Introduction
With the development of the economy and changes in
lifestyle, the incidence of diabetes, particularly type 2 diabetes,
is increasing in China, and has become a major public health
problem threatening the health of the Chinese population (1). The latest data released by the
International Diabetes Federation in 2019 revealed that the number
of patients with diabetes worldwide had reached 463 million, and
the number of patients with diabetes in China was 116.4 million,
accounting for ~1/4 of the total number of patients worldwide
(2). Diabetic nephropathy (DN) is
not only a serious complication of diabetes, but is also one of the
most important causes of end-stage renal disease (ESRD) (3). Some investigators have reported that
DN has become the leading cause of chronic kidney disease among
hospitalized patients in China (4).
At present, there is a large number of studies and recommended
treatment approaches to diabetic nephropathy (DN); however, studies
in the United States over the past 20 years indicate that the
incidence of ESRD caused by diabetes has not significantly
decreased (5). Thus, effective
intervention measures to delay the progression of DN are currently
lacking. Therefore, studying the pathogenesis of DN is crucial for
further identifying effective treatments.
The pathological changes of DN include
glomerulosclerosis, tubulointerstitial fibrosis and renal vascular
lesions. At present, studies on the mechanism of DN are mostly
focused on glomerular injury, and the clinical indices used to
assess the severity of DN are based on the changes of glomerular
structure and function (6).
However, the renal tubulointerstitium accounts for >90% of the
renal parenchyma and serves a variety of important functions
(7). Renal tubular interstitial
injury plays a key role in the progression of DN and, therefore,
its study is important.
In recent years, it has been reported that
inflammasomes serve an important role in the inflammatory response
and cellular injury of DN (8-10).
Inflammasomes are a class of large polyprotein complexes, the
receptor proteins of which include Nod-like receptors (NLR) family
pyrin domain containing (NLRP)1, NLRP3 and NLR family CARD domain
containing 4 (NLRC4) from the NLR family, and absent in melanoma 2
from the HIN-200 family, which can activate caspase-1, regulate
IL-1β and IL-18 maturation and secretion, and trigger inflammation
and cell injury (11). Some
bacterial infections can activate NLRC4 inflammatory bodies,
causing an inflammatory response (12). Moderate inflammation is beneficial
for the clearance of bacteria and can inhibit bacterial infection,
while excessive inflammation may lead to host cell death. Previous
studies reported that NLRC4 inflammasomes are also involved in the
pathological process of kidney disease. For example, Yuan et
al (13) observed a significant
increase in NLRC4 expression in the renal tubules and interstitium
of patients with DN compared with that of control cases.
Quantitatively, NLRC4 staining intensity exhibited a one-fold
increase in the renal tubulointerstitium of patients with DN
(13). In addition, it has been
revealed that the expression of NLRC4 in intestinal epithelial
cells is increased and it is involved in intestinal inflammation
(14,15). The main aim of the present study was
to examine the role of the NLRC4 inflammasome in renal tubular
epithelial cell (RTEC) injury in DN.
Mitophagy is a type of selective autophagy, which
was first proposed by Lemasters in 2005(16). Mitophagy selectively removes damaged
or unwanted mitochondria via PTEN-induced kinase 1 (Pink1)/parkin
and NIP-3-like protein X/BCL2 interacting protein 3 signaling
(17). This process involves
autophagosome fusion with lysosomes, degradation of damaged or
dysfunctional mitochondria and maintenance of reactive oxygen
species (ROS) balance, and it participates in a variety of
pathophysiological processes and diseases (18).
Mitochondrial ROS (mROS) produced by mitophagy can
activate the NLRP3 inflammasome (19). As a member of the NLR family, NLRC4
has a similar structure to NLRP3 and may also be activated by mROS.
Recent studies have reported that mitophagy can activate the NLRC4
inflammasome, causing an inflammatory response and cell death in
macrophages infected by Pseudomonas aeruginosa and in
myocardial cells following myocardial infarction in a high-glucose
(HG) environment (20,21). Therefore, the NLRC4 inflammasome may
be an important factor mediating mitophagy in DN; however, to the
best of our knowledge, there is no related research at present. The
present study was undertaken to investigate whether mitophagy
dysfunction and NLRC4 inflammasome activation in RTECs are involved
in the inflammatory response and apoptosis in a HG environment.
Materials and methods
Morphological analysis of human kidney
samples
Human kidney biopsy tissues of patients (n=42) with
type 2 DN were obtained from the Department of Nephrology of The
First Affiliated Hospital of Zhengzhou University (Zhengzhou,
China), and normal kidney tissues from nephrectomies performed for
renal hamartoma (n=10) served as the control (the kidney samples in
paraffin blocks were obtained retrospectively between
01/2016-10/2018 and the experiments were performed in 11/2018). All
renal tissues were cut into sections ~2 µm thick and examined via
immunofluorescence, light microscopy (LM) and electron microscopy
(EM), according to standard methods. The sections were evaluated
according to the pathological classification standard of DN
published in the Journal of American Society of Nephrology in
2010(22) as follows: Class I: Mild
or non-specific LM changes and EM-proven glomerular basement
membrane (GBM) thickening, biopsy does not meet any of the criteria
mentioned below for class II, III or IV, GBM>395 nm in female
and >430 nm in male individuals aged ≥9 years; class IIa: Mild
mesangial expansion in >25% of the observed mesangium; class
IIb: Severe mesangial expansion in >25% of the observed
mesangium; class III: At least one convincing Kimmelstiel-Wilson
lesion, does not meet criteria for class IV; and class IV: Global
glomerular sclerosis in >50% of glomeruli. The present study has
been approved by the Ethics Committee of The First Affiliated
Hospital of Zhengzhou University (Zhengzhou, China; approval no.
