Introduction
Obstructive sleep apneahypopnea syndrome (OSAHS)
poses a serious threat to human health, affecting 3.7-97.3% of
Asian adults (1). The
pathophysiological mechanism underpinning OSAHS includes repeated
upper airway stenosis and collapse in the sleep state, resulting in
recurrent apnea, hypopnea and chronic intermittent hypoxia (CIH)
during sleep (2). Based on current
knowledge, OSAHS is considered to be closely associated with
structural stenosis of the airway, reduced muscle tension and
abnormal function of the upper airway. The genioglossus muscle, as
the main upper airway dilator, is crucial for ensuring that the
upper airway remains unobstructed, and is referred to as the
‘safety muscle of the upper airway’ (3). Zhang et al (3) reported that CIH induced genioglossus
myocyte apoptosis through activating endoplasmic reticulum (ER)
stress (ERS). Wang et al (4)
reported that CIH induced ROS production and cell apoptosis in the
genioglossus through downregulating and upregulating mitophagy by
adiponectin to improve the CIH-induced genioglossus myocyte injury.
It was previously reported that stimulation of rats with CIH led to
an increase in the fatigability of upper airway muscles and caused
hypoadiponectinemia, disrupted genioglossal ultrastructure and
mitochondrial dysfunction (5,6).
Mitochondrial dysfunction, in turn, affects the
major pathways implicated in the pathophysiology of airway disease,
including airway contractility, response to oxidative stress and
apoptosis (7). In total, three
apoptotic pathways have been described: The ER, mitochondrial and
death receptor pathways (8).
External stressors can cause unfolded or misfolded proteins to
accumulate in the ER, resulting in ERS. In order to maintain
homeostasis, the unfolded protein response (UPR) pathway activates
three transcription factors, including inositolrequiring enzyme
(IRE1), type I PKRlike endoplasmic reticulum kinase (PERK) and
activating transcription factor 6 (ATF6) (9,10).
Under normal conditions, these three transcription factors are
bound to glucose-regulated protein 78 (GRP78) and remain inactive.
Under stress conditions, however, GRP78 and the transcription
factors dissociate, leading to activation of the UPR (11). The activated IRE1, PERK and ATF6
pathways all upregulate C/EBP homologous protein (CHOP), which, in
turn, regulates Bcl-2 family proteins, increasing the rate of
synthesis of the pro-apoptotic protein Bim, reducing the synthesis
of the anti-apoptotic protein Bcl-2 and causing
mitochondrialassociated apoptosis (12,13).
In addition, during ERS, caspase-12 is activated on the ER membrane
and, as an ERS apoptosis-specific protein, activates downstream
caspase-3, leading to apoptosis (14,15). A
previous study by our research group demonstrated that CIH
upregulates ERS-associated proteins in rat genioglossus myocytes,
activates ERS-associated apoptosis pathways and causes genioglossus
dysfunction (3).
The klotho gene is located on chromosome 13q12, and
encodes two types of proteins: Membrane-bound and secreted klotho
proteins. A previous study revealed that secreted klotho protein
exerts cytoprotective effects, inhibiting oxidative stress and
apoptosis (16). In exploring the
underlying mechanisms, Maekawa et al (17) determined that klotho both promoted
MEK/ERK pathway activation and led to a significant decrease in
apoptosis of human umbilical vein endothelial cells stimulated by
hydrogen peroxide. Furthermore, Yamamoto et al (18) demonstrated that in vitro
supplementation of soluble klotho led to a marked reduction in
paraquat-induced lipid peroxidation in HeLa cells via inhibiting
the insulin-like growth factor-1 (IGF-1) pathway. A previous study
also revealed reduced klotho protein levels in serum samples
collected from patients with obstructive sleep apnea syndrome
(OSAS), and the klotho protein level was found to be negatively
correlated with disease severity (19). Navarro-González et al
(20) demonstrated that the
protective role of pentoxifylline was associated with increased
levels of klotho in patients with diabetes and chronic kidney
disease. Therefore, taken together, the finding of these studies
suggested that supplementary klotho may protect against
OSAS-induced injury; however, the mechanisms underpinning these
effects have yet to be elucidated. To meet this end, the present
study aimed to assess the effects of exogenous klotho on
CIH-induced genioglossus muscle injury in a mouse model, and
determine the involvement of ERS in this process.
Materials and methods
Mice and grouping
In total, 36 male adult C57BL/6 mice, aged 8 weeks,
weighing 18-20 g, were purchased from and housed at the Animal
Center of Southeast University. The animals were provided with
access to water and standard food ad libitum under a 12-h
light/dark cycle at 24˚C and 60% humidity. The present study was
approved (approval no. 201704025) by the Experimental Animal Ethics
Committee of Southeast University. The mice were assigned to three
groups according to the random number table method as follows: The
normal control (NC), CIH and CIH + klotho groups (n=12 mice per
group; the different treatments and conditions of the experimental
groups are explained in detail below).
Establishment of the mouse CIH model
and intraperitoneal injection of klotho
The mouse hypoxia box (Nanjing Xinfei Analytical
Instrument Co., Ltd.) was used to establish the mouse CIH model, as
described previously (6). Briefly,
the hypoxia cycle time was set to 1 min, and the chamber was filled
with nitrogen for the first 30 sec to reduce oxygen concentration
to 6-7%; subsequently, air was allowed into the chamber for the
second 30 sec to gradually increase the oxygen concentration to
21%. A total of 60 cycles were performed per h, thereby simulating
severe human OSAHS. The CIH and CIH + klotho groups underwent
intermittent hypoxia treatment for 8 h (8:00 a.m. - 4:00 p.m.)
every day, for a total of 12 weeks, whereas the NC group was placed
in the hypoxia box that was only filled with air in parallel.
Recombinant mouse klotho protein was purchased from
R&D Systems, Inc. and dissolved in sterile normal saline
solution (1 µg/ml). Mice in the CIH + klotho group received an
intraperitoneal injection of klotho protein (10 µg/kg/day)
(21,22), whereas the NC and CIH groups were
administered normal saline (0.5 ml/day) intraperitoneally during
model establishment.
