Introduction
Stroke, a common neurological disorder associated
with a high risk of disability and mortality (1), disrupts the blood-brain barrier (BBB),
where it can induce neuronal injury through brain edema and
inflammation (2). There is
increasing evidence that inflammation contributed to the
progression of ischemia-induced secondary brain damage, leading to
aggravated dysregulation of the BBB, as well as cerebral edema
(3,4). Thus, identification of novel drugs to
protect against ischemic stroke and to understand the underlying
mechanisms involved is important to combat this pathology (5).
The BBB consists of a variety of cell types and acts
as a border between the brain and the blood circulating through the
body (6). It is well-known that BBB
impairment is a consequence of ischemic stroke (7,8).
Controlling the BBB during stroke has both neuronal and vascular
protective effects (9). Previous
studies have suggested that NF-κB and MMP-9, which are central
mediators of inflammation, play pivotal roles in inflammatory
damage following experimental ischemic stroke (10,11).
There are numerous types of MMPs. The MMP that
damages the BBB following stroke is gelatinase MMP-9(12). Under normal, steady state
conditions, MMP-9 protein expression levels in endothelial cells
are low and are present as inactive zymogens. In animals
experiencing cerebral ischemia and reperfusion, inflammatory
mediators are upregulated and activate leukocytes, which can
secrete MMP-9(13). When MMP-9
protein levels in the endothelial cells are elevated, extracellular
matrix components and substrates in the BBB are damaged, primarily
through degradation of type IV collagen. Structural damage to the
BBB will increase permeability and result in vascular-derived brain
edema, and toxic substances are introduced into the BBB and harm
brain tissue (14). Notably, NF-κB
directly regulates the transcription of MMP-9, and inhibition of
NF-κB activity reduces the protein expression level of MMP-9, as
well as inflammation (15,16). Reduced MMP-9 protein expression in
endothelial cells, as well as decreased inflammation, reduces BBB
disruption and cerebral injury caused by ischemic stroke (17).
Curcumin, a pleiotropic agent extracted from the
rhizome of Curcuma longa (18), exerts pharmacological effects
against stroke via its anti-oxidative and anti-inflammatory action
(19). Previous research has
revealed that curcumin could pass through the BBB and significantly
decreased water content, infarct size and BBB leakage in a middle
cerebral artery occlusion/reperfusion (MCAO/R) rat model (20-23).
The post-ischemic neuroprotective effects of curcumin have been
investigated; however, the potential mechanisms behind these
effects are not fully known.
The aim of the present study was to assess how
curcumin affected brain injury, the BBB status and the protein
expression level of phosphorylated (p)NF-κBp65 and MMP-9 in a rat
MCAO/R stroke model. We hypothesized that pretreatment with
curcumin would protect BBB integrity after MCAO/R in the brain via
its anti-inflammatory effects, including the attenuation of
pNF-κBp65 and MMP-9 in endothelial cells and increase the
expression level of tight junction proteins.
Materials and methods
Animal and establishment of the stroke
model
A total of 90 3-month-old male Sprague-Dawley (SD)
rats, weighing 250-280 g were provided by Xuzhou Medical University
(Jiangsu, China). The rats were housed in a temperature-controlled
(22±2˚C) setting, including 55±10% humidity and a 12 h light/dark
cycle with free access to food and water. All rats were
sufficiently adapted to their environments before subjected to
surgery. The MCAO/R stroke model used in the present study was
established as previously described (24). Briefly, rats were intraperitoneally
anesthetized with ketamine (60 mg/kg) and xylazine (5 mg/kg). The
ventral midline neck was cut to expose the right common (CCA),
right internal (ICA) and right external (ECA) carotid arteries. The
ECA branches were then ligated. Next, a 4-0 nylon monofilament
containing a round tip (Guangzhou Jialing Biotechnology Co., Ltd.)
was inserted along the CCA into the ICA. This was performed until
resistance was detected. Approximately 90 min following MCAO
surgery, the monofilament was removed, followed by reperfusion for
24 h, and 3 and 7 days post-MCAO. The exact same surgery was
performed in the sham group rats; however, filament insertion was
not performed.
The SD rats were randomized into three individual
groups, including the sham (n=30), the MCAO/R with vehicle (n=30)
and the MCAO/R plus curcumin groups (n=30). Curcumin (300 mg/kg;
Sigma-Aldrich; Merck KGaA) was dissolved in 2% dimethyl sulfoxide
(vehicle) and intraperitoneally administered 30 min prior to MCAO/R
surgery (25). All the experiments
were performed following guidelines written by the Institutional
Animal Care and Use Committee of China. The studies were also
approved by the Ethics Committee for the Use of Experimental
Animals at Xuzhou Medical University (assurance nos. 2015-46 and
2015-47).
Assessing neurological deficits
The modified Neurological Severity Scale (mNSS) has
a total score of 18 points and is divided into 4 parts as follows:
Motion, sensation, balance, and reflex. The score of normal rats was
0. The higher the score, the more severe the symptoms of neural
power defciency. Neurological functions were evaluated 24 h, and 3
and 7 days following MCAO/R using a modified Neurological Severity
Scale (mNSS), as previously described (26). This scale includes measurement of
balance, reflex, sensory and motor skills. The mNSS is similar to
the contralateral neglect tests performed for humans. Neurological
functions were measured on a scale from 0 to 18, where normal was
scored as 0 and maximum deficit was scored as 18. A mNSS score of 0
was indicative of the sham group.
Measuring infarct size
The rats were anesthetized 24 h post-MCAO/R and 2 mm
coronal slices were prepared. The issues were stained at 37˚C for
30 min using 2% solution 2, 3, 5-triphenyltetrazolium chloride
(TTC; Sigma-Aldrich; Merck KGaA) diluted in PBS. Following which,
the tissues were fixed using 4% paraformaldehyde in a 4˚C
refrigerator overnight. Red staining was associated with undamaged
regions after MCAO/R and white stained areas revealed regions
experiencing an infarct. The relative infarction volume percentage
(RIVP) was calculated using the following formula: RIVP=total
infarct area/total area x100%. Infarct size was quantified using
ImageJ software 1.8.0 (National Institutes of Health).
Rapid golgi staining
The brains were removed from the rats and processed
using the rapid Golgi staining kit (FD Neurotechnologies, Inc.),
based on the instructions provided by the manufacturer. Briefly,
serial sections (100-µm) from the hippocampus were prepared on a
freezing microtome and dehydrated in an ascending absolute ethanol
series (50, 70 and 90%), washed in xylene and mounted in neutral
balsam. Next, 5 pyramidal neurons extracted from each rat (3
rats/group; 20 brain sections from each group) were measured from
area CA1 of the hippocampus. A camera lucida drawing tube, attached
to an Olympus BX51 microscope (x400; Olympus Corporation) was used
to select neurons for analyses. To analyze the neurons, the soma
center was considered as the reference dot. Total dendritic length
and number were measured every 50 µm.
