Introduction
Gestational diabetes mellitus (GDM) is defined as
hyperglycaemia that is initially diagnosed during pregnancy
(1). The prevalence of GDM in
developed countries is 2-10% of all pregnancies and its incidence
rate continues to rise annually (2). GDM is known to significantly increase
the incidence of macrosomia, dystocia and operative delivery, and
is closely associated with various adverse pregnancy outcomes and
long-term health risks, such as miscarriage, foetal malformations,
intrauterine death and development of type 2 diabetes in the mother
and child (3-5).
Bone morphogenetic protein-4 (BMP-4) is a member of
the transforming growth factor-β superfamily that plays important
roles in embryonic development, angiogenesis and chondrogenesis
(6-9).
In various types of tissue, BMP-4 signalling has been associated
with regulatory processes, such as cell development,
differentiation, proliferation and apoptosis. Previous studies have
found that hyperglycaemia stimulates the synthesis and release of
BMP-4 in the vascular endothelium (10,11).
BMP-4 acts as an autocrine and paracrine ligand to its receptor, a
serine/threonine kinase receptor classified as types 1 and 2.
Specifically, BMP-4 has been demonstrated to exhibit high affinity
for type 1 receptors (8). After
binding, activation of Nox1-dependent reduced nicotinamide adenine
dinucleotide phosphate (NADPH) oxidase (NOX-1) stimulates the
synthesis of reactive oxygen species (ROS) (12,13).
Increased intracellular ROS upregulates cyclooxygenase-2 (COX-2)
expression via a p38 mitogen-activated protein kinase
(MAPK)-dependent mechanism, as well as releasing prostaglandin 2α
(PGF2α), thereby causing endothelium-dependent vasoconstriction and
blocking endothelium-dependent vasodilation (14-17).
Additionally, the increase in BMP-4 activates the expression of
vascular cell adhesion molecule 1 (VCAM-1), which induces vascular
endothelial inflammation and subsequent exacerbation of vascular
endothelial dysfunction, thereby causing systemic vasoconstriction
and multi-organ ischaemia, which results in elevated blood pressure
and multiple organ damage (14,18).
Preeclampsia is the main cause of maternal and
foetal morbidity and mortality (19) and affects 2-7% of pregnancies in
non-diabetic women in developed countries (20,21).
GDM further increases the risk of preeclampsia. The aetiology of
preeclampsia remains elusive. However, GDM and preeclampsia share
many risk factors (22). It is
unclear whether a common aetiology underlies GDM and preeclampsia;
however, various studies suggest that endothelial dysfunction
occurs in GDM, as well as in preeclampsia (23-25).
Therefore, the present study hypothesized that BMP-4
is involved in GDM-related hypertension through impairment of
endothelial function. The results indicated that the
BMP-4/NOX-1/COX-2 signalling pathway may be involved in GDM-related
hypertension, but that VCAM-1 may be substantially associated with
GDM-related hypertension. Furthermore, overexpression of BMP-4
could lead to hypertension by impairing endothelial function in
pregnancy.
Materials and methods
Establishing an animal model of
GDM
The experimental protocol was approved by the
Institutional Review Board of Shengjing Hospital of China Medical
University (Shenyang, China). All the experiments were conducted
according to the institutional guidelines for the humane treatment
of laboratory animals. In the present study, a high-fat diet
combined with intraperitoneal injection of streptozotocin (STZ; an
antibiotic that produces pancreatic islet β-cell destruction) was
employed to generate a rat model of GDM (26-28).
A total of 70 (10 male and 60 female) Sprague-Dawley rats aged 9-10
weeks were purchased from Changsheng Biotechnology Co., Ltd. The
mean weight of the rats was 301±52 g. All the animals were
specific-pathogen free, housed at 21-22˚C, under a relative
humidity of 60-70% and a 12-h light/dark cycle. The female rats
were randomly divided into two groups (30 rats per group), namely a
high-fat diet group and a normal diet group. The high-fat diet
group was fed a high-fat diet (Huafukang Medical Technology Co.,
Ltd.) with a daily fat content of 60%, whereas the normal diet
group was fed a normal diet. All rats had free access to food and
autoclaved water.
After one month, female and male rats were placed in
cages at a 2:1 ratio and the vaginal plug or vaginal secretion
smear of the female rats was examined the next morning. The day on
which sperm or vaginal plugs were observed in the rats was recorded
as pregnancy day 0. Following the detection of pregnancy in the
high-fat diet group, rats were starved for 12 h with provision of
water ad libitum and then administered with an
intraperitoneal injection of STZ (cat. no. S0130; MilliporeSigma)
at a dosage of 30 mg/kg body weight (dissolved in 0.1 mmol/l sodium
citrate, ready to use). The rats were then fed the high-fat diet.
One week later, 100 µl blood from each rat was collected through
the tail vein to evaluate fasting blood glucose and non-fasting
blood glucose; measurements were conducted three times separately.
Rats with ≥120 mg/dl fasting blood glucose and at the same time,
≥300 mg/dl non-fasting blood glucose were diagnosed with GDM
(29-32);
measurements were conducted three times separately. The normal diet
group continued to receive the normal diet.
Monitoring blood pressure
After confirming pregnancy, the blood pressure of
each rat was monitored every week using the tail-cuff method (using
a Softron BP-98A and a Softron TMC-203). Three days before the
formal measurement, the blood pressure was measured at a fixed time
every day for adaptation to measurement conditions. The first three
measurements were not recorded as a formal measurement and the
measurement commenced only after the rats were stabilized. Blood
pressure in each rat was measured three times and the mean value
was recorded (14). Tail pressure
was monitored every week until pregnancy day 20, as the average
gestational period of rats is 21 days. Currently, no uniform
standards exist for normal blood pressure and hypertension in
pregnant rats. In the present study, hypertension was defined as
systolic blood pressure elevated by >20 mmHg compared with the
initial measurement (33-35).