After modeling, the mice were anesthetized with 1%
pentobarbital (50 mg/kg) via intraperitoneal injection, and 1 ml
blood was collected by cardiac puncture and centrifuged at 1,500 x
g for 15 min at 4˚C for serum preparation. The resulting serum was
stored at -80˚C prior to analysis. The mice were euthanized by
exsanguination after anesthesia with pentobarbital (1%; 50 mg/kg),
the genioglossus muscle was isolated, and a portion of the muscle
was placed in 4% paraformaldehyde. After fixation for 48 h at 4 ˚C,
the samples were dehydrated with xylene, waxed, embedded in
paraffin and cut into 5-µm sections for subsequent analysis. The
remaining genioglossus muscle samples were stored at -80˚C.
Detection of klotho protein levels in
serum and genioglossus muscle samples
ELISA was performed to detect the serum klotho
protein levels in the mice using the klotho protein kit (cat. no.
DL-KL-Mu; Wuxi Donglin Sci & Tech Development Co., Ltd.),
following the manufacturer's protcols. For assessment of the klotho
protein level in the tissue, 20 mg fresh genioglossus tissue was
placed in 500 µl PBS, homogenized, and cleared by centrifugation at
5,000 x g for 5 min. The supernatant was collected and tested as
described for the serum samples. Klotho protein levels in serum and
tissue samples were assessed by reading the absorbance at 450 nm on
a spectrophotometer.
Apoptosis detection
A TUNEL assay was performed to detect apoptosis of
genioglossus myocytes in mice. The TUNEL kit (cat. no. 11684817910)
was purchased from Roche Diagnostics GmbH and experiments were
performed according to the manufacturer's instructions. The
genioglossus sections were treated with protease K (20 µg/ml) for
15 min at room temperature after deparaffinization and rehydration.
The sections were then treated with the TUNEL reaction mixture for
1 h at 37˚C in the dark and humidified atmosphere. Next, the
sections were treated with DAPI for 5 min at room temperature in
the dark before being mounted in neutral resin. A fluorescence
microscope was used for observation of the cells. Blue and green
signals represented normal and apoptotic nuclei, respectively. In
total, five random fields per tissue section were captured at x200
magnification. The extent of cell apoptosis was quantified using
ImageJ 1.52 software (National Institutes of Health).
Hematoxylin and eosin (H&E)
staining
The genioglossus muscle was fixed in 4%
paraformaldehyde for 48 h at 4˚C, after which the samples were
dehydrated, embedded in paraffin and cut into 5-µm sections prior
to H&E staining. Subsequently, the sections were
deparaffinized, rehydrated and stained. Each section was subjected
to hematoxylin and eosin staining (1 min each) at room temperature.
Finally, the sections were observed under a light microscope
(magnification, x400) after dehydration and preparation of cover
slips.
Western blot analysis
Total protein was extracted from the genioglossus
muscle using the protein extraction kit (cat. no. KGP2100) of
Nanjing KeyGen Biotech Co., Ltd. Genioglossus muscle tissue samples
were homogenized on ice in lysis buffer containing 10 µl
phosphatase inhibitor, 1 µl protease inhibitor and 10 µl 100 mM
PMSF. The supernatant was then collected by centrifugation at
12,000 x g and 4˚C for 5 min. The protein concentration was
measured using the BCA method (Thermo Fisher Scientific, Inc.).
Equal amounts of protein (20 µg) were resolved by SDS-PAGE (10%)
followed by electro-transfer onto a PVDF membrane (Roche
Diagnostics). In total, 5% bovine serum albumin (Beyotime Institute
of Biotechnology) was used to block the membranes for 1 h at room
temperature; subsequently, the samples were successively incubated
with primary (overnight at 4˚C) and secondary (37˚C for 1 h)
antibodies diluted in 5% bovine serum albumin in TBS with 0.1%
Tween-20 at pH 7.6. Antibodies against CHOP (cat. no. 2895;
monoclonal antibody), GRP78 (cat. no. 3183; rabbit polyclonal
antibody), GAPDH (cat. no. 5174; rabbit monoclonal antibody) and
cleaved caspase-3 (cat. no. 9664; rabbit monoclonal antibody) were
purchased from Cell Signaling Technology, Inc., whereas the
antibody against cleaved caspase-12 (cat. no. ab62463; rabbit
polyclonal antibody) was obtained from Abcam. The horseradish
peroxidase-linked secondary antibodies (Anti-mouse, 1:1,000, cat.
no. 7076; Anti-rabbit, 1:1,000, cat. no. 7074) were purchased from
Cell Signaling Technology. ECL solution was evenly applied on to
the PVDF membrane, and the membranes were subsequently photographed
with a fluorescence imaging machine. The densitometric evaluation
of the bands was performed using Image Lab™ 6.0 software (Bio-Rad
Laboratories, Inc.).
Detection of the mRNA levels of
ERS-associated genes in genioglossus muscle samples
Reverse transcriptionquantitative PCR (RT-qPCR)
analysis was performed to detect the mRNA expression levels of
GRP78 and CHOP. Total RNA was obtained by lysing the genioglossus
muscle with TRIzol® lysis buffer (Thermo Fisher
Scientific, Inc.). Subsequently, 1 µg total RNA was
reversetranscribed using the Transcriptor First Strand cDNA
Synthesis Kit (cat. no. 04897030001) of Roche Diagnostics GmbH
using the following protocol: 30 min at 55˚C and at 85˚C for 5 min.