Evaluation of BBB permeability
To measure BBB permeability, Evans blue (EB) dye was
used as a tracer, as previously described (27). Briefly, the rats were injected with
2% EB solution diluted in normal saline through the tail vein (2
ml/kg of body weight; Sigma-Aldrich; Merck KGaA) 24 h post-surgery.
The EB dye was allowed to circulate for 2 h. Next, the rats were
anesthetized and transcardially perfused using 0.9% sodium
chloride. The brains were removed, divided into left and right
hemispheres, then the right hemisphere was immersed in formamide
(10 ml/kg; Sigma-Aldrich; Merck KGaA) at 60˚C for 24 h. Cortical
proteins were formamide extracted and centrifuged (2,500 x g) for
10 min at 4˚C. A total of 1 ml supernatant was measured in a
spectrophotometer at 620 nm to compare EB content in the brain
tissue with standard EB solution.
Immunofluorescence
Following brain perfusion, the tissues were further
post-fixed overnight in 4% paraformaldehyde and 4˚C refrigerator,
then dehydrated in 30% sucrose. Cerebral peri-ischemic cortices
were sliced into 20-µm slices and washed in PBS. The slices were
then incubated in blocking solution 0.1% Triton X-100 in 0.1M PBS,
10% bovine serum albumin (cat. no. Pro-422, pH 7.6; Prospec-Tany
Technogene Ltd.) in a 4˚C refrigerator for 2 h, followed by
overnight incubation at 4˚C with anti-ZO-1 (1:100; cat. no.
ab96587; Abcam) and anti-claudin-5 (1:100; cat. no. 352588; Thermo
Fisher Scientific, Inc.) antibodies. After washing in PBS, the
tissues were incubated with goat biotin-conjugated anti-cat IgG
antibody (1:10,000, cat. no. LS-C68537; LifeSpan BioSciences, Inc.)
and counterstained with 4',6-diamidino-2-phenylindole (Beyotime
Institute of Biotechnology). Lastly, the sections were analyzed
using a spectral confocal microscope DMI6000 (Leica Microsystems
GmbH).
Western blot analysis
Peri-ischemic cortical proteins were extracted from
the rat brains by homogenizing the tissues in RIPA lysis buffer
(cat. no. P0013B; Beyotime Institute of Biotechnology),
supplemented with protease and phosphatase inhibitors. The samples
were centrifuged at 7,000 x g (EP5417R; Eppendorf) for 20 min at
4˚C. A BCA assay kit (Thermo Fisher Scientific, Inc.) was used to
measure the concentration of each sample. Nuclear and cytoplasmic
extraction kits (cat. no. 78833; Thermo Fisher Scientific, Inc.)
for nuclear protein extraction. An equal amount (0.06 mg) of
protein was separated using 6 and 10% SDS-PAGE before being
transferred to a PVDF membrane (EMD Millipore). Membranes were
blocked with 5% skimmed milk for 90 min. Following blocking, the
membranes were incubated overnight at 4˚C with anti-pNF-κBp65
(1:1,000; cat. no ab222494; Abcam), anti-NF-κBp65 (1:1,000; cat.
no. ab228497; Abcam), anti-MMP-9 (1:1,000; cat. no. ab38898;
Abcam), anti-β-actin (1:5,000; cat. no. ab11003; ABclonal Biotech
Co., Ltd.), anti-occludin (1:1,000; cat. no. ab216327; Abcam),
anti-claudin-5 (1:500; cat. no. 35288; Thermo Fisher Scientific,
Inc.) and anti-ZO-1 (1:1,000; cat. no. ab96587; Abcam) antibodies.
After several washes with TBS/0.1% Tween-20 and incubation with the
goat anti-cat IgG antibody (1:10,000, LS-C68537, LSBio) for 2 h at
room temperature, the immuno-reactive bands were visualized using
an ECL kit (Beyotime Institute of Biotechnology). The protein bands
were then analyzed using ImageJ software version 1.8.0 (National
Institutes of Health).
Statistical analysis
All the data are presented as the mean ± SEM.
Shapiro-Wilk normality test was used to determine if the data was
normally distributed. Statistically significant differences were
analyzed using one-way ANOVA for multiple groups or an unpaired
Student's t-test for comparisons of 2 groups were applied. All
statistical analyses were performed using GraphPad prism v5
(GraphPad Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Curcumin ameliorates early brain
injury and improves neurological performance in MCAO/R rats
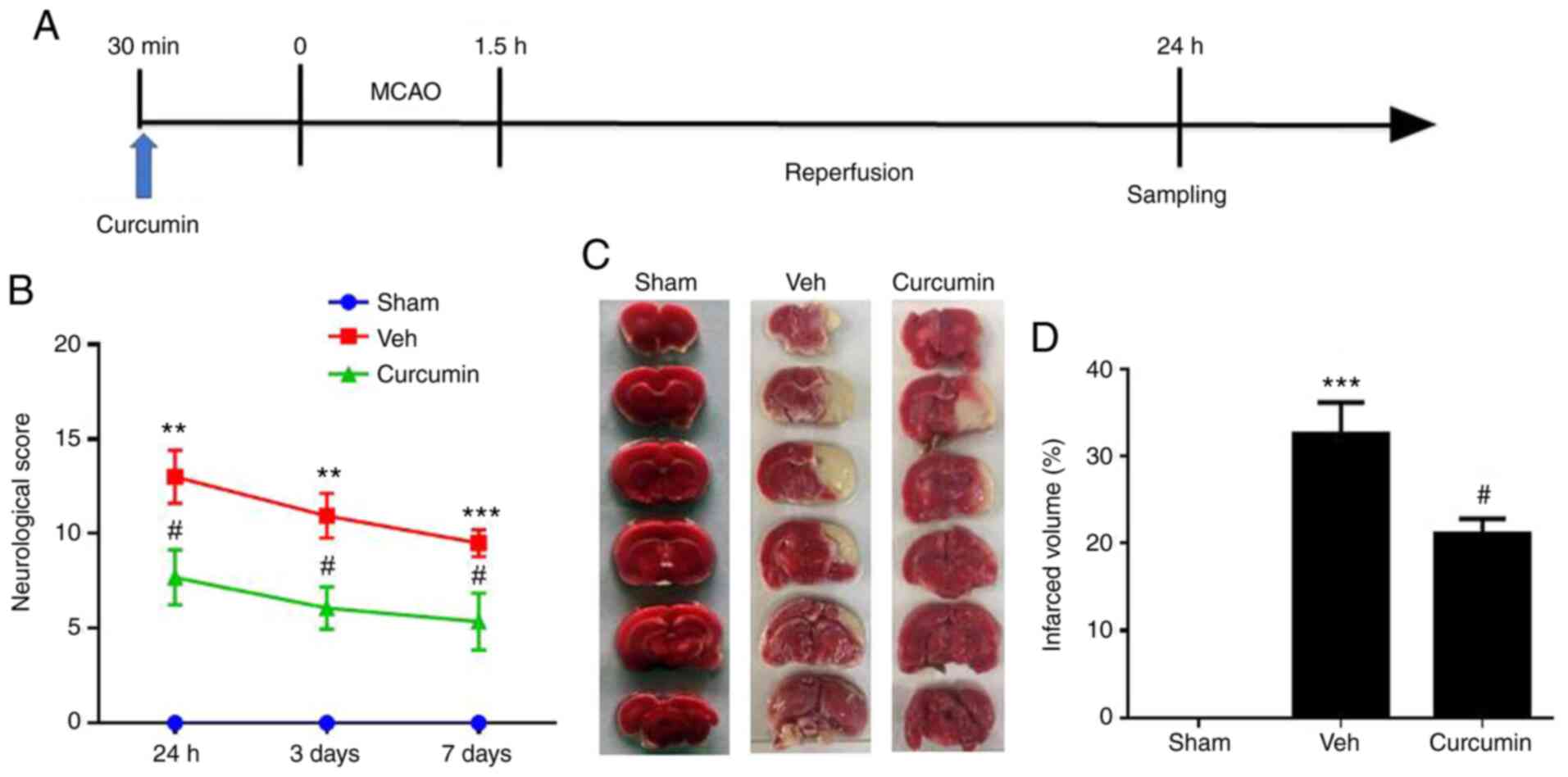
A schematic diagram representing experimental
procedures is shown in Fig. 1A
(reperfusion was performed 24 h, and 3 and 7 days following
MCAO/R). As depicted in Fig. 1B,
vehicle-treated MCAO/R rats exhibited poor and reduced neurological
function as compared with that in the sham group. Based on the mNSS
scale, the MCAO/R rats treated with curcumin showed a significant
improvement in neurological performance at all the measured time
points (24 h, and 3 and 7 days following reperfusion), where the
most optimal effect was noted 24 h post-surgery (P<0.05;
Fig. 1B). As the lowest mNSS score
was observed 24 h following reperfusion, this time point was
selected for subsequent experiments.
