Introduction
End-stage liver disease (ESLD) is caused by hepatic
injury and leads to an irreversible loss of liver function, and
changes in liver architecture and blood supply (1). The presenting symptoms vary widely
from impaired synthesis of blood proteins to the loss of glucose or
ammonia control and impaired bile acid production (1). While the incidence of ESLD in the
pediatric population is low, the condition is severe and requires
tertiary care (1,2). Biliary atresia was the most common
cause of pediatric ESLD in 2001 in Canada and the USA (43.4%),
followed by fulminant liver failure (15.0%), cirrhosis (9.1%) and
metabolic diseases (8.0%) (3).
Liver transplant is the only curative treatment in numerous cases
(4).
Parental donor liver transplantation has become the
most effective treatment for pediatric patients with ESLD (5), with a worldwide postoperative 5-year
survival rate of 70-90% in 2011 (6,7).
Although marked survival rate, complications after liver
transplantation still occur (8).
The incidence of neurological complications can be 20%, which
severely affects postoperative survival rates compared to those
without (50 vs. 7%) (9). The
optimal timeframe for pediatric liver transplantation coincides
with the peak time of cerebral development in children (10,11).
Therefore, pediatric patients undergoing transplantation during
this period are at high risk of neurological complications,
imposing challenges to the anesthesiologist for the protection of
nervous system function and the reduction of neurological damage
during the perioperative period.
Currently, anesthetic drugs and surgical trauma have
the potential to cause the death of developing neurons, causing
long-term injury to nerve function (12-15).
S100 calcium-binding protein β (S-100β) is expressed by astrocytes
and neuron-specific enolase (NSE) is expressed by ganglia cells
(16,17). S-100β is secreted by astrocytes and
can be leaked into the circulation by injured astrocytes; blood
levels of S-100β have been reported to be increased during the
acute phase of brain damage (18-20).
Similarly, leakage of NSE into the circulation indicates injured
ganglia cells (21,22). A meta-analysis revealed that NSE had
a moderate predictive value for brain injury in pediatric patients
(23). High postoperative levels of
S-100β and NSE are associated with brain injury in pediatric
patients who have undergone major surgeries (24,25).
However, to the best of our knowledge, perioperative changes in the
levels of S-100β and NSE in pediatric patients with ESLD undergoing
parental donor liver transplantation remain unknown.
Therefore, the present study investigated the
effects of parental donor liver transplantation on the changes of
serum NSE and S-100β during the perioperative period, on brain
injury and on postoperative cognitive function. The results may aid
in improving perioperative management in these pediatric
patients.
Materials and methods
Study design and patients
The present prospective observational study
investigated infants with congenital biliary atresia who underwent
selective liver transplantation between January and December 2017
at Tianjin First Central Hospital (Tianjin, China). The present
study was approved by the Medical Ethics Committee of Tianjin First
Central Hospital (approval no. 2016N0039KY) and written informed
consent was obtained from the infants' parents. The privacy rights
of human patients were observed.
The inclusion criteria were as follows: i) 4-12
months of age; ii) an American Society of Anesthesiologists
physical status of III or IV (26);
and iii) scheduled to undergo elective pediatric living related
donor liver transplantation. The exclusion criteria were: i) known
or suspected allergies to propofol, soy and/or egg; ii) congenital
heart disease, or impairment of renal or pulmonary function prior
to liver transplantation; or iii) treatment with any other type of
operation. All living donors were parents (father or mother). Every
transplantation case received ethical review and approval from
Tianjin First Center Hospital.
Anesthesia and intraoperative
management
All infants underwent combined intravenous and
inhalation anesthesia. Preoperatively, routine fasting was
performed (formula and milk were not provided for 6 h, breast milk
for 4 h and water or sugary drinks for 2 h prior to surgery).
Atropine (0.01 mg/kg) was intramuscularly injected 30 min prior to
anesthesia. After being transferred to the operating room, routine
monitoring of pulse oxygen saturation (SpO2) and
electrocardiograms were conducted. Peripheral venous access was
opened. Rapid induction of anesthesia was performed using the
following: 1 mg/kg methylprednisolone, 0.05 mg/kg midazolam, 0.2
mg/kg etomidate, 2 µg/kg fentanyl and 0.08 mg/kg vecuronium
bromide. Auscultation of both lungs was performed following oral
tracheal intubation to ensure clear breathing sounds in both lungs.
The ventilator was connected to mechanical ventilation to observe
the normal waveform of patient end-tidal carbon dioxide
(PETCO2). The fraction of inspired oxygen was 50-60%
(100% at the anhepatic phase), tidal volume was 8-10 ml/kg,
respiratory rate was 20-26 breaths/min and the inspiration and
expiration ratio was 1.0:1.5-2.0. A PETCO2 partial
pressure of 30-35 mmHg and airway pressure of 18-25
cmH2O (1 cm H2O=0.098 kPa) was maintained.
