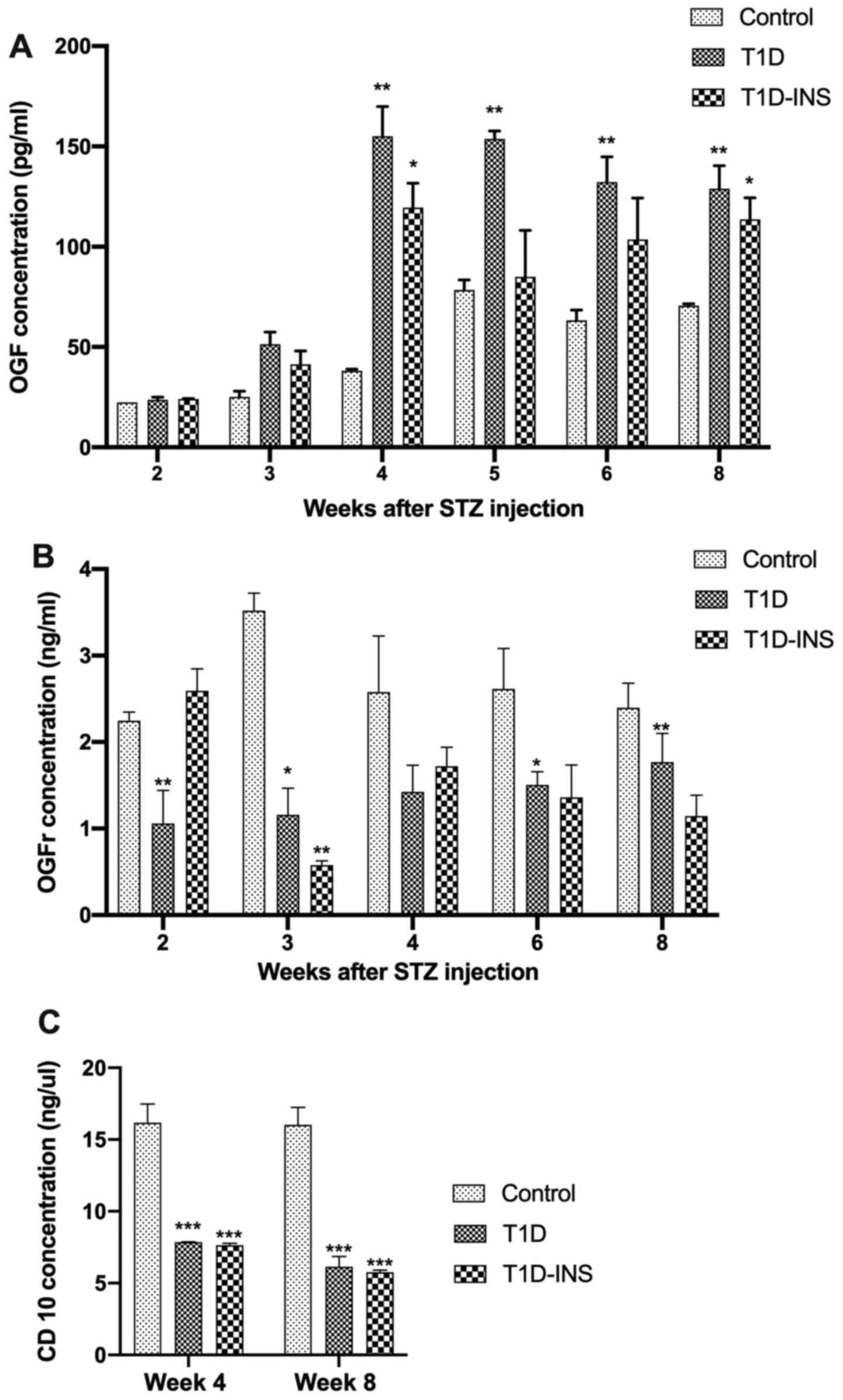 | Figure 4Serum levels of female T1D, T1D-INS
