Introduction
Atherosclerosis (AS) is one the main pathological
causes of cardiovascular disease and is characterized by the
accumulation of large numbers of lipids, inflammatory cells and
fibrous materials in the vascular endothelium (1). Thrombosis and plaque rupture during AS
can result in serious cardiovascular diseases, including coronary
heart disease and myocardial infarction (2). Previous epidemiological studies have
reported that, in developing countries, especially in China, the
prevalence of AS is increasing rapidly (3-5).
During the progression of AS, foam cells are one of the main
components of the atherosclerotic plaque, where their formation is
considered to be the hallmark a key step in AS pathogenesis
(6). With the exception of a small
subset derived from vascular endothelial cells and smooth muscle
cells, the vast majority of foam cells are derived from macrophages
that can phagocytose lipids (7).
Under physiological conditions, there is a balance between the
cholesterol uptake and efflux out of foam cells (7). However, in advanced atherosclerotic
plaques, this balance is perturbed and cholesterol efflux decreases
(8). This continuous accumulation
of free cholesterol (FC) in foam cells can cause inflammatory
responses and ultimately lead to foam cell apoptosis (7). Therefore, homeostasis of cholesterol
uptake and efflux has a critical importance in AS, where
information on the underlying mechanism of foam cell formation and
lipid metabolism in macrophages may reveal novel strategies for the
treatment of AS.
The peroxisome proliferator activated receptor (PPAR
γ/liver X receptor (LXR)α/ATP binding cassette subfamily A member 1
(ABCA1)/ATP binding cassette subfamily G member 1 (ABCG1) pathway
in macrophages serves a crucial role in controlling cholesterol
efflux (9). Both PPARγ and LXRα are
transcription factors that can be activated by oxidized low-density
lipoprotein (ox-LDL), which directly increases the expression
levels of the membrane ATP-binding cassette transporters ABCA1 and
ABCG1, leading to cholesterol excretion from macrophages (10). In addition to regulating cholesterol
homeostasis, PPARγ and LXRα also exhibit anti-inflammatory
properties by promoting the release of anti-inflammatory factors,
such as TNFα, IL-1β and IL-6(11).
All these findings aforementioned suggest that activation of PPARγ
and LXRα can alleviate AS. In addition, activation of the
PPARγ/LXRα pathway in foam cells may serve to be a promising
anti-AS treatment strategy.
Septin 4 is a member of the septin family that
possesses GTPase activity and is widely expressed in eukaryotic
cells (12). Septin 4 is considered
to be a major component of the cytoskeleton and is involved in
numerous important physiological processes, including cell
differentiation, vesicle trafficking and apoptosis (12). Septin 4 has also been reported to be
a tumor suppressor that can promote the apoptosis of cancer cells.
For example, Septin 4 could promote cell death in human colon
cancer cells by increasing apoptosis (13). Another study revealed that silencing
Septin 4 expression increased platelet-derived growth factor
(PDGF)-BB-induced human aortic vascular smooth muscle cell (HAVSMC)
proliferation, migration and phenotypic transformation, whilst the
overexpression of Septin 4 had the opposite effects, implicating
the involvement of Septin 4 in AS (14). However, the role of Septin 4 on foam
cell formation, lipid accumulation and lipid metabolism remain
poorly understood.
Therefore, the present study intended to investigate
the role of Septin 4 in macrophage formation and lipid metabolism
and to study any potential underlying mechanism. The expression
levels of Septin 4 in ox-LDL-induced foam cells were also measured,
following which the effect of Septin 4 knockdown and overexpression
on cholesterol accumulation, ABCA1, ABCG and PPARγ expression was
evaluated in foam cells. Mechanistically, PPARγ and LXRα was
knockdown was used to explore the underlying function of Septin 4
on cholesterol accumulation.
Materials and methods
Cell culture and treatment
Human THP-1 monocytes (The Cell Bank of Type Culture
Collection of Chinese Academy of Sciences) were cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) with 10%
FBS (Gibco; Thermo Fisher Scientific, Inc.) in a humidified
atmosphere with 5% CO2 at 37˚C.
Macrophages were obtained from THP-1 cells
stimulated with 100 ng/ml phorbol 12-myristate-13-acetate (PMA;
Sigma-Aldrich; Merck KGaA) at 37˚C for 48 h. Cells were exposed to
ox-LDL (50 µg/ml) for 24 h.
Lentiviral particles expressing short hairpin RNA
(shRNA/sh) targeting human Septin 4 or LXRα, the corresponding
negative control (NC; sh-NC) and the recombinant pcDNA3.0 vector
overexpressing Septin 4 (ov-Septin 4) along with the empty pcDNA3.0
vector (empty vector for ov-Septin 4) were designed and
successfully constructed by Shanghai GenePharma Co., Ltd. After PMA
and ox-LDL treatment, cells were transfected using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) as
described previously (15,16). For PPARγ inhibition, its selective
antagonist T0070907 (10 µM; Sigma-Aldrich; Merck KGaA) was used to
treat transfected or untransfected macrophages at 37˚C for 24
h.
Oil red O staining (ORO)
THP-1 derived macrophages were exposed to ox-LDL (50
µg/ml) for 24 h. The cells were washed with a buffer solution (PBS)
and directly fixed with 10% formalin for 20 min. Cells were then
incubated with 60% isopropanol for 5 min and stained with ORO
(Sigma-Aldrich; Merck KGaA) for 30 min. After counterstaining with
crystal violet, the cells were imaged using a phase contrast light
microscope (magnification, x200; Leica Microsystems GmbH). All
operations were performed at room temperature.