Following induction of stable anesthesia, bispectral index (BIS)
values were monitored. B-mode ultrasound-guided radial artery
catheterization for invasive blood pressure monitoring, and
placement of a triple-lumen central venous catheter through the
right internal jugular vein for monitoring of central venous
pressure (CVP) and intraoperative infusion were performed.
Anesthesia maintenance was performed using the following:
Continuous intravenous infusion of 1% propofol at 9-15 mg/kg/h,
0.1-0.2 µg/kg/min remifentanil and 0.12 mg/kg/h cisatracurium
besylate. Additionally, 1-3 µg/kg fentanyl was administered
intermittently to maintain the depth of anesthesia. The
intraoperative fluid infusion was warmed. Sodium lactate, glucose
and albumin solution were intravenously infused. Body temperature
was maintained at 35.5-37.5˚C. According to the results of
intraoperative blood gas analysis and coagulation function
monitoring, the appropriate amount of concentrated red blood cells
and fresh frozen plasma were infused. By adjusting the transfusion
speed and continuous intravenous infusion of small doses of
dopamine, a mean arterial pressure (MAP) of 40-65 mmHg (1
mmHg=0.133 kPa), a CVP of 6-8 mmHg, a heart rate (HR) of 110-170
beats/min, a SpO2 of 95-100%, a body temperature of
35.5-37.5˚C, a BIS of 40-60, a PETCO2 of 35-45 mmHg,
hemoglobin >80 g/l and a urine volume >1 ml/kg/h were
maintained. According to the results of arterial blood gas
analysis, the breathing parameters were adjusted throughout the
surgery. A heating blanket and infusion heating device were used to
maintain constant body temperature.
Data collection and examination
methods
A total of 1 ml central venous blood was collected
into coagulation tubes a following anesthesia (T1), 30 min after
the anhepatic phase (T2), and 1 h (T3) and 24 h (T4) after the
neohepatic phase. The samples were placed at 37˚C for 10 min,
centrifuged at 3,960 x g rpm, 22-24˚C for 10 min and stored at
-80˚C. NSE and S-100β were detected using ELISA kits (cat. nos.
E0lN0025 and S100 E0lS0042, respectively; Shanghai Lanji
Biotechnology Co., Ltd.). HR, MAP, CVP and BIS were recorded at
each time point (T1-T4).
Two doctors independently conducted evaluations 1
day prior to surgery and 3 months after surgery. Pediatric
end-stage liver disease model (PELD) score was calculated as
follows: PELD Score=[0.436x (age <1 year)]-0.687x log[albumin
(g/dl)] +0.480x log[total bilirubin (mg/dl)] +1.875x
log[international normalized ratio (INR)] +0.667x (growth failure
height or weight ≥2-SD below the age- and sex-adjusted mean)
(27). The Bayley Scales of Infant
Development (BSID) is a standardized technique and measurement tool
for evaluating the psychomotor behaviors of children aged between 2
months and 3 years (28). BSID
revised by the Hunan Medical University in 1990 was used to assess
the psychomotor and behavior development conditions of all of the
infants (29). According to the raw
score, the corresponding mental development index (MDI) and
psychomotor development index (PDI) were calculated to analyze the
effect of liver transplantation on the neurocognitive behaviors of
infants. All examinations were carried out in a quiet environment.
The MDI and PDI are standard scores obtained from the conversion
table based on the corresponding raw score of age and other values.
The average number is 100 and the standard deviation is 16; >90
points indicated a normal level and <90 indicated poor
development. Furthermore, the postoperative delirium of infants was
independently evaluated by two physicians at 30 min, 2 and 4 h
following extubation using the pediatric anesthesia emergence
delirium (PAED) scale (30).
Statistical analysis
Statistical analyses were performed using SPSS
software (version 20.0; IBM Corp.). Continuous data were tested
with the Kolmogorov-Smirnov test and are presented as mean ± SD or
medians (first and third quartiles), as appropriate. Data were
analyzed using repeated-measures ANOVA with the Bonferroni post-hoc
test across different time points. Categorical data are presented
as n (%) and were compared with the χ2 test or Fisher's
exact test with the Bonferroni post-hoc test, as appropriate.
Pearson's correlation analysis was used to analyze the correlation
between NSE and S-100β at T3, PAED scores at 30 min and MDI and PDI
at 1 month following surgery. P<0.05 was considered to indicate
a statistically significant difference.