After mixing the primers of GRP78, CHOP, and GAPDH (for the
sequences, see Table I; Invitrogen,
Thermo Fisher Scientific, Inc.), 1 µg cDNA, and Power
SYBR® Green PCR Master Mix (cat. no. 4367659; Applied
Biosystems; Thermo Fisher Scientific, Inc.), RT-qPCR was performed
on an ABI7900 instrument, and the thermocycling conditions were as
follows: Initial denaturation for 10 min at 95˚C, 40 cycles of 15
sec at 95˚C and 1 min at 60˚C. The 2-ΔΔCq method
(23) was used for quantitative
analysis of the mRNA levels.
 | Table IPCR primers for detecting endoplasmic
reticulum stress-related genes in genioglossus muscle samples. |
Table I
PCR primers for detecting endoplasmic
reticulum stress-related genes in genioglossus muscle samples.
| Gene | Primer sequences
(5'-3') |
|---|
| GRP78 | Forward:
CTCGGATCCACCATGATGAAGTTCACTGTGGTG |
| | Reverse:
TGCTCTAGAGCTCAACTCATCTTTTTCTGATG |
| CHOP | Forward:
CTCGCTCTCCAGATTCCAGT |
| | Reverse:
CTGCTCCTTCTCCTTCATGC |
| GAPDH | Forward:
AGCAGTCCCGTACACTGGCAAAC |
| | Reverse:
TCTGTGGTGATGTAAATGTCCTCT |
Detection of reactive oxygen species
(ROS)
OCT (cat. no. 4583; Sakura Finetek USA, Inc.)
embedding solution was used to embed fresh genioglossus muscle
tissue samples, which were cut using a frozen microtome for
subsequent use. Frozen sections (6 µm) were treated with
dihydroethidium assay kit (cat. no. S0063; Beyotime Institute of
Biotechnology) according to the manufacturer's instructions, prior
to analysis with fluorescence microscopy (magnification, x200). In
total, five random fields per tissue section were captured. ImageJ
1.52 software (National Institutes of Health) was used to detect
the fluorescence intensity, which represented the reactive oxygen
species level.
Statistical analysis
GraphPad 7.0 software (GraphPad Software, Inc.) was
used for data analysis. Data are presented as the mean ± standard
deviation, and were analyzed by oneway ANOVA followed by the
Student-Newman-Keuls post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Serum and genioglossus muscle klotho
protein levels
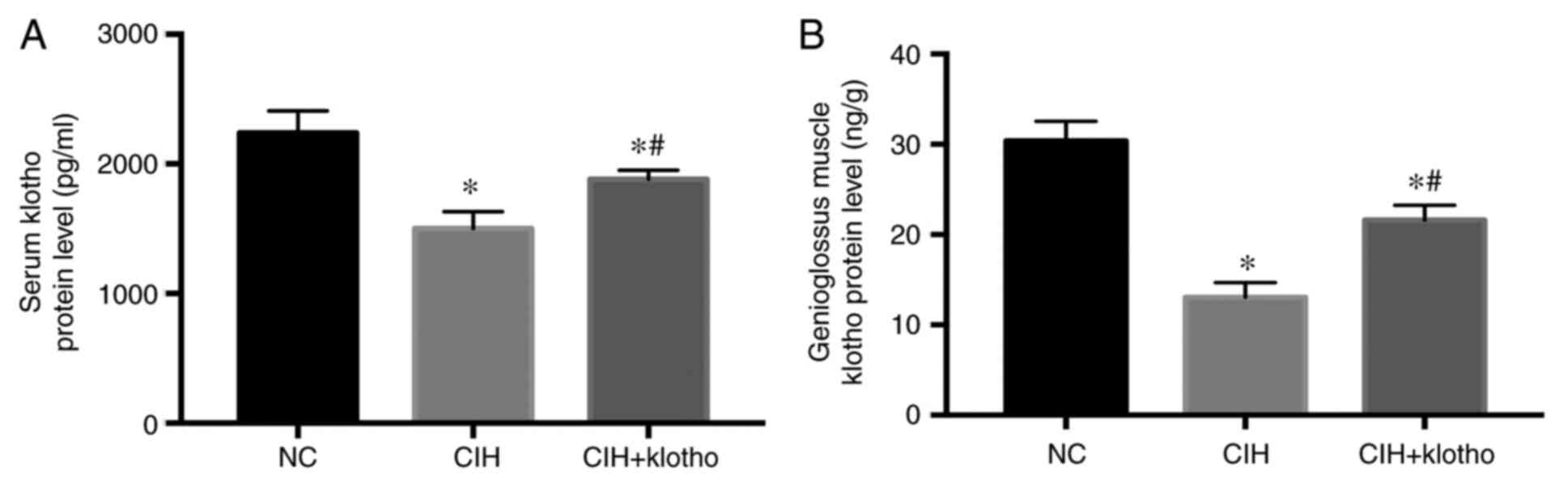
At 12 weeks after initiating establishment of the
mouse CIH model, the levels of the klotho protein in the serum and
genioglossus muscle samples of the C57BL/6 mice were found to be
significantly decreased in the CIH group compared with those in the
NC group (P<0.05). Following exogenous klotho protein
administration, however, the levels of klotho protein in the serum
and genioglossus muscle tissue were significantly increased
compared with those in the CIH group (P<0.05), although they
remained lower in comparison with those in the NC group (P<0.05;
Fig. 1). These results suggested
that klotho protein injected intraperitoneally was distributed
systemically, including its delivery to the genioglossus muscle
tissue.
