Introduction
Esophageal cancer, one of the most common
malignancies worldwide, can be stratified according to its
pathology into two subtypes: Esophageal squamous cell carcinoma
(ESCC) and esophageal adenocarcinoma (1,2). At
present, the majority of esophageal cancers take the form of ESCC
(3). The early clinical symptoms of
ESCC are not obvious, while at the late stages the disease is
associated with lymphatic and hematogenous metastasis (4). In recent years, progress has been made
in the screening, diagnosis and treatment of ESCC; however, its
incidence is still very high and the prognosis remains not ideal,
accompanied by a lower survival rate (5). Due to the lack of biomarkers for early
detection and prognosis (6), the
5-year survival rate is <10% after treatment with surgery and
chemoradiotherapy (7). Therefore,
it is important to identify accurate clinical diagnosis and
prognostic markers for ESCC, and to establish new targets for the
treatment of these tumors.
The spermatogenesis associated serine rich 2
(SPATS2) gene product is a predicted cytoplasmic RNA binding
protein that has been reported to serve a tumorigenic role in
several types of tumors, such as colorectal cancer and prostate
cancer (8,9). A previous study found that SPATS2 was
significantly upregulated in colorectal cancer and promoted cancer
cell survival by targeting small nucleolar RNA host gene 5(9). Ngollo et al (10) reported that SPATS2 was enriched and
interacted with H3K27me3 to promote prostate cancer aggressiveness.
In lung cancer, Takamochi et al (11) discovered that SPATS2 acted as a
marker to discriminate squamous cell carcinoma and adenocarcinoma.
SPATS2L, an important paralog of SPATS2, is potentially associated
with ribosomal processes and translational control (12), and ribosomal biogenesis has been
demonstrated to regulate cell growth and proliferation (13). Meanwhile, SPATS2L was also
established to serve a role in the occurrence and progression of
hepatocellular carcinoma, in combination with microRNA-1269a
(14). However, the function of
SPATS2 in ESCC remains largely unclear.
The present study aimed to investigate the
relationship between the expression of SPATS2 and the prognosis of
patients with ESCC. In addition, the regulation of SPATS2 on the
proliferation, apoptosis, invasion and migration of ESCC cells was
also explored in this study.
Materials and methods
Data acquisition
Expression patterns of SPATS2 in ESCC tissues were
assessed using data obtained from the Oncomine database (version
4.5, https://www.oncomine.org; dataset
GSE20347) including 17 esophageal and 17 ESCC tissues. Additional
data from The Cancer Genome Atlas (TCGA; https://cancergenome.nih.gov/) database containing 182
ESCC tissues and 13 normal tissues (adjacent to the tumor) were
used to confirm the expression of SPATS2 in ESCC. The expression of
SPATS2 in ESCC was further compared in an analysis using tumor
tissue data from the TCGA database (n=182) and normal tissues from
the TCGA database (n=13) and samples from the Genotype-Tissue
Expression (GTEx) database (n=273). The data from the TCGA and GTEx
databases was processed using the Gene Expression Profiling
Interactive Analysis (GEPIA1; http://gepia.cancer-pku.cn/) website. Prognostic
analysis directed at SPATS2 in patients with ESCC was acquired from
the GEPIA website.
Cell culture
Cell lines, including the ESCC cell lines Eca109,
KYSE-150 and TE-1, and normal control cell line human esophageal
epithelial cells (HEEC) were purchased from The Cell Bank of Type
Culture Collection of the Chinese Academy of Sciences. Cells were
incubated in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.)
culture solution with 10% fetal bovine serum (FBS, Gibco; Thermo
Fisher Scientific, Inc.), 100 U/ml penicillin (Shanghai Qiaoxing
Trading Co., Ltd.; http://www.bridge-star.com/qiaoxing2012-Products-18067360/)
and 0.1 mg/ml streptomycin (Shanghai Qiaoxing Trading Co., Ltd,
China) at 37˚C and 5% CO2.
Cell transfection
Lipofectamine 2000 transfection reagent (Thermo
Fisher Scientific, Inc.) was used to transfect Eca109 and KY-SE150
cells in the logarithmic growth stage, according to the
manufacturer's guidelines. After 24 h, transfection efficiency was
determined and subsequent experiments were performed. The sequences
of small interfering si-SPATS2 (20 nM) and si-control (con; 25 nM)
were synthesized by Shanghai GenePharma Co., Ltd., and the primer
sequences were as follows: si-SPATS2#1:
5'-GCACTTTGTTAGTGAACGTAA-3'; si-SPATS2#2:
5'-CCCGATGTAGCTCAGTTACAT-3' and si-con:
5'-AATTCTCCGAACGGTCACGT-3'.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from Eca109 and KY-SE150
cells using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and then reverse transcribed into cDNA using
SuperScript III reverse transcriptase (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
thermocycling conditions of the RT reaction was as follows: Initial
annealing at 37˚C for 8 min, extension at 42˚C for 1 h and
termination at 85˚C for 2 min. SYBR Green Master Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.) was used to determine
the relative expression of mRNA. The thermocycling procedure was
incubation at 95˚C for 5 min, followed by 40 cycles of 95˚C for 30
sec and 60˚C for 45 sec before a further 72˚C incubation for 30
min. GAPDH was used as an internal control to evaluate mRNA
expression. The relative expression of mRNA was analyzed using the
2-ΔΔCq method (15) and
the primer sequences were as follows: SPATS2 forward,
5'-CTTTGTCCCCAACCCTCTCC-3' and reverse, 5'-GATCCTCCACCTCCCCTTCT-3';
and GAPDH forward, 5'-GCTCTCTGCTCCTCCTGTTC-3' and, reverse:
5'-AAGTGGTCGTTGAGGGCAATG-3'.