Measurement of cholesterol
To measure total cholesterol (TC) and FC levels,
THP-1-derived macrophages (1x106) were exposed to ox-LDL
(50 µg/ml) at 37˚C for 24 h in six-well plates as aforementioned.
TC and FC levels were determined using a TC/FC quantitative method
(Abcam; cat. no. ab65359) according to the manufacturer's
protocols. Fluorescence measurement was conducted using a
microplate reader (Thermo Fisher Scientific, Inc.) at
excitation/emission=535/587 nm. TC and FC results are expressed in
mg/dl.
Reverse transcription-quantitative
qPCR (RT-qPCR)
Total RNA were extracted from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) following the manufacturer's protocols. Total RNA (~1 µg)
from each sample was then reverse transcribed into cDNA using the
PrimeScript™ RT kit (Takara Bio, Inc.). The temperature protocol
for RT was as follows: 37˚C for 15 min, followed by 85˚C for 5 sec
and stop at 4˚C. Quantitative assessment of mRNA expression was
performed by qPCR based using Fast SYBR™ Green Master Mix (Thermo
Fisher Scientific, Inc.) in an Applied Biosystems (ABI 7500 system;
Thermo Fisher Scientific, Inc.). The primers were synthesized by
Nanjing Genscript Biotechnology Co., Ltd. The thermocycling
conditions were as follows: 95˚C for 2 min, followed by 40 cycles
of 95˚C for 20 sec and 65˚C for 40 sec. mRNA expression levels were
compared after normalization with β-actin. The primer sequences
used were as follows: Septin 4 forward,
5'-CTGCTTTCTTCCTGGATGTCTCT-3' and reverse,
5'-AGGTTCCAAGCCCCAAA-GAAA-3'; LXRα forward,
5'-CCTGGGGATTTGGACAGTGC-3' and reverse, 5'-GCCCCTTTTTCCGCTTTTGT-3'
and β-actin forward, 5'-CTTCTACAATGAGCTGCGTGTG-3' and reverse,
5'-AGT CATAGTCCGCCTAGAAGC-3'. Expression levels of target genes
were normalized to the endogenous control GAPDH using the
2-ΔΔCq method (17)
Western blotting
Total protein was extracted using RIPA lysis buffer
(Beyotime Institute of Biotechnology) supplemented with protease
and phosphatase inhibitors. After determining the protein
concentration using a BCA kit (Thermo Fisher Scientific, Inc.), the
proteins (30 µg per lane) were separated by 10% SDS-PAGE and
transferred onto PVDF membranes. The membranes were then blocked
with 5% BSA (Beyotime Institute of Biotechnology) solution at room
temperature for 1 h and incubated with primary antibodies (All
Abcam) against Septin 4 (cat. no. ab166788; 1:2,000), ABCA1 (cat.
no. ab66217, 1:1,000), ABCG1 (cat. no. ab52617; 1:5,000), PPARγ
(cat. no. ab178860; 1:1,000), LXRα (cat. no. ab176323; 1:2,000) and
GAPDH (ab8245; 1:10,000) overnight at 4˚C. After incubation, the
membranes were washed, incubated with the corresponding horseradish
peroxidase-conjugated secondary antibodies (goat anti-mouse IgG,
cat. no. ab205719, 1:10,000; goat anti-rabbit, cat. no. ab205718,
1:10,000; both Abcam) at room temperature for 2 h and the bands
were visualized using an ECL system (Thermo Fisher Scientific,
Inc.). Protein expression levels were semi-quantified using
Image-Pro Plus software version 6.0 (Media Cybernetic, Inc.).