Apoptosis of genioglossus
myocytes
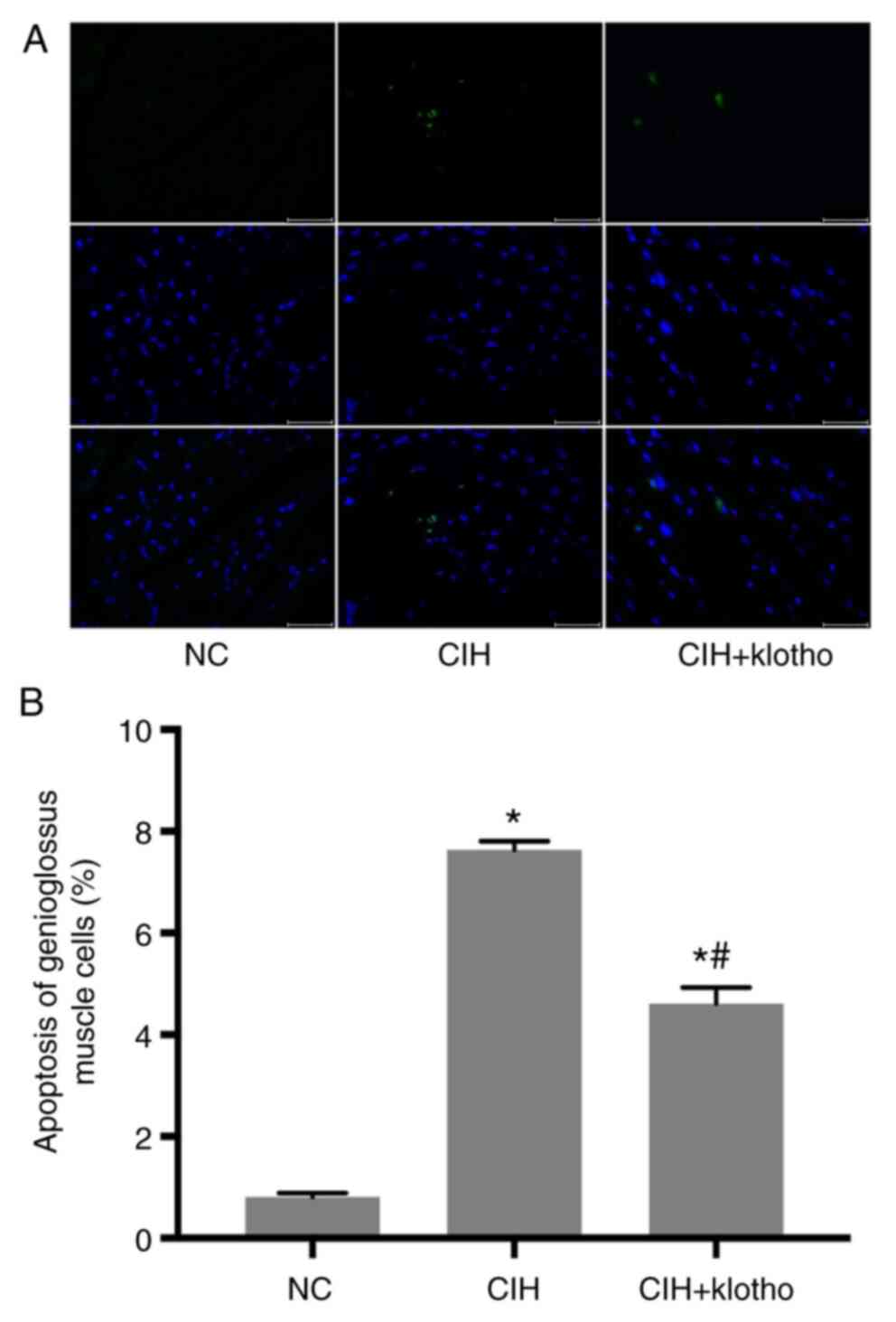
After 12 weeks of CIH modeling, the apoptotic rate
of the genioglossus myocytes was found to be significantly higher
in the CIH group (7.633±0.1672%) compared with that in the NC group
(0.813±0.0719%; P<0.05). Following administration of the klotho
protein, however, the apoptotic rate was markedly reduced
(4.609±0.3164%) compared with that in the CIH group (P<0.05),
although this remained higher compared with the control value
(P<0.05; Fig. 2). These results
suggested that administration of exogenous klotho protein could
alleviate apoptosis in genioglossus myocytes.
Histological changes in the
genioglossus
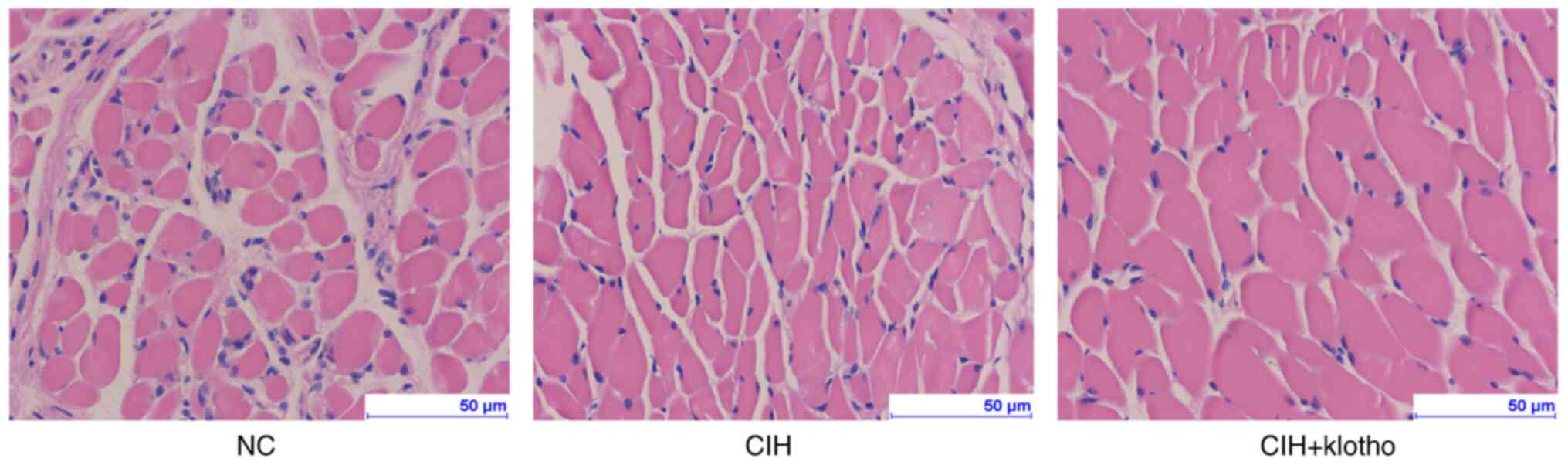
After 12 weeks of CIH modeling, the H&E staining
experiments revealed no significant differences in terms of
histological changes among the three groups (Fig. 3).
