Introduction
Morphine is widely used as an analgesic and acts by
activating opioid receptors in the central nervous system (1). In addition to providing pain relief,
morphine has also been reported to modulate apoptosis in various
types of cells, including immune, neuronal and cancer cells,
suggested its potential effects on immunomodulation, nerve damage
and tumor progression (2-4).
Although opioid receptors are essential for opioid-induced effects,
an increasing number of studies have demonstrated that other
mechanisms beyond opioid receptors may be involved in
morphine-mediated cell apoptosis (5-7).
However, the underlying molecular mechanisms remain to be fully
elucidated.
Apoptosis, a type of programmed cell death, is
crucial for maintaining growth, development and homeostasis within
the body (8). Macrophages, which
are important innate immune cells, have been reported to exert
regulatory effects on various pathological processes beyond
immunomodulation, and the dysregulation of macrophage apoptosis was
found to contribute to multiple diseases including atherosclerosis,
diabetic kidney disease and hepatitis (9-11).
Previous studies have demonstrated that lipopolysaccharide (LPS) is
an activator of apoptosis in macrophages (12-14).
Morphine was also reported to induce macrophage apoptosis; however,
the specific mechanisms were not elucidated (15). To the best of our knowledge, the
effects of morphine on LPS-induced macrophage apoptosis have not
been investigated to date.
Peroxisome proliferator-activated receptor (PPAR)γ,
which belongs to the steroid-lipid nuclear receptor family, is
predominantly found in adipose tissue and plays a crucial role in
adipocyte differentiation, lipid metabolism and insulin resistance
(16,17). PPARs regulate gene expression by
heterodimerizing with retinoid X receptors and binding to specific
PPAR response elements in the promoter regions of specific target
genes (16,17). PPARγ was also discovered to be
highly expressed in macrophages and the activation of PPARγ
triggered the apoptosis of macrophages through activation of the
proliferator-activated receptors (18-20).
In addition, a previous study indicated that the development of
analgesic tolerance to morphine may be regulated by PPARγ (21). However, whether PPARγ is involved in
morphine-induced apoptosis remains unclear.
The present study was undertaken to investigate the
possible effects of morphine on LPS-induced bone marrow
(BM)-derived macrophage (BMDM) apoptosis and to determine the role
of the PPARγ signaling pathway in this process, hoping to uncover
novel potential therapeutic and clinical applications for
morphine.
Materials and methods
BMDM isolation
All animals were maintained under specific
pathogen-free (SPF) barrier conditions. All animal experiments were
carried out in accordance with the guidelines of the Institutional
Animal Care and Use Committee of Wuhan University. A total of 8
male BALB/c mice (age, 7-8 weeks; weight, 20.23±1.01 g) were
sacrificed by cervical dislocation, and the femurs and tibias were
removed. After flushing the medullar cavity, BM cells were
collected and cultured in DMEM, high glucose (cat. no. 11965092;
Gibco; Thermo Fisher Scientific, Inc.) supplemented with
L-glutamine (Gibco; Thermo Fisher Scientific, Inc.), 10%
heat-inactivated FBS (HyClone; Cytiva), 1% penicillin/streptomycin
(Gibco; Thermo Fisher Scientific, Inc.) and 20 ng/ml mouse
macrophage colony-stimulating factor recombinant protein
(eBioscience; Thermo Fisher Scientific, Inc.). The cells were
incubated at 37˚C in an atmosphere containing 5% CO2 for
7 days to induce macrophage differentiation. BMDMs were
subsequently harvested (22) and
the purity was analyzed by flow cytometry for the expression of
F4/80 (>90%). All animal experimental protocols were approved by
the Institutional Animal Care and Use Committee of the Zhongnan
Hospital of Wuhan University (Wuhan, China; approval no.
AF165).
Chemicals and BMDM treatment
Morphine sulfate was obtained from the National
Institutes for Food and Drug Control. LPS and GW9662 were purchased
from Sigma-Aldrich; Merck KGaA. For the exposure to morphine, BMDMs
were subjected to overnight incubation with 1 µM morphine, followed
by stimulation with 0.5 µg/ml LPS for 24 h. For the treatment with
the PPARγ inhibitor, BMDMs were pretreated with 1 µM GW9662 for 4 h
prior to morphine and/or LPS stimulation.
Hoechst 33342 staining
To evaluate the morphological changes of apoptotic
BMDMs, Hoechst staining was performed as described previously
(23). Briefly, BMDMs cells were
seeded on chamber slides, treated with 1 µM GW9662 for 24 h. BMDMs
were then fixed with 4% formaldehyde for 15 min at room temperature
and washed three times with PBS. Subsequently, the cells were
incubated with Hoechst 33342 dye for 5 min in the dark. The cells
were washed three times with PBS, and the slides were then
visualized using a fluorescence microscope (Olympus Corporation;
magnification, x40 and x100). For semi-quantification, apoptotic
scores were counted from five randomly selected fields by direct
counting of 500 cells in each sample using a blinded method
(23). The percentage of apoptotic
cells was calculated as the number of apoptotic cells divided by
the number of total cells.
Flow cytometric analysis of
apoptosis
Apoptosis was measured using the FITC-Annexin V
Apoptosis Detection kit (BD Biosciences) as previously described
(24). BMDMs were harvested by
centrifugation at 1,000 x g for 5 min at 4˚C and washed twice with
ice-cold PBS. The cells were resuspended in binding buffer [10 mM
HEPES/NaOH (pH 7.4), 140 mM NaCl, 2.5 mM CaCl2] at a
concentration of 1x106 cells/ml, and then gently mixed
and incubated with 5 µl Annexin V-FITC (BD Biosciences) and 10 µl
propidium iodide (PI; BD Biosciences) in the dark at room
temperature for 15 min. After washing the cells with 1X binding
buffer to remove the excess FITC-Annexin V and PI, apoptotic cells
were analyzed using flow cytometry (FACSVia Flow Cytometer; BD
Biosciences) within 1 h to determine the levels of apoptosis. The
fluorescence of FITC and PI were measured using the FL-1 and FL-2
channels, respectively. Apoptosis was quantified by the percentage
of the population shifting to fluorescence positivity. The
percentage of apoptotic cells was calculated as the percentage of
early and late apoptotic cells. The data were analyzed using
CytExpert software 2.0 (Beckman Coulter, Inc.).
Caspase activity assay
The activities of caspase-3, -8 and -9 in BMDMs
subjected to the indicated treatments were detected using
caspase-Glo 3/7, caspase-Glo 8 and caspase-Glo assay kits (cat.
nos. G8090, G8200 and G8210, respectively; Promega Corporation)
according to the manufacturer's protocols. BMDMs were seeded at a
density of 1x103 cells in 96-well plates and allowed to
adhere overnight. Following incubation with the indicated drugs,
caspase-Glo reagent was added to the cells at a 1:1 ratio. The
contents were mixed and the cells were incubated for 1 h at room
temperature. The luminescence signal of each sample was then
detected using a Veritas plate-reading luminometer (Turner
BioSystems) according to the manufacturer's instructions (25). The relative percentage of
luminescence intensity was calculated by comparison to the vehicle
control (26).
