Introduction
Esophageal squamous cell carcinoma (ESCC) is one of
the most common types of malignant disease in the world and
particularly in China (1). It is
the fourth most common cause of cancer-associated death in China
(1). Xinjiang is one of the areas
with a high incidence of ESCC. Of note, in Kazakh, the mortality
rate for ESCC is as high as 68.88% (2,3). Due
to extensive lymphatic drainage, the majority of patients with ESCC
are diagnosed at a late stage and the malignancy easily
metastasizes at an early stage (4,5). The
treatment of ESCC is based on surgery combined with radiotherapy or
chemotherapy (4,5). The discovery of genes associated with
the occurrence and development of the disease and investigation of
the underlying mechanisms are of great significance for the
development of specific targeted drugs that may be used for the
treatment of this disease.
Our team has been investigating the classical Wnt
signaling pathway as a molecular mechanism associated with the
occurrence and development of ESCC (6). In cervical cancer, enhancer of zeste
homolog 2 (EZH2) promotes cell proliferation and tumor formation in
cervical cancer through activation of the Wnt/β-catenin pathway via
GSK-3β- and TP53-mediated epigenetic silencing (7). However, the association of EZH2 with
the development of ESCC and associated molecular mechanisms have
remained largely elusive.
EZH2 is a key component of the polycomb repressive
complex 2 complex, which catalyzes the trimethylation of histone H3
lysine 27 to promote transcriptional silencing that maintains cell
integrity during development by promoting chromatin modifications
(7,8). EZH2 promotes cancer formation and
progression through epigenetic activation of oncogenic signaling
cascades and inhibition of pro-differentiation pathways (9). Studies have suggested that EZH2 is
overexpressed in cancer and functions as an oncogene. Its
expression correlates with poor patient prognosis in various cancer
types by mediating the expression of target genes involved in
tumorigenesis (10-12).
In addition, EZH2 has been indicated to act as an epigenetic
modifier during the TGF-β-induced epithelial-mesenchymal transition
(EMT) (12). It was also reported
that EZH2 is a member of the SRY-related HMG box (SOX) family
signaling cascade and SOX4 directly activates EZH2 (13-15).
SOX4 is the core protein involved in ESCC and its
overexpression is attributed to both gene amplification and
activation of the PI3K, Wnt and TGF-β pathways (16). It has been identified as a master
regulator of invasion and metastasis, acting upstream of several
EMT inducers; EMT is a cellular biological process involved in the
migration of primary cancer cells to secondary sites to cause
metastasis (13,14). SOX4 regulates several processes,
such as the induction of cell survival, stemness, EMT, migration
and metastasis (16). Studies
including that by Tiwari et al (14) delineated a pathway wherein TGF-β
stimulates SOX4 expression, thereby reprogramming the epigenome to
elicit metastasis of cancers (17).
SOX4 overexpression is elevated in a wide variety of human cancer
types and correlates with cancer progression and poor prognosis in
prostate cancer (18), cutaneous
melanoma (19) and breast cancer
(20), suggesting the potential
role of SOX4 in tumor progression. It has been previously reported
that SOX4 activates EZH2 in a variety of cancers (10,13,15).
Above all, the effects of the SOX4-EZH2 signaling pathway were
indicated to be able to predict the presence of metastasis and
invasion of ESCC, as well as poor prognosis of affected patients
(21).
Metastasis-associated protein 1 (MTA1) is a
well-known oncogene that drives the metastasis of various cancer
types (22). The
metastasis-associated proteins are a family of co-regulators, which
include MTA1, MTA2 and MTA3. MTA members are primarily involved in
regulating target gene expression through the deacetylation of
histones (23). The effects of MTA1
are attributed to the regulation of various cancer-promoting
processes. These include the canonical Wnt1/β-catenin signaling
pathway, the stabilization of hypoxia-inducible factor-1α and the
regulation of invasion and metastasis via repression of adhesion
molecules including E-cadherin (24-27).
In ESCC, MTA1 has been reported to promote tumor metastasis and
invasion (21). MTA1 is able to
regulate the expression of EMT-associated factors in both normal
and cancerous cells (8,28) and predict cancer aggressiveness and
adverse clinical outcomes in a wide range of tumor types, as
evidenced by previous studies by our and other groups (29-31).
It was predicted that the role of MTA1 in ESCC may be associated
with SOX4. The purpose of the present study was to determine the
expression levels of MTA1, SOX4 and EZH2 in ESCC and to investigate
their association with clinicopathological parameters. The effects
of MTA1, SOX4 and EZH2 on the prognosis of ESCC were analyzed in
detail. The association between MTA1, SOX4 and EZH2 was analyzed by
Spearman correlation analysis. The aim of the present study was to
identify target genes that inhibit the proliferation of ESCC cells
to facilitate the development of targeted drugs for the treatment
of ESCC. Determination of the function of the MTA1/SOX4/EZH2 axis
in ESCC and its influence on clinicopathological parameters and
disease prognosis may lead to the development of strategies to
predict and improve outcomes of ESCC.
Materials and methods
Patients and tissue samples
A total of 229 cases of ESCC were collected from
January 2008 to December 2018 at the First Affiliated Hospital of
Xinjiang Medical University (Urumqi, China). The inclusion criteria
were as follows: Patients diagnosed with ESCC between January 2008
and December 2018, the presence of SCC of the esophagus, the
absence of radiotherapy or chemotherapy prior to surgery,
esophageal malignancy as the major treatment focus and subjects of
the Han and Kazakh ethnicities. The exclusion criteria were as
follows: Adenocarcinoma of the esophagus, patients who had received
radiotherapy or chemotherapy prior to surgery, tumor metastases to
the esophagus and other ethnicities, such as Uygur and Mongolian. A
total of 229 patients with ESCC were randomly selected for the
present study. Paraffin-embedded ESCC tissues and matched
noncancerous tissues were collected from a total of 229 cases, 119
of which were of Han and 110 of Kazak ethnicity. The resected
specimens of these 229 patients were diagnosed as ESCCs at the
pathology department. All 229 patients were treated by surgery and
without any preoperative radiochemotherapy. The following
information was recorded for each patient: Age, sex, ethnicity,
tumor location, tumor size, degree of differentiation and TNM
staging, lymph node status, vascular invasion, nerve invasion,
postoperative radio-chemotherapy and progression of disease
(32). The present study was
approved by the Ethics committee of the First Affiliated Hospital
of Xinjiang Medical University (Urumqi, China). The follow-up ended
in July 2020 and information was obtained from the patients'
medical records and telephone calls.
Tissue microarray (TMA) and
immunohistochemistry (IHC)
Paraffin-embedded tissue blocks of representative
tumor and normal control tissue samples were selected by reviewing
the hematoxylin and eosin-stained slides. Tissue cores with a
diameter of 1.5 mm were extracted from each donor block and
precisely arrayed into a new paraffin receptacle block with a
maximum of 200 cores by using the Organization Microarrayer
(Pathology Devices, Inc.). The sections (4 µm) were obtained from
formalin-fixed and paraffin-embedded TMA blocks, mounted on
poly-L-lysine-coated glass slides and used for IHC.
