Introduction
Hepatocellular carcinoma (HCC) is estimated to be
the sixth most common primary malignant tumor and the second
leading cause of cancer-associated mortality worldwide (1-3).
The most common etiological factor for HCC is the cirrhotic liver,
which provides a pro-carcinogenic intrahepatic environment
(4,5). The most notable risk factors,
including chronic viral hepatitis caused by hepatitis B virus (HBV)
or hepatitis C virus infection, alcohol abuse, non-alcoholic fatty
liver disease and other abnormal metabolic conditions, contribute
to the development of cirrhosis, particularly in East Asian
countries where HBV infection is endemic (6-8).
HCC is generally diagnosed in individuals at an advanced stage due
to the lack of early symptoms (6).
Despite advancements in the comprehensive treatments based on
surgery, the postoperative recurrence of HCC seriously threatens
the quality of life of ~30% those affected, with limited treatment
options (9). Thus, it remains
crucial to develop novel therapeutic strategies to improve the
outcome of patients with HCC.
The onset and development of HCC involves multi-step
biological processes, which are regulated by multiple factors and
signaling molecules (10).
Ribosomal protein LP1 (RPLP1) consists of 114 amino acids and is a
member of the ribosomal protein L12P family (11). Increasing evidence suggests that
RPLP1 plays a crucial role in the elongation step of protein
synthesis (12,13). Upregulated RPLP1 expression
facilitates tumorigenesis and immortalizes primary cells,
contributing to cellular transformation (14). It has been reported that RPLP1 may
act as a prognostic biomarker and anti-metastatic candidate
therapeutic target in triple-negative breast cancer (14). In addition, RPLP1 expression has
been demonstrated to be upregulated in biopsy specimens taken from
patients with colon cancer (11). A
previous study demonstrated that RPLP1 expression is significantly
associated with the progression of gynecological tumors, including
serous ovarian cancers and endometrial carcinomas (15). However, the role of RPLP1 in HCC
progression remains unknown. Thus, the present study aimed to
investigate the role of RPLP1 in HCC progression.
The present study aimed to investigate the role of
RPLP1 in HCC progression and assess its effect on the cellular
behaviors of human HCC cells. The present study provided novel
insight into understanding the HCC pathogenesis and indicating
RPLP1 may be of value as a promising therapeutic target for HCC
treatment.
Materials and methods
Cell culture and transfection
Human liver HHL-5 cells and the HCC cell lines,
SK-HEP-1, Hep3b and Huh-7, were purchased from The Cell Bank of
Type Culture Collection of the Chinese Academy of Sciences. All
cells were maintained in RPMI-1640 medium supplemented with 10%
fetal bovine serum, 100 µg/ml streptomycin and 100 U/ml penicillin
(all Gibco; Thermo Fisher Scientific, Inc.), at 37˚C with 5%
CO2.
Hep3b cells were seeded into 96-well
(2x103 cells/well) or six-well (1x105
cells/well) plates and transfected with short hairpin (sh)
RNA-RPLP1 (100 nM) using Lipofectamine® 3000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37˚C for 12 h.
Cells were cultured until they reached 60-70% confluence. RPLP1
shRNA-1 (5'-CATTAAAGCAGCCGGTGTAAATGTTGAGC-3') and RPLP1 shRNA-2
(5'-GAAGAAAGTGGAAGCATTCAAGAGATGCT-3') or the negative control RNA
(non-targeting) were synthesized by Shanghai GeneChem Co., Ltd.
Reverse transcription-quantitative (RT-q)PCR analysis was performed
to assess transfection efficiency. Hep3b cells were used for
subsequent experimentation 48 h post-transfection. RPLP1 expression
was assessed by screening the Gene Expression Profiling Interactive
Analysis database (http://gepia.cancer-pku.cn) to determine the
association between RPLP1 expression and HCC progression. Moreover,
the overall survival (OS) analyzed using the GEPIA database
(http://gepia.cancer-pku.cn/) to
investigate the role of RPLP1 in HCC progression and the UALCAN
database (http://ualcan.path.uab.edu) was used
to determine the association between RPLP1 expression and tumor
grade of HCC.
RT-qPCR
Total RNA was extracted from transfected Hep3b cells
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Total RNA was reverse transcribed into cDNA
using the High-Capacity cDNA Reverse Transcription kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The prepared reaction
mixture was incubated at 37˚C for 60 min after brief centrifugation
(14,000 x g for 5 min at 4˚C), followed by incubation at 85˚C for 5
min for RT. qPCR was subsequently performed using the SYBR Premix
EX Taq™ II kit (Takara Bio, Inc.) on an ABI Prism 7500 Real-Time
PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The thermocycling conditions were as follows: Pre-denaturation at
95˚C for 5 min, followed by 40 cycles at 95˚C for 15 sec, 60˚C for
30 sec and 72˚C for 30 sec. The following primer sequences were
used for qPCR: RPLP1, Forward: 5'-TGGCCTGGCTTGTTTGC-3' and reverse:
5'-CTCGGATTCTTCTTTCTTTGCTT-3'; and GAPDH, forward:
5'-CTCCTCCACCTTTGACGCTG-3' and reverse: 5'-TCCTCTTGTGCTCTTGCTGG-3'.