Western blotting
Total protein was extracted from cells using RIPA
lysis buffer (Sigma-Aldrich; Merck KGaA) and stored at -80˚C. Total
protein concentration was determined using a BCA assay and proteins
(20 µg per lane) were separated via SDS-PAGE (12%). The separated
proteins were subsequently electrotransferred onto nitrocellulose
membranes (Amersham; Cytiva) and blocked in TBS solution containing
5% non-fat milk for 1 h at room temperature. The membranes were
then incubated with the appropriate primary antibodies overnight at
4˚C. Following incubation with the primary antibodies anti-PPARγ
(1:1,000; cat. no. 95128; Cell Signaling Technology, Inc.),
anti-caspase-3 (1:1,000; cat. no. 9661; Cell Signaling Technology,
Inc.), anti-caspase-8 (1:1,000; cat. no. 8592; Cell Signaling
Technology, Inc.), and anti-β-actin (1:1,000; cat. no. 4970, Cell
Signaling Technology, Inc.), the membranes were incubated with the
relevant species-specific horseradish peroxidase-conjugated
secondary antibodies (1:1,000; cat. nos. 7074 and 7076; Cell
Signaling Technology, Inc.) for 1 h at room temperature. The
membranes were subsequently washed and protein bands were
visualized using a chemiluminescence detection system (Amersham;
Cytiva). The expression levels of specific proteins were normalized
to the expression levels of β-actin.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism software, version 8.0 (GraphPad Software, Inc.). Data are
presented as the mean ± SD of three independent experiments.
Comparisons among multiple groups were assessed by one-way or
two-way ANOVA followed by Bonferroni's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
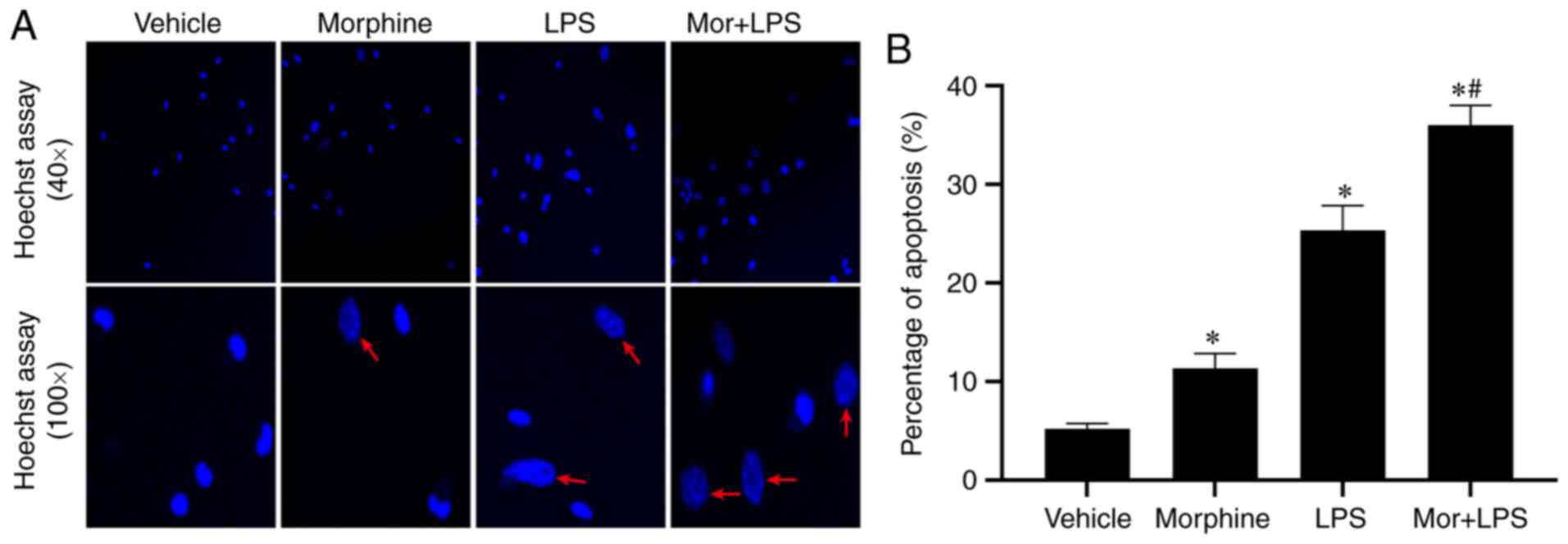
Morphine promotes apoptosis in
LPS-induced BMDMs
To investigate the effects of morphine on
LPS-induced BMDMs, the cells were incubated with morphine and/or
LPS, and the levels of apoptosis in each group were analyzed by
Hoechst 33342 staining. In the vehicle control group, few apoptotic
cells were observed, and the majority of the nuclei displayed
uniform morphology, intact membrane and even chromatin distribution
(Fig. 1A). Following treatment with