Western blot analysis
Protein was extracted from treated cells including
Eca109 and KY-SE150 using RIPA buffer (Beijing Solarbio Science
& Technology Co., Ltd.) with protease inhibitor (PMSF; Abcam)
and quantified using a bicinchoninic acid protein assay kit (Thermo
Fisher Scientific, Inc.). Equal amounts of protein (20 µg) were
separated using 12% SDS-PAGE, and then transferred onto PVDF
membranes. Subsequently, the membranes were incubated in 5% skimmed
milk for 1 h at room temperature and then incubated with primary
antibodies (Abcam), including SPATS2 (cat. no. ab122495; 1:500),
cyclin E (cat. no. ab33911; 1:1,000), P53 (cat. no. ab32389;
1:1,000), matrix metalloproteinase (MMP-9; cat. no. ab73734; 1
µg/ml), N-cadherin (cat. no. ab202030; 1:500), and GAPDH (cat. no.
ab181602; 1:10,000) at 4˚C overnight. After washing the membranes
with TBS-Tween-20 (0.1%) three times, they were incubated with
secondary antibody goat anti-rabbit IgG H&L (cat. no. ab205718;
1:2,000; Abcam) at room temperature for 1 h. After washing the
membranes three times with TBS-T, the signals were detected using
enhanced chemiluminescent reagent (Beyotime Institute of
Biotechnology) and densitometry was estimated using Quantity One
software (v4.6.6; Bio-Rad Laboratories, Inc.). GAPDH was used as a
normalization control.
Cell proliferation assays
Cell Counting Kit-8 (CCK-8) reagent (Dojindo
Molecular Technologies, Inc.) was used to assess the viability of
Eca109 and KY-SE150 cells according to the manufacturer's protocol.
Cells in the logarithmic phase were transfected with si-SPATS2 for
24 h, and then cultured in 96-well plates at a density of
1x103 cells/well under the aforementioned culture
conditions (37˚C with 5% CO2). A total of 10 µl of CCK-8
reagent was added into each well and the cells were cultured for
1.5 h at 37˚C. The optical density values were measured at 0, 24,
48 and 72 h at a wavelength of 450 nm using a microplate
reader.
Colony-formation assays
Cell colony-formation capabilities were estimated in
the logarithmic phase. Cells including Eca109 and KY-SE150 were
inoculated into a 60 mm culture dish at a density of 400 cells/dish
and incubated at 37˚C and 5% CO2 for 1-2 weeks. When
visible clones appeared, the culture was terminated. Colonies were
fixed with 4% paraformaldehyde for 20 min and stained with 0.1%
crystal violet for 30 min at room temperature. At least three
images of the colonies were captured under a light microscope
(magnification, x400) and counted.
Transwell assay
The invasion and migration abilities of Eca109 and
KY-SE150 cells were determined using Transwell chambers (Corning,
Inc.) with or without Matrigel (BD Biosciences). For cell invasion
assays, the chambers were pre-coated with Matrigel. Coating was
performed by adding 100 µl Matrigel into the upper chamber of the
Transwell chamber of the 24-well plate, shaken evenly and placed in
a CO2 incubator at 37˚C for 4-6 h to form a gel. Cell
migration assays did not require the chambers to be pre-coated. A
total of 100 µl cell suspension (containing 5x103 cells,
excluding serum) was added into the upper chamber and 500 µl
complete RPMI-1640 medium was added into the lower chamber. After
incubation at 37˚C for 24 h and removal of the residual cells on
the surface of upper chamber, the invasive and migratory cells were
fixed with 4% paraformaldehyde for 30 min and stained with 0.1%
crystal violet for 20 min at room temperature. A total of 5 fields
were randomly selected and images captured by a light microscope
(magnification, x400).
Statistical analysis
All experiments were repeated at least three times.
Experimental data was analyzed using SPSS version 22.0 (IBM Corp.)
and GraphPad Prism version 5.0 (GraphPad Software, Inc.). Unpaired
Student's t-test was performed to analyze the difference between
two groups and one-way ANOVA analysis with Dunnett's post hoc test
was performed for comparison among multiple samples. Survival
curves were analyzed by the Kaplan-Meier method and log-rank test.
In survival analysis, ESCC patients were divided into a high
expression group and low expression group according to SPATS2
expression level. The median expression level values were used to
define high and low expression of SPATS2 in ESCC, the median value
in tumor was calculated to be 14.92 whereas in that the normal
group is 6.31. All experiments were independently repeated three
times. *P<0.05 was considered statistically
significant.
