Introduction
Inflammation is a complex pathological response
caused by harmful stimuli to the internal and external environment
(1). In vitro inflammatory
models are typically established by lipopolysaccharide (LPS) or
interferon-γ induction in macrophages. Macrophages, which are
central cells that produce inflammatory mediators and modulate
inflammatory responses in vivo, can be immunomodulated by
the secretion of various cytokines or lysosome release (2,3).
The RAW264.7 cell line is derived from mouse
mononuclear macrophage leukemia cells (4). RAW264.7 cells are widely used to
evaluate the immunomodulatory effects of mononuclear macrophages on
nitric oxide (NO) secretion and associated inflammatory signaling
pathways (5). LPS stimulates cells
to secrete a variety of inflammatory mediators, including nitric
oxide (NO), tumor necrosis factor (TNF)-α and interleukin (IL)-1β,
via binding to the corresponding receptors on the cell membrane to
regulate immune response (6).
NO is the major mediator of oxidative stress, which
exacerbates inflammatory responses. Therefore, NO levels are
closely associated with the pathogenesis of numerous inflammatory
diseases (7). Inducible NO synthase
(iNOS) is a vital indicator of the inflammatory response (8). At present, the regulation of NO
synthesis and iNOS expression is considered to be a novel
therapeutic strategy for inflammatory diseases. Proinflammatory
cytokines, including TNF-α, IL-1β and IL-6, can interact with the
anti-inflammatory cytokine IL-10 to participate in inflammation
regulation (9).
LPS is a major component of the cell wall of
Gram-negative bacteria; identification and signal transduction of
LPS is an essential step in the self-defense response of the body
(10). Previous studies have
reported that LPS can promote the development of acute kidney
injury by inducing the production of proinflammatory cytokines,
including TNF-α, IL-6 and IL-1β (11,12).
LPS induction in tyrosine hydroxylase immunoreactive cells
selectively inhibit cell viability and increases the culture medium
contents of IL-1β, TNF-α and NO (13). Toll-like receptor (TLR)-4 mediates
LPS-induced inflammatory responsesin human coronary artery
endothelial cells (13). Moreover,
LPS can induce inflammatory effects by regulating the nuclear
factor (NF)-κB signaling pathway in A549 cells (14). The primary downstream signaling
pathways involved in LPS-induced inflammatory responses include the
NF-κB, MAPK and JAK-STAT signaling pathways. Activation of the
aforementioned signaling pathways further regulates a variety of
inflammatory mediators (15).
Previous studies have reported that LPS-induced
production of proinflammatory cytokines is associated with the
NF-κB signaling pathway (16-19).
It is considered to be the central step in LPS-induced macrophage
inflammation that exerts a crucial role in promoting iNOS and
proinflammatory cytokine expression (20). LPS activates TLR-4 and binds to heat
shock protein 60 via activating the NF-κB signaling pathway
(21). LPS also induces the
production of proinflammatory cytokines by macrophages, thus
leading to myocardial hypertrophy and ischemia (22).
LPS-induced inflammation is also associated with
peroxisome proliferator-activated receptors (PPARs). PPARγ belongs
to the nuclear hormone receptor superfamily and is a
ligand-activated transcription factor. PPARγ regulates cell
proliferation, differentiation, carbohydrate lipid metabolism and
inflammatory responses. PPARs can be divided into three types:
PPARα, PPARβ and PPARγ, among which PPARγ is primarily distributed
in adipose tissue and the immune system, suggesting its role in fat
metabolism and body immunity (23).
Recent studies have demonstrated that PPARγ
activation downregulates the expression of NOS, matrix
metalloproteinases and adhesion molecules in the mononuclear
phagocyte cell line, thereby inhibiting the inflammatory response
(24-26).
PPARγ agonists are capable of inhibiting the production of
proinflammatory cytokines in mononuclear macrophages (23). Pretreatment with a PPARγ ligand can
significantly decrease the expression of proinflammatory cytokines
in tissues, and alleviate tissue damage at local and distant sites
of inflammation (27). PPARγ
agonist ligands are split into two major classes, natural ligands
and synthetic ligands. Natural ligands are primarily 15-deoxy
prostaglandin J2 (15d-PGJ2) and linoleic acid oxidation products,
whereas synthetic ligands are primarily thiazolidinedione (TZDs),
including pioglitazone, troglitazone and rosiglitazone.
Rosiglitazone is the most commonly used drug with the highest
bioavailability, strongest drug effect and fewest side effects
(28). Previous studies have
demonstrated the anti-inflammatory effects of rosiglitazone in
diverse models (29). Rosiglitazone
upregulatesheme oxygenase-1 expression via the reactive oxygen
species-dependent nuclear factor, erythroid 2 like 2-antioxidant
response elements axis (30).
Moreover, rosiglitazone could also impair colonic
inflammation in mice with experimental colitis (31). However, the mechanism underlying the
anti-inflammatory effects of rosiglitazone is not completely
understood.
The present study aimed to explore the role of the
PPARγ agonist rosiglitazone in the regulation of LPS-induced
inflammatory responses and decreases in viability in RAW264.7
cells, as well as its potential underlying mechanisms.
Materials and methods
Cell culture
The RAW264.7 cell line is a mouse mononuclear
macrophage leukemia cell line that was obtained from the American
Type Culture Collection. Cells were cultured in DMEM (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml
streptomycin in a 5% CO2 incubator at 37˚C. Culture
medium was replaced every 2 days.
MTT assay
RAW264.7 cells at the logarithmic growth phase were
digested with PBS supplemented with 0.25% EDTA and prepared for
cell suspension. After the cell density was adjusted to
2x105/ml, 100 µl cell suspension was added to each well
of a 96-well plate. RAW264.7 cells were treated with 100 ng/ml LPS
(Sigma-Aldrich; Merck KGaA; L4391), or 1, 2, 5, 10 or 20 µM
rosiglitazone (Sigma-Aldrich; Merck KGaA; cat. no. R2408) for 48 or
72 h at 37˚C. Each group consisted of three replicates.