Statistical analysis
Data are presented as the mean ± SD from ≥ three
independent experiments and were analyzed using one-way ANOVA
followed by Tukey's test for comparisons. All statistical analyses
were performed using GraphPad Prism 6 software (GraphPad Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Septin 4 expression upregulated in
THP-1 macrophages following ox-LDL stimulation
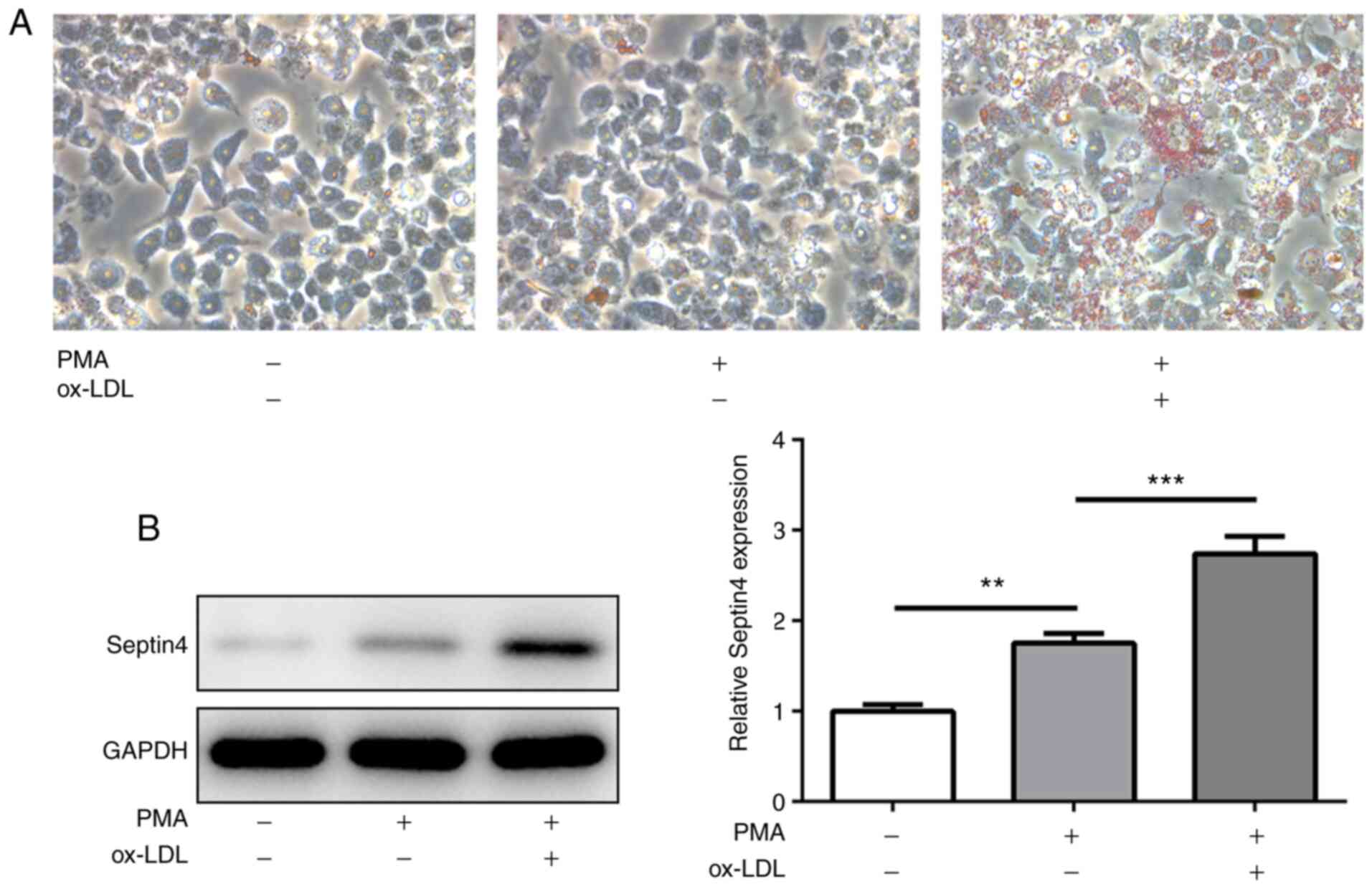
To determine the formation of foam cells derived
from THP-1 macrophages, THP-1 monocytes were stimulated with PMA
for 48 h to differentiate them into macrophages. Subsequently, the
level of intracellular lipid accumulation after ox-LDL treatment
was observed using ORO staining. A marked increase in lipid
accumulation in PMA-induced THP-1 macrophages was observed upon
ox-LDL stimulation compared with cells that were exposed to PMA
alone (Fig. 1A), suggesting uptake
of lipids in ox-LDL-treated macrophages and the formation of foam
cells.
The expression of Septin 4 was
subsequently measured
PMA treatment significantly increased Septin 4
expression compared with that in untreated cells, but the
additional presence of ox-LDL increased the expression level of
Septin 4 further in a significant manner (Fig. 1B). These observations suggest that
Septin 4 may serve a role in the formation of foam cells derived
from THP-1.
Septin 4 knockdown promotes but Septin
4 overexpression attenuates formation of foam cells derived from
THP-1 macrophages
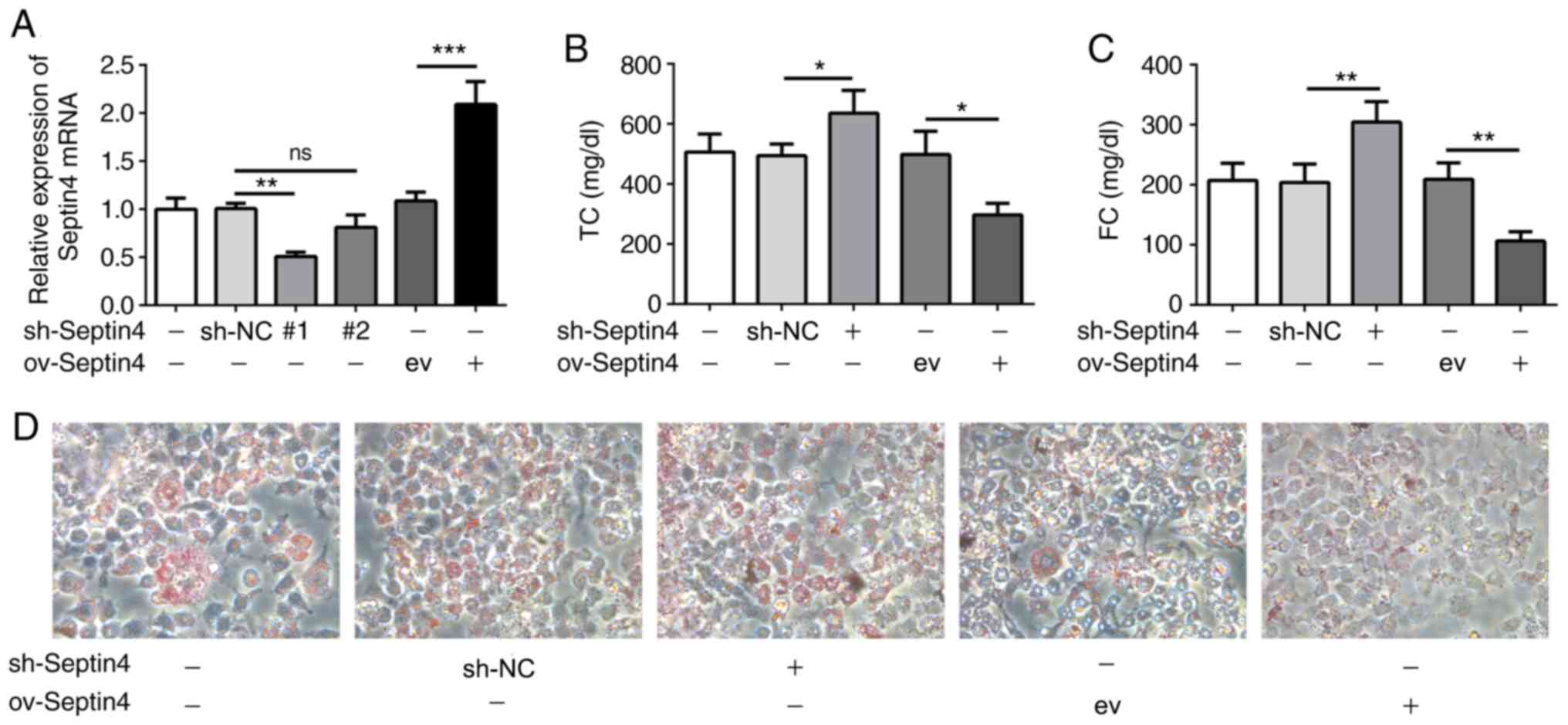
Subsequently, Septin 4 expression was knocked down
or overexpressed in THP-1-derived foam cells using the
corresponding plasmids. Transfection efficacy was then verified