morphine or LPS alone, an increasing number of apoptotic cells with
morphological changes and uneven staining were detected,
particularly in the LPS group. Moreover, the characteristics of
apoptosis were more prominent in BMDMs challenged with LPS and
morphine in combination. In the combined treatment group, Hoechst
33342 staining revealed a larger number of blue fluorescent cells
with fragmented nuclei, chromatin condensation and apoptotic body
formation. Statistical analysis of the percentage of apoptotic
cells revealed that, compared with the control group, LPS
stimulation significantly increased the levels of apoptosis in
BMDMs, which were further stimulated by morphine treatment
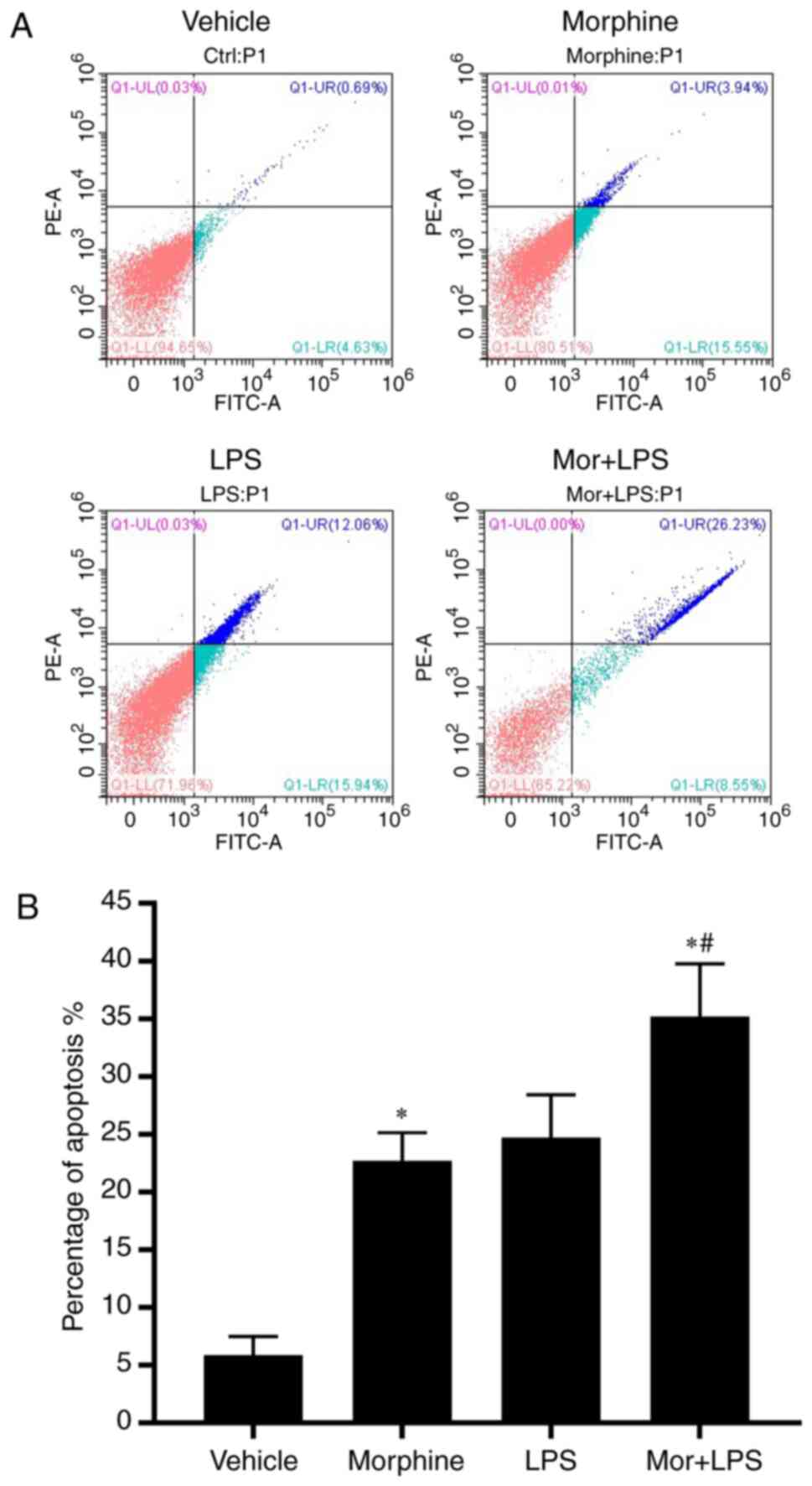
(Fig. 1B and C). The apoptosis of BMDMs was further
quantified by flow cytometric analysis following Annexin V-FITC and
PI double staining. As shown in Fig.
2, morphine treatment significantly increased the percentage of
apoptotic cells. Taken together, these findings indicated the
potential stimulatory effects of morphine on LPS-induced BMDM
apoptosis.
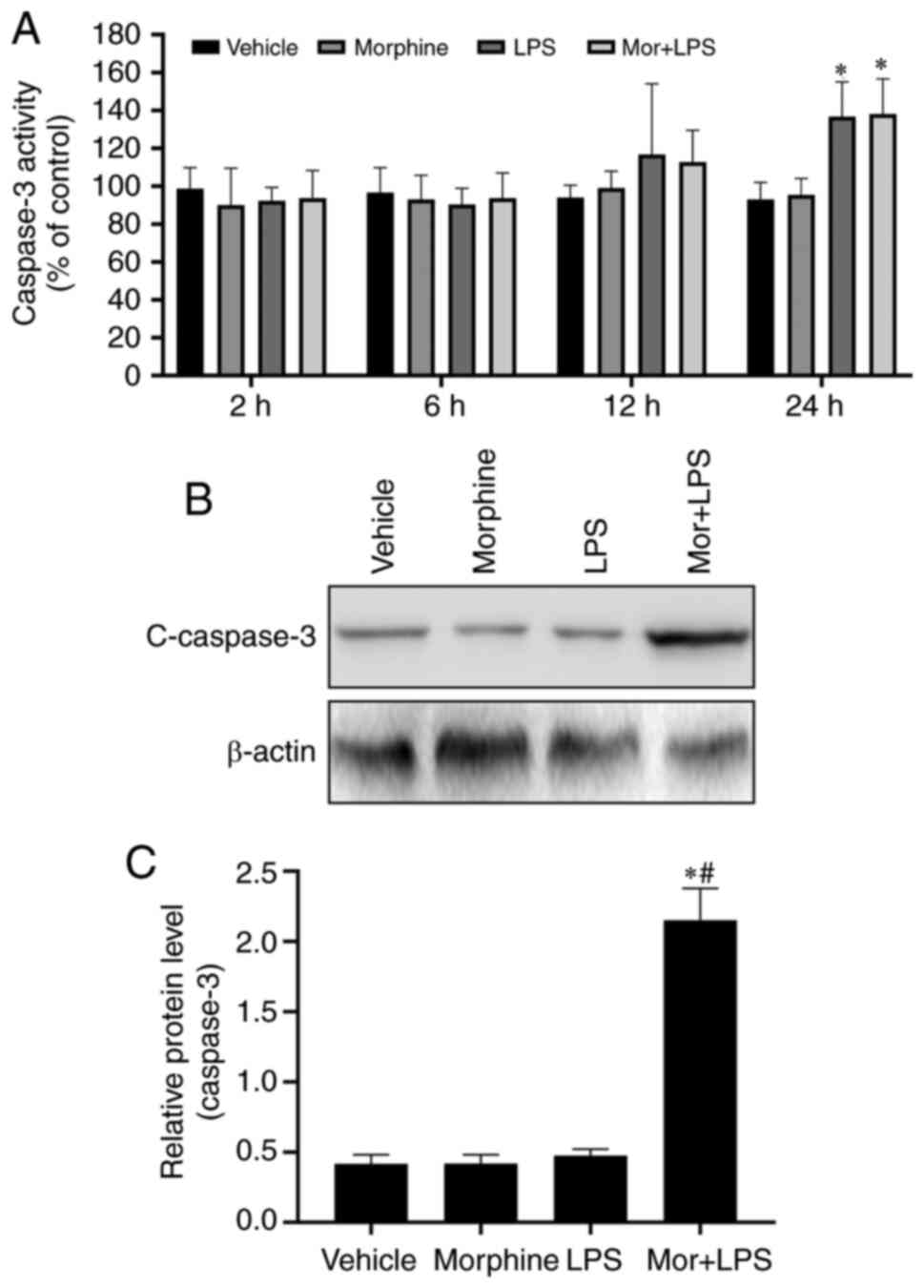
Morphine promotes the activation of
caspase-3 in LPS-induced BMDMs
The caspase-3 protease is the predominant effector
caspase involved in the execution of apoptosis, and may be
activated by both the extrinsic and intrinsic apoptotic pathways
(27). To determine whether
morphine treatment affected the activation of caspase-3 in
LPS-induced BMDMs, caspase-3 activity was determined using the
caspase-Glo 3/7 assay kit. The results revealed that morphine
treatment further promoted the LPS-induced activation of caspase-3
in a time-dependent manner (Fig.
3A). To validate these findings, the expression levels of
caspase-3 were analyzed using western blotting. Exposure to
morphine or LPS alone did not markedly upregulated the expression
levels of cleaved caspase-3, whereas the expression levels were
significantly higher in the combined treatment group compared with
either treatment alone (Fig. 3B and
C). These results indicated the
potentially important role of caspase-3 in the effects of morphine
on LPS-induced BMDMs.
