Introduction
Colorectal cancer (CRC) is the second most common
type of cancer and the leading cause of cancer-associated mortality
in China (1). It is associated with
obesity, type 2 diabetes and chronic inflammatory disease (2). Although progress has been achieved in
the traditional treatment of CRC, it remains one of the most lethal
malignancies globally due to the limited therapeutics, high
recurrence rate and poor prognosis (3). Thus, identification of safer and more
effective treatments for CRC is required.
Mammalian thioredoxin (Trx) reductase 1 (TrxR1) is a
selenoprotein that functions to maintain redox homeostasis in cells
via transferring electrons from NADPH to its substrate Trx
(4,5). Both Trx and TrxR1 are reported to be
upregulated in CRC cells and have been associated with poor
prognosis and resistance to chemotherapy (6-9).
Increasing evidence has suggested that TrxR1 is involved in
multiple steps of tumorigenesis (10-14).
Therefore, TrxR1 is a potential target for anticancer drug
development for CRC.
Natural products and their synthetic analogues are a
major source of therapeutic agents and have been used for centuries
to treat a variety of human ailments (15,16).
Hydroxytyrosol (HT) is a major bioactive component of olive leaves
and oil with a polyphenol scaffold, which has been demonstrated to
exhibit a wide variety of pharmacological activities, including
anti-inflammatory (17),
neuroprotective (18),
immunomodulatory (19) and
antimicrobial activities (20-22).
HT has been indicated to exhibit anticancer properties due to its
important antiproliferative and pro-apoptotic effects in different
types of cancer cells, including colon cancer cells. This effect
could be mediated via numerous signaling pathways, for example,
inhibition of ERK1/2(23),
antagonization of the G-protein-coupled estrogen receptor (24), dysregulated expression of catalase
(25-27)
and inhibition of the Akt, NF-κB, STAT3 and EGFR signaling pathways
(28-30).
Notably, the majority of the cellular actions of HT are associated
with the induction of reactive oxygen species (ROS) production in
colon cancer cells (25-27).
In addition, the polyphenol scaffold has been revealed to be a
useful scaffold for the TrxR1 inhibitor development (31). Therefore, the molecular mechanisms
of ROS induction by HT require further elucidation.
The present study reported that HT is a potent
inhibitor of TrxR1 with the ability to inhibit both the recombinant
and the cellular TrxR1 in colon cancer cells. Inhibition of TrxR1
by HT induced accumulation of ROS, promoted apoptosis and inhibited
proliferation. Silencing of TrxR1 expression by RNA interference
enhanced the cytotoxicity and the TrxR1 inhibitory activity of HT,
supporting that TrxR1 is involved in the cellular actions of HT.
The discovery of TrxR1 targeting by HT provided insight into the
pharmacological activity of HT and indicated ability of HT to
induce cellular ROS. Thus, HT could potentially be used for the
development of novel TrxR1 inhibitors. Together with evidence from
previously published data (26), HT
could be developed as a clinical treatment for patients with
CRC.
Materials and methods
Materials
The natural compound library (cat. no. L6000) was
purchased from Shanghai Topscience Co., Ltd. DMSO, NADPH, insulin,
5,5'-dithiobis-2-nitrobenzoic acid (DTNB), EDTA and recombinant
human Trx (expressed in E. coli) were purchased from
Sigma-Aldrich (Merck KGaA). Cell Counting Kit-8 (CCK-8; cat. no.
CK04) and caspase-3 activity detection kit (cat. no. C551) were
purchased from Dojindo Molecular Technologies, Inc. Rabbit
polyclonal anti-TrxR1 antibody (cat. no. 11117-1-AP) was purchased
from Wuhan Sanying Biotechnology. Mouse monoclonal anti-GAPDH
antibody (cat. no. MA515738), HRP-conjugated goat anti-rabbit (cat.
no. 31460) and goat anti-mouse (cat. no. 31430) IgG (H+L) secondary
antibodies were purchased from Thermo Fisher Scientific, Inc. ROS
assay kit (cat. no. S0033) and RIPA lysis buffer (cat. no. P0013B)
were purchased from Beyotime Institute of Biotechnology. FITC
Annexin V Apoptosis Detection Kit I (cat. no. 556547) was purchased
from BD Biosciences. Protease and phosphatase inhibitor cocktail
(cat. no. 78440) and Pierce BCA Protein Assay Kit (cat. no. 23225)
were purchased from Thermo Fisher Scientific, Inc.
Cell culture
All human cell lines were purchased from American
Type Culture Collection (ATCC). The normal colon epithelial cell
line FHC was cultured in DMEM/F12 medium (cat. no. 30-2006; ATCC)
with a final concentration of 25 mM HEPES, 10 ng/ml cholera toxin
(cat. no. C8052; Sigma-Aldrich; Merck KGaA), 0.005 mg/ml insulin
(cat. no. 91077C; Sigma-Aldrich; Merck KGaA), 0.005 mg/ml
transferrin (cat. no. T8158; Sigma-Aldrich; Merck KGaA), 100 ng/ml
hydrocortisone (cat. no. HY-N0583; MedChemExpress), 20 ng/ml human
recombinant EGF (cat. no. PHG0311; Thermo Fisher Scientific, Inc.)
and 10% (v/v) FBS (cat. no. 10099141C; Thermo Fisher Scientific,
Inc.). HCT-8 and HCT-116 cells were cultured in RPMI-1640 medium
(Thermo Fisher Scientific, Inc.) supplemented with 10% (v/v) FBS;
SW620 cells were cultured in Leibovitz's L-15 medium (Thermo Fisher
Scientific, Inc.) supplemented with 10% (v/v) FBS. All cells except
SW620 (cultured with no CO2 at 37˚C) were maintained in
a humidified atmosphere with 5% CO2 at 37˚C. All treated
cells were incubated at 37˚C in the following experiments.
Recombinant protein production
Plasmid pET-TRSter was a gift from Elias Arnér (cat.
no. 78865; Addgene, Inc.; http://n2t.net/addgene:78865) (32), and recombinant rat TrxR1 protein
(TrxR-wt) was expressed in E. coli BL21 (DE3) strain.
TrxR-wt protein was purified by 2',5'-ADP-Sepharose column (Cytiva)
with protein purification system (AKTA pure 25; Cytiva).
Recombinant mutant human TrxR1 (residues 1-496; lack
of core sites Cys497 and Sec498 residues in the redox active center
of the C-terminal) with an N-terminal His tag (pET-28a-TrxR1-ΔC)
was expressed in the E. coli BL21 (DE3) strain and purified
by immobilized metal affinity chromatography with protein
purification system (AKTA pure 25; Cytiva).
Plasmid pET-28a-TrxR1-ΔC was constructed by cloning
DNA encoding human TrxR1 protein (residues 1-496) into pET28a
vector (cat. no. 69864; Sigma-Aldrich; Merck KGaA) using
EcoRI and NcoI restriction sites. Recombinant rat
TrxR1 (TrxR-wt) and mutant human TrxR1 enzyme activity were
measured via DTNB assay and calculated using the following formula:
TrxR1 U/mg protein=(ΔApro-ΔAblank)/(ε x d)
x109 x Vtotal/(Cpr x
Vpro)/T. ΔApro was the absorbance of wells
with TrxR1 protein, ΔAblank was the absorbance of wells
without TrxR1 protein, ε was the molar extinction coefficient, d
was the optical path length of the 96-well plate, Vtotal
was the total reaction volume (unit, µl), Cpr was the
concentration of TrxR1 protein (unit, µl/µg), Vpro was
the TrxR1 protein volume in the reaction (unit, µl), T was the
reaction time (unit, min) and U was the enzyme units generating 1
nM 2-nitro-5-thiobenzoate per mg TrxR1 protein per min. The
activity of recombinant rat TrxR1 was 511 U/mg protein, while that
of recombinant mutant TrxR1 was 23 U/mg protein.