Relative expression levels were calculated using the
2-ΔΔCq method (16) and
normalized to the internal reference gene GAPDH.
Cell Counting Kit-8 (CCK-8) assay
The CCK-8 (Dojindo Molecular Technologies, Inc.)
assay was performed to assess the viability of transfected Hep3b
cells, according to the manufacturer's instructions. Briefly, Hep3b
cells transfected with or without RPLP1 shRNA-1 were seeded into
96-well plates at a density of 2x103 cells/well and
incubated for 0, 24, 48 and 72 h at 37˚C. Following incubation, 10
µl CCK-8 solution was added to each well for another 2 h and cell
viability was analyzed at a wavelength of 450 nm using a microtiter
plate reader (Bio-Rad Laboratories, Inc.).
Colony formation assay
Transfected Hep3b cells were seeded into six-well
plates at a density of 1x103 cells/well and cultured for
4 weeks at 37˚C, without disturbing. Cell colonies were
subsequently fixed with 4% paraformaldehyde for 10 min and stained
with 0.1% crystal violet for 10 min at room temperature. Stained
cells were counted in five randomly selected fields of view by eye
and images were captured.
Western blotting
Total lysates were extracted from transfected Hep3b
cells using RIPA buffer with protease inhibitors cocktail (both
Sigma-Aldrich; Merck KGaA). Protein concentration was measured
using the BCA assay kit (Bio-Rad Laboratories, Inc.) and 25 µg
protein/lane was separated using 10% SDS-PAGE gels. The separated
proteins were subsequently transferred onto PVDF membranes (EMD
Millipore) and blocked with 5% skimmed milk at room temperature for
2 h. The membranes were incubated with primary antibodies against:
Minichromosome maintenance (MCM; cat. no. #3228), proliferating
cell nuclear antigen (PCNA; cat. no. #13110), vimentin (cat. no.
#5741,), snail (cat. no. #3895), slug (cat. no. #9585) (all
1:1,000), β-catenin (1:500; cat. no. #9582), N-cadherin (cat. no.
#13116), E-cadherin (cat. no. #5296) (all 1:500), claudin-1
(1:1,000, #4933, Cell Signaling Technology, Inc.), matrix
metalloproteinase (MMP)-2 (cat. no. #4022,), matrix
metalloproteinase (MMP)-9 (cat. no. #3852), tissue inhibitor of
MMP-1 (TIMP-1; cat. no. #8946) (all 1:1,000) and GAPDH (1:2,000;
cat. no. #5174) (all Cell Signaling Technology, Inc.) overnight at
4˚C. After washing with PBS, the membranes were incubated with
secondary HRP-conjugated goat anti-rabbit IgG (1:5,000; cat. no.
#7074; Cell Signaling Technology, Inc.) at room temperature for 2
h, and then visualized using an ECL chemiluminescence (Pierce;
Thermo Fisher Scientific, Inc.). At last, the gray values of bands
were detected using ImageJ software (version 1.48; National
Institutes of Health) and normalized to GAPDH.
Immunofluorescence
When Hep3b cells were cultured to the appropriate
densities, cells in co-culture plates were fixed with 4%
paraformaldehyde for 10 min at room temperature and subsequently
permeabilized with 0.1% Triton X-100 for 10 min. Following blocking
with 5% BSA and 10% horse serum (Sigma-aldrich; Merck KGaA) in PBST
for 1 h at room temperature, Hep3b cells were incubated with
primary antibody against Ki-67 (1:200; cat. no. #12075; Cell
Signaling Technology, Inc.) overnight at 4˚C. Following the primary
incubation, cells were incubated with Alexa Fluor 488-conjugated
goat anti-rabbit IgG (1:200; cat. no. ab150077; Abcam) for 1 h at
room temperature. Cell nuclei were stained with DAPI for 5 min at
room temperature. Hep3b cells were washed three times with PBST
prior to observation under a light microscope at x200 magnification
and images were captured (Olympus Corporation).
Wound healing assay
Hep3b cells were seeded into six-well plates at a
density of 1x105 cells/well and transfected with or
without shRNA-RPLP1. Once cells reached 100% confluence, the
monolayers were scratched using 200-µl sterile pipette tips to
create straight linear wounds. Cells were cultured in serum-free
medium for 24 h at 37˚C, with 5% CO2. Wounds were
observed in five randomly selected fields using an inverted
fluorescence microscope at 100x magnification and images were
captured. The recovered wound area (%) at the indicated time point
(24 h) was calculated according to the following formula: [(Wound
width at 0 h)-(wound width at 24 h)]/wound width at 0 h.
Transwell assay
Following transfection, Hep3b cells were resuspended
in serum-free medium and plated in the upper chambers of Transwell
plates precoated with Matrigel at 37˚C for 30 min. Complete medium
(600 µl) was plated in the lower chambers. Following incubation for
24 h at 37˚C with 5% CO2, cells in the upper chambers
were gently removed using a wet cotton swab. Cells in the lower
chambers were fixed with 4% paraformaldehyde for 30 min and stained
with 0.1% crystal violet for 30 min, both at room temperature.
Stained cells were counted in five randomly selected fields using
an inverted light microscope and images were captured
(magnification, x200).