TTC staining was used to measure infarct volume 24 h
post-reperfusion. The results showed an absence of infarct in the
sham group, but significant ischemic injury in the MCAO/R group
compared with that in the sham group (P<0.001; Fig. 1C). However, curcumin pre-treatment
significantly reduced infarct size compared with that in the
vehicle treated MCAO/R group (P<0.05; Fig. 1D). Therefore, it was concluded that
pretreatment with curcumin protected rats against cerebral injury
and improved neurological deficits in MCAO/R rats.
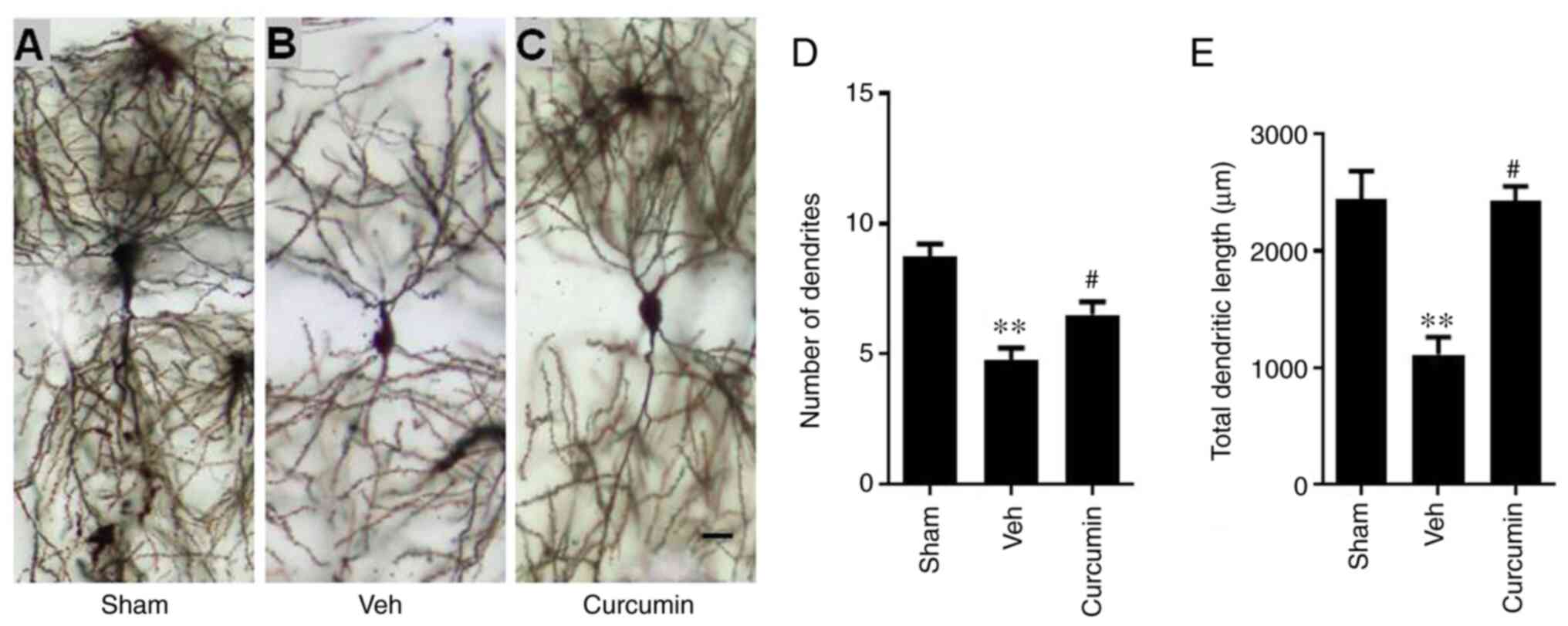
Curcumin protects synaptic remodeling
of hippocampal neurons against MCAO/R
As shown in Fig.
2A-E, neurons were selected according to the following
criteria: The body and dendrites of the neuron were completely
impregnated, the neuron was relatively separated from the
surrounding neurons and the neuron was located in the hippocampal
CA1 area. Golgi staining and quantitative analysis indicated that
rats in the vehicle-treated MCAO/R group showed a significant
decrease in the number of hippocampal dendrites, as well as total
dendritic length as compared with that in the sham group
(P<0.01). The rats that were treated with curcumin before MCAO/R
had significantly reduced MCAO/R-induced atrophy in the hippocampal
neurons, as there was an increase in both dendrite number and
length (P<0.05). These data revealed that curcumin could protect
hippocampal neuron remodeling when subjected to MCAO/R.