TrxR1 enzyme assay
TrxR1 activity was determined at room temperature
using a microplate reader. The NADPH-reduced recombinant rat TrxR1
(100 nM) or mutant human TrxR1 (1 µM) was incubated with different
concentrations of HT for 1 h at room temperature (the final volume
of the mixture was 50 µl) in a 96-well plate. A mixture in
Tris-EDTA buffer (50 mM Tris-HCl pH 7.5; 1 mM EDTA; 50 µl)
containing DTNB and NADPH was added (final concentration 2 mM and
200 µM, respectively), and the linear increase in absorbance (AB)
at 412 nm during the initial 3 min was recorded. The same amount of
DMSO (0.1%; v/v) was added to the control experiments, and the
TrxR1 inhibitory rate was calculated using the following formula:
TrxR1 Inhibitory rate=[1- (AB value of test at 3 min - AB value of
test at 0 min)/(AB value of control at 3 min - AB value of control
at 0 min)] x 100%.
Cellular TrxR1 activity assay
After HCT-116 or SW620 cells were treated with
different concentrations (2-fold dilution for 11 concentrations
starting at 100 µM) of HT for 24 h, the cells were harvested and
washed twice with PBS. Total cellular proteins were extracted using
RIPA buffer for 30 min on ice and quantified using the BCA method.
TrxR1 activity in cell lysates was measured via the endpoint
insulin reduction assay. Briefly, the cell extract containing 20 µg
total protein was incubated in a final reaction volume of 50 µl
containing 100 mM Tris-HCl (pH 7.6), 0.3 mM insulin, 660 µM NADPH,
3 mM EDTA and 1.3 µM recombinant human Trx for 30 min at 37˚C. The
reaction was terminated by adding 200 µl of 1 mM DTNB in 6 M
guanidine hydrochloride, pH 8.0 at 25˚C for 5 min. A blank sample,
containing everything except Trx, was treated in the same manner.
The AB at 412 nm was measured, and the blank value was subtracted
from the corresponding absorbance value of the sample. The same
amount of DMSO was added to the control experiments and the TrxR1
inhibitory rate was calculated using the following formula: TrxR1
Inhibitory rate=[1-(AB value of sample-AB value of blank)/(AB value
of control-AB value of blank)] x100%.
Cell proliferation assay
The effect of drug treatments on cell proliferation
was quantified using a CCK-8 assay in HCT-116, SW620, HCT-8 and FHC
cell lines. A total of 5,000 cells per well were seeded in 96-well
plates overnight and then treated with HT (2-fold dilution for 12
concentrations starting at 200 µM). Complete culture medium without
drug was added to the blank wells. Control cells were treated with
DMSO only. After culture for 24 h, 10 µL CCK-8 was added to each
well and cells were incubated at 37˚C for 4 h. Subsequently, the
plate was gently shaken at 25˚C for 10 min. The AB at 450 nm was
measured using a Cytation 5 microplate reader (BioTek Instruments,
Inc.), and the cell cytotoxicity inhibition rate was calculated
using the following formula: Growth inhibition rate=(AB value of
control - AB value of test)/(AB value of control - AB value of
blank) x 100%. Cells were also pretreated with 3 mM
N-acetylcysteine (NAC) for 2 h prior to HT exposure, followed by
analysis of the effect of NAC on the inhibitory effect of HT on
cell proliferation.
Imaging TrxR1 activity in HCT-116 and
SW620 cells by TRFS-green
Cells were treated with the indicated concentrations
(5, 10 and 20 µM for HCT-116 cells; 15, 30 and 60 µM for SW620
cells) of HT for 24 h followed by treatment with TRFS-green (10 µM)
(33) for 4 h. Phase contrast and
fluorescence images were captured using fluorescence microscopy
(EVOS FL). A total of 10 cells were randomly selected, and the
fluorescence intensity in individual cells was quantified using
ImageJ software (version 1.8; National Institutes of Health).
Western blotting
HCT-116 or SW620 cells were treated with HT at the
indicated concentrations for 24 h (5, 10 and 20 µM for HCT-116
cells; 15, 30 and 60 µM for SW620 cells). Cells were lysed with
RIPA lysis buffer. Protein quantification was performed using a BCA
Protein Assay Kit, following which equal quantities of proteins (20
µg/lane) were separated via a 4-20% gradient SDS-PAGE gel and
transferred onto a PVDF membrane. The membranes were blocked with
5% BSA (cat. no. V900933; Sigma-Aldrich; Merck KGaA) at room
temperature for 2 h. TrxR1 antibody was used at a 1:2,000 dilution
and GAPDH antibody was used at a 1:5,000 dilution in 5% BSA and the
membranes were incubated at 4˚C overnight. Following three washes
in TBS/0.1% Tween 20, the membranes were probed with the a
forementioned HRP-conjugated secondary antibodies at a 10,000-fold
dilution in 5% BSA. Following six washes with TBS/0.1% Tween 20,
the immune complexes were incubated with SuperSignal™ West Pico
PLUS reagent (cat. no. 34577; Thermo Fisher Scientific, Inc.) and
detected using the ChemiDoc™ Touch Imaging system (Bio-Rad
Laboratories, Inc.). The intensity of the resulting bands on the
membranes was calculated and normalized to GAPDH in each sample
using ImageJ software (version 1.8; National Institutes of Health).
For the detection of the knockdown efficiency of TrxR1, cells were
pre-transfected with small interfering (si)TrxR1 for 60 h prior to
lysis as described below.
Apoptosis assay
HCT-116 and SW620 cells were plated in six-well
plates at an initial density of 2.4x105 cells/well for 8
h in complete RPMI-1640 medium. Cells were divided into the
following three treatment groups: i) DMSO (Control); ii) HT
treatment (5, 10 and 20 µM in HCT-116 cells; 15, 30 and 60 µM in
SW620 cells); and iii) a combination of HT and 3 mM NAC. Cells were
starved in serum-free RPMI-1640 medium for 16 h, except that groups
with NAC treatment were pretreated with 3 mM NAC for 2 h after
starvation in serum-free medium for 14 h, followed by HT treatment
for 24 h. Cells were harvested, washed twice with PBS (Thermo
Fisher Scientific, Inc.), evaluated for apoptosis using FITC
Annexin V Apoptosis Detection kit I according to the manufacturer's
protocol and analyzed using Novocyte® flow cytometer
with NovoExpress® version 1.2.4 software (ACEA
Bioscience, Inc.).
Caspase-3 activity assay
This experiment was performed using the
aforementioned caspase-3 activity detection kit according to the
manufacturer's instructions. Briefly, HCT-116 cells were treated
with the indicated concentrations of HT for 24 h. Subsequently, the
cells were collected and lysed with RIPA lysis buffer for 15 min at
0˚C, using the Bradford method to quantify the protein content. A
total of 50 µg protein were incubated with 10 µl Ac-DEVD-pNA
substrate and 40 µl assay buffer at 37˚C for 2 h in a final volume
of 100 µl. The absorbance at 405 nm was read on a microplate
reader.
Cell cycle assay
HCT-116 and SW620 cells were seeded in a six-well
plate at an initial density of 2.4x105 cells/well and
allowed to attach overnight in complete culture medium. Cells were
divided into three treatment groups, as aforementioned in the
apoptosis assay. Cells were starved in serum-free culture medium
for 24 h, except that groups with NAC treatment were pretreated
with 3 mM NAC for 2 h after starvation in serum-free medium for 22
h, followed by HT treatment for 12 h. Cells were harvested, washed
twice with PBS, evaluated for cell cycle using the cell cycle assay
kit (cat. no. BB-4104; BestBio) and analyzed using the
Novocyte® flow cytometer with NovoExpress®
version 1.2.4 software (ACEA Bioscience, Inc.).
RNA interference analysis
TrxR1 siRNA and a scramble non-targeting negative
control siRNA (siNC) were purchased from Biomics Biotechnologies
Co., Ltd. The siRNAs (final concentration, 50 nM) were transfected
into cells using Lipofectamine® 3000 reagent (Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. The siRNA sequences were as follows: siTrxR1 sense,
5'-GCAUCAAGCAGCUUUGUUAdTdT-3', siTrxR1 antisense,
5'-UAACAAAGCUGCUUGAUGCdTdT-3'; siNC sense,
5'-UUCUCCGAACGUGUCACGUdTdT-3', siNC antisense,
5'-ACGUGACACGUUCGGAGAAdTdT-3'. For the loss-of-function analysis,
HCT-116 or SW620 cells were pre-transfected with siNC or siTrxR1
for 36 h prior to HT exposure, followed by the same process as in
CCK-8 assay, cellular TrxR1 activity assay and TRFS-green
imaging.