Gene expression levels of
ERS-associated molecules in genioglossus muscle samples
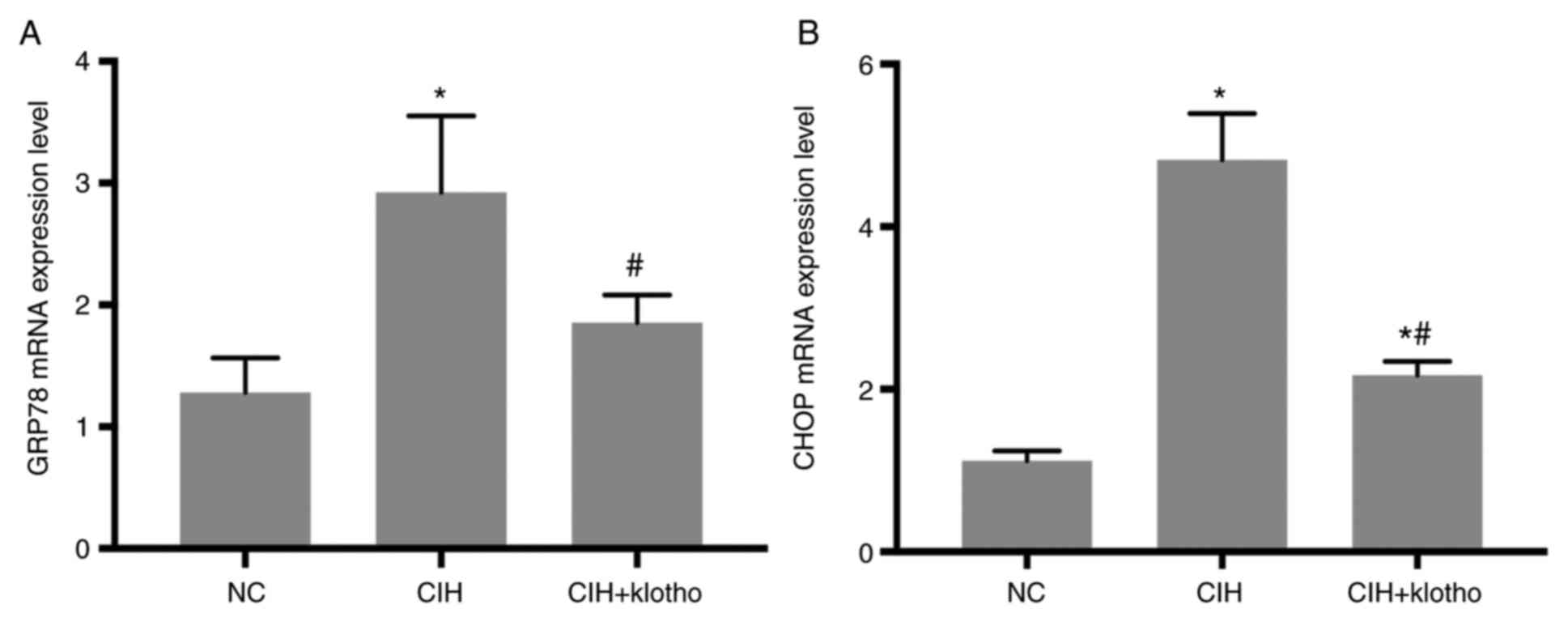
After 12 weeks of CIH modeling, the GRP78 and CHOP
mRNA levels in the genioglossus muscle samples were significantly
increased in the CIH group compared with those in the NC group (all
P<0.05). Compared with the CIH group, the CIH + klotho group
revealed markedly reduced mRNA levels of GRP78 and CHOP (all
P<0.05). The CHOP mRNA levels in the CIH + klotho group remained
higher compared with those of the NC group (P<0.05), whereas the
GRP78 gene expression levels in the CIH + klotho and NC groups were
comparable (P>0.05; Fig. 4).
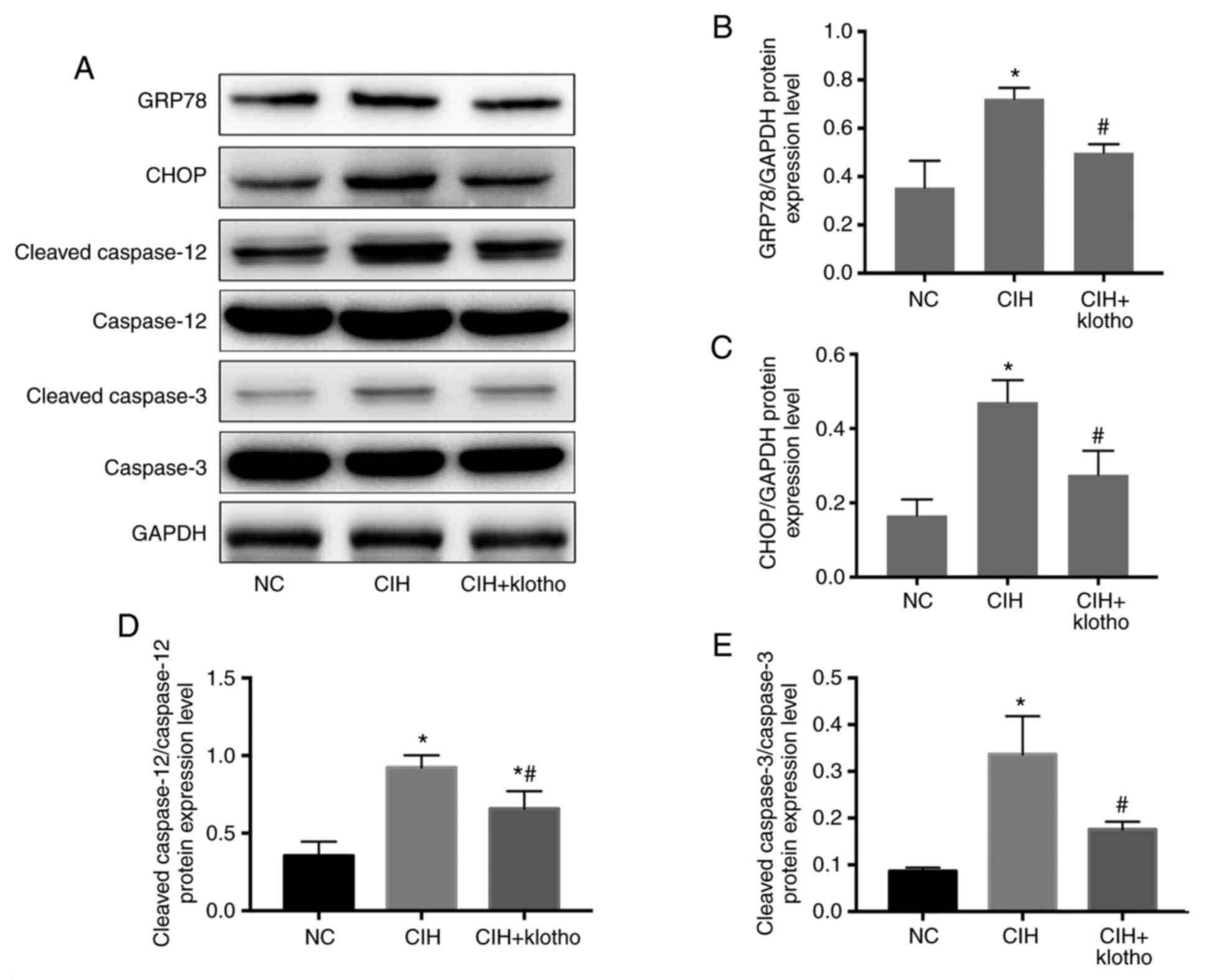
Protein expression levels of
ERS-associated molecules in genioglossus muscle samples
After 12 weeks of CIH modeling, the protein
expression levels of ERS-associated proteins (GRP78, CHOP, cleaved
caspase-12 and cleaved caspase-3) in the genioglossus muscle
samples were significantly higher in the CIH group compared with
those in the NC group (all P<0.05). Administration of klotho,
however, led to a significant reduction in these levels in the
model mice (all P<0.05). The cleaved caspase-12/caspase-12 ratio
remained significantly lower in the CIH + klotho group compared
with the NC group (both P<0.05), whereas the levels of GRP78,