2018-KY-023).
Blood and urine examination
The blood and urine samples of the patients were
collected for the detection of 24 h urinary protein quantity,
hemoglobin, glycosylated hemoglobin (HbA1c), serum creatinine,
serum albumin and estimated glomerular filtration rate (eGFR). The
eGFR was calculated using the chronic kidney disease epidemiology
collaboration formula (23) based
on serum creatinine.
Cell culture
Human RTECs (HK2 cells) were obtained from The Cell
Bank of Type Culture Collection of The Chinese Academy of Sciences,
and cultured in DMEM (Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with 5.6 mM D-glucose, 10% FBS (Invitrogen; Thermo
Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml
streptomycin in a 5% CO2 incubator at 37˚C.
To detect the effect of HG on NLRC4 inflammasome and
mitophagy, cells were cultured under normal glucose (NG) conditions
(5.6 mM glucose), hyperosmotic (HO) conditions (5.6 mM glucose +
24.4 mM mannitol) or HG conditions (30 mM glucose) for 48 h.
Small interfering (si)RNA
transfection
HK2 cells were transfected with scramble small
interfering RNA (siRNA) and NLRC4 siRNA (Sangon Biotech Co., Ltd.)
using Lipofectamine® 2000 (Thermo Fisher Scientific,
Inc.) according to the instructions of the manufacturer. In total,
0.1 nmol siRNA and 5 µl Lipofectamine® 2000 were diluted
in 250 µl Opti-MEM (Invitrogen; Thermo Fisher Scientific, Inc.),
and incubated for 5 min at room temperature. The sequence of the
siRNAs was as follows: NLRC4 siRNA forward,
5'-CCUUAUUACCUCAUCGAAUTT-3' and reverse,
5'-AUUCGAUGAGGUAAUAAGGTT-3'; control siRNA forward,
5'-UUCUCCGAACGUGUCACGUTT-3' and reverse,
5'-ACGUGACACGUUCGGAGAATT-3'. Then, the two diluents were mixed and
incubated for 20 min at room temperature. The resulting
DNA-Lipofectamine® 2000 complex was added to
5x105 cells and incubated at 37˚C for 6 h. Then, the
medium was replaced with DMEM supplemented with 2 mmol/l glutamine
and 10% FBS.
Western blotting
After cleavage, the renal homogenates and cells in
different groups was extracted using a protein extraction kit
(Qiagen China Co., Ltd.), and the total protein content was
determined using the BCA method. The 10% gel for SDS-PAGE was
prepared, the target protein sample was collected and adjusted to
the same concentration. The lanes on both sides of the sample were
sampled with 1X loading buffer at an equal volume, and the marker
was adjusted to the same volume as the sample using 1X loading
buffer. Electrophoresis was performed and the target protein was
moved to the end at 1 cm above the lower edge of the gel. After
electrophoresis, the proteins were transferred to a PVDF membrane,
followed by blocking at room temperature for 1 h and incubated
overnight at 4˚C with the primary antibodies. The membrane was
washed three times with TBS with 0.05% Tween-20 (TBST) and then
incubated at room temperature for 2 h with the secondary antibody.
The membrane was washed again with TBST to elute the primary
antibody. HRP HR and the ECL method (Wuhan Boster Biological
Technology, Ltd.) were used to visualize the bands. The following
antibodies were used for protein detection: Anti-NLRC4 (cat. no.
ab99860; 1:1,000), anti-parkin (cat. no. ab233434; 1:500),
anti-phosphorylated (p)-parkin (cat. no. ab73015; 1:500),
anti-PINK1 (cat. no. 23707; 1:500) and anti-β-actin (cat. no.
ab8227; 1:1,000). All antibodies were purchased from Abcam. Each
experiment was performed in triplicate.
Reverse transcription-quantitative
(RT-q)PCR analysis
The renal homogenates and cells of different groups
were cleaved and RNA was extracted using an RNA extraction kit
(Qiagen China Co., Ltd.). Total RNA was determined using a micro
ultraviolet spectrophotometer. Using total RNA as the template, a
random primer hexamer (Shenggong Bioengineering Shanghai Co., Ltd.)
was used as the reverse primer, and a total of 40 µl cDNA was
synthesized using PrimeScript™ II High Fidelity RT-PCR Kit (Takara
Biotechnology Co, Ltd.) according to the manufacturer's protocol.
Fluorescence qPCR amplification was performed as follows: The PCR
amplification reaction system was 50 µl, and the ratio of each
detection index to the internal reference GAPDH was used to
indicate the mRNA expression level of the gene. The expression
levels of tested genes were normalized to that of human GAPDH and
calculated using the 2-ΔΔCq method (24). The assay was performed in
triplicate. The primers for the NLRC4 gene were as follows:
Forward, 5'-TCTACCTGATCCAGCATTAGTCAG-3' and reverse,
5'-TGCCACCCAACAAGCCTAGC-3'. GAPDH was used as a reference gene, and
the primers were as follows: Forward, 5'-TCAACAGCGACACCCACTCC-3'
and reverse, 5'-TGAGGTCCACCACCCTGTTG-3' (25).
ELISA
IL-1β and IL-18 levels in cell culture supernatants
and urine were determined using ELISA kits, according to the
manufacturer's instructions (Genmed Scientifics, Inc.). Each
experiment was performed in triplicate.
Measurement of superoxide generation
and apoptosis
Briefly, mitochondrial superoxide generation was
detected using MitoSOX Red indicator (Invitrogen; Thermo Fisher
Scientific, Inc.). Mitochondrial-associated ROS levels were
measured by staining cells with MitoSOX (2.5 mM) for 30 min at
37˚C. Cells were then washed with PBS solution and resuspended in
cold PBS solution containing 1% FBS. TUNEL assay was used to
measure cell apoptosis, according to the manufacturer's
instructions (cat. no. GS0246; Beijing Baiao Laibo Technology Co.,
Ltd.).