Subsequently, cells were incubated with 200 µl 0.5% MTT solution
(0.5 mg/ml) for 4 h. The purple formazan was dissolved with DMSO
solution. Absorbance was measured at a wavelength of 490 nm using a
microplate reader (Bio-Rad Laboratories, Inc.).
Enzyme-linked immunosorbent assay
(ELISA)
RAW264.7 cells in the logarithmic growth phase were
seeded (1x104/ml; 100 µl/well) into 96-well plates.
Following culture for 24 h, cells were pretreated with
rosiglitazone at different concentration for 1 h and then treated
with LPS for 24 h. Subsequently, the culture medium was collected
and cytokine levels were detected using an IL-6 (cat. no. M6000B)
and TNFα (cat. no. MTA00B) ELISA kit (R&D Systems, Inc.)
according to the manufacturer's protocol.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from cells using
TRIzol® (Thermo Fisher Scientific, Inc.). The RNA
concentration of each sample was detected using a NanoDrop 2000
spectrophotometer (Thermo Fisher Scientific, Inc.). Total RNA was
reverse transcribed into cDNA using a first-strand cDNA synthesis
kit (Takara Bio, Inc.) at 42˚C for 30 min. Subsequently, qPCR was
performed for 40 cycles using Hieff™ qPCR
SYBR®-Green Master Mix (with ROX; Yeasen Technology,
Inc.). The sequences of the primers used for qPCR are presented in
Table I. The 2-ΔΔCq
method was used for quantitative analysis (32).
 | Table ISequences of primers used for
quantitative reverse transcription PCR. |
Table I
Sequences of primers used for
quantitative reverse transcription PCR.
| Gene | Sequence,
5'-3' |
|---|
| GAPDH | F:
CGGAGTCAACGGATTTGGTCGTAT |
| | R:
AGCCTTCTCCATGGTGGTGAAGAC |
| IL-1β | F:
GAAAGACGGCACACCCACCCT |
| | R:
GCTCTGCTTGTGAGGTGCTGATGTA |
| TNF-α | F:
TTCTGTCTACTGAACTTCGGGGTGAT CGGTCC |
| | R:
GTATGAGATAGCAAATCGGCTGACGG TGTGGG |
| IL-10 | F:
ATAACTGCACCCACTTCCCA |
| | R:
GGGCATCACTTCTACCAGGT |
| iNOS | F:
CCTTGTTCAGCTACGCCTTC |
| | R:
CTGAGGGCTCTGTTGAGGTC |
Western blotting
Total protein was extracted from cells using RIPA
solution (cat. no. 89900; Thermo Fisher Scientific, Inc.). The BCA
method was used to determine the protein concentration. A total of
20 µg protein was separated via 10% SDS-PAGE gel and transferred to
PVDF membranes. Following blocking with 5% skimmed milk at room
temperature for 1 h, the membranes were incubated overnight at 4˚C
with primary antibodies (1:1,000 dilution). Subsequently, the
membranes were incubated with a HRP-conjugated anti-mouse (1:5,000;
cat. no. #7076; Cell Signaling Technology, Inc.) or HRP-conjugated
anti-rabbit IgG (1:5,000; cat. no. #7074; Cell Signaling
Technology, Inc.) at room temperature for 1 h. p65 (cat. no.
66535-1-Ig), IkB-α (cat. no. 10268-1-AP), PPAR (cat. no.
16643-1-AP) and β-actin (cat. no. 60008-1-Ig) primary antibodies
were purchased from ProteinTech Group, Inc. Thephosphorylated
(p)-p65 (cat. no. 3033) primary antibody was purchased from Cell
Signaling Technology, Inc. Protein bands were visualized using an
ECL system with an ImageQuant LAS 500 imager (GE Healthcare). The
protein bands were quantified by ImageQuant TL version 8.0 (GE
Healthcare).
Cell transfection
Small interfering RNA (si)-PPARγ-1, si-PPARγ-2 and
si-negative control were purchased from Shanghai GenePharma Co.,
Ltd. Briefly, 0.8 µg si RNA or 3 µl Viromer blue transfection
reagent (Lipocalyx GmbH) were diluted in 350 µl buffer blue, mixed
and stored at room temperature for 15 min. Subsequently, cells were
seeded at 1x105 cells/well in a six-well plate and then
were incubated with the reagent mixture for 48 h. Culture medium
was replaced every 2 days. The siRNA sequences were as follows:
si-PPARγ-1:
5'-CCGGGCTCCACACTATGAAGACATTCTCGAGAATGTCTTCATAGTGTGGAGCTTTTT-3';
si-PPARγ-2:
5'-CCGGGCCTCCCTGATGAATAAAGATCTCGAGATCTTTATTCAGGGAGGCTTTTT-3'.
Determination of NO secretion
NO secretion levels were determined using the Griess
reagent system kit (Beyotime Institute of Biotechnology). Cells
were seeded (1x104/ml) into 96-well plates and incubated
for 24 h. Following different treatments for 24 h, 50 µl cell
supernatant was collected and plated into 96-well plates at room
temperature. Subsequently, 50 µl Griess I and Griess II reagent
were added in order at room temperature. Absorbance was measured at
a wavelength of 540 nm with microplate reader.
Dual-luciferase reporter gene
assay
A total of 2x104 cells were
co-transfected with 1 µg mass luciferase-coupled reporter gene for
NF-κB and 1 µg Renilla luciferase reporter (Beyotime
Institute of Biotechnology) using Viromer blue transfection reagent
(High-Tech Gründerfonds). At 48 h post-transfection, cells were
pre-treated with rosiglitazone for 1 h and then treated with LPS
for 5 h at 37˚C. Following washes with cold PBS, cells were lysed
with luciferase lysis buffer (Beyotime Institute of Biotechnology)
and luciferase activities were measured using the Dual Luciferase
Assay System and a Victor luminometer (Promega Corporation)
according to the manufacturer's instructions. Relative NF-κB
luciferase activity was normalized to Renilla activity.
Statistical analysis
Statistical analyses were performed using GraphPad
software (version 7; GraphPad Software, Inc.). Data are presented
as the mean ± SD. Comparisons between groups were analyzed using
Kruskal-Wallis test and Dunn's post hoc test. P<0.05 was
considered to indicate a statistically significant difference. All
experiments were performed three times.