The sections were deparaffinized with xylene,
rehydrated in a graded alcohol series and heated in a microwave
oven for antigen retrieval. To enhance antigen retrieval, the
slides were autoclaved for 20 min in 1% sodium citrate buffer (pH
6.0) and subsequently left at room temperature. Endogenous
peroxidase activity was blocked with 3% H2O2
at 37˚C for 20 min. The sections were incubated with primary
antibodies against SOX4 (cat. no. bs-11208R; 1:200 dilution;
BIOSS), MTA1 (cat. no. bs-1412R; 1:200 dilution; BIOSS) and EZH2
(cat. no. 5246; 1:50 dilution; Cell Signaling Technology, Inc.)
overnight at 4˚C. After the primary antibodies were rinsed off, the
sections were incubated with biotinylated secondary antibody (cat.
no. SP-9001; OriGene Technologies, Inc.) for 30 min at 37˚C. The
sections were then incubated with streptavidin horseradish
peroxidase for an additional 30 min (LSAB kit; Dako; Agilent
Technologies, Inc.) and stained with 3,3'-diaminobenzidine. The
sections were counterstained with hematoxylin, dehydrated and
mounted. Any sections in which primary antibodies were omitted were
used as negative controls.
Evaluation of IHC
Immunostaining was examined under a light microscope
by two pathologists who were blinded to the experimental
conditions. The intensity of immunoreactivity was scored as
follows: 0, no staining; 1, weak staining; 2, moderate staining;
and 3, strong staining. The percentage of stained cells ranged from
0 to 100%. The percentage of positive cells was scored for SOX4
expression according to the following criteria: 0 (0% positively
stained tumor cells), 1 (1-25% positively stained tumor cells), 2
(26-50% positively stained tumor cells), 3 (51-75% positively
stained tumor cells) and 4 (76-100% positively stained tumor
cells). MTA1 was located in the cytoplasm and nucleus and its
expression intensity was scored as follows: 0 (none), 1 (<10%
positively stained tumor cells), 2 (11-50% positively stained tumor
cells), 3 (51-80% positively stained tumor cells) and 4 (81-100%
positively stained tumor cells). The staining index was calculated
using the following formula: Percentage of positive cells x
staining intensity score. The possible staining indexes calculated
were 0, 1, 2, 3, 4, 6, 9 and 12. For MTA1 and SOX4, samples with a
score of ≥4 were considered positive. For EZH2 expression, tumor
cells with nuclear staining were considered positive and all scores
were applied to discriminate between positive (score ≥3) and
negative (score <3) staining (18,33,34).
Statistical analysis
The association of expression data with
clinicopathological characteristics was determined with the
χ2 and the Fisher's exact tests. The association between
the expression levels of MTA1, SOX4 and EZH2 was assessed by
Spearman correlation analysis. As survival outcomes, overall
survival (OS) and progression-free survival (PFS) were determined.
PFS was defined as the time from diagnosis of ESCC to the time of
tumor progression or death. Univariate analyses were used to assess
the impact of various parameters on survival in the Kaplan-Meier;
univariate and multivariate analyses were used to assess the impact
of various parameters on survival in via Cox hazard regression
analysis. The Kaplan-Meier method was used to analyze the
clinicopathological parameters and tumor marker expression
associated with the prognosis of ESCC. Cox hazard regression
analysis was an additional analysis of independent factors
associated with the prognosis of ESCC on the basis of the
Kaplan-Meier method, log-rank tests were used to determine
significant differences between Kaplan-Meier curves. The covariates
of the Cox hazard regression analysis included MTA1, SOX4 and EZH2
expression, age, sex, ethnicity, tumor location, tumor size, degree
of differentiation, TNM stage, lymph node status, vascular
invasion, nerve invasion and postoperative radiochemotherapy. The
results of the Cox hazard regression analysis and multinomial
logistic regression analysis are expressed as the hazard ratio (HR)
and 95% CI. P<0.05 was considered to indicate a statistically
significant difference. All data were processed and statistically
analyzed using SPSS statistics 23.0 software (IBM Corp.).
Results
Expression of MTA1, SOX4 and EZH2 and
its association with clinicopathological parameters of ESCC
Clinicopathological data for the cohort are listed
in Table SI. The results of the
IHC staining demonstrated that EZH2 expression was localized in the
nucleus of tumor cells. Two patterns of expression of MTA1 and SOX4
were evident, namely in the cytoplasm and nucleus (Fig. 1A-I). In normal esophageal tissues,
MTA1, SOX4 and EZH2 were not expressed or only expressed in the
basal layer cells. Among the 229 cases of ESCC, 194 were positive
for MTA1 expression and 35 cases were negative, resulting in a
positive rate of MTA1 expression in ESCC of 84.72%. A total of 152
cases were positive for SOX4, while 76 cases were negative
(positive rate for SOX4, 66.81%). EZH2 expression was positive in
95 and negative in 134 cases of ESCC (positive rate, 41.48%).
However, they were completely negative in normal control tissues.
The association between the positive expression of the three
proteins and the clinicopathological parameters was assessed in the
229 cases of ESCC. The association of expression data with
clinicopathological characteristics was determined. High expression
of MTA1 was associated with ethnicity (P<0.001) and lymph node
metastasis (P=0.037); high expression of SOX2 was associated with
age (P=0.016) and ethnicity (P=0.035); and high expression of EZH2
was associated with degree of differentiation (P=0.003). The
results are presented in Table
I.