CHOP and cleaved caspase-3/caspase-3 ratio were similar between the
NC and CIH + klotho groups (all P>0.05; Fig. 5).
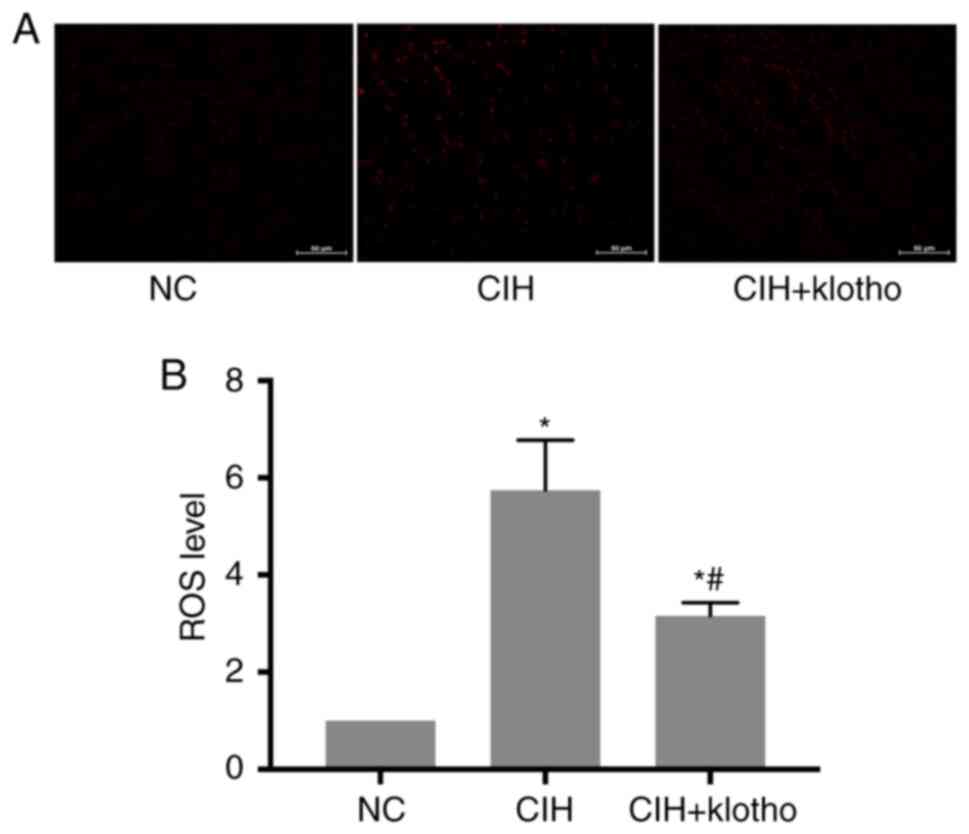
ROS levels in the genioglossus muscle
samples
At the end of the 12-week study, compared with the
NC group, the CIH and CIH + klotho treatment groups were found to
have significantly increased ROS levels in the genioglossus muscle
samples (all P<0.05). Compared with the CIH group, however, the
CIH + klotho group exhibited significantly decreased ROS levels,
although they remained higher compared with those of the NC group
(P<0.05; Fig. 6).
Discussion
The present study demonstrated that exogenous klotho
may alleviate apoptosis of genioglossus myocytes in mice by
inhibiting ROS-associated ERS. As revealed by the experiments
described in the present study, the serum and genioglossus muscle
tissue klotho protein levels in the CIH group were significantly
lower compared with those of the control group, suggesting that the
CIH model had indeed been successfully established, also
corroborating previous findings of reduced klotho protein amounts
in OSAS (19). Interestingly, after
repeated treatment with klotho, the protein was identified both in
the serum and in the genioglossus muscle, although at levels lower
than normal physiological levels.