Statistical analysis
Statistical analysis was performed using SPSS 13.0
software (SPSS Inc.). All experiments were performed in triplicate
and data are presented as the mean ± standard error of mean (unless
otherwise shown). A two-tailed unpaired Student's t-test was used
to compare differences between two groups, while one-way ANOVA
followed by Dunnett's post hoc tests were used to compare
differences between multiple groups. Survival curves were plotted
using the Kaplan-Meier method and compared using the log-rank test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
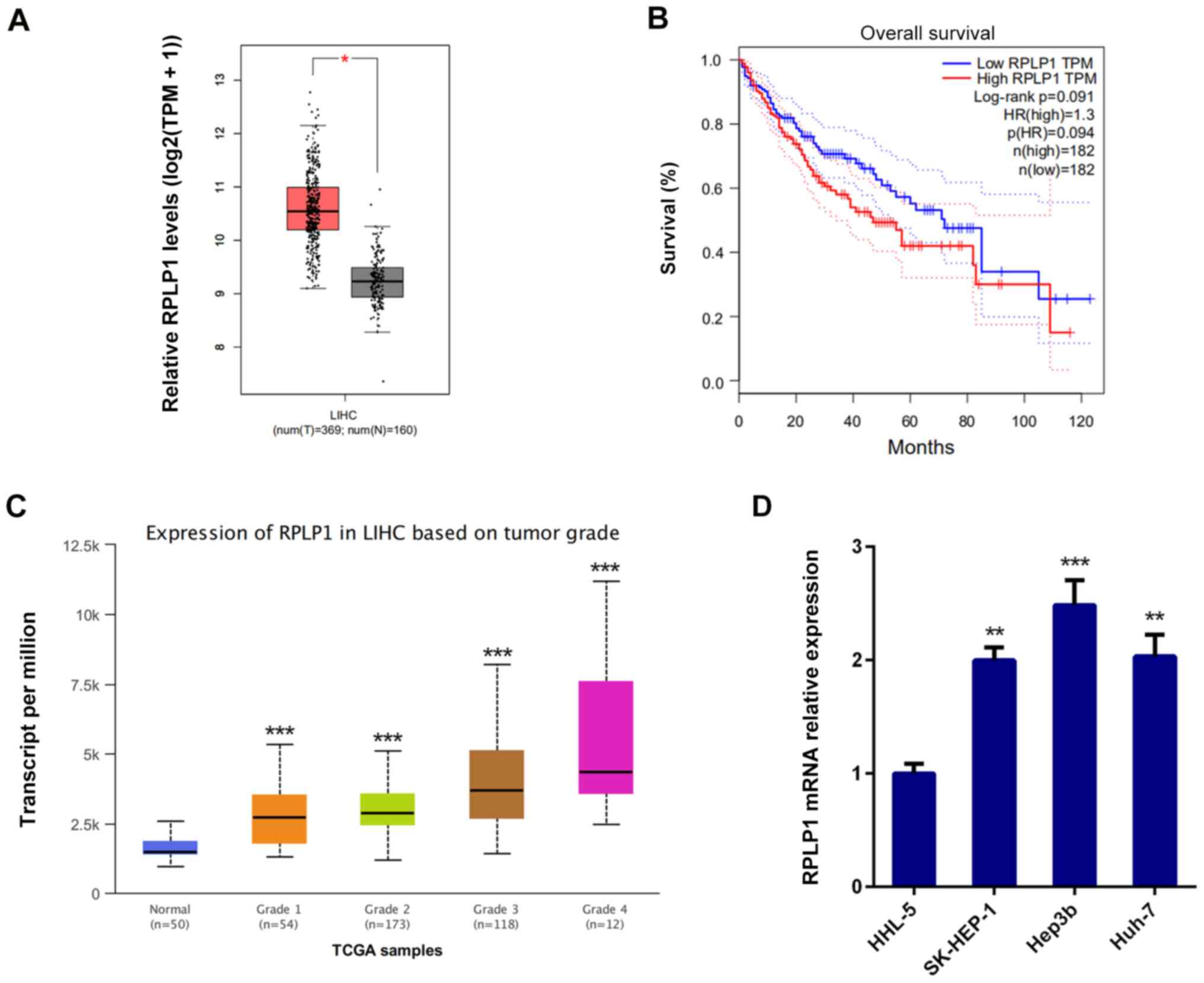
RPLP1 is highly expressed in HCC
tissues and cells, and overexpression of RPLP1 is associated with
the prognosis of patients with HCC
RPLP1 expression was assessed by screening the Gene
Expression Profiling Interactive Analysis database (http://gepia.cancer-pku.cn) to determine the
association between RPLP1 expression and HCC progression. The
results demonstrated that RPLP1 was highly expressed in HCC tissues
compared with adjacent liver tissues (Fig. 1A) (529 cases;
P=1.6x10-12).
To further investigate the role of RPLP1 in HCC
progression, the overall survival (OS) datasets was analyzed by
GEPIA database (http://gepia.cancer-pku.cn/). As presented in Fig. 1B, high RPLP1 expression was
associated with poor prognosis and high mortality, and the OS rate
(log-rank P=0.091; hazard ratio=1.3) were less favorable in
patients with high RPLP1 expression levels.
