Introduction
Relatively little is known regarding the
relationship between autophagy and the Wnt/β-catenin signaling
pathway in membranous nephropathy, in which both serve an important
role in the repair of injured podocytes (1-3).
Idiopathic membranous nephropathy is characterized by the diffuse
deposition of immune complexes under the glomerular basement
membrane of epithelial cells and diffuse thickening of the basement
membrane, which is a disease mediated by in situ immune
complexes (4). Phospholipase A2
receptor (PLA2R) and thrombospondin type-1 domain-containing 7A,
expressed in podocytes, have been identified as the main pathogenic
antigens in this condition (5), and
the source of PLA2R antibodies may be circulating neutrophils
(6). Podocytes are epithelial cells
of the glomerular viscera, located outside the glomerular basement
membrane, and autoantibodies bind to antigens on the surface of
podocytes, forming an in situ immune complex that results in
disease (2,5). In situ immune complexes differ
from circulating immune complexes in that they do not come into
contact with circulating inflammatory mediators and generally do
not trigger inflammatory reactions (7). Therefore, they are more likely to
trigger podocyte lesion formation by activating complement, leading
to proteinuria and renal tissue damage (8). The membrane attack complex, as the
final product of complement activation, attacks glomerular
podocytes primarily through stimulating membrane phospholipids via
phospholipase A2 to generate arachidonic acid and through
cyclo-oxygenase to stimulate endoplasmic reticulum stress (9). In addition, oxidative stress,
cytoskeletal protein migration and other factors are involved in
the occurrence and development of podocyte injury (8,10).
Autophagy is an important evolutionarily conserved
mechanism which allows eukaryotic cells to maintain homeostasis and
recycle intracellular components (11). During autophagy, cells form a
double-layered membrane structure in the cytoplasm to form
autophagosomes around damaged or aging organelles and
biomacromolecules, and bind with lysosomes to form an autolysosome,
in which the material is degraded and the contents reused for
synthesis of cellular components (11). Mature podocytes, which are
terminally-differentiated cells, have a high basal level of
autophagy (12). The formation of
autophagosomes to remove excess or damaged proteins and organelles
is a vital injury response mechanism on which podocytes rely for
survival (13). When mature
podocytes are injured by the membrane attack complex complement
5b-9 (C5b-9), multiple injury signaling pathways are activated in
the cells, leading to podocyte lesions, and a series of defense
mechanisms are activated to limit the damage and promote repair. Lv
et al successfully established an in vitro model of
C5b-9 complex injury in podocytes (14). This revealed that C5b-9 can cause
abnormal podocyte morphology and autophagy activation (14). Wang et al (15) found that the expression of ER
stress-related proteins GRP78 and GRP94 in podocytes was abnormally
distributed, and the expression of autophagy-related protein LC3
was significantly increased in a rat model of passive Heymann
nephritis. In these autophagy related studies, 3-methyladenine
(3-MA) is a commonly used inhibitor and rapamycin is a commonly
used activator (16,17).
The Wnt/β-catenin signaling pathway serves a crucial
role in the adhesion, differentiation and survival of podocytes
(18). Activation of the
Wnt/β-catenin signaling pathway can lead to podocyte damage, both
in vitro and in vivo (2). For example, in diabetic kidney
disease, doxorubicin nephropathy and other glomerular diseases,
which can lead to podocyte injury, the expression of Wnt in
glomerular epithelial cells is significantly increased and the
downstream β-catenin activated, resulting in activation of the
Wnt/β-catenin signaling pathway. However, specific inhibition of
this pathway, such as through the knockout of β-catenin or the use
of pathway inhibitors, can alleviate podocyte damage, suggesting a
critical role for this pathway in kidney protection (18-20).
To the best of our knowledge, the relationship between autophagy
and the Wnt/β-catenin signaling pathway has not been elucidated in
the repair of podocytes. In our previous study, it was shown that
treatment with curcumin protected podocyte cells against
leptin-induced damage and that its protective effects were mediated
by inhibition of the Wnt/β-catenin signaling pathway in
vitro (21). Dickkopf-related
protein 1 (DKK1), a specific inhibitor of the Wnt/β-catenin
signaling pathways, decreased LC3II significantly (3,22).
These results suggest a close relationship between the
Wnt/β-catenin signaling pathway and autophagic inhibition in
podocytes. However, this observation cannot fully explain the
correlation between the Wnt/β-catenin signal pathway and autophagy.
Thus, C5b-9 was used to further study the relationship between the
canonical Wnt/β-catenin signal pathway and autophagy.
In the present study, podocytes were incubated with
C5b-9 serum and treated with DKK1 and changes in autophagy were
observed. These results may help us to more fully elucidate the
potential mechanism of the influence of Wnt/β-catenin signaling
pathway on the autophagy-lysosome pathway. The results of the
present study highlight the potential existence of a critical
relationship between the canonical Wnt/β-catenin signaling pathway
and autophagy.
Materials and methods
Cell culture and treatments
A conditioned immortal mouse podocyte cell line was
kindly provided by Professor Maria Pia Rastaldi (S.C. Hospital of
the University of Milan, Milan, Italy). Cells were cultured in
RPMI-1640 medium (cat. no. 11875093; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (cat. no. 10100147;
Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin-streptomycin (cat. no. SV30010; Hyclone; Cytiva) and 10
U/ml of mouse recombinant interferon-γ (cat. no. 39127S; Cell
Signaling Technology, Inc.) with 5% CO2, at 33˚C to
induce proliferation. The podocytes were then cultured with 5%
CO2 at 37˚C without interferon-γ when they reached
60-70% confluence. The cells changed from spindle-liked to
star-liked after 7-10 days, indicating that they had fully
differentiated, At this point, they were used in subsequent
experiments.
The complement membrane attack complex, C5b-9, was
established using normal human serum as a complement source and
treated with zymosan (cat. no. Z4250; Sigma-Aldrich; Merck KGaA) as
described by Liu et al (23). The normal human serum used in this
study was provided by a 23-year-old male in November 2018. The
serum was provided by healthy volunteers that provided written
informed consent. The study received ethical approval from the
Medical Ethics Committee of Beijing Hospital of Traditional Chinese
Medicine Affiliated to Capital Medical University (Beijing, China).
To evaluate the effect of C5b-9, mouse podocyte cells (MPC) were
treated with different concentrations of zymosan activated serum
(ZAS) for 1, 2, 4, 8, 12 or 24 h (C5b-9 group), and heat
inactivated serum was used as the control (blank group). The volume
of zymosan required to increase lactate dehydrogenase (LDH) release
by <10% was used as a sublethal dose to induce podocyte injury.
The cells were also pretreated with 3-MA (cat. on. 3977; R&D
Systems, Inc.) rapamycin (cat. no. 1292; R&D Systems, Inc.) or
DKK1 (cat. no. 5897-DK; R&D Systems, Inc.) before treatment
with ZAS. The group preincubated with 3-MA before using ZAS was
called the 3-MA group, the group preincubated with rapamycin was
called the rapamycin group, and the group preincubated with DKK1
was called the DKK1 group.
C5b-9 ELISA
Following the manufacturer's protocol, a C5b-9 ELISA
kit (cat. no. A020; Quidel Corporation, Inc) was used to determine
the levels of C5b-9 in the serum of samples incubated with zymosan.
The optical density was measured at 450 nm using a plate
reader.