using RT-qPCR. shRNA-Septin 4-1 was chosen to knock down Septin 4
expression due to higher transfection efficacy, which significantly
reduced Septin 4 expression compared that in cells transfected with
sh-NC (Fig. 2A).
Cellular cholesterol content was then detected,
where it was found that Septin 4 knockdown significantly increased
intracellular TC and FC concentrations but Septin 4 overexpression
resulted in the opposite trend being observed compared with those
in their corresponding transfection controls (Fig. 2B and C). To further confirm these results, the
uptake of lipids was measured using ORO staining (Fig. 2D). Compared with that in
non-transfected ox-LDL-treated THP-1 macrophages, knocking down
Septin 4 expression caused a notable increase in the uptake of
ox-LDL. By contrast, ORO staining was markedly reduced by the
overexpression of Septin 4. These data suggest that Septin 4 can
inhibit the formation of foam cells derived from ox-LDL-induced
THP-1 macrophages.
Septin 4 knockdown reduces but Septin 4 upregulation
enhances the expression levels of ABCA1, ABCG1, PPARγ and LXRα. The
molecular mechanism underlying the actions of Septin 4 was further
investigated. The protein expression levels of ABCA1 and ABCG1 were
significantly decreased by Septin 4 knockdown in THP-1 macrophages
compared with those in cells in the sh-NC group (Fig. 3A). By contrast, ABCA1 and ABCG1
protein expression levels were significantly increased by the
overexpression of Septin 4 when compared with the empty vector
ov-Septin 4 group (Fig. 3A). These
results suggest that Septin 4 may inhibit cholesterol accumulation
by upregulating ABCA1 and ABCG1 expression, which can assist in
excreting cholesterol from the cell.