Measurement of ROS generation
Cellular ROS content was measured by flow cytometry
and fluorescence microscopy for quantitative and qualitative
evaluation. HCT-116 or SW620 cells were plated in six-well plates
at a density of 2.0x105 cells/well and allowed to attach
overnight, and were then exposed to the indicated concentrations of
HT for 4 h (5, 10 and 20 µM for HCT-116 cells; 15, 30 and 60 µM for
SW620 cells). Cells were stained with 10 µM
2',7'-dichlorofluorescein diacetate (DCFH-DA; Beyotime Institute of
Biotechnology) at 37˚C for 30 min and then washed three times in
serum-free culture medium. For flow cytometry, cells were
collected, and fluorescence was analyzed at excitation and emission
wavelengths of 488 and 525 nm, respectively, using the
Novocyte® flow cytometer with NovoExpress®
version 1.2.4 software (ACEA Bioscience, Inc.). The mean value of
DCFH-DA fluorescence intensity was utilized for quantitative
analysis. For fluorescence microscopy, cell images were captured
using the EVOS FL Imaging System (Thermo Fisher Scientific,
Inc.).
Time course of HT activity assay
For the time course assay, HCT-116 cells were
cultured as aforementioned, and then exposed to 20 µM HT for 16, 24
and 32 h followed by apoptosis assay, western blotting and
TRFS-green imaging. For ROS generation measurement, cells were
exposed to 20 µM HT for 2, 4 and 6 h, followed by DCFH-DA
fluorescence assay.
Docking of HT to the TrxR1 structural
model
To further study the interaction between HT and
TrxR1, a docking study was implemented by Molecular Operating
Environment (MOE) version 2019.0102 (https://www.chemcomp.com/index.htm). The crystal
structure of human TrxR1 (Protein Data Bank code 2ZZ0, chain A;
https://www.rcsb.org/structure/2ZZ0)
was used for the present docking study. The central coordination of
the dock pocket was examined by Site Finder through MOE software,
which was calculated by selecting residues Cys497 and Sec498. The
default parameters were used for implementing the docking
simulation.
Statistical analysis
All statistical analyses were performed using
GraphPad Prism software version 9.0 (GraphPad Software, Inc.).
Results are presented as the mean ± standard deviation of at least
three independent experiments for each group. Statistical
differences for the datasets of apoptosis rate and cell cycle
distribution were analyzed via two-way ANOVA, while other data sets
consisting of >2 groups were analyzed via one-way ANOVA,
followed by Tukey-Kramer post hoc test for multiple comparisons if
significance was determined. For data not conforming to a normal
distribution, Kruskal-Wallis test was used for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
HT inhibits TrxR1 activity in
vitro
To screen potent inhibitors of TrxR1, a TrxR1
activity assay was performed with a natural compound library
against the recombinant TrxR1 enzyme (4,457 compounds; purity,
>90%). Out of these, eight compounds were indicated to exhibit
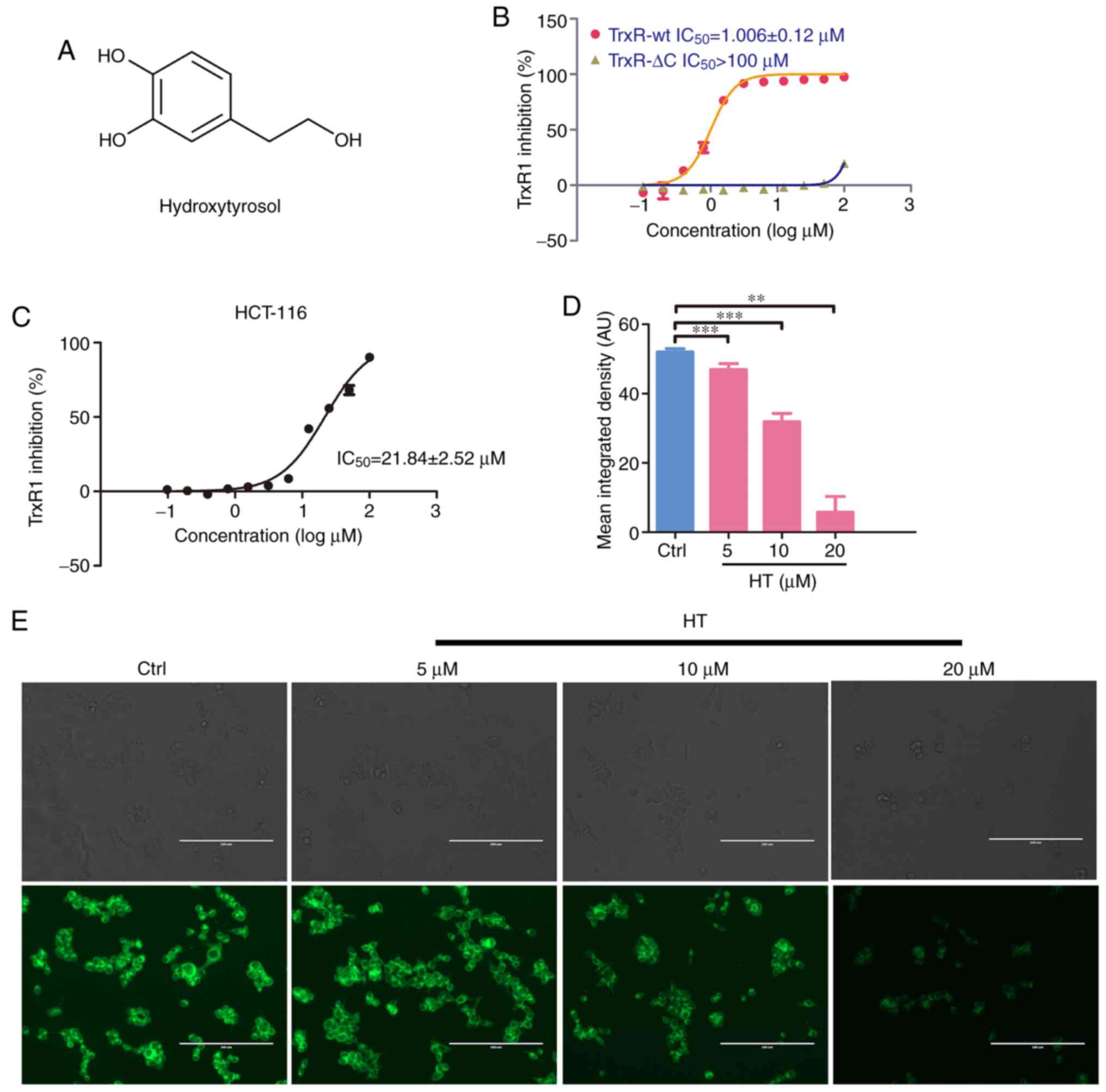
TrxR1 enzyme inhibitory activity. Among them, HT (the structure of
this compound is depicted in Fig.
1A) could effectively inhibit TrxR1-wt activity with an
IC50 ~1 µM, but exhibited little effect on the TrxR1-ΔC
enzyme, a mutant TrxR1 lacking Cys497 and Sec498 residue in the
C-terminal of wt TrxR1 (Fig. 1B).
These results indicated that the Sec residue in TrxR1 was
associated with the inhibition of TrxR1 by HT. The inhibition of
cellular TrxR1 by HT was further confirmed using an insulin
reduction assay and image-based live cell TrxR1 activity assay. As
illustrated in Fig. 1C, treating
HCT-116 cells with HT led to a notable inhibition of cellular TrxR1
activity, with an IC50 of ~21.84 µM. By measuring and
quantifying the fluorescence signal of this probe, a decrease in
TrxR1 activity in living HCT-116 cells treated with HT compared
with control cells was confirmed (Fig.
1D and E). Both assays produced
consistent results revealing a dose-dependent inhibition of TrxR1
by HT in HCT-116 cells. Taken together, these data demonstrated
that HT is a potent TrxR1 inhibitor.
HT treatment inhibits proliferation
and induces accumulation of ROS
The antiproliferative effects of HT were tested in
cultured human CRC cells (HCT-116, SW620 and HCT-8 cells) and
normal colon cells (FHC cells). It was observed that HT treatment
preferentially suppressed proliferation of all three CRC cells
tested in a dose-dependent manner (Fig.