LDH assay
The optimum concentration and incubation time for
C5b-9 was determined using an LDH release test, which utilized a
non-radioactive cytotoxicity assay (cat. no. G1780; Promega
Corporation). Mature and differentiated MPCs suspended in RPMI1640
medium supplemented with 10% inactivated FBS, were plated in 96
well cell plates (7x103 cells/well). Podocytes were
measured following exposure to zymosan for different periods of
time at 490 nm using a plate reader, according to the
manufacturer's protocol. The formula used to calculate LDH release
was: LDH release rate (%) = (experimental-target
spontaneous)/(target maximum-target spontaneous) x100. Experimental
group refers to the OD value of cell pore after relevant
intervention stimulation. Target spontaneous group refers to the OD
value of normal cell pores without intervention or stimulation.
Target maximum group refers to the OD value of the cell pore after
adding the lysate provided by the kit.
Immunofluorescence staining of
cultured podocytes
The podocyte samples were fixed with 4%
paraformaldehyde for 20 min at room temperature, rinsed with PBS
(cat. no. KGB5001; Nanjing KeyGen Biotech Co., Ltd.) and then
permeabilized with 0.5% Triton X-100 (Applygen Technologies, Inc.)
in PBS for 30 min at room temperature. Non-specific binding was
blocked with 5% BSA (cat. no. SW3015; Beijing Solarbio Science
& Technology Co., Ltd.) for 1 h at room temperature. The
podocytes were incubated with primary antibodies overnight at 4˚C.
The following day, the podocytes were washed with PBS, labeled with
secondary antibodies using 2 drops/ml for 30 min at room
temperature and incubated with Alexa Fluor 488 Phalloidin (cat. no.
A12379 Invitrogen; Thermo Fisher Scientific, Inc.) for 30 min at
room temperature. Finally, podocytes were washed with PBS and
sealed using antifade mounting reagent containing DAPI (cat. no.
S2110; Beijing Solarbio Science & Technology Co.).
The primary antibodies used were: Anti-C5b-9 rabbit
polyclonal antibody (pAb) (1:1,000; cat. no. ab55811; Abcam),
anti-podocin rabbit monoclonal antibody (mAb) (1:500; cat. no.
ab181143; Abcam), anti-beclin-1 rabbit mAb (1:50; cat. no.
ab217179; Abcam), anti-LC3A/B rabbit antibody (1:200; cat. no.
4108; Cell Signaling Technology, Inc.), anti-SQSTM1 mouse mAb
(1:100; cat. no. ab109012; Abcam), anti-β-catenin rabbit mAb
(1:100; cat. no. 8480; Cell Signaling Technology, Inc.),
anti-GSK-3β rabbit mAb (1:50; cat. no. 12456; Cell Signaling
Technology, Inc.) and anti-Akt rabbit mAb (1:400; cat. no. 4691;
Cell Signaling Technology, Inc. ). The secondary antibodies used
were goat anti-rabbit IgG (H+L) Alexa Fluor 594 (cat. no. R37117;
Invitrogen; Thermo Fisher Scientific, Inc.) and goat anti-mouse IgG
(H+L) Alexa Fluor 594 (cat. no. R37121; Invitrogen; Thermo Fisher
Scientific, Inc.).
Capillary western immunoassay
(WES)
According to the manufacturer's protocols, the
12-230 kDa separation module (ProteinSimple; Bio-Techne
Corporation) and anti-mouse detection module (ProteinSimple;
Bio-Techne Corporation) were used for analysis using a WES system
(ProteinSimple; Bio-Techne Corporation) as previously described
(24). Proteins were extracted from
cells using RIPA lysate and protease inhibitor, The concentration
of the extracted protein was determined using a BCA kit (cat. no.
PC0200, Beijing Solarbio Science & Technology Co.). Extracted
cell proteins were diluted with 5x master mix and 0.1x sample
buffer provided with the kit. Antibody diluent II provided in the
kit was then used to dilute the primary antibody. Diluted protein,
antibody diluent II, diluted primary antibody, secondary HRP
conjugate, luminol-conjugate mix and wash buffer were added to each
row of the plate provided in the kit. Finally, a WES dedicated
instrument (ProteinSimple; Bio-Techne Corporation) and Compass
software 4.0.0 (ProteinSimple; Bio-Techne Corporation) was used to
process the plate. After 2.5 h, the corresponding band of each
protein sample was obtained. The concentration of the band was
analyzed to determine the corresponding protein content using
Compass software.
Anti-nephrin rabbit mAb (1:50; cat. no. ab216341;
Abcam), anti-podocin rabbit mAb (1:50; cat. no. ab181143; Abcam),
anti-podocalyxin-like 1 mouse antibody (1:10; cat. no. sc-23903;
Santa Cruz Biotechnology, Inc.), anti-podoplanin mouse antibody
(1:10; cat. no. sc-166906; Santa Cruz Biotechnology, Inc.),
anti-beclin-1 rabbit mAb (1:50; cat. no. ab217179; Abcam),
anti-LC3A/B rabbit pAb (1:50; cat. no. ab62721; Abcam), anti-SQSTM1
mouse mAb (1:50; cat. no. ab109012; Abcam), anti-β-catenin rabbit
mAb (1:50; cat. no. 8480; Cell Signaling Technology, Inc.),
anti-phosphorylated (p)-β-catenin rabbit mAb (S675; 1:50; cat. no.
4176; Cell Signaling Technology, Inc.), anti-p-β-catenin rabbit mAb
(S552; 1:50; cat. no. 5651; Cell Signaling Technology, Inc.),
anti-GSK-3β rabbit mAb (1:50; cat. no. 12456; Cell Signaling
Technology, Inc.), anti-p-GSK-3β rabbit mAb (S9; 1:50; cat. no.
5558; Cell Signaling Technology, Inc.), anti-p-GSK-3β rabbit pAb
(Y216; 1:50; ab75745; Abcam), anti-Akt rabbit mAb (1:50; cat. no.
4691; Cell Signaling Technology, Inc.) and anti-GAPDH rabbit mAb
(1:50; cat. no. 5174; Cell Signaling Technology, Inc.) were used as
primary antibodies to detect the expression of their respective
protein targets in the different cell groups.
Statistical analysis
SPSS version 20.0 (IBM, Corp.) was used for
statistical analysis. Data are expressed as the mean ± standard
deviation, or as counts or percentages as relevant. Comparisons
between two-groups were performed using an independent-sample
t-test unless otherwise indicated. Comparisons between
multiple-groups were performed using an ANOVA followed by a least
significant difference or Student-Newman-Keuls tests for ≤3 groups
or Tukey's test for >3 groups to verify the data. P<0.05 was
considered to indicate a statistically significant difference.
Results
C5b-9 serum-mediates injury of
podocytes
Firstly, to mimic the formation of the membrane
attack complex in vitro, using human blood as the complement
source, the content of C5b-9 in the extracted serum was measured
using ELISA (Fig. 1A). Glomerular
epithelial cells were incubated with C5b-9 serum for differing
periods of time, and their respective LDH release rates were
calculated (Fig. 1B). A duration of
2 h was used as the model of hypo-lysis.