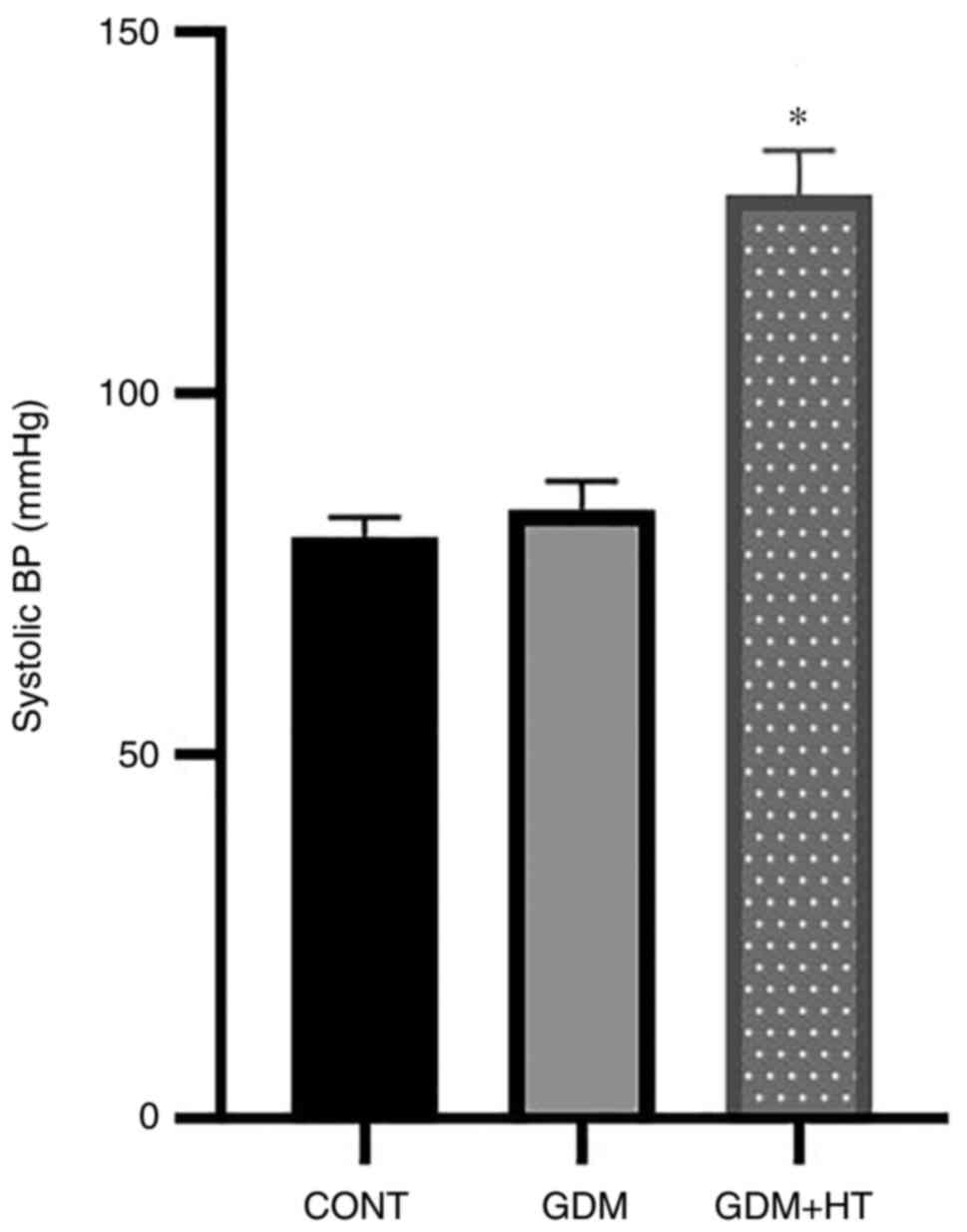
Based on the blood pressure values, rats with GDM were divided into
a GDM group (GDM) and a GDM with hypertension group (GDM + HT). The
blood pressure measurements were performed repeatedly to reduce
error. The normal diet group served as the control group (CONT;
Fig. 1).
Immunohistochemistry
Rats in all three groups were euthanized by
exsanguination via abdominal aorta after anaesthetising by
isoflurane inhalation (2.5-3.5% for the induction phase and
2.5-3.0% for the maintenance phase) on pregnancy day 20. Successful
euthanasia was confirmed via respiratory and cardiac arrest.
Following euthanasia, the abdominal aorta was collected
immediately.
During sample collection, care was taken to avoid
damage to the vascular endothelium. The tissue samples from the
three groups of rats were fixed in 10% formaldehyde and ethanol at
25˚C for 24 h. Following dehydration and paraffin embedding, 4-µm
serial sections were obtained. After heating of paraffin-embedded
sections in a 60˚C incubator for 0.5-1 h, sections were
deparaffinized with xylene at 25˚C, rehydrated in a concentration
descending ethanol series and immersed in endogenous peroxidase
blocking buffer for 10 min. Next, antigen retrieval was performed
using high-pressure antigen retrieval. Diluted primary antibodies,
BMP-4 (cat. no. ab39973; 1:100), COX-2 (cat. no. ab15191; 1:2,000),
VCAM-1 (cat. no. ab134047; 1:500) and NOX-1 (cat. no. ab131088;
1:1,000; all obtained from Abcam) were added and slides were
incubated overnight at 4˚C. PBS treatment served as a negative
control. Slides were re-heated at 37˚C for 20 min and rinsed three
times with PBS solution. The reaction enhancer was added and slides
were incubated at 25˚C for 20 min and rinsed three times with PBS
solution. The horseradish enzyme labelled goat anti-rabbit IgG
(cat. no. ZB-2301; 1:5,000; OriGene Technologies, Inc.) was added
and slides were incubated at 25˚C for 20 min. Slides were then
rinsed three times with PBS solution. Subsequently,
3,3'-diaminobenzidine was used for colour development. Slides were
rinsed with running water and haematoxylin was used for
counterstaining of nuclei, while hydrochloric acid and ethanol were
used for differentiation. Finally, slides were rinsed with running
water, dehydrated and dried using an ethanol gradient, cleared with
xylene and mounted with neutral gum.
Scoring criteria
The BMP-4, NOX-1, COX-2 and VCAM-1 proteins are
located primarily in the cytoplasm of vascular endothelial cells
and positive expression is indicated by brown or tan colouration in
immunohistochemistry. Five high-magnification (x400) fields of view
were randomly selected for each slide, and the staining intensity
and number of positive cells were observed. A score was assigned
based on the staining intensity as follows: 0, No staining; 1,
light yellow; 2, brown; 3, tan. Another score was assigned based on
the percentage of positive cells as follows: 0, All cells negative;
1, 1-10% positive cells; 2, 11-50% positive cells; 3, 51-75%
positive cells; and 4, >76% positive cells. A positive immune
reaction was defined as a product of the staining intensity score
and the percentage of positive cells >3.
Western blotting
Rat aortas were harvested after the indicated
treatment. Protein was extracted using 300 µl lysis buffer (cat.
no. WLA019a; Wanleibio Co., Ltd.) supplemented with protease and
phosphatase inhibitors. BCA method was used to determine the
concentration of the protein and 50 µg protein was loaded per lane.
