Introduction
Urinary tract infections (UTIs) represent one of the
most frequent infectious diseases affecting humans, as well as an
important public health issue with a significant economic burden
(1). UTIs represent one of the most
common bacterial infections in children and one of the main reasons
for fever and antibiotic prescription (2,3).
The incidence of the disease reaches 3% in neonates
and is around 0.7% in infants up to 1 year. The prevalence of UTI
in febrile infants is around 5% (2). Up to 11% of girls and 7% of boys will
have had a UTI by the age of 16 years, and recurrence of infection
is common. Vesicoureteral reflux (VUR) is identified in up to 40%
of children being investigated for a first UTI and it represents a
risk factor but a weak predictor for renal parenchymal defects
(4).
UTI is defined as a significant bacteriuria growth
of a single pathogen: At least 104 colony forming units
(CFU) for catheter specimens and at least 105 CFU for
midstream clean catch specimens) or 5x104 CFU and
significant pyuria in a patient with fever or other clinical
symptoms (5-9).
Recurrent UTIs are defined as repeated infections with a different
pathogen agent, while relapsing UTIs represent repeated infections
with the same pathogen (5-7,10,11).
Up to 30% of infants and children experience
recurrent infections during the first 6-12 months after the initial
UTI. In the youngest infants, UTI symptoms differ significantly
compared to older infants and children (1,2).
UTIs are primarily caused by Gram-negative bacteria.
The main pathogen responsible for uncomplicated cystitis and
pyelonephritis is Escherichia coli followed by other species
of Enterobacteriaceae, such as Proteus mirabilis and mostly
Klebsiella pneumoniae, and by Gram-positive pathogens, such
as Enterococcus faecalis and Staphylococcus
saprophyticus (1).
Empiric antibiotic treatment should be initiated for
suspected UTI in a sick child, and if necessary, changed later
according to the sensitivity results for the isolated uropathogen.
Guidelines recommend that empiric antibiotic treatment for
suspected UTI should be based on local susceptibilities derived
from available local epidemiological information (5,9,12).
In recent years, effective antibiotic treatment of
UTIs in young children alleviates acute symptoms and may also limit
long-term sequelae. Antibiotics should ideally be prescribed only
to those who have a UTI, using an antibiotic with the narrowest
effective spectrum. Treating pediatric UTIs in less than 3 days
reduces the risk of acquiring kidney scars by 50% (13).
Currently, there is an alarming level of
antimicrobial resistance which has developed in UTI pathogens as a
result of improper and widespread use of antibiotics (1,14).
Antimicrobial resistance is an internationally
recognized threat to public health. The contribution of primary
healthcare is of significant importance as this is where around 80%
of all antibiotics used within the health service are prescribed
(15). Antibiotic resistance in
pediatric patients is increasing. Less than 50% of all pediatric
UTIs are susceptible to commonly used antibiotics (16,17).
Antibiotic-resistant infections are most likely to be associated
with greater morbidity and mortality and are associated with
increased healthcare costs (15).
As for E. coli, resistance to
third-generation cephalosporins and combined resistance to
third-generation cephalosporins, fluoroquinolones, and
aminoglycosides has increased significantly at the European
Union/European Economic Area level between 2013 and 2016.
Carbapenems are an important group of last-line antibiotics for the
treatment of infections with multidrug-resistant (MDR)
gram-negative bacteria such as Klebsiella pneumoniae and
E. coli. In 2016, carbapenem resistance in E. coli
remained rare, and most countries reported low resistant levels for
Klebsiella pneumonia (18).
MDR is increasing worldwide, especially for commonly
used antibiotics. Bacterial resistance to at least one
antimicrobial in three or more classes defines MDR (19).
Patients and methods
Study sample and data source
A retrospective, transversal study was performed
using 331pediatric patients diagnosed with UTI, aged between 2
weeks and 17 years, admitted to the Pediatric Clinic 1, Nephrology
Department of the Emergency Clinical County Hospital (TârguMureș)
and the Nephrology Department of the Emergency Clinical Hospital
for Children (Cluj-Napoca, Romania), between January 2016 and
December 2018.
Inclusion and exclusion criteria
We included all children with clinical and
paraclinical signs of UTI. Exclusion criteria consisted of
incomplete anamnestic, clinical or paraclinical data. If fever was
absent, the UTI was classified as afebrile UTI (aUTI).
Laboratory methods
In general, antibiogram results were considered as
susceptible, intermediate, or resistant; for the purpose of our
study, intermediate and resistant isolates were considered
collectively as non-susceptible. Extended-spectrum β-lactamase
(ESBL)-producing strains were identified using double-disk synergy
test.
Ethics
All mothers signed informed consent for their
children. Our study was approved by the Ethics Committee of the
University of Medicine and Pharmacy of Târgu Mureș (no. 259/July
11, 2019), and it was accepted according to the principles of the
Helsinki Declaration.
Statistical analysis
Microsoft Office Excel package was used for data
collection and GraphPadPrism v.5 (GraphPad Software, Inc.) for
statistical analysis. We used discrete quantitative and binary
qualitative variables. For the comparison of means, we used the
Student's t-test with a significance threshold of 95% confidence
interval (CI). In addition, inferential statistical test, such as
Chi-square and analysis of variance (ANOVA) were applied.