Statistical analysis
Statistical analyses were performed with SPSS 20.0
software (IBM Corp). Data are presented as the mean ± SD, unless
otherwise indicated. The difference between means was assessed
using the unpaired Student's t-test for two-group comparisons.
Classification variables are described as percentages. Other data
from experiments were analyzed using one-way ANOVA where
appropriate. Both LSD and SNK post hoc tests were used for the
ANOVA analysis of the PCR results of mitophagy-related indices,
NLRC4, IL-1β, IL-18 and cell apoptosis in different groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinical characteristics of patients
with type 2 DN and controls
The pathological types of DN were classified as
class I (n=8), IIa (n=11), IIb (n=10), III (n=9) and IV (n=4). The
levels of urinary protein and HbA1C in the DN group were higher
compared with those in the control group. Moreover, patients with
DN class IV had the most severely compromised renal function and
the lowest hemoglobin levels. With the aggravation of the
pathological injury of DN, renal function deteriorated, and anemia
worsened (Table I).
 | Table IClinical characteristics of patients
with type 2 DN and control subjects. |
Table I
Clinical characteristics of patients
with type 2 DN and control subjects.
| Characteristics | Control group
(n=10) | Patients with DN
(n=42) |
|---|
| Sex, male/female
(n) | 6/4 | 25/17 |
| Age (years) | 53.0±6.3 | 47.6±5.7 |
| Duration of diabetes
(years) | - | 8.1±7.4 |
| Glycosylated
hemoglobin (%) | 5.2±0.7 | 6.8± 2.2a |
| Hemoglobin (g/l) | 143.33±20.13 | 116±26.2a |
| Serum creatinine
(µmol/l) | 82.67±16.26 |
142.1±112.85a |
| Serum albumin
(g/l) | 41.2±2.08 |
35.80±9.02a |
| 24-h urinary
protein (g/24 h) | 0.10±0.04 |
3.95±3.11a |
| Estimated
glomerular filtration rate (ml/min/1.73 m2) | 91.67±19.22 |
67.84±36.96a |
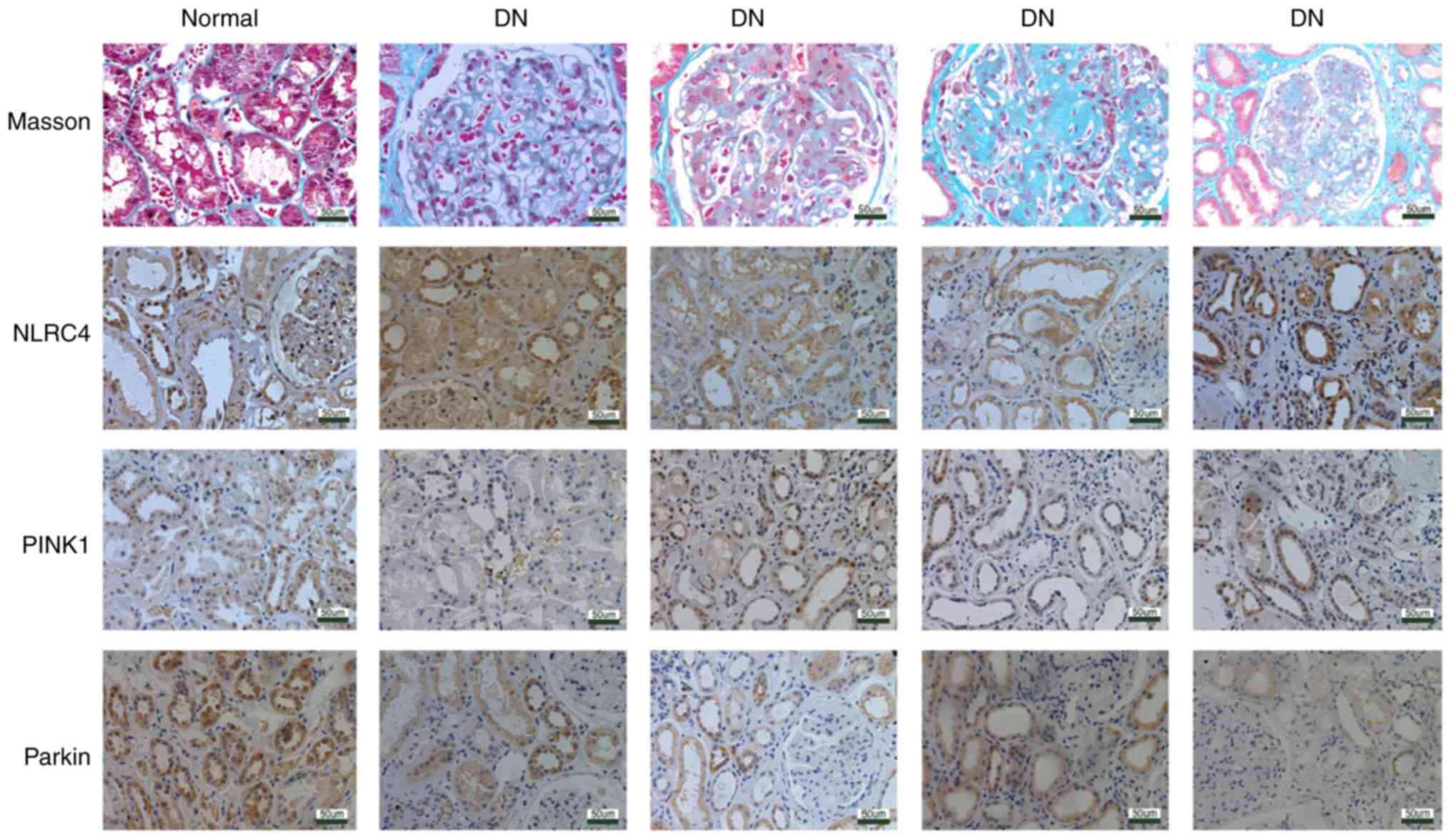
NLRC4 expression is increased, while
the expression levels of PINK1 and parkin are decreased in the
renal tissues of patients with DN
A total of 42 renal tissue samples were collected
from patients with DN confirmed by renal biopsy, and 10 normal
renal tissue samples obtained at a distance of 3-5 cm from the edge
of renal hamartoma served as the control sample. Renal tubular
atrophy and interstitial fibrosis were observed via LM, and
immunohistochemistry was used to observe the expression and
localization of NLRC4 in the renal tissues of patients with DN. The
results of immunohistochemistry demonstrated that the expression of
NLRC4 in the RTECs of patients with DN was higher compared with
that of normal controls. In addition, the expression levels of
PINK1, parkin and p-parkin, which are associated with mitophagy,
were lower in the renal tissues of patients with DN compared with
those in normal controls (Fig. 1).