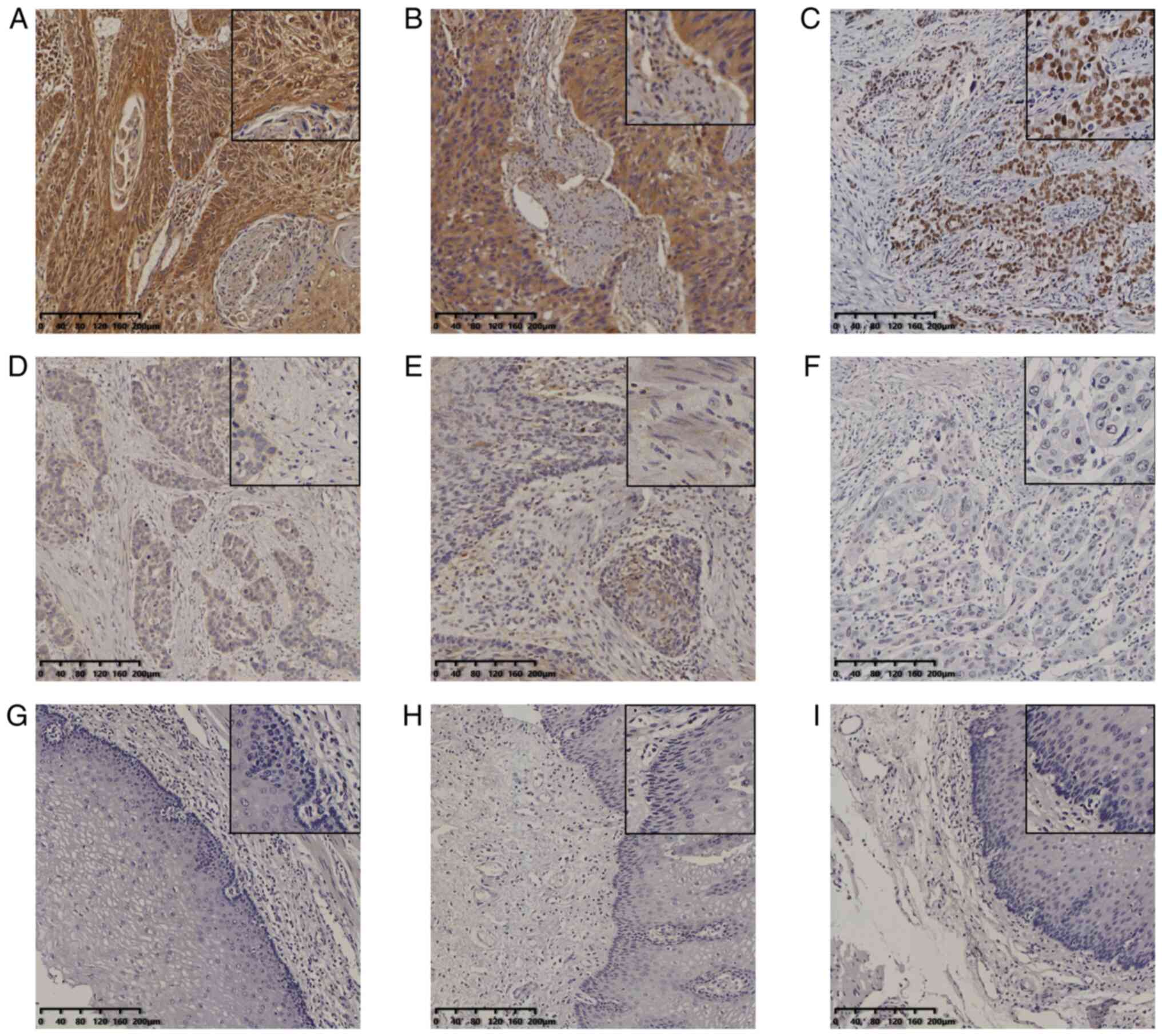 | Figure 1Expression of MTA1, SOX4 and EZH2 in
ESCC and normal esophageal tissues. Immunohistochemical staining of
ESCC for (A) MTA1 (positive), (B) SOX4 (positive), (C) EZH2
(positive), (D) SOX4 (negative), (E) SOX4 (negative) and (F) EZH2
(negative). Staining of normal esophageal tissues for (G and H)
SOX4 (negative) and (I) EZH2 (negative) (magnification, x100 or
x400 in magnified window; scale bar, 200 µm). ESCC, esophageal
squamous cell carcinoma; MTA1, metastasis-associated protein 1;
EZH2, enhancer of zeste homolog 2. |
 | Table IAssociation between MTA1, SOX4 and
EZH2 expression and clinicopathological parameters in esophageal
squamous cell carcinoma. |
Table I
Association between MTA1, SOX4 and
EZH2 expression and clinicopathological parameters in esophageal
squamous cell carcinoma.
| | MTA1 | SOX4 | EZH2 |
|---|
| Item | Negative | Positive | P-value | Negative | Positive | P-value | Negative | Positive | P-value |
|---|
| Age (years) | | | 0.557 | | | 0.016 | | | 0.092 |
|
<60 | 11 (4.8) | 71 (31.0) | | 19 (8.3) | 63 (27.5) | | 54 (23.6) | 28 (12.2) | |
|
≥60 | 24 (10.5) | 123 (53.7) | | 57 (24.9) | 90 (39.3) | | 80 (34.9) | 67 (29.3) | |
| Ethnicity | | | <0.001 | | | 0.035 | | | 0.130 |
|
Han | 7 (3.1) | 112 (48.9) | | 32 (14.0) | 87 (38.0) | | 64 (27.9) | 55 (24.0) | |
|
Kazakh | 28 (12.2) | 82 (35.8) | | 44 (19.2) | 66 (28.8) | | 70 (30.6) | 40 (17.5) | |
| Degree of
differentiation | | | 0.395 | | | 0.725 | | | 0.003 |
|
PD | 10 (4.4) | 36 (15.7) | | 13 (5.7) | 33 (14.4) | | 35 (15.3) | 11 (4.8) | |
|
MD | 17 (7.4) | 106 (46.3) | | 42 (18.3) | 81 (35.4) | | 73 (31.9) | 50 (21.8) | |
|
WD | 8 (3.5) | 52 (22.7) | | 21 (9.2) | 39 (17.0) | | 26 (11.4) | 34 (14.8) | |
| Lymph node
metastasis | | | 0.037 | | | 0.665 | | | 0.289 |
|
No | 29 (12.7) | 126 (55.0) | | 50 (21.8) | 105 (45.9) | | 87 (38.0) | 68 (29.7) | |
|
Yes | 6 (2.6) | 68 (29.7) | | 26 (11.4) | 48 (21.0) | | 47 (20.5) | 27 (11.8) | |
Association between the expression
levels of MTA1, SOX4 and EZH2
The correlations among the expression levels of
MTA1, SOX4 and EZH2 determined by IHC staining were assessed in the
229 cases of ESCC. The expression of MTA1 was positively correlated
with SOX4 expression, as determined using Spearman's correlation
analysis (ρ=0.139; P=0.036; Table
II). The expression of MTA1 was not significantly correlated
with that of EZH2 (ρ=0.087; P=0.191; Table III); he expression of SOX4 did not
exhibit any correlation with that of EZH2 (ρ=-0.122; P=0.066;
Table II). This was different from
the experimental results reported in other studies (14-15),
possibly due to inter-individual differences, the sample size of
the cohort and the ethnic groups Kazak that were included (Table III).
 | Table IICorrelation of SOX4 with MTA1 and
EZH2. |
Table II
Correlation of SOX4 with MTA1 and
EZH2.
| | SOX4 | |
|---|
| Item | - | + | ρ | P-value |
|---|
| MTA1 | | | 0.139 | 0.036 |
|
- | 17 (7.4) | 18 (7.9) | | |
|
+ | 59 (25.8) | 135 (59.0) | | |
| EZH2 | | | -0.122 | 0.066 |
|
- | 38 (16.6) | 96 (41.9) | | |
|
+ | 38 (16.6) | 57 (24.9) | | |
 | Table IIICorrelation of MTA1 with EZH2. |
Table III
Correlation of MTA1 with EZH2.
| | MTA1 | |
|---|
| Item | - | + | ρ | P-value |
|---|
| EZH2 | | | 0.087 | 0.191 |
|
- | 24 (10.5) | 110 (48.0) | | |
|
+ | 11 (48.0) | 84 (36.7) | | |
Prognostic factors for OS and PFS
The 3-year and 5-year survival rates were calculated
separately in order to obtain more accurate data on prognosis.
Kaplan-Meier and Cox hazard regression analysis were proposed to
assess disease prognosis. The 5-year survival rate was 24.4% and
the 3-year survival rate was 37.1% for the 229 cases of ESCC.