Results
High expression of SPATS2 is
associated with a less favorable overall survival time in patients
with ESCC
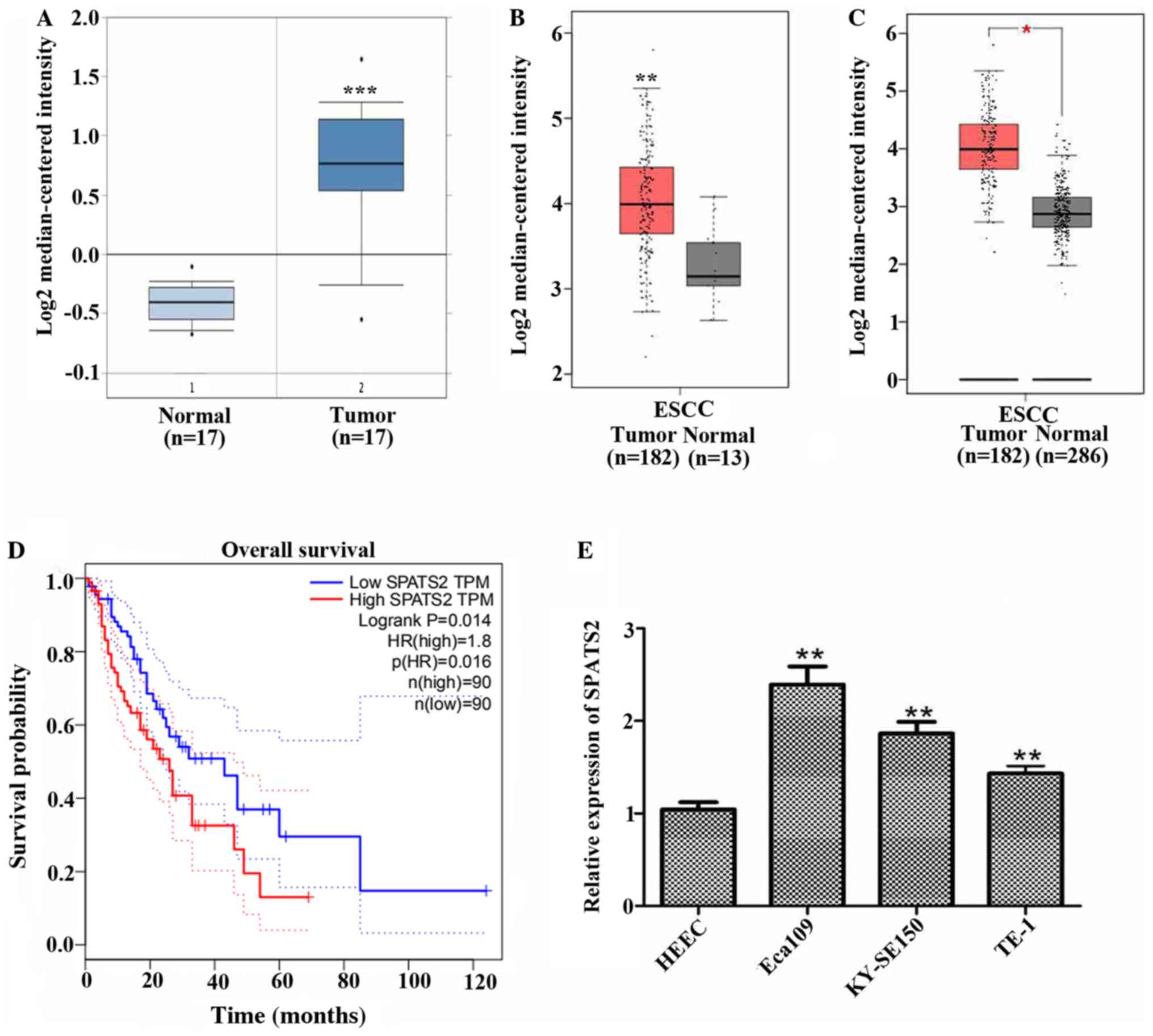
In order to explore the function of SPATS2 in ESCC,
SPATS2 expression patterns in patient samples were derived from a
number of public databases. Data acquired from Oncomine indicated
that SPATS2 was expressed at higher levels in ESCC tissues (n=17)
compared with normal tissues (n=17; Fig. 1A; P<0.0001). SPATS2 was also
upregulated in patients with ESCC compared with normal tissues
according to data from TCGA, including 182 tumor tissues and 13
normal tissues (Fig. 1B;
P<0.01). A comparison of cancer samples and normal tissue data
derived from TCGA including 182 tumor tissues and 13 normal tissues
and GTEx containing 273 normal tissues revealed that SPATS2 was
upregulated in tumor tissues by contrast to normal tissues
(Fig. 1C; P<0.05). Moreover,
high expression of SPATS2 was associated with a shorter survival
time in patients with ESCC (Fig.
1D; P=0.014).
Subsequently, the mRNA expression of SPATS2 was
investigated in three ESCC cell lines, Eca109, KY-SE150 and TE-1,
and the normal cell line HEEC. As indicated in Fig. 1E, the expression of SPATS2 was
significantly increased in ESCC cell lines compared with a normal
cell line (P<0.01). These data indicate that SPATS2 was
upregulated in ESCC tissues and cell lines and was associated with
a poor prognosis in patients with ESCC.