2A). By contrast, HT treatment exhibited a weak effect on
normal FHC cells compared with that on cancer cells. In addition,
the TrxR1 expression levels of HCT-116 cells were the highest among
all three CRC cell lines, while those of SW620 cells were the
lowest (data not shown). Thus, HCT-116 and SW620 cell lines were
selected to investigate the cellular function of HT. TrxR1 protein
levels were significantly decreased after the cells were treated
with HT for 24 h (Fig. 2B),
indicating that the decreased TrxR1 protein expression levels may
account for the decreased TrxR1 enzyme activity after HT
treatment.
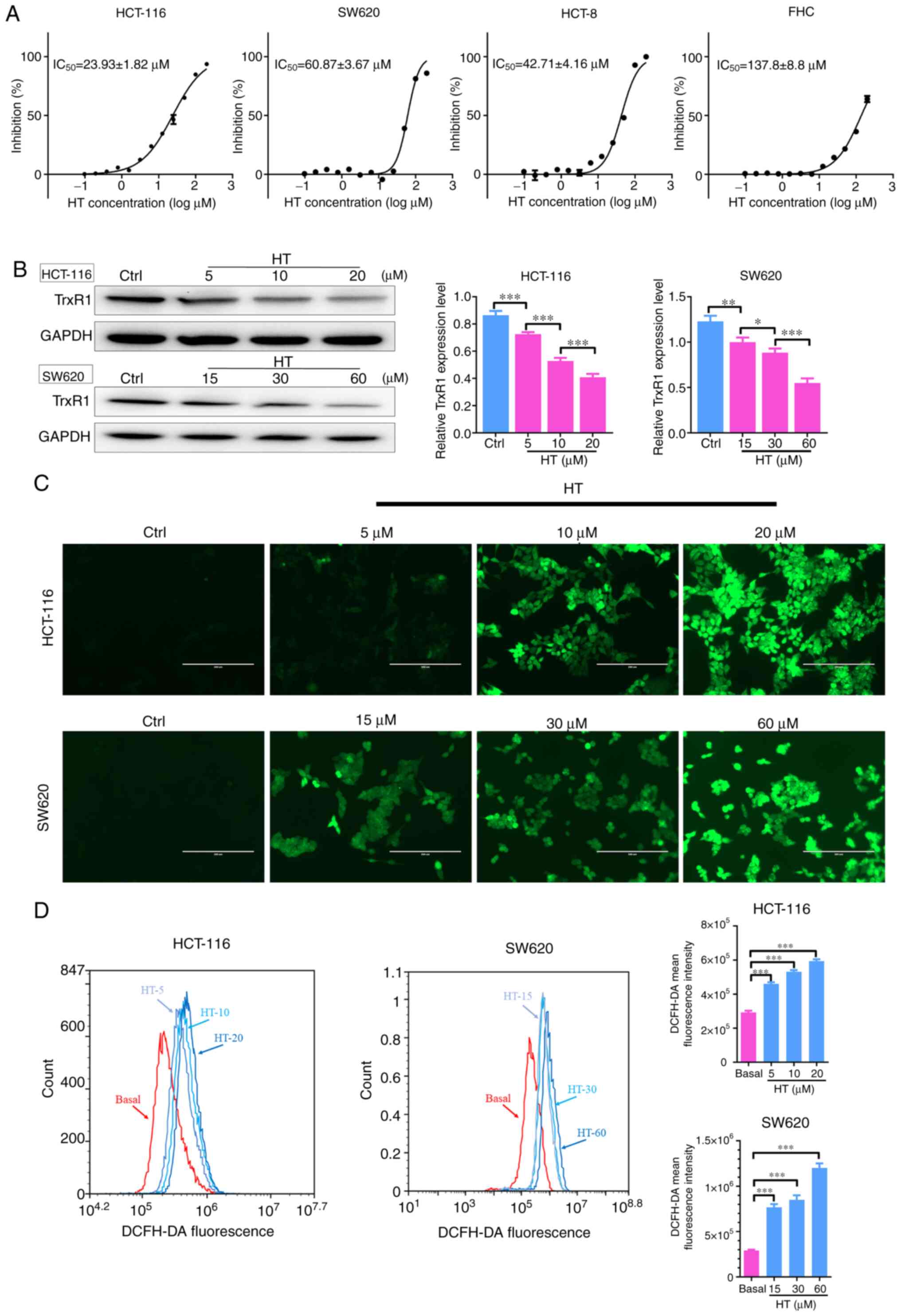 | Figure 2Effects of HT treatment on cell
proliferation, TrxR expression levels and ROS accumulation. (A)
Effect of increasing doses of HT on the proliferation of human
colorectal cancer cell lines (HCT-116, HCT-8, SW620) and the normal
FHC cell line. (B) Cells were treated with the indicated
concentrations of HT for 24 h, and cell extracts were prepared and
analyzed via western blotting with an antibody against TrxR1. TrxR1
expression levels were quantified and normalized to GAPDH.
Intracellular ROS generation was induced by increasing doses of HT,
and it was detected via staining with 10 µM DCFH-DA and examined
via (C) fluorescence microscopy and (D) flow cytometry in HCT-116
and SW620 cells. DCFH-DA mean fluorescence intensity was quantified
by flow cytometry. DMSO was used as the negative control. Scale
bars, 200 µm. *P<0.05, **P<0.01 and
***P<0.005. TrxR, thioredoxin reductase; HT,
hydroxytyrosol; ROS, reactive oxygen species; DCFH-DA,
2',7'-dichlorofluorescein diacetate. |
The major function of TrxR1 is to maintain Trx in a
reduced state and prevent oxidative stress (34). Having confirmed that HT was a potent
TrxR1 inhibitor, the levels of ROS were then determined in HCT-116
and SW620 cells. ROS levels in the two cell lines were assessed by
flow cytometry and cell imaging using the redox-sensitive
fluorescent probe DCFH-DA. As presented in Fig. 2C and D, treatment with HT for 4 h significantly
increased ROS levels in a dose-dependent manner, indicating that HT
exhibited the ability to induce cellular ROS accumulation. Taken
together, these results suggested that HT treatment inhibited
proliferation and promoted accumulation of ROS in CRC cells.