The increased expression of NLRC4 in the renal tubular epithelium
of DN suggests that it may be involved in the injury of RTECs in
DN. Previous studies have reported that mitophagy is dysregulated
in DN RTECs (17,26,27),
and the present study also confirmed this finding. Furthermore, the
expression of NLRC4 was found to be inversely proportional to the
expression of mitochondrial autophagy-related proteins, suggesting
that these may interact with each other.
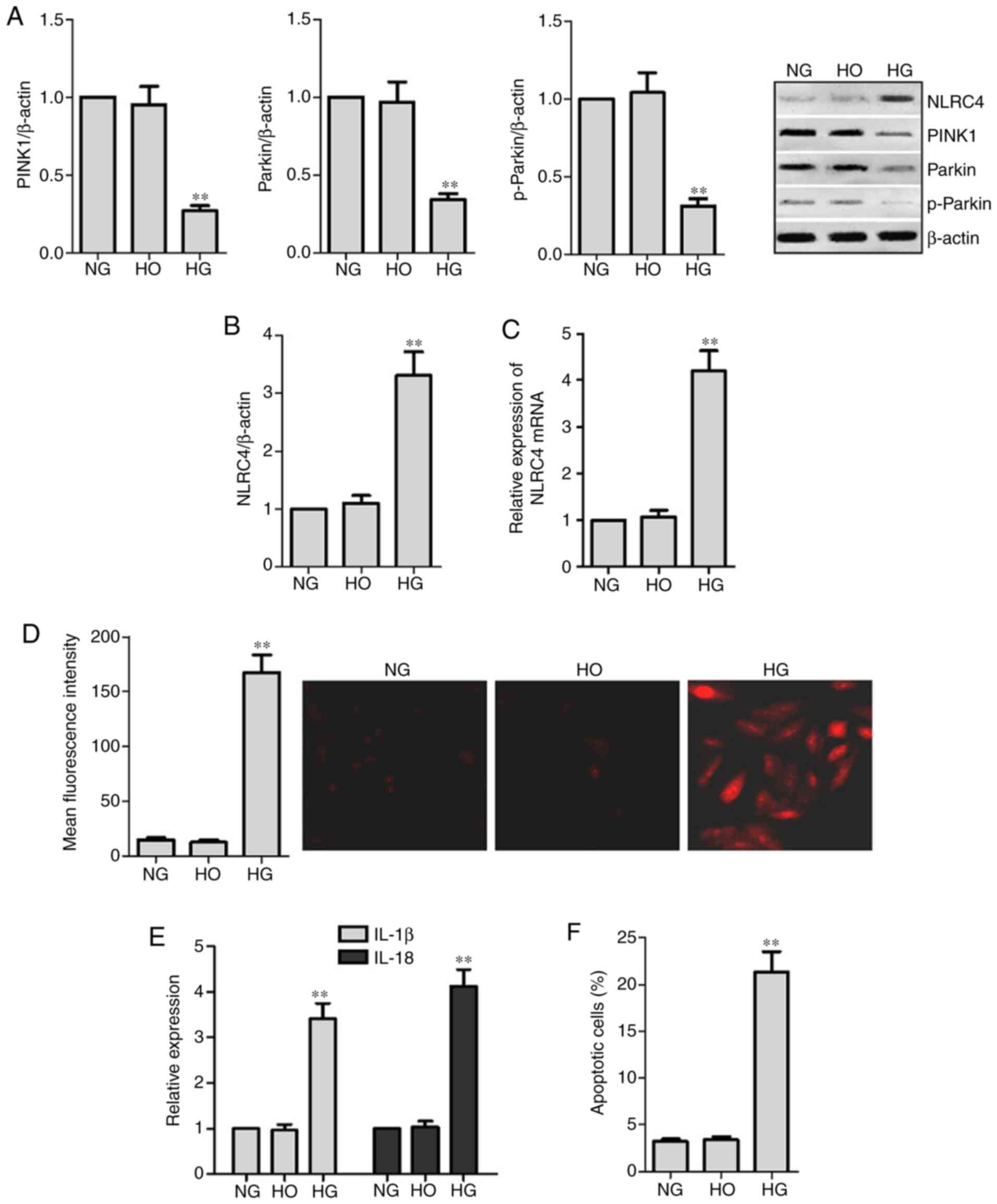
HG-induced NLRC4 expression and
secretion of the cytokines IL-1 β and IL-18 are increased in HK2
cells
In order to investigate the effect of HG on the
mitophagy of RTECs, PINK1, parkin and p-parkin expression levels
were analyzed. MitoSOX was used to detect intracellular mROS. The
results demonstrated that the expression levels of PINK1, parkin
and p-parkin in HK2 cells treated with 30 mM glucose (HG) for 48 h
were lower compared with those in the NG and HO groups (P<0.05).