Subsequently, the extent of apoptosis of the
genioglossus muscle was assessed. Significantly higher apoptotic
rates in the CIH group were identified compared with the control
animals, a phenomenon that was slightly, although not completely,
reversed by administration of exogenous klotho protein in
vivo. These findings confirmed the antiapoptotic effects of
klotho (24,25).
Klotho is an anti-aging gene, which has been
identified at reduced levels in patients with OSAS, type 2 diabetes
mellitus, and in subjects who are smokers (20,26,27).
High klotho expression levels in the plasma are associated with
good response in patients with acute ischemia stroke (28). Furthermore, it has been reported
that increases in the klotho protein level may have a
renoprotective function in patients with diabetes and chronic
kidney disease (20). Liu et
al (22) reported that klotho
reduced lipopolysaccharide-induced acute cardiorenal injury in
mice. Therefore, it was hypothesized that supplementation with
klotho may also protect against CIH-induced injury.
It has been demonstrated that klotho protein is
involved in ERS regulation, reducing the pathophysiological injury
caused by an increased UPR and abnormal ER activation (29). A recent study also demonstrated that
activated IRE-1 is coupled with c-Jun N-terminal kinase activation
through interaction with TRAF-2 and apoptotic signal-regulated
kinase-1(30). Therefore, whether
ERS is involved in CIH-induced apoptosis was the major objective of
the present study.
In the present study, establishment of CIH led to an
increase in the mRNA expression levels of ERS-associated genes,
including GRP78 and CHOP, as well as the protein levels of GRP78,
CHOP, cleaved caspase-12 and cleaved caspase-3 in genioglossus
muscle samples from mice. These findings were in line with previous
reports showing that ERS mediates cell apoptosis to cause cognitive
dysfunction in OSAS (31,32). As demonstrated above, administration
of exogenous klotho protein significantly reduced the levels of
ERS-associated genes and proteins, indicating that klotho protein
is involved in regulating CIH-induced, ERS-associated
apoptosis.
As oxidative stress is also an important factor in
the pathophysiology of airway diseases (7), the present study sought to determine
whether the latter was affected by klotho protein treatment in the
current model. As shown above, the ROS levels were increased after
CIH modeling, but decreased by klotho protein administration. These
results indicated that klotho protein alleviated oxidative stress
in the CIH model. Taken together, the present findings have
demonstrated that exogenous klotho protein inhibits the apoptosis
levels of genioglossus myocytes in mice with CIH via suppression of
the ROS-associated ERS pathways, and this should be further
assessed in order to improve the clinical treatment of OSAS.
The limitations of the present study should,
however, be mentioned. First, these experiments were conducted in a
mouse CIH model, and whether similar findings would be obtained in
a human study remains unclear. In addition, the animals were
treated for a relatively long time, and the observed effects were
still not complete. Furthermore, the mice were not assessed for
disease characteristics or evaluated after discontinuation of the
treatment. Finally, tauroursodeoxycholate (TUDCA) is an ER stress
inhibitor, where it remains unknown whether TUDCA decreased
klotho-induced oxidative stress and apoptosis. Therefore, the lack
of a TUDCA group is another limitation of this study. Therefore,
further studies are required to confirm these findings before
performing clinical trials that may pave the way for the use of
klotho protein in treatment of OSAS in the future.
In conclusion, the present study has demonstrated
that exogenous klotho protein may reduce CIH-induced genioglossus
muscle injury in mice, at least in part by regulating
ERS-associated apoptotic pathways. Further studies, however, are
required to corroborate these findings and to help determine
whether klotho protein administration may be a feasible option for
the treatment of OSAS.
Acknowledgements
Not applicable.
Funding
Funding: The present study supported by the Nanjing Science and
Technology Development Plan Project (20150409-2).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
QZ conceived and supervised the study; ZX and QZ
designed the experiments; WD and LG performed the experiments; QZ
provided new tools and reagents; ZX developed new software and
performed simulation studies; ZX analyzed the data; ZX wrote the
manuscript; ZX made manuscript revisions. All authors have seen and
can confirm the authenticity of the raw data. All authors have
reviewed the results and approved the final version of the
manuscript.
Ethics approval and consent to
participate
This study was approved by the Experimental Animal
Ethics Committee of Southeast University (Nanjing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mirrakhimov AE, Sooronbaev T and
Mirrakhimov EM: Prevalence of obstructive sleep apnea in Asian
adults: A systematic review of the literature. BMC Pulm Med.
13(10)2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
De Backer W: Obstructive sleep
apnea/hypopnea syndrome. Panminerva Med. 55:191–195.
2013.PubMed/NCBI
|
|
3
|
Zhang XF, Huang HP, Ding WX, Ding N, Lu G,
Liu JN and Zhang XL: Adiponectin protects the genioglossus of rats
against chronic intermittent hypoxia-induced injury via inhibition
of endoplasmic reticulum stress. Chin Med J (Engl). 126:3270–3275.
2013.PubMed/NCBI
|
|
4
|
Wang W, Ding W, Huang H, Zhu Y, Ding N,
Feng G and Zhang X: The role of mitophagy in the mechanism of
genioglossal dysfunction caused by chronic intermittent hypoxia and
the protective effect of adiponectin. Sleep Breath 2020 (Epub ahead
of print).
|
|
5
|
Ding WH, Li W, Chen XY and Shi JJ: The
study of genistein attenuating genioglossus muscle fatigue under
chronic intermittent hypoxia. Zhonghua Kou Qiang Yi Xue Za Zhi.
51:46–50. 2016.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
6
|
Huang H and Zhang X, Ding N, Li Q, Min Y
and Zhang X: Effects of chronic intermittent hypoxia on
genioglossus in rats. Sleep Breath. 16:505–510. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Prakash YS, Pabelick CM and Sieck GC:
Mitochondrial dysfunction in airway disease. Chest. 152:618–626.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tabas I and Ron D: Integrating the
mechanisms of apoptosis induced by endoplasmic reticulum stress.