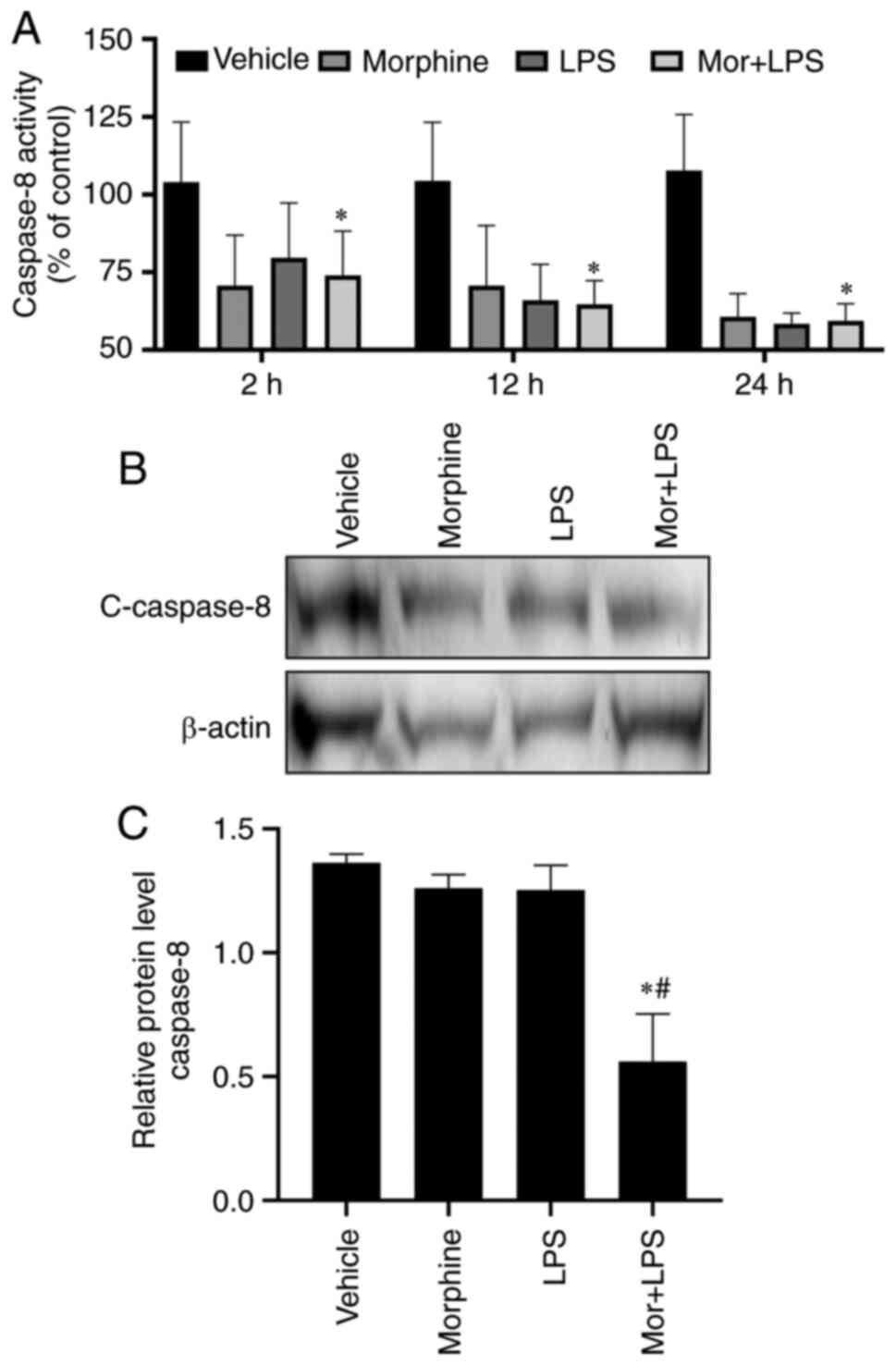
Morphine treatment reduces caspase-8
activity in LPS-activated BMDMs
Caspase-8 is a crucial upstream initiator that
cleaves and activates the effector caspase-3 in the death
receptor-triggered extrinsic pathway (27). Therefore, the activity and
expression levels of caspase-8 were investigated in the present
study. Notably, morphine or LPS treatment, alone or in combination,
significantly reduced the activity of caspase-8 at 2, 6, 8 and 12 h
(Fig. 4A). Western blotting
demonstrated that the expression levels of active caspase-8 subunit
p18 were downregulated in the LPS, morphine and combined treatment
groups (Fig. 4B and C). These results suggested that the
effects of morphine on BMDM apoptosis may be independent of
caspase-8 activation, indicating the potential involvement of the
intrinsic pathway in the apoptotic events.
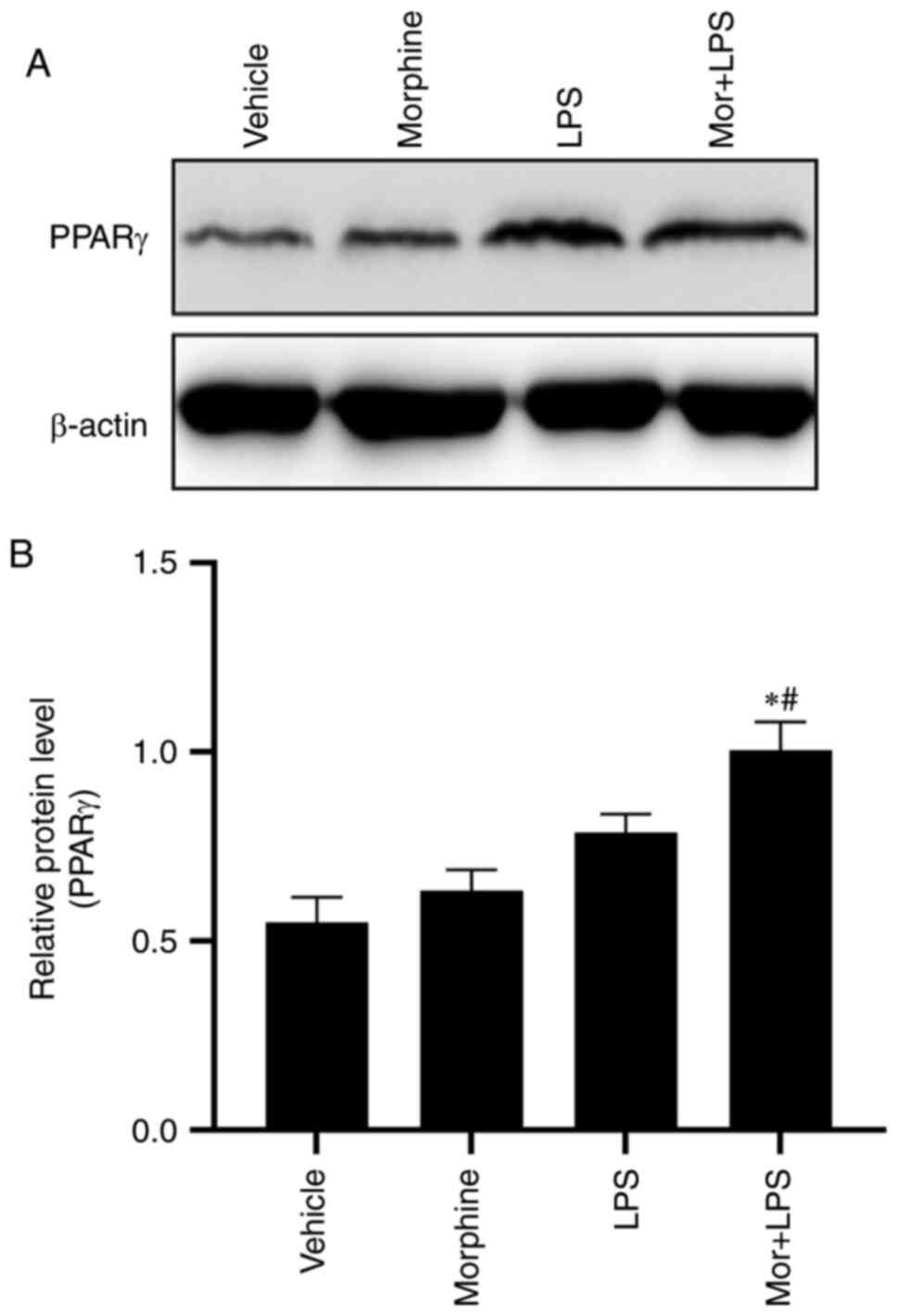
Morphine treatment upregulates
LPS-induced PPARγ expression in BMDMs
PPARγ was previously reported to be involved in the
tolerance to morphine analgesia (21). Considering the crucial role of PPARγ
in the regulation of macrophage apoptosis (18-20),
the present study hypothesized that PPARγ may participate in the
proapoptotic effect of morphine in LPS-stimulated BMDMs. Western
blotting was performed to determine the protein expression levels
of PPARγ in each group. Compared with the vehicle control group,
exposure to morphine or LPS treatment alone upregulated the protein
expression levels of PPARγ, although the observed difference was
not deemed statistically significant. Moreover, the upregulation of
PPARγ was significantly enhanced in the BMDMs challenged with
morphine in combination with LPS, suggesting a potential role of
PPARγ in the regulatory effect of morphine on LPS-stimulated BMDMs
(Fig. 5A and B).