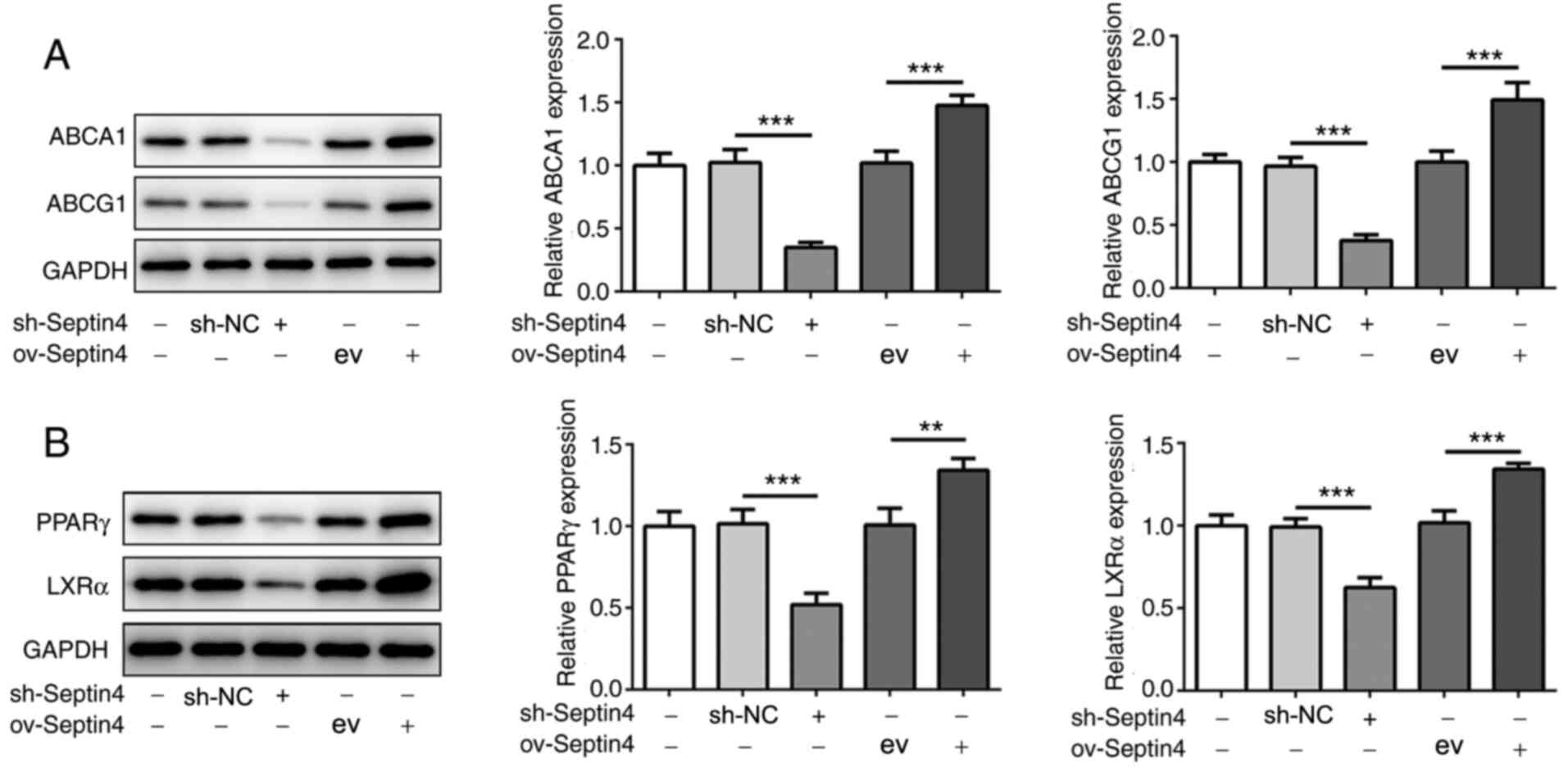 | Figure 3Effect of Septin4 knockdown and
overexpression on PPARγ, LXRα and ABCA1/G1 expression in THP-1
macrophage-derived foam cells. (A) ABCA1, ABCG1, (B) PPARγ and LXRα
protein expression levels in THP-1 macrophage-derived foam cells
following Septin 4 knockdown or overexpression were analyzed using
western blotting. **P<0.01 and
***P<0.001. PPARγ, proliferator activated receptor γ;
LXRα, liver X receptor α; ABCA1, ATP binding cassette subfamily A
member 1; ABCG1, ATP binding cassette subfamily G member 1; NC,
negative control; sh, short hairpin; ov, overexpression; ev, empty
vector. |
To further investigate whether PPARγ and LXRα are
involved in the effects of Septin 4, the protein expression levels
of PPARγ and LXRα were also measured. Compared with those in their
corresponding transfection controls, Septin 4 knockdown and
overexpression significantly reduced and increased the expression
levels of PPARγ and LXRα, respectively, compared with those in
their corresponding transfection controls (Fig. 3B), consistent with the findings
aforementioned. These results suggest that the effects mediated by
Septin 4 may involve the PPARγ/LXRα-related cholesterol metabolism
pathway.
Inhibition of PPARγ or LXRα reverses
the effect of Septin 4 overexpression on foam cell formation and
ABCA1 and ABCG1 expression
Subsequently, the PPARγ inhibitor T0070907 and shRNA
targeting LXRα were used to inhibit PPARγ and LXRα, respectively.
To avoid off-target effects, two LXRα shRNA candidates were
designed and synthesized. Both shRNA-1 and shRNA-2 significantly
decreased the mRNA expression levels of LXRα compared with those
transfected with sh-NC (Fig. 4A).
In subsequent experiments, shRNA-LXRα-1 was selected to knockdown
LXRα expression in THP-1 macrophages that were overexpressing
Septin 4. It was found that the inhibitory effects of Septin 4
overexpression on TC and FC content were significantly reversed by
both PPARγ inhibition or LXRα knockdown (Fig. 4B and C). In addition, similar results were
observed in terms of lipid accumulation, where it was found that
both PPARγ inhibition and LXRα knockdown canceled the suppressive
effects of Septin 4 overexpression on lipid accumulation (Fig. 4D). PPARγ inhibition or LXRα
knockdown also significantly reversed Septin 4
overexpression-induced increments in ABCA1 and ABCG1 expression
(Fig. 4E). Collectively, these
findings suggest that the actions of Septin 4 on foam cell
formation were dependent on the PPARγ/LXRα-related cholesterol
metabolism pathway.