Results
Characteristics of the
transplantations
Parental donor piggyback liver transplantations were
performed. The left lateral lobe of the donor liver was used for
transplantation, with a graft-to-recipient weight ratio of
0.83-5.16%. The mean operation time was 8.2±1.2 h. The mean
anhepatic phase was 45.5±12.4 min. The median cold ischemia time of
the donor livers was 95 (64, 178) min. The infusion of red blood
cells was 2.00 (2.00, 3.68) units. The infusion of plasma was 400
(210, 400) ml. Data are presented in Table I.
 | Table IPatient characteristics and operation
indicators. |
Table I
Patient characteristics and operation
indicators.
| Variables | Values |
|---|
| Age, months | 8.1 (6.6, 10.2) |
| Sex | |
|
Male | 51 (59.3%) |
|
Female | 35 (40.7%) |
| Weight, kg | 7.6±1.7 |
| Height, cm | 66.3±7.2 |
| ASA status | |
|
III | 62 (72.1%) |
|
IV | 24 (27.9%) |
| PELD score | 16.6±2.2 |
| Preoperative serum
creatinine, µmol/l | 17.34±4.68 |
| Preoperative ALT,
U/l | 114.46±48.25 |
| Preoperative AST,
U/l | 206.80±85.26 |
| γ-glutaryl
transferase, U/l | 435.8±95.66 |
| Total bilirubin,
mg/dl | 205.42±90.48 |
| Anhepatic time,
min | 45.5±12.4 |
| Operation time,
h | 8.2±1.2 |
| Anesthesia
duration, h | 10.1±1.3 |
| Bleeding volume,
ml | 127±30 |
| Urine volume,
ml | 424±62 |
| Intraoperative
blood transfusions, units | 2.0 (2.0, 3.7) |
| Intraoperative
frozen plasma transfusions, ml | 400 (210, 400) |
| Graft cold ischemia
time, min | 95 (64, 178) |
Significant hemodynamic changes
observed during surgery
The anhepatic and hepato-reperfusion phases (T2 and
T3) MAP and CVP decreased significantly compared with T1 and T2
(P<0.05 at all time points). MAP and CVP gradually recovered
during the hepato-reperfusion phase (Table II).
 | Table IIChanges in hemodynamics, serum pH and
biomarkers. |
Table II
Changes in hemodynamics, serum pH and
biomarkers.
| Variable | T1 | T2 | T3 | T4 |
|---|
| HR, beat/min | 118.42±12.25 |
132.25±14.55a |
112.18±10.12a,b |
105.28±7.16a-c |
| MAP, mmHg | 46.23±7.18 |
40.35±8.16a |
49.25±7.36a,b |
53.62±3.28a-c |
| CVP,
cmH2O | 4.36±1.42 |
2.84±1.06a |
6.42±1.91a,b |
7.42±2.23a-c |
| pH | 7.45±0.46 |
7.32±0.44a |
7.39±0.65a,b |
7.42±0.51b,c |
| NSE, ng/ml | 23.46±3.74 |
28.85±4.14a |
35.57±7.06a,b |
29.25±4.90a,c |
| S100-β (ng/ml) | 3.97±0.79 |
7.69±1.92a |
12.36±3.29a,b |
6.87±2.11a,c |
NSE and S100β levels are elevated
during and following transplantation
Levels of NSE and S100β were increased significantly
at T2, T3 and T4 compared with T1 (P<0.05) (Table II). Levels gradually increased at
T2 and peaked at T3 compared with T1 (P<0.05). Furthermore,
S-100β and NSE levels decreased gradually at T4 compared with T3
(P<0.05). These results indicated that liver transplantation
caused a certain degree of brain injury.
Rate of delirium is high following
extubation and decreases with time
The rate of delirium was 17.4% at 30 min
post-extubation (Table III). The
incidence of delirium was significantly lower at 2 h (6.9%) and 4 h
(3.4%) post-extubation (P<0.05 vs. 30 min post-extubation) The
PAED score was 9.6±2.4 at 30 min post-extubation (Table III). The PAED score was
significantly lower at 2 h (6.6±1.8) and 4 h (4.0±1.1)
post-extubation (P<0.05 vs. 30 min post-extubation).
 | Table IIIComparison of delirium values
following extubation. |
Table III
Comparison of delirium values
following extubation.
| Indicator | 30 min
post-extubation | 2 h
post-extubation | 4 h
post-extubation |
|---|
| PAED score | 9.6±2.4 |
6.6±1.8a |
4.0±1.1a |
| Rate of
delirium | 15 (17.4%) | 6
(6.9%)a | 3
(3.4%)a |
A certain degree of brain injury
occurs during liver transplantation
MDI and PDI were decreased at 3 months after surgery
compared with 1 day prior to surgery (MDI, 87.7±8.4 vs. 84.5±8.5,
P=0.015; PDI, 82.9±8.7 vs. 79.6±8.8, P=0.016; Table IV). These results indicated that a
certain degree of brain injury occurred in the infants during or
following liver transplantation.
 | Table IVChanges in MDI and PDI prior to and
after liver transplantation. |
Table IV
Changes in MDI and PDI prior to and
after liver transplantation.