Western blotting was performed according to standard methods:
Protein separation by 10% gel electrophoresis, transferring the
protein from the gel onto polyvinylidene fluoride membranes
(Bio-Rad Laboratories, Inc.), the membranes were blocked using 3%
bovine serum albumin (cat. no. B2064; Sigma-Aldrich; Merck KGaA) at
25˚C for 1 h, and then were probed with BMP-4, COX-2, VCAM-1 and
NOX-1 antibodies (dilution of all the antibodies was 1:1,000),
β-actin antibody (cat. no. sc-1616; Santa Cruz Biotechnology, Inc.;
1:1,000) was used for the reference protein, the membranes were
incubated overnight at 4˚C, then the goat anti-rabbit IgG (cat. no.
sc-2004; Santa Cruz Biotechnology, Inc.; 1:1,000) was added in the
membranes and then the membranes were incubated at 25˚C for 1 h,
then an enhanced chemiluminescence method using chemiluminescent
substrate (cat. no. 34577; Thermo Fisher Scientific, Inc.) was
performed. The molecular weight of each protein shown on the
immunoblot was estimated based on the Rainbow 245 Marker for
Western Blotting Protein Standard (Beijing Solarbio Science &
Technology Co., Ltd.) on a 10% SDS-PAGE gel. Quantification was
performed based on the density of bands obtained using ImageJ
v1.8.0 Software (National Institutes of Health). β-actin served as
the loading control.
Assessment of blood pressure in rats
infused with BMP-4
Following fertilization, female rats that were fed
with a normal diet were randomly divided into four groups (n=4 rats
per group) designated as A, B, C and D. Blood pressure was measured
using the tail-cuff method on the day pregnancy was discovered and
recorded as the initial blood pressure. In order to evaluate
whether BMP-4 affected the blood pressure of pregnant rats, osmotic
minipumps (Alzet®Osmotic Pumps; Durect Corp.) were
implanted using 1% sodium pentobarbital as the anaesthetic (3 ml/kg
via intraperitoneal injection) on the third day of pregnancy. The
solution was composed of an infusion vehicle, recombinant human
BMP-4 (cat. no. 314-BP-050; R&D Systems, Inc.) or recombinant
human noggin (cat. no. 6057-NG-100; R&D Systems, Inc.)
supplemented with 0.1% bovine serum albumin (cat. no. A8020,
Solarbio) in 4 mmol/l HCl. Four groups were evaluated as follows:
Group A was infused with 0.45 mg/kg BMP-4 + vehicle, group B was
infused with 0.45 mg/kg noggin + vehicle, group C was infused with
0.45 mg/kg BMP-4 + vehicle and 0.45 mg/kg noggin + vehicle, two
osmotic minipumps were used and implanted at different sites for
infusion to avoid mixing, and group D was infused with vehicle
alone and served as a control (14). These four groups of rats were
administered with infusions continuously for 2 weeks. Blood
pressure was monitored and recorded again on pregnancy day 18 and
the changes in blood pressure among the four groups were
compared.
Vascular reactivity testing
Rats in all four groups were euthanised by
exsanguination via abdominal aorta after anaesthetising by 2.5-3.0%
isoflurane inhalation on pregnancy day 20. Following euthanasia,
the thoracic aorta was collected immediately for vascular
reactivity testing. During sample collection, care was taken to
avoid damage to the vascular endothelium. After collection, the
aorta was immediately placed in a petri dish containing
Krebs-Henseleit (KH) solution (cat. no. PB180348; Procell Life
Science & Technology Co., Ltd.) at 37˚C. A mixture of 95%
oxygen + 5% carbon dioxide gas was continuously injected into the
KH solution. The fatty tissue on the surface of the blood vessels
was removed and blood vessels were cut into rings (length, 4 mm).
Two L-shaped stainless-steel hooks were passed through the lumen,
with one end fixed and the other end connected to the tension
transducer of a physiological recorder (PL3508 PowerLab 8/35;
ADInstruments, Ltd.). Blood vessels were immersed in the KH
solution and the physiological recorder was adjusted to apply a
baseline tension of 1 g to the blood vessels to simulate
physiological tension. The tension was equilibrated for 60 min,
during which the KH solution was replaced every 15 min. After
stabilisation of the tension, 10-5 mol/l phenylephrine
was added to the container, and 10-9-10-6
mol/l acetylcholine was added after vasoconstriction was
equilibrated. The time interval between additions of different
concentrations of acetylcholine was 2 min. The rings were
continuously aerated while phenylephrine and acetylcholine were
added. The relaxation of the thoracic aorta vascular ring of each
group of rats was recorded.
Statistical analysis
SPSS 23.0 software (IBM Corp.) was used for
statistical analysis and data are expressed as means ± standard
error. Student's t-test was used for comparisons between two groups
and one-way analysis of variance was used for comparisons between
multiple groups. Tukey's post hoc test was performed for pairwise
comparisons between groups. The χ2 test or Fisher's
exact probability test was performed for comparisons of countable
data. P<0.05 was considered to indicate a statistically
significant difference.
Results
Vascular endothelium in GDM rats shows
higher positive expression rates of VCAM-1
As shown in Fig. 2,
the positive expression rates of BMP-4 in the GDM, GDM + HT and
control groups were 100% (11/11), 100% (4/4) and 72.7% (8/11),
respectively. The positive expression rates of NOX-1 in the GDM,
GDM + HT and control groups were 100% (11/11), 100% (4/4) and 81.8%
(9/11), respectively. The positive expression rates of COX-2 in the
GDM, GDM + HT and control groups were 100% (11/11), 100% (4/4) and
72.7% (8/11), respectively. The positive expression rates of VCAM-1
in the GDM, GDM + HT and control groups were 100% (11/11), 100%
(4/4) and 10% (1/10), respectively. The expression levels of all
four proteins in the control group were lower than those in the GDM
and GDM + HT groups. The positive expression rate of VCAM-1 in the
control group was significantly lower than that in the GDM and GDM
+ HT groups; the differences were statistically significant
(χ2=17.325, P<0.05 and χ2=10.080,
P<0.05, respectively).