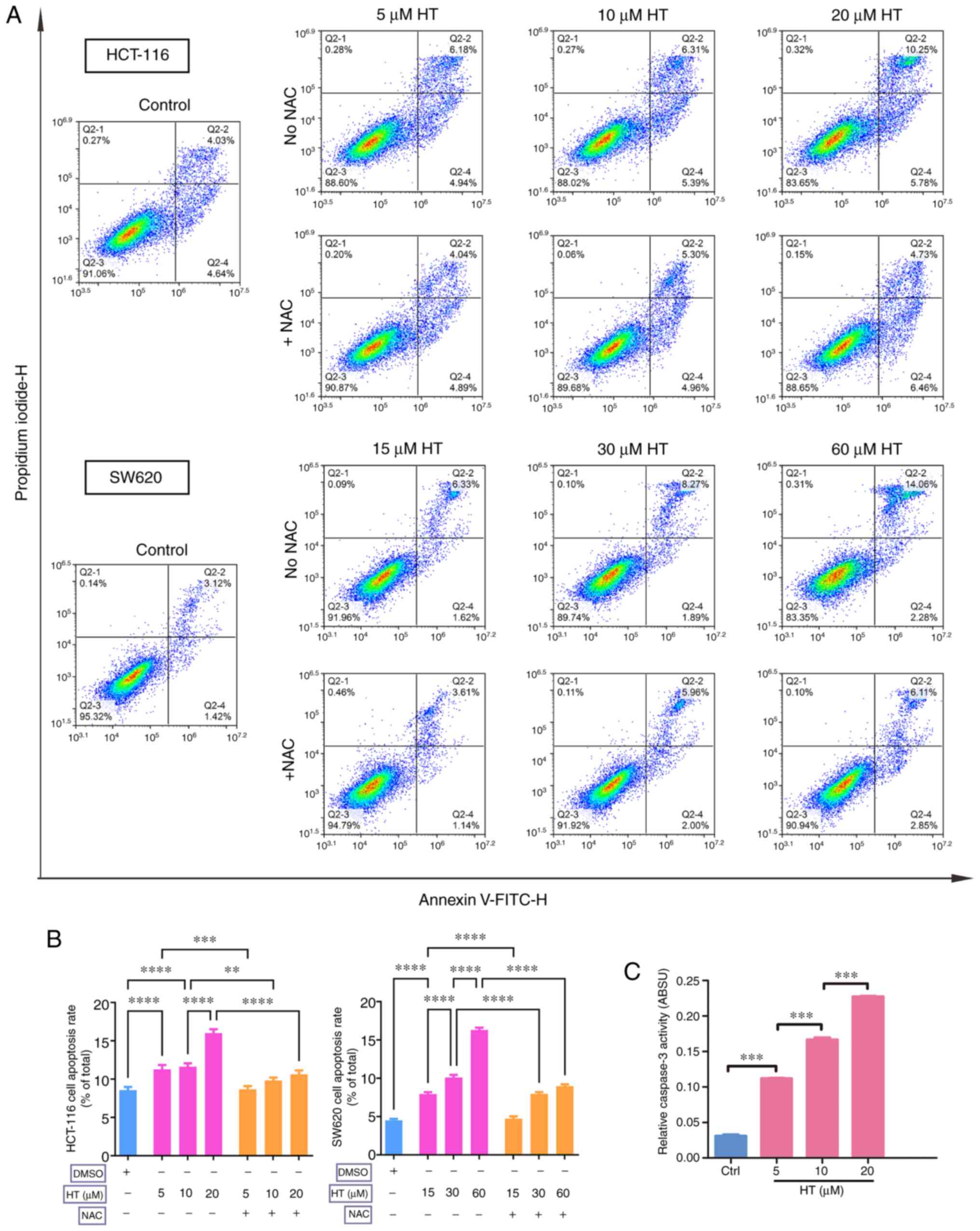
HT treatment induces apoptosis and
G1/S cell cycle arrest in HCT-116 and SW620 cells
Since excess ROS levels could be cytotoxic (35), apoptosis was examined using Annexin
V/PI double staining assay. A significant increase in the
percentage of apoptotic cells was detected after HT treatment for
24 h in HCT-116 and SW620 cells (Fig.
3A and B). Similar results were
observed in the caspase-3 activity assay, where a significant
concentration-dependent activation of caspase-3 was observed after
a 24-h treatment with HT in HCT-116 cells (Fig. 3C).
By contrast, NAC inhibited the apoptosis-inducing
effect of HT treatment in HCT-116 and SW620 cells, and the effects
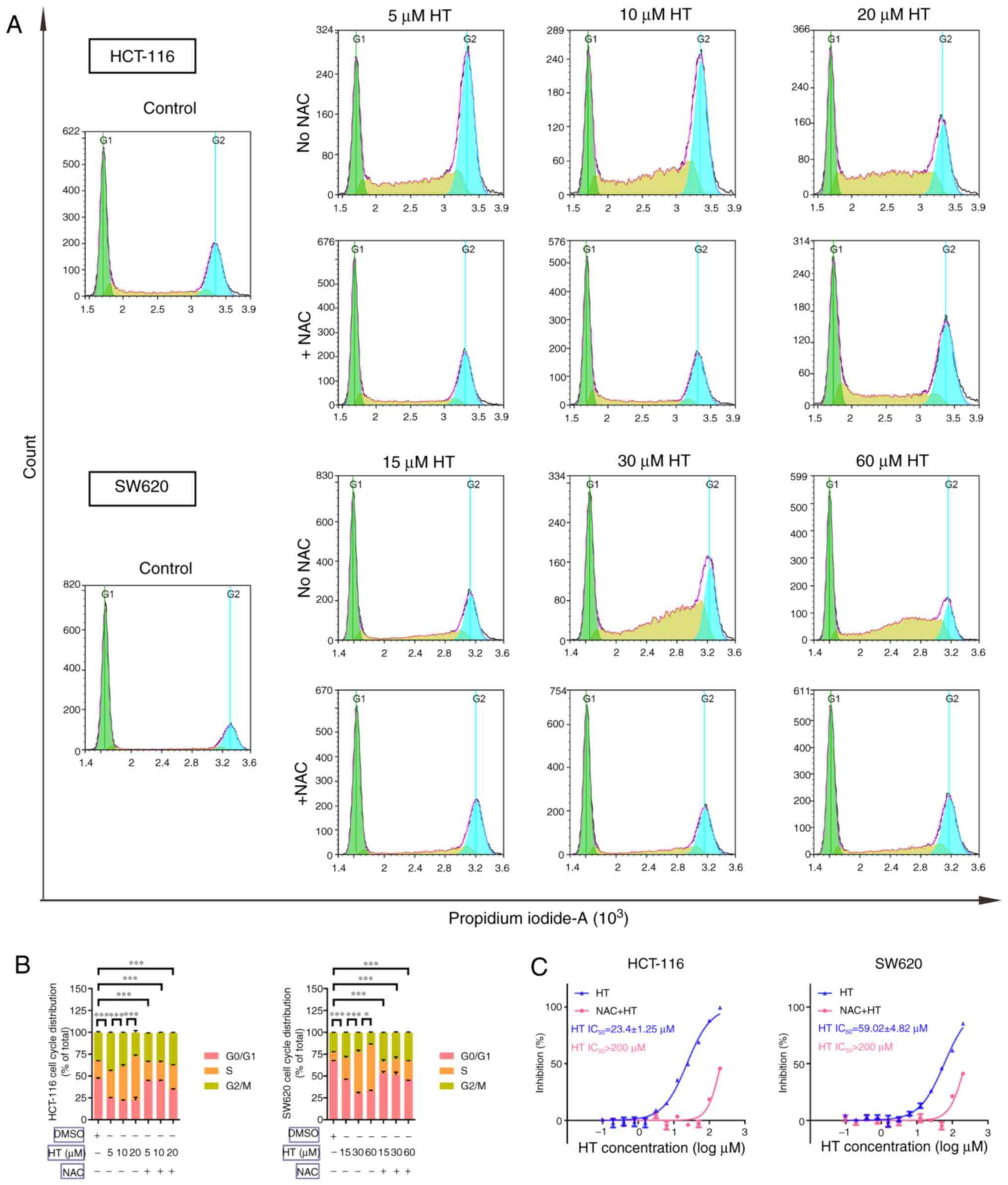
of ROS induction on cell cycle arrest were also determined. Cells
were treated with various concentrations of HT with or without NAC
for 24 h. The results in Fig. 4A
and B indicated that HT treatment
significantly induced G1/S cell cycle arrest in HCT-116
and SW620 cells. NAC significantly inhibited the cell cycle arrest
effect of HT treatment in both cell lines. In addition, NAC
pre-treatment inhibited the antiproliferative effect of HT in
HCT-116 and SW620 cells (Fig. 4C).
These results demonstrated that HT treatment promoted apoptosis and
G1/S cell cycle arrest in CRC cells via its effect on
ROS generation.