Results
Rosiglitazone reverses LPS-induced
decrease in cell viability
Rosiglitazone is an insulin sensitizer and is
commonly used for the treatment of type 2 diabetes (33). In order to explore the effect of
different concentrations of rosiglitazone on LPS-induced decrease
in cell viability, RAW264.7 cells were treated with 1, 2, 5, 10 or
20 µM rosiglitazone to detect the cell cytotoxicity of
rosiglitazone. Following treatment for 48 h, cell viability was
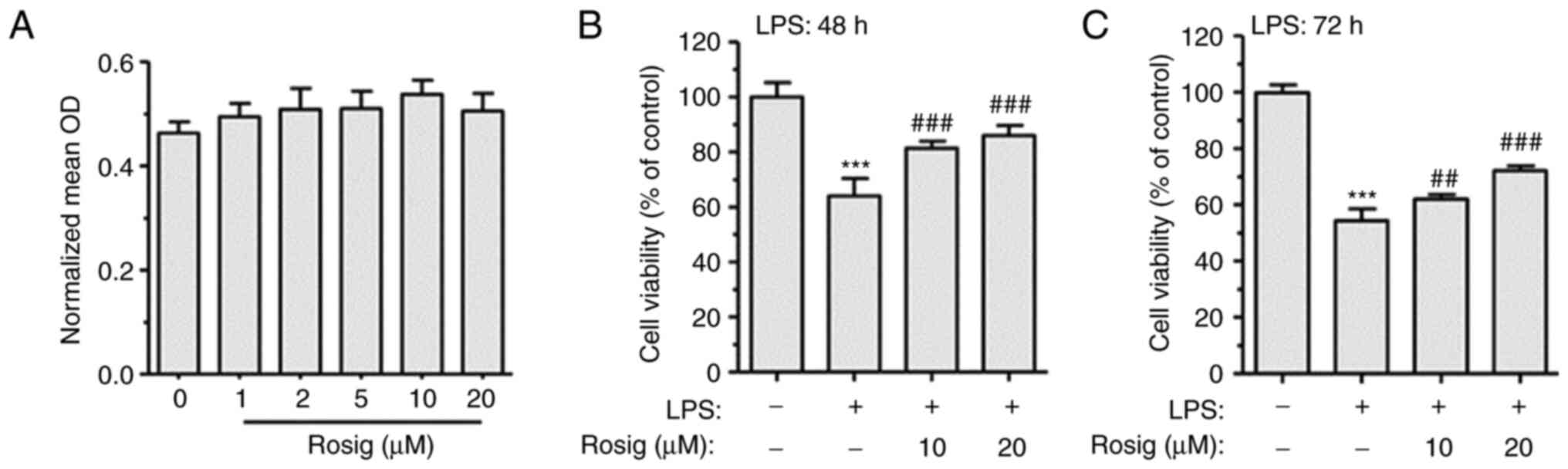
measured using the MTT assay. Compared with the control group, 1-20
µM rosiglitazone showed no obvious cytotoxic effect on RAW264.7
cells (Fig. 1A). Therefore, 1, 5,
10 and 20 µM rosiglitazone were selected as the extremely low-,
low-, middle- and high-dose rosiglitazone groups, respectively.
Subsequently, RAW264.7 cells were treated with 100 ng/ml LPS for 48
h. LPS treatment decreased RAW264.7 cell viability compared with
the control group. However, middle- and high-dose rosiglitazone
treatment for 48 h reversed LPS-induced decrease in cell viability
(Fig. 1B); similar results were
observed following treatment for 72 h (Fig. 1C).
Effect of rosiglitazone on LPS-induced
proinflammatory and anti-inflammatory cytokine expression
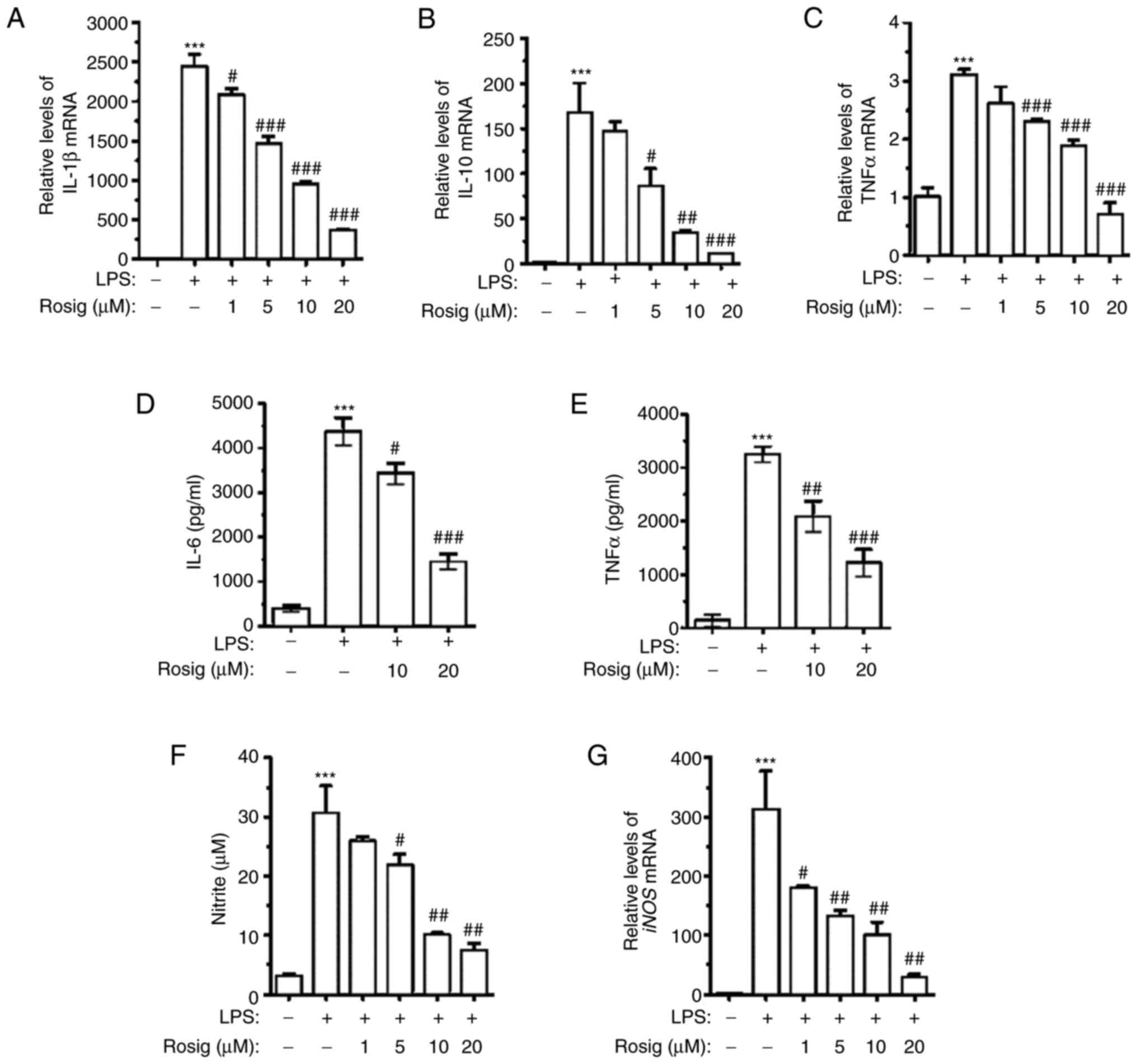
In order to explore the effect of rosiglitazone on
LPS-induced alterations to the expression of proinflammatory and
anti-inflammatory cytokines, mRNA expression levels of IL-1β, TNF-α
and IL-10 were detected via RT-qPCR. The results demonstrated that
treatment with 100 ng/ml LPS for 48 h remarkably upregulated IL-1β,
TNF-α and IL-10 mRNA expression levels. Compared with the LPS
group, rosiglitazone treatment downregulated IL-1β, IL-10 and TNF-α