| Indicator | 1 day prior to
surgery | 3 months after
surgery | P-value |
|---|
| MDI | 87.7±8.4 | 84.5±8.5 | 0.015 |
| PDI | 82.9±8.7 | 79.6±8.8 | 0.016 |
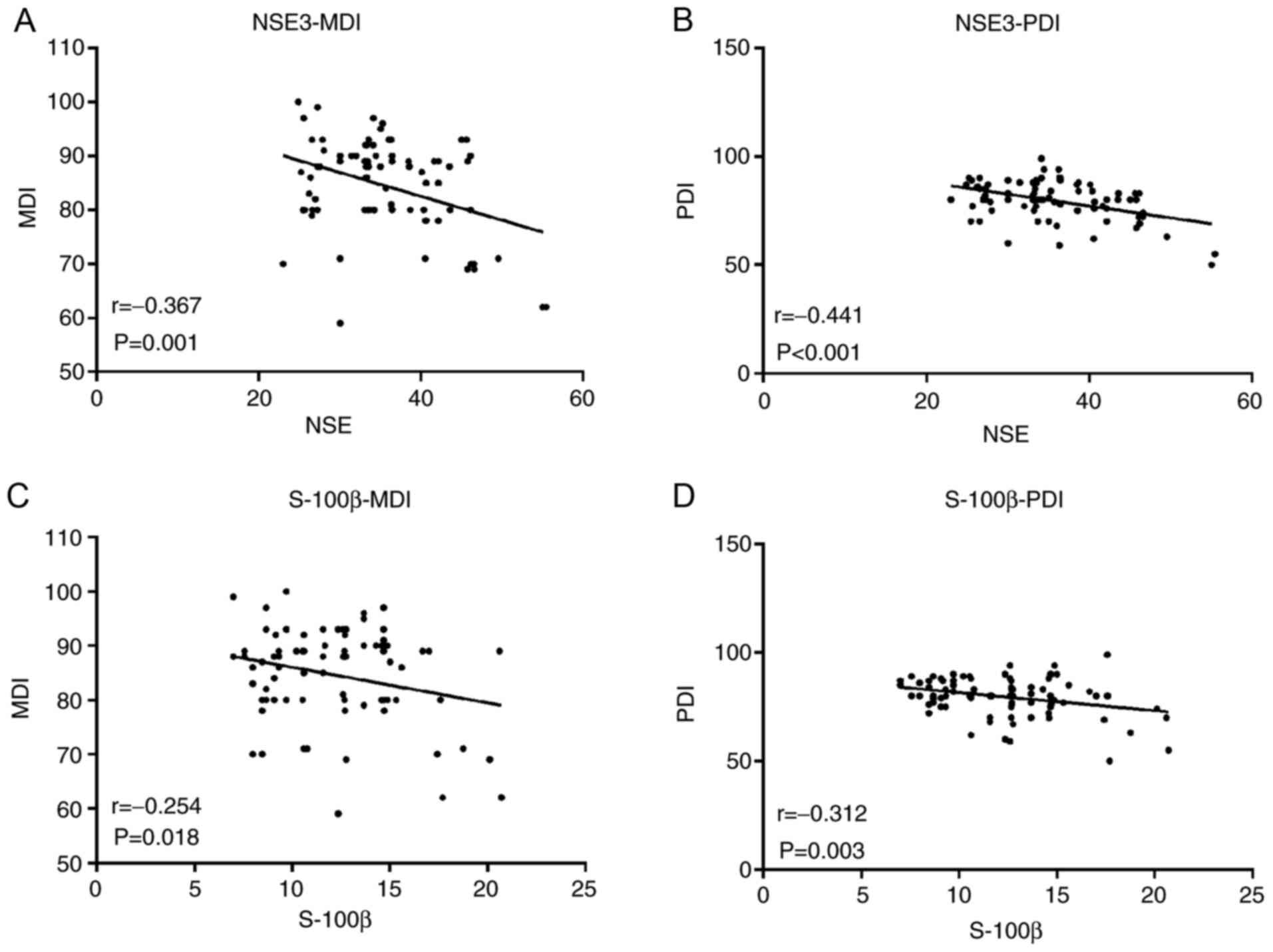
Brain injury markers are correlated
with the developmental indexes
NSE and S-100β (T3) were linearly correlated with
MDI and PDI 3 months after surgery. NSE was moderate negatively
correlated with postoperative MDI (r=-0.367; P=0.001; Fig. 1A) and PDI (r=-0.441; P<0.001;
Fig. 1B). Furthermore, S-100β was
weakly negatively correlated with MDI (r=-0.254; P=0.018; Fig. 1C) and moderate negatively with PDI
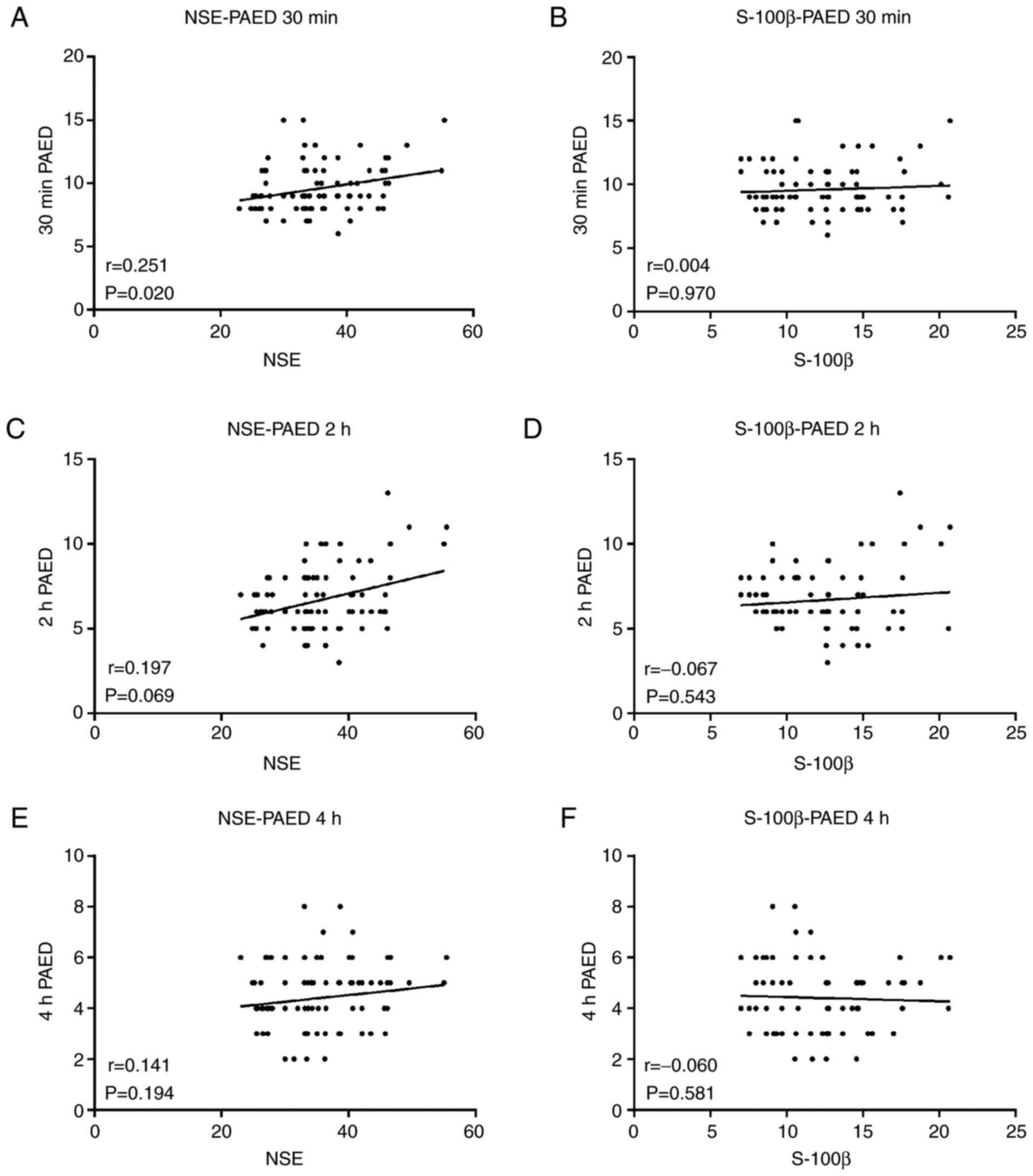
(r=-0.312; P=0.003 Fig. 1D). NSE
was weak correlated with PAED 30 min after surgery (r=0.251;
P=0.020; Fig. 2A). These results
indicated that S-100β was not correlated with PAED 30 min (r=0.004;
P=0.970; Fig. 2B), 2 h (r=0.067;
P=0.543; Fig. 2D) and 4 h (r=0.060;
P=0.581; Fig. 2F) after surgery.
NSE was not correlated with PAED 2 (r=0.197; P=0.069; Fig. 2C) and 4 h (r=0.141; P=0.194;
Fig. 2E) after surgery, and also
the elevated markers of neurological injury during or after surgery
may be correlated with delayed development.
Discussion
The present study evaluated the effect of liver
transplantation on the brains of infants according to the
perioperative serum brain injury markers S-100β and NSE. The
results indicated that hemodynamic fluctuations were significant
during the anhepatic and hepato-reperfusion phases (T2-T4). The
levels of brain injury markers S-100β and NSE increased and peaked
at the ischemia-reperfusion phase (T3), indicating that
ischemia-reperfusion may affect the brains of the pediatric
patients to a certain degree. In addition, the incidence of
delirium was highest at 30 min post-extubation. MDI and PDI were
decreased at 3 months after surgery compared with 1 day prior to
surgery. Furthermore, S-100β and NSE were negatively correlated
with MDI and PDI, indicating that elevated markers of neurological
injury during or after surgery may be correlated with delayed brain
development. These results strongly suggested that there was a
certain degree of brain injury following liver transplantation in
children with biliary atresia.