and control rats. Serum levels of (A) OGF, (B) OGFr and (C) CD10
were measured weekly following hyperglycemia in control, T1D and
T1D-INS female rats. The data are presented as the mean ± SE for
values from 4 different ELISA tests, and were analyzed using
one-way ANOVA and Tukey's post-hoc test. *P<0.05,
**P<0.01 and ***P<0.001 vs. control.
T1D, type 1 diabetes; INS, insulin; STZ, streptozotocin; OGF,
opioid growth factor; OGFr, OGF receptor. |
Treatment of ocular surface
complications by blocking the OGF-OGFr pathway
The second part of this study focused on the impact
of topical NTX treatment to block the interaction between OGF and
OGFr, and to determine whether dysregulation of this pathway led to
ocular surface defects. At 2, 3 and 4 weeks post-STZ injection,
corneal surface sensitivity (Fig.
5A) and tear production (Fig.
5B) were evaluated. All groups recorded a required pressure of
0.38 g/mm2 at week 2 for eliciting a blink response. T1D
rats demonstrated significantly decreased corneal sensitivity on
weeks 3 and 4 relative to the control, requiring twice the pressure
to elicit a positive blink response. T1D-INS rats had comparable
sensitivity to the control rats in the first 4 weeks post-STZ.
However, T1DTopNTX-2Drop rats were comparable to the
control rats throughout the entirety of the study. Measurement of
tear volume showed the rats in the control group had Schirmer strip
wetting measurements of 7-8 mm over the course of 4 weeks. Female
T1D and T1D-INS rats exhibited reduced tear production beginning on
week 2, and it ranged between 4-5 mm in week 4 post-STZ. However,
topical administration of NTX restored tear production, such that
rats in the T1DTopNTX-2Drop group had tear volume
production levels comparable to the control rats at all time
points, ranging from 6.8-8.0 mm. T1DTopNTX-2Drop values
were significantly higher than that in the T1D rats at weeks 2, 3
and 4, and from T1D-INS rats at week 4. Serum levels of OGF
(Fig. 5C) and OGFr (Fig. 5D) were monitored only at week 4. The
results showed that all diabetic groups exhibited significantly
increased serum levels of OGF relative to the levels observed in
the control rats (~47 pg/ml). Rats in the T1D, T1D-INS and
T1DTopNTX-2Drop groups exhibited a >2-fold increase
in the serum levels of OGF, with values of ~154, ~125 and 137
pg/ml, respectively. OGFr serum concentrations did not differ
amongst groups (Fig. 5D).