mRNA expression levels in a dose-dependent manner (Fig. 2A-C). In order to further verify the
aforementioned results, IL-6 and TNF-α contents in the culture
medium of different groups were assessed. The ELISA results
demonstrated that IL-6 and TNF-α contents in the culture medium of
the LPS group were remarkably elevated. However, IL-6 and TNF-α
contents were downregulated in the middle- and high-dose groups in
a dose-dependent manner (Fig. 2D
and E). NO and iNOS mRNA expression
levels in RAW264.7 cells, following exposure to LPS and different
concentrations of rosiglitazone, were also detected. The results
demonstrated that different concentrations of rosiglitazone
treatment decreased NO secretion in a dose-dependent manner
(Fig. 2F). Similar results were
obtained for the detection of iNOS mRNA expression levels via
RT-qPCR (Fig. 2G).
Rosiglitazone inhibits the NF-κB
signaling pathway in LPS-treated RAW264.7 cells
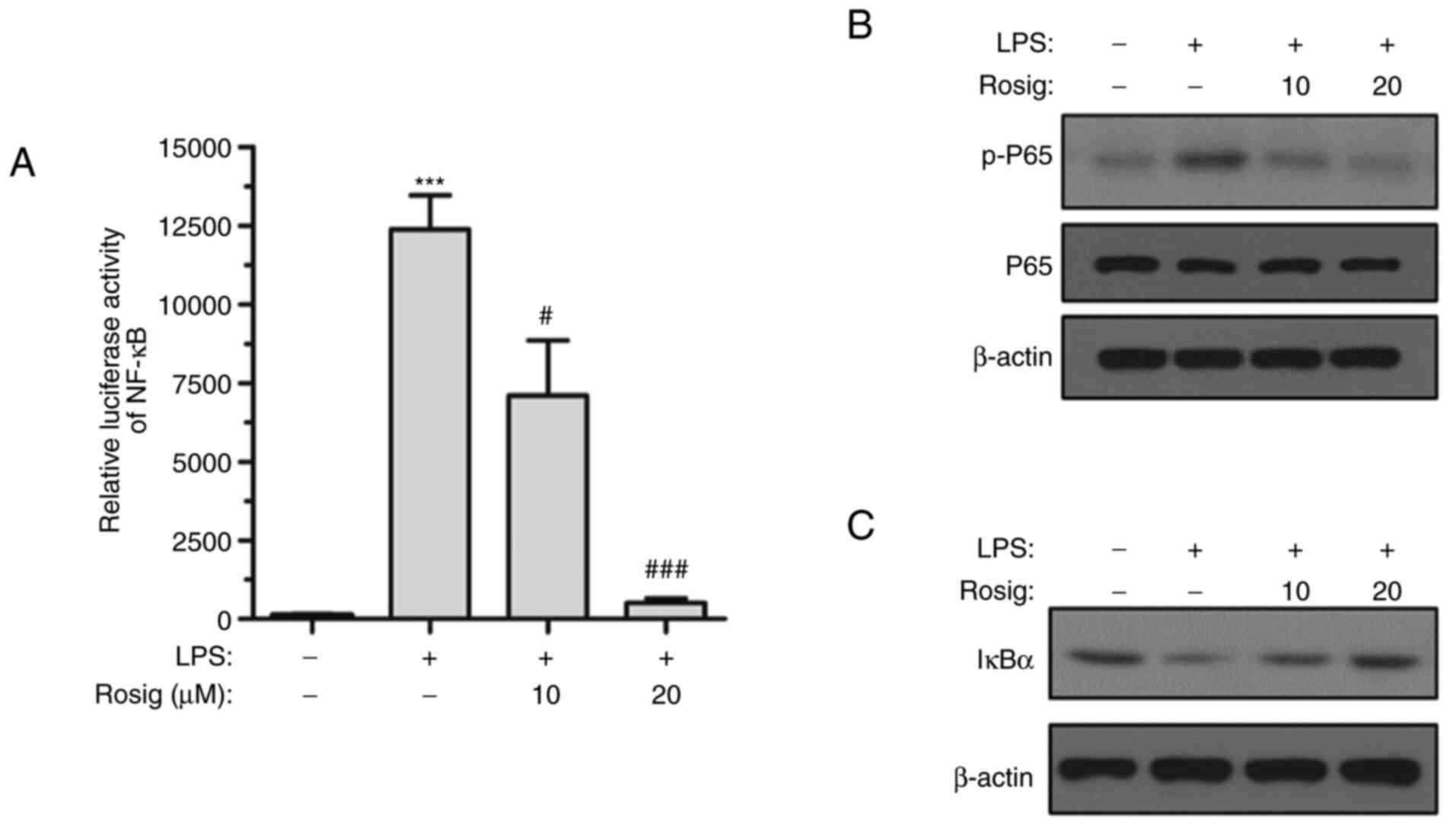
It has been reported that the NF-κB signaling
pathway is greatly involved in inflammatory responses (34). Therefore, whether rosiglitazone
could regulate RAW264.7 cell inflammation via the NF-κB signaling
pathway was investigated. The activity of the NF-κB-driven
luciferase reporter gene was markedly elevated after LPS induction
for 48 h. However, middle- and high-dose rosiglitazone treatment
inhibited the activity of the NF-κB-driven luciferase reporter gene
(Fig. 3A). Moreover, the
phosphorylation level of p65 was gradually decreased and IκBα
expression was increased with increasing concentrations of
rosiglitazone (Fig. 3B and C), indicating that the NF-κB signaling
pathway was inhibited by rosiglitazone in a concentration-dependent
manner.