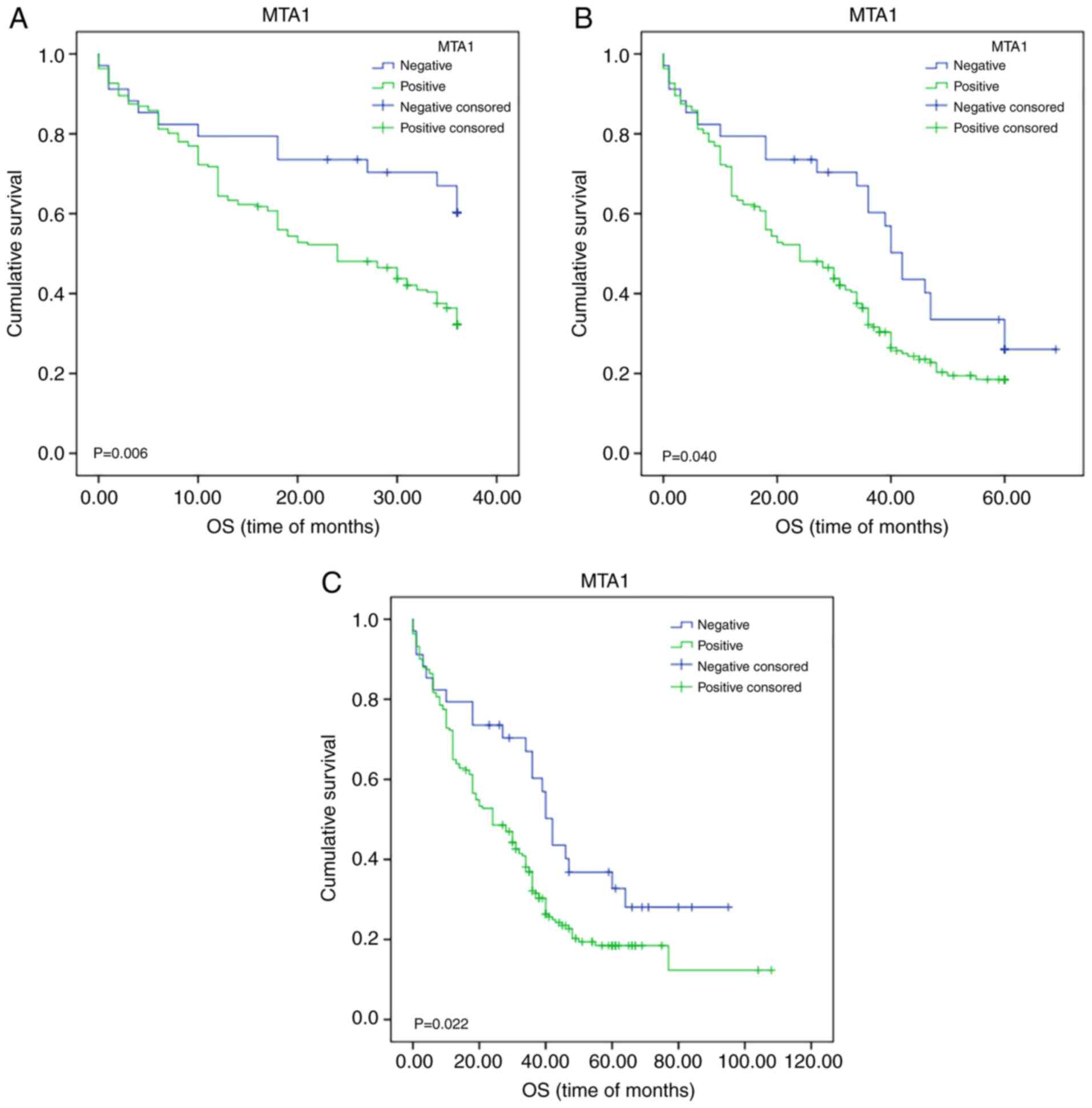
Kaplan-Meier survival analysis indicated that MTA1
expression had an impact on the OS of patients with ESCC.
Statistical analysis of 3-year survival demonstrated that it was
significantly associated with MTA1 expression (χ2=7.460;
P=0.006; Fig. 2A). Further subgroup
analysis (Table IV) indicated that
MTA1 expression also significantly affected survival in patients
based on age (≥60 group; P=0.024), sex (male group; P=0.001),
ethnicity (Kazakh group; P=0.013), tumor size (<3 cm group;
P=0.031), degree of differentiation (moderate degree of
differentiation group; P=0.014), lymph node metastasis (no specific
group; P=0.043), depth of invasion (full-thickness group; P=0.006),
TNM stage (IVA+B group; P=0.043), vascular invasion (no invasion
group; P=0.034), nerve invasion (no invasion group; P=0.006),
hematogenous metastasis (no metastasis group; P=0.009) and in
postoperative chemoradiotherapy (no chemotherapy group; P=0.031).
Statistical analysis of 5-year survival revealed a significant
association of the latter with MTA1 expression levels in all groups
(χ2=4.198; P=0.040; Fig.
2B). Further subgroup analysis indicated that MTA1 expression
also significantly affected survival in patients of a specific sex
(male group; P=0.020), ethnicity (Kazakh group; P=0.010), depth of
invasion (full-thickness group; P=0.029) degree of differentiation
(moderate degree of differentiation group; P=0.012) and in nerve
invasion (no invasion group; P=0.022); χ2 analysis of
the influence of MTA1 expression (positive vs. negative) on
survival in the above subgroups is presented in Table IV. The use of the survival rate
based on different clinicopathological parameters is more
beneficial to accurately predict the prognosis and survival time of
patients and the conclusions drawn are more reliable. By using this
comparison, specific patients with different clinicopathological
parameters were selected to target MTA1 and achieve precision
therapy.
 | Table IVAssociation of MTA1 expression
(positive vs. negative) with 3-year and 5-year OS in patient
subgroups. |
Table IV
Association of MTA1 expression
(positive vs. negative) with 3-year and 5-year OS in patient
subgroups.