In addition, the production of mROS was increased in the HG group.
Thus, it was suggested that HG stimulation can cause mitophagy
disorder and increase the production of mROS in RTECs. The results
also indicated that HG stimulated the expression of NLRC4,
secretion of IL-1β and IL-18 and HK2 cell death (P<0.05;
Fig. 2).
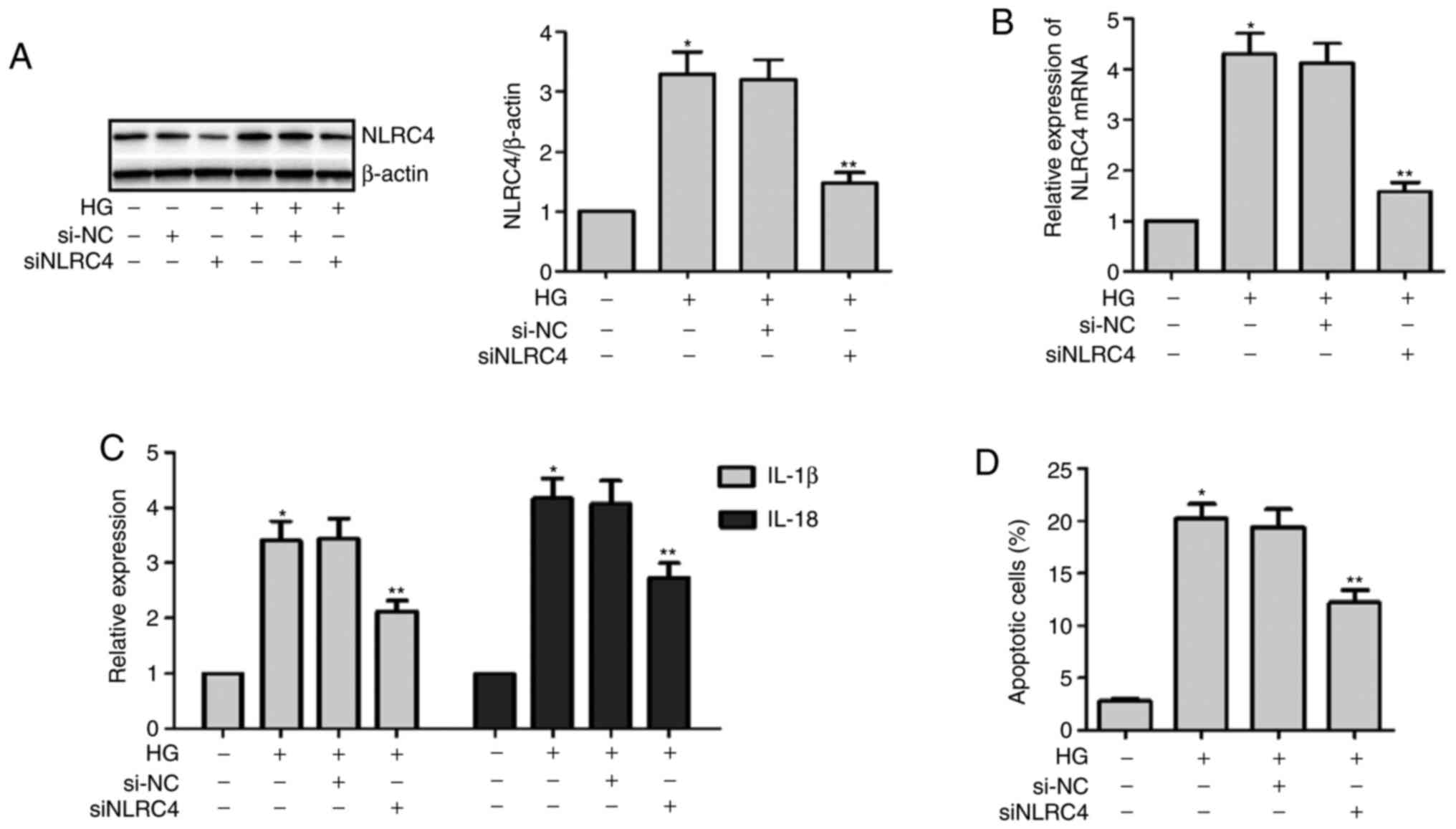
Knockdown of NLRC4 expression in HK2
cells treated with HG can decrease the secretion of the
inflammatory cytokines IL-1β and IL-18
Transfecting NLRC4 siRNA into HK2 cells
downregulated the expression of NLRC4. First, the success of the
transfection experiments of siNLRC4 was verified via western
blotting in an NG environment (Fig.
3A). Then, these cells were divided into three groups: HG +
siNLRC4, HG and control group. The cells were cultured for 48 h.
The results demonstrated that silencing NLRC4 expression in a HG
environment could reduce the secretion of IL-1β and IL-18 and
decrease HK2 cell death (Fig. 3).