PPARγ knockdown impairs the
anti-inflammatory effect of rosiglitazone on RAW264.7 cells
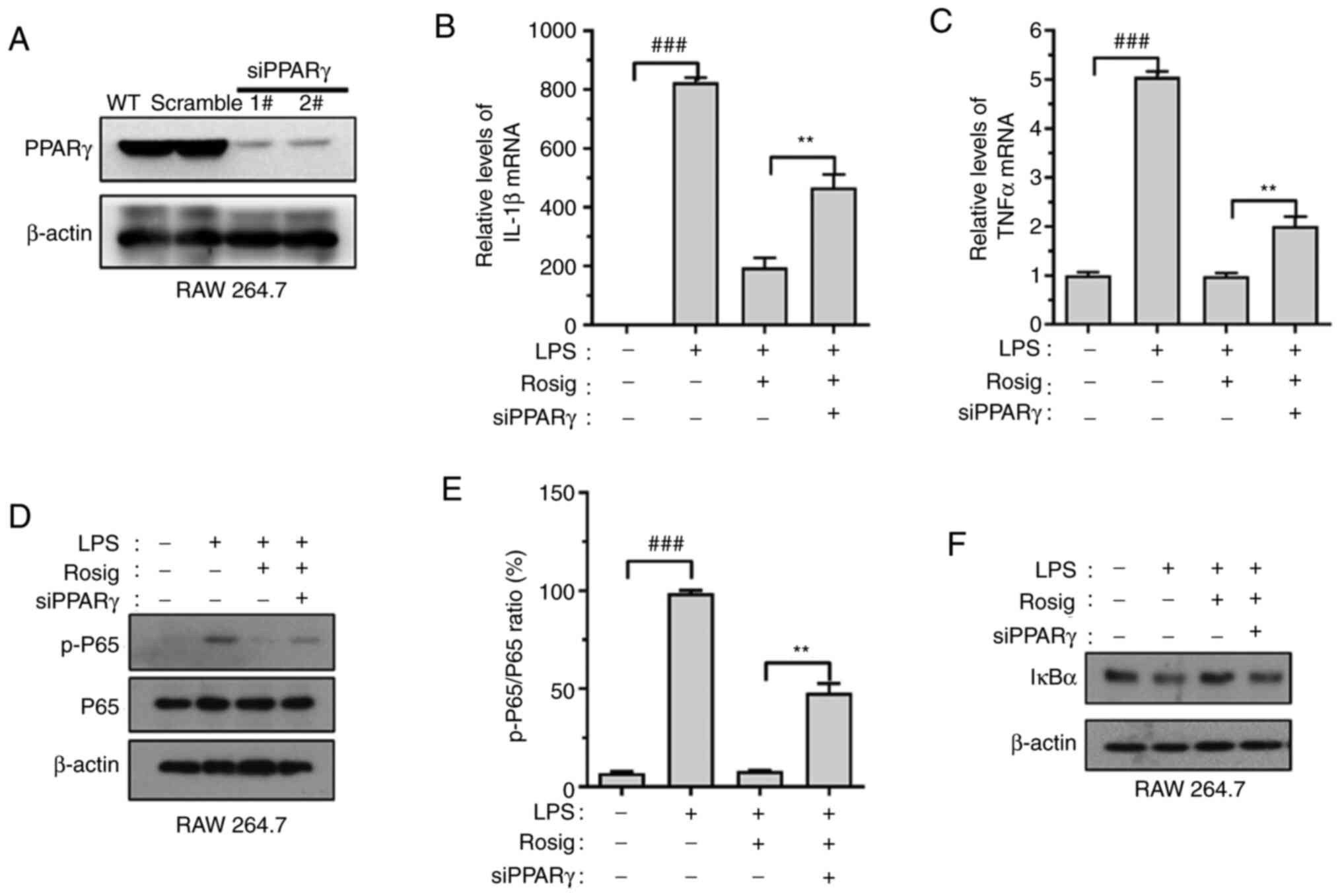
In order to verify whether the effect of
rosiglitazone on inflammation regulation was mediated via PPARγ,
si-PPARγ-RAW264.7 cell lines were constructed. The transfection
efficacy of si-PPARγ was verified via western blotting (Fig. 4A). The results indicated that PPARγ
knockdown upregulated IL-1β and TNF-α mRNA expression levels
(Fig. 4B and C). Similarly, PPARγ knockdown reversed
rosiglitazone-induced decrease in p65 phosphorylation levels and
increased IκBα expression (Fig.
4D-F).