Antitumor effect of HT treatment is
associated with its interaction with TrxR1
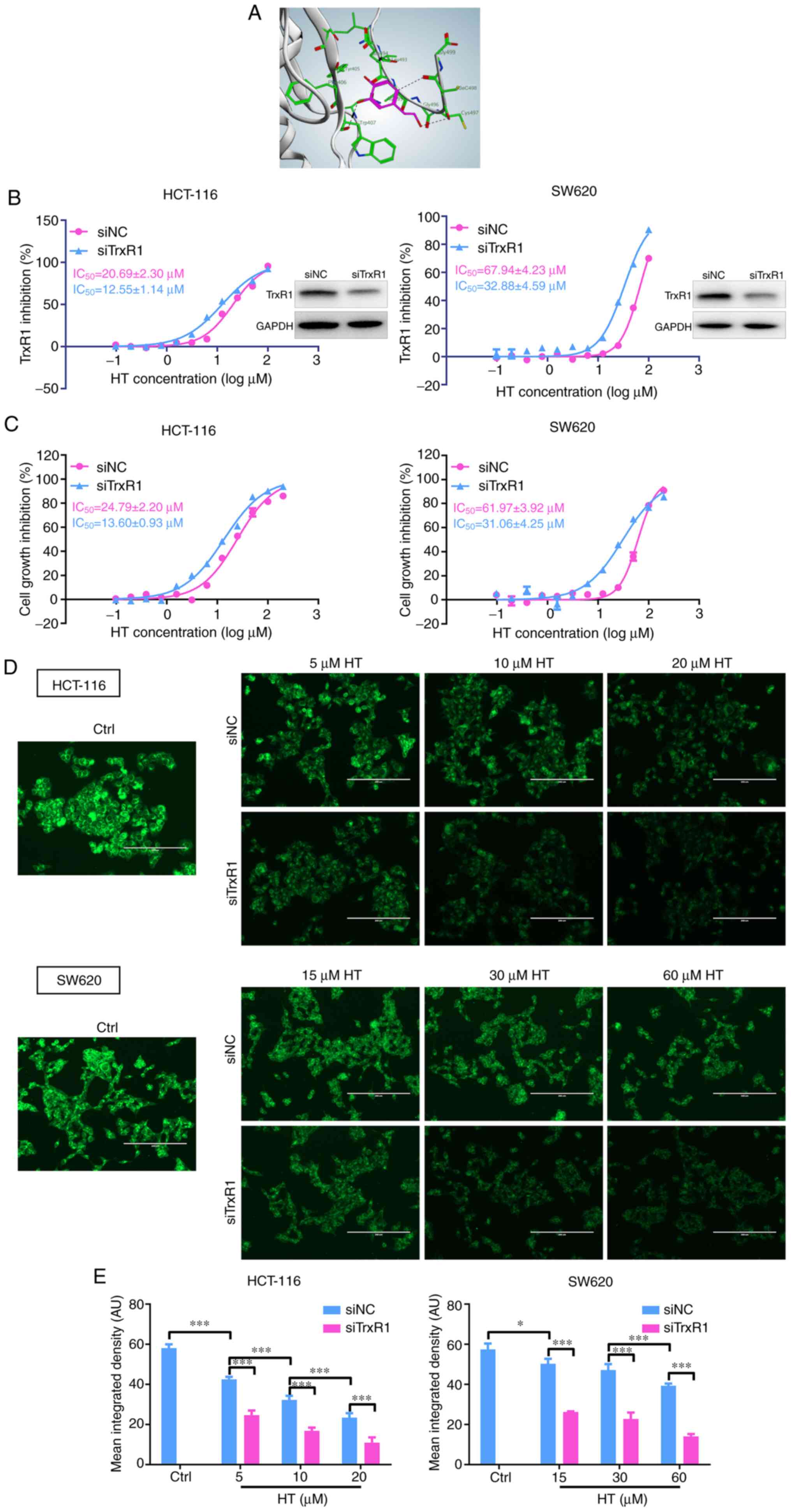
To investigate the underlying structural mechanism
of HT binding to the TrxR1 protein, a molecular simulation of the
HT-TrxR1 complex was performed using docking software. As presented
in Fig. 5A, HT was indicated to
form hydrogen bonds with Sec498, Cys497, Gln494 and Trp407 residues
of the C-terminal active site of the redox center of TrxR1
(34,36-39).
Thus, the proposed reaction mechanism for HT is the simultaneous
inhibition of the adjacent C-terminal active site residues of
TrxR1, which is expected to effectively suppress TrxR1 activity.
This mechanism is consistent with the difference in the HT-mediated
TrxR1 inhibition of the TrxR-wt and TrxR-ΔC enzymes, as the TrxR-ΔC
enzyme lacks the core site Sec498 and Cys497 residues for HT
binding.
As HT inhibition of both the recombinant and the
cellular TrxR1 enzyme was demonstrated (Fig. 1), the physiological significance of
TrxR1 inhibition in the cellular actions of HT was further
investigated. TrxR1 expression levels were reduced in HCT-116 and
SW620 cells following transfection of an siRNA specifically
targeting TrxR1, and the knockdown efficiency was validated
(Fig. 5B). As presented in Fig. 5C, HT exhibited a higher
antiproliferative effect in siNC-transfected cells compared with
siTrxR1-transfected cells. Knocking down TrxR1 was helpful for
better characterizing the TrxR1 inhibitory activity of HT, which
was further confirmed via the Trx-mediated insulin reduction assay
(Fig. 5B) and the image-based assay
using the specific TrxR1 probe TRFS-green in HCT-116 and SW620
cells (Fig. 5D and E). Taken together, the data supported the
conclusion that the antiproliferative effect of HT was associated
with its interaction with TrxR1.
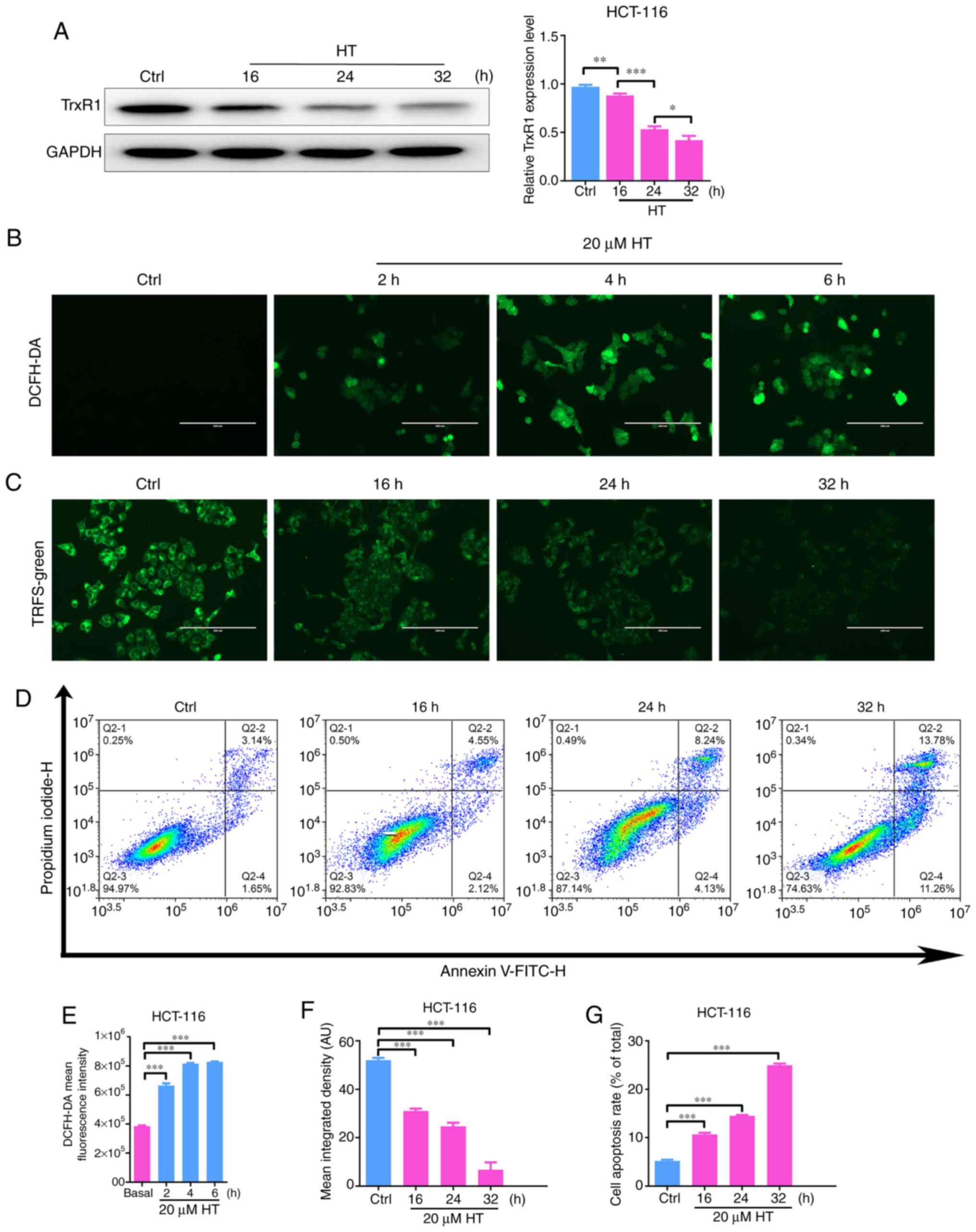
In addition, a time course assay was performed to
further confirm the association between the antitumor effects and
the TrxR1 inhibitory activity of HT. As illustrated in Fig. 6A, C
and F, HT treatment could reduce
the expression level and activity of TrxR1 protein in a time
course-dependent manner. However, HT treatment also induced ROS
accumulation and apoptosis in a time course-dependent manner
(Fig. 6B, D, E and
G). These results partly
demonstrated the antitumor effect of HT and revealed its
association with TrxR1.