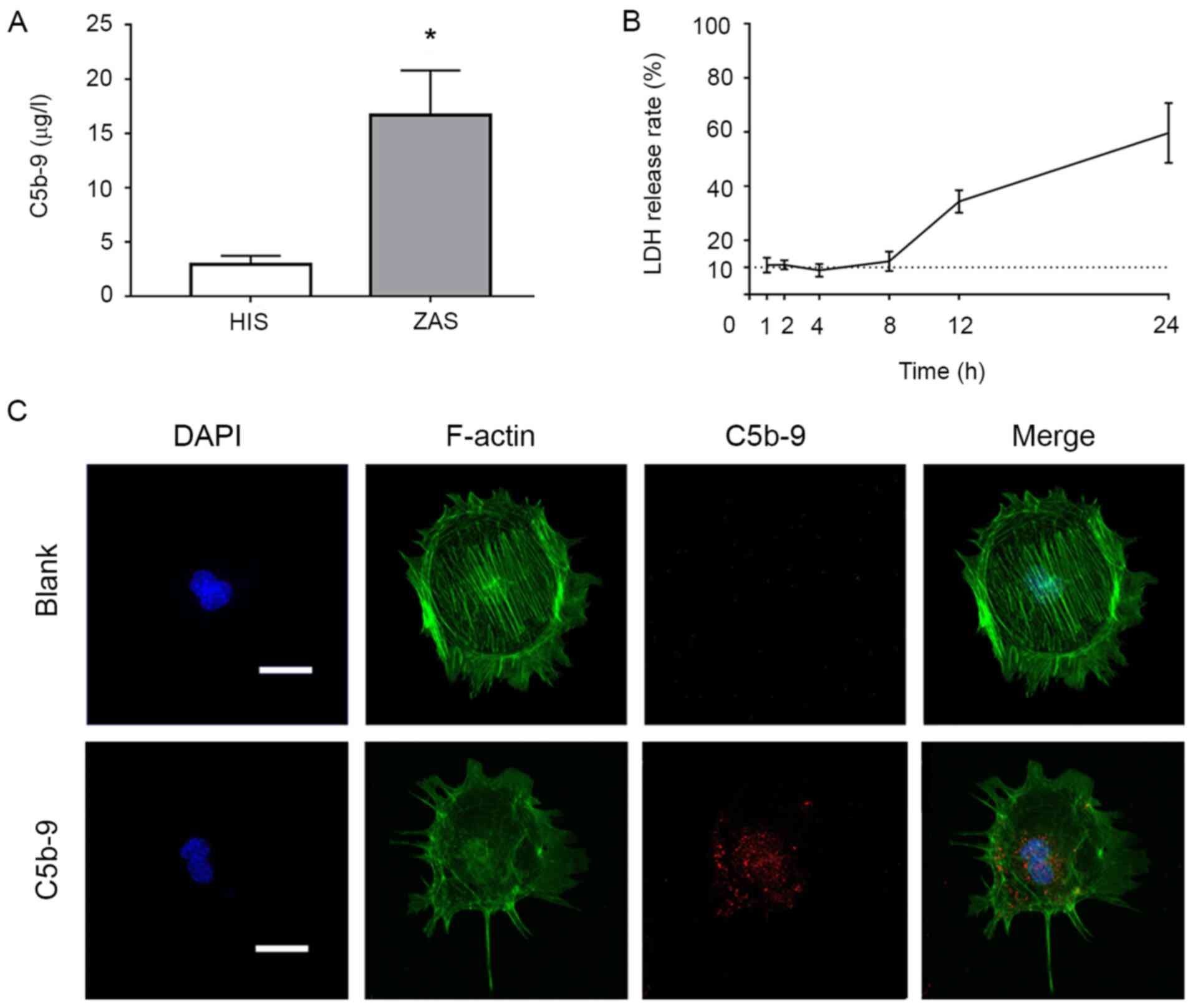 | Figure 1Effects of C5b-9 and different
concentrations of Rapamycin, 3-MA and DKK1 on podocytes. (A) The
content of C5b-9 in human serum after incubation with Zymosan was
determined by ELISA. (B) LDH release rates at different time points
were determined to reflect the cytotoxicity of C5b-9. (C) C5b-9
damaged podocytes. Immunofluorescence staining of the cytoskeleton
(green) and C5b-9 (red) in glomerular epithelial cells indicated
that cell size in the C5b-9 group was smaller than that of the
blank group, that fluorescence intensity was weaker and the
cytoskeleton was more disordered. Scale bar, 10 µm.
*P<0.05 (Student's t-test). C5b-9, complement 5b-9;
3-MA, 3-methyladenine; DKK1, Dickkopf-related protein 1; LDH,
lactate dehydrogenase; F-actin, filamentous actin, HIS, human
inactivated serum; ZAS, zymosan activated serum. |
Immunofluorescence staining was performed on the
cytoskeleton of podocytes in each group with using phalloidin, and
it was demonstrated that the cells in the C5b-9 group exhibited
weaker fluorescence intensity and a more disordered cytoskeleton in
comparison with the blank group (Fig.
1C).
3-MA, rapamycin and DKK1 protect
against C5b-9-mediated injury of podocytes
In order to better understand the protective effects
of the autophagy and Wnt/β-catenin pathways on podocytes, 3-MA,
rapamycin and DKK1 were used to preculture podocytes that were
subsequently damaged using C5b-9. Firstly, LDH release rates of
podocytes incubated with different concentrations of 3-MA,
rapamycin and DKK1 were calculated, and then the maximum
concentration possible with minimal cell damage to cells was
selected. As shown in Fig. 2A, the
concentration of 3-MA used for subsequent experiments was 10
mmol/l, 100 nmol/l for rapamycin and 100 ng/ml for DKK1.
Subsequently, the above concentrations were used to pretreat the
podocytes prior to C5b-9 injury, and the LDH release rates were
measured. DKK1 reduced the release rate of LDH in comparison to
that with C5b-9 alone, whereas 3-MA and rapamycin did not. This
suggested that 3-MA and rapamycin pretreatment did not protect
podocytes, whereas DKK1 pretreatment did (Fig. 2A). Immunofluorescence staining of
podocin was used to detect changes in the treated cells. The
results of injury analysis were consistent with LDH release rates
(Fig. 2B). Finally, WES was used to
determine the effects of DKK1 on podocin, nephrin, podoplanin and
podocalyxin in podocytes. The results indicated that DKK1 protected
podocytes from damage caused by C5b-9 (Fig. 2C).