Results
Patient characteristics
Among the 331 patients included in our study, the
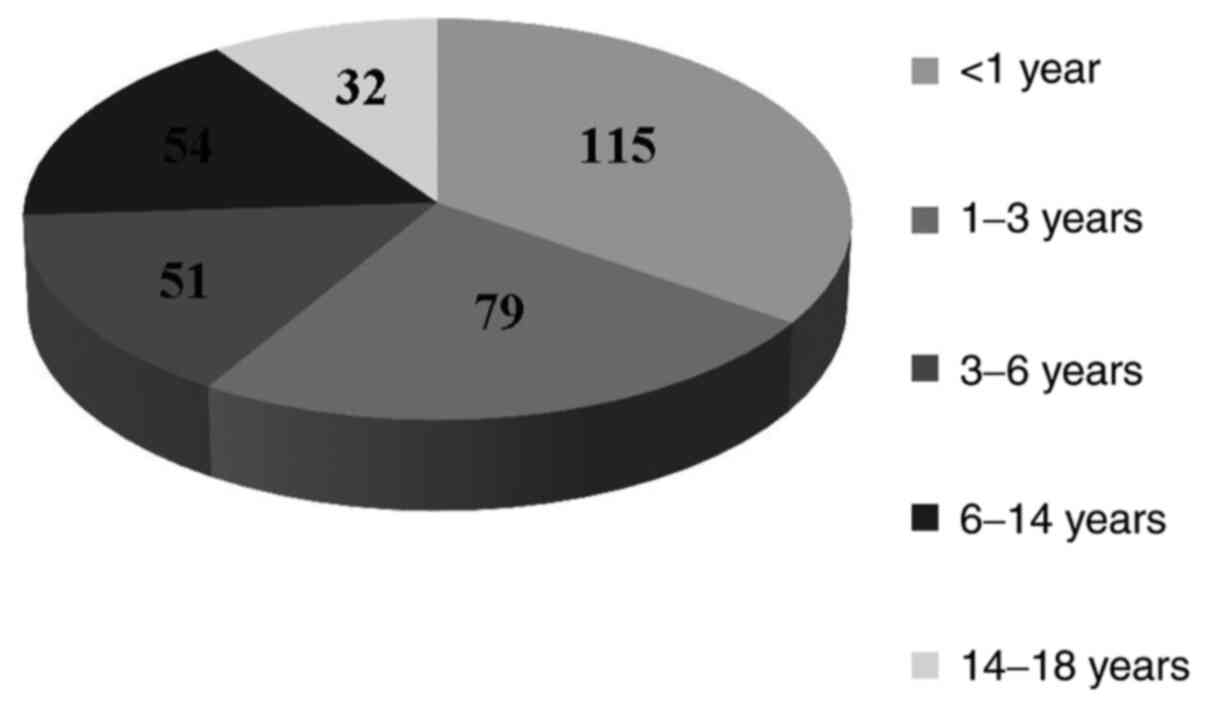
mean age was 4.13±4.48 years. The study group was divided into age
groups (<1, 1-3, 3-6, 6-14 and 14-18 years. More than a third of
isolates (n=115, 34.74%) were from patients younger than 1 year,
followed by the group of age 1-3 years (n=79, 23.86%), and the
groups of 3-6 and 6-14 years with similar frequency (n=51, 15.42%
and n=54, 16.32% respectively), while the lowest number of cases
was within the 14-18 year group (n=32, 9.66%) (Fig. 1).
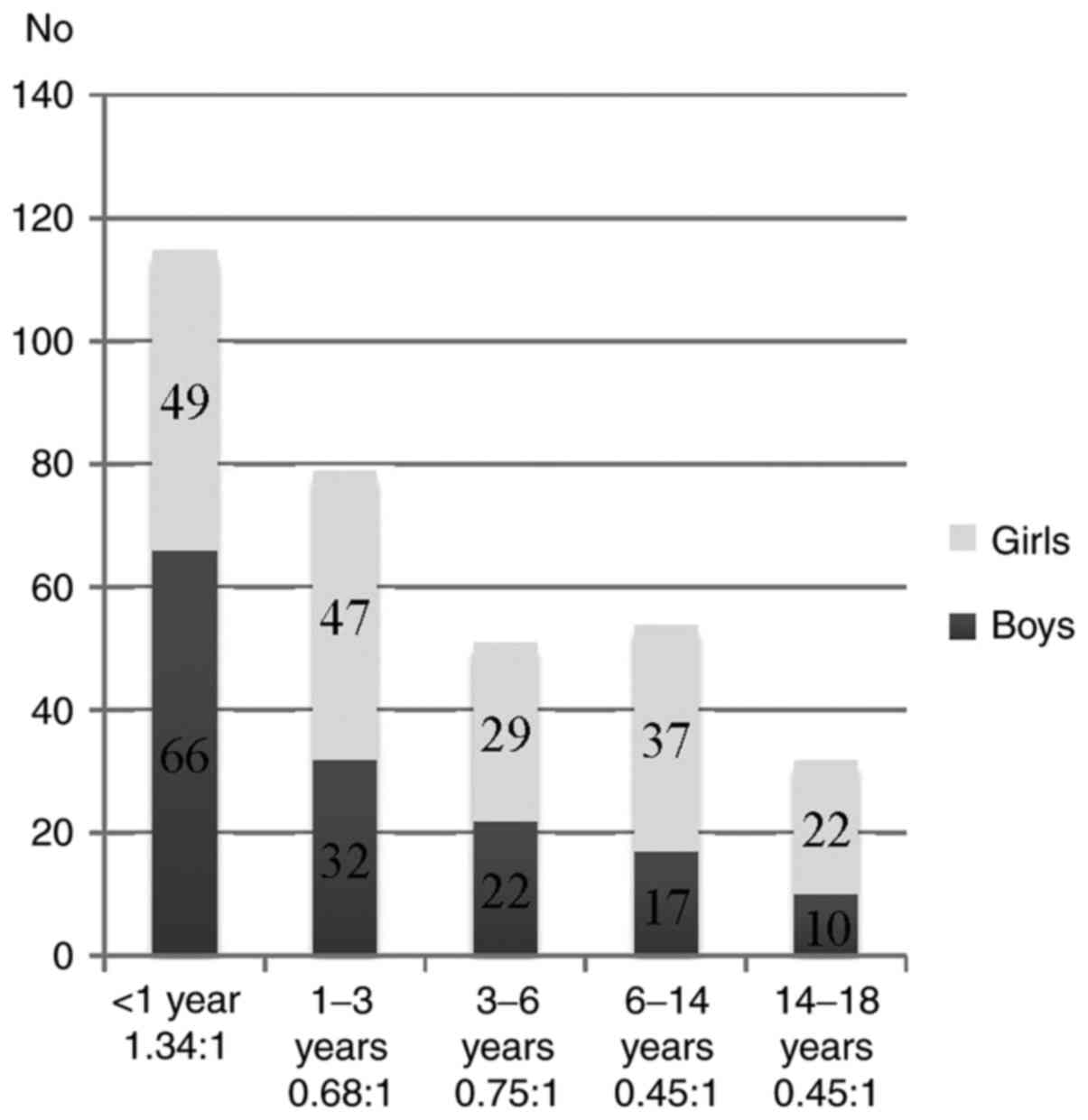
With respect to sex distribution, urine samples were
processed from 147 boys and184 girls (44.41/55.59%). The sex ratio
was 1:1.25, high lighting that UTIs are a more frequent pathology
in girls. The male to female ratio varied according to age as
follows: In the <1 year of age group, sex ratio favored boys
1.34:1 while in the other age groups, sex ratio was in favor of
female patients: [1-3 year age group, 0.68:1; 3-6 year age group,
0.75:1; 6-14 year age group, 0.45:1; 14-18 year age group, 0.45:1
(Fig. 2).