The UALCAN database (http://ualcan.path.uab.edu) was used to determine the
association between RPLP1 expression and tumor grade of HCC. As
presented in Fig. 1C, RPLP1
expression was positively associated with tumor grade. Taken
together, these results suggested that RPLP1 may promote HCC
development. In addition, RT-qPCR analysis was performed to detect
RPLP1 expression in human liver HHL-5 cells and the HCC cell lines,
SK-HEP-1, Hep3b and Huh-7. The results demonstrated that RPLP1 was
highly expressed in the HCC cell lines compared with normal liver
cells, and the most significant overexpression was observed in
Hep3b cells (Fig. 1D). Thus, Hep3b
cells were selected for subsequent experiments.
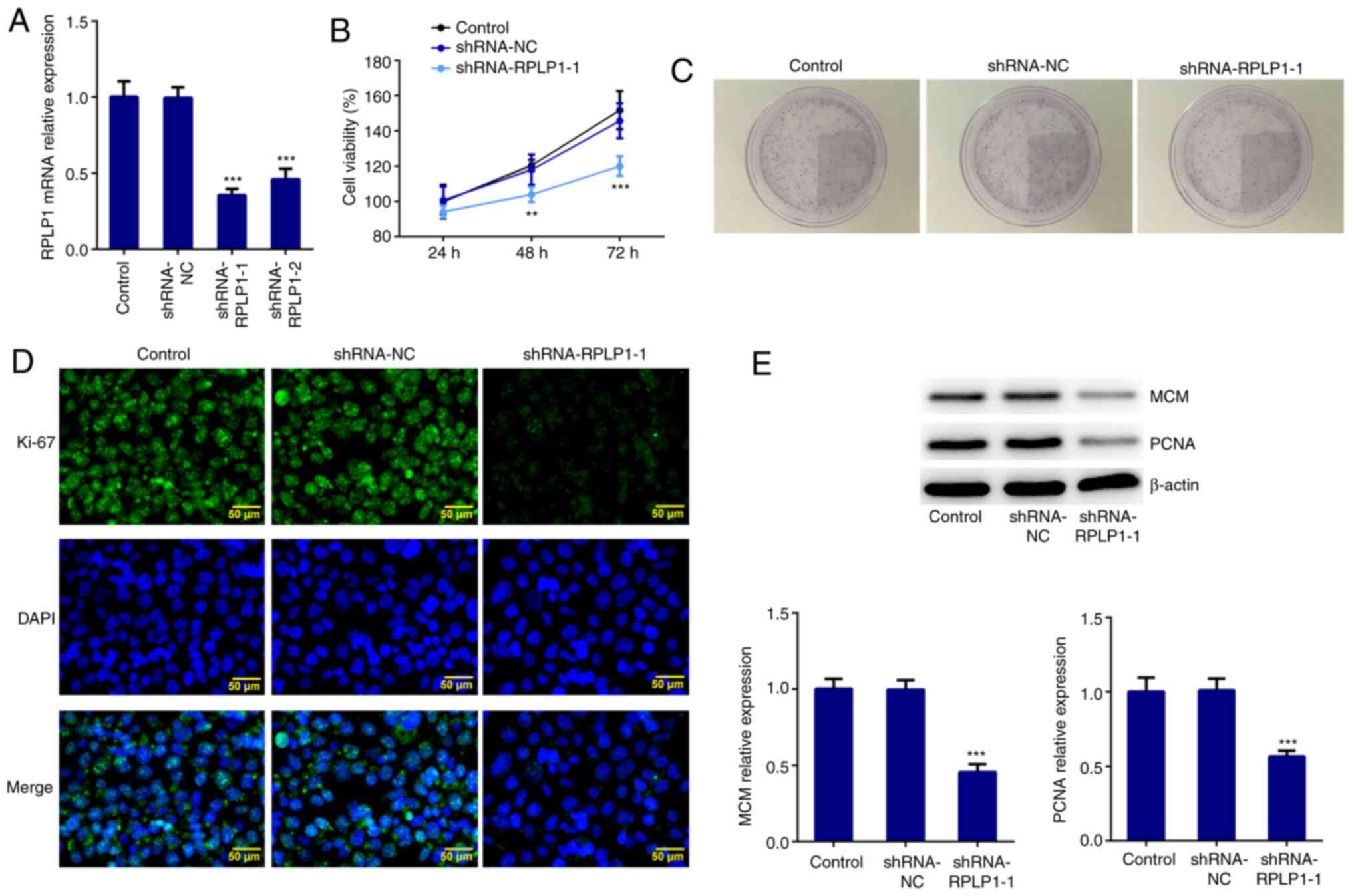
Downregulation of RPLP1 inhibits the
proliferation of Hep3b cells
RPLP1 expression was downregulated using
shRNA-RPLP1-1, shRNA-RPLP1-2 and shRNA-NC. Due to the low
expression level induced by shRNA-RPLP1-1 (Fig. 2A), this shRNA was selected for
subsequent experimentation. The results of the CCK-8 assay
demonstrated that downregulation of RPLP1 significantly suppressed
Hep3b cell viability (Fig. 2B). The
results of the colony formation assay revealed that the
proliferation rate of Hep3b cells transfected with shRNA-RPLP1-1
markedly decreased compared with the control and shRNA-NC groups
(Fig. 2C). Furthermore, the
expression levels of the standard markers of proliferation, Ki-67,
PCNA and MCM (17) were assessed
via western blotting and immunofluorescence. The results
demonstrated that the expression levels of PCNA, MCM and Ki-67
decreased in the shRNA-RPLP1-1 group compared with the control and
shRNA-NC groups (Fig. 2D and
E). Taken together, these results
suggested that downregulation of RPLP1 suppresses the proliferation
of Hep3b cells.