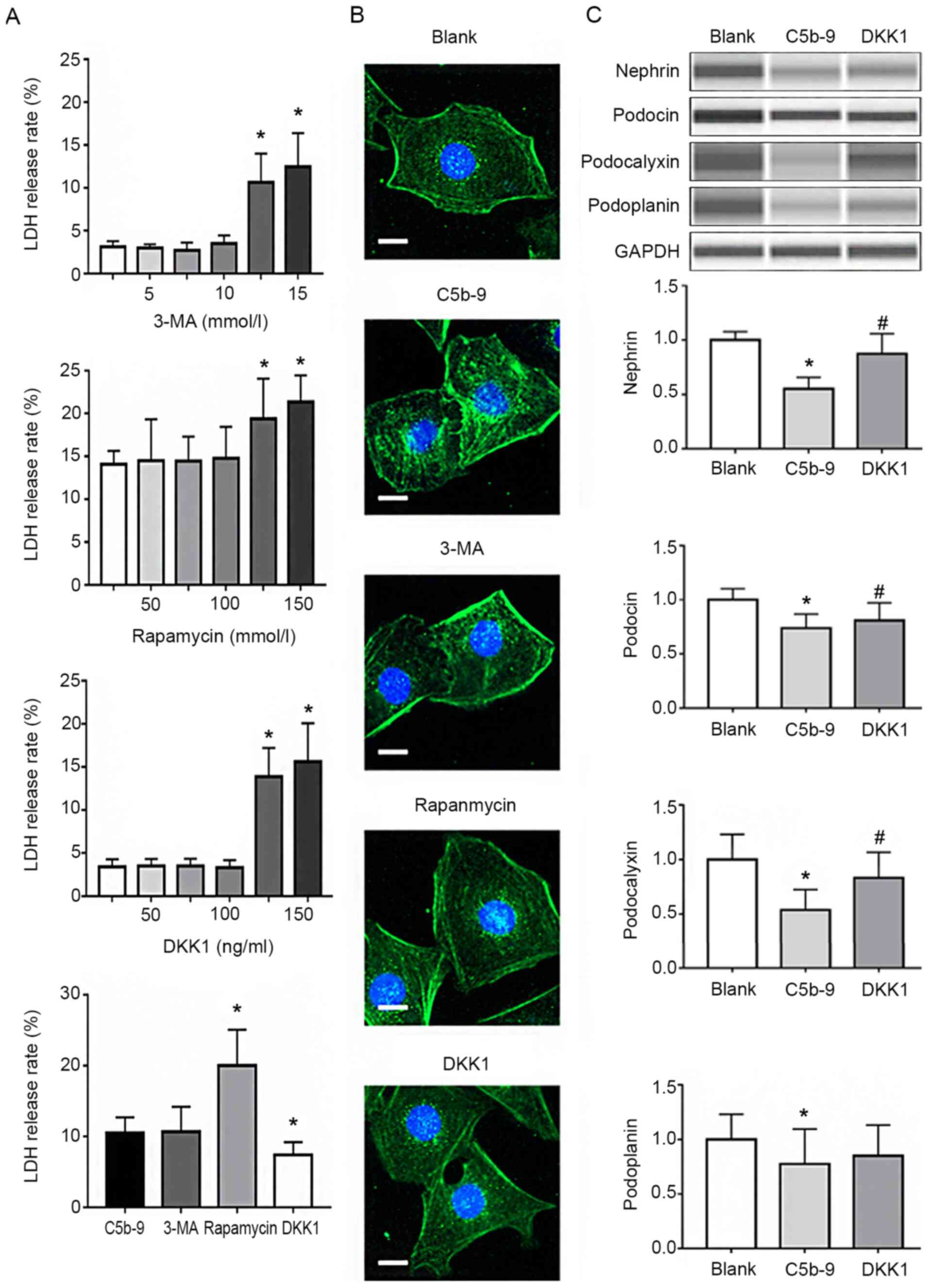 | Figure 2C5b-9 can damage podocytes and DKK1
can repair the damage. (A) In order to select the optimal
concentration of DKK1, normal podocytes were incubated with
different concentrations of Rapamycin, 3-MA and DKK1 and their LDH
release rates calculated. The maximum concentration with the least
damage was selected. *P<0.05 (Student's t-test). (B)
Immunofluorescence staining for podocin in the blank, C5b-9,
rapamycin, 3-MA and DKK1 groups. Scale bar, 1 µm. (C) WES was used
to determine the levels of nephrin, podocin, podocalyxin and
podoplanin of glomerular epithelial cells in the blank, C5b-9 and
DKK1 groups. *P<0.05 vs. blank group;
#P<0.05 vs. C5b-9 group. C5b-9, complement 5b-9;
3-MA, 3-methyladenine; DKK1, Dickkopf-related protein 1; LDH,
lactate dehydrogenase. |
Effects of C5b-9 serum on
autophagy
The levels of beclin-1, LC3 and SQSTM1 expression in
podocytes of the blank group, C5b-9 group and DKK1 group were
detected using immunofluorescence.
The arrangement of the cytoskeleton in the C5b-9
group was more disordered compared with the blank group, and the
cytoskeleton of the DKK1 group did not exhibit the disorganization
of the C5b-9 group. The fluorescence intensity of beclin-1 showed
no significant difference among the three groups (Fig. 3A), and LC3 in the C5b-9 group was
stronger compared with the blank group and DKK1 group (Fig. 3B), and the fluorescence intensity of
SQSTM1 was stronger compared with the blank group, whereas in the
DKK1 group, the fluorescence intensity was slightly stronger
compared with the other two groups (Fig. 3C). These results suggested that
C5b-9 activated autophagy.
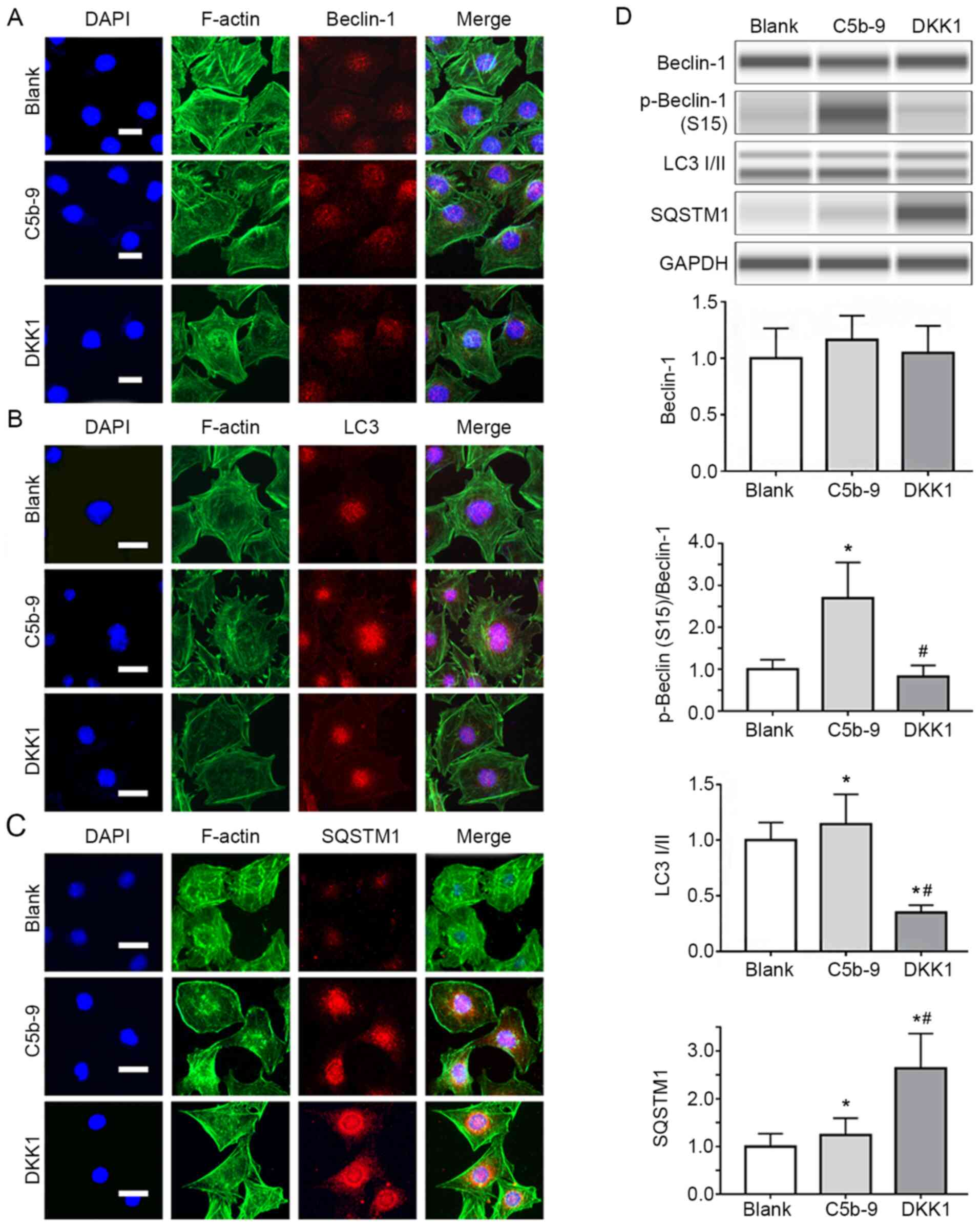 | Figure 3DKK1 successfully inhibited autophagy
activated by C5b-9. (A) Immunofluorescence staining for Beclin-1 in
blank, C5b-9 and DKK1 groups. Scale bar, 10 µm. (B)
Immunofluorescence staining for LC3 in blank, C5b-9 and DKK1
groups. Scale bar, 10 µm. (C) Immunofluorescence staining for
SQSTM1 in blank, C5b-9 and DKK1 groups. Scale bar, 10 µm. (D)
Beclin-1, phospho-beclin-1 (S15), LC3I/II and SQSTM1 proteins were
determined by WES in blank, C5b-9 and DKK1 groups.