Discussion
As innate immune cells, macrophages trigger
inflammatory and immune responses for self-defense. LPS is a potent
inducer of monocyte and macrophage immune responses. When activated
by LPS, macrophages release a variety of proinflammatory cytokines
and anti-inflammatory cytokines (35). Excessive release of cytokines may
lead to extensive tissue damage and pathological alterations
(36). Macrophages produce a number
of inflammatory mediators, including IL-1β, IL-6, TNF-α and NO
(37). LPS induction stimulates the
secretion of proinflammatory mediators by macrophages, eventually
leading to cell injury and even cell death (38). Therefore, the present study used LPS
as an in vitro model of inflammation.
PPARγ is a type of ligand-dependent transcription
factor that regulates the proliferation, invasion, differentiation
and apoptosis of various cells at the transcriptional level. PPARγ
serves a crucial role in various inflammatory injury processes
(39-40). Rosiglitazone is a synthetic PPARγ
agonist and is widely used for the treatment of type 2 diabetes
(41). Previous studies have
demonstrated that rosiglitazone serves a neuroprotective role via
anti-inflammatory and antioxidant mechanisms after brain trauma
(41-43).
In the present study, 1-20 µM rosiglitazone showed no obvious
cytotoxic effect on RAW264.7 cells. However, rosiglitazone reversed
the inhibitory effect of LPS on cell viability, potentially via
inhibiting cytokine expression. Moreover, rosiglitazone inhibited
LPS-induced proinflammatory cytokine and enzyme expression,
including IL-1β, TNF-α, IL-6 and iNOS in RAW264.7 cells.
Interestingly, LPS also elevated the expression of IL-10, an
anti-inflammatory cytokine, potentially to overcome the
proinflammatory cytokines, which is a phenomenon derived from cell
self-protective mechanisms (44).
Rosiglitazone not only inhibited proinflammatory cytokines, but
also repressed anti-inflammatory cytokines, suggesting that it
might serve a vital role in balancing the process of
inflammation.
To confirm whether the anti-inflammatory effect of
rosiglitazone was mediated via PPARγ, si-PPARγ-RAW264.7 cells were
constructed. The results indicated that PPARγ knockdown attenuated
the inhibitory effect of rosiglitazone on proinflammatory
cytokines. Therefore, the aforementioned results suggested that
rosiglitazone regulated inflammation via PPARγ activation.
NF-κB is an important transcription factor that
regulates the expression of immune and inflammatory response
factors (45). Previous studies
have demonstrated that the PPARγ/NF-κB signaling pathway is
involved in the dynamic balance of the inflammatory response
(46-48).
Besides, PPARγ agonists, including rosiglitazone, were reported to
inhibit the activity of the NF-κB signaling pathway in
osteoclastogenesis. The aforementioned studies suggested that PPARγ
could partly regulate the level of the NF-κB signaling pathway.
NF-κB signaling pathway activation may be the control point for the
expression of abundant inflammatory response genes (49). In the present study, rosiglitazone
inhibited NF-κBp65 phosphorylation and increased IKBα expression,
reversing LPS-induced activation of NF-κB. PPARγ knockdown impaired
the effect of rosiglitazone on NF-κB activation. Therefore, the
results suggested that the PPARγ/NF-κB signaling pathway might
serve as a crucial target for controlling inflammatory
responses.
NF-κB is a transcription factor family that
regulates a number of genes that are involved in several
physiological and pathological processes. In the canonical pathway,
NF-κB dimers and molecules of IκB family form a stable complex
which prevent dimers translocating to the nucleus. When stimulated
by extracellular stimuli, IκB kinase (IKK) is phosphorylated
causing the dimers to translocate to the nucleus and activate
downstream gene expression (50).
Due to the limitation of funding, p-IKKβ as well as the
translocation of cytosolic p65 to the nucleus, and other signaling
such as MAPK substances were not detected. The effect of IL-1β,
TNF-α, IL-6 on NF-κB transcriptional activity were not studied.
In conclusion, the present study demonstrated that
rosiglitazone significantly inhibited the LPS-induced inflammatory
response in RAW264.7 cells and improved cell viability.
Rosiglitazone inhibited the expression level of proinflammatory
cytokines, potentially via activating PPARγ and inhibiting NF-κB.
The results of the present study provided an experimental basis for
the new application of old drugs.
Acknowledgements
The authors would like to thank Dr Changsheng Yan
(School of Medical, Xiamen University, Xiamen, China) who offered
some suggestions and help with the experiment design.
Funding
Funding: Funding was received from Health and Family Planning
Commission (grant no. 2014-2-72), the Natural Science Foundation of
Fujian Province (grant no. 2015J01534) and the National Natural
Science Foundation of China (grant no. 81702428).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JPZ and XNY contributed to the study design. YQH,
LGC and JJL contributed to the interpretation of data. FZ performed
the experiments and YS was responsible for statistical analysis.
All authors read and approved the final manuscript. JPS and XNY
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen L, Shen X, Chen G, Cao X and Yang J:
Effect of Three-spot seahorse petroleum ether extract on
lipopolysaccharide induced macrophage RAW264.7 inflammatory
cytokine nitric oxide and composition analysis. J Oleo Sci.
64:933–942. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dong D, Zhou NN, Liu RX, Xiong JW, Pan H,
Sun SQ, Ma L and Wang R: Sarsasapogenin-AA13 inhibits LPS-induced
inflammatory responses in macrophage cells in vitro and relieves
dimethylbenzene-induced ear edema in mice. Acta Pharmacol Sin.
38:699–709. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ti D, Hao H, Tong C, Liu J, Dong L, Zheng
J, Zhao Y, Liu H, Fu X and Han W: LPS-preconditioned mesenchymal
stromal cells modify macrophage polarization for resolution of
chronic inflammation via exosome-shuttled let-7b. J Transl Med.
13(308)2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Schmidt HH, Warner TD, Nakane M,
Förstermann U and Murad F: Regulation and subcellular location of
nitrogen oxide synthases in RAW264.7 macrophages. Mol Pharmacol.