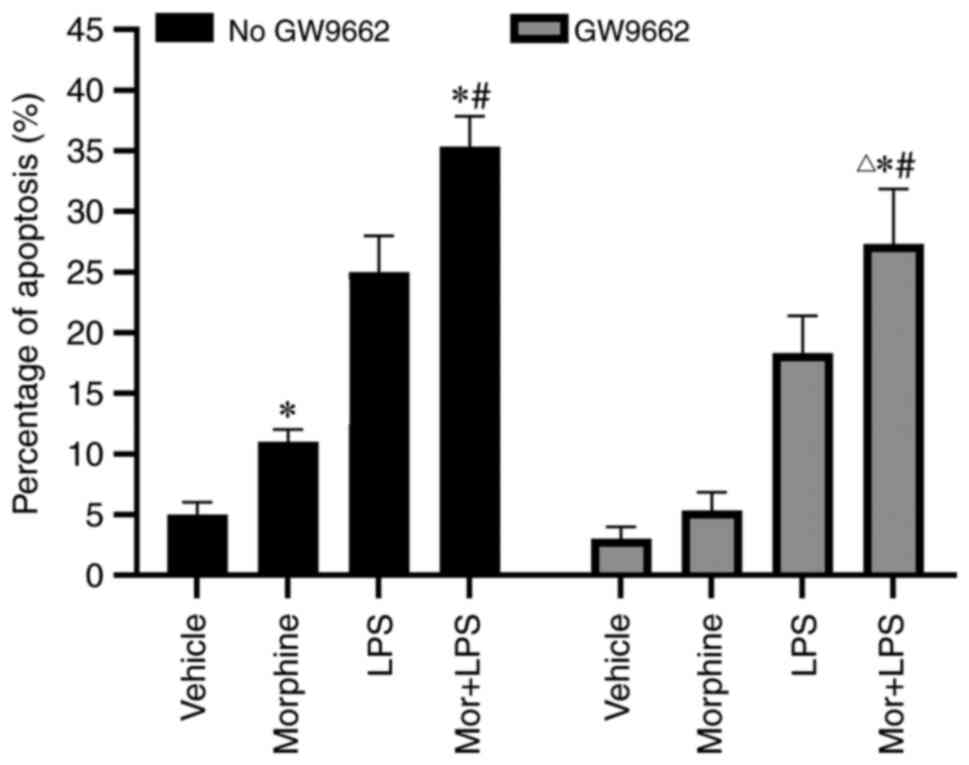
Proapoptotic effect of morphine is
dependent on PPARγ activation
To validate the role of PPARγ in morphine-induced
BMDM apoptosis, BMDMs were pre-incubated with GW9662, a specific
PPARγ antagonist, for 4 h prior to the treatment with morphine
and/or LPS. As shown in Fig. 6, the
morphine-induced increased percentage of apoptotic cells was
significantly reduced by GW9662 treatment, suggesting that
morphine-induced apoptosis of LPS-activated BMDMs may be mediated
via PPARγ activation.
PPARγ antagonist reverses
morphine-induced activation of caspase-3/9 in LPS-treated
BMDMs
The effects of PPARγ on morphine-induced caspase
activation were further evaluated. As mentioned above, morphine
treatment enhanced LPS-induced caspase-3 activation, which was
subsequently reversed by GW9662 pretreatment (Fig. 7A and B). A similar result was observed regarding
the activation of caspase-9, a major upstream initiator of
caspase-3 in the intrinsic pathway of apoptosis (28,29).
In addition, LPS stimulation significantly increased the activity
of caspase-9, which was further enhanced by LPS + morphine
treatment (Fig. 7C and D). GW9662 significantly abrogated the
stimulatory effect of morphine on LPS-induced caspase-9 activation
(Fig. 7C and D). However, GW9662 treatment exerted no
statistically significant effect on caspase-8 activity (Fig. 7E and F). Taken together, these results provided
further evidence to suggest that the intrinsic pathway of apoptosis
may be involved in the proapoptotic effects of morphine on
LPS-induced BMDMs, which may be dependent, at least partially, on
PPARγ activation.