Downregulation of RPLP1 affects the
expression of epithelial-to-mesenchymal (EMT)-associated
proteins
To further investigate the effect of RPLP1 on
cellular biological behaviors, proteins related to EMT were
detected via western blot analysis. The results demonstrated that
downregulation of RPLP1 significantly decreased the expression
levels of vimentin, snail and slug, and increased β-catenin
expression (Fig. 3A). In addition,
N-cadherin expression (mesenchymal cell marker) (18) notably decreased following
downregulation of RPLP1 expression, while the expression levels of
E-cadherin (epithelial cell marker) (18) and claudin-1 significantly increased
following downregulation of RPLP1 (Fig.
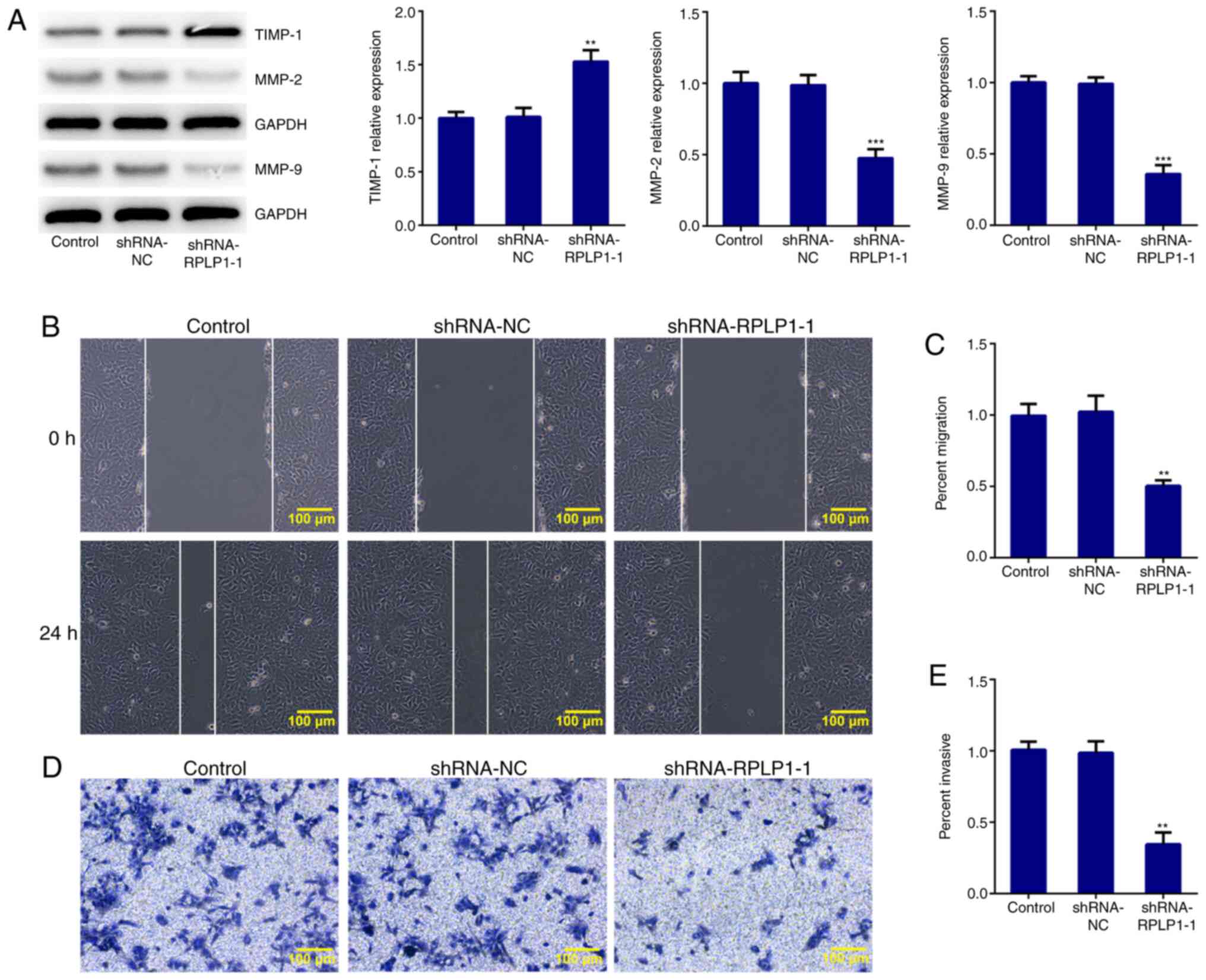
3B). Downregulation of RPLP1 also suppressed the expression
levels of MMP-2 and MMP-9, and notably increased TIMP-1 expression
(Fig. 4A). These results implied
that downregulation of RPLP1 inhibited the EMT process of Hep3b
cells.