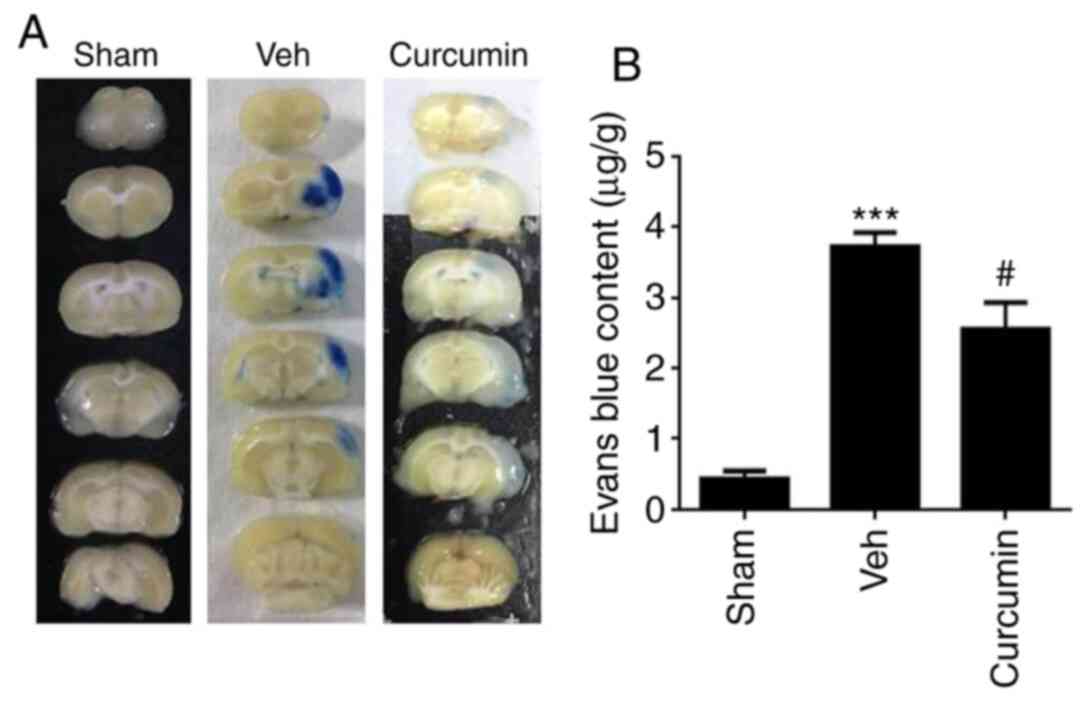
Curcumin pretreatment protects against
BBB permeability induced by MCAO/R
The brain sections were viewed using a EB tracer, as
shown in Fig. 3A. Leakage of EB dye
was significantly attenuated in the curcumin group compared with
that in the MCAO/R vehicle group. Levels of formamide extracted
from EB dye were further analyzed. Fig.
3B demonstrated that MCAO/R induced significant EB
extravasation as compared with that in the sham group at 24 h
(P<0.001). Curcumin pretreatment significantly reduced EB
extravasation levels (P<0.05) compared with that in the
vehicle-treated MCAO/R group. This indicated that curcumin could
protect MCAO/R rats from disrupted BBB permeability resulting from
ischemic damage.
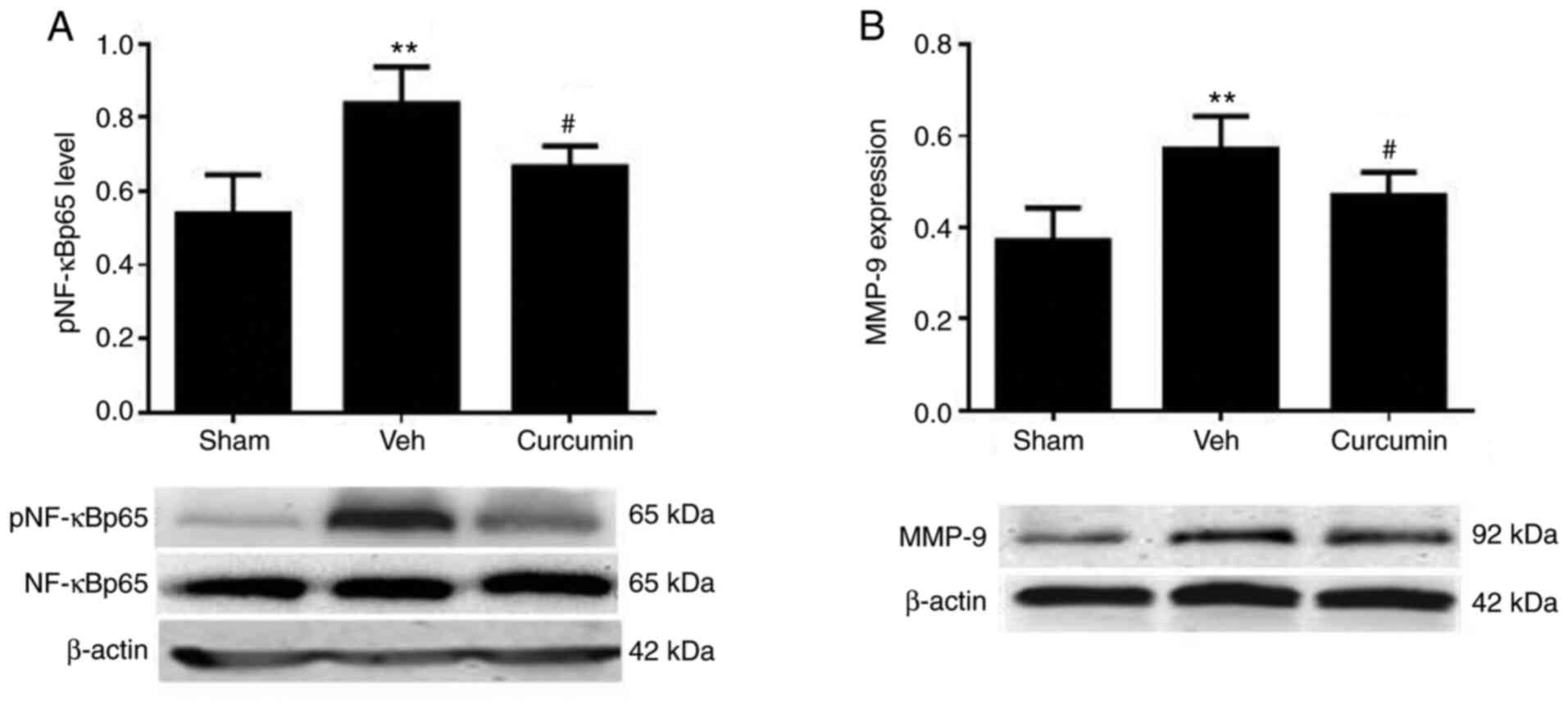
Curcumin suppresses the MCAO/R-induced
inflammatory response
The inflammatory response is activated as a result
of BBB injury (28); therefore, it
was determined whether curcumin pretreatment could affect the
protein expression levels of known inflammatory mediators.
Following reperfusion for 24 h, the protein expression levels of
both pNF-κBp65 and MMP-9 were increased. However, when exposed to
curcumin pretreatment, there was a significant reduction in the
expression levels of both pNF-κBp65 (P<0.01; Fig. 4A) and MMP-9 (P<0.05; Fig. 4B) compared with the vehicle groups.