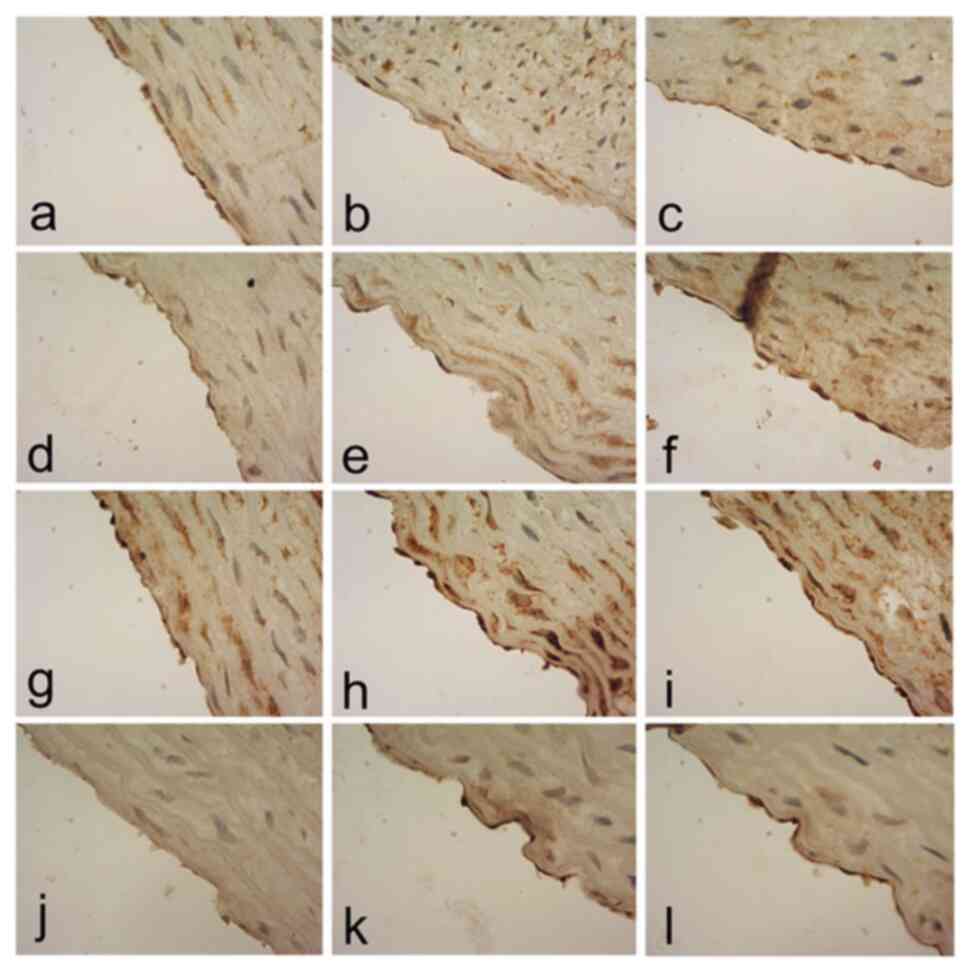 | Figure 2Immunohistochemistry of BMP-4, COX-2,
NOX-1 and VCAM-1 expression in the vascular endothelium of rats in
CONT group (n=11), GDM group (n=11) and GDM+HT group (n=4). Panels
a, b and c demonstrate the expression of BMP-4 in the CONT, GDM and
GDM + HT groups, respectively. Panels d, e and f demonstrate the
expression of COX-2 in the CONT, GDM, and GDM + HT groups,
respectively. Panels g, h and i demonstrate the expression of NOX-1
in the CONT, GDM and GDM + HT groups, respectively. Panels j, k and
l demonstrate the expression of VCAM-1 in the CONT, GDM and GDM +
HT groups, respectively. Magnification, x400. BMP-4, bone
morphogenetic protein-4; COX-2, cyclooxygenase-2; NOX-1,
nicotinamide adenine dinucleotide phosphate oxidase 1; VCAM-1,
vascular cell adhesion molecule 1; CONT, control; GDM, gestational
diabetes mellitus; HT, hypertension. |
The expression levels of BMP-4, COX-2
and VCAM-1 proteins in rats with GDM related hypertension are
higher compared with those without hypertension
The expression level of the BMP-4 protein in the
control, GDM and GDM + HT groups exhibited a sequentially
increasing tendency; however, the difference observed among the
groups was not statistically significant (F=2.651, P=0.13).
Similarly, the difference in the expression levels of the NOX-1
protein among the three groups was not statistically significant
(F=2.122, P=0.18). By contrast, the expression levels of COX-2
protein in the three groups exhibited a sequential increase and the
difference was observed to be statistically significant (F=8.560,
P<0.05). The difference in the expression levels of the COX-2
protein between the control (1.000±0.255) and the GDM (1.434±0.344)
groups was not identified to be statistically significant (P=0.78),
but the expression levels in the control and GDM groups were
significantly lower than those in the GDM+ HT group (3.358±1.286,
P<0.05 and P<0.05, respectively). The expression levels of
the VCAM-1 protein in the three groups also exhibited a sequential
increase, with a statistically significant difference (F=31.732,
P≤0.001). The difference in the expression levels of the VCAM-1
protein between the control (1.000±0.297) and GDM (1.525±0.347)
groups was not statistically significant (P=0.11), but the
expression levels in the control and GDM groups were significantly
lower than those in the GDM + HT group (2.698±0.223, P≤0.001 and
P≤0.001, respectively; Fig. 3).
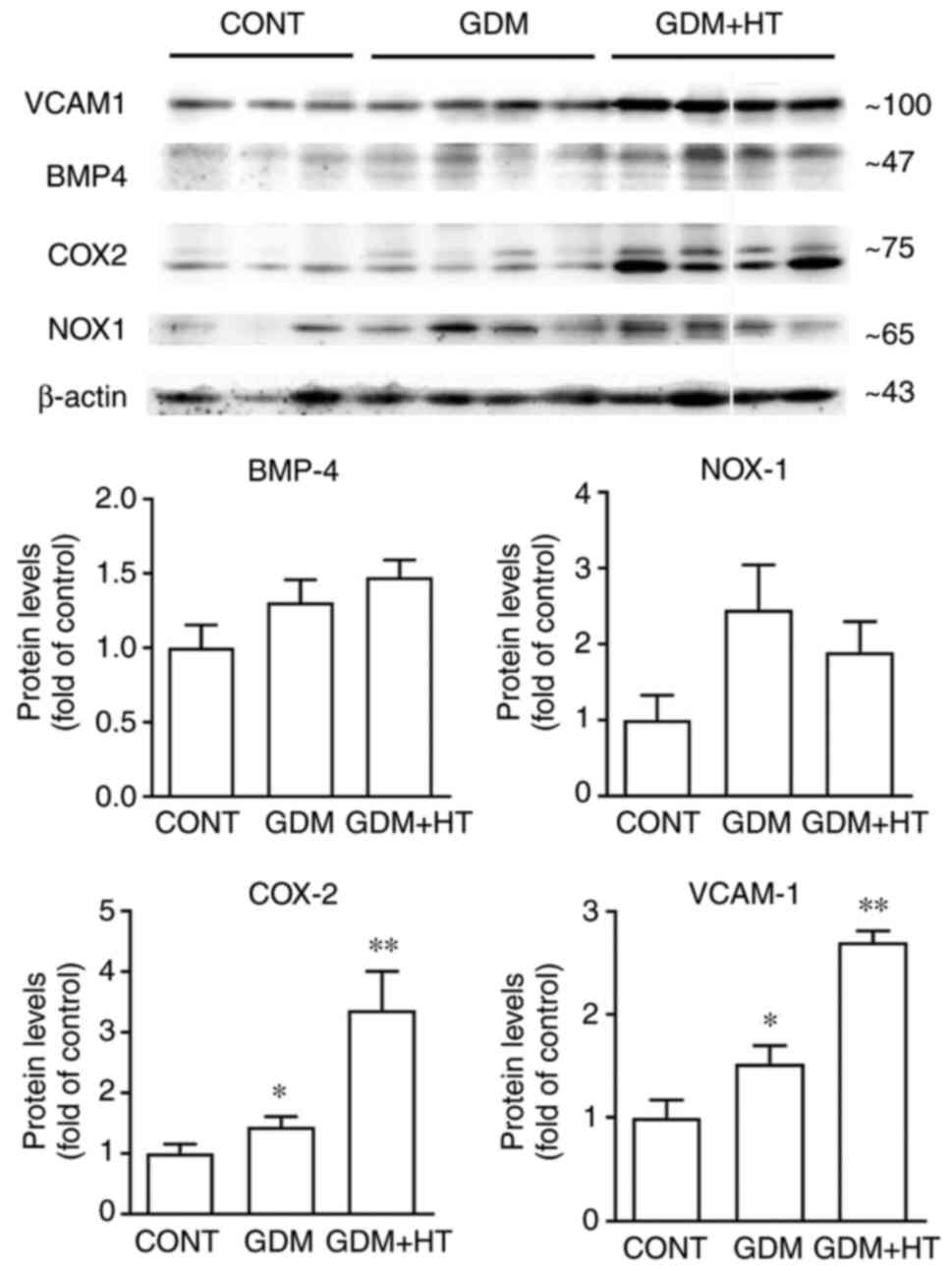 | Figure 3Western blot analysis of BMP-4, COX-2,
NOX-1 and VCAM-1 expression levels in the vascular endothelium of
rats in CONT group (n=3), GDM group (n=4) and GDM+HT group (n=4).