Currently, it is hypothesized that multiple factors
are involved in perioperative brain injury during liver
transplantation. The suggested pathogenesis is that intraoperative
liver ischemia/reperfusion leads to the impaired autoregulation of
cerebral blood flow, and intraoperative hemorrhage, infusion and
inferior vena cava blockage, which may cause significant
hemodynamic changes, and result in drastic fluctuation of cerebral
blood perfusion and oxygenation, particularly at the neohepatic
phase (31,32). Furthermore, the release of
inflammatory factors induced by liver transplantation may result in
large amounts of oxygen-free radicals, which may lead to cerebral
edema, delayed neuronal death and other serious injuries (31,32).
S-100β is a nervous tissue protein with high
concentrations in the brain (18).
Following necrosis of nerve cells, S-100β is released into the
cerebrospinal fluid and enters the bloodstream through the damaged
blood-brain barrier (18-20).
The levels of S-100β in body fluids have been used to monitor
perinatal asphyxia in infants and to guide clinical treatment
(16). NSE is mainly present in
neurons and neurosecretory cells, and is an important marker of
brain injury (20,21). The physical and chemical properties
of NSE are quite stable and changes in the external environments
have little effect on levels (23).
NSE is closely associated with neuronal injury and can be used as
an important parameter to assess the severity of neuronal injury
(23). High postoperative levels of
S-100β and NSE are well known to be associated with brain injury in
pediatric patients who have undergone major surgeries (24,25).
However, to the best of our knowledge, the changes in S-100β and
NSE levels in pediatric patients with biliary atresia undergoing
parental donor liver transplantation remains unknown. The current
study demonstrated that the serum levels of S-100β and NSE in
infants undergoing liver transplantation after induction of
anesthesia were significantly higher compared with normal
preoperative values. The possible underlying mechanism was that
preoperative abnormal liver function and liver failure led to the
disturbance of electrolyte levels and the acid-base equilibrium,
metabolic disorders. Preoperative brain functions in patients with
end-stage diseases are usually manifested as serious abnormal
infections (17). Additionally,
biliary atresia can result in ischemia, sepsis, acid-base imbalance
and electrolyte disturbance, which can cause different degrees of
damage to the central nervous system (17).
Parental donor liver transplantation is complicated.
In all donor transplants, when anastomosing the inferior vena cava
and donor hepatic vein, the inferior vena cava needs to be blocked
(33). After entering the anhepatic
phase, HR is increased, MAP and CVP are decreased and tissue
hypoperfusion and hypoxic metabolism produce a numerous acidic
metabolites. Ultimately, S-100β and NSE levels are increased and
peak following liver transplantation and reperfusion. Cardiac
output does not recover rapidly in the early neohepatic phase
(31,32); instead, it is decreased further, and
numerous acidic metabolites, endotoxins, vasoactive substances and
inflammatory factors can cause damage to the nervous system in the
early stage (31,32). If liver transplantation is
successful, the new liver can gradually recover these imbalances
and there is a gradual reducing trend in the aforementioned
products following surgery.
The use of elevated serological indicators for the
assessment of brain injury is uncertain; therefore, the present
study further investigated neurobehavioral cognition and
postoperative delirium in infants. BSID is a standardized technique
and measurement tool used to evaluate the psychomotor behaviors of
children aged between 2 months and 3 years, and can be used to
evaluate the sensitivity, discriminability and ability to respond
to external factors of sensory perception, learning and memorizing
and psychomotor abilities (28). In
the present study, BSID was applied to evaluate the psychomotor
behavior conditions of infants prior to and following liver
transplantation. The preoperative MDI and PDI scores were
significantly lower compared with normal levels (data not shown),
indicating that there was a certain neurocognitive dysfunction in
infants with biliary atresia. The MDI and PDI scores at 3 months
after liver transplantation were lower compared with those prior to
surgery. The postoperative delirium conditions indicated that the
incidence of delirium was higher in a short time period (30 min)
following extubation, which strongly suggested that liver
transplantation may cause a certain degree of brain injury in
infants. Furthermore, the MDI and PDI scores from the correlation
analysis were consistent with the results for S-100β and NSE
levels.
NSE and S-100β are well-known markers of brain
injury (18-23).
High postoperative levels of S-100β and NSE have been reported to
be associated with brain injury in pediatric patients who have
undergone major surgeries (24,25).
However, to the best of our knowledge, the perioperative changes in
S-100β and NSE levels in pediatric patients with ESLD undergoing
parental donor liver transplantation is unknown. The present study
indicated that pediatric patients with ESLD undergoing parental
donor liver transplantation suffered from brain injury. The
elevation of S-100β and NSE peaked at 1 h in the neohepatic phase.