Taken together, pretreatment with curcumin suppressed the
inflammatory response, which was revealed by the reduction of key
inflammatory mediators.
Curcumin preserves in-situ protein
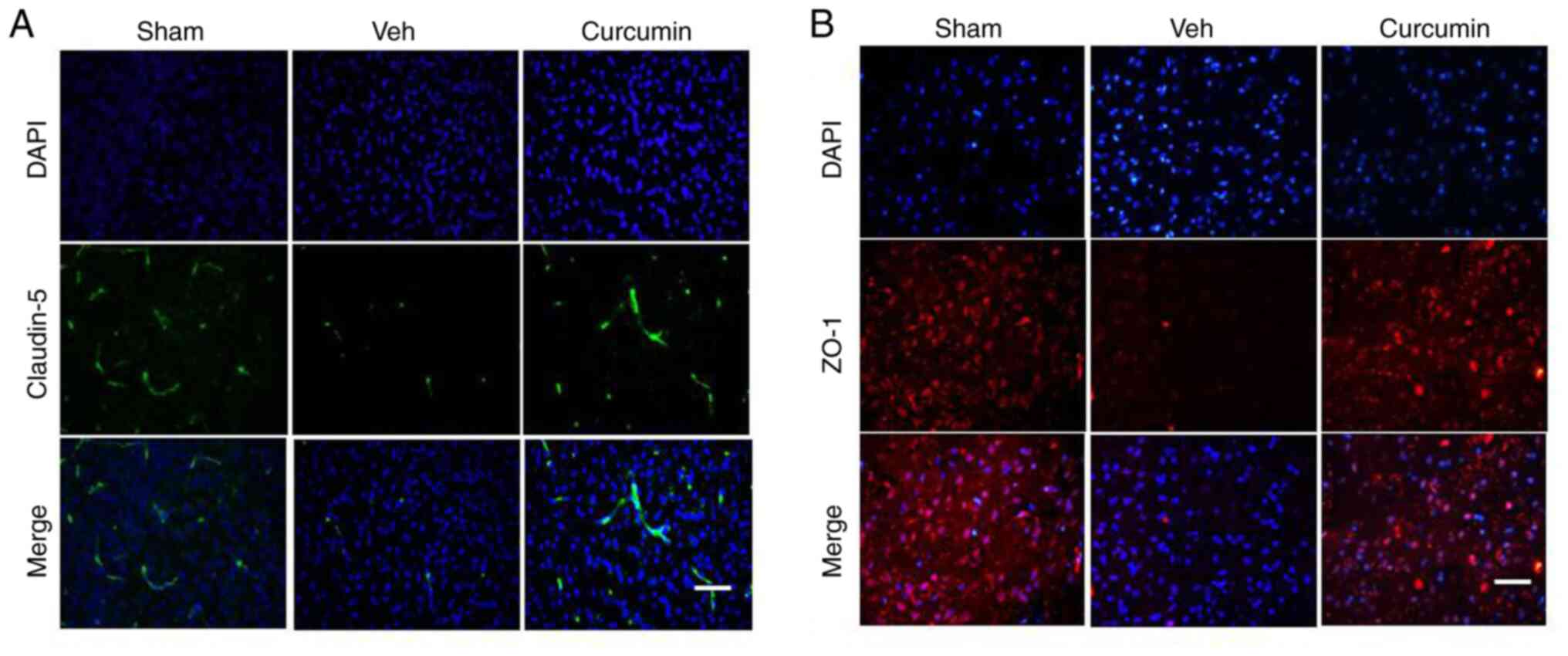
expression level of claudin-5 and ZO-1 in MCAO/R rats
Subsequently, the effects of curcumin pretreatment
on BBB permeability were further investigated to confirm the
earlier result. Immunohistochemistry, using claudin-5 and ZO-1
antibodies, was performed on the tissues extracted from the rats in
the sham and MCAO/R groups, 24 h following reperfusion. As seen in
Fig. 5A and B, claudin-5 and ZO-1 protein expression
levels were markedly decreased in the cortex of the rats, 24 h
following MCAO/R injury as compared with that in the sham group.
However, in the group of curcumin pretreated rats, both claudin-5
and ZO-1 expression levels were increased as compared with that in
the MCAO/R rats treated with vehicle. These data suggested that
curcumin could reverse the attenuation of claudin-5 and ZO-1, which
typically occurs when the BBB is disrupted following ischemic brain
injury.
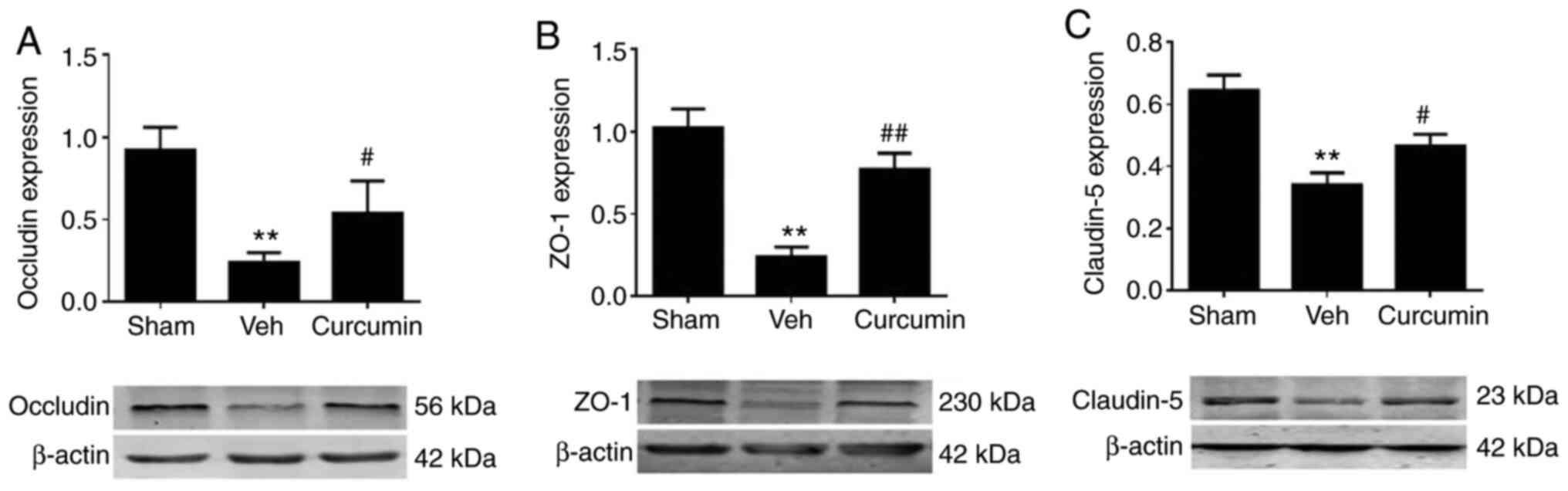
Curcumin treatment prevents the
degradation of BBB tight junction proteins
Lastly it was investigated how curcumin pretreatment
affected BBB integrity and permeability in rats, which had
experienced a stroke. The protein expression levels of tight
junction proteins, ZO-1, occludin and claudin-5 were analyzed,
which play an important role in BBB integrity (29). Western blot analysis followed by
densitometry analysis revealed that MCAO/R induced significant
reduction of ZO-1, occludin and claudin-5 protein expression
levels, 24 h following reperfusion as compared with that in the
sham rats. Notably, the protein expression levels of these tight
junction proteins were significantly increased following curcumin
pretreatment (P<0.05; Fig.