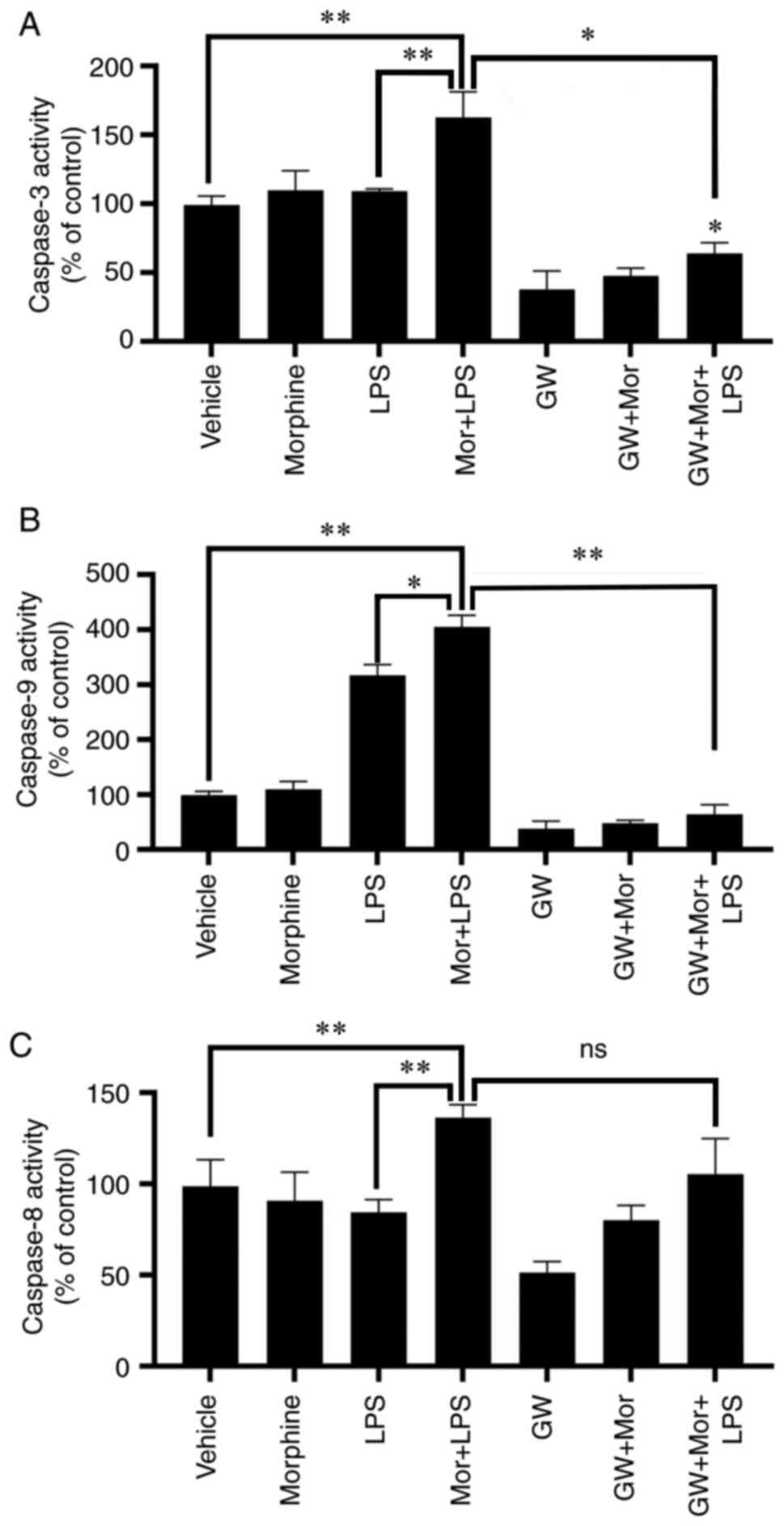 | Figure 7PPARγ antagonist reverses
morphine-induced activation of caspase-3 and -9 in LPS-induced
BMDMs. (A) Caspase-3, (B) caspase-9 and (C) caspase-8 activities
were analyzed in BMDMs treated with vehicle, morphine, LPS or
morphine + LPS, with or without treatment with the PPARγ inhibitor,
GW9662 (1 µM). Data are presented as the mean ± SD. Data are
presented as the mean ± SD and were determined by one-way ANOVA and
Bonferroni's post hoc test. *P<0.05,
**P<0.01 and ns indicated no significance (P>0.05)
vs. vehicle or the two groups connected by the umbrella lines in
the figure. BMDMs, bone marrow-derived macrophages; LPS,
lipopolysaccharide; PPARγ, peroxisome proliferator-activated
receptor-γ; ns, not significant. |
Discussion
Morphine has been widely used in the clinical
setting as an analgesic, and numerous studies have supported a role
for morphine in the regulation of apoptosis (30,31).
Although opioid receptors are crucial for opioid-mediated effects,
the molecular mechanisms underlying opioid-induced cell apoptosis
remain to be fully elucidated. The results of the present study
demonstrated that morphine enhanced LPS-induced apoptosis. Further
investigations revealed that morphine treatment potentiated
LPS-induced caspase-3 and caspase-9 activation, but inhibited the
activity of caspase-8, suggesting the involvement of the intrinsic
apoptosis pathway in the apoptotic events. In addition, morphine
exposure resulted in the upregulation of PPARγ expression levels.
More importantly, the stimulatory effects of morphine on
LPS-induced apoptosis and caspase-3/9 activation were significantly
reduced by GW9662, a PPARγ antagonist. Taken together, these
results revealed a regulatory role for morphine in LPS-induced BMDM
apoptosis and provided evidence supporting the involvement of PPARγ
in the mechanisms underlying these observed effects.
To date, at least two major mechanisms have been
discovered to be involved in the initiation of apoptosis: The
extrinsic (death receptor-induced) and intrinsic
(mitochondria-mediated) pathways (8,32).
Both pathways are dependent on the activation of the caspase family
of the cysteine proteases, which together act as a proteolytic
cascade to dismantle and remove dying cells (8,30). In
the extrinsic pathway, death receptor stimulation leads to the
recruitment and activation of caspase-8, which subsequently
promotes the proteolysis of the downstream effector caspase-3 or
other caspases, such as caspase-1 and -7. In addition, caspase-8
cleaves and activates the BH3-only protein, Bid, which can
translocate to the mitochondria and promote cytochrome c
release, resulting in caspase-9 and caspase-3 activation (8,32).
LPS, a well-established ligand of Toll-like receptor 4, is a
powerful stimulator of macrophages, which plays a key role in
regulating macrophage activation and the resultant inflammatory
response (33). It was also
reported that LPS may play a proapoptotic role by enhancing
caspase-3 activity and, thus, inducing the apoptosis of macrophages
(12,14). The results of the present study
revealed that morphine treatment acted synergistically with LPS and
potentiated LPS-induced caspase-3/9 activation in BMDMs.
Conversely, morphine or LPS treatment, alone or in combination,
significantly reduced the activity and expression levels of
caspase-8. Taken together, these results indicated the involvement
of the intrinsic pathway in the proapoptotic effects of morphine in
coordination with LPS. Considering the potent effects of LPS on
BMDM-mediated inflammation, future studies should aim to further
determine the potential function of morphine in LPS-induced
inflammation in BMDMs.
PPARγ, a member of the nuclear hormone receptor
superfamily, regulates cell proliferation, differentiation and
death (16,17). Previous studies have demonstrated
that the activation of PPARγ by agonists promoted macrophage
apoptosis, and the proapoptotic effect may be dependent on
negatively regulating NF-κB signaling, induction of cathepsin L and
regulation of vitamin D3-upregulated protein-1 expression levels
(18-20).
Although the mechanisms underlying PPARγ-mediated apoptosis remain
to be fully elucidated, recent evidence has demonstrated that PPARγ
activation promoted caspase-3 activity, while the transfection with
PPARγ antisense oligonucleotides resulted in the downregulation of
caspase-9 activity, indicating that the proapoptotic effects of
PPARγ may be dependent, at least in part, on the caspase-mediated
pathway (18-20).
The findings of the present study demonstrated that the exposure to
morphine upregulated PPARγ expression levels in LPS-induced BMDMs.