| | 3-year survival
rate | 5-year survival
rate | OS (at follow-up
date) |
|---|
| Parameter | χ2 | P-value | χ2 | P-value | χ2 | P-value |
|---|
| Age (years) | | | | | | |
|
<60 | 2.196 | 0.138 | 3.325 | 0.068 | 3.325 | 0.068 |
|
≥60 | 5.062 | 0.024 | 1.314 | 0.252 | 1.922 | 0.166 |
| Sex | | | | | | |
|
Male | 11.981 | 0.001 | 5.435 | 0.020 | 6.685 | 0.010 |
|
Female | 0.269 | 0.604 | 0.001 | 0.980 | 0.001 | 0.980 |
| Ethnicity | | | | | | |
|
Han | 3.094 | 0.079 | 0.512 | 0.474 | 1.289 | 0.256 |
|
Kazakh | 6.199 | 0.013 | 6.632 | 0.010 | 6.541 | 0.011 |
| Tumor location | | | | | | |
|
Up | 1.525 | 0.217 | 1.525 | 1.525 | 1.525 | 0.217 |
|
M | 3.851 | 0.050 | 2.541 | 2.541 | 2.917 | 0.088 |
|
L | 2.361 | 0.124 | 0.666 | 0.666 | 1.115 | 0.291 |
| Tumor size
(cm) | | | | | | |
|
<3 | 4.664 | 0.031 | 1.545 | 0.214 | 2.361 | 0.124 |
|
≥3 | 3.233 | 0.072 | 2.589 | 0.108 | 2.748 | 0.097 |
| Degree of
differentiation | | | | | | |
|
PD | 3.724 | 0.054 | 1.377 | 0.241 | 2.027 | 0.155 |
|
MD | 6.064 | 0.014 | 6.366 | 0.012 | 6.663 | 0.010 |
|
WD | 0.342 | 0.559 | 0.001 | 0.976 | 0.009 | 0.923 |
| Lymph node
metastasis | | | | | | |
|
No | 4.100 | 0.043 | 2.591 | 0.107 | 3.103 | 0.078 |
|
Yes | 1.655 | 0.198 | 0.552 | 0.457 | 0.506 | 0.477 |
| Depth of
invasion | | | | | | |
|
MA | 0.616 | 0.432 | 0.616 | 0.432 | 0.616 | 0.432 |
|
MS | 0.588 | 0.443 | 0.183 | 0.669 | 0.546 | 0.460 |
|
FT | 7.691 | 0.006 | 4.760 | 0.029 | 5.066 | 0.024 |
| TNM stage | | | | | | |
|
IA+B | 0.863 | 0.353 | 0.863 | 0.353 | 0.732 | 0.392 |
|
IIA+B | 2.085 | 0.149 | 1.238 | 0.266 | 1.751 | 0.186 |
|
IIIA+B | 1.782 | 0.182 | 0.882 | 0.348 | 0.882 | 0.348 |
|
IVA+B | 4.101 | 0.043 | 2.482 | 0.115 | 2.482 | 0.115 |
| Vascular
invasion | | | | | | |
|
No | 4.470 | 0.034 | 2.553 | 0.110 | 2.999 | 0.083 |
|
Yes | 3.328 | 0.068 | 2.746 | 0.098 | 3.648 | 0.056 |
| Nerve invasion | | | | | | |
|
No | 7.591 | 0.006 | 5.215 | 0.022 | 6.476 | 0.011 |
|
Yes | 0.371 | 0.542 | 0.012 | 0.913 | 0.012 | 0.913 |
| Hematogenous
metastasis | | | | | | |
|
No | 4.666 | 0.031 | 1.720 | 0.190 | 2.404 | 0.121 |
|
Yes | 3.164 | 0.075 | 2.869 | 0.090 | 2.893 | 0.089 |
Furthermore, the HR was analyzed in the present
study. By using Kaplan-Meier survival analysis, the OS at the
follow-up date of patients with ESCC was assessed depending on MTA1
expression (positive vs. negative; χ2=5.229; P=0.022;
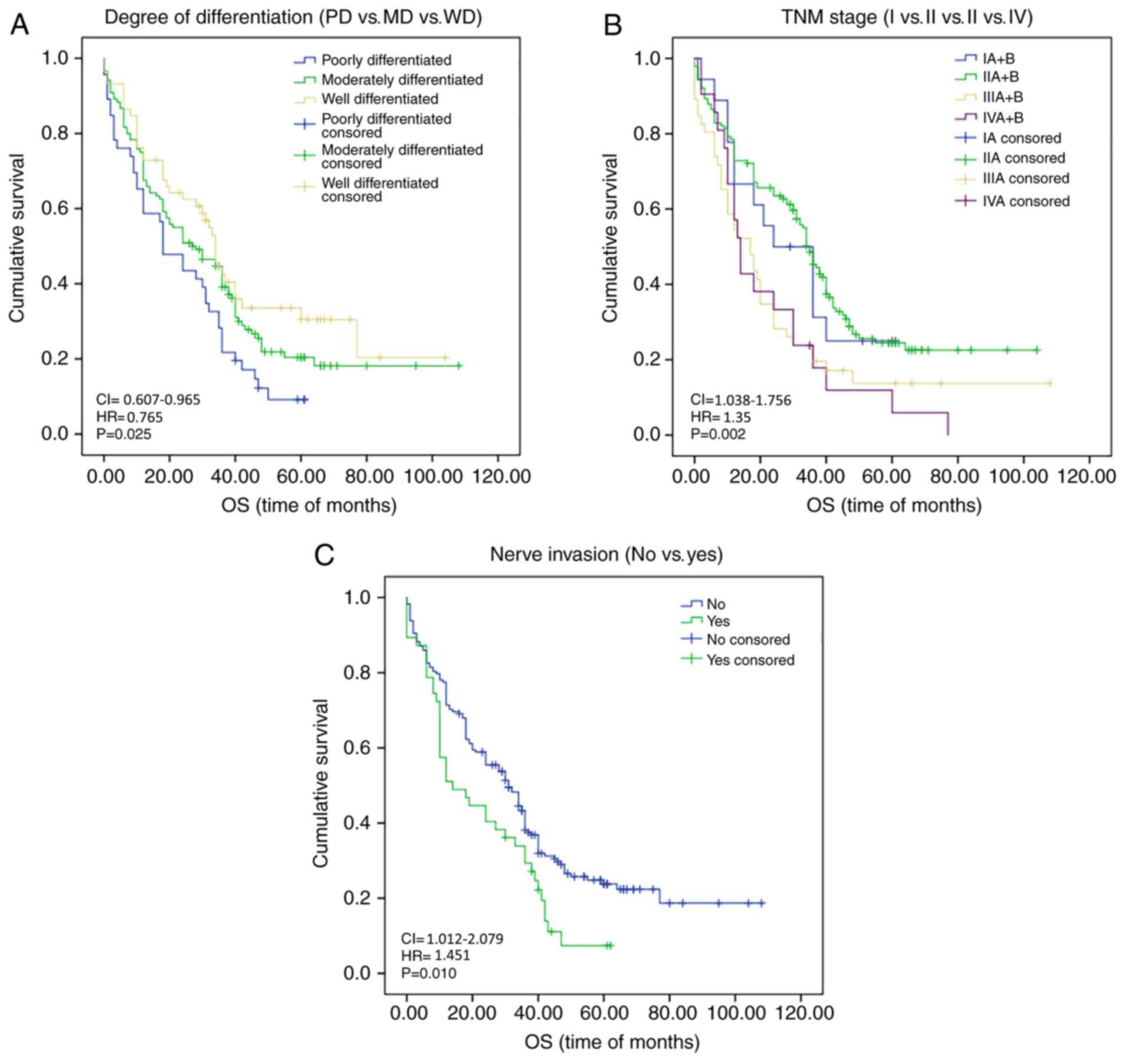
Fig. 2C). The degree of
differentiation (PD vs. MD vs. WD; P=0.025), TNM staging (I vs. II
vs. III vs. IV; P=0.002), lymph node metastasis (no vs. yes;
P=0.003) and nerve invasion (no vs. yes; P=0.010) also had a
significant impact on OS at the follow-up date (Fig. 3A-C). Kaplan-Meier survival analysis
and log-rank results are presented in Table V. However, further multivariate
regression analysis indicated that only the degree of
differentiation (HR: 0.765; 95% CI: 0.607-0.965; P=0.023), nerve
invasion (HR: 1.451; 95% CI: 1.012-2.079; P=0.043) and MTA1
expression (HR: 1.565; 95% CI: 0.355-0.899; P=0.016) had an impact
on OS (Table V). With regard to
PFS, significant differences were noted between subjects with
different tumor size (≥3 cm vs. <3 cm; χ2=4.432;
P=0.035), degree of differentiation (PD vs. MD vs. WD;
χ2=12.282; P=0.002), TNM stage (I vs. II vs. III vs. IV;
χ2=15.805; P=0.001), lymph node metastasis (no vs. yes;
χ2=17.046; P<0.001), nerve invasion (no vs. yes;
χ2=4.938; P=0.026) and hematogenous metastasis (no vs.