6A-C). Therefore, curcumin may protect the integrity of the BBB
by inhibiting the degradation of tight junction proteins induced by
MCAO/R.
Discussion
In the present study, the effects of curcumin on
reperfusion-induced BBB disruption in a rat model of MCAO/R was
investigated. The results revealed that pretreatment with curcumin
protected rats from MCAO/R and the resulting neurological deficits,
reduced infarct size, as well as nerve injury. To obtain a
mechanistic insight behind these effects, it was identified that
curcumin repressed the inflammatory response and restored levels of
tight junction proteins early enough to protect against BBB
deterioration.
Curcumin belongs to a class of yellow polyphenols
derived from turmeric medicinal plants (25). A previous study has shown that
curcumin exerted an array of biological functions, including
anti-tumor, anti-immunity, anti-oxidation, anti-fibrosis and
anti-inflammatory effects (30).
Recently, a study has indicated that curcumin harbored protective
effects against neuronal damage following cerebral ischemia
(31). In addition, previous
studies have shown that following curcumin treatment, neurological
scores were improved, early brain injury was ameliorated, and NF-κB
and MMP-9 protein expression levels in brain tissue of injured rats
were decreased (25,32,33).
Furthermore, both the number of hippocampal neuronal dendrites and
total dendritic length were increased and EB content in the brain
tissue was significantly decreased (34-36).
The results from the present study provides additional results by
revealing the beneficial effects of curcumin pretreatment against
MCAO/R injury. Abnormal hippocampal neurotransmitters may affect
neural plasticity, which may be associated with the pathophysiology
of ischemic cerebral infarction (37). In the present study, it was found
that in the MCAO/R rat model, hippocampal neuronal remodeling could
be significantly disrupted following ischemia and reperfusion. It
was also confirmed that the administration of curcumin protected
the BBB structure, inhibited the inflammatory response of the
hippocampus, and remodeled hippocampal neurons against MCAO/R
(37-39).
The protective effect of curcumin on hippocampal neurons provides
evidence of its advantage in the treatment of ischemic stroke.
In most unstimulated cells, the inactive p65
component of NF-κB remains in the cytoplasm as a dimer, which can
bind to IkB. The protein expression levels of tumor necrosis factor
(TNF)-α and IL-1β were increased at the early stages of the
inflammatory response following cerebral ischemia and reperfusion
(30,40). As the initiating factors of the
inflammatory response, both TNF-α and IL-1β can stimulate
leukocytes and activate the c signaling pathway (33,41).
The IkB protein is phosphorylated and targeted for degradation via
the proteasome pathway, where the p65 component of NF-κB is
translocated to the nucleus to form pNF-κBp65 and increases the
secretion of adhesion molecules via microvascular endothelial cells
and leukocytes (42). Leukocytes
can adhere to vascular endothelial cells and produce
neutrophil-derived oxidant and MMP-9(13), resulting in BBB damage from MMP-9
(25,32). Previous studies have also showed
that MMP-9 protein was expressed in endothelial cells and the main
cellular source of brain MMP-9 are brain microvascular endothelial
cells in the initial phase following focal cerebral ischemia
(43,44). Based on this, it could be concluded
that inhibition of NF-κB activation could reduce
ischemia-reperfusion injury in rats (45). The protein expression levels of
pNF-κBp65 were analyzed in the nucleus. The results showed that
curcumin pretreatment effectively reduced the protein expression
levels of pNF-κBp65, inhibited the transcriptional activity of
NF-κB in cerebral ischemia/reperfusion (I/R) and may have a strong
anti-inflammatory effect.
Activation of MMP-9 leads to the degradation of key
proteins located in the cerebral blood vessels, mainly through the
degradation of type IV collagen. The extracellular matrix
components and substrates in the BBB are destroyed, resulting in
the extravasation of water in the capillaries and plasma proteins
in the peripheral blood, resulting in an increase of water in the
cell space and formation of vascular-derived brain edema. There is
an introduction of toxic substances into the BBB and damage to
brain tissue, which destroys the integrity of the vascular
structure (43,46,47).
Previously, the association between MMP-9 and ischemic brain injury
has gained interest (46,47). When an inflammatory reaction occurs
in the body, activated white blood cells secrete substances that
can be toxic, including IL-8, TNF, MMPs, nitric oxide and reactive
oxygen species. Among these, MMP-9 is a key protein, which causes
damage to the BBB. Inhibition of MMP-9 has been reported to prevent
damage to the brain by maintaining BBB integrity in elderly humans
(48). In the present study,
curcumin pretreatment was found to significantly reduce MMP-9
protein expression levels induced following MCAO/R. This is
consistent with previous reports showing that MMP-9 inhibition
mediated BBB protection in a mouse model of ischemic stroke
(16). Therefore, it is reasonable
to conclude that curcumin pretreatment could reduce BBB damage at
least via the reduction of MMP-9 protein expression level to
protect against damage from cerebral ischemia.
The BBB is composed of highly selective tight
junctions between endothelial cells, made primarily of the
membrane-associated accessory proteins, including occludin, ZO-1
and claudin-5 (4,49,50).
The results from the present study revealed that pretreatment with
curcumin blocked the decrease of tight junction protein expression,
which is instrumental in maintaining BBB integrity and
function.
In summary, the present study revealed that
pretreatment with curcumin prior to stroke could inhibit the
central pro-inflammatory mediator NF-κB, reduce the protein
expression level of MMP-9 and attenuate BBB damage, indicating its
neuroprotective effects. These data provide novel targets to
investigate its protective effects underlying cerebral ischemic
injury and provide a new direction to determine therapeutics for
brain insults by restoring the BBB.
Acknowledgements
Not applicable.