*P<0.05 vs. blank group; #P<0.05 vs.
C5b-9 group. C5b-9, complement 5b-9; DKK1, Dickkopf-related protein
1; LDH, lactate dehydrogenase; F-actin, filamentous actin; .SQSTM1,
sequestosome 1; LC3, microtubule-associated protein light chain
3. |
Beclin-1, p-beclin-1 (S15), LC3I/II and SQSTM1
expression levels were detected using WES to reflect the changes in
autophagy in podocytes in each group. Beclin-1 expression slightly
increased in the C5b-9 group compared with the blank group, and in
the DKK1 group, beclin-1 expression was slightly lower than that of
the C5b-9 group, and similar to that of the blank group, though the
difference was not statistically significant. LC3II and p-beclin-1
(S15) levels increased in the C5b-9 group compared with the blank
group and were lower in the DKK1 group compared with the C5b-9
group and similar to that in the blank group. SQSTM1 levels in the
C5b-9 group were slightly higher than that in the blank group,
while in the DKK1 group its expression was significantly higher
compared with the C5b-9 group. This suggests that C5b-9 activates
autophagy in glomerular epithelial cells and that this can be
inhibited by DKK1 (Fig. 3D).
Effect of C5b-9 serum on the
Wnt/β-catenin signaling pathway
The levels of β-catenin, GSK-3β and Akt in podocytes
of blank group, C5b-9 group and DKK1 group were assessed based on
immunofluorescence staining. The fluorescence intensity of
β-catenin in the C5b-9 group was stronger than that of the blank
group and DKK1 group (Fig. 4A), and
the fluorescence intensity of the GSK-3β was weaker than that of
the blank group and DKK1 group (Fig.
4B). This suggests that C5b-9 activates the Wnt/β-catenin
signaling pathway.
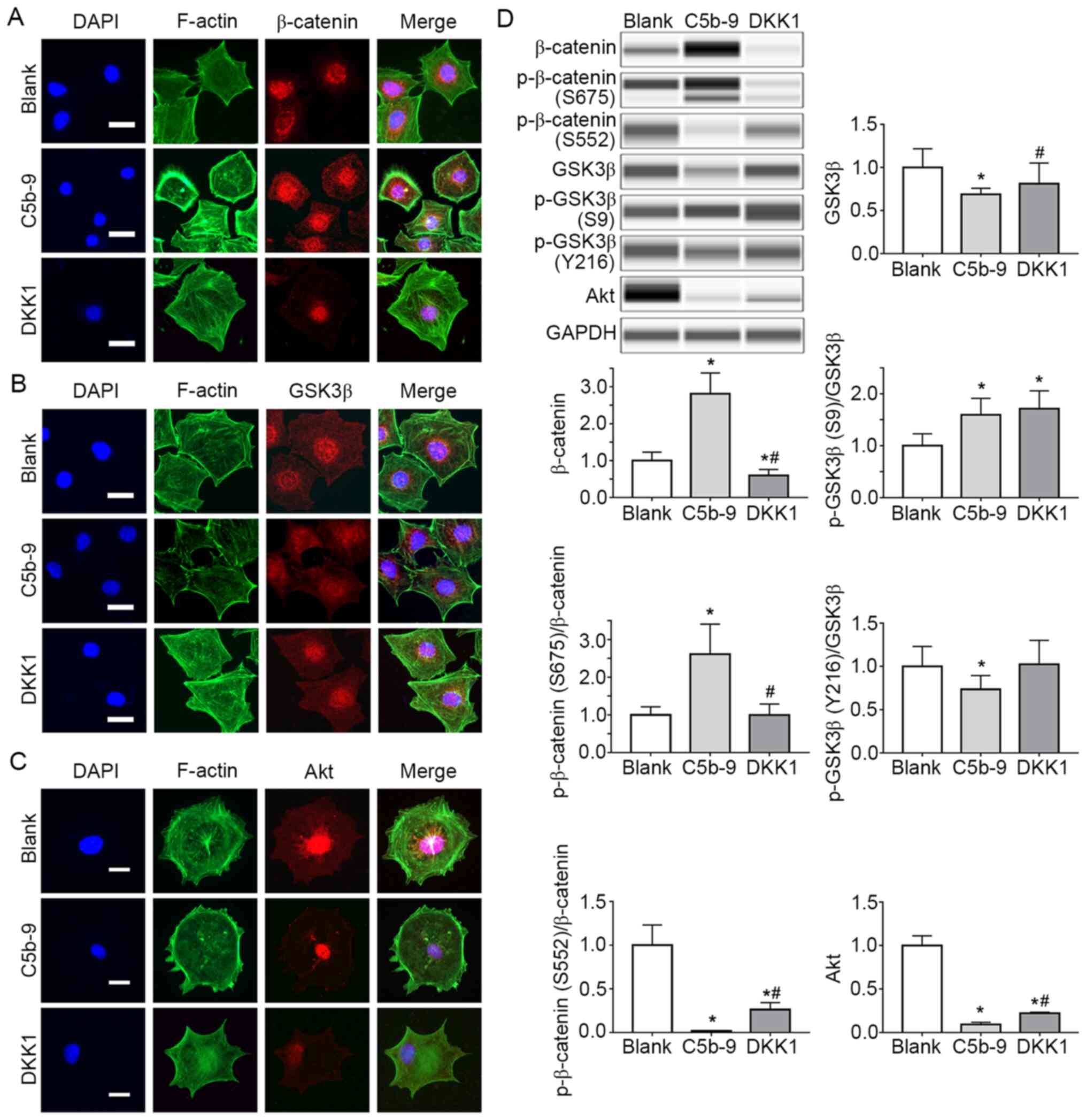 | Figure 4DKK1 successfully inhibited the
Wnt/β-catenin signal pathway activated by C5b-9 and simultaneously
increased the Akt. (A) Immunofluorescence staining for β-catenin in
blank, C5b-9 and DKK1 groups. Scale bar, 10 µm. (B)
Immunofluorescence staining for GSK-3β in blank, C5b-9 and DKK1
groups. Scale bar, 10 µm. (C) Immunofluorescence staining for Akt
in blank, C5b-9 and DKK1 groups. Scale bar, 10 µm. (D) β-catenin,
phosphorylayed-β-catenin (S675, S552), GSK-3β,
phosphorylated-GSK-3β (S9, Y216) and Akt proteins were determined
by WES in blank, C5b-9 and DKK1 groups. *P<0.05 vs.
blank group; #P<0.05 vs. C5b-9 group. C5b-9,
complement 5b-9; DKK1, Dickkopf-related protein 1; F-actin,
filamentous actin; GSK3, glycogen synthase kinase 3. |
At the same time, the changes in the levels of
canonical Wnt pathway members [GSK-3β, p-GSK-3β (S9), β-catenin and
p-β-catenin (S675, S552)] were detected. GSK-3β expression in the
C5b-9 group was significantly lower compared with the blank group,
whereas in the DKK1 group, GSK3-β expression was significantly
higher than the C5b-9 group and similar to that in the blank group.