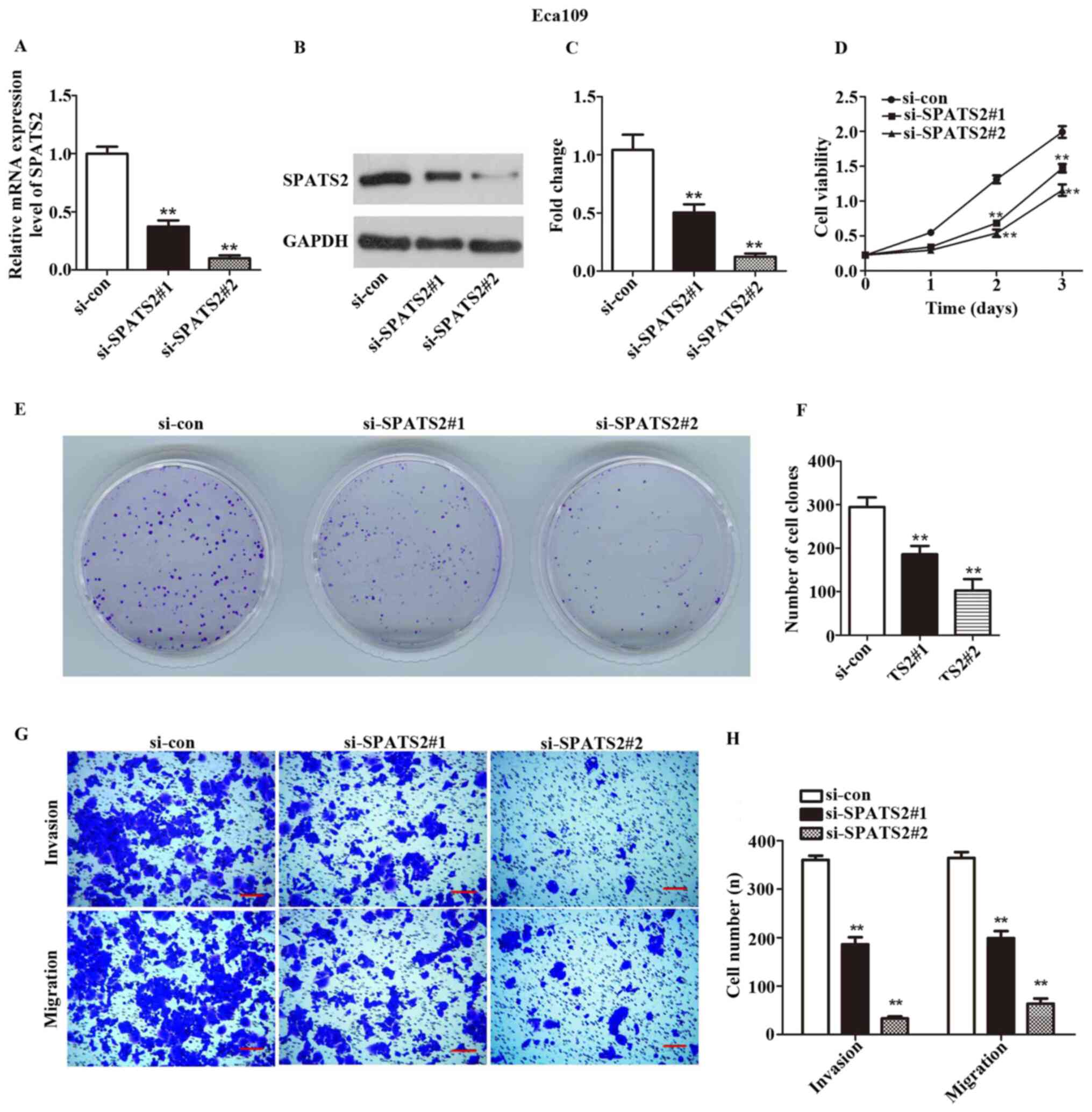
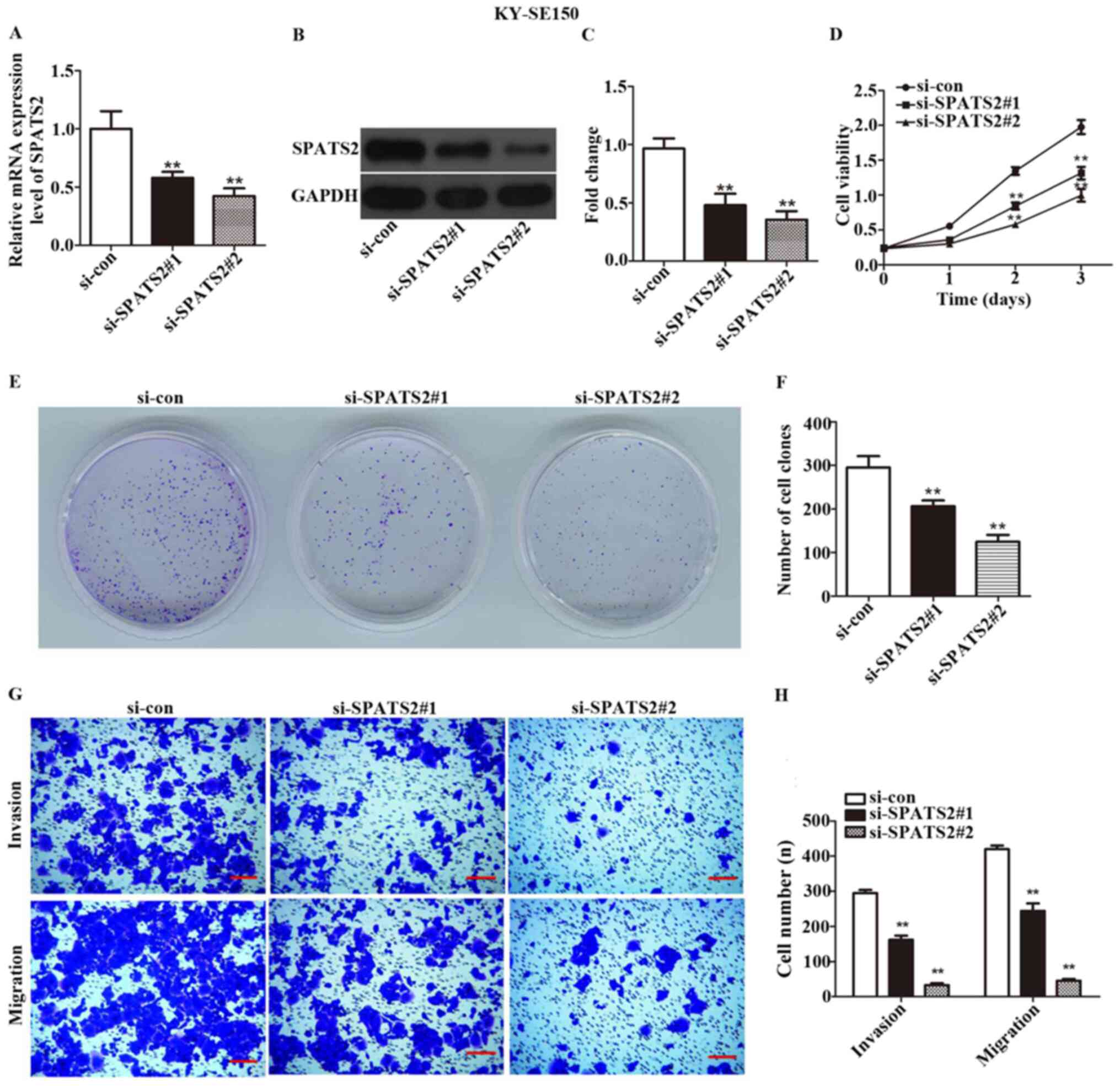
Knockdown of SPATS2 suppresses cell
proliferation in ESCC
To further investigate the association between
SPATS2 and ESCC progression, two ESCC cell lines, Eca109 and
KYSE-150, were examined. Of the ESCC lines investigated, SPATS2 was
expressed at the highest levels in these two cell lines (Fig. 1E). siRNA was transfected in order to
silence SPATS2 in ESCC cells. As indicated in Figs. 2A-C and 3A-C, the expression of SPATS2 was
suppressed at both the mRNA and protein level after transfection
with si-SPATS2 when compared with si-con, providing a basis for
subsequent experiments (P<0.01).
A CCK-8 kit was used to determine ESCC cell
viability. The results indicated that Eca109 and KYSE-150 cell
proliferation was significantly inhibited at 48 and 72 h after
knockdown of SPATS2 when compared with the control (Figs. 2D and 3D; P<0.01).
According to the observed link between cell
viability and SPATS2 expression, a colony formation assay was
designed to assess the influence of SPATS2 on ESCC cells. The
results indicated that colony formation ability was significantly
decreased after knockdown of SPATS2, compared with the control
(Figs. 2E-F and 3E-F; P<0.01). Taken together, the
current findings indicate that downregulation of SPATS2 repressed
the proliferation of ESCC cells.
SPATS2 serves an active role in
migration and invasion of ESCC cells
Next, a Transwell assay was used to explore the
migration and invasion abilities of ESCC cells, after silencing of
SPATS2. As revealed in Figs. 2G-H,
and 3G-H, migratory and invasive
cells numbers declined after knockdown of SPATS2 in contrast with
the si-con group (P<0.01). The results suggested that
downregulation of SPATS2 suppressed the migratory and invasive
capacity of ESCC cells.