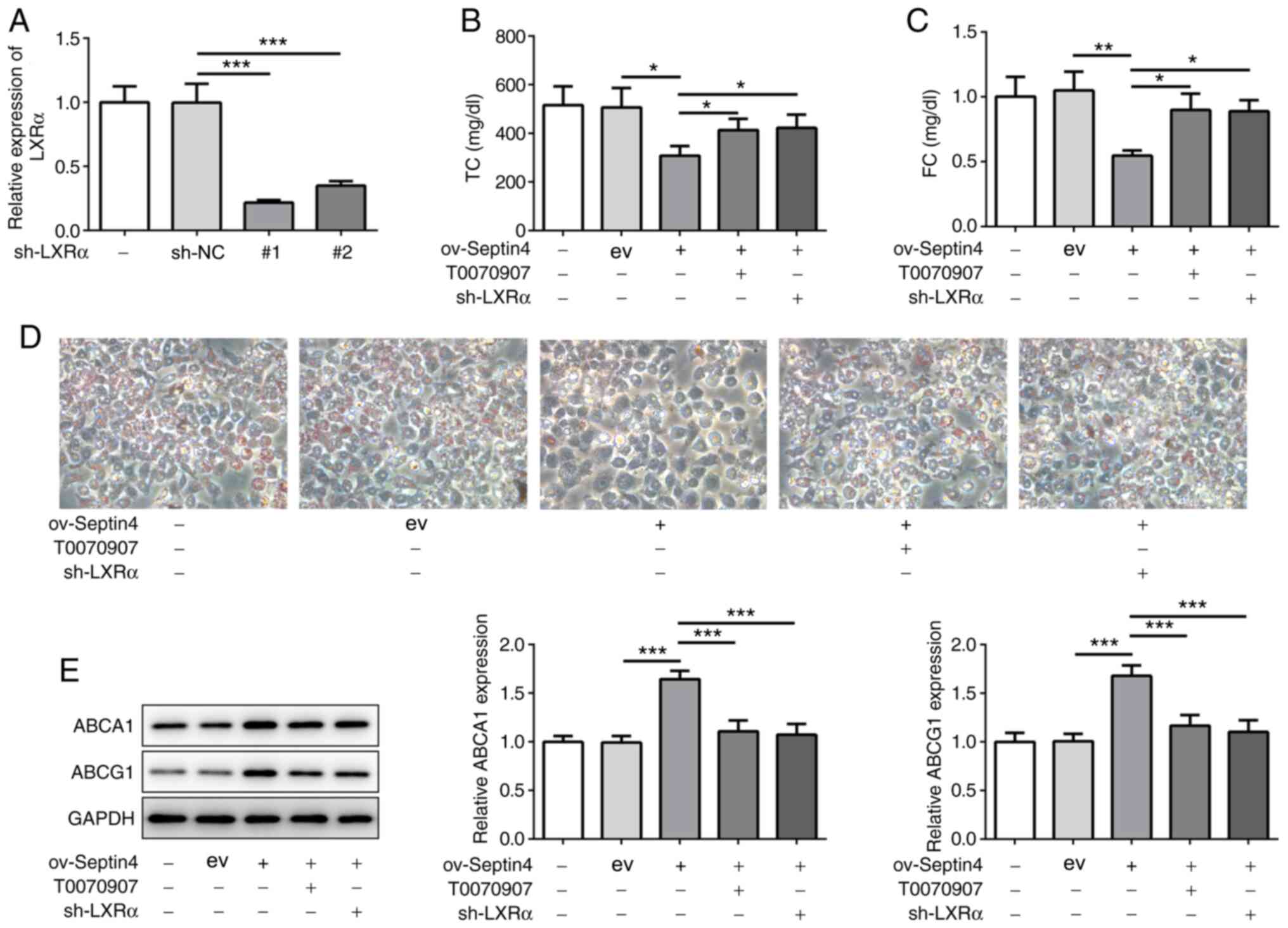 | Figure 4PPARγ or LXRα inhibition reverses the
effect of Septin 4 overexpression on cholesterol accumulation. (A)
Transfection efficiency analysis for LXRα knockdown in THP-1 cells
was conducted by reverse transcription-quantitative PCR.
Measurements of (B) TC and (C) FC content in THP-1
macrophage-derived foam cells following PPARγ or LXRα inhibition in
the presence of Septin 4 overexpression. (D) THP-1 cells following
PPARγ or LXRα inhibition in the presence of Septin 4 overexpression
were treated with PMA to differentiate them into macrophages. The
macrophages were then incubated with or without 50 µg/ml ox-LDL for
24 h and stained with Oil Red O. Magnification, x200. (E) ABCA1 and
ABCG1 protein expression levels in THP-1 macrophage-derived foam
cells following PPARγ or LXRα inhibition, in the presence of Septin
4 overexpression, were measured by western blotting.
*P<0.05, **P <0.01 and
***P<0.001. PPARγ, proliferator activated receptor γ;
LXRα, liver X receptor α; ABCA1, ATP binding cassette subfamily A
member 1; ABCG1, ATP binding cassette subfamily G member 1; PMA,
phorbol-12-myristate-13-acetate; ox-LDL, oxidized low-density
lipoprotein; sh, short hairpin; NC, negative control; ov,
overexpression; ev, empty vector. |
Discussion
AS is a chronic and maladaptive inflammatory disease
that is caused by the accumulation of modified lipoproteins, such
as ox-LDL, in the arterial wall (18,19).