More than half of the children [57.71%, n=191/331]
had no other comorbidities while in 42.29% cases (n=140/331) a
urinary tract-abnormality was detected.
Uropathogens
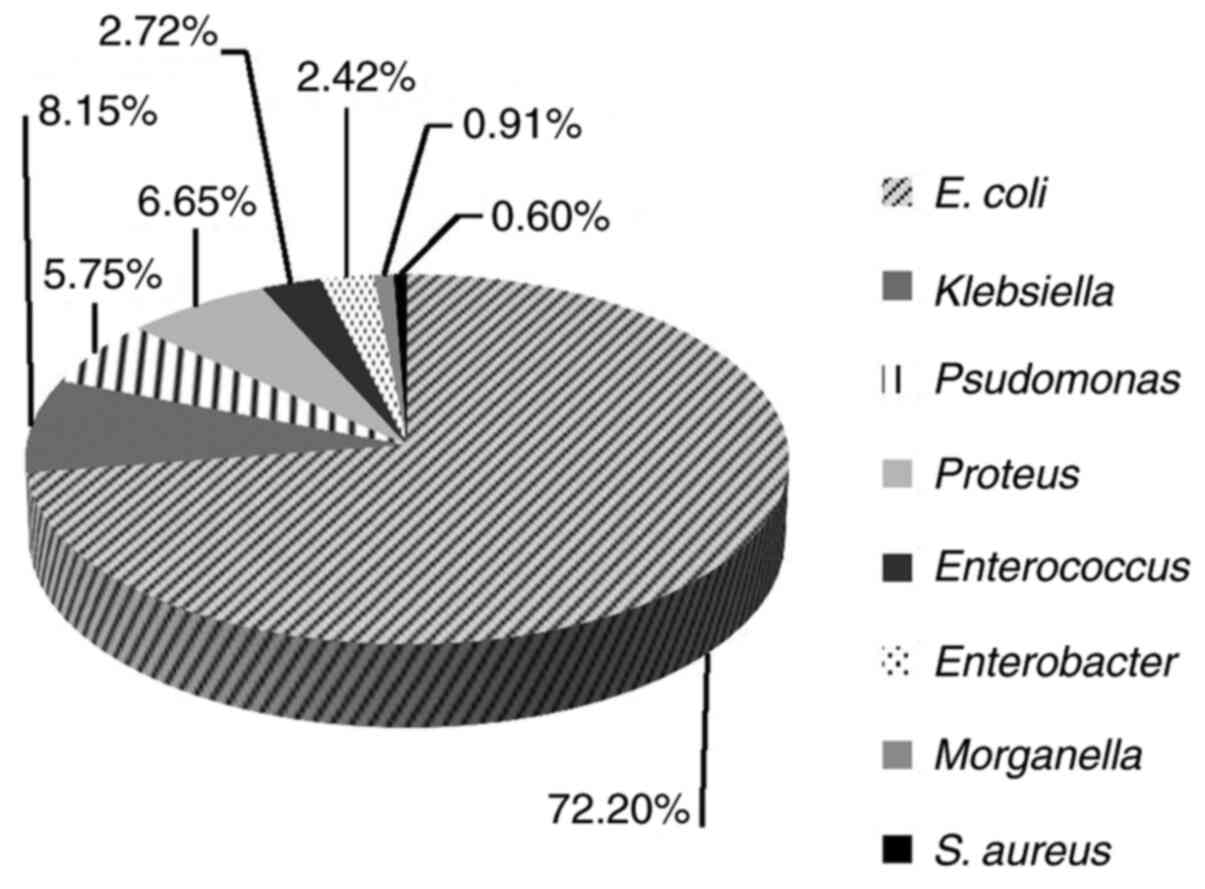
E. coli was the most frequently identified
uropathogen (72.2%, 239/331), followed by Klebsiella spp.
(8.15%, 27/331), Proteus spp. (6.65%, 22/331) and
Pseudomonas aeruginosa (5.75%; 19/331) (Fig. 3). In contrast, the lowest frequency
in our study group was noted for Enterococcus (2.72%;
9/331), Enterobacter spp. (2.42%; 8/331), Morganella
morganii (0.91%; 3/331), Staph. aureus (0.6%; 2/331),
and others (0.6%; 2/331). Extended spectrum beta-lactamase (ESBL)
producing bacteria were also detected in a high percentage in our
samples, 7.85% (26/331). The characteristics of these pathogens are
presented in Table I.
 | Table IDistribution of the uropathogens. |
Table I
Distribution of the uropathogens.
| Uropathogens | Total (N=331) n
(%) | ESBL n (%) | Girls n (%) | Boys n (%) | P-value |
|---|
| E. coli | 239 (72.20) | 19 (7.95) | 151 (63.17) | 88 (36.83) | 0.0001 |
| Klebsiella
spp. | 27 (8.15) | 5 (18.51) | 10 (37.04) | 17 (62.96) | 0.0400 |
| Pseudomonas
aeruginosa | 19 (5.75) | - | 9 (47.36) | 10 (52.64) | - |
| Proteus
spp. | 22 (6.65) | 2 (0.60) | 9 (40.90) | 13 (59.10) | 0.1500 |
|
Enterococcus | 9 (2.72) | - | 3 (33.33) | 6 (66.67) | - |
| Enterobacter
spp. | 8 (2.42) | - | - | 8 | - |
| Morganella
morgani | 3 (0.91) | - | 1 (33.33) | 2 (66.67) | - |
| Staph.