β-catenin, p-β-catenin (S675) and p-GSK-3β (S9) levels in the C5b-9
group were significantly higher than those in the blank group.
However, the β-catenin levels in the DKK1 group were significantly
lower than the other two groups. p-β-catenin (675) levels were
lower in the C5b-9 group and similar to that of the blank group.
p-GSK-3β (S9) levels were significantly higher in the DKK1 group in
comparison with the other two groups. These results suggest that
the membrane attack complex activates the Wnt/β-catenin pathway in
glomerular epithelial cells. However, the p-β-catenin (S552) level
in the C5b-9 group was significantly lower compared with the blank
group. In addition, p-β-catenin levels in the DKK1 group were lower
than in the blank group, but higher than that in the C5b-9 group
(Fig. 4D).
In order to explain this phenomenon,
immunofluorescence staining and WES were used to detect Akt levels
in each group. The results indicated that the fluorescence
intensity was weaker in the C5b-9 group, and slightly stronger in
the DKK1 group I comparison with the C5b-9 group (Fig. 4A-D). WES showed that Akt expression
in the C5b-9 group was significantly lower compared with the blank
group, whereas Akt levels in the DKK1 group were higher than those
in the C5b-9 group, but still lower than those in the blank group
(Fig. 4D).
Discussion
In the present study, three important observations
were made: Firstly, the results suggested that C5b-9 activated
autophagy and the Wnt/β-catenin signaling pathway. Secondly, it was
indicated that DKK1-mediated inhibition of the Wnt/β-catenin
signaling pathway reduced C5b-9-induced damage to glomerular
epithelial cells. Thirdly, it appeared that autophagy was inhibited
and DKK1 protected podocytes.
The purpose of the present study was to observe
changes to autophagy and the Wnt/ catenin signaling pathway in
podocytes after C5b-9 injury. Therefore, podocytes were pretreated
with 3-MA, rapamycin and DKK1 before C5b-9 treatment, and the
condition of podocytes was observed by immunofluorescence. The
Wnt/β-catenin signaling pathway is associated with autophagy and it
has been reported that activation of GSK-3β can inhibit mammalian
target of rapamycin complex 1 (mTORC1) to activate autophagy by
promoting the binding of tuberous sclerosis 1/2(25). It has also been reported that
β-catenin enucleation inhibits autophagy by inhibiting SQSTM1
transcription (22). Thus,
inhibition of the Wnt/β-catenin signaling pathway can activate
autophagy (26,27). However, the majority of these
previous studies were carried out in tumor cells. To the best of
our knowledge, the present study is the first to evaluate the
effects of C5b-9 on podocyte Wnt/β-catenin signaling pathway and
autophagy, mimicking the process in the human body. The aim of the
present study was to determine whether damage of glomerular
epithelial cells could be reduced, and thus highlight a potential
treatment strategy for idiopathic membranous nephropathy.
C5b-9, via the lectin complement pathway, is the
primary cause of podocyte injury in idiopathic membranous
nephropathy (28). Studies on the
pathogenesis of idiopathic membranous nephropathy have revealed the
molecular mechanism underlying complement activation (29). IgG4 is a major subtype of IgG
deposited in the glomeruli of idiopathic membranous nephropathy
(30,31). IgG4 does not activate the classical
complement pathway (32).
Therefore, there may be other complement activation mechanisms.
Preliminary evidence suggests that specific IgG4 anti-PLA2R
antibody activates the alternative complement pathway or the
mannose-lectin pathway (33,34).
The results of the present study are consistent with those commonly
seen in patients with idiopathic membranous nephropathy, where C4
is deposited on the glomeruli in the absence of Complement 1q.
Eventually, C5b-9 dissolves the glomerular epithelial cells,
leading to the formation of proteinuria (10,35).
In addition, zymosan, a polysaccharide prepared from the cell walls
of saccharomyces cerevisiae, activates the alternative complement
pathway (36). Therefore, this
complement pathway was stimulated in the present study, and
incubated in normal serum with zymosan to reduce the injury to
podocytes as much as possible.
Results of the DKK1 pretreatment of podocytes showed
that expression of beclin-1 and LC3II were decreased, whereas
SQSTM1 expression was significantly increased. DKK1 activates
GSK-3β by inhibiting the Wnt/β-catenin pathway, which was
hypothesized to indirectly inhibit mTORC1 to activate beclin-1 and
thus activate autophagy. However, completely opposing results were
obtained in the present experiment. In addition, when the
Wnt/β-catenin signaling pathway was activated by complement, GSK-3β
(S9) phosphorylation levels were increased, whereas β-catenin
(S552) phosphorylation was significantly reduced, and thus, this
phenomenon may be explained simply by Akt protein abnormalities
(37). As Akt phosphorylates both
GSK-3β and β-catenin, GSK3β is inhibited and β-catenin is
activated, thereby activating the Wnt/β-catenin pathway (38). Thus, it was hypothesized that this
anomaly was related to Akt, and this was demonstrated in subsequent
experiments. Thus, the canonical Wnt signaling pathway and other
related signal proteins may have variations in the cell line used.
However, it is also possible that complement attack mediated damage
activated or inhibited the targets of multiple signaling pathways,
thus resulting in the aforementioned phenomenon.
The results of the present study indicated that Akt
protein was damaged by C5b-9, and that DKK1 inhibited the canonical
Wnt pathway, which can repair cell damage and restore Akt, which
in-turn phosphorylates β-catenin (S552) and reduces the levels of
p-GSK-3β (S9). Thus, autophagy may be inhibited due to the
activation of Akt (39). This
suggests that C5b-9, while activating the Wnt/β-catenin signaling
pathway, acts directly on low density lipoprotein receptor-related
protein 5/6 (LRP5/6), which is the target of DKK1(40), and other proteins together to cause
damage to Akt. DKK1 inhibits LRP5/6 and restores Akt function to
some extent, but not completely (Fig.
5). In addition, variations in signaling pathways in various
cells and in complement, which are common pathogenic factors in the
human body that are not commonly assessed in cellular experimental
methods, require further study.