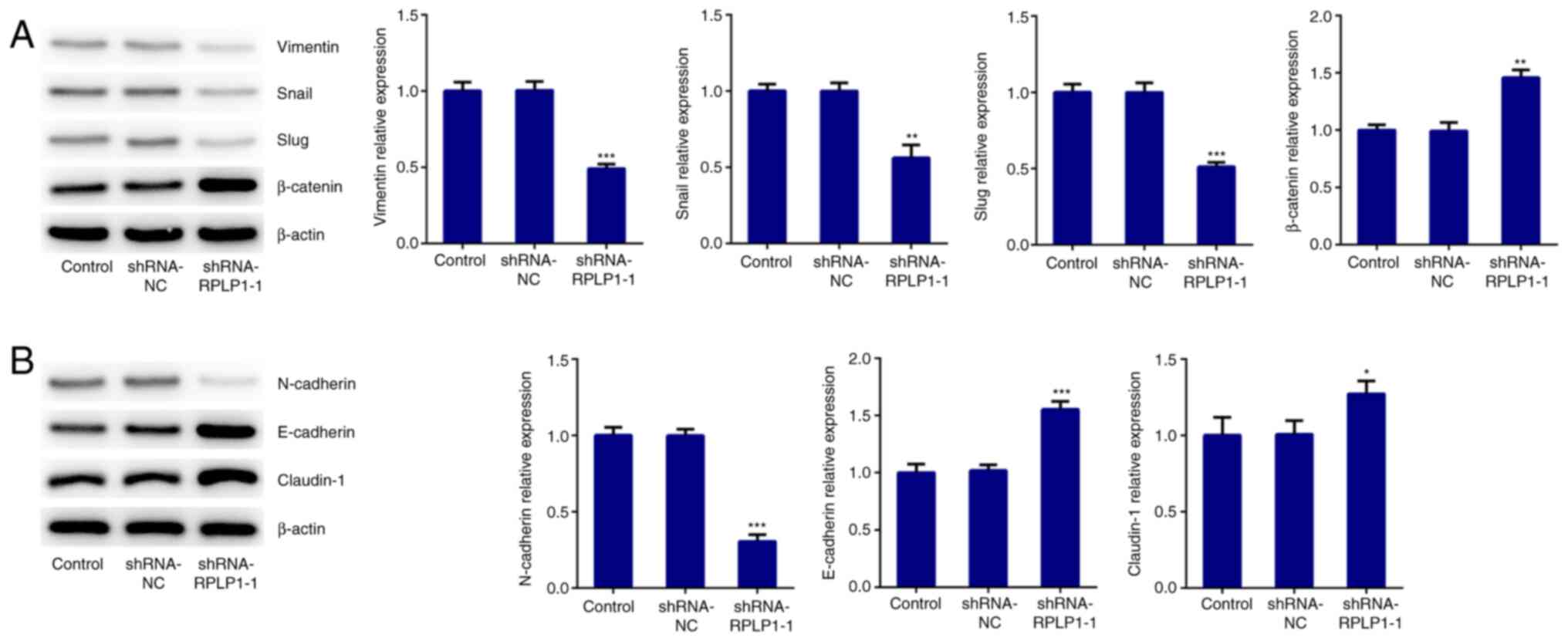 | Figure 3Downregulation of RPLP1 suppresses the
expression levels of EMT-associated proteins in Hep3b cells. (A and
B) Western blot analysis was performed to detect the protein
expression levels of vimentin, snail, slug, β-catenin, N-cadherin,
E-cadherin and claudin-1. *P<0.05,
**P<0.01 and ***P<0.001 vs. control.
RPLP1, ribosomal protein LP1; EMT, epithelial-to-mesenchymal
transition; sh, short hairpin; NC, negative control. |
Downregulation of RPLP1 suppresses the
migration and invasion of Hep3b cells
The wound healing and Transwell assays were
performed to confirm the effect of RPLP1 downregulation on the
migratory and invasive abilities of Hep3b cells. As presented in
Fig. 4B-E, the migratory and
invasive abilities of Hep3b cells were significantly inhibited
following downregulation of RPLP1. Taken together, these results
suggested that downregulation of RPLP1 inhibits the potency of
invasion and migration of Hep3b cells.
Discussion
HCC is the sixth most common primary malignant
tumor, which poses a great threat to human health and life, with
~800,000 mortalities annually worldwide (19,20).
Increasing evidence suggests that several biomarkers can be used to
predict cancer progression and are associated with the prognosis of
patients with different types of cancer (21,22).
Notably, the results of the present study demonstrated that RPLP1
expression was upregulated in HCC tissues compared with adjacent
liver tissues and was significantly associated with less favorable
prognosis of patients with HCC. In addition, the results
demonstrated an association between RPLP1 expression and HCC
pathogenesis, which merits further investigation for use of RPLP1
as a potential target for HCC treatment.