Moreover, pretreatment with GW9662, a selective PPARγ antagonist,
markedly abolished the stimulatory effects of morphine on
LPS-induced apoptosis and caspase-3/9 activation. It has been
reported that PPARγ plays a key role in the modulation of morphine
tolerance (34). In the present
study, PPARγ was found to be involved in the regulation of
morphine-induced cell apoptosis, which may provide novel insight
into the possible mechanisms underlying the biological function of
morphine. However, to the best of our knowledge, the mechanism
through which morphine upregulates PPARγ expression in BMDMs has
not been reported to date. Thus, further research is required to
elucidate the exact mechanism underlying the regulatory role of
morphine in PPARγ expression.
Morphine is the most well-characterized and commonly
used analgesic in the clinical setting (1). Following in-depth analysis of the
pharmacological effects of morphine, a large number of studies have
reported its numerous biological functions, including regulation of
autophagy and neuroimmune modulation (35,36).
Morphine is recommended for the alleviation of chest pain during
acute coronary syndromes (37,38).
In addition to pain relief, several previous studies have
demonstrated a cardioprotective effect of morphine treatment
(39-41).
In animal studies, morphine was reported to protect against
ischemia-reperfusion injury by significantly reducing infarct size
and improving heart function (42,43).
The results of the present study revealed a regulatory effect of
morphine on PPARγ expression and the resultant apoptosis of BMDMs,
which may expand the potential clinical applications of morphine to
include acute myocardial infarction (AMI). Numerous previous
studies have suggested an anti-atherogenic role of PPARγ in
macrophages by reducing the inflammatory response (14,18,44).
In addition, macrophage apoptosis, as the predominant pathway for
macrophage removal from the plaque, was found to play a crucial
role in atherosclerosis progression and plaque stability (45,46).
During the early stages of AMI, the administration of morphine was
found to not only exert an analgesic effect, but also improve
myocardial injury, alleviate the local inflammatory response and
promote plaque stability, which may be attributed to its regulatory
function over PPARγ expression and macrophage apoptosis. Therefore,
further studies are required to provide novel insight into the
potential therapeutic and clinical applications of morphine beyond
its analgesic properties.
In conclusion, the findings of the present study
demonstrated that morphine treatment enhanced the LPS-induced
apoptosis of BMDMs. Regarding the molecular mechanisms underlying
morphine-mediated apoptosis of LPS-activated BMDMs, it was
demonstrated that the co-administration of morphine facilitated the
LPS-induced activation of caspase-3/9, but inhibited caspase-8
activity, indicating the involvement of the intrinsic pathway in
the apoptotic events. In addition, morphine treatment upregulated
LPS-induced PPARγ expression levels in BMDMs, while the PPARγ
antagonist, GW9662, markedly abrogated the stimulatory effects of
morphine on LPS-induced apoptosis and caspase activation. Taken
together, these results provide what is, to the best of our
knowledge, the first evidence to suggest that the intrinsic pathway
of apoptosis may be involved in the proapoptotic effects of
morphine on LPS-induced BMDMs, which may be dependent, at least
partially, on PPARγ activation.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Natural Science
Foundation (grant no. 81670409).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW designed the study; MYL and YL performed the
experiments; KQD analyzed the data; MYL performed the biological
analysis; MYL and KQD collected and analyzed the data; MYL, KQD and
YL sacrificed the mice and performed the caspase assays; MYL
performed the western blot experiments; KQD determined the quality
of the BMDMs and performed the western blot experiments; MYL and JW
wrote the manuscript. MYL and JW confirm the authenticity of all
the raw data. All the authors have read and approved the final
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee of the Zhongnan Hospital of Wuhan
University (Wuhan, China; approval no. AF165).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Puig S and Gutstein HB: Opioids: Keeping
the good, eliminating the bad. Nat Med. 23:272–273. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Tuerxun H and Cui J: The dual effect of
morphine on tumor development. Clin Transl Oncol. 21:695–701.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Plein LM and Rittner HL: Opioids and the
immune system-friend or foe. Br J Pharmacol. 175:2717–2725.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bajic D, Commons KG and Soriano SG:
Morphine-enhanced apoptosis in selective brain regions of neonatal
rats. Int J Dev Neurosci. 31:258–266. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li Y, Li H, Zhang Y, Sun X, Hanley GA,
LeSage G, Zhang Y, Sun S, Peng Y and Yin D: Toll-like receptor 2 is
required for opioids-induced neuronal apoptosis. Biochem Bioph Res
Commun. 391:426–430. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Osmanloglu O, Yldirim MK, Akyuva Y,
Yildizhan K and Naziroglu M: Morphine induces apoptosis,
inflammation, and mitochondrial oxidative stress via activation of
TRPM2 channel and nitric oxide signaling pathways in the
hippocampus. Mol Neurobiol. 57:3376–3389. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Weng HL and Wang MJ: Effects of
microRNA-338-3p on morphine-induced apoptosis and its underlying
mechanisms. Mol Med Rep. 14:2085–2092. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Singh R, Letai A and Sarosiek K:
Regulation of apoptosis in health and disease: The balancing act of
BCL-2 family proteins. Nat Rev Mol Cell Biol. 20:175–193.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gonzalez L and Trigatti BL: Macrophage
apoptosis and necrotic core development in atherosclerosis: A
rapidly advancing field with clinical relevance to imaging and
therapy. Can J Cardiol. 33:303–312. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Davis TME, Peters KE and Lipscombe R:
Apoptosis inhibitor of macrophage and diabetic kidney disease. Cell
Mol Immunol. 16(521)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li Z and Weinman SA: Regulation of hepatic
inflammation via macrophage cell death. Semin Liver Dis.
38:340–350. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
George L, Ramasamy T, Sirajudeen KNS and
Manickam V: LPS-induced apoptosis is partially mediated by hydrogen
sulphide in RAW 264.7 murine macrophages. Immunol Invest.
48:451–465. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xaus J, Comalada M, Valledor AF, Lloberas
J, López-Soriano F, Argilés JM, Bogdan C and Celada A: LPS induces
apoptosis in macrophages mostly through the autocrine production of
TNF-alpha. Blood. 95:3823–3831. 2000.PubMed/NCBI
|
|
14
|
Du P, Li SJ, Ojcius DM, Li KX, Hu WL, Lin
X, Sun AH and Yan J: A novel Fas-binding outer membrane protein and
lipopolysaccharide of Leptospira interrogans induce macrophage
apoptosis through the Fas/FasL-caspase-8/-3 pathway. Emerg Microbes
Infec. 7(135)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bhat RS, Bhaskaran M, Mongia A, Hitosugi N
and Singhal PC: Morphine-induced macrophage apoptosis: Oxidative
stress and strategies for modulation. J Leukocyte Biol.
75:1131–1138. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ahmadian M, Suh JM, Hah N, Liddle C,
Atkins AR, Downes M and Evans RM: PPARγ signaling and metabolism:
The good, the bad and the future. Nat Med. 19:557–566.
2013.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Wang SB, Dougherty EJ and Danner RL: PPARγ
signaling and emerging opportunities for improved therapeutics.