Nat Cell Biol. 13:184–190. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hetz C: The unfolded protein response:
Controlling cell fate decisions under ER stress and beyond. Nat Rev
Mol Cell Biol. 13:89–102. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Bouvier N, Fougeray S, Beaune P, Thervet E
and Pallet N: The unfolded protein response regulates an angiogenic
response by the kidney epithelium during ischemic stress. J Biol
Chem. 287:14557–14568. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gardner BM, Pincus D, Gotthardt K,
Gallagher CM and Walter P: Endoplasmic reticulum stress sensing in
the unfolded protein response. Cold Spring Harb Perspect Biol.
5(a013169)2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Marciniak SJ, Yun CY, Oyadomari S, Novoa
I, Zhang Y, Jungreis R, Nagata K, Harding HP and Ron D: CHOP
induces death by promoting protein synthesis and oxidation in the
stressed endoplasmic reticulum. Genes Dev. 18:3066–3077.
2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zong WX, Li C, Hatzivassiliou G, Lindsten
T, Yu QC, Yuan J and Thompson CB: Bax and Bak can localize to the
endoplasmic reticulum to initiate apoptosis. J Cell Biol.
162:59–69. 2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shore GC, Papa FR and Oakes SA: Signaling
cell death from the endoplasmic reticulum stress response. Curr
Opin Cell Biol. 23:143–149. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shi Z, Xu L and Zhou R:
Tauroursodeoxycholic acid suppresses endoplasmic reticulum stress
in pulmonary tissues of intermittent hypoxia mice. Zhong Nan Da Xue
Xue Bao Yi Xue Ban. 40:1165–1172. 2015.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
16
|
Cui W, Leng B, Liu W and Wang G:
Suppression of apoptosis in human umbilical vein endothelial cells
(HUVECs) by klotho protein is associated with reduced endoplasmic
reticulum oxidative stress and activation of the PI3K/AKT pathway.
Med Sci Monit. 24:8489–8499. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Maekawa Y, Ohishi M, Ikushima M, Yamamoto
K, Yasuda O, Oguro R, Yamamoto-Hanasaki H, Tatara Y, Takeya Y and
Rakugi H: Klotho protein diminishes endothelial apoptosis and
senescence via a mitogen-activated kinase pathway. Geriatr Gerontol
Int. 11:510–516. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yamamoto M, Clark JD, Pastor JV, Gurnani
P, Nandi A, Kurosu H, Miyoshi M, Ogawa Y, Castrillon DH, Rosenblatt
KP and Kuro-o M: Regulation of oxidative stress by the anti-aging
hormone klotho. J Biol Chem. 280:38029–38034. 2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pákó J, Kunos L, Mészáros M, Tárnoki DL,
Tárnoki ÁD, Horváth I and Bikov A: Decreased levels of anti-aging
klotho in obstructive sleep apnea. Rejuvenation Res. 23:256–261.
2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Navarro-González JF, Sánchez-Niño MD,
Donate-Correa J, Martín-Núñez E, Ferri C, Pérez-Delgado N, Górriz
JL, Martínez-Castelao A, Ortiz A and Mora-Fernández C: Effects of
pentoxifylline on soluble klotho concentrations and renal tubular
cell expression in diabetic kidney disease. Diabetes Care.
41:1817–1820. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Song S, Gao P, Xiao H, Xu Y and Si LY:
Klotho suppresses cardiomyocyte apoptosis in mice with
stress-induced cardiac injury via downregulation of endoplasmic
reticulum stress. PLoS One. 8(e82968)2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu X, Niu Y, Zhang X, Zhang Y, Yu Y,
Huang J, Li J and Yu C: Recombinant α-klotho protein alleviated
acute cardiorenal injury in a mouse model of
lipopolysaccharide-induced septic cardiorenal syndrome type 5. Anal
Cell Pathol (Amst). 2019(5853426)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cui W, Leng B and Wang G: Klotho protein
inhibits H2O2-induced oxidative injury in
endothelial cells via regulation of PI3K/AKT/Nrf2/HO-1 pathways.
Can J Physiol Pharmacol. 97:370–376. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mencke R and Hillebrands JL: NIGRAM
consortium. The role of the anti-ageing protein Klotho in vascular
physiology and pathophysiology. Ageing Res Rev. 35:124–146.
2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang L and Liu T: Clinical implication of
alterations in serum Klotho levels in patients with type 2 diabetes
mellitus and its associated complications. J Diabetes
Complications. 32:922–930. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Patel MS, Donaldson AV, Lewis A, Natanek
SA, Lee JY, Andersson YM, Haji G, Jackson SG, Bolognese BJ, Foley
JP, et al: Klotho and smoking-An interplay influencing the skeletal
muscle function deficits that occur in COPD. Respir Med. 113:50–56.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lee JB, Woo HG, Chang Y, Jin YM, Jo I, Kim
J and Song TJ: Plasma Klotho concentrations predict functional
outcome at three months after acute ischemic stroke patients. Ann
Med. 51:262–269. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Banerjee S, Zhao Y, Sarkar PS, Rosenblatt
KP, Tilton RG and Choudhary S: Klotho ameliorates chemically
induced endoplasmic reticulum (ER) stress signaling. Cell Physiol
Biochem. 31:659–672. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Song J, Park KA, Lee WT and Lee JE:
Apoptosis signal regulating kinase 1 (ASK1): Potential as a
therapeutic target for Alzheimer's disease. Int J Mol Sci.
15:2119–2129. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cai XH, Li XC, Jin SW, Liang DS, Wen ZW,
Cao HC, Mei HF, Wu Y, Lin ZD and Wang LX: Endoplasmic reticulum
stress plays critical role in brain damage after chronic
intermittent hypoxia in growing rats. Exp Neurol. 257:148–156.
2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhou L, Chen P, Peng Y and Ouyang R: Role
of oxidative stress in the neurocognitive dysfunction of
obstructive sleep apnea syndrome. Oxid Med Cell Longev.
2016(9626831)2016.PubMed/NCBI View Article : Google Scholar
|