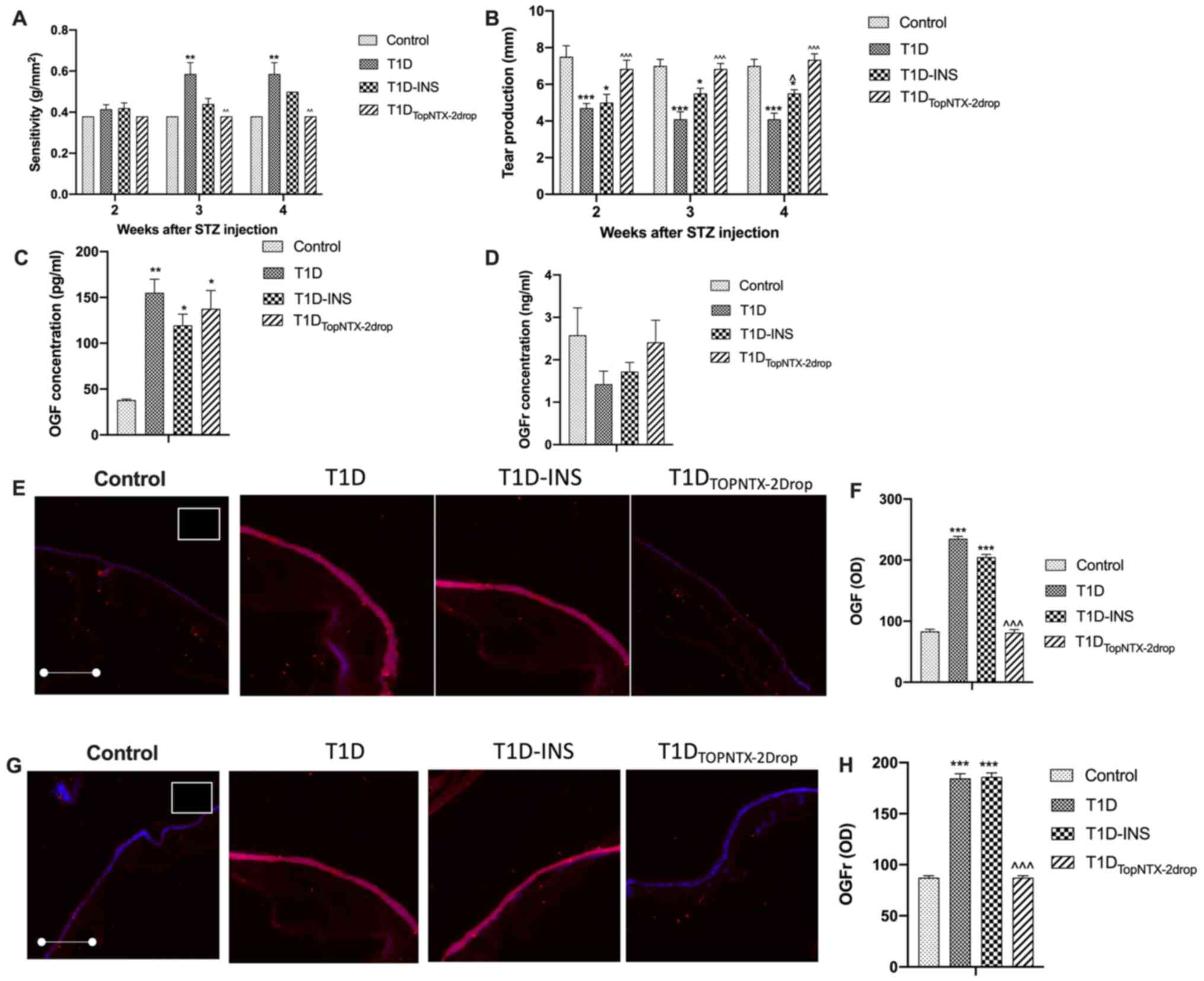 | Figure 5Effects of receptor blockade by NTX on
corneal surface defects in female T1D, T1D-INS,
T1DTopNTX-2Drop and control rats. (A) Corneal surface
sensitivity was measured with an aesthesiometer and 4 readings of
pressure were averaged at each time point for each rat and
converted to sensitivity using the conversion table provided by the
manufacturer (Cochet-Bonnet; Boca Raton). (B) Tear production was
measured by the Schirmer tear test. Values are presented as the
mean ± SE for female rates randomly chosen at each time point from
all groups (n≥13 per group). Serum levels of (C) OGF and (D) OGFr
were measured at 4 weeks following hyperglycemia in control, T1D,
T1D-INS and T1DTopNTX-2Drop female rats. (E and F) OGF
and (G and H) OGFr expression in intact corneal epithelium at 4
weeks following hyperglycemia in control, T1D, T1D-INS and
T1DTopNTX-2Drop female rats. Images were taken at x10
magnification. Tissue stained with only secondary antibody is shown
in the white outlined insert located in control images in the upper
right corner. Quantitative evaluation of OGF and OGFr expression
was based on scanned confocal images and evaluated using ImageJ
software. Values are presented as the mean ± SE for 18-20 readings
collected from ≥4 tissue specimens. *P<0.05,
**P<0.01 and ***P<0.001 vs. control;
^P<0.05, ^P<0.01 and
^^^P<0.001 vs. T1D. T1D, type 1 diabetes; INS,
insulin; STZ, streptozotocin; OGF, opioid growth factor; OGFr, OGF
receptor; NTX, naltrexone; OD, optical density. |
OGF (Fig. 5E and
F) and OGFr (Fig. 5G and H) expression levels in the corneal
epithelial tissue were also assessed on week 4 in the corneal
epithelium treated topically with NTX. Compared with the expression
levels of the control rats, the levels of OGF in the T1D and
T1D-INS rat tissues were significantly increased, with levels of
~219 and 202 OD units, respectively, whereas the
T1DTopNTX-2Drop NTX-treated rats had OGF levels
comparable to that of the control rats (~87 OD units). The value of
the T1DTopNTX-2Drop group was 92 OD units, and this was
significantly lower compared with the T1D rats. Similarly, OGFr
expression levels were elevated in the corneal epithelium of T1D
and T1D-INS rats (180 and 192 OD units, respectively) compared with
the control rats (80 OD units). T1DTopNTX-2Drop treated
rats had a substantially reduced OGFr value of 88 OD units, similar
to that of the control rats, and significantly lower than that of
the T1D rats.