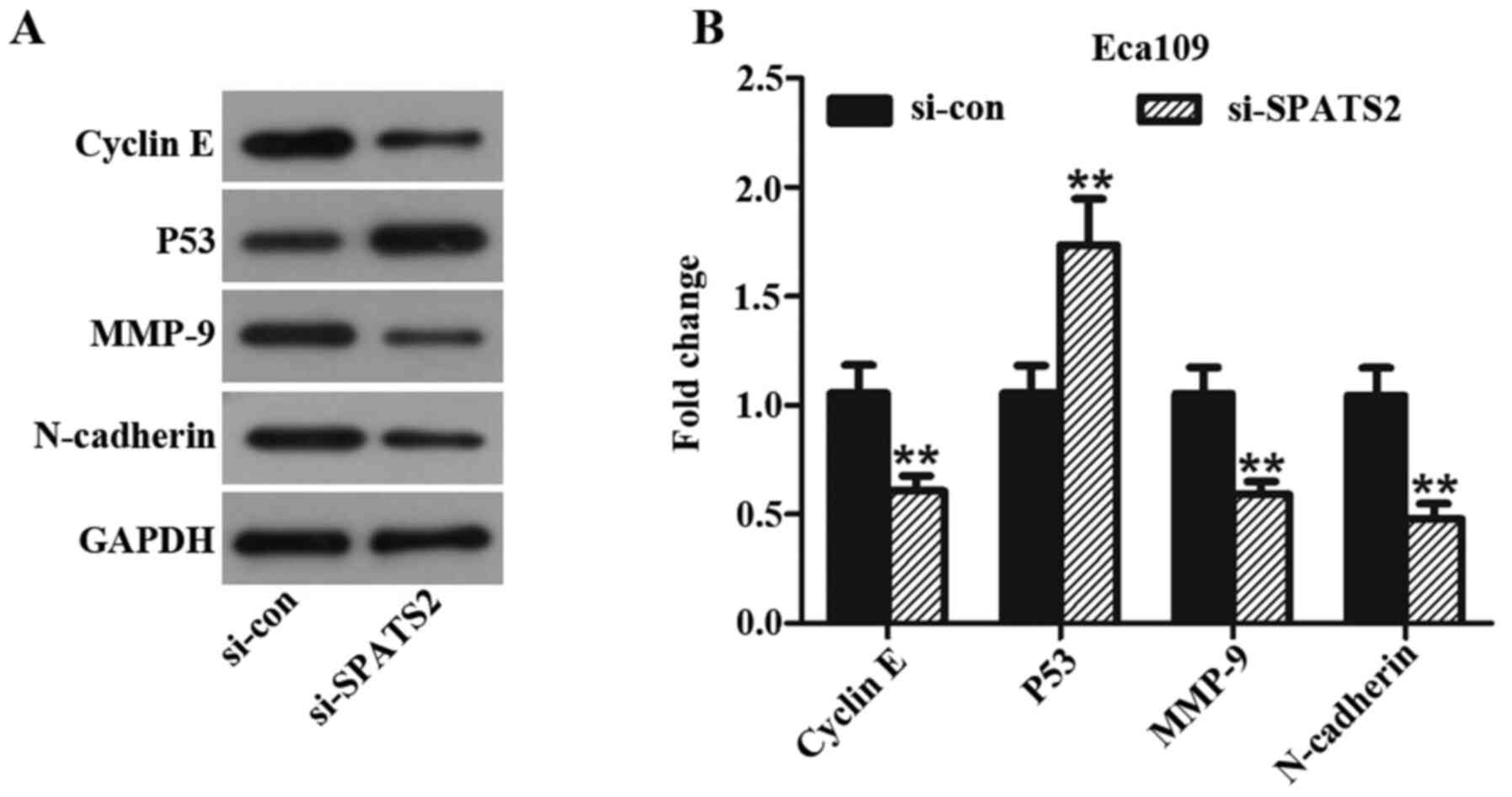
Silencing SPATS2 has a significant effect on key
proteins in ESCC progression. In the present study, Eca109 cells
were used to assess changes in the expression of proteins
associated with ESCC progression after knockdown of SPATS2 with
si-SPATS2#2. Following knockdown, the expression levels of cyclin
E, MMP-9 and N-cadherin were significantly decreased compared to
cells treated with si-con group, while P53 was significantly
upregulated (Fig. 4A and B, P<0.01). Taken together, the data
suggest that SPATS2 may influence ESCC cell proliferation, invasion
and migration abilities, partly via regulating expression of cyclin
E, MMP-9, N-cadherin and P53.
Discussion
In the present study, SPATS2 was determined to be
upregulated in ESCC tissues and cells, and this was significantly
associated with a less favorable prognosis in patients with ESCC.
Subsequently, RNA interference experiments revealed that knockdown
of SPATS2 inhibited ESCC cell proliferation, migration and
invasion. In addition, knockdown of SPATS2 influenced the
expression of key proteins associated with the progression of ESCC.
Taken together, the present data indicate that SPATS2 may
facilitate tumorigenesis in ESCC via its influence on cell
proliferation, migration and invasion.
ESCC has inconspicuous early symptoms, high
incidence and poor prognosis, and for these reasons developing new
approaches to treatment is important (16). Numerous possible biomarkers of ESCC
have been reported, but none have been widely applied to guide
clinical studies (6). As SPATS2 is
a relatively recently discovered gene, limited research into its
potential roles has been performed (8). The earliest study into SPATS2 reported
that its expression was induced by bisphenol A downregulation in
oral epithelial cells (17).
Recently, increased expression of SPATS2 was found both in
colorectal and prostate cancer (9,10). As
the upstream interaction protein of SPATS2, neuroblastoma RAS viral
oncogene homolog (NRAS) plays an important role in the occurrence
and metastasis of various cancers, including colorectal (18), primary thyroid (19), lung (20) and bladder cancer (21). In the present study, SPATS2 was
revealed to be highly expressed in ESCC tissues and cells, and
silencing of SPATS2 had negative effects on cell proliferation,
invasion and migration. The present data together with previous
reports indicates that SPATS2 may serve an active role in the
occurrence and progression of ESCC.