High potency of invasion and migration of cancer
cells promotes cancer progression and is associated with poor
prognosis of patients with cancer (23,24). A
previous study reported that overexpression of RPLP1 promoted the
proliferation, migration and invasion of cervical cancer cells
(25). The results of the present
study demonstrated that RPLP1 was highly expressed in HCC cell
lines, which is consistent with the previous study. The
postoperative quality of life and prognosis of patients with HCC is
restrained by the strong invasion and migration potential of tumor
cell (26). Thus, the
proliferative, migratory and invasive abilities of Hep3b cells were
assessed in the present study. RPLP1 expression was downregulated
following transfection of Hep3b cells with shRNA-RPLP1. Ki67, PCNA
and MCM proteins are the standard proliferative markers that are
commonly applied to analyze the proliferative activity of a cell
population (27). The results of
the present study demonstrated that downregulation of RPLP1
significantly decreased the expression levels of Ki67, PCNA and
MCM, and suppressed the viability and proliferation of Hep3b cells.
Collectively, these results suggested that downregulation of RPLP1
inhibits the proliferation of HCC cells. In addition, the
expression levels of the EMT-related proteins, and the migratory
and invasive abilities of Hep3b cells, were assessed by a series of
functional experiments. The loss of epithelial marker, E-cadherin,
accompanied by the gain of the key mesenchymal markers, N-cadherin
and vimentin, suggests that the cells underwent EMT (28). As a component of the cadherin
complex, β-catenin plays a key role in localization (29). During the process of EMT, β-catenin
dissociates from the cadherin complex and is translocated into the
nucleus where it functions as a transcription factor, which
regulates the expression of several genes associated with cancer
metastasis including Wnt and p53(29). In addition, MMP-2 and MMP-9
expression are associated with tumor invasion and metastasis of
malignant tumors (30). In the
present study, RPLP1 silencing decreased the expression levels of
vimentin, snail, slug, N-cadherin, MMP-2 and MMP-9, and increased
the expression levels of β-catenin, E-cadherin, claudin-1 and
TIMP-1, suggesting that RPLP1 may contribute to the induction of
EMT. Hence, increased RPLP1 expression may promote HCC metastasis
via regulation of EMT and enhanced cell motility. Moreover, the
results of the wound healing and Transwell assays demonstrated that
RPLP1 silencing remarkably suppressed the migratory and invasive
abilities of Hep3b cells. Taken together, these results suggested
that downregulation of RPLP1 significantly inhibits the
proliferation, migration and invasion of Hep3b cells. Thus, RPLP1
may play a key role in HCC occurrence, progression, invasion and
metastasis and so may be used as a target for HCC treatment.
A previous study reported that the RPLP protein
deficiency resulted in reactive oxygen species accumulation and
MAPK1/ERK2 signaling pathway activation in colon cancer cells
(11). However, the detailed
mechanism underlying the role of RPLP remains unclear. Hence,
future studies will be performed to further investigate the role of
RPLP1 in HCC progression.
In conclusion, the results of the present study
demonstrated that RPLP1 was highly expressed in HCC tissues and
cells, and overexpression of RPLP1 was associated with the
prognosis of patients with HCC. Notably, downregulation of RPLP1
suppressed the proliferation, migration and invasion of Hep3b
cells. Collectively, the results of the present study suggested
that RPLP1 acts as an oncogene in HCC, and thus may be used to
treat patients with HCC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CX and LQ designed the experiment and drafted the
manuscript. CX, KC and DP performed the experiments and analyzed
the data. CX and LQ reviewed the manuscript. All authors read and
approved the final manuscript. CX and LQ confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xiao JX, Xu W, Fei X, Hao F, Wang N, Chen
Y and Wang J: Anillin facilitates cell proliferation and induces
tumor growth of hepatocellular carcinoma via miR-138/SOX4 axis
regulation. Transl Oncol. 13(100815)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Vilgrain V, Pereira H, Assenat E, Guiu B,
Ilonca AD, Pageaux GP, Sibert A, Bouattour M, Lebtahi R, Allaham W,
et al: Efficacy and safety of selective internal radiotherapy with
yttrium-90 resin microspheres compared with sorafenib in locally
advanced and inoperable hepatocellular carcinoma (SARAH): An
open-label randomised controlled phase 3 trial. Lancet Oncol.
18:1624–1636. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Coskun M: Hepatocellular carcinoma in the
cirrhotic liver: Evaluation using computed tomography and magnetic
resonance imaging. Exp Clin Transplant. 15 (Suppl 2):S36–S44.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Granito A and Bolondi L: Non-transplant
therapies for patients with hepatocellular carcinoma and
Child-Pugh-Turcotte class B cirrhosis. Lancet Oncol. 18:e101–e112.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ghouri YA, Mian I and Rowe JH: Review of
hepatocellular carcinoma: Epidemiology, etiology, and
carcinogenesis. J Carcinog. 16(1)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mahtab MA, Chaudhury M, Uddin MH, Noor
EASM, Rahim MA, Alam MA, Moben AL, Khondaker FA, Choudhury MF,
Sarkar MJ, et al: Cost assessment of Hepatitis B virus-related
hepatitis in Bangladesh. Euroasian J Hepatogastroenterol.
6:163–166. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sarma MP, Bhattacharjee M, Kar P and Medhi
S: Detection of HBV genotype C in hepatocellular carcinoma patients
from north east India: A brief report. Asian Pac J Cancer Prev.