Aureus | 2 (0.60) | - | - | 2 | - |
| Others | 2 (0.60) | - | - | 2 | - |
UTIs caused by E. coli were more frequent in
female patients (n=151, 63.17%) than in males (n=88, 36.83%) with
statistical significance (P=0.0001), while those caused by
Klebsiella and Proteus spp. were more frequent in
boys [n=17 (62.96%) and n=13 (59.09%)] than in girls [n=10 (37.04%)
and n=9 (40.91%)], but with no statistical significance (P=0.04 and
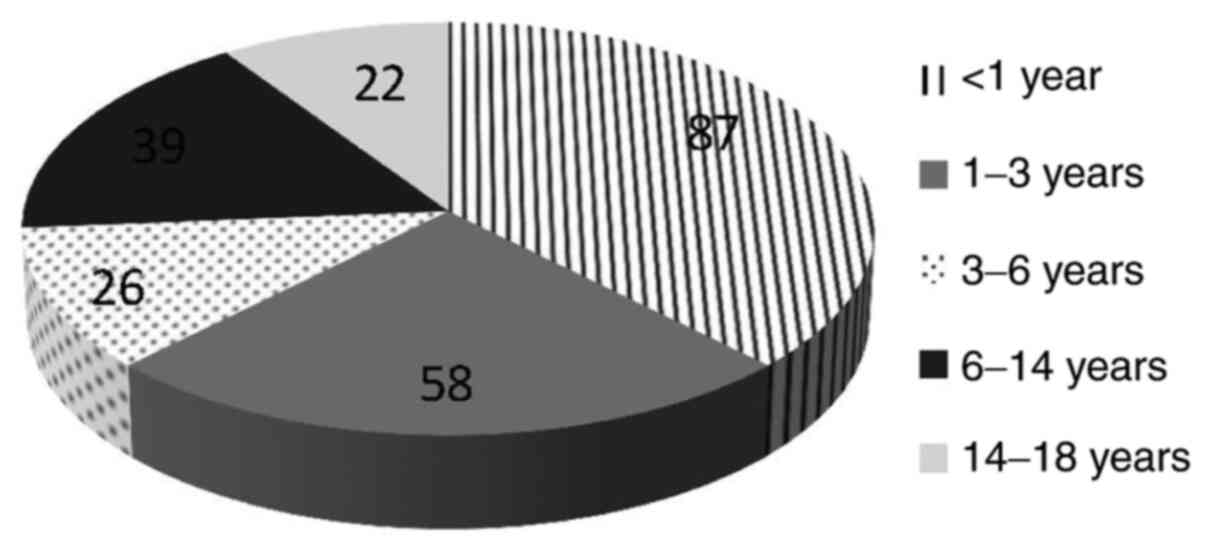
=0.15) (Table I). Age distribution
of the patients who presented with UTIs with E. coli is