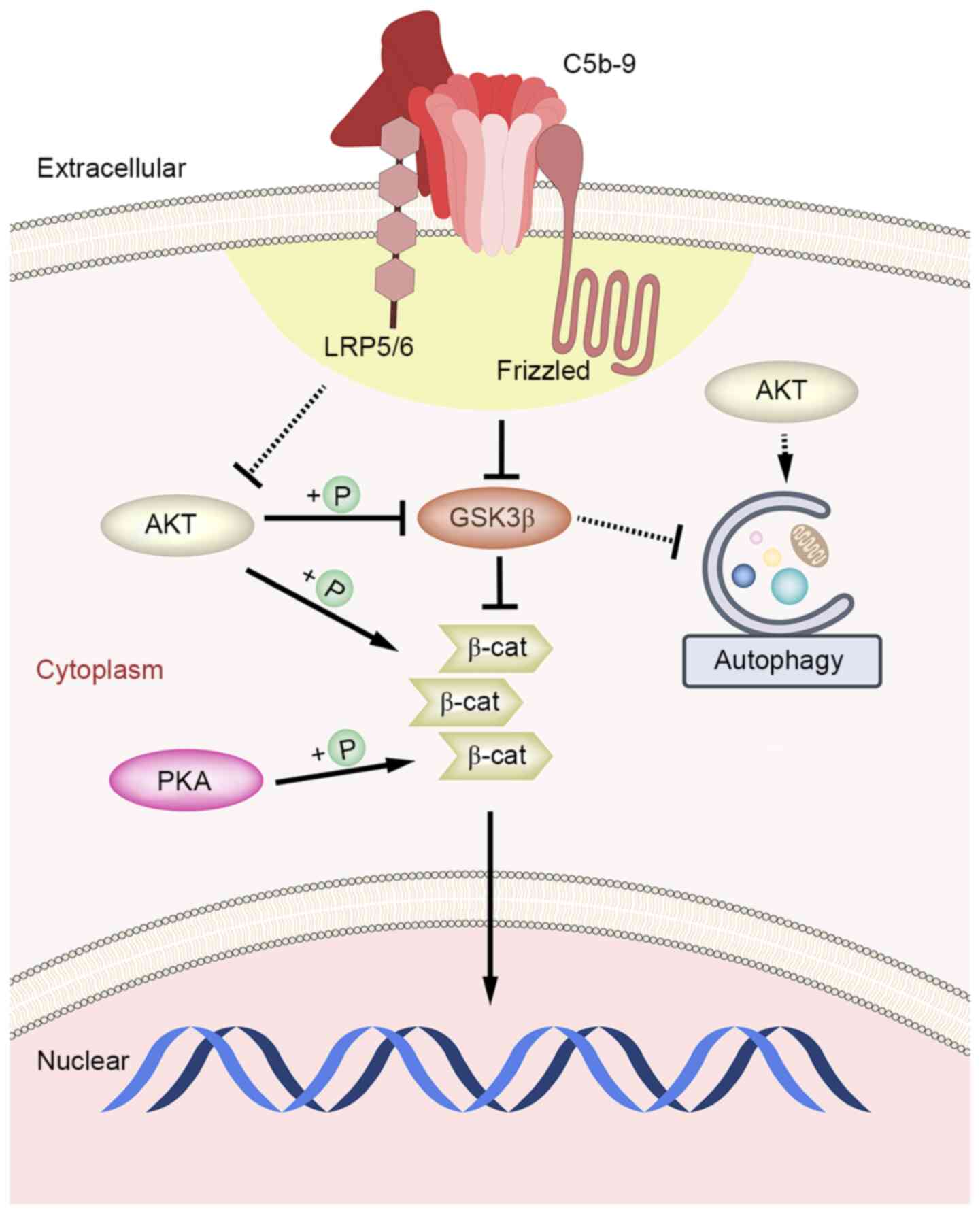 | Figure 5Schematic diagram of the mechanism of
the effect of C5b-9 on Wnt/β-catenin signaling pathway and
autophagy in glomerular epithelial cells. After the activation of
Wnt/β-catenin signaling pathway by C5b-9, GSK3 was weakened, thus
might playing an indirect inhibitory effect on autophagy. However,
C5b-9 also damaged Akt while activating the Canonical Wnt pathway
in glomerular epithelial cells, leading to the activation of
autophagy ultimately. C5b-9 group. C5b-9, complement 5b-9; GSK3,
glycogen synthase kinase 3; AKT, protein kinase B; PKA, protein
kinase A; +P, phosphorylation; β-cat, β-catenin. |
In conclusion, inhibition of the Wnt/β-catenin
signaling pathway may protect podocytes from damage caused by
complement within a certain period of time, and one of the
mechanisms of this is through inhibition of autophagy. However, the
specific role of signaling proteins, such as GSK-3β and β-catenin
remains unclear and requires further study. The results of the
present study also suggest that complement aggravates the damage to
glomerular epithelial cells in idiopathic membranous nephropathy
through over-activation of the Wnt/β-catenin signaling pathway, and
pathway inhibitors such as DKK1 may serve as a novel approach to
treat this disease.
Acknowledgements
The authors are grateful for associate researcher
Yan Lin and Lei Zhang and assistant researcher Lu Zhang as staff of
the Institute of Traditional Chinese Medicine of Beijing Hospital
of Traditional Chinese Medicine for their guidance on the
experimental operation.
Funding
Funding: This work was supported by grants from the National
Natural Science Foundation of China (grant nos. 81673907 and
81973793 to LB), Natural Science Foundation of Beijing Municipality
(grant no. 7182070 to LB) and Beijing Municipal Administration of
Hospitals Clinical Medicine Development of special funding support
(grant no. XLMX201833 to LB).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BL, HD and WeiL were responsible for conception of
the study. BL, ZD, FL, YG, ZZha and FM were responsible for the
cell experiments and the detection of related indicators. HD, XX
and ZZhu were responsible for data analysis and interpretation. ZD
and FL participated in the drafting of the manuscript. BL, ZF and
WenL were responsible for conception of the study and responsible
for critical revision of important content, and undertook part of
the data analysis and interpretation work. BL and HD are
responsible for approving the final version to be published and
agree to be responsible for all aspects of the work and to ensure
that issues relating to the accuracy or completeness of any part of
the work are properly investigated and resolved. The authenticity
of the original data in this paper has been confirmed by BL and HD
and they are responsible for this. All authors read and approved
this manuscript.
Ethics approval and consent to
participate
Ethical approval was obtained from the Medical
Ethics Committee of Beijing Hospital of Traditional Chinese
Medicine Affiliated to Capital Medical University. Blood donors
provided their written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhou L and Liu Y: Wnt/β-catenin signalling
and podocyte dysfunction in proteinuric kidney disease. Nat Rev
Nephrol. 11:535–545. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dai H, Liu Q and Liu B: Research progress
on mechanism of podocyte depletion in diabetic nephropathy. J
Diabetes Res. 2017(2615286)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Dai H, Liu F, Qiu X, Liu W, Dong Z, Jia Y,
Feng Z, Liu Z, Zhao Q, Gao Y, et al: Alleviation by Mahuang Fuzi
and Shenzhuo decoction in high glucose-induced podocyte injury by
inhibiting the activation of Wnt/β-catenin signaling pathway,
resulting in activation of podocyte autophagy. Evid Based
Complement Alternat Med. 2020(7809427)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Couser WG: Primary membranous nephropathy.
Clin J Am Soc Nephrol. 12:983–997. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ronco P and Debiec H: Pathophysiological
advances in membranous nephropathy: Time for a shift in patient's
care. Lancet. 385:1983–1992. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu W, Gao C, Dai H, Zheng Y, Dong Z, Gao
Y, Liu F, Zhang Z, Liu Z, Liu W, et al: Immunological pathogenesis
of membranous nephropathy: Focus on PLA2R1 and its role. Front
Immunol. 10(1809)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu W, Gao C, Liu Z, Dai H, Feng Z, Dong
Z, Zheng Y, Gao Y, Tian X and Liu B: Idiopathic membranous
nephropathy: Glomerular pathological pattern caused by extrarenal
immunity activity. Front Immunol. 11(1846)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nangaku M, Shankland SJ and Couser WG:
Cellular response to injury in membranous nephropathy. J Am Soc
Nephrol. 16:1195–1204. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cybulsky AV, Takano T, Papillon J and
McTavish AJ: Complement-induced phospholipase A2 activation in
experimental membranous nephropathy. Kidney Int. 57:1052–1062.
2000.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Takano T, Elimam H and Cybulsky AV:
Complement-mediated cellular injury. Semin Nephrol. 33:586–601.