yes; χ2=3.952; P=0.047), as determined by univariate
analysis. However, multivariate analysis indicated that only the
degree of differentiation (HR: 0.723; 95% CI: 0.577-0.907; P=0.005)
and nerve invasion (HR: 1.465; 95% CI: 1.029-2.087; P=0.034) had an
independent impact on PFS (Table
V). These data provided ample evidence that MTA1 may be used as
an independent prognostic and diagnostic marker for ESCC and for
the prediction of survival of patients with ESCC.
 | Table VUnivariate and multivariate analysis
of the influence of variables on OS and PFS. |
Table V
Univariate and multivariate analysis
of the influence of variables on OS and PFS.
| | OS | PFS |
|---|
| | Univariate | Multivariate | Univariate | Multivariate |
|---|
| Variable | χ2 | P-value | HR (95% CI) | P-value | χ2 | P-value | HR (95% CI) | P-value |
| Sex (female vs.
male) | 2.146 | 0.143 | - | - | 1.762 | 0.184 | - | - |
| Age (≥60 years vs.
<60 years) | 0.679 | 0.410 | - | - | 0.247 | 0.619 | - | - |
| Ethnicity (Han vs.
Kazakh) | 0.384 | 0.535 | - | - | 0.015 | 0.903 | - | - |
| Tumor location (Up
vs. M vs. L) | 0.271 | 0.873 | - | - | 0.771 | 0.680 | - | - |
| Tumor size (≥3 cm
vs. <3 cm) | 3.826 | 0.050 | - | - | 4.432 | 0.035 | 1.327
(0.942-1.870) | 0.106 |
| Degree of
differentiation (PD vs. MD vs. WD) | 7.398 | 0.025 | PD 0.245
(0.072-0.841) | 0.025 | 12.282 | 0.002 | PD 0.106
(0.020-0.548) | 0.007 |
| Depth of invasion
(MA vs. MS vs. FT) | 2.400 | 0.301 | - | - | 3.346 | 0.188 | - | - |
| TNM stage (I vs. II
vs. III vs. IV) | 15.350 | 0.002 | - | - | 15.805 | 0.001 | - | - |
| Lymph node
metastasis (yes vs. no) | 8.707 | 0.003 | 1.085
(0.716-1.642) | 0.701 | 17.046 | <0.001 | 1.479
(0.968-2.259) | 0.071 |
| Vascular invasion
(yes vs. no) | 0.924 | 0.337 | - | - | 1.187 | 0.276 | - | - |
| Nerve invasion (yes
vs. no) | 6.720 | 0.010 | 1.451
(1.012-2.079) | 0.043 | 4.938 | 0.026 | 1.465
(1.029-2.087) | 0.034 |
|
Postradiochemotherapy (yes vs. no) | 0.003 | 0.953 | - | - | 0.224 | 0.636 | - | - |
| Hematogenous
metastasis (yes vs. no) | 1.425 | 0.433 | - | - | 3.952 | 0.047 | 1.485
(0.995-2.217) | 0.053 |
| MTA1 expression
(positive vs. negative) | 5.229 | 0.022 | 1.565
(0.355-0.899) | 0.016 | 2.004 | 0.157 | - | - |
| SOX4 expression
(positive vs. negative) | 0.013 | 0.908 | - | - | 0.006 | 0.941 | - | - |
| EZH2 expression
(positive vs. negative) | 0.807 | 0.369 | - | - | 0.657 | 0.481 | - | - |
Discussion
In the present study, the expression levels of MTA1,
SOX4 and EZH2 were investigated in clinical specimens and the
association between the expression of these markers and several
histopathological factors related to clinical outcome was assessed.
Cancer metastasis represents a major challenge in ESCC treatment.
The change in the expression levels of certain factors is a
characteristic feature of ESCC and is a prerequisite for tumor
metastasis. Therefore, a clear understanding of the molecular
mechanisms underlying the progression of ESCC is of pivotal
importance for the prevention of its metastasis. MTA1 is a
constitutive component of the NuRD complex and is able to regulate
gene transcription in both NuRD-dependent and NuRD-independent
manners (33). MTA1 overexpression
has been reported in various cancer types and leads to metastasis
and poor prognosis (35). The
mechanism of the role of MTA1 in ESCC metastasis remains elusive. A
previous study analyzed data from the ESCC survival database and
the results indicated that patients with ESCC, high MTA1 expression
exhibited a significant association with tumor metastasis and poor
prognosis (36). It has also been
indicated that MTA1 is associated with tumor recurrence and
metastasis in cervical and prostate cancers (26,27).
The present study demonstrated that the expression levels of MTA1
were significantly associated with prognosis of patients with ESCC,
which is consistent with previously reported results (21).
Furthermore, it was indicated that the MTA1-SOX4
axis is associated with ESCC progression. In the present study, the
correlation between MTA1 and SOX4 expression was assessed in 229
ESCC tissues. In addition, the association with clinicopathological
parameters and poor prognosis was assessed. Li et al
(37) performed an expression
profile analysis and verified the role of the MTA1-SOX4 regulatory
axis in three different cancer cell lines. The migratory and
invasive abilities of cancer cells overexpressing MTA1 were also
assessed (37). Widespread
co-expression of MTA1 and SOX4 has been determined in various
cancer types using The Cancer Genome Atlas database. To the best of
our knowledge, the present study was the first to demonstrate
MTA1-SOX4 signaling in ESCC. Targeting a single factor provides
less data than targeting a specific pathway. Therefore, the
discovery of the MTA1-SOX4 signaling pathway may provide potential
treatment options for ESCC.
SOX4 has been associated with metastasis and poor
prognosis and is induced by TGF-β. SOX4 acts upstream of the EMT in
order to promote invasion and metastasis (13,19).
Its mechanism is independent of the canonical TGF-β signaling
effector SMAD4, since short hairpin RNA-mediated ablation of SMAD4
did not substantially affect SOX4 expression in NMuMG cells
(16). Li et al (37) demonstrated that both SOX4 and MTA1
were downstream effectors of TGF-β, whereas the deletion of either
one substantially impaired the ability of TGF-β to induce
metastasis and invasion. MTA1 overexpression alone was sufficient
to induce metastasis and invasion by activating SOX4 in the absence
of TGF-β (37). This also suggests
that other factors leading to MTA1 upregulation in cancer may
contribute to metastasis and invasion by activating the MTA1-SOX4
signaling pathway. The present data demonstrated a significant
correlation between the expression of SOX4 and MTA1, which also
suggested that MTA1 may be used as a prognostic and diagnostic
marker of ESCC, which was consistent with previous findings
(37). In the present study, the
data indicated that the expression status of SOX4 was closely
associated with age (P=0.016) and ethnicity (P=0.035). However,
SOX4 expression was not significantly associated with sex, tumor
size, tumor location, degree of differentiation, AJCC stage, lymph
node metastasis, vascular invasion, nerve invasion and hematogenous
metastasis in ESCC. Age and ethnicity were assessed in the present
analysis and the results suggested that the expression levels of
MTA1 were significantly different between subjects with different
sexes and among subjects with different ethnicity, which may aid in
determining suitable recipients of different targeted
therapies.