emphasized in Fig. 4.
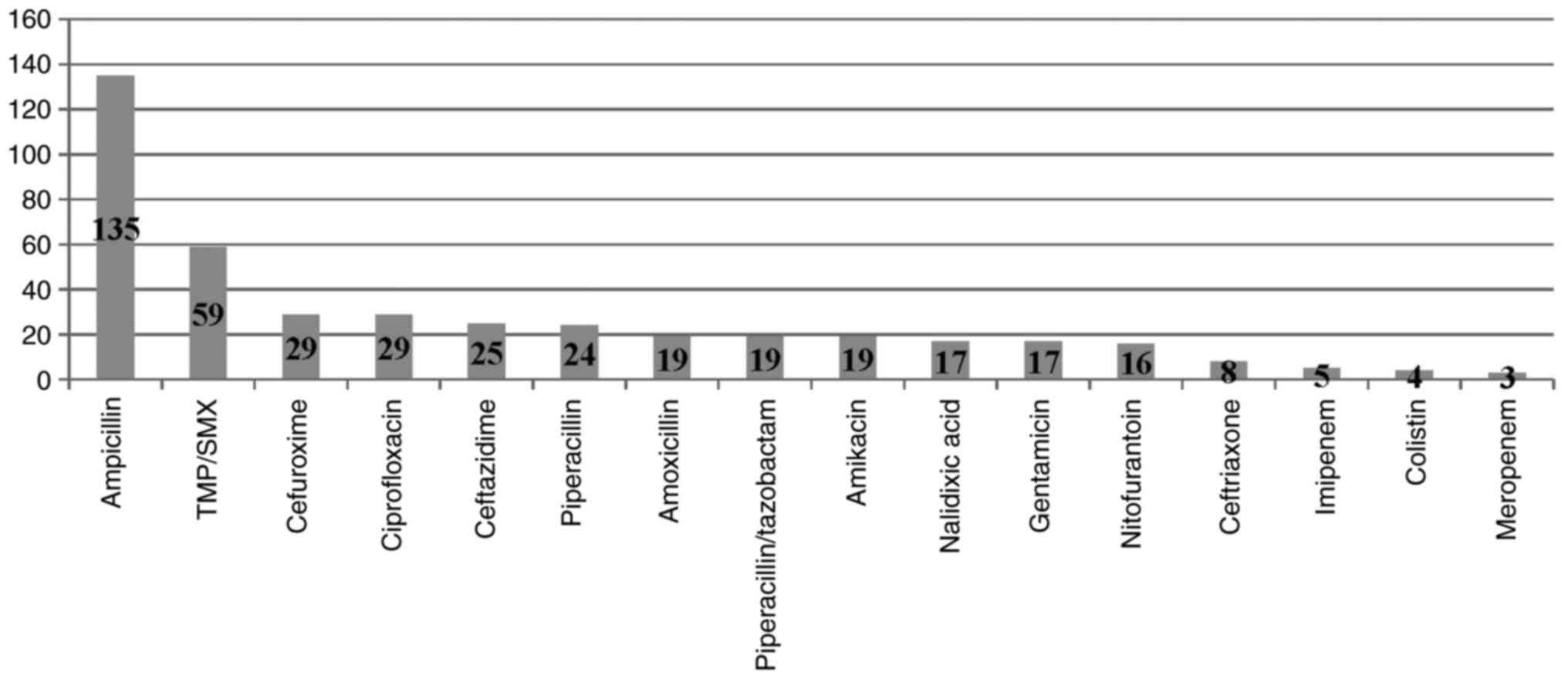
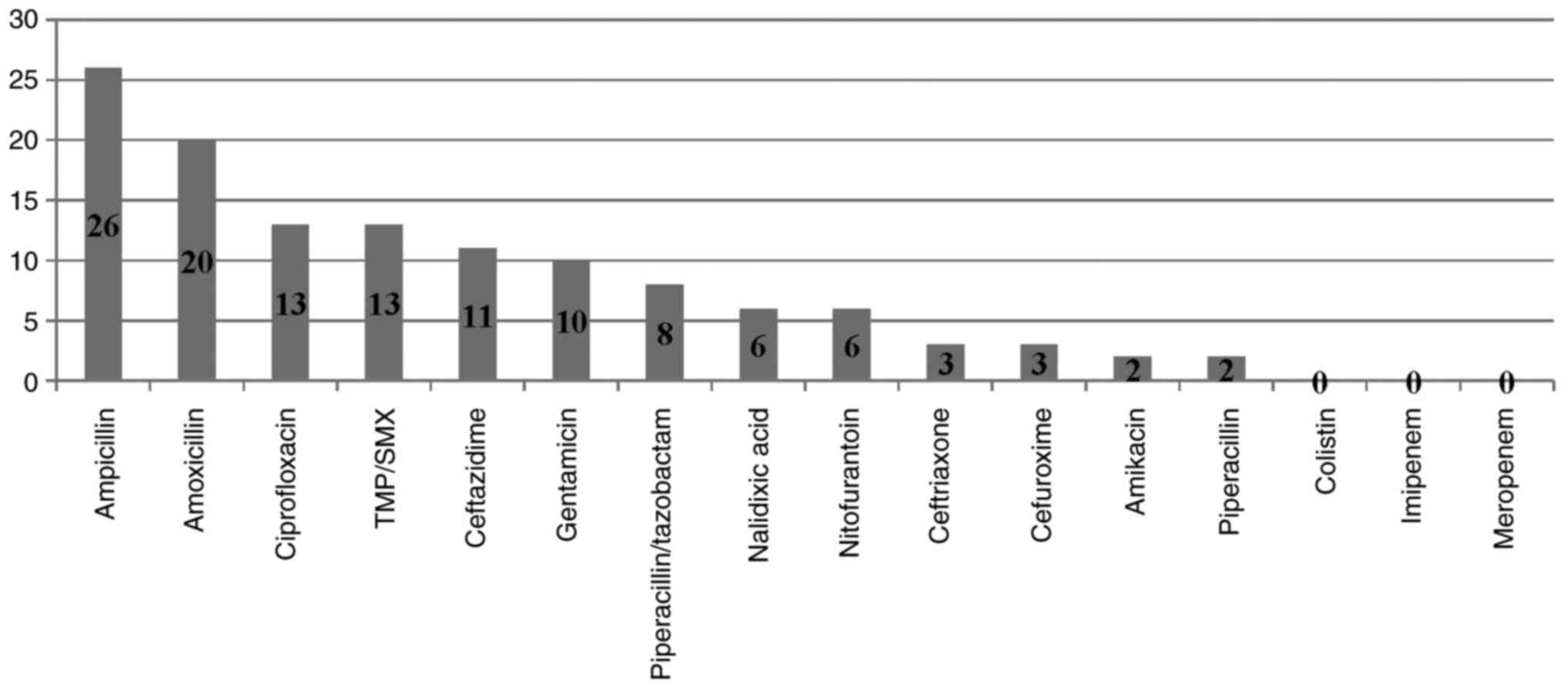
The antimicrobial resistance pattern of the
uropathogens can be observed in Table
II. Both E. coli and Klebsiella showed high
resistance to ampicillin, amoxicillin, TMP/SMX, cefuroxime and
ciprofloxacine, respectively. E. coli remained susceptible
to nitrofurantoin, ceftriaxone, meropenem while Klebsiella
to amikacin, colistin and meropenem (Figs. 5 and 6).
 | Table IIResistance patterns of the
uropathogens. |
Table II
Resistance patterns of the
uropathogens.