Discussion
HT has been indicated to induce ROS generation in
cancer cells (26). To the best of
our knowledge, the mechanism responsible for ROS induction by HT
remains unclear. The results of the present study demonstrated that
HT treatment inactivated TrxR1, induced accumulation of ROS and
eventually resulted in oxidative stress-mediated apoptosis in CRC
cells.
As both Trx and TrxR1 have been reported to be
upregulated in numerous human cancer types, including leukemia,
lung cancer, breast cancer and colorectal cancer among others, and
be associated with increased tumor growth, drug resistance and poor
patient prognosis, the thioredoxin system has been recognized as an
attractive target for anticancer drug development (4,6-10,40).
This system plays a role in maintaining H2O2
levels in cells via the conversion of H2O2 to
H2O. Trx contains two adjacent thiols in its reduced
form that are converted to a disulfide bond in the oxidized form
(39). The oxidized Trx is then
recycled back into the reduced form by TrxR1 and NADPH (35). The present study demonstrated that
HT could significantly inhibit both recombinant and cellular TrxR1
enzyme activity. The mammalian TrxR1 enzyme uniformly harbors a
critical Sec residue in its C-terminal active site. The high
nucleophilicity of the Sec residue renders the enzyme vulnerable to
be modified by electrophiles, based on which numerous selective
TrxR1 inhibitors have been developed (4,5,40-44).
The results of the current study revealed strong inhibition of the
TrxR-wt enzyme but not the enzyme lacking the Sec residue
(TrxR-ΔC), thus revealing the potential interaction site between
TrxR1 and HT. The docking results were also supportive of this
hypothesis. Therefore, further investigation on HT could lead to
the development of a novel strategy for treating CRC.
HT is a phenolic phytochemical with numerous medical
properties (45). It is considered
a chemopreventive agent due to its antioxidative properties
(46). Recently, HT was indicated
to be a promising anticancer compound. For, instance, previous
studies have demonstrated that HT can induce oxidative stress in
various cell lines, and the induction of ROS production is involved
in the biological functions of HT (25,26,47-49).
However, to the best of our knowledge, there is no detailed
mechanism of how HT elevates oxidative stress. In the current
study, TrxR1 was identified as a target of HT, and it was
demonstrated that HT induced apoptosis and cell cycle arrest
through a previously uncharacterized mechanism by targeting TrxR1.
Binding of HT to TrxR1 inhibits the physiological functions of
TrxR1, which leads to the conversion of H2O2
to H2O and ROS accumulation within cells, and finally
elicits oxidative stress. However, elevated TrxR1 activity has been
observed in HT-treated PC12 cells (50), which indicated HT may exhibit a
two-sided effect on ROS production. HT produces
H2O2 in the auto-oxidation process, which is
a common event in cell culture media; however, different cell types
exhibit various sensitivities to HT-induced
H2O2 and this leads to different cell fates
(51). This depends on both the
capability of cells to eliminate H2O2 by
specific enzymes, such as TrxR1, catalase and glutathione
peroxidase, and the effectiveness by which HT releases
H2O2 in different cell culture media
(52-54).
Therefore, different concentrations of HT treatment in various cell
types may lead to different levels of oxidative stress and,
therefore, stimulate various effects of TrxR1. The cellular
mechanisms by which HT exerts these effects are still poorly
understood and require further elucidation.
To the best of our knowledge, there are no TrxR1
inhibitors used clinically to date. As a HT is novel TrxR1
inhibitor with a unique scaffold, it is difficult to select another
inhibitor for comparison against HT. Therefore, a TrxR1 inhibitory
experiment was performed to compare HT and the current most
effective TrxR1 inhibitor, auranofin. Compared with the results of
our previous study, auranofin exhibited better TrxR1 inhibitory
activity compared with HT in HCT-116 cells (55). However, auranofin has insufficient
physiological stability and this limits its clinical development
(10). HT exhibited cellular enzyme
inhibitory activity and inhibited proliferation of several
colorectal cancer cells (HCT-116, SW620 and HCT-8) at the µmol
levels, which demonstrated that it was an effective antitumor
agent. Although its TrxR1 inhibitory activity is poor at present,
its activity may be improved by further modification of its
scaffold.
In conclusion, TrxR1 was investigated as a target of
HT in vitro in human CRC cells, and it was demonstrated that
HT induced oxidative stress-mediated apoptosis through a yet
unclear mechanism. Furthermore, the results of the present study
investigated the binding model between HT and TrxR1, which could
serve as a new scaffold to develop novel TrxR1 inhibitors for CRC
treatment.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Natural Science
Foundation of Anhui Province (grant no. 1808085QH262), University
Natural Science Research Project of Anhui Province (grant no.
KJ2018A0259), Key Research and Development Project of Anhui
Province (grant no. 202004a07020041), Major University Natural
Science Research Project of Anhui Province (grant no. KJ2019ZD30)
and Foundation for Excellent Talents in Higher Education of Anhui
Province (grant no. gxbjZD18).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SPZ and JZ performed the experiments, wrote the
manuscript and analyzed the data. CZ and YMZ designed the
experiments, wrote the manuscript and confirmed the authenticity of
all the raw data. QZF and XML purified recombinant TrxR1 protein
and performed TrxR1 enzyme activity assays. TW, FW, YC and SYH
performed the apoptosis, cell cycle and western blotting assays.
XPL performed the RNA interference assays. BSX and LH carried out
the cell culture and treatment with the drug solutions. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Feng RM, Zong YN, Cao SM and Xu RH:
Current cancer situation in China: Good or bad news from the 2018
global cancer statistics? Cancer Commun (Lond).
39(22)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lucas C, Barnich N and Nguyen HT:
Microbiota, inflammation and colorectal cancer. Int J Mol Sci.
18(1310)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang X and Li T: Development of a 15-gene
signature for predicting prognosis in advanced colorectal cancer.
Bioengineered. 11:165–174. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bian M, Wang X, Sun Y and Liu W: Synthesis
and biological evaluation of gold(III) Schiff base complexes for
the treatment of hepatocellular carcinoma through attenuating TrxR
activity. Eur J Med Chem. 193(112234)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yao J, Duan D, Song ZL, Zhang J and Fang
J: Sanguinarine as a new chemical entity of thioredoxin reductase
inhibitor to elicit oxidative stress and promote tumor cell
apoptosis. Free Radic Biol Med. 20:659–667. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kim SJ, Miyoshi Y, Taguchi T, Tamaki Y,
Nakamura H, Yodoi J, Kato K and Noguchi S: High thioredoxin
expression is associated with resistance to docetaxel in primary
breast cancer. Clin Cancer Res. 11:8425–8430. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Peng W, Zhou Z, Zhong Y, Sun Y, Wang Y,
Zhu Z, Jiao W, Bai M, Sun J, Lu J and Yin H: Plasma activity of
thioredoxin reductase as a novel biomarker in gastric cancer. Sci
Rep. 9(19084)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xu L, Zhao Y, Pan F, Zhu M, Yao L, Liu Y,
Feng J, Xiong J, Chen X, Ren F, et al: Inhibition of the Nrf2-TrxR
axis sensitizes the drug-resistant chronic myelogenous leukemia
cell line K562/G01 to imatinib treatments. Biomed Res Int.
2019(6502793)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhu B, Ren C, Du K, Zhu H, Ai Y, Kang F,
Luo Y, Liu W, Wang L, Xu Y, et al: Olean-28,13b-olide 2 plays a
role in cisplatin-mediated apoptosis and reverses cisplatin
resistance in human lung cancer through multiple signaling
pathways. Biochem Pharmacol. 170(113642)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bian M, Fan R, Zhao S and Liu W: Targeting
the thioredoxin system as a strategy for cancer therapy. J Med
Chem. 62:7309–7321. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang J, Li X, Han X, Liu R and Fang J:
Targeting the thioredoxin system for cancer therapy. Trends
Pharmacol Sci. 38:794–808. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Arnér ES: Targeting the selenoprotein
thioredoxin reductase 1 for anticancer therapy. Adv Cancer Res.