Funding
Funding: This research was supported by the Jiangsu Provincial
Commission of Health and Family Planning, Genera Programs (grant.
no. H201527), Open Project Program of Jiangsu Key Laboratory of
Anesthesiology (grant no. KJS1704) and Jiangsu Social Development
Foundation (grant no.BE2017641).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
XZ and SH conceived the design of the study. SW and
TG performed the experiments and analyzed the data. TG, WQ, YL, JG
and CL performed the experiments and analyzed the data. YS and BY
collected the data. YS, BY, SH and XZ performed data analysis
and/or wrote part of the paper. SW, XZ and SH confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All the experiments were performed following
guidelines written by the Institutional Animal Care and Use
Committee of China. The studies were also approved by the Ethics
Committee for the Use of Experimental Animals at Xuzhou Medical
University (assurance nos. 2015-46 and 2015-47).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bai X, Zhang YL and Liu LN: Inhibition of
TRIM8 restrains ischaemia-reperfusion-mediated cerebral injury by
regulation of NF-κB activation associated inflammation and
apoptosis. Exp Cell Res. 388(111818)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Li W, Suwanwela NC and Patumraj S:
Curcumin prevents reperfusion injury following ischemic stroke in
rats via inhibition of NF-κB, ICAM-1, MMP-9 and caspase-3
expression. Mol Med Rep. 47:4710–4720. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang L, Geng J, Qu M, Yuan F, Wang Y, Pan
J, Li Y, Ma Y, Zhou P, Zhang Z and Yang GY: Oligodendrocyte
precursor cells transplantation protects blood-brain barrier in a
mouse model of brain ischemia via Wnt/β-catenin signaling. Cell
Death Dis. 11(9)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Beard RS Jr, Reynolds JJ and Bearden SE:
Hyperhomocysteinemia increases permeability of the blood-brain
barrier by NMDA receptor-dependent regulation of adherens and tight
junctions. Blood. 118:2007–2014. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yang E, Cai Y, Yao X, Liu J, Wang Q, Jin
W, Wu Q, Fan W, Qiu L, Kang C and Wu J: Tissue plasminogen
activator disrupts the blood-brain barrier through increasing the
inflammatory response mediated by pericytes after cerebral
ischemia. Aging (Albany NY). 11:10167–10182. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cao C, Zhou J, Wu X, Qian Y, Hong Y, Mu J,
Jin L, Zhu C and Li S: Activation of CRHR1 contributes to cerebral
endothelial barrier impairment via cPLA2 phosphorylation in
experimental ischemic stroke. Cell Signal.
66(109467)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu ZJ, Liu W, Liu L, Xiao C, Wang Y and
Jiao JS: Curcumin protects neuron against cerebral ischemia-induced
inflammation through improving PPAR-Gamma function. Evid Based
Complement Alternat Med. 2013(470975)2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang DD, Jin C, Zhang YT, Gan XD, Zou MJ,
Wang YY, Fu WL, Xu T, Xing WW, Xia WR and Xu DG: A novel IL-1RA-PEP
fusion protein alleviates blood-brain barrier disruption after
ischemia-reperfusion in male rats. J Neuroinflammation.
15(16)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Guo P, Jin Z, Wu H, Li X, Ke J, Zhang Z
and Zhao Q: Effects of irisin on the dysfunction of blood-brain
barrier in rats after focal cerebral ischemia/reperfusion. Brain
Behav. 9(e01425)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bai X, Zhang X, Chen L, Zhang J, Zhang L,
Zhao X, Zhao T and Zhao Y: Protective effect of naringenin in
experimental ischemic stroke: Down-regulated NOD2, RIP2, NF-κB,
MMP-9 and up-regulated claudin-5 expression. Neurochem Res.
39:1405–1415. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang J, Fu B, Zhang X, Chen L, Zhang L,
Zhao X, Bai X, Zhu C, Cui L and Wang L: Neuroprotective effect of
bicyclol in rat ischemic stroke: Down-regulates TLR4, TLR9, TRAF6,
NF-κB, MMP-9 and up-regulates claudin-5 expression. Brain Res.
1528:80–88. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Vandooren J, Van Damme J and Opdenakker G:
On the structure and functions of gelatinase B/matrix
metalloproteinase-9 in neuroinflammation. Prog Brain Res.
214:193–206. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Turner RJ and Sharp FR: Implications of
MMP9 for blood brain barrier disruption and hemorrhagic
transformation following ischemic stroke. Front Cell Neurosci.
10(56)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Riabinska A, Zille M, Terzi MY, Cordell R,
Nieminen-Kelhä M, Klohs J and Piña AL: Pigment epithelium-derived
factor improves paracellular blood-brain barrier integrity in the
normal and ischemic mouse brain. Cell Mol Neurobiol. 40:751–764.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Song Y, Yang Y, Cui Y, Gao J, Wang K and
Cui J: Lipoxin A4 methyl ester reduces early brain injury by
inhibition of the nuclear factor Kappa B (NF-κB)-dependent matrix
metallopeptidase 9 (MMP-9) pathway in a rat model of intracerebral
hemorrhage. Med Sci Monit. 25:1838–1847. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ludewig P, Sedlacik J, Gelderblom M,
Bernreuther C, Korkusuz Y, Wagener C, Gerloff C, Fiehler J, Magnus
T and Horst AK: Carcinoembryonic antigen-related cell adhesion
molecule 1 inhibits MMP-9-mediated blood-brain-barrier breakdown in
a mouse model for ischemic stroke. Circ Res. 113:1013–1022.
2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li XF, Zhang XJ, Zhang C, Wang LN, Li YR,
Zhang Y, He TT, Zhu XY, Cui LL and Gao BL: Ulinastatin protects
brain against cerebral ischemia/reperfusion injury through
inhibiting MMP-9 and alleviating loss of ZO-1 and occludin proteins
in mice. Exp Neurol. 302:68–74. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ding R, Feng L, He L, Chen Y, Wen P, Fu Z,
Lin C, Yang S, Deng X, Zeng J and Sun G: Peroxynitrite
decomposition catalyst prevents matrix metalloproteinase-9
activation and neurovascular injury after hemoglobin injection into
the caudate nucleus of rats. Neuroscience. 297:182–193.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Han L, Liu DL, Zeng QK, Shi MQ, Zhao LX,
He Q, Kuang X and Du JR: The neuroprotective effects and probable
mechanisms of Ligustilide and its degradative products on
intracerebral hemorrhage in mice. Int Immunopharmacol. 63:43–57.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kumari A, Singh DK, Dash D and Singh R:
Intranasal curcumin protects against LPS-induced airway remodeling
by modulating toll-like receptor-4 (TLR-4) and
matrixmetalloproteinase-9 (MMP-9) expression via affecting MAP
kinases in mouse model. Inflammopharmacology. 27:731–748.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang YF, Gu YT, Qin GH, Zhong L and Meng
YN: Curcumin ameliorates the permeability of the blood-brain
barrier during hypoxia by upregulating heme oxygenase-1 expression
in brain microvascular endothelial cells. J Mol Neurosci.
51:344–351. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yavarpour-Bali H, Ghasemi-Kasman M and
irzadeh M: Curcumin-loaded nanoparticles: A novel therapeutic
strategy in treatment of central nervous system disorders. Int J
Nanomedicine. 14:4449–4460. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tsai YM, Chien CF, Lin LC and Tsai TH:
Curcumin and its nano-formulation: The kinetics of tissue
distribution and blood-brain barrier penetration. Int J Pharm.
416:331–338. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang Y, Luo J and Li SY: Nano-curcumin
simultaneously protects the blood-brain barrier and reduces M1
microglial activation during cerebral ischemia-reperfusion injury.