|
ATB/Uropathogen | E. coli
(%) | Klebsiella
(%) | Pseudomonas
(%) | Proteus
(%) | Enterococus
(%) | Morganella
(%) | Enterobacter
(%) |
|---|
| Nalidixic acid | 7.11 | 1.81 | <1 | <1 | <1 | <1 | <1 |
| Amikacin | 7.94 | <1 | <1 | <1 | <1 | <1 | <1 |
| Amoxicillin | 33.05 | 6.04 | <1 | 3.62 | <1 | 1.25 | 1.81 |
| Ampicillin | 56.48 | 7.85 | <1 | 5.1.3 | <1 | 1.25 | 2.09 |
| Cefepime | 7.94 | 4.53 | 1.25 | 2.09 | <1 | <1 | 2.09 |
| Cefotaxime | 10.04 | 4.53 | <1 | 2.09 | 1.25 | <1 | 1.81 |
| Ceftazidime | 10.46 | 3.32 | 1.67 | 1.25 | 1.25 | <1 | 2.09 |
| Ceftriaxone | 3.34 | 1.25 | <1 | <1 | 1.25 | <1 | 1.67 |
| Cefuroxime | 12.13 | 1.25 | 1.25 | 1.67 | 1.25 | <1 | 2.09 |
| Ciprofloxacin | 12.13 | 3.92 | <1 | 1.25 | 1.67 | <1 | 1.25 |
| Colistin | 1.67 | <1 | <1 | <1 | <1 | <1 | <1 |
| Ertapenem | 0.83 | 2.09 | <1 | <1 | <1 | <1 | 1.25 |
| Gentamicin | 7.11 | 3.02 | 3.32 | 1.67 | 1.81 | <1 | 1.25 |
| Imipenem | 2.09 | <1 | <1 | <1 | <1 | <1 | <1 |
| Linezolid | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| Meropenem | 1.25 | <1 | <1 | <1 | <1 | <1 | <1 |
| Netilmicin | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| Nitrofurantoin | 6.69 | 1.81 | <1 | 1.81 | <1 | <1 | <1 |
| Norfloxacin | 15.06 | 4.23 | <1 | 1.67 | <1 | <1 | <1 |
| Oxacillin | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| Piperacillin | 7.94 | 0.83 | <1 | <1 | <1 | <1 | <1 |
|
Piperacillin/tazobactam | 10.04 | 2.41 | 1.25 | <1 | <1 | <1 | 2.09 |
| Streptomycin | <1 | <1 | <1 | <1 | 1.67 | <1 | <1 |
| Teicoplanin | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| Tetracycline | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| Ticarcillin | 0.41 | <1 | <1 | <1 | <1 | <1 | <1 |
|
Ticarcillin/clavulanic acid | 0.83 | <1 | 1.25 | <1 | <1 | <1 | <1 |
| Tigecycline | 1.25 | <1 | <1 | <1 | <1 | <1 | <1 |
| Trimethoprim | 24.68 | 3.92 | 1.67 | 2.71 | 1.25 | 1.25 | 1.25 |
| Vancomycin | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
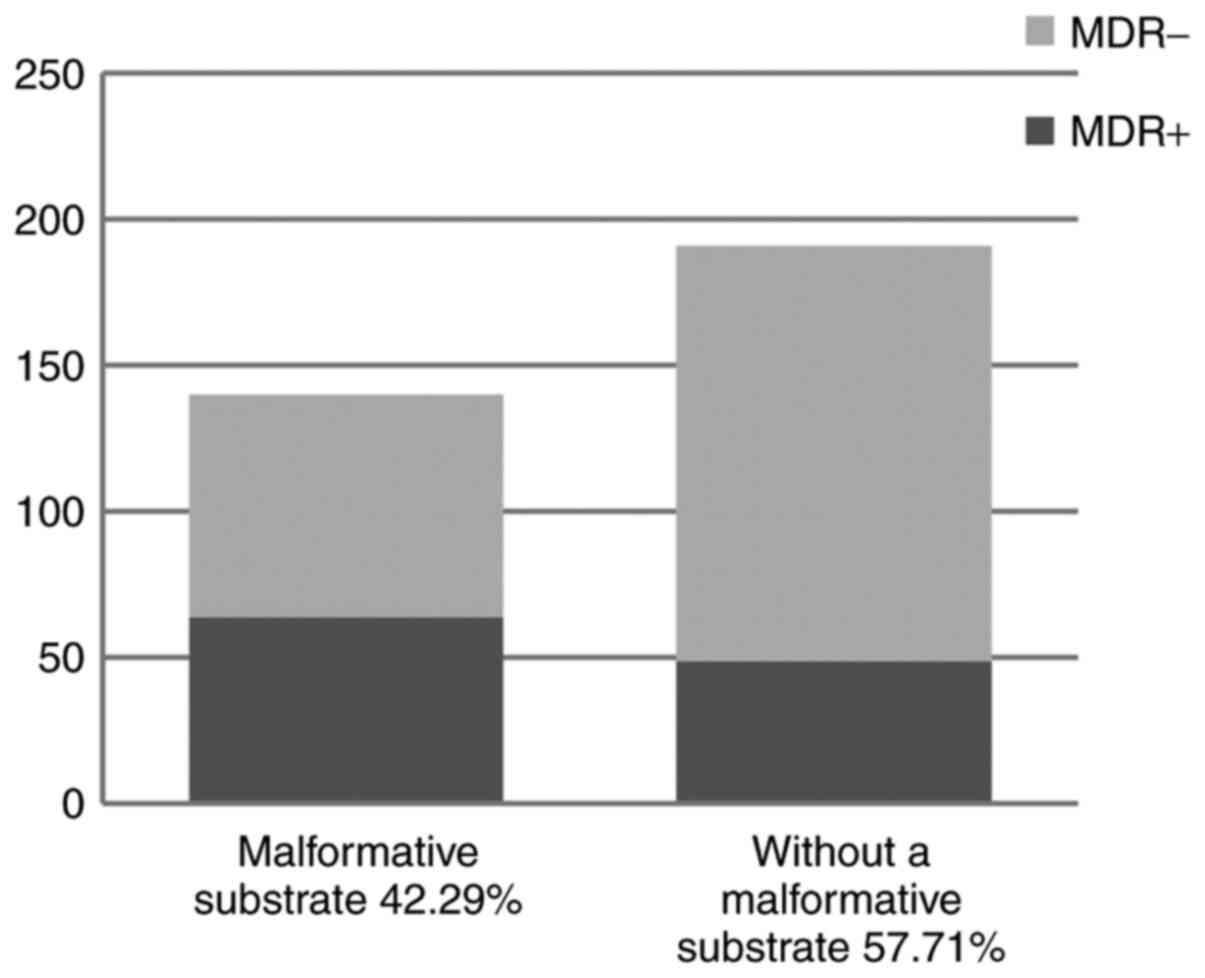
Out of the total of 331 UTIs, a significant
percentage of cases presented with associated urinary tract
malformations (n=140, 42.29%). This condition entailed a higher
proportion of MDR of the involved uropathogens. In other words, MDR
was detected in 34.13% of the uropathogens, among which 56.63% were
isolated in patient with urinary tract malformation (Fig. 7). Analyzing a contingence table, we
obtained a positive correlation between these variables (P=0.0001,
OR 2.44), meaning that urinary tract malformations are a
predisposing factor for UTIs with MDR uropathogens.
From the 239 cases diagnosed with E. coli
infection, 32.63% (78/239) presented a urinary tract abnormality,
and 39.74% (31/78) with associated MDR. Also, in this instance,
statistically, urinary tract abnormalities significantly influence
the occurrence of a UTI caused by a MDR E. coli (P=0.005, OR
2.29).