Previous studies have indicated that EZH2 is one of
the direct transcriptional targets of SOX4 and that ablation of
EZH2 function affects SOX4 expression (13). In addition, SOX4 is able to enhance
the expression of EZH2 via binding to the promoter region (13), whereas it may also interact with
EZH2 and histone deacetylase 3 in order to form a corepressor
complex, which silences microRNA expression and affects metastasis
and invasion (20). Studies have
suggested that increased expression or activity of SOX4-EZH2 is a
marker of advanced and metastatic disease in various solid tumor
types and the SOX4-EZH2 axis is closely associated with disease
progression in ovarian and pancreatic cancers (13,20).
Li et al (37) demonstrated
that EZH2 is a downstream target of the TGF-β-MTA1-SOX4 signaling
axis in human small cell lung cancer, colorectal carcinoma and
ovarian cancer. In the present study, the MTA1-positive group
exhibited poor prognosis based on the OS rates. However, this does
not affect the interpretation of the results, since SOX4 affects
metastasis and invasion by regulating the expression of EZH2 in
ESCCs, which has been previously reported (20). However, this was not confirmed by
the results of the present study, which is inconsistent with
previous conclusions. Therefore, the clinical significance of
SOX4-EZH2 requires further verification. In ESCC, the MTA1-SOX4
interaction may be associated with the improvement of clinical
outcomes (37). In summary, the
data of the present study validated MTA1 as a predictive marker for
poor OS and PFS in patients with metastatic ESCC. In conclusion,
the MTA1-SOX4 axis was associated with ESCC progression and MTA1
was identified as a novel, independent prognostic factor in ESCC.
The results of the present study contradicted those of previous
studies. The results may be different from those reported by other
studies due to the small sample size and confounding bias.
MTA1-SOX4 may serve as a key cascade in ESCC, which is important
for metastasis, invasion and prognosis. In conclusion, high
expression of MTA1 was associated with ethnicity and lymph node
metastasis, whereas high expression of SOX4 was closely associated
with age and ethnicity and high expression of EZH2 with the degree
of differentiation. A positive correlation between MTA1 and SOX4
was identified. The results of univariate and multivariate analyses
indicated that these two markers had an effect on prognosis.
Therefore, MTA1 may be used as a molecular marker for screening for
ESCC and prognostication of patients. It may be concluded that the
activation of the MTA1-SOX4 axis may have a role in the development
of ESCC and is associated with poor prognosis. The identification
of targeted drugs that are able to inhibit MTA1 expression may
provide a potential strategy to prolong the survival of patients
with ESCC.
The present study was the first to propose the
application of MTA1 expression as a marker in ESCC. Further studies
are required to confirm these findings and facilitate the clinical
application of MTA1 in ESCC.
Supplementary Material
Clinicopathological data of the
cohort.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by grants from The Natural
Science Foundation of Xinjiang Uygur Autonomous Region (grant no.
2018D01C182), The National Natural Science Foundation of China
(grant no. 81860422), State Key Laboratory of Pathogenesis,
Prevention and Treatment of High Incidence Diseases in Central Asia
Fund (grant no. SKL-HIDCA-2020-4) and Xinjiang Medical University
Clinical Medicine Peak Discipline supporting funds within the
school (grant no. 33-0104006020801#).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WYL, YX and YQM conceived and designed the study;
GLA, MYL, HW and LPS performed the experiments; CL, YS, WJZ and SSX
analyzed the data; WZ and ZM interpreted the data. WYL wrote the
manuscript; WYL, YX and YQM gave final approval of the version to
be published. All authors read and approved the final manuscript.
All authors have checked and confirmed the authenticity of the raw
data.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics Review
Board of The First Affiliated Hospital of Xinjiang Medical
University (approval no. 20180223-08). Written informed consent was
obtained from all participants. All of the procedures were
performed in accordance with the Declaration of Helsinki and
relevant policies in China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lin DC, Hao JJ, Nagata Y, Xu L, Shang L,
Meng X, Sato Y, Okuno Y, Varela AM, Ding LW, et al: Genomic and
molecular characterization of esophageal squamous cell carcinoma.
Nat Genet. 46:467–473. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Jain R, Gupta S, Pasricha N, Faujdar M,
Sharma M and Mishra P: ESCC with metastasis in the young age of
caustic ingestion of shortest duration. J Gastrointest Cancer.
41:93–95. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Liu Q, Liang M, Liu T, Vuitton L, Zheng S,
Gao X, Lu M, Li X, Sheyhidin I and Lu X: M2 isoform of pyruvate
kinase (PKM2) is upregulated in Kazakh's ESCC and promotes
proliferation and migration of ESCC cells. Tumour Biol.
37:2665–2672. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Encinas de la Iglesia J, Corral de la
Calle MA, Fernandez Perez GC, Ruano Perez R and Alvarez Delgado A:
Esophageal cancer: Anatomic particularities, staging, and imaging
techniques. Radiologia. 58:352–365. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liu B, Bo Y, Wang K, Liu Y, Tang X, Zhao
Y, Zhao E and Yuan L: Concurrent neoadjuvant chemoradiotherapy
could improve survival outcomes for patients with esophageal
cancer: A meta-analysis based on random clinical trials.
Oncotarget. 8:20410–20417. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhou YX, Liu Q, Wang H, Ding F and Ma YQ:
The expression and prognostic value of SOX2, β-catenin and survivin
in esophageal squamous cell carcinoma. Future Oncol. 15:4181–4195.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen Q, Zheng PS and Yang WT:
EZH2-mediated repression of GSK-3β and TP53 promotes Wnt/β-catenin
signaling-dependent cell expansion in cervical carcinoma.
Oncotarget. 7:36115–36129. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Moon HE, Cheon H, Chun KH, Lee SK, Kim YS,
Jung BK, Park JA, Kim SH, Jeong JW and Lee MS:
Metastasis-associated protein 1 enhances angiogenesis by
stabilization of HIF-1alpha. Oncol Rep. 16:929–935. 2006.PubMed/NCBI
|
|
9
|
Ezhkova E, Pasolli HA, Parker JS, Stokes
N, Su IH, Hannon G, Tarakhovsky A and Fuchs E: Ezh2 orchestrates
gene expression for the stepwise differentiation of tissue-specific
stem cells. Cell. 136:1122–1135. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Su IH, Basavaraj A, Krutchinsky AN, Hobert
O, Ullrich A, Chait BT and Tarakhovsky A: Ezh2 controls B cell
development through histone H3 methylation and Igh rearrangement.