Thus, it was suggested that NLRC4 may be involved in HK2 cell death
and the secretion of inflammatory cytokines in a HG environment. A
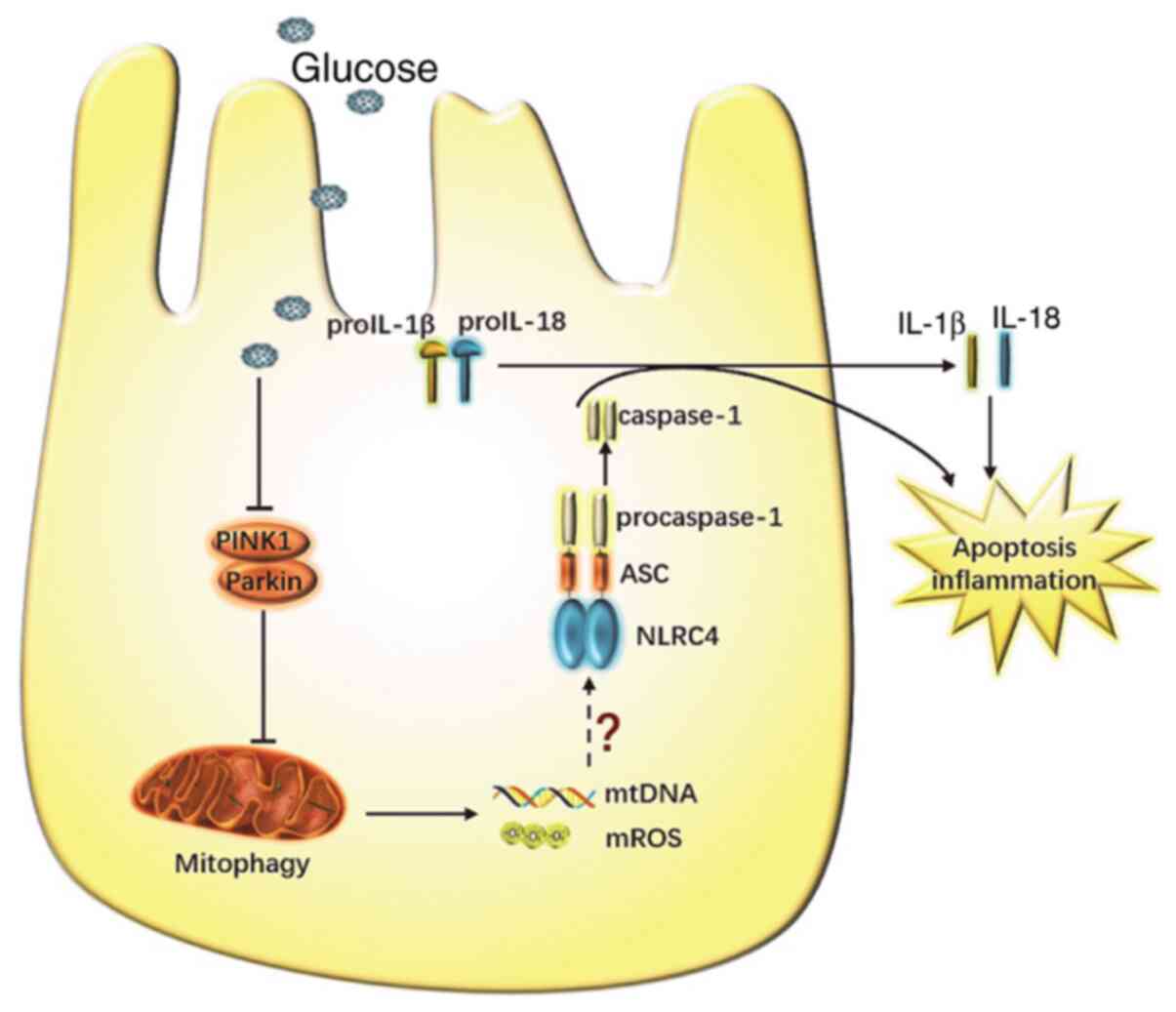
schematic representation of the possible molecular mechanism
linking disrupted mitophagy with NLRC4 inflammasome activation in
RTECs under HG conditions is shown in Fig. 4.
Discussion
Several hypotheses have been suggested to explain
the development and progression of DN; however, previous studies on
DN mainly focused on glomerular injury (6). Recent studies have reported that DN
glomerulopathy is important, but the role of renal tubular injury
cannot be ignored (6,28,29).
Renal tubular injury may be an early lesion of DN, and may predict
and participate in the progression of DN (6). It was previously reported that, when
the urinary albumin excretion rate of diabetic patients is normal,
renal tubular injury is likely, indicating that renal tubular
lesions may play an important role in the occurrence of DN
(7). Brezniceanu et al
(7) also found that RTEC apoptosis
and renal tubular atrophy may be present in the early stage of DN,
and the proximal renal tubule may be the earliest involved site.
Therefore, the study of renal tubular injury in DN may uncover
novel targets for the treatment of DN.
Most previous studies (3,8,30)
reported that DN is the result of the interaction of numerous
factors, such as hemodynamic changes, metabolic disorders,
oxidative stress and genetic factors, amongst others. In recent
years, the role of inflammatory activation in the pathogenesis of
DN has been attracting increasing attention. The inflammatory
response and activation of innate immunity serve an important role
in the pathogenesis of DN (29). It
has also been reported that the activation of the NLRP3
inflammasome is involved in the injury of RTECs in DN (31,32),
but the role of other inflammasomes in DN remains unknown. It has
also been confirmed that bacterial products can activate the NLRC4
inflammasome (12). Moreover, some
studies (14,15,25)
have observed that the NLRC4 inflammasome is involved in the
occurrence of tumors, psoriasis and ulcerative colitis, indicating
that the NLRC4 inflammasome plays an important role in the systemic
inflammatory response and self-inflammatory diseases. The present
study identified a causal link between NLRC4 inflammasome
activation and RTEC injury.