Discussion
Strengths and limitations
To the best of our knowledge, this is the first
widespread research that evaluates antibiotic resistance in
childhood UTIs, and their association with urinary tract
abnormalities in Romania.
E. coli and Klebsiella represented
common uropathogens in children admitted to both nephrology
departments involved. In this study, the frequency of infection
with E. coli and Klebsiella were similar with studies
from Nepal and Turkey (20,21).
Of the 331 patients, 184 (55.58%) were female
compared with a study of Vazouras et al where the female
proportion was 72.2% (22). The
cause may be the higher rate of urinary tract abnormalities in our
study group.
In the study performed by Vazouras et al,
11.3% (26/230) of the evaluated children had urinary
tract-associated abnormalities (22) compared with our study where a
3.5-time higher rate of urinary tract-associated abnormalities were
observed. The explanation may be that our study group was from two
nephrology centers.
Similar to other pediatric studies on the same
subject (2,20,22),
E. coli was the most frequently encountered etiological
agent in our research as well.
In a recent research in our country, E. coli,
Klebsiella spp., Enterococcus spp., Morganella
morganii, Proteus spp., and Enterobacter spp.
represented the leading etiology for UTIs (53.3, 10.6, 5.2, 5.2,
4.5, and 3.9%, respectively), results that are in part similar to
our findings (2). When compared
with our age-matched group, we found an occurrence of 87/115
(75.65%) for E. coli, 11/115 (9.56%) for Klebsiella
spp., 5/115 (4.34%) for Enterococcus spp., 3/115 (2.6%)
for Proteus spp., and 3/115 (2.6%) for
Enterobacter spp.
In their research, Vazouras et al found that
the main causative organism was E. coli (79.2%) with high
reported resistance rates to ampicillin (42.0%), TMP/SMX (26.5%),
and amoxicillin/clavulanic acid (12.2%); lower resistance rates
were identified for third-generation cephalosporins (1.7%),
nitrofurantoin (2.3%), ciprofloxacin (1.4%) and amikacin (0.9%)
(22). These results are only
partly similar to ours.
Low resistance rates of E. coli and
Klebsiella to piperacillin/tazobactam, meropenem, nalidixic
acid, chloramphenicol and colistin were found by Falup-Pecurariu
et al, these results being comparable with the present study
(2).
In our cohort, the ESBL-positive UTIs were 7.85%
(n=26) compared with a lower incidence in a recent study (1.7%)
(22), while another study in
Northern Greece exposed an incidence of ESBL-positive UTIs of 10.4%
(23).
Our research depicted that there is a higher chance
for UTIs due to ESBL-producing pathogens in children with urinary
tract abnormalities and those receiving antimicrobial prophylaxis.
This finding is comparable with the results of another study
(23).
In the pediatric group with non-ESBL-positive UTIs,
a higher ratio of urinary tract anomalies as well as antimicrobial
prophylaxis were observed (P<0.05), compared to those from
ESBL-positive UTI group. This is in disagreement with the
conclusion of a recent study (23).
A high incidence of ESBL-positive uropathogens was
revealed by Falup-Pecurariu et al (2) compared to our research [55 of 68
(80.9%) E. coli vs. 5 of 87 (5.75%)] were ESBL-positive;
15/35 (42.9%) of the Klebsiella spp. vs. 2 of 11 (18.2%)
were ESBL-positive.
Regarding the MDR UTI cases, a prolonged
antimicrobial prophylaxis and presence of urinary tract anomalies
have been considered as risk factors (24).
Current literature data suggest the effectiveness of
fosfomycin against MDR and drug-resistant bacteria (25). This may be extrapolated to us as
there is very low use of this drug in our centers.
Our study indicates that nitrofurantoin, ceftriaxon,
amikacin, and carbapenem may be used for the empirical treatment of
febrile or complicated UTIs in children. Our study results are
comparative with those achieved by Raya et al (20).
These results are however contradicted by a local
study (2), where only 6% of infants
had previous renal anomalies compared to our study where we had a
significant higher frequency.
Current findings emphasize that male patients are
more often diagnosed with UTIs in the first year of life while
female patients are diagnosed at approximately 3 years of age
(26). Our study sustains this
idea, statistically confirming that in the first year of life
almost 60% of the patients diagnosed with UTIs (57.39%, 66/115)
were male, thus obtaining a 1.34:1 sex ratio. However, our overall
ratio of male and female patients with UTIs was in favor of girls
1:1.25, this result being comparable with other studies (26).
In conclusion, initial therapy for UTI is originally
empirical until the results of a urine culture are ready.
Therefore, it is mandatory to know the local resistance of
uropathogens to antimicrobial agents as well as the risk factors
for UTI due to resistant pathogens such as ESBL.
The results of this study may influence empirical
therapy for UTIs until the ESBL production has been confirmed.
Since local antimicrobial sensitivities vary significantly, local
guidelines and close monitoring should be provided to coordinate
empiric antibiotic treatment.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Further information regarding the data of the
present study is available from the corresponding author upon
reasonable request.
Authors' contributions
CD and IC contributed to the conception and design
of the research. CD and IC wrote the first draft of the manuscript.
CD, IC, DD, CA wrote sections of the manuscript. DD, AAA, and CA
analyzed previous literature studies and revised the manuscript
critically for important intellectual content. All authors
contributed to manuscript revision, read and approved the submitted
version.
Ethics approval and consent to
participate
Our study was approved by the Ethics Committee of
the University of Medicine and Pharmacy of Târgu Mureș (no.
259/July 11, 2019). All mothers signed informed consent for their
children.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mazzariol A, Bazaj A and Cornaglia G:
Multi-drug-resistant gram-negative bacteria causing urinary tract
infections: A review. J Chemother. 29 (Suppl 1):S2–S9.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Falup-Pecurariu O, Leibovitz E, Bucur M,
Lixandru R, Bleotu L and Falup-Pecurariu C: High resistance rates
to 2nd and 3rd generation cephalosporins, ciprofloxacin and
gentamicin of the uropathogens isolated in young infants
hospitalized with first urinary tract infection. Biomed Res.