41:615–624. 1992.PubMed/NCBI
|
|
5
|
Tian LX, Tang X, Zhu JY, Zhang W, Tang WQ,
Yan J, Xu X and Liang HP: Corrigendum to ‘Cytochrome P450 1A1
enhances Arginase-1 expression, which reduces LPS-induced mouse
peritonitis by targeting JAK1/STAT6’ [Cell. Immunol 349 (2020)
104047]. Cell Immunol. 351(104084)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Araya AV, Pavez V, Perez C, Gonzalez F,
Columbo A, Aguirre A, Schiattino I and Aguillón JC: Ex vivo
lipopolysaccharide (LPS)-induced TNF-alpha, IL-1beta, IL-6 and PGE2
secretion in whole blood from type 1 diabetes mellitus patients
with or without aggressive periodontitis. Eur Cytokine Netw.
14:128–133. 2003.PubMed/NCBI
|
|
7
|
Chiang SS, Chen LS and Chu CY: Active food
ingredients production from cold pressed processing residues of
Camellia oleifera and Camellia sinensis seeds for regulation of
blood pressure and vascular function. Chemosphere.
267(129267)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kim YJ, Hwang SY, Oh ES, Oh S and Han IO:
IL-1beta, an immediate early protein secreted by activated
microglia, induces iNOS/NO in C6 astrocytoma cells through p38 MAPK
and NF-kappaB pathways. J Neurosci Res. 84:1037–1046.
2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nie Z, Xia X, Zhao Y, Zhang S, Zhang Y and
Wang J: JNK selective inhibitor, IQ-1S, protects the mice against
lipopolysaccharides-induced sepsis. Bioorg Med Chem.
30(115945)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lamping N, Dettmer R, Schröder NW, Pfeil
D, Hallatschek W, Burger R and Schumann RR: LPS-binding protein
protects mice from septic shock caused by LPS or gram-negative
bacteria. J Clin Invest. 101:2065–2071. 1998.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Shi M, Zeng X, Guo F, Huang R, Feng Y, Ma
L, Zhou L and Fu P: Anti-inflammatory pyranochalcone derivative
attenuates LPS-induced acute kidney injury via inhibiting
TLR4/NF-κB pathway. Molecules. 22(1683)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hou C, Mei Q, Song X, Bao Q, Li X, Wang D
and Shen Y: Mono-macrophage-derived MANF protects against
lipopolysaccharide-induced acute kidney injury via inhibiting
inflammation and renal M1 macrophages. Inflammation. 44:693–703.
2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gayle DA, Ling Z, Tong C, Landers T,
Lipton JW and Carvey PM: Lipopolysaccharide (LPS)-induced dopamine
cell loss in culture: Roles of tumor necrosis factor-alpha,
interleukin-1beta, and nitric oxide. Brain Res Dev Brain Res.
133:27–35. 2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Feng T, Yunfeng N, Jinbo Z, Zhipei Z,
Huizhong Z, Li L, Tao J and Yunjie W: Single immunoglobulin IL-1
receptor-related protein attenuates the lipopolysaccharide-induced
inflammatory response in A549 cells. Chem Biol Interact.
183:442–449. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang J, Pan Y, Cao Y, Zhou W and Lu J:
Salidroside regulates the expressions of IL-6 and defensins in
LPS-activated intestinal epithelial cells through NF-κB/MAPK and
STAT3 pathways. Iran J Basic Med Sci. 22:31–37. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Pan MH, Lin-Shiau SY and Lin JK:
Comparative studies on the suppression of nitric oxide synthase by
curcumin and its hydrogenated metabolites through down-regulation
of IkappaB kinase and NFkappaB activation in macrophages. Biochem
Pharmacol. 60:1665–1676. 2000.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Karin M and Ben-Neriah Y: Phosphorylation
meets ubiquitination: The control of NF-[kappa]B activity. Annu Rev
Immunol. 18:621–623. 2000.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chow JC, Young DW, Golenbock DT, Christ WJ
and Gusovsky F: Toll-like receptor-4 mediates
lipopolysaccharide-induced signal transduction. J Biol Chem.
274:10689–10692. 1999.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Faure E, Equils O, Sieling PA, Thomas L,
Zhang FX, Kirschning CJ, Polentarutti N, Muzio M and Arditi M:
Bacterial lipopolysaccharide activates NF-kappaB through toll-like
receptor 4 (TLR-4) in cultured human dermal endothelial cells.
Differential expression of TLR-4 and TLR-2 in endothelial cells. J
Biol Chem. 275:11058–11063. 2000.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang G and Ghosh S: Molecular mechanisms
of NF-kappaB activation induced by bacterial lipopolysaccharide
through Toll-like receptors. J Endotoxin Res. 6:453–457.
2000.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li X, Huang R, Liu K, Li M, Luo H, Cui L,
Huang L and Luo L: Fucoxanthin attenuates LPS-induced acute lung
injury via inhibition of the TLR4/MYD88 signaling axis. Aging
(Albany NY). 12:2655–2667. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hernesniemi J, Lehtimäki T, Rontu R, Islam
MS, Eklund C, Mikkelsson J, Ilveskoski E, Kajander O, Goebeler S,
Viiri LE, Hurme M and Karhunen PJ: Toll-like receptor 4
polymorphism is associated with coronary stenosis but not with the
occurrence of acute or old myocardial infarctions. Scand J Clin Lab
Invest. 66:667–675. 2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wagner KD and Wagner N: PPARs and
myocardial infarction. Int J Mol Sci. 21(9436)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Daynes RA and Jones DC: Emerging roles of
PPARs in inflammation and immunity. Nat Rev Immunol. 2:748–759.
2002.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Ricote M, Li AC, Willson TM, Kelly CJ and
Glass CK: The peroxisome proliferator-activated receptor-gamma is a
negative regulator of macrophage activation. Nature. 391:79–82.
1998.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Jiang C, Ting AT and Seed B: PPAR-gamma
agonists inhibit production of monocyte inflammatory cytokines.
Nature. 391:82–86. 1998.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Azuma Y, Shinohara M, Wang PL and Ohura K:
15-Deoxy-delta(12,14)-prostaglandin J(2) inhibits IL-10 and IL-12
production by macrophages. Biochem Biophys Res Commun. 283:344–346.