*P<0.05 vs. GDM + HT group; **P<0.05
vs. CONT group. The white line on the blot indicates gels that were
cropped due to loading of the incorrect sample. BMP-4, bone
morphogenetic protein-4; COX-2, cyclooxygenase-2; NOX-1,
nicotinamide adenine dinucleotide phosphate oxidase 1; VCAM-1,
vascular cell adhesion molecule 1; GDM, gestational diabetes
mellitus; HT, hypertension; CONT, control. |
BMP-4 induces elevated blood pressure
in pregnant SD rats
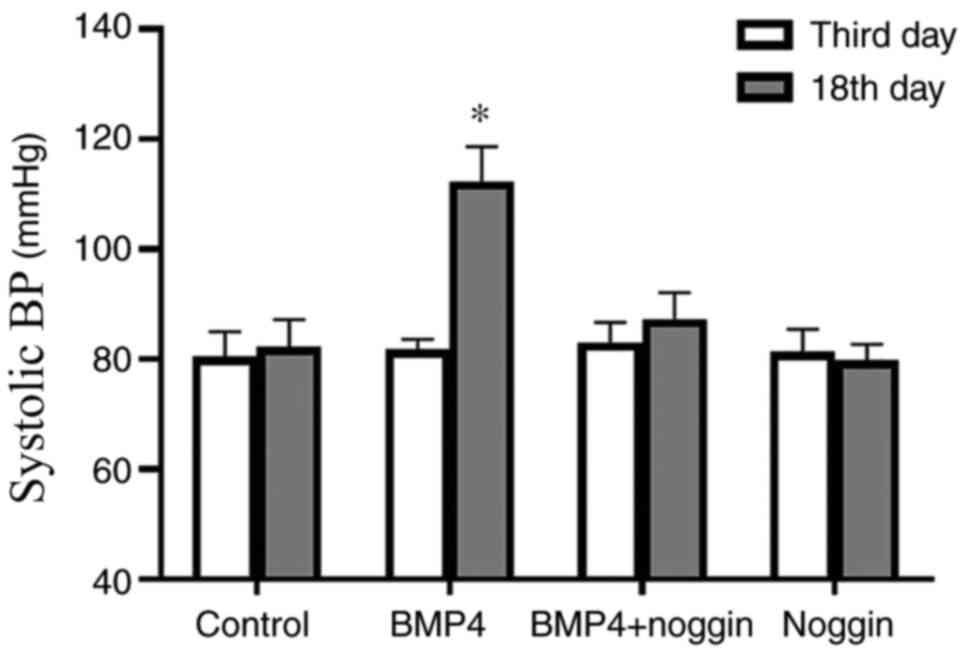
Fig. 4 demonstrates
that infusion of BMP-4 significantly increased the blood pressure
of rats by 30 mmHg above the control values (from 82.3±5.0 to
112.3±6.3 mmHg; P<0.05).
The specificity of the hypertensive effect of BMP-4
was evaluated by infusing noggin, a BMP-4 antagonist, either alone
or in combination with BMP-4. As shown in Fig. 4, noggin co-delivery prevented the
BMP-4-induced hypertension in pregnant SD rats.
BMP-4 abrogates the
endothelium-dependent vasodilation of the vascular endothelium in
pregnant rats
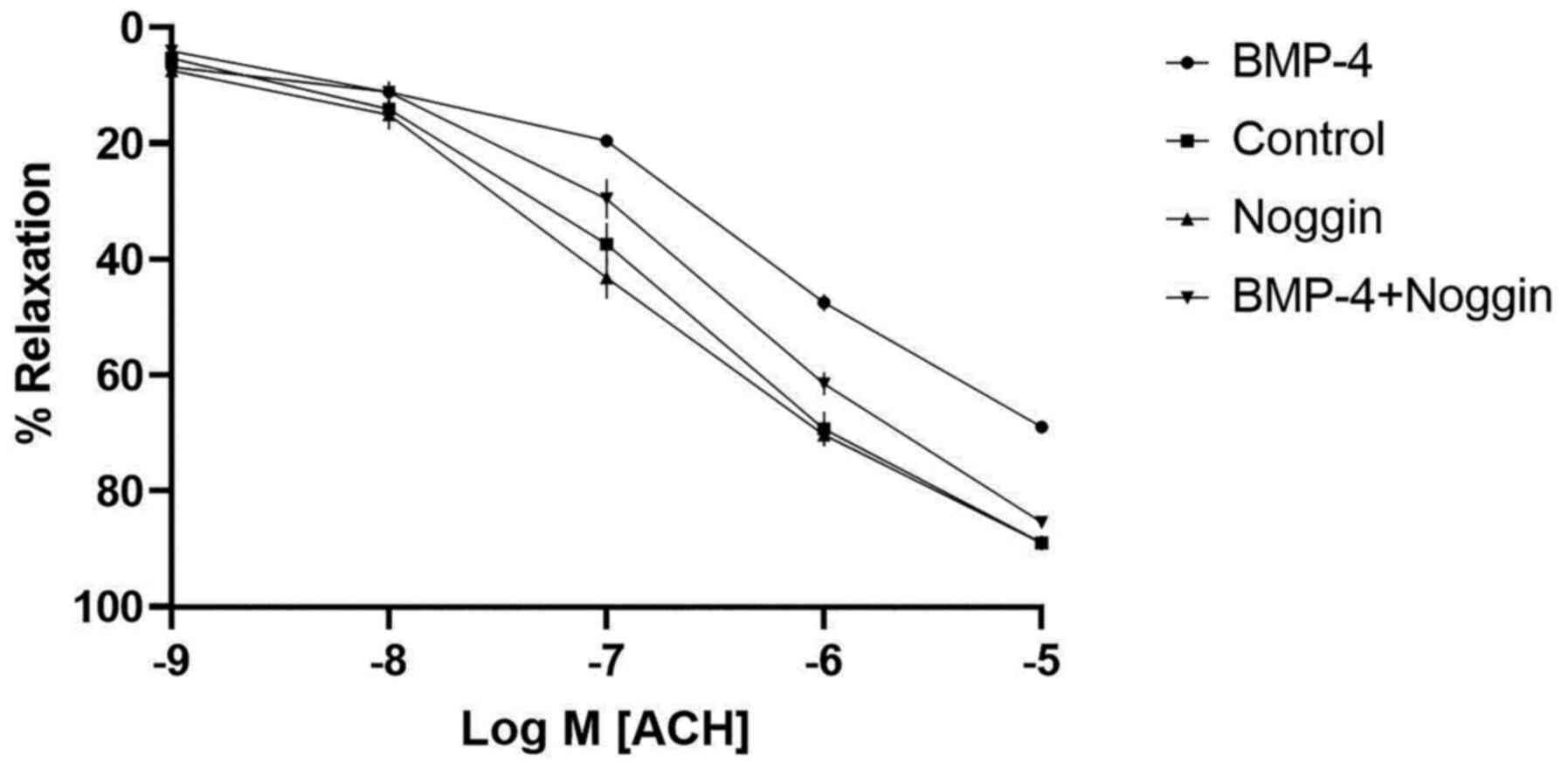
To evaluate the effect of BMP-4 on vascular
endothelial function in pregnant rats, the diastolic activity of
the thoracic aorta of rats in each group were assessed. The results
demonstrated that the maximum relaxation in the control group after
adding acetylcholine was 88.90±1% of the maximum contractile
tension, whereas the maximum relaxation in the BMP-4-infused group
after adding acetylcholine was 68.96±0.8% of the previous maximum
contractile tension, which was significantly smaller than that in
the control group (P<0.05). Accordingly, it was observed that
the blood vessels in the noggin-infused group were nearly the same
as those in the control group (Fig.
5 and Table I).
 | Table IEffects of BMP-4 and noggin on
vasorelaxation responses in pregnant Sprague-Dawley rats. |
Table I
Effects of BMP-4 and noggin on
vasorelaxation responses in pregnant Sprague-Dawley rats.
| Group | Maximal relaxation
(%) |
|---|
| Vehicle | 88.90±1 |
| BMP-4 |
68.96±1a |
| BMP-4 + noggin | 85.41±1 |
| Noggin | 88.99±1 |
Discussion
BMP-4, as a member of the transforming growth
factor-β superfamily, was first discovered to mediate bone growth
and serves as an essential signalling molecule for human embryonic
development (6,7). Noggin is an endogenous antagonist that
counteracts with BMP-4(7). BMP-4
interacts with type 1 BMP receptors to form a binding complex and
trigger downstream signalling (8),
which stimulates SMAD-dependent and -independent pathways,
including the activation of NADPH oxidases that result in ROS
overproduction, p38 MAPK activation and COX-2 upregulation
(14-16).
Previous in vivo and in vitro studies have
demonstrated that BMP-4 activates the NOX1-dependent NADPH oxidase
pathway in vascular endothelial cells and promotes the expression
of inflammatory factors, which leads to vascular endothelial
dysfunction and elevated blood pressure (14,16,36-45);
however, to the best of our knowledge, there have been no studies
on the role of BMP-4 during pregnancy. The present study attempted
to identify the role of BMP-4 in GDM-related hypertension and
endothelial dysfunction. In order to investigate the effect of
BMP-4 on endothelial function, the change in vasorelaxation after
adding BMP-4 and its antagonist requires measurement (14,16).
However, in order to change endothelial function, the duration of