This may indicate the timing of brain protection methods in these
pediatric patients. This result indicated that timely and effective
brain protection measures, taken during the 1st hour of the
neohepatic phase, may decrease the level of S-100β and NSE
The present study had limitations. Firstly, it was a
single-center study and only included infants with simple biliary
atresia undergoing parental donor liver transplantation. Only two
markers of brain injury and hemodynamics were assessed, and
systemic inflammation and oxidative stress were not measured.
Additionally, blood samples were available only for the four time
points examined, and multiple samples for the dynamic assessment of
NSE and S-100β were not available. Therefore, multicenter studies
involving more samples are required to determine the association of
NSE and S-100β with brain injury.
In conclusion, brain injury was observed in the
perioperative period in pediatric patients during parental donor
liver transplantation, as indicated by elevated serum NSE and
S-100β levels. The anhepatic phase and ischemia-reperfusion caused
a certain degree of brain injury. NSE and S-100β levels were also
correlated with infant development scores. The detection of
perioperative brain injury markers may be used to determine and
predict postoperative brain injury, thus providing guidance for
clinical cerebral protection. Clinical cerebral protection is
important for the prevention of postoperative neurological
complications in infants following parental donor liver
transplantation.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the 2018 Tianjin
Natural Science Foundation Project (grant no. 2016N0039KY), the
Tianjin Clinical Key Discipline Project (Anesthesiology; grant no.
NCT03024840), the College Program of Tianjin First Central Hospital
(grant no. CF201819), the Tianjin Health and Family Planning
Commission of Science and Technology Research Projects (grant no.
16KG101), the Tianjin Health and Family Planning Commission of
Chinese and Western Medicine of Traditional Chinese Medicine
Combined with Scientific Research Subject (grant no. 2017056) and
the 2017 Tianjin Natural Science Fund Project (grant no.
17JCYBJC28000). The funding bodies had no role in the design of the
present study or the collection, analysis, data interpretation or
the writing of the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WY and HY conceptualized the study. YSh contributed
to the design of the present study. GZ and YSu contributed to
performing the experiments, acquiring results and the analysis. MZ
interpreted the data. HY drafted and substantially revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of Tianjin First Central Hospital, Tianjin, China
(approval no. 2016N0039KY) and written informed consent was
obtained from the infants' parents or guardians. The privacy rights
of human subjects were observed.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sokal EM, Goldstein D, Ciocca M, Lewindon
P, Ni YH, Silveira T, Sibal A, Dhawan A, Mack C and Bucuvalas J:
End-Stage Liver Disease Working Group. End-stage liver disease and
liver transplant: Current situation and key issues. J Pediatr
Gastroenterol Nutr. 47:239–246. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Protheroe SM and Kelly DA: Cholestasis and
end-stage liver disease. Baillieres Clin Gastroenterol. 12:823–841.
1998.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Group SR: Studies of Pediatric Liver
Transplantation (SPLIT): Year 2000 outcomes. Transplantation.
72:463–476. 2001.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Young S, Kwarta E, Azzam R and Sentongo T:
Nutrition assessment and support in children with end-stage liver
disease. Nutr Clin Pract. 28:317–329. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Stanescu AL, Hryhorczuk AL, Chang PT, Lee
EY and Phillips GS: Pediatric abdominal organ transplantation:
Current indications, techniques, and imaging findings. Radiol Clin
North Am. 54:281–302. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tannuri AC, Gibelli NE, Ricardi LR, Silva
MM, Santos MM, Pinho-Apezzato ML, Maksoud-Filho JG, Velhote MC,
Ayoub AA, Andrade WC, et al: Orthotopic liver transplantation in
biliary atresia: A single-center experience. Transplant Proc.
43:181–183. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Colledan M, Torri E, Bertani A, Corno V,
Guizzetti M, Lucianetti A, Maldini G, Pinelli D, Zambelli M,
Giovanelli M, et al: Orthotopic liver transplantation for biliary
atresia. Transplant Proc. 37:1153–1154. 2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ameres M, Melter M, Zant R, Schilling S
and Geis T: Liver transplantation during infancy: No increased rate
of neurological complications. Pediatr Transplant.
22(e13304)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Menegaux F, Keeffe EB, Andrews BT, Egawa
H, Monge H, Concepcion W, So SK and Esquivel CO: Neurological
complications of liver transplantation in adult versus pediatric
patients. Transplantation. 58:447–450. 1994.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nemati H, Kazemi K and Mokarram AT:
Neurological complications associated with pediatric liver
transplant in Namazi hospital: One-year follow-up. Int J Organ
Transplant Med. 10:30–35. 2019.PubMed/NCBI
|
|
11
|
Squires RH, Ng V, Romero R, Ekong U,
Hardikar W, Emre S and Mazariegos GV: Evaluation of the pediatric
patient for liver transplantation: 2014 practice guideline by the
American Association for the Study of Liver Diseases, American
Society of Transplantation and the North American Society for
Pediatric Gastroenterology, Hepatology and Nutrition. Hepatology.