Nat Immunol. 4:124–131. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Nienstedt JC, Schroeder C, Clauditz T,
Simon R, Sauter G, Muenscher A, Blessmann M, Hanken H and Pflug C:
EZH2 overexpression in head and neck cancer is related to lymph
node metastasis. J Oral Pathol Med. 47:240–245. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Feng H, Yu Z, Tian Y, Lee YY, Li MS, Go
MY, Cheung YS, Lai PB, Chan AM, To KF, et al: A CCRK-EZH2
epigenetic circuitry drives hepatocarcinogenesis and associates
with tumor recurrence and poor survival of patients. J Hepatol.
62:1100–1111. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Parvani JG and Schiemann WP: Sox4, EMT
programs, and the metastatic progression of breast cancers:
Mastering the masters of EMT. Breast Cancer Res.
15(R72)2013.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Tiwari N, Tiwari VK, Waldmeier L, Balwierz
PJ, Arnold P, Pachkov M, Meyer-Schaller N, Schubeler D, van
Nimwegen E and Christofori G: Sox4 is a master regulator of
epithelial-mesenchymal transition by controlling Ezh2 expression
and epigenetic reprogramming. Cancer Cell. 23:768–783.
2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lin L, Wang Z, Jin H, Shi H, Lu Z and Qi
Z: miR-212/132 is epigenetically downregulated by
SOX4/EZH2-H3K27me3 feedback loop in ovarian cancer cells. Biol: Nov
3, 2016 (Epub ahead of print).
|
|
16
|
Moreno CS: SOX4: The unappreciated
oncogene. Semin Cancer Biol. 67:57–64. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
David CJ, Huang YH, Chen M, Su J, Zou Y,
Bardeesy N, Iacobuzio-Donahue CA and Massague J: TGF-β tumor
suppression through a lethal EMT. Cell. 164:1015–1030.
2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang L, Zhang J, Yang X, Chang YW, Qi M,
Zhou Z, Zhang J and Han B: SOX4 is associated with poor prognosis
in prostate cancer and promotes epithelial-mesenchymal transition
in vitro. Prostate Cancer Prostatic Dis. 16:301–307.
2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jafarnejad SM, Wani AA, Martinka M and Li
G: Prognostic significance of Sox4 expression in human cutaneous
melanoma and its role in cell migration and invasion. Am J Pathol.
177:2741–2752. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang J, Liang Q, Lei Y, Yao M, Li L, Gao
X, Feng J, Zhang Y, Gao H, Liu DX, et al: SOX4 induces
epithelial-mesenchymal transition and contributes to breast cancer
progression. Cancer Res. 72:4597–4608. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Koumangoye RB, Andl T, Taubenslag KJ,
Zilberman ST, Taylor CJ, Loomans HA and Andl CD: SOX4 interacts
with EZH2 and HDAC3 to suppress microRNA-31 in invasive esophageal
cancer cells. Mol Cancer. 14(24)2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Nan P, Wang T, Li C, Li H, Wang J, Zhang
J, Dou N, Zhan Q, Wang H and Qian H: MTA1 promotes tumorigenesis
and development of esophageal squamous cell carcinoma via
activating the MEK/ERK/p90RSK signaling pathway. Carcinogenesis.
41:1263–1272. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hannafon BN, Gin AL, Xu YF, Bruns M,
Calloway CL and Ding WQ: Metastasis-associated protein 1 (MTA1) is
transferred by exosomes and contributes to the regulation of
hypoxia and estrogen signaling in breast cancer cells. Cell Commun
Signal. 17(13)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Deng L, Tang J, Yang H, Cheng C, Lu S,
Jiang R and Sun B: MTA1 modulated by miR-30e contributes to
epithelial-to-mesenchymal transition in hepatocellular carcinoma
through an ErbB2-dependent pathway. Oncogene. 36:3976–3985.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu J, Xu D, Wang H, Zhang Y, Chang Y,
Zhang J, Wang J, Li C, Liu H, Zhao M, et al: The subcellular
distribution and function of MTA1 in cancer differentiation.
Oncotarget. 5:5153–5164. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Xue H, Wang H, Liu J, Liu H, Li C, Han L,
Lin C, Zhan Q, Zhao Z and Qian H: MTA1 downregulation inhibits
malignant potential in a small cell lung cancer cell line. Oncol
Rep. 33:885–892. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Dhar S, Kumar A, Gomez CR, Akhtar I,
Hancock JC, Lage JM, Pound CR and Levenson AS: MTA1-activated
Epi-microRNA-22 regulates E-cadherin and prostate cancer
invasiveness. FEBS Lett. 591:924–933. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Guo N, Shen G, Zhang Y, Moustafa AA, Ge D
and You Z: Interleukin-17 promotes migration and invasion of human
cancer cells through upregulation of MTA1 expression. Front Oncol.
9(546)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yoo YG, Kong G and Lee MO:
Metastasis-associated protein 1 enhances stability of
hypoxia-inducible factor-1alpha protein by recruiting histone
deacetylase 1. EMBO J. 25:1231–1241. 2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Weng W, Yin J, Zhang Y, Qiu J and Wang X:
Metastasis-associated protein 1 promotes tumor invasion by
downregulation of E-cadherin. Int J Oncol. 44:812–818.
2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Song Q, Wang B, Liu M, Ren Z, Fu Y, Zhang
P and Yang M: MTA1 promotes the invasion and migration of oral
squamous carcinoma by inducing epithelial-mesenchymal transition
via the hedgehog signaling pathway. Exp Cell Res.
382(111450)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Edge S, Byrd DR and Compton CC: AJCC
Cancer Staging Manual (7th edition). Springer International
Publishing: American Joint Commission on Cancer, 2009.
|
|
33
|
Zhou N, Wang H, Liu H, Xue H, Lin F, Meng
X, Liang A, Zhao Z, Liu Y and Qian H: MTA1-upregulated EpCAM is
associated with metastatic behaviors and poor prognosis in lung
cancer. J Exp Clin Cancer Res. 34(157)2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Matsubara T, Toyokawa G, Takada K,
Kinoshita F, Kozuma Y, Akamine T, Shimokawa M, Haro A, Osoegawa A,
Tagawa T and Mori M: The association and prognostic impact of
enhancer of zeste homologue 2 expression and epithelial-mesenchymal
transition in resected lung adenocarcinoma. PLoS One.
14(e0215103)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Toh Y and Nicolson GL: Properties and
clinical relevance of MTA1 protein in human cancer. Cancer
Metastasis Rev. 33:891–900. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang Q, Wang F, Lv J, Xin J, Xie L, Zhu W,
Tang Y, Li Y, Zhao X, Wang Y, et al: Interactive online consensus
survival tool for esophageal squamous cell carcinoma prognosis
analysis. Oncol Lett. 18:1199–1206. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Li L, Liu J, Xue H, Li C, Liu Q, Zhou Y,
Wang T, Wang H, Qian H and Wen T: A TGF-β-MTA1-SOX4-EZH2 signaling
axis drives epithelial-mesenchymal transition in tumor metastasis.
Oncogene. 39:2125–2139. 2020.PubMed/NCBI View Article : Google Scholar
|