treatment with BMP-4 or its antagonist must be more than two weeks
and even up to four weeks. Unfortunately, the gestational period of
rats is only 21 days and the duration for establishing the GDM
animal model accounts for 7-10 days, meaning there is insufficient
time to infuse BMP-4 and its antagonist to evaluate their effect on
endothelial function in GDM rats. Accordingly, the present study
was divided into two parts; one part evaluated the correlation
between BMP-4 and GDM-related hypertension and the other
investigated the role of BMP-4 on endothelial function during
pregnancy.
During the first part of the study, a GDM rat model
was established and the expression levels of BMP-4 pathway-related
proteins and VCAM-1 proteins were evaluated in the endothelium of
the abdominal aorta of rats. The results indicated that BMP-4,
NOX-1, COX-2 and VCAM-1 proteins were expressed in the endothelium
of the abdominal aorta of pregnant rats, which, to the best of our
knowledge, has not previously been shown. Moreover, the expression
levels of these proteins in the endothelium of the abdominal aorta
of rats with GDM were higher than those in pregnant rats without
GDM. Additionally, the expression levels of BMP-4, COX-2 and VCAM-1
proteins were higher in rats with GDM-related hypertension when
compared with those in rats with GDM with normal blood pressure,
and the level of VCAM-1 expression was the highest among all three
proteins. Therefore, it is hypothesized that BMP-4 pathway-related
proteins are involved in GDM-related hypertension and VCAM-1 may be
substantially associated with GDM-related hypertension.
In the second part of the present study, pregnant
rats were subjected to slow infusions of BMP-4. The results
revealed that the blood pressure of the BMP-4 group was
significantly higher than that of the vehicle group, thereby
indicating that BMP-4 could cause elevated blood pressure in
pregnant rats. Noggin inhibited the BMP-4-induced increase in blood
pressure. Further analysis of the effect of BMP-4 on vascular
reactivity showed that BMP-4 abrogated the endothelium-dependent
vasodilation in the vascular endothelium in pregnant rats, which
led to vascular endothelial dysfunction and further hypertension.
Whereas noggin blocked the destructive effect of BMP-4 on the
endothelium-dependent vasodilation in the vascular endothelium.
Accordingly, it is hypothesized that BMP-4 pathway proteins and
VCAM-1 may participate in the hypertension associated with GDM,
with VCAM-1 potentially playing a substantial role in hypertension.
Therefore, the aetiology of GDM-related hypertension may be the
endothelial dysfunction induced by overexpression of BMP-4 pathway
proteins and VCAM-1.
However, there were certain limitations of the
present study. Since the specific pathogenesis of GDM remains
unclear, the GDM animal model that was established may not entirely
simulate human GDM. Furthermore, data obtained from the aorta may
not apply to other vascular beds, such as mesenteric arteries,
which are resistance vessels and contribute to blood pressure
markedly more than the aorta. Therefore, further studies on smaller
arterial vessels are required in future. In addition, noggin, as a
well-known inhibitor of BMP-4, may also have numerous other effects
such as reducing the serum glucose levels (46), that could influence the experiments.