Alterations in the mechanism controlling apoptosis
and cell cycle progression influence the pathogenesis of different
human neoplasia (22). P53, as a
tumor suppressor gene, is influences numerous types of cancer,
including ESCC (23). P53
dysfunction is frequently observed in tumors and regulates
molecular mechanisms underlying tumor progression (24). Cyclin E is a key regulatory factor
of the cell cycle (25), promoting
DNA duplication and centrosome replication in the late
G1 stage, and is highly expressed in cancers of the
digestive system (26). MMPs are
important contributors to metastasis and have been revealed to
promote cell invasion in numerous human cancers (27). MMP-9 is a protease, which is
upregulated in the majority of malignancies (28), including ESCC, and has been
demonstrated to enhance cell invasion and metastasis (29). N-cadherin promotes the invasion of
cancer cells and is highly expressed in a range cancer types.
Expression of N-cadherin in epithelial cells causes changes in
morphology to a fibroblastic phenotype, causing the cells to become
more motile and invasive (30). In
addition, N-cadherin has been reported to promote cell adhesion and
regulate tumor progression (31).
All of these genes are crucial to the development of tumors and are
regarded as makers for tumor progression. Therefore, in the present
study, in order to explore the molecular mechanism of ESCC
following SPATS2 depletion, P53, MMP-9, cyclin E and N-cadherin
levels were assessed. The results indicated that protein expression
of MMP-9, cyclin E and N-cadherin decrease compared with the
control, after silencing of SPATS2. By contrast, P53 was
significantly upregulated. The aforementioned results indicate that
SPATS2 may affect the proliferation, migration and invasion
abilities of ESCC cells via regulating the expression of these
proteins. Notably, a limitation of the present research was the
lack of in vivo experiments to confirm the current
results.
In summary, SPATS2 was highly expressed in ESCC
tissues and cells, and its upregulation was significantly
associated with a poor prognosis in patients with ESCC.
Furthermore, knockdown of SPATS2 suppressed cell proliferation,
invasion and migration abilities by regulating key proteins
involved in ESCC progression. The current findings suggest that
SPATS2 expression may be an important factor in the prognosis of
patients with ESCC, providing a new potential target for the
treatment of ESCC tumors.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YPL analyzed the data from biological websites, and
performed the biological experiments, and edited the manuscript.
QHC performed the biological experiments and edited the manuscript.
LL analyzed the experimental data and edited the manuscript. MZ
processed the experimental data and revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hu JM, Liu K, Liu JH, Jiang XL, Wang XL,
Chen YZ, Li SG, Zou H, Pang LJ, Liu CX, et al: CD163 as a marker of
M2 macrophage, contribute to predict aggressiveness and prognosis
of Kazakh esophageal squamous cell carcinoma. Oncotarget.
8:21526–21538. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sakai NS, Samia-Aly E, Barbera M and
Fitzgerald RC: A review of the current understanding and clinical
utility of miRNAs in esophageal cancer. Semin Cancer Biol.
23:512–521. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Murphy G, McCormack V, Abedi-Ardekani B,
Arnold M, Camargo MC, Dar NA, Dawsey SM, Etemadi A, Fitzgerald RC,
Fleischer DE, et al: International cancer seminars: A focus on
esophageal squamous cell carcinoma. Ann Oncol. 28:2086–2093.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gao S, Li S, Duan X, Gu Z, Ma Z, Yuan X,
Feng X and Wang H: Inhibition of glycogen synthase kinase 3 beta
(GSK3beta) suppresses the progression of esophageal squamous cell
carcinoma by modifying STAT3 activity. Mol Carcinog. 56:2301–2316.
2017.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Wang C, Wang J, Chen Z, Gao Y and He J:
Immunohistochemical prognostic markers of esophageal squamous cell
carcinoma: A systematic review. Chin J Cancer.
36(65)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ekman S, Dreilich M, Lennartsson J,
Wallner B, Brattstrom D, Sundbom M and Bergqvist M: Esophageal
cancer: Current and emerging therapy modalities. Expert Rev
Anticancer Ther. 8:1433–1448. 2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Senoo M, Hoshino S, Mochida N, Matsumura Y
and Habu S: Identification of a novel protein p59(scr), which is
expressed at specific stages of mouse spermatogenesis. Biochem
Biophys Res Commun. 292:992–998. 2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Damas ND, Marcatti M, Come C, Christensen
LL, Nielsen MM, Baumgartner R, Gylling HM, Maglieri G, Rundsten CF,
Seemann SE, et al: SNHG5 promotes colorectal cancer cell survival
by counteracting STAU1-mediated mRNA destabilization. Nat Commun.
7(13875)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ngollo M, Lebert A, Daures M, Judes G,
Rifai K, Dubois L, Kemeny JL, Penault-Llorca F, Bignon YJ, Guy L
and Bernard-Gallon D: Global analysis of H3K27me3 as an epigenetic
marker in prostate cancer progression. BMC Cancer.
17(261)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Takamochi K, Ohmiya H, Itoh M, Mogushi K,
Saito T, Hara K, Mitani K, Kogo Y, Yamanaka Y, Kawai J, et al:
Novel biomarkers that assist in accurate discrimination of squamous
cell carcinoma from adenocarcinoma of the lung. BMC Cancer.