28:8774–8779. 2017.
|
|
3
|
Ismaili K, Wissing KM, Lolin K, Le PQ,
Christophe C, Lepage P and Hall M: Characteristics of first urinary
tract infection with fever in children: A prospective clinical and
imaging study. Pediatr Infect Dis J. 30:371–374. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Larcombe J: Urinary tract infection in
children: Recurrent infections. BMJ Clin Evid.
2015(0306)2015.PubMed/NCBI
|
|
5
|
NICE. Urinary tract infection in under
16s: Diagnosis and management, clinical guideline CG54. National
Institute for Health and care Excellence, 2017. Available from:
https://www.nice.org.uk/guidance/cg54/resources/urinary-tract-infection-in-under-16s-diagnosis-and-management-pdf-975507490501.
|
|
6
|
Stein R, Dogan HS, Hoebeke P, Kočvara R,
Nijman RJ, Radmayr C and Tekgül S: European association of urology;
European society for pediatric urology: Urinary tract infections in
children: EAU/ESPU guidelines. Eur Urol. 67:546–558.
2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Armean I, Meliț LE and Duicu C: A rare
case of urinary tract infection with Burkholderia cepacia in a male
child. Ro J Infect Dis. 21:70–73. 2018.
|
|
8
|
Primack W, Bukowski T, Sutherland R,
Gravens-Mueller L and Carpenter M: What urinary colony count
indicates a urinary tract infection in children? J Pediatr.
191:259–261.e1. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Duicu C, Armean I and Aldea C: New
insights in treatment options in pediatric urinary tract infection.
Acta Med Marisiensis. 65:7–11. 2019.
|
|
10
|
Gorczyca D, Augustyniak D,
Basiewicz-Worsztynowicz B and Karnas-Kalemba W: Serum and urinary
MIP-1α and IP-10 levels in children with urinary tract infections.
Adv Clin Exp Med. 23:933–938. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Becknell B, Schober M, Korbel L and
Spencer JD: The diagnosis evaluation and treatment of acute and
recurrent pediatric urinary tract infections. Expert Rev Anti
Infect Ther. 13:81–90. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Butler CC, O'Brien K, Wootton M, Pickles
T, Hood K, Howe R, Waldron CA, Thomas-Jones E, Dudley J, Van Der
Voort J, et al: Empiric antibiotic treatment for urinary tract
infection in preschool children: Susceptibilities of urine sample
isolates. Fam Pract. 33:127–132. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Coulthard MG, Lambert HJ, Vernon SJ,
Hunter WE, Keir MJ and Matthews JN: Does prompt treatment of
urinary tract infection in preschool children prevent renal
scarring: Mixed retrospective and prospective audits. Arch Dis
Child. 99:342–347. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bonkat G, Bartoletti R, Bruyère F, Cai T,
Geerlings SE, Köves B, Schubert S and Wagenlehner F: EAU guidelines
on urological infections, 2017. Available from: https://uroweb.org/wp-content/uploads/19-Urological-infections_2017_web.pdf.
|
|
15
|
Bryce A, Hay AD, Lane IF, Thornton HV,
Wootton M and Costelloe C: Global prevalence of antibiotic
resistance in paediatric urinary tract infections caused by
Escherichia coli and association with routine use of
antibiotics in primary care: Systematic review and meta-analysis.
BMJ. 352(i939)2016.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Saperston KN, Shapiro DJ, Hersh AL and
Copp HL: A comparison of inpatient versus outpatient resistance
patterns of pediatric urinary tract infection. J Urol. 191 (Suppl
5):S1608–S1613. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Prabhu A, Taylor P, Konecny P and Brown
MA: Pyelonephritis: What are the present day causative organisms
and antibiotic susceptibilities? Nephrology (Carlton). 18:463–467.
2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Summary of the latest data on antibiotic
resistance in the European Union. EARS-Net surveillance data. ECDC
November, 2017. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/EAAD%20EARS-Net%20summary.pdf.
|
|
19
|
Magiorakos AP, Srinivasan A, Carey RB,
Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter
G, Olsson-Liljequist B, et al: Multidrug-resistant, extensively
drug-resistant and pandrug-resistant bacteria: An international
expert proposal for interim standard definitions for acquired
resistance. Clin Microbiol Infect. 18:268–281. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Raya GB, Dhoubhadel BG, Shrestha D, Raya
S, Laghu U, Shah A, Raya BB, Kafle R, Parry CM and Ariyoshi K:
Multidrug-resistant and extended-spectrum beta-lactamase-producing
uropathogens in children in Bhaktapur, Nepal. Trop Med Health.
48(65)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shakya P, Shrestha D, Maharjan E, Sharma
VK and Paudyal R: ESBL production among E. coli and
Klebsiella spp. causing urinary tract infection: A hospital
based study. Open Microbiol J. 11:23–30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Vazouras K, Velali K, Tassiou I,
Anastasiou-Katsiardani A, Athanasopoulou K, Barbouni A, Jackson C,
Folgori L, Zaoutis T, Basmaci R and Hsia Y: Antibiotic treatment
and antimicrobial resistance in children with urinary tract
infections. J Glob Antimicrob Resist. 20:4–10. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Dotis J, Printza N, Marneri A, Gidaris D
and Papachristou F: Urinary tract infections caused by
extended-spectrum betalactamase-producing bacteria in children: A
matched casecontrol study. Turk J Pediatr. 55:571–574.
2013.PubMed/NCBI
|
|
24
|
Duicu C, Kiss E, Simu I and Aldea C: A
rare case of double-system with ectopic ureteral openings into
vagina. Front Pediatr. 6(176)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Williams PC: Potential of fosfomycin in
treating multidrug-resistant infections in children. J Paediatr
Child Health. 56:864–872. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Woo B, Jung Y and Kim HS: Antibiotic
sensitivity patterns in children with urinary tract infection:
Retrospective study over 8 years in a single center. Child Kidney
Dis. 23:22–28. 2019.
|