Discussion
In the present study, an association between ocular
surface abnormalities and the dysregulation of the OGF-OGFr pathway
in female diabetic rats was demonstrated. In STZ-induced
hyperglycemic female rats, systemic administration of insulin
prevented elevations in blood glucose levels and weight loss
associated with hyperglycemia over an 8-week period. Systemic
insulin treatment in female diabetic rats resulted in Control rates
of corneal epithelial wound healing, but did not protect against
other ocular surface complications. The present study is the first
to report the relative expression of OGF and OGFr in the corneal
epithelium over the course of 8 weeks, in uncontrolled and insulin
controlled diabetic female rats. Systemic insulin treatment did not
prevent the increase in OGF or OGFr expression in corneal
epithelium, nor did it reduce the magnitude of ocular
complications. The efficacy of OGF-OGFr blockade with NTX was
assessed in female rats. NTX treatment was capable of restoring
tear production and corneal surface sensitivity, and appeared to be
more effective than insulin treatment in addressing the ocular
surface abnormalities.
In a previous study using the diabetic male rat
model, tear production and corneal sensitivity were altered 4 weeks
after STZ injection, with a corresponding increase in the serum
levels of OGF (16), whereas the
onset of these ocular surface complications occurred earlier in the
diabetic female animals. Female T1D and T1D-INS rats exhibited
significant abnormalities with regard to corneal sensitivity and
tear production 2 weeks after STZ injections relative to the male
rats after 4 weeks (16). In the
present study, female T1D rats healed in ~50% of the time it took
for the male T1D rats to heal.
The interaction between insulin and the OGF-OGFr
pathway is unknown. The findings in the present study, as well as
data on male rats (16), suggest
that there are potentially two pathways modulating corneal surface
complications in diabetes. One pathway is related to the delayed
epithelial wound healing, which appears to benefit from systemic
administration of insulin, as female rats in the T1D-INS group
exhibited relatively normal rates of repair. In the present and
previous studies (20,21), insulin provided a level of
protection against delayed re-epithelialization. However, control
of hyperglycemia through insulin did not affect the decrease in
tear production or altered corneal sensitivity. Moreover, OGF and
OGFr levels were dysregulated in both controlled and uncontrolled
T1D, suggesting that a separate upstream pathway caused
dysregulation of the OGF-OGFr axis, which then subsequently altered
peptide and receptor levels.
The elevated levels of OGF and OGFr in epithelial
tissue occurred earlier in diabetic female animals compared with
the male T1D animals, and the severity of the ocular defects were
increased 3-fold compared with the magnitude recorded in male T1D
animals (16). There were
differences in the serum OGF and OGFr levels based on sex; baseline
serum OGF levels in female animals were twice that of males
(16), and hyperglycemia resulted
in an increase in OGF levels in female T1D animals that were almost
3-fold greater than the increase observed in the male T1D rats, and
>6-fold higher than that of the baseline. These data suggest
that sex serves a role in the onset and magnitude of diabetic
ocular complications, and this observation is supported by previous
studies (16,19,21,22).
Thus, additional investigations into the role that male and female
hormones serve in the regulation of the OGF-OGFr pathway are
required.