2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ayza MA, Zewdie KA, Tesfaye BA,
Gebrekirstos ST and Berhe DF: Anti-diabetic effect of telmisartan
through its partial PPARγ-agonistic activity. Diabetes Metab Syndr
Obes. 13:3627–3635. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Paschoal VA, Walenta E, Talukdar S,
Pessentheiner AR, Osborn O, Hah N, Chi TJ, Tye GL, Armando AM,
Evans RM, et al: Positive reinforcing mechanisms between GPR120 and
PPARγ modulate insulin sensitivity. Cell Metab. 31:1173–1188.e5.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cho RL, Yang CC, Tseng HC, Hsiao LD, Lin
CC and Yang CM: Haem oxygenase-1 up-regulation by rosiglitazone via
ROS-dependent Nrf2-antioxidant response elements axis or PPARγ
attenuates LPS-mediated lung inflammation. Br J Pharmacol.
175:3928–3946. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Celinski K, Dworzanski T, Fornal R,
Korolczuk A, Madro A, Brzozowski T and Slomka M: Comparison of
anti-inflammatory properties of peroxisome proliferator-activated
receptor gamma agonists rosiglitazone and troglitazone in
prophylactic treatment of experimental colitis. J Physiol
Pharmacol. 64:587–595. 2013.PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2000.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Akimoto H, Negishi A, Oshima S, Wakiyama
H, Okita M, Horii N, Inoue N, Ohshima S and Kobayashi D:
Antidiabetic drugs for the risk of alzheimer disease in patients
with type 2 DM using FAERS. Am J Alzheimers Dis Other Demen.
35(1533317519899546)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wu Y, Xiao W, Pei C, Wang M, Wang X, Huang
D, Wang F and Wang Z: Astragaloside IV alleviates PM2.5-induced
lung injury in rats by modulating TLR4/MyD88/NF-κB signalling
pathway. Int Immunopharmacol. 91(107290)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Islam SU, Lee JH, Shehzad A, Ahn EM, Lee
YM and Lee YS: Decursinol angelate inhibits LPS-induced macrophage
polarization through modulation of the NFκB and MAPK signaling
pathways. Molecules. 23(1880)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
O'Connell RM, Taganov KD, Boldin MP, Cheng
G and Baltimore D: MicroRNA-155 is induced during the macrophage
inflammatory response. Proc Natl Acad Sci USA. 104:1604–1609.
2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Hirano S, Zhou Q, Furuyama A and Kanno S:
Differential regulation of IL-1β and IL-6 release in murine
macrophages. Inflammation. 40:1933–1943. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
de la Haba C, Morros A, Martínez P and
Palacio JR: LPS-induced macrophage activation and plasma membrane
fluidity changes are inhibited under oxidative stress. J Membr
Biol. 249:789–800. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zingarelli B and Cook JA: Peroxisome
proliferator-activated receptor-gamma is a new therapeutic target
in sepsis and inflammation. Shock. 23:393–399. 2005.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Han X, Wu Y, Yang Q and Cao G: Peroxisome
proliferator-activated receptors in the pathogenesis and therapies
of liver fibrosis. Pharmacol Ther. 222(107791)2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
K C S, Kakoty V, Marathe S, Chitkara D and
Taliyan R: Exploring the neuroprotective potential of rosiglitazone
embedded nanocarrier system on streptozotocin induced mice model of
Alzheimer's disease. Neurotox Res. 39:240–255. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yi JH, Park SW, Brooks N, Lang BT and
Vemuganti R: PPARgamma agonist rosiglitazone is neuroprotective
after traumatic brain injury via anti-inflammatory and
anti-oxidative mechanisms. Brain Res. 1244:164–172. 2008.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Peng Y, Chen L, Qu Y, Wang D and Zhu Y and
Zhu Y: Rosiglitazone prevents autophagy by regulating
Nrf2-antioxidant response element in a rat model of
Lithium-pilocarpine-induced status epilepticus. Neuroscience.
455:212–222. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Arcalis E, Ibl V, Hilscher J, Rademacher
T, Avesani L, Morandini F, Bortesi L, Pezzotti M, Vitale A, Pum D,
et al: Russell-like bodies in plant seeds share common features
with prolamin bodies and occur upon recombinant protein production.
Front Plant Sci. 10(777)2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Song C, Chen J, Li X, Yang R, Cao X, Zhou
L, Zhou Y, Ying H, Zhang Q and Sun Y: Limonin ameliorates dextran
sulfate sodium-induced chronic colitis in mice by inhibiting
PERK-ATF4-CHOP pathway of ER stress and NF-κB signaling. Int
Immunopharmacol. 90(107161)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kaplan J, Cook JA, O'Connor M and
Zingarelli B: Peroxisome proliferator-activated receptor gamma is
required for the inhibitory effect of ciglitazone but not
15-deoxy-Delta 12,14-prostaglandin J2 on the NFkappaB pathway in
human endothelial cells. Shock. 28:722–726. 2007.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Xia H, Ge Y, Wang F, Ming Y, Wu Z, Wang J,
Sun S, Huang S, Chen M, Xiao W and Yao S: Protectin DX ameliorates
inflammation in sepsis-induced acute lung injury through mediating
PPARγ/NF-κB pathway. Immunol Res. 68:280–288. 2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Liu WC, Wu CW, Fu MH, Tain YL, Liang CK,
Hung CY, Chen IC, Lee YC, Wu CY and Wu KLH: Maternal high
fructose-induced hippocampal neuroinflammation in the adult female
offspring via PPARγ-NF-κB signaling. J Nutr Biochem.
81(108378)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Gonzalez Segura G, Cantelli BA, Peronni K,
Rodrigo Sanches P, Komoto TT, Rizzi E, Beleboni RO, Junior WADS,
Martinez-Rossi NM, Marins M and Fachin AL: Cellular and molecular
response of macrophages THP-1 during Co-culture with inactive
Trichophyton rubrum conidia. J Fungi (Basel).
6(363)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Pires BRB, Silva R, Ferreira GM and
Abdelhay E: NF-kappaB: Two sides of the same coin. Genes (Basel).
9(24)2018.PubMed/NCBI View Article : Google Scholar
|