Finally, due to the limited sample size, the results of the present
study require confirmation by further studies using a larger sample
size.
However, the present study demonstrated that BMP-4
may cause vascular endothelial dysfunction by disrupting the
endothelium-dependent vasodilation in pregnant rats, thereby
causing elevated blood pressure. Noggin, as an antagonist of BMP-4,
may block the destructive effect of BMP-4 on endothelium-dependent
vasodilation. Therefore, it is hypothesized that upregulation of
the BMP-4 pathway proteins induced by hyperglycaemia in GDM may be
involved in the mechanism of GDM-related vascular endothelial
dysfunction and hypertension, and VCAM-1 may be substantially
associated with GDM-related vascular endothelial dysfunction and
hypertension. The present results may provide insight into the
aetiology and treatment of GDM-related hypertension or
preeclampsia. The action of BMP-4 raises the possibility that
noggin and its related compounds may serve as therapeutic agents
for GDM-related hypertension or preeclampsia. In the future, the
authors aim to conduct clinical studies, evaluating the expression
levels of BMP-4 and its related proteins in humans to test our
hypothesis, in order to identify a novel therapeutic strategy for
GDM-related hypertension or preeclampsia.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Youth Backbone
Support Program of China Medical University (grant no.
QGZ2018054).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BC conceptualized and designed the study. He also
performed the experiments and wrote the initial draft of the
manuscript. JD supervised the design of the study and critically
reviewed the manuscript drafts. BC and JD confirm the authenticity
of all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The experimental protocol was approved by the Ethics
Committee of Shengjing Hospital of China Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
American Diabetes Association. 2.
Classification and diagnosis of diabetes: Standards of medical care
in diabetes-2018. Diabetes Care. 41 (Suppl 1):S13–S27.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jiwani A, Marseille E, Lohse N, Damm P,
Hod M and Kahn JG: Gestational diabetes mellitus: Results from a
survey of country prevalence and practices. J Matern Fetal Neonatal
Med. 25:600–610. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li Z, Cheng Y, Wang D, Chen H, Chen H,
Ming WK and Wang Z: Incidence rate of type 2 diabetes mellitus
after gestational diabetes mellitus: A systematic review and
meta-analysis of 170,139 women. J Diabetes Res.
2020(3076463)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Catalano PM and Ehrenberg HM: The short-
and long-term implications of maternal obesity on the mother and
her offspring. BJOG. 113:1126–1133. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ehrenberg HM, Mercer BM and Catalano PM:
The influence of obesity and diabetes on the prevalence of
macrosomia. Am J Obstet Gynecol. 191:964–968. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li RH and Wozney JM: Delivering on the
promise of bone morphogenetic proteins. Trends Biotechnol.
19:255–265. 2001.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hogan BL: Bone morphogenetic proteins in
development. Curr Opin Genet Dev. 6:432–438. 1996.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Miyazono K, Maeda S and Imamura T: BMP
receptor signaling: Transcriptional targets, regulation of signals,
and signaling cross-talk. Cytokine Growth Factor Rev. 16:251–263.
2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cao S, Reece EA, Shen WB and Yang P:
Restoring BMP4 expression in vascular endothelial progenitors
ameliorates maternal diabetes-induced apoptosis and neural tube
defects. Cell Death Dis. 11(859)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bostrom KI, Jumabay M, Matveyenko A,
Nicholas SB and Yao Y: Activation of vascular bone morphogenetic
protein signaling in diabetes mellitus. Circ Res. 108:446–457.
2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hu J, Liu J, Kwok MW, Wong RH, Huang Y and
Wan S: Bone morphogenic protein-4 contributes to venous endothelial
dysfunction in patients with diabetes undergoing coronary
revascularization. Ann Thorac Surg. 95:1331–1339. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sorescu GP, Song H, Tressel SL, Hwang J,
Dikalov S, Smith DA, Boyd NL, Platt MO, Lassègue B, Griendling KK
and Jo H: Bone morphogenic protein 4 produced in endothelial cells
by oscillatory shear stress induces monocyte adhesion by
stimulating reactive oxygen species production from a nox1-based
NADPH oxidase. Circ Res. 95:773–779. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sanchez-de-Diego C, Valer JA,
Pimenta-Lopes C, Rosa JL and Ventura F: Interplay between BMPs and
reactive oxygen species in cell signaling and pathology.
Biomolecules. 9(534)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Miriyala S, Gongora Nieto MC, Mingone C,
Smith D, Dikalov S, Harrison DG and Jo H: Bone morphogenic
protein-4 induces hypertension in mice: Role of noggin, vascular
NADPH oxidases, and impaired vasorelaxation. Circulation.
113:2818–2825. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yang X, Long L, Southwood M,
Rudarakanchana N, Upton PD, Jeffery TK, Atkinson C, Chen H,
Trembath RC and Morrell NW: Dysfunctional Smad signaling
contributes to abnormal smooth muscle cell proliferation in
familial pulmonary arterial hypertension. Circ Res. 96:1053–1063.
2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wong WT, Tian XY, Chen Y, Leung FP, Liu L,
Lee HK, Ng CF, Xu A, Yao X, Vanhoutte PM, et al: Bone morphogenic
protein-4 impairs endothelial function through oxidative
stress-dependent cyclooxygenase-2 upregulation: Implications on
hypertension. Circ Res. 107:984–991. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Luo JY, Zhang Y, Wang L and Huang Y:
Regulators and effectors of bone morphogenetic protein signalling
in the cardiovascular system. J Physiol. 593:2995–3011.
2015.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Youn JY, Zhou J and Cai H: Bone
morphogenic protein 4 mediates NOX1-dependent eNOS uncoupling,
endothelial dysfunction, and COX2 induction in type 2 diabetes
mellitus. Mol Endocrinol. 29:1123–1133. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Altman D, Carroli G, Duley L, Farrell B,
Moodley J, Neilson J and Smith D: Magpie Trial Collaboration Group.
Do women with pre-eclampsia, and their babies, benefit from
magnesium sulphate? The magpie trial: A randomised
placebo-controlled trial. Lancet. 359:1877–1890. 2002.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Knuist M, Bonsel GJ, Zondervan HA and
Treffers PE: Intensification of fetal and maternal surveillance in
pregnant women with hypertensive disorders. Int J Gynaecol Obstet.
61:127–133. 1998.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hauth JC, Ewell MG, Levine RJ, Esterlitz
JR, Sibai B, Curet LB, Catalano PM and Morris CD: Pregnancy
outcomes in healthy nulliparas who developed hypertension. Calcium
for preeclampsia prevention study group. Obstet Gynecol. 95:24–28.
2000.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Schneider S, Freerksen N, Rohrig S, Hoeft
B and Maul H: Gestational diabetes and preeclampsia-similar risk
factor profiles? Early Hum Dev. 88:179–184. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Roberts JM and Lain KY: Recent Insights
into the pathogenesis of pre-eclampsia. Placenta. 23:359–372.