Information on the OGF-OGFr pathway in female rats
is limited, and the impact of dysregulation of the OGF-OGFr pathway
in female T1D rats remains to be assessed. Blockade of the OGF-OGFr
pathway using the opioid receptor antagonist NTX, reversed ocular
surface abnormalities in a male diabetic animal model (10-12,16),
leading to the hypothesis that corneal surface abnormalities and
the dysregulation of the OGF-OGFr pathway may also be correlated
with a female diabetic rat model. However, there are sex
differences that result in varied responses between male and female
mice. To the best of our knowledge, the present study was the first
to show an association between the onset of ocular surface
abnormalities and elevated OGF serum levels in female diabetic
rats. Furthermore, it was the first study to investigate continuous
blockade of OGFr using topical administration of NTX as a treatment
for ocular surface complications in female T1D rats. These findings
extend and corroborate those of a previous study, which used a male
diabetic animal model (16), and
suggest that both male and female rats with hyperglycemia are
vulnerable to the effects of a dysregulated OGF-OGFr pathway.
Moreover, topically administered NTX is a safe and effective
treatment modality (8,23), and based on the results of the
present study, may also be an effective means of preventing and/or
treating ocular surface complications associated with diabetes in
female rats.
In conclusion, the results of the present study
support the hypothesis that dysregulation of the OGF-OGFr pathway
in female T1D rats is associated with the onset of ocular surface
complications, and that blockade of this pathway via topical
administration of NTX prevents and/or reduces the magnitude of dry
eye and corneal surface sensitivity. These results may assist in
the discovery of specialized treatment options for diabetic female
patients, who are disproportionately affected by corneal surface
complications.
Acknowledgements
The authors would like to thank Dr Chirag L. Patel
from Penn State College of Medicine (Hershey, USA) for his support
during this study.
Funding
Funding: The present study was supported by a grant from the
National Institutes of Health [grant no. EY029223 (ISZ)].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ISZ, PJM and JWS conceived the study. IP performed
the experiments, and acquired and analyzed the data. PJM and ISZ
confirm the authenticity of all the raw data. IP, JWS, ISZ and PJM
interpreted the data, and wrote and revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study (protocol 47207) was approved by
the Penn State College of Medicine Institutional Animal Care and
Use Committee (Hershey, USA).
Patient consent for publication
Not applicable.
Competing interests
PJM, ISZ and JSW have intellectual property owned by
Penn State Research Foundation that involves naltrexone treatment
of the ocular surface, but receive no financial compensation or
royalties. IP declares to have no competing interests.
References
|
1
|
Cho NH, Shaw JE, Karuranga S, Huang Y, da
Rocha Fernandes JD, Ohlrogge AW and Malanda B: IDF diabetes atlas:
Global estimates of diabetes prevalence for 2017 and projections
for 2045. Diabetes Res Clin Pract. 138:271–281. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
International Diabetes Federation.
Worldwide toll of diabetes. IDF Diabetes Atlas, 9th edition. 2019.
https://www.diabetesatlas.org/en/.
Accessed December 2020.
|
|
3
|
Centers for Disease Control and
Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA:
Centers for Disease Control and Prevention, U.S. Department of
Health and Human Services. Available from: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
Accessed January 2021.
|
|
4
|
Moeineslam M, Amiri P, Karimi M,
Jalali-Farahani S, Shiva N and Azizi F: Diabetes in women and
health-related quality of life in the whole family: A structural
equation modeling. Health Qual Life Outcomes.
17(178)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
American Diabetes Association.
Complications. https://www.diabetes.org/diabetes/complications
Accessed December 2020.
|
|
6
|
Vieira-Potter VJ, Karamichos D and Lee DJ:
Ocular complications of diabetes and therapeutic approaches. Biomed
Res Int. 2016(3801570)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zagon IS, Sassani JW and McLaughlin PJ:
Reepithelialization of the human cornea is regulated by endogenous
opioids. Invest Ophthalmol Vis Sci. 41:73–81. 2000.PubMed/NCBI
|
|
8
|
Sassani JW, McLaughlin PJ and Zagon IS:
The yin and yang of the opioid growth regulatory system: Focus on
diabetes-the Lorenz E. Zimmerman tribute lecture. J Diabetes Res.