These lipoproteins can activate the endothelium, following which
activated endothelial cells recruit circulating monocytes to
differentiate into macrophages, which then uptake lipoprotein and
transform into foam cells to aggravate damage to the blood vessel
wall (19). Therefore, preventing
the accumulation of cholesterol in macrophages in addition to the
transformation of macrophages into foam cells may be beneficial in
alleviating AS.
The present study demonstrated that the expression
of Septin 4 was upregulated in PMA-stimulated THP-1 macrophages,
which was increased further upon ox-LDL stimulation, suggesting
that Septin 4 expression was gradually enhanced during the
formation of THP-1-derived foam cells. This result was consistent
with another recent finding, which revealed that Septin 4 was
highly expressed in ApoE-/- AS mice and PDGF-BB-induced HAVSMCs,
where it could prevent PDGF-BB-induced HAVSMC phenotypic
transformation, proliferation and migration (14). In addition, Wang et al
(20) previously reported that
Septin 4 expression was upregulated in the mouse aorta and cultured
vascular smooth muscle cells (VSMCs) following angiotensin-II
stimulation, thus regulating Angiotensin-II mediated VSMCs
proliferation and migration. The proliferation and migration of
VSMCs are the core processes leading to AS (21). In response to certain stimuli, VSMCs
exhibit aberrant proliferation, migration and extracellular matrix
(ECM) synthesis, which will promote plaque formation and contribute
to the progression of hypertension, AS and other vascular diseases
(22). Therefore, in accordance
with previous reports, the present findings provided evidence that
Septin 4 served an inhibitory role in the development of AS, such
that upregulation of Septin 4 may protect cells against
cholesterol-induced injury. The present study also knocked down and
overexpressed Septin 4 in THP-1 macrophages in the presence of
ox-LDL treatment. The results demonstrated that knocking down
Septin 4 expression can promote cholesterol accumulation in THP-1
macrophages and then formation of foam cells. By contrast, Septin 4
overexpression resulted in the opposite effects being observed.
Therefore, it can be suggested that Septin 4 served as an inhibitor
of the formation of foam cells from THP-1 macrophages.
In the present study, Septin 4 overexpression
elevated, whilst its knockdown reduced ABCA1 and ABCG1 expression.
Previous studies reported that ABCA1/G1 serves a key role in
promoting cellular cholesterol efflux and regulating lipid
metabolism (9,23). In total, >50% cholesterol is
excreted from macrophages by ABCA1 and ABCG1, whereby in advanced
plaques, reduced expression levels of ABCA1 and ABCG1 typically
leads to an 80% reduction in cholesterol efflux and increases in
lipid accumulation (8). The
expression of ABCA1/G1 can be regulated by a large network of
transcription factors, including PPARγ and LXRα, which can be
activated by ox-LDL and directly increases the expression of
ABCA1/G1(24). It has been
previously reported that PPARγ and LXRα agonists can promote
cholesterol efflux from macrophages and relieve AS (25). In addition, the present study
suggests that overexpression of Septin 4 could upregulate PPARγ and
LXRα expression levels but Septin 4 knockdown exerted opposite
effects. These findings support notion that Septin 4 may enhance
ABCA1/G1 expression by activating PPARγ/LXRα signaling.
To further verify the present hypothesis, T0070907
was used to inhibit PPARγ and shRNA was utilized to silence LXRα
expression in ox-LDL treated THP-1 macrophages in the presence of
Septin 4 overexpression. The inhibitory effects of Septin 4
overexpression on cholesterol accumulation and its positive effects
on ABCA1/G1 expression were reversed by both PPARγ and LXRα
inhibition. This finding supports the notion of an important role
of PPARγ/LXRα signaling in mediating the actions of Septin 4 on
cholesterol accumulation in THP-1 macrophages. However, the present
study only displayed in vitro findings. In vivo animal models or
human samples will need to be applied in in future studies to
validate the conclusions in the present study.
In conclusion, the present study suggests that
Septin 4 was involved in preventing THP-1 macrophage transformation
into foam cells stimulated by ox-LDL through the activation of
PPARγ/LXRα signaling, thereby increasing ABCA1/G1 expression. The
present study provided a potentially novel regulation pathway of
ox-LDL-induced foam cell formation and demonstrated that Septin 4
upregulation may be a new target for preventing foam cell formation
during the development of AS.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Medical guidance
(TCM) science and technology-supported project of Science and
Technology Commission of Shanghai Municipality (grant no.
17401933700), Scientific research project of Shanghai Municipal
Health Commission (grant no. 201640039) and Peak plateau subject of
Shanghai University of Traditional Chinese Medicine (Special
project for clinical talents; grant no. 171319).
Availability of data and materials
All datasets generated and/or analyzed during the
present study are included in this published article.
Authors' contributions
XYS and GLY conceived, designed the study and
acquired the data. HHW analyzed the data. DFL drafted the
manuscript and revised it for critically important intellectual
content. All authors read and approved the final manuscript. XYS
and GLY confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing
interests.
References
|
1
|
Geovanini R and Libby P: Atherosclerosis
and inflammation: Overview and updates. Clin Sci (Lond).
132:1243–12522. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Palasubramaniam J, Wang X and Peter K:
Myocardial Infarction-From Atherosclerosis to Thrombosis.