2002.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Conti E, Zezza L, Ralli E, Caserta D,
Musumeci MB, Moscarini M, Autore C and Volpe M: Growth factors in
preeclampsia: A vascular disease model. A failed vasodilation and
angiogenic challenge from pregnancy onwards? Cytokine Growth Factor
Rev. 24:411–425. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
de Resende Guimarães MFB, Brandão AHF, de
Lima Rezende CA, Cabral ACV, Brum AP, Leite HV and Capuruço CAB:
Assessment of endothelial function in pregnant women with
preeclampsia and gestational diabetes mellitus by flow-mediated
dilation of brachial artery. Arch Gynecol Obstet. 290:441–447.
2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Abdul Aziz SH, John CM, Mohamed Yusof NI,
Nordin M, Ramasamy R, Adam A and Fauzi FM: Animal model of
gestational diabetes mellitus with pathophysiological resemblance
to the human condition induced by multiple factors (nutritional,
pharmacological, and stress) in rats. Biomed Res Int.
2016(9704607)2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mdaki KS, Larsen TD, Wachal AL,
Schimelpfenig MD, Weaver LJ, Dooyema SD, Louwagie EJ and Baack ML:
Maternal high-fat diet impairs cardiac function in offspring of
diabetic pregnancy through metabolic stress and mitochondrial
dysfunction. Am J Physiol Heart Circ Physiol. 310:H681–H692.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gutierrez JC, Bahamonde J, Prater MR, Yefi
CP and Holladay SD: Production of a type 2 maternal diabetes rodent
model using the combination of high-fat diet and moderate dose of
streptozocin. Endocr Res. 35:59–70. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Vesentini G, Marini G, Piculo F, Damasceno
DC, Matheus SMM, Felisbino SL, Calderon IMP, Hijaz A, Barbosa AMP
and Rudge MVC: Morphological changes in rat rectus abdominis muscle
induced by diabetes and pregnancy. Braz J Med Biol Res.
51(e7035)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Suckow MA, Gobbett TA and Peterson RG:
Wound healing delay in the ZDSD rat. In Vivo. 31:55–60.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Domon A, Katayama K, Tochigi Y and Suzuki
H: Characterization of novel nonobese type 2 diabetes rat model
with enlarged kidneys. J Diabetes Res. 2019(8153140)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Laurino LF, Viroel FJM, Caetano E, Spim S,
Pickler TB, Rosa-Castro RM, Vasconcelos EA, Jozala AF, Hataka A,
Grotto D and Gerenutti M: Lentinus edodes exposure before and after
fetus implantation: Materno-fetal development in rats with
gestational diabetes mellitus. Nutrients. 11(2720)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Danda RS, Habiba NM, Rincon-Choles H,
Bhandari BK, Barnes JL, Abboud HE and Pergola PE: Kidney
involvement in a nongenetic rat model of type 2 diabetes. Kidney
Int. 68:2562–2571. 2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Catena C, Giacchetti G, Novello M, Colussi
G, Cavarape A and Sechi LA: Cellular mechanisms of insulin
resistance in rats with fructose-induced hypertension. Am J
Hypertens. 16:973–978. 2003.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Elmarakby AA and Imig JD: Obesity is the
major contributor to vascular dysfunction and inflammation in
high-fat diet hypertensive rats. Clin Sci (Lond). 118:291–301.
2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Singh VP, Le B, Khode R, Baker KM and
Kumar R: Intracellular angiotensin II production in diabetic rats
is correlated with cardiomyocyte apoptosis, oxidative stress, and
cardiac fibrosis. Diabetes. 57:3297–3306. 2008.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Feldman DL, Jin L, Xuan H, Contrepas A,
Zhou Y, Webb RL, Mueller DN, Feldt S, Cumin F, Maniara W, et al:
Effects of aliskiren on blood pressure, albuminuria, and (pro)renin
receptor expression in diabetic TG(mRen-2)27 rats. Hypertension.
52:130–136. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Imanishi T, Tsujioka H, Ikejima H, Kuroi
A, Takarada S, Kitabata H, Tanimoto T, Muragaki Y, Mochizuki S,
Goto M, et al: Renin inhibitor aliskiren improves impaired nitric
oxide bioavailability and protects against atherosclerotic changes.
Hypertension. 52:563–572. 2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Corvol P, Michaud A, Soubrier F and
Williams TA: Recent advances in knowledge of the structure and
function of the angiotensin I converting enzyme. J Hypertens Suppl.
13:S3–10. 1995.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wei L, Alhenc-Gelas F, Soubrier F, Michaud
A, Corvol P and Clauser E: Expression and characterization of
recombinant human angiotensin I-converting enzyme. Evidence for a
C-terminal transmembrane anchor and for a proteolytic processing of
the secreted recombinant and plasma enzymes. J Biol Chem.
266:5540–5546. 1991.PubMed/NCBI
|
|
41
|
Linz W, Gohlke P, Unger T and Scholkens
BA: Experimental evidence for effects of ramipril on cardiac and
vascular hypertrophy beyond blood pressure reduction. Arch Mal
Coeur Vaiss. 2:31–34. 1995.PubMed/NCBI
|
|
42
|
Csiszar A, Ahmad M, Smith KE, Labinskyy N,
Gao Q, Kaley G, Edwards JG, Wolin MS and Ungvari Z: Bone
morphogenetic protein-2 induces proinflammatory endothelial
phenotype. Am J Pathol. 168:629–638. 2006.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Csiszar A, Labinskyy N, Jo H, Ballabh P
and Ungvari Z: Differential proinflammatory and prooxidant effects
of bone morphogenetic protein-4 in coronary and pulmonary arterial
endothelial cells. Am J Physiol Heart Circ Physiol. 295:H569–H577.
2008.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Frank DB, Abtahi A, Yamaguchi DJ, Manning
S, Shyr Y, Pozzi A, Baldwin HS, Johnson JE and de Caestecker MP:
Bone morphogenetic protein 4 promotes pulmonary vascular remodeling
in hypoxic pulmonary hypertension. Circ Res. 97:496–504.
2005.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yang J, Davies RJ, Southwood M, Long L,
Yang X, Sobolewski A, Upton PD, Trembath RC and Morrell NW:
Mutations in bone morphogenetic protein type II receptor cause
dysregulation of Id gene expression in pulmonary artery smooth
muscle cells: Implications for familial pulmonary arterial
hypertension. Circ Res. 102:1212–1221. 2008.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Koga M, Engberding N, Dikalova AE, Chang
KH, Seidel-Rogol B, Long JS, Lassègue B, Jo H and Griendling KK:
The bone morphogenic protein inhibitor, noggin, reduces glycemia
and vascular inflammation in db/db mice. Am J Physiol Heart Circ
Physiol. 305:H747–H755. 2013.PubMed/NCBI View Article : Google Scholar
|