2016(9703729)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zagon IS, Sassani JW, Verderame MF and
McLaughlin PJ: Particle-mediated gene transfer of OGFr cDNA
regulates cell proliferation of the corneal epithelium. Cornea.
24:614–619. 2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zagon IS, Sassani JW, Immonen JA and
McLaughlin PJ: Ocular surface abnormalities related to type 2
diabetes are reversed by the opioid antagonist naltrexone. Clin Exp
Ophthalmol. 42:159–168. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zagon IS, Klocek MS, Sassani JW and
McLaughlin PJ: Dry eye reversal and corneal sensation restoration
with topical naltrexone in diabetes mellitus. Arch Ophthalmol.
127:1468–1473. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Klocek MS, Sassani JW, McLaughlin PJ and
Zagon IS: Topically applied naltrexone restores corneal
reepithelialization in diabetic rats. J Ocul Pharmacol Ther.
23:89–102. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Negri M, Fallucca F, Tonnarini G, Mariani
P, D'alessandro M and Pachí A: High levels of circulating
met-enkephalin in pregnant and menstruating type 1 diabetic women.
Gynecol Endocrinol. 4:25–31. 1990.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fallucca F, Tonnarini G, Di Biase N,
D'Alessandro M and Negri M: Plasma met-enkephalin levels in
diabetic patients: Influence of autonomic neuropathy. Metabolism.
45:1065–1068. 1996.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Negri M, Tonnarini G, D'Alessandro M and
Fallucca F: Plasma met-enkephalin in type I diabetes. Metabolism.
41:460–461. 1992.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zagon IS, Sassani JW, Purushothaman I and
McLaughlin PJ: Dysregulation of the OGF-OGFr pathway correlates
with elevated serum OGF and ocular surface complications in the
diabetic rat. Exp Biol Med (Maywood). 245:1414–1421.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Vistisen D, Witte DR, Tabák AG, Brunner
EJ, Kivimäki M and Færch K: Sex differences in glucose and insulin
trajectories prior to diabetes diagnosis: The Whitehall II study.
Acta Diabetol. 51:315–319. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bowden MA, Tesch GH, Julius TL, Rosli S,
Love JE and Ritchie RH: Earlier onset of diabesity-induced adverse
cardiac remodeling in female compared to male mice. Obesity (Silver
Spring). 23:1166–1177. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gagliano C, Caruso S, Napolitao G,
Malaguarnera G, Cicinelli NV, Amato R, Reibaldi M, Incarbone G,
Bucolo C, Drago F and Avitabile T: Low levels of 17-β-oestradiol,
oestrone and testosterone correlate with severe evaporative
dysfunctional tear syndrome in postmenopausal women: A case-control
study. Br J Ophthalmol. 98:371–376. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Klocek MS, Sassani JW, McLaughlin PJ and
Zagon IS: Naltrexone and insulin are independently effective but
not additive in accelerating corneal epithelial healing in type I
diabetic rats. Exp Eye Res. 89:686–692. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zagon IS, Sassani JW and McLaughlin PJ:
Insulin treatment ameliorates impaired corneal reepithelialization
in diabetic rats. Diabetes. 55:1141–1147. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chandramouli C, Reichelt ME, Curl CL,
Varma U, Bienvenu LA, Koutsifeli P, Raaijmakers AJA, De Blasio MJ,
Qin CX, Jenkins AJ, et al: Diastoli dysfunction is more apparent in
STZ-induced diabetic female mice, despite less pronounced
hyperglycemia. Sci Rep. 8(2346)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
McLaughlin PJ, Sassani JW, Titunick MB and
Zagon IS: Efficacy and safety of a novel naltrexone treatment for
dry eye in type 1 diabetes. BMC Ophthalmol. 19(35)2019.PubMed/NCBI View Article : Google Scholar
|