60:362–398. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Schifilliti D, Mondello S, D'Arrigo MG,
Chille G and Fodale V: Genotoxic effects of anesthetic agents: An
update. Expert Opin Drug Saf. 10:891–899. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Istaphanous G and Loepke AW: General
anesthetics and the developing brain. Curr Opin Anaesthesiol.
22:368–373. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Vutskits L: Anesthetic-related
neurotoxicity and the developing brain: Shall we change practice?
Paediatr Drugs. 14:13–21. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Stratmann G: Review article: Neurotoxicity
of anesthetic drugs in the developing brain. Anesth Analg.
113:1170–1179. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wainwright MS, Craft JM, Griffin WS, Marks
A, Pineda J, Padgett KR and Van Eldik LJ: Increased susceptibility
of S100B transgenic mice to perinatal Hypoxia-ischemia. Ann Neurol.
56:61–67. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chiaretti A, Barone G, Riccardi R,
Antonelli A, Pezzotti P, Genovese O, Tortorolo L and Conti G: NGF,
DCX, and NSE upregulation correlates with severity and outcome of
head trauma in children. Neurology. 72:609–616. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Egea-Guerrero JJ, Revuelto-Rey J,
Murillo-Cabezas F, Muñoz-Sánchez MA, Vilches-Arenas A,
Sánchez-Linares P, Domínguez-Roldán JM and León-Carrión J: Accuracy
of the S100β protein as a marker of brain damage in traumatic brain
injury. Brain Inj. 26:76–82. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yao B, Zhang LN, Ai YH, Liu ZY and Huang
L: Serum S100β is a better biomarker than neuron-specific enolase
for Sepsis-associated encephalopathy and determining its prognosis:
A prospective and observational study. Neurochem Res. 39:1263–1269.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Oris C, Pereira B, Durif J, Simon-Pimmel
J, Castellani C, Manzano S, Sapin V and Bouvier D: The Biomarker
S100B and Mild Traumatic Brain Injury: A Meta-analysis. Pediatrics.
141(e20180037)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Thelin EP, Jeppsson E, Frostell A,
Svensson M, Mondello S, Bellander BM and Nelson DW: Utility of
neuron-specific enolase in traumatic brain injury; relations to
S100B levels, outcome, and extracranial injury severity. Crit Care.
20(285)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Rech TH, Vieira SR, Nagel F, Brauner JS
and Scalco R: Serum neuron-specific enolase as early predictor of
outcome after in-hospital cardiac arrest: A cohort study. Crit
Care. 10(R133)2006.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Nakhjavan-Shahraki B, Yousefifard M, Oraii
A, Sarveazad A and Hosseini M: Meta-analysis of neuron specific
enolase in predicting pediatric brain injury outcomes. EXCLI J.
16:995–1008. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Schmitt B, Bauersfeld U, Schmid ER,
Tuchschmid P, Molinari L, Fanconi S and Bandtlow C: Serum and CSF
levels of neuron-specific enolase (NSE) in cardiac surgery with
cardiopulmonary bypass: A marker of brain injury? Brain Dev.
20:536–539. 1998.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu Y, Xu Y, Li DZ, Shi Y and Ye M:
Comparison of S100B and NSE between cardiac surgery and
interventional therapy for children. Pediatr Cardiol. 30:893–897.
2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Saklad M: Grading of patients for surgical
procedures. Anesthesiology. 2:281–284. 1941.
|
|
27
|
Freeman RB Jr, Wiesner RH, Roberts JP,
McDiarmid S, Dykstra DM and Merion RM: Improving liver allocation:
MELD and PELD. Am J Transplant. 4 (Suppl 9):S114–S131.
2004.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bayley N: Bayley scales of infant
development. 2nd edition. Psychological Corporation, San Antonio,
1993.
|
|
29
|
Bai Y, Shang G, Wang L, Sun Y, Osborn A
and Rozelle S: The relationship between birth season and early
childhood development: Evidence from northwest rural China. PLoS
One. 13(e0205281)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sikich N and Lerman J: Development and
psychometric evaluation of the pediatric anesthesia emergence
delirium scale. Anesthesiology. 100:1138–1145. 2004.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Weiss N and Thabut D: Neurological
complications occurring after liver transplantation: Role of risk
factors, hepatic encephalopathy, and acute (on Chronic) brain
injury. Liver Transpl. 25:469–487. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Singh S, Nasa V and Tandon M:
Perioperative monitoring in liver transplant patients. J Clin Exp
Hepatol. 2:271–278. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Cuenca AG, Kim HB and Vakili K: Pediatric
liver transplantation. Semin Pediatr Surg. 26:217–223.
2017.PubMed/NCBI View Article : Google Scholar
|