Pharmacol Res. 111:76–85. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mahmood DFD, Jguirim-Souissi I, Khadija
EH, Blondeau N, Diderot V, Amrani S, Slimane MN, Syrovets T, Simmet
T and Rouis M: Peroxisome proliferator-activated receptor gamma
induces apoptosis and inhibits autophagy of human monocyte-derived
macrophages via induction of cathepsin L: Potential role in
atherosclerosis. J Biol Chem. 286:28858–28866. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Billiet L, Furman C, Larigauderie G, Copin
C, Page S, Fruchart JC, Brand K and Rouis M: Enhanced VDUP-1 gene
expression by PPARgamma agonist induces apoptosis in human
macrophage. J Cell Physiol. 214:183–191. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chinetti G, Griglio S, Antonucci M, Torra
IP, Delerive P, Majd Z, Fruchart JC, Chapman J, Najib J and Staels
B: Activation of proliferator-activated receptors alpha and gamma
induces apoptosis of human monocyte-derived macrophages. J Biol
Chem. 273:25573–25580. 1998.PubMed/NCBI View Article : Google Scholar
|
|
21
|
de Guglielmo G, Kallupi M, Scuppa G,
Stopponi S, Demopulos G, Gaitanaris G and Ciccocioppo R: Analgesic
tolerance to morphine is regulated by PPARγ. Br J Pharmacol.
171:5407–5416. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Weischenfeldt J and Porse B: Bone
marrow-derived macrophages (BMM): Isolation and applications. CSH
Protoc 2008: pdb.prot5080, 2008.
|
|
23
|
Wan J, Xiao Z, Chao S, Xiong S, Gan X, Qiu
X, Xu C, Ma Y and Tu X: Pioglitazone modulates the proliferation
and apoptosis of vascular smooth muscle cells via peroxisome
proliferators-activated receptor-gamma. Diabetol Metab Syndr.
6(101)2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Pazár B, Ea HK, Narayan S, Kolly L,
Bagnoud N, Chobaz V, Roger T, Lioté F, So A and Busso N: Basic
calcium phosphate crystals induce monocyte/Macrophage IL-1β
secretion through the NLRP3 inflammasome in vitro. J Immunol.
186:2495–2502. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li CG, Yan L, Mai FY, Shi ZJ, Xu LH, Jing
YY, Zha QB, Ouyang DY and He XH: Baicalin inhibits NOD-like
receptor family, pyrin containing domain 3 inflammasome activation
in murine macrophages by augmenting protein kinase a signaling.
Front Immunol. 8(1409)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Guo S, Sun X, Cheng J, Xu H, Dan J, Shen
J, Zhou Q, Zhang Y, Meng L, Cao W and Tian Y: Apoptosis of THP-1
macrophages induced by protoporphyrin IX-mediated sonodynamic
therapy. Int J Nanomedicine. 8:2239–2246. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Brentnall M, Rodriguez-Menocal L, De
Guevara RL, Cepero E and Boise LH: Caspase-9, caspase-3 and
caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell
Biol. 14(32)2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kurokawa M and Kornbluth S: Caspases and
kinases in a death grip. Cell. 138:838–854. 2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yenikomshian HA, Curtis EE, Carrougher GJ,
Qiu Q, Gibran NS and Mandell SP: Outpatient opioid use of burn
patients: A retrospective review. Burns. 45:1737–1742.
2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Maher DP, Walia D and Heller NM: Morphine
decreases the function of primary human natural killer cells by
both TLR4 and opioid receptor signaling. Brain Behavior Immunity.
83:298–302. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kroemer G and Martin SJ:
Caspase-independent cell death. Nat Med. 11:725–730.
2005.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Shapouri-Moghaddam A, Mohammadian S,
Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi
A, Afshari JT and Sahebkar A: Macrophage plasticity, polarization,
and function in health and disease. J Cell Physiol. 233:6425–6440.
2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Javadi S, Ejtemaeimehr S, Keyvanfar HR,
Moghaddas P, Aminian A, Rajabzadeh A, Mani AR and Dehpour AR:
Pioglitazone potentiates development of morphine-dependence in
mice: Possible role of NO/cGMP pathway. Brain Res. 1510:22–37.
2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhao L, Zhu Y, Wang D, Chen M, Gao P, Xiao
W, Rao G, Wang X, Jin H, Xu L, et al: Morphine induces Beclin 1-and
ATG5-dependent autophagy in human neuroblastoma SH-SY5Y cells and
in the rat hippocampus. Autophagy. 6:386–394. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Koob GF: Neurobiology of opioid addiction:
Opponent process, hyperkatifeia, and negative reinforcement. Biol
Psychiat. 87:44–53. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Task Force on the management of ST-segment
elevation acute myocardial infarction of the European Society of
Cardiology (ESC). Steg PG, James SK, Atar D, Badano LP,
Blömstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq
G, et al: ESC guidelines for the management of acute myocardial
infarction in patients presenting with ST-segment elevation. Eur
Heart J. 33:2569–2619. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
O'Gara PT: 2013 ACCF/AHA guideline for the
management of ST-elevation myocardial infarction: A report of the
American College of Cardiology Foundation/American Heart
Association Task Force on Practice Guidelines (vol 127, pp e362,
2013). Circulation. 128:e362–e425. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Murphy GS, Szokol JW, Marymont JH, Avram
MJ and Vender JS: Opioids and cardioprotection: The impact of
morphine and fentanyl on recovery of ventricular function after
cardiopulmonary bypass. J Cardiothor Vasc Anesth. 20:493–502.
2006.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Tanaka K, Kersten JR and Riess ML:
Opioid-induced cardioprotection. Curr Pharm Design. 20:5696–5705.
2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Rentoukas I, Giannopoulos G, Kaoukis A,
Kossyvakis C, Raisakis K, Driva M, Panagopoulou V, Tsarouchas K,
Vavetsi S, Pyrgakis V and Deftereos S: Cardioprotective role of
remote ischemic periconditioning in primary percutaneous coronary
intervention enhancement by opioid action. JACC Cardiovasc Interv.
3:49–55. 2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wang Y, Wang L, Li JH, Zhao HW and Zhang
FZ: Morphine alleviates myocardial ischemia/reperfusion injury in
rats by inhibiting TLR4/NF-κB signaling pathway. Eur Rev Med
Pharmaco. 23:8616–8624. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chen ZL, Li TZ and Zhang BX: Morphine
postconditioning protects against reperfusion injury in the
isolated rat hearts. J Surg Res. 145:287–294. 2008.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Oppi S, Nusser-Stein S, Blyszczuk P, Wang
X, Jomard A, Marzolla V, Yang K, Velagapudi S, Ward LJ, Yuan XM, et
al: Macrophage NCOR1 protects from atherosclerosis by repressing a
pro-atherogenic PPARγ signature. Eur Heart J. 41:995–1005.
2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Subramanian M, Thorp E and Tabas I:
Identification of a non-growth factor role for GM-CSF in advanced
atherosclerosis promotion of macrophage apoptosis and plaque
necrosis through IL-23 signaling. Circ Res. 116:e13–e24.
2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Linton MF, Babaev VR, Huang JS, Linton EF,
Tao H and Yancey PG: Macrophage apoptosis and efferocytosis in the
pathogenesis of atherosclerosis. Circ J. 80:2259–2268.
2016.PubMed/NCBI View Article : Google Scholar
|