Arterioscler Thromb Vasc Biol. 39:e176–e185. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yang X, Li J, Hu D, Chen J, Li Y, Huang J,
Liu X, Liu F, Cao J, Shen C, et al: Predicting the 10-year risks of
atherosclerotic cardiovascular disease in Chinese population: The
China-PAR Project (Prediction for ASCVD Risk in China).
Circulation. 134:1430–1440. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
He J, Gu D, Wu X, Reynolds K, Duan X, Yao
C, Wang J, Chen CS, Chen J, Wildman RP, et al: Major causes of
death among men and women in China. N Engl J Med. 353:1124–1134.
2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhou M, Wang H, Zhu J, Chen W, Wang L, Liu
S, Li Y, Wang L, Liu Y, Yin P, et al: Cause-specific mortality for
240 causes in China during 1990-2013: A systematic subnational
analysis for the Global Burden of Disease Study 2013. Lancet.
387:251–272. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang D, Yang Y, Lei Y, Tzvetkov NT, Liu X,
Yeung AWK, Xu S and Atanasov AG: Targeting foam cell formation in
atherosclerosis: Therapeutic potential of natural products.
Pharmacol Rev. 71:596–670. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chistiakov DA, Melnichenko AA, Myasoedova
VA, Grechko AV and Orekhov AN: Mechanisms of foam cell formation in
atherosclerosis. J Mol Med (Berl). 95:1153–1165. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chistiakov DA, Bobryshev YV and Orekhov
AN: Macrophage-mediated cholesterol handling in atherosclerosis. J
Cell Mol Med. 20:17–28. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yu XH, Fu YC, Zhang DW, Yin K and Tang CK:
Foam cells in atherosclerosis. Clin Chim Acta. 424:245–252.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang JM, Wang D, Tan YY, Zhao G and Ji ZL:
22(R)-hydroxycholesterol and pioglitazone synergistically decrease
cholesterol ester via the PPARγ-LXRα-ABCA1 pathway in cholesterosis
of the gallbladder. Biochem Biophys Res Commun. 447:152–157.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kim JS, Lee YH, Chang YU and Yi HK: PPARγ
regulates inflammatory reaction by inhibiting the MAPK/NF-κB
pathway in C2C12 skeletal muscle cells. J Physiol Biochem.
73:49–57. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sun X, Yang Y, Zhu D, Qian H, Duan Y, He
X, Gu X, Sun W and Zhu Y: Expression of Septin4 in human hepatic
stellate cells LX-2 stimulated by LPS. Inflammation. 36:539–548.
2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhao X, Feng H, Wang Y, Wu Y, Guo Q, Feng
Y, Ma M, Guo W, Song X, Zhang Y, et al: Septin4 promotes cell death
in human colon cancer cells by interacting with BAX. Int J Biol
Sci. 16:1917–1928. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang N, Zhang Y, You S, Tian Y, Lu S, Cao
L and Sun Y: Septin4 Prevents PDGF-BB-induced HAVSMC Phenotypic
Transformation, Proliferation and Migration by Promoting
SIRT1-STAT3 Deacetylation and Dephosphorylation. Int J Biol Sci.
16:708–718. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sun Y and Xu J: TCF-4 Regulated
lncRNA-XIST promotes M2 polarization of macrophages and is
associated with lung cancer. OncoTargets Ther. 12:8055–8062.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ma MM, Jin CC, Huang XL, Sun L, Zhou H,
Wen XJ, Huang XQ, Du JY, Sun HS, Ren ZX, et al: Clcn3 deficiency
ameliorates high-fat diet-induced obesity and adipose tissue
macrophage inflammation in mice. Acta Pharmacol Sin. 40:1532–1543.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhu Y, Xian X, Wang Z, Bi Y, Chen Q, Han
X, Tang D and Chen R: Research progress on the relationship between
atherosclerosis and inflammation. Biomolecules.
8(80)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kattoor AJ, Kanuri SH and Mehta JL: Role
of Ox-LDL and LOX-1 in Atherogenesis. Curr Med Chem. 26:1693–1700.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang N, Xu F, Lu S, Zhang N and Sun Y:
Septin4 as an autophagy modulator regulates Angiotensin-II mediated
VSMCs proliferation and migration. Biochem Biophys Res Commun.
525:272–279. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chistiakov DA, Orekhov AN and Bobryshev
YV: Vascular smooth muscle cell in atherosclerosis. Acta Physiol
(Oxf). 214:33–50. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bennett MR, Sinha S and Owens GK: Vascular
smooth muscle cells in Atherosclerosis. Circ Res. 118:692–702.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Westerterp M, Fotakis P, Ouimet M, Bochem
AE, Zhang H, Molusky MM, Wang W, Abramowicz S, la Bastide-van
Gemert S, Wang N, et al: Cholesterol efflux pathways suppress
inflammasome activation, NETosis, and atherogenesis. Circulation.
138:898–912. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lin XL, Hu HJ, Liu YB, Hu XM, Fan XJ, Zou
WW, Pan YQ, Zhou WQ, Peng MW and Gu CH: Allicin induces the
upregulation of ABCA1 expression via PPARγ/LXRα signaling in THP-1
macrophage-derived foam cells. Int J Mol Med. 39:1452–1460.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Grygiel-Górniak B: Peroxisome
proliferator-activated receptors and their ligands: Nutritional and
clinical implications - a review. Nutr J. 13(17)2014.PubMed/NCBI View Article : Google Scholar
|