2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hartleben B, Gödel M, Meyer-Schwesinger C,
Liu S, Ulrich T, Köbler S, Wiech T, Grahammer F, Arnold SJ,
Lindenmeyer MT, et al: Autophagy influences glomerular disease
susceptibility and maintains podocyte homeostasis in aging mice. J
Clin Invest. 120:1084–1096. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Asanuma K, Tanida I, Shirato I, Ueno T,
Takahara H, Nishitani T, Kominami E, Tomino Y, Arnold SJ,
Lindenmeyer MT, et al: MAP-LC3, a promising autophagosomal marker,
is processed during the differentiation and recovery of podocytes
from PAN nephrosis. FASEB J. 17:1165–1167. 2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lv Q, Yang F, Chen K and Zhang Y:
Autophagy protects podocytes from sublytic complement induced
injury. Exp Cell Res. 341:132–138. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang L, Hong Q, Lv Y, Feng Z, Zhang X, Wu
L, Cui S, Hou K, Su H, Huang Z, et al: Autophagy can repair
endoplasmic reticulum stress damage of the passive Heymann
nephritis model as revealed by proteomics analysis. J Proteomics.
75:3866–3876. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fan T, Chen L, Huang Z, Wang W, Zhang B,
Xu Y, Mao Z, Hu H and Geng Q: Autophagy activation by rapamycin
before hypoxia-reoxygenation reduces endoplasmic reticulum stress
in alveolar epithelial cells. Cell Physiol Biochem. 41:79–90.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Unno R, Kawabata T, Taguchi K, Sugino T,
Hamamoto S, Ando R, Okada A, Kohri K, Yoshimori T and Yasui T:
Deregulated MTOR (mechanistic target of rapamycin kinase) is
responsible for autophagy defects exacerbating kidney stone
development. Autophagy. 16:709–723. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kato H, Gruenwald A, Suh JH, Miner JH,
Barisoni-Thomas L, Taketo MM, Faul C, Millar SE, Holzman LB and
Susztak K: Wnt/β-catenin pathway in podocytes integrates cell
adhesion, differentiation, and survival. J Biol Chem.
286:26003–26015. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Dai C, Stolz DB, Kiss LP, Monga SP,
Holzman LB and Liu Y: Wnt/β-catenin signaling promotes podocyte
dysfunction and albuminuria. J Am Soc Nephrol. 20:1997–2008.
2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
He W, Kang YS, Dai C and Liu Y: Blockade
of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria
and kidney injury. J Am Soc Nephrol. 22:90–103. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liu BL, Chen YP, Cheng H, Wang YY, Rui HL,
Yang M, Dong HR, Han DN and Dong J: The protective effects of
curcumin on -obesity-related glomerulopathy are associated with
inhibition of Wnt/β-catenin signaling activation in podocytes. Evid
Based Complement Alternat Med. 2015(827472)2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Petherick KJ, Williams AC, Lane JD,
Ordóñez-Morán P, Huelsken J, Collard TJ, Smartt HJ, Batson J, Malik
K, Paraskeva C, et al: Autolysosomal β-catenin degradation
regulates Wnt-autophagy-p62 crosstalk. EMBO J. 32:1903–1916.
2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liu WJ, Li ZH, Chen XC, Zhao XL, Zhong Z,
Yang C, Wu HL, An N, Li WY and Liu HF: Blockage of the
lysosome-dependent autophagic pathway contributes to complement
membrane attack complex-induced podocyte injury in idiopathic
membranous nephropathy. Sci Rep. 7(8643)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Beekman C, Janson AA, Baghat A, van
Deutekom JC and Datson NA: Use of capillary Western immunoassay
(Wes) for quantification of dystrophin levels in skeletal muscle of
healthy controls and individuals with Becker and Duchenne muscular
dystrophy. PLoS One. 13(e0195850)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cao J, Tyburczy ME, Moss J, Darling TN,
Widlund HR and Kwiatkowski DJ: Tuberous sclerosis complex
inactivation disrupts melanogenesis via mTORC1 activation. J Clin
Invest. 127:349–364. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Zhang Z, Liu T, Yu M, Li K and Li W: The
plant alkaloid tetrandrine inhibits metastasis via
autophagy-dependent Wnt/β-catenin and metastatic tumor antigen 1
signaling in human liver cancer cells. J Exp Clin Cancer Res.
37(7)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li RN, Liu B, Li XM, Hou LS, Mu XL, Wang H
and Linghu H: DACT1 overexpression in type I ovarian cancer
inhibits malignant expansion and cis-platinum resistance by
modulating canonical Wnt signalling and autophagy. Sci Rep.
7(9285)2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Luo W, Olaru F, Miner JH, Beck LH Jr, van
der Vlag J, Thurman JM and Borza DB: Alternative pathway is
essential for glomerular complement activation and proteinuria in a
mouse model of membranous nephropathy. Front Immunol.
9(1433)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ronco P and Debiec H: Pathogenesis of
membranous nephropathy: Recent advances and future challenges. Nat
Rev Nephrol. 8:203–213. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Beck LH Jr, Bonegio RG, Lambeau G, Beck
DM, Powell DW, Cummins TD, Klein JB and Salant DJ: M-type
phospholipase A2 receptor as target antigen in idiopathic
membranous nephropathy. N Engl J Med. 361:11–21. 2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ruggenenti P, Debiec H, Ruggiero B,
Chianca A, Pellé T, Gaspari F, Suardi F, Gagliardini E, Orisio S,
Benigni A, et al: Anti-phospholipase A2 receptor antibody titer
predicts post-rituximab outcome of membranous nephropathy. J Am Soc
Nephrol. 26:2545–2558. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Borza DB: Alternative pathway
dysregulation and the conundrum of complement activation by IgG4
immune complexes in membranous Nephropathy. Front Immunol.
7(157)2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Glassock RJ: Pathogenesis of membranous
nephropathy: A new paradigm in evolution. Contrib Nephrol.
181:131–142. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ma H, Sandor DG and Beck LH Jr: The role
of complement in membranous nephropathy. Semin Nephrol. 33:531–542.
2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Couser WG and Nangaku M: Cellular and
molecular biology of membranous nephropathy. J Nephrol. 19:699–705.
2006.PubMed/NCBI
|
|
36
|
Paréj K, Kocsis A, Enyingi C, Dani R,
Oroszlán G, Beinrohr L, Dobó J, Závodszky P, Pál G and Gál P:
Cutting edge: A new player in the alternative complement pathway,
MASP-1 is essential for LPS-induced, but not for zymosan-induced,
alternative pathway activation. J Immunol. 200:2247–2252.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sastre-Perona A, Riesco-Eizaguirre G,
Zaballos MA and Santisteban P: β-catenin signaling is required for
RAS-driven thyroid cancer through PI3K activation. Oncotarget.
7:49435–49449. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Soutto M, Peng D, Katsha A, Chen Z,
Piazuelo MB, Washington MK, Belkhiri A, Correa P and El-Rifai W:
Activation of β-catenin signalling by TFF1 loss promotes cell
proliferation and gastric tumorigenesis. Gut. 64:1028–1039.
2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wiza C, Nascimento EB and Ouwens DM: Role
of PRAS40 in Akt and mTOR signaling in health and disease. Am J
Physiol Endocrinol Metab. 302:E1453–E1460. 2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Katoh M and Katoh M: Molecular genetics
and targeted therapy of WNT-related human diseases (Review). Int J
Mol Med. 40:587–606. 2017.PubMed/NCBI View Article : Google Scholar
|



