The present study demonstrated that the expression
of NLRC4 was increased in the RTECs of patients with DN and was
associated with the grade of DN, as the higher the grade, the
higher the expression of NLRC4. In addition, the study of HK2 cells
found that, under HG conditions, NLRC4 expression, IL-1β and IL-18
secretion and cell death were increased. Furthermore, it was found
that knocking down the expression of NLRC4 in HK2 cells under HG
conditions reduced the secretion of the inflammatory cytokines
IL-1β and IL-18, and decreased cell death. Of note, flow cytometry
may be more suitable for detecting apoptosis, and it will be
perform in future experiments. It was also demonstrated that the
NLRC4 inflammasome may be involved in the occurrence and
development of DN, and its activation may not be solely caused by
bacterial flagella or bacterial secreted substances. However, the
factors that cause NLRC4 activation in DN remain to be fully
determined.
Giacco and Brownlee (30) reported that in primary arterial
endothelial cells in culture, intracellular hyperglycemia increases
the voltage across the mitochondrial membrane to above the critical
necessary threshold, which increases superoxide formation and,
subsequently, enhances the production of ROS. It has been also
revealed that dynamic changes in mitochondrial morphology are
associated with HG-induced overproduction of ROS. Furthermore,
Eleftheriadis et al (28)
observed that HG increases solute carrier family 5 member 2
expression and glucose consumption, resulting in ROS
overproduction. Thus, it was suggested that ROS may serve an
important role in the pathogenesis of DN.
It was previously demonstrated that Pseudomonas
aeruginosa infection can cause macrophage autophagy
disturbance, accumulation of damaged mitochondria and increased
production of mROS and mitochondrial DNA, which activates the NLRC4
inflammasome and increases the expression levels of caspase-1 and
IL-1β. Moreover, enhanced autophagy clearance of damaged
mitochondria may significantly inhibit the activation of the NLRC4
inflammasome (20). Recently, it
was reported that the disturbance of mitophagy in cardiomyocytes
with heart failure after myocardial infarction in type 2 diabetic
mice can promote the activation of the NLRC4 inflammasome, leading
to an increase in IL-18 and cell death (21). The present study also demonstrated
that mitophagy in RTECs was decreased in patients with DN,
mitophagy in HK2 cells was impaired and NLRC4 expression was
increased in a HG environment. These findings suggest that
mitophagy may lead to the activation of the NLRC4 inflammasome and
may be involved in cell injury via the production of mROS and
mitochondrial RNA. However, additional research is required to
validate these findings.
In conclusion, the present findings provided a
rationale for developing targeted treatment methods for patients
with DN by preventing inflammasome activation. It was suggested
that mitophagy disorder may promote the activation of the NLRC4
inflammasome, but additional experiments are required to further
confirm this result.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Fund (grant no. U1904134).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW, LT and RG designed, performed and analyzed the
experiments. YW wrote and revised the manuscript. LY, LW, ZY and YG
carried out the data collection, data analysis and revised the
manuscript. LT revised the manuscript and applied for funding
support. YW and LT confirm the authenticity of the raw data. All
the authors have reviewed the results and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The First Affiliated Hospital of Zhengzhou University
(Zhengzhou, China). Written informed consent was obtained from each
patient prior to participation in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li Y, Teng D, Shi X, Qin G, Qin Y, Quan H,
Shi B, Sun H, Ba J, Chen B, et al: Prevalence of diabetes recorded
in mainland China using 2018 diagnostic criteria from the American
Diabetes Association: National cross sectional study. BMJ.
369(m997)2020.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Saeedi P, Petersohn I, Salpea P, Malanda
B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA,
Ogurtsova K, et al: Global and regional diabetes prevalence
estimates for 2019 and projections for 2030 and 2045: Results from
the International Diabetes Federation Diabetes Atlas, 9th edition.
Diabetes Res Clin Pract. 157(107843)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yang D, Livingston MJ, Liu Z, Dong G,
Zhang M, Chen JK and Dong Z: Autophagy in diabetic kidney disease:
Regulation, pathological role and therapeutic potential. Cell Mol
Life Sci. 75:669–688. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhang L, Zhao MH, Zuo L, Wang Y, Yu F,
Zhang H and Wang H: CK-NET Work Group. China kidney disease network
(CK-NET) 2015 annual data report. Kidney Int Suppl (2011).
9:e1–e81. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liu M, Liu SW, Wang LJ, Bai YM, Zeng XY,
Guo HB, Liu YN, Jiang YY, Dong WL, He GX, et al: Burden of
diabetes, hyperglycaemia in China from to 2016: Findings from the
1990 to. 2016, global burden of disease study. Diabetes Metab.
45:286–293. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gilbert RE: Proximal Tubulopathy: Prime
mover and key therapeutic target in diabetic kidney disease.
Diabetes. 66:791–800. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Brezniceanu ML, Liu F, Wei CC, Chénier I,
Godin N, Zhang SL, Filep JG, Ingelfinger JR and Chan JS:
Attenuation of interstitial fibrosis and tubular apoptosis in db/db
transgenic mice overexpressing catalase in renal proximal tubular
cells. Diabetes. 57:451–459. 2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tang SCW and Yiu WH: Innate immunity in
diabetic kidney disease. Nat Rev Nephrol. 16:206–222.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wu M, Han W, Song S, Du Y, Liu C, Chen N,
Wu H, Shi Y and Duan H: NLRP3 deficiency ameliorates renal
inflammation and fibrosis in diabetic mice. Mol Cell Endocrinol.