ACS Appl Mater Interfaces. 11:3763–3770. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bavarsad K, Barreto GE, Hadjzadeh MA and
Sahebkar A: Protective effects of curcumin against
ischemia-reperfusion injury in the nervous system. Mol Neurobiol.
56:1391–1404. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Xue X, Wang H and Su J: Inhibition of
MiR-122 decreases cerebral ischemia-reperfusion injury by
upregulating DJ-1-phosphatase and tensin homologue deleted on
chromosome 10 (PTEN)/Phosphonosinol-3 kinase (PI3K)/AKT. Med Sci
Monit. 26(e915825)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Guo T, Wang Y, Guo Y, Wu S, Chen W, Liu N
and Geng D: 1,25-D3 protects from cerebral ischemia by
maintaining BBB permeability via PPAR-γ activation. Front Cell
Neurosci. 12(480)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Danielson M, Reinsfelt B, Westerlind A,
Zetterberg H, Blennow K and Ricksten SE: Effects of
methylprednisolone on blood-brain barrier and cerebral inflammation
in cardiac surgery-a randomized trial. J Neuroinflammation.
15(283)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Jiao H, Wang Z, Liu Y, Wang P and Xue Y:
Specific role of tight junction proteins claudin-5, occludin, and
ZO-1 of the blood-brain barrier in a focal cerebral ischemic
insult. J Mol Neurosci. 44:130–139. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sikora E, Scapagnini G and Barbagallo M:
Curcumin, inflammation, ageing and age-related diseases. Immun
Ageing. 7(1)2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Huang L, Chen C, Zhang X, Li X, Chen Z,
Yang C, Liang X, Zhu G and Xu Z: Neuroprotective effect of curcumin
against cerebral ischemia-reperfusion via mediating autophagy and
inflammation. J Mol Neurosci. 64:129–139. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jiang J, Wang W, Sun YJ, Hu M, Li F and
Zhu DY: Neuroprotective effect of curcumin on focal cerebral
ischemic rats by preventing blood-brain barrier damage. Eur J
Pharmacol. 561:54–62. 2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang C, Yang YH, Zhou L, Ding XL, Meng YC
and Han K: Curcumin alleviates OGD/R-induced PC12 cell damage via
repressing CCL3 and inactivating TLR4/MyD88/MAPK/NF-κB to suppress
inflammation and apoptosis. J Pharm Pharmacol. 72:1176–1185.
2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zong X, Wu S, Li F, Lv L, Han D, Zhao N,
Yan X, Hu S and Xu T: Transplantation of VEGF-mediated bone marrow
mesenchymal stem cells promotes functional improvement in a rat
acute cerebral infarction model. Brain Res. 1676:9–18.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yang Y and Rosenberg GA: Blood-brain
barrier breakdown in acute and chronic cerebrovascular disease.
Stroke. 42:3323–3328. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gerace E, Scartabelli T,
Pellegrini-Giampietro DE and Landucci E: Tolerance induced by
(S)-3,5-dihydroxyphenylglycine postconditioning is mediated by the
PI3K/Akt/GSK3β signalling pathway in an in vitro model of cerebral
ischemia. Neuroscience. 433:221–229. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Eghbaliferiz S, Farhadi F, Barreto GE,
Majeed M and Sahebkar A: Effects of curcumin on neurological
diseases: Focus on astrocytes. Pharmacol Rep. 72:769–782.
2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Nery-Flores SD, Mendoza-Magaña ML,
Ramírez-Herrera MA, Ramírez-Vázquez JJ, Romero-Prado MMJ,
Cortez-Álvarez CR and Ramírez-Mendoza AA: Curcumin exerted
neuroprotection against ozone-induced oxidative damage and
decreased NF-κB activation in rat hippocampus and serum levels of
inflammatory cytokines. Oxid Med Cell Longev.
2018(9620684)2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kodali M, Hattiangady B, Shetty GA, Bates
A, Shuai B and Shetty AK: Curcumin treatment leads to better
cognitive and mood function in a model of Gulf War Illness with
enhanced neurogenesis, and alleviation of inflammation and
mitochondrial dysfunction in the hippocampus. Brain Behav Immun.
69:499–514. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lambertsen KL, Biber K and Finsen B:
Inflammatory cytokines in experimental and human stroke. J Cereb
Blood Flow Metab. 32:1677–1698. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kar F, Hacioglu C, Senturk H, Donmez DB,
Kanbak G and Uslu S: Curcumin and LOXblock-1 ameliorate
ischemia-reperfusion induced inflammation and acute kidney injury
by suppressing the semaphorin-plexin pathway. Life Sci.
256(118016)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Tang X, Sun L, Wang G, Chen B and Luo F:
RUNX1: A regulator of NF-κB signaling in pulmonary diseases. Curr
Protein Pept Sci. 19:172–178. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhu H, Dai R, Fu H and Meng Q: MMP-9
upregulation is attenuated by the monoclonal TLR2 antagonist T2.5
after oxygen-glucose deprivation and reoxygenation in rat brain
microvascular endothelial cells. J Stroke Cerebrovasc Dis.
28:97–106. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zhu H, Dai R, Zhou Y, Fu H and Meng Q:
TLR2 ligand Pam3CSK4 regulates MMP-2/9 expression by MAPK/NF-κB
signaling pathways in primary brain microvascular endothelial
cells. Neurochem Res. 43:1897–1904. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yang SL, Chen LJ, Kong Y, Xu D and Lou YJ:
Sodium nitroprusside regulates mRNA expressions of LTC4 synthesis
enzymes in hepatic ischemia/reperfusion injury rats via NF-kappaB
signaling pathway. Pharmacology. 80:11–20. 2007.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zhang S, An Q, Wang T, Gao S and Zhou G:
Autophagy- and MMP-2/9-mediated reduction and redistribution of
ZO-1 contribute to hyperglycemia-increased blood-brain barrier
permeability during early reperfusion in stroke. Neuroscience.
377:126–137. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chang JJ, Emanuel BA, Mack WJ, Tsivgoulis
G and Alexandrov AV: Matrix metalloproteinase-9: Dual role and
temporal profile in intracerebral hemorrhage. J Stroke Cerebrovasc
Dis. 23:2498–2505. 2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Liu WC, Wang X, Zhang X, Chen X and Jin X:
Melatonin supplementation, a strategy to prevent neurological
diseases through maintaining integrity of blood brain barrier in
old people. Front Aging Neurosci. 9(165)2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Xiong D, Deng Y, Huang B, Yin C, Liu B,
Shi J and Gong Q: Icariin attenuates cerebral ischemia-reperfusion
injury through inhibition of inflammatory response mediated by
NF-κB, PPARα and PPARγ in rats. Int Immunopharmacol. 30:157–162.
2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Sun J, Guo W, Ben Y, Jiang J, Tan C, Xu Z,
Wang X and Bai C: Preventive effects of curcumin and dexamethasone
on lung transplantation-associated lung injury in rats. Crit Care
Med. 36:1205–1213. 2008.PubMed/NCBI View Article : Google Scholar
|