16(760)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhu CH, Kim J, Shay JW and Wright WE:
SGNP: An essential Stress Granule/Nucleolar Protein potentially
involved in 5.8s rRNA processing/transport. PLoS One.
3(e3716)2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Donati G, Montanaro L and Derenzini M:
Ribosome biogenesis and control of cell proliferation: p53 is not
alone. Cancer Res. 72:1602–1607. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Min P, Li W, Zeng D, Ma Y, Xu D, Zheng W,
Tang F, Chen J, Shi J, Hu H, et al: A single nucleotide variant in
microRNA-1269a promotes the occurrence and process of
hepatocellular carcinoma by targeting to oncogenes SPATS2L and
LRP6. Bull Cancer. 104:311–320. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–8. 2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Baba Y, Saeki H, Nakashima Y, Oki E,
Shigaki H, Yoshida N, Watanabe M, Maehara Y and Baba H: Review of
chemotherapeutic approaches for operable and inoperable esophageal
squamous cell carcinoma. Dis Esophagus. 30:1–7. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Seki K, Koshi R, Sugano N, Masutani S,
Yoshinuma N and Ito K: Microarray analysis of bisphenol A-induced
changes in gene expression in human oral epithelial cells. Acta
Biochim Biophys Sin (Shanghai). 39:879–884. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cicenas J, Tamosaitis L, Kvederaviciute K,
Tarvydas R, Staniute G, Kalyan K, Meskinyte-Kausiliene E,
Stankevicius V and Valius M: KRAS, NRAS and BRAF mutations in
colorectal cancer and melanoma. Med Oncol. 34(26)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Melo M, Gaspar da Rocha A, Batista R,
Vinagre J, Martins MJ, Costa G, Ribeiro C, Carrilho F, Leite V,
Lobo C, et al: TERT, BRAF, and NRAS in primary thyroid cancer and
metastatic disease. J Clin Endocrinol Metab. 102:1898–1907.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Giannou AD, Marazioti A, Kanellakis NI,
Giopanou I, Lilis I, Zazara DE, Ntaliarda G, Kati D, Armenis V,
Giotopoulou GA, et al: NRAS destines tumor cells to the lungs. EMBO
Mol Med. 9:672–686. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li Y, Shan Z, Liu C, Yang D, Wu J, Men C
and Xu Y: MicroRNA-294 promotes cellular proliferation and motility
through the PI3K/AKT and JAK/STAT pathways by Upregulation of NRAS
in bladder cancer. Biochemistry (Mosc). 82:474–482. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
De Amicis F, Perri A, Vizza D, Russo A,
Panno ML, Bonofiglio D, Giordano C, Mauro L, Aquila S, Tramontano D
and Ando S: Epigallocatechin gallate inhibits growth and
epithelial-to-mesenchymal transition in human thyroid carcinoma
cell lines. J Cell Physiol. 228:2054–2062. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bellini MF, Cadamuro AC, Succi M, Proenca
MA and Silva AE: Alterations of the TP53 gene in gastric and
esophageal carcinogenesis. J Biomed Biotechnol.
2012(891961)2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang Y, Zhu C, Sun B, Lv J, Liu Z, Liu S
and Li H: Integrated high throughput analysis identifies GSK3 as a
crucial determinant of p53-mediated apoptosis in lung cancer cells.
Cell Physiol Biochem. 42:1177–1191. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Dai L, Liu Y, Liu J, Wen X, Xu Z, Wang Z,
Sun H, Tang S, Maguire AR, Quan J, et al: A novel
cyclinE/cyclinA-CDK inhibitor targets p27(Kip1) degradation, cell
cycle progression and cell survival: Implications in cancer
therapy. Cancer Lett. 333:103–112. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Huang L, Ren F, Tang R, Feng Z and Chen G:
Prognostic value of expression of Cyclin E in gastrointestinal
cancer: A systematic review and meta-analysis. Technol Cancer Res
Treat. 15:12–19. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Thaker PH, Han LY, Kamat AA, Arevalo JM,
Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori
M, et al: Chronic stress promotes tumor growth and angiogenesis in
a mouse model of ovarian carcinoma. Nat Med. 12:939–944.
2006.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Grunwald B, Vandooren J, Locatelli E,
Fiten P, Opdenakker G, Proost P, Kruger A, Lellouche JP, Israel LL,
Shenkman L and Comes Franchini M: Matrix metalloproteinase-9
(MMP-9) as an activator of nanosystems for targeted drug delivery
in pancreatic cancer. J Control Release. 239:39–48. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bai X, Li YY, Zhang HY, Wang F, He HL, Yao
JC, Liu L and Li SS: Role of matrix metalloproteinase-9 in
transforming growth factor-β1-induced epithelial-mesenchymal
transition in esophageal squamous cell carcinoma. Onco Targets
Ther. 10:2837–2847. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Derycke LD and Bracke ME: N-cadherin in
the spotlight of cell-cell adhesion, differentiation,
embryogenesis, invasion and signalling. Int J Dev Biol. 48:463–476.
2004.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Blaschuk OW: N-cadherin antagonists as
oncology therapeutics. Philos Trans R Soc Lond B Biol Sci.
370(20140039)2015.PubMed/NCBI View Article : Google Scholar
|