19:1741–1746. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Reig M, Mariño Z, Perelló C, Iñarrairaegui
M, Ribeiro A, Lens S, Díaz A, Vilana R, Darnell A, Varela M, et al:
Unexpected high rate of early tumor recurrence in patients with
HCV-related HCC undergoing interferon-free therapy. J Hepatol.
65:719–726. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dituri F, Mancarella S, Cigliano A, Chieti
A and Giannelli G: TGF-β as multifaceted orchestrator in HCC
progression: Signaling, EMT, immune microenvironment, and novel
therapeutic perspectives. Semin Liver Dis. 39:53–69.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Artero-Castro A, Kondoh H,
Fernandez-Marcos PJ, Serrano M, Ramon y Cajal S and Lleonart ME:
Rplp1 bypasses replicative senescence and contributes to
transformation. Exp Cell Res. 315:1372–1383. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Artero-Castro A, Perez-Alea M, Feliciano
A, Leal JA, Genestar M, Castellvi J, Peg V, Ramon y Cajal S and
Lleonart ME: Disruption of the ribosomal P complex leads to
stress-induced autophagy. Autophagy. 11:1499–1519. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Campos RK, Wong B, Xie X, Lu YF, Shi PY,
Pompon J, Garcia-Blanco MA and Bradrick SS: RPLP1 and RPLP2 are
essential flavivirus host factors that promote early viral protein
accumulation. J Virol. 91(e01706)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
He Z, Xu Q, Wang X, Wang J, Mu X, Cai Y,
Qian Y, Shao W and Shao Z: RPLP1 promotes tumor metastasis and is
associated with a poor prognosis in triple-negative breast cancer
patients. Cancer Cell Int. 18(170)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Artero-Castro A, Castellvi J, Garcia A,
Hernandez J, Ramon y Cajal S and Lleonart ME: Expression of the
ribosomal proteins Rplp0, Rplp1, and Rplp2 in gynecologic tumors.
Hum Pathol. 42:194–203. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Roels S, Tilmant K and Ducatelle R: PCNA
and Ki67 proliferation markers as criteria for prediction of
clinical behaviour of melanocytic tumours in cats and dogs. J Comp
Pathol. 121:13–24. 1999.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang H, Wang Y and Ding H: COL4A1,
negatively regulated by XPD and miR-29a-3p, promotes cell
proliferation, migration, invasion and epithelial-mesenchymal
transition in liver cancer cells. Clin Transl Oncol: Apr 23, 2021
(Epub ahead of print).
|
|
19
|
Beudeker BJB and Boonstra A: Circulating
biomarkers for early detection of hepatocellular carcinoma. Therap
Adv Gastroenterol. 13(1756284820931734)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yang JD, Hainaut P, Gores GJ, Amadou A,
Plymoth A and Roberts LR: A global view of hepatocellular
carcinoma: Trends, risk, prevention and management. Nat Rev
Gastroenterol Hepatol. 16:589–604. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Leiphrakpam PD, Lazenby AJ, Chowdhury S,
Smith LM, Mathiesen M, Brattain MG, Wang J, Black JD and Are C:
Prognostic and therapeutic implications of NHERF1 expression and
regulation in colorectal cancer. J Surg Oncol. 121:545–560.
2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang XJ, Yu Q, Chi P, Lin HM, Lu XR, Huang
Y, Xu ZB, Huang SH, Sun YW and Ye DX: Identification of gene
biomarkers to predict responses to neoadjuvant chemoradiotherapy in
patients with rectal cancer and pathways enrichment analysis.
Zhonghua Wei Chang Wai Ke Za Zhi. 22:1183–1187. 2019.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
23
|
Liu YP, Chen WD, Li WN and Zhang M:
Overexpression of FNDC1 relates to poor prognosis and its knockdown
impairs cell invasion and migration in gastric cancer. Technol
Cancer Res Treat. 18(1533033819869928)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yu ZH, Wang YM, Jiang YZ, Ma SJ, Zhong Q,
Wan YY and Wang XW: NID2 can serve as a potential prognosis
prediction biomarker and promotes the invasion and migration of
gastric cancer. Pathol Res Pract. 215(152553)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xia L, Yue Y, Li M, Zhang YN, Zhao L, Lu
W, Wang X and Xie X: CNN3 acts as a potential oncogene in cervical
cancer by affecting RPLP1 mRNA expression. Sci Rep.
10(2427)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang G and Zhang G: Upregulation of FoxP4
in HCC promotes migration and invasion through regulation of EMT.
Oncol Lett. 17:3944–3951. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Juríková M, Danihel Ľ, Polák S and Varga
I: Ki67, PCNA, and MCM proteins: Markers of proliferation in the
diagnosis of breast cancer. Acta Histochem. 118:544–552.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Serrano-Gomez SJ, Maziveyi M and Alahari
SK: Regulation of epithelial-mesenchymal transition through
epigenetic and post-translational modifications. Mol Cancer.
15(18)2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tafrihi M and Nakhaei Sistani R:
E-Cadherin/β-catenin complex: A target for anticancer and
antimetastasis plants/plant-derived compounds. Nutr Cancer.
69:702–722. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bjorklund M and Koivunen E:
Gelatinase-mediated migration and invasion of cancer cells. Biochim
Biophys Acta. 1755:37–69. 2005.PubMed/NCBI View Article : Google Scholar
|