478:115–125. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shahzad K, Bock F, Al-Dabet MM, Gadi I,
Kohli S, Nazir S, Ghosh S, Ranjan S, Wang H, Madhusudhan T, et al:
Caspase-1, but not caspase-3, promotes diabetic nephropathy. J Am
Soc Nephrol. 27:2270–2275. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Song F, Ma Y, Bai XY and Chen X: The
expression changes of inflammasomes in the aging rat kidneys. J
Gerontol A Biol Sci Med Sci. 71:747–756. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Duncan JA and Canna SW: The NLRC4
inflammasome. Immunol Rev. 281:115–123. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yuan F, Kolb R, Pandey G, Li W, Sun L, Liu
F, Sutterwala FS, Liu Y and Zhang W: Involvement of the
NLRC4-Inflammasome in diabetic nephropathy. PLoS One.
11(e164135)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rauch I, Deets KA, Ji DX, von Moltke J,
Tenthorey JL, Lee AY, Philip NH, Ayres JS, Brodsky IE, Gronert K
and Vance RE: NAIP-NLRC4 inflammasomes coordinate intestinal
epithelial cell expulsion with eicosanoid and IL-18 release via
activation of caspase-1 and -8. Immunity. 46:649–659.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hiruma J, Harada K, Motoyama A, Okubo Y,
Maeda T, Yamamoto M, Miyai M, Hibino T and Tsuboi R: Key component
of inflammasome, NLRC4, was identified in the lesional epidermis of
psoriatic patients. J Dermatol. 45:971–977. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lemasters JJ: Selective mitochondrial
autophagy, or mitophagy, as a targeted defense against oxidative
stress, mitochondrial dysfunction, and aging. Rejuvenation Res.
8:3–5. 2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xiao L, Xu X, Zhang F, Wang M, Xu Y, Tang
D, Wang J, Qin Y, Liu Y, Tang C, et al: The mitochondria-targeted
antioxidant MitoQ ameliorated tubular injury mediated by mitophagy
in diabetic kidney disease via Nrf2/PINK1. Redox Biol. 11:297–311.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hamacher-Brady A and Brady NR: Mitophagy
programs: Mechanisms and physiological implications of
mitochondrial targeting by autophagy. Cell Mol Life Sci.
73:775–795. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen K, Dai H, Yuan J, Chen J, Lin L,
Zhang W, Wang L, Zhang J, Li K and He Y: Optineurin-mediated
mitophagy protects renal tubular epithelial cells against
accelerated senescence in diabetic nephropathy. Cell Death Dis.
9(105)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jabir MS, Hopkins L, Ritchie ND, Ullah I,
Bayes HK, Li D, Tourlomousis P, Lupton A, Puleston D, Simon AK, et
al: Mitochondrial damage contributes to Pseudomonas
aeruginosa activation of the inflammasome and is downregulated
by autophagy. Autophagy. 11:166–182. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Durga Devi T, Babu M, Mäkinen P, Kaikkonen
MU, Heinaniemi M, Laakso H, Ylä-Herttuala E, Rieppo L, Liimatainen
T, Naumenko N, et al: Aggravated postinfarct heart failure in type
2 diabetes is associated with impaired mitophagy and exaggerated
inflammasome activation. Am J Pathol. 187:2659–2673.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tervaert TW, Mooyaart AL, Amann K, Cohen
AH, Cook HT, Drachenberg CB, Ferrario F, Fogo AB, Haas M, de Heer
E, et al: Pathologic classification of diabetic nephropathy. J Am
Soc Nephrol. 21:556–563. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Delanaye P and Pottel H: New equation to
estimate glomerular filtration rate in China: A reference issue.
Kidney Int. 96(521)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Janowski AM, Colegio OR, Hornick EE,
McNiff JM, Martin MD, Badovinac VP, Norian LA, Zhang W, Cassel SL
and Sutterwala FS: NLRC4 suppresses melanoma tumor progression
independently of inflammasome activation. J Clin Invest.
126:3917–3928. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Zhan M, Usman IM, Sun L and Kanwar YS:
Disruption of renal tubular mitochondrial quality control by
Myo-inositol oxygenase in diabetic kidney disease. J Am Soc
Nephrol. 26:1304–1321. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Huang C, Zhang Y, Kelly DJ, Tan CY, Gill
A, Cheng D, Braet F, Park JS, Sue CM, Pollock CA and Chen XM:
Thioredoxin interacting protein (TXNIP) regulates tubular autophagy
and mitophagy in diabetic nephropathy through the mTOR signaling
pathway. Sci Rep. 6(29196)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Eleftheriadis T, Pissas G, Tsogka K,
Nikolaou E, Liakopoulos V and Stefanidis I: A unifying model of
glucotoxicity in human renal proximal tubular epithelial cells and
the effect of the SGLT2 inhibitor dapagliflozin. Int Urol Nephrol.
52:1179–1189. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gou R, Chen J, Sheng S, Wang R, Fang Y,
Yang Z, Wang L and Tang L: KIM-1 mediates high glucose-induced
autophagy and apoptosis in renal tubular epithelial cells. Cell
Physiol Biochem. 38:2479–2488. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Giacco F and Brownlee M: Oxidative stress
and diabetic complications. Circ Res. 107:1058–1070.
2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen K, Feng L, Hu W, Chen J, Wang X, Wang
L and He Y: Optineurin inhibits NLRP3 inflammasome activation by
enhancing mitophagy of renal tubular cells in diabetic nephropathy.
FASEB J. 33:4571–4585. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Song S, Qiu D, Luo F, Wei J, Wu M, Wu H,
Du C, Du Y, Ren Y, Chen N, et al: Knockdown of NLRP3 alleviates
high glucose or TGFB1-induced EMT in human renal tubular cells. J
Mol Endocrinol. 61:101–113. 2018.PubMed/NCBI View Article : Google Scholar
|