136:139–151. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Scalcon V, Bindoli A and Rigobello MP:
Significance of the mitochondrial thioredoxin reductase in cancer
cells: An update on role, targets and inhibitors. Free Radic Biol
Med. 127:62–79. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Stafford WC, Peng X, Olofsson MH, Zhang X,
Luci DK, Lu L, Cheng Q, Trésaugues L, Dexheimer TS, Coussens NP, et
al: Irreversible inhibition of cytosolic thioredoxin reductase 1 as
a mechanistic basis for anticancer therapy. Sci Transl Med.
10(eaaf7444)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kingston DG: Modern natural products drug
discovery and its relevance to biodiversity conservation. J Nat
Prod. 74:496–511. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mishra BB and Tiwari VK: Natural products:
An evolving role in future drug discovery. Eur J Med Chem.
46:4769–4807. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Stefanon B and Colitti M: Original
research: Hydroxytyrosol, an ingredient of olive oil, reduces
triglyceride accumulation and promotes lipolysis in human primary
visceral adipocytes during differentiation. Exp Biol Med.
241:1796–1802. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Goldstein DS, Jinsmaa Y, Sullivan P,
Holmes C, Kopin IJ and Sharabi Y: 3,4-dihydroxyphenylethanol
(hydroxytyrosol) mitigates the increase in spontaneous oxidation of
dopamine during monoamine oxidase inhibition in PC12 cells.
Neurochem Res. 41:2173–2178. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Persia FA, Mariani ML, Fogal TH and
Penissi AB: Hydroxytyrosol and oleuropein of olive oil inhibit mast
cell degranulation induced by immune and non-immune pathways.
Phytomedicine. 21:1400–1405. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bedoya LM, Beltrán M, Obregón-Calderón P,
García-Pérez J, de la Torre HE, González N, Pérez-Olmeda M, Auñón
D, Capa L, Gómez-Acebo E and Alcamí J: Hydroxytyrosol: A new class
of microbicide displaying broad anti-HIV-1 activity. AIDS.
30:2767–2776. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Walter WM Jr, Fleming HP and Etchells JL:
Preparation of antimicrobial compounds by hydrolysis of oleuropein
from green olives. Appl Microbiol. 26:773–776. 1973.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Medina E, de Castro A, Romero C and Brenes
M: Comparison of the concentrations of phenolic compounds in olive
oils and other plant oils: Correlation with antimicrobial activity.
J Agric Food Chem. 54:4954–4961. 2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sirianni R, Chimento A, De Luca A,
Casaburi I, Rizza P, Onofrio A, Iacopetta D, Puoci F, Andò S,
Maggiolini M and Pezzi V: Oleuropein and hydroxytyrosol inhibit
MCF-7 breast cancer cell proliferation interfering with ERK1/2
activation. Mol Nutr Food Res. 54:833–840. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chimento A, Casaburi I, Rosano C, Avena P,
De Luca A, Campana C, Martire E, Santolla MF, Maggiolini M, Pezzi V
and Sirianni R: Oleuropein and hydroxytyrosol activate GPER/
GPR30-dependent pathways leading to apoptosis of ER-negative SKBR3
breast cancer cells. Mol Nutr Food Res. 58:478–489. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Luo C, Li Y, Wang H, Cui Y, Feng Z, Li H,
Li Y, Wang Y, Wurtz K, Weber P, et al: Hydroxytyrosol promotes
superoxide production and defects in autophagy leading to
anti-proliferation and apoptosis on human prostate cancer cells.
Curr Cancer Drug Targets. 13:625–639. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sun L, Luo C and Liu J: Hydroxytyrosol
induces apoptosis in human colon cancer cells through ROS
generation. Food Funct. 5:1909–1914. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Toteda G, Lupinacci S, Vizza D, Bonofiglio
R, Perri E, Bonofiglio M, Lofaro D, La Russa A, Leone F, Gigliotti
P, et al: High doses of hydroxytyrosol induce apoptosis in
papillary and follicular thyroid cancer cells. J Endocrinol Invest.
40:153–162. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Terzuoli E, Giachetti A, Ziche M and
Donnini S: Hydroxytyrosol, a product from olive oil, reduces colon
cancer growth by enhancing epidermal growth factor receptor
degradation. Mol Nutr Food Res. 60:519–529. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhao B, Ma Y, Xu Z, Wang J, Wang F, Wang
D, Pan S, Wu Y, Pan H, Xu D, et al: Hydroxytyrosol, a natural
molecule from olive oil, suppresses the growth of human
hepatocellular carcinoma cells via inactivating AKT and nuclear
factor-kappa B pathways. Cancer Lett. 347:79–87. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zubair H, Bhardwaj A, Ahmad A, Srivastava
SK, Khan MA, Patel GK, Singh S and Singh AP: Hydroxytyrosol induces
apoptosis and cell cycle arrest and suppresses multiple oncogenic
signaling pathways in prostate cancer cells. Nutr Cancer.
69:932–942. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang J, Zhang B, Li X, Han X, Liu R and
Fang J: Small molecule inhibitors of mammalian thioredoxin
reductase as potential anticancer agents: An update. Med Res Rev.
39:5–39. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Arnér ES, Sarioglu H, Lottspeich F,
Holmgren A and Böck A: High-level expression in Escherichia coli of
selenocysteine-containing rat thioredoxin reductase utilizing gene
fusions with engineered bacterial-type SECIS elements and
co-expression with the selA, selB and selC genes. J Mol Biol.
292:1003–1016. 1999.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang L, Duan D, Liu Y, Ge C, Cui X, Sun J
and Fang J: Highly selective off-on fluorescent probe for imaging
thioredoxin reductase in living cells. J Am Chem Soc. 136:226–233.
2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhang J, Duan D, Osama A and Fang J:
Natural molecules targeting thioredoxin system and their
therapeutic potential. Antioxid Redox Signal. 34:1083–1107.
2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang J, Duan D, Song ZL, Liu T, Hou Y and
Fang J: Small molecules regulating reactive oxygen species
homeostasis for cancer therapy. Med Res Rev. 41:342–394.
2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhong L and Holmgren A: Essential role of
selenium in the catalytic activities of mammalian thioredoxin
reductase revealed by characterization of recombinant enzymes with
selenocysteine mutations. J Biol Chem. 275:18121–18128.
2000.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhong L, Arnér ES and Holmgren A:
Structure and mechanism of mammalian thioredoxin reductase: The
active site is a redox-active selenolthiol/selenenylsulfide formed
from the conserved cysteine-selenocysteine sequence. Proc Natl Acad
Sci U S A. 97:5854–5859. 2000.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cai W, Zhang L, Song Y, Wang B, Zhang B,
Cui X, Hu G, Liu Y, Wu J and Fang J: Small molecule inhibitors of
mammalian thioredoxin reductase. Free Radic Biol Med. 52:257–265.
2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lo YC, Ko TP, Su WC, Su TL and Wang AH:
Terpyridine-platinum(II) complexes are effective inhibitors of
mammalian topoisomerases and human thioredoxin reductase 1. J Inorg
Biochem. 103:1082–1092. 2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ng HL, Ma X, Chew EH and Chui WK: Design,
synthesis, and biological evaluation of coupled bioactive scaffolds
as potential anticancer agents for dual targeting of dihydrofolate
reductase and thioredoxin reductase. J Med Chem. 60:1734–1745.
2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Liu Y, Duan D, Yao J, Zhang B, Peng S, Ma
H, Song Y and Fang J: Dithiaarsanes induce oxidative
stress-mediated apoptosis in HL-60 cells by selectively targeting
thioredoxin reductase. J Med Chem. 57:5203–5211. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhang B, Duan D, Ge C, Yao J, Liu Y, Li X
and Fang J: Synthesis of xanthohumol analogues and discovery of
potent thioredoxin reductase inhibitor as potential anticancer
agent. J Med Chem. 58:1795–1805. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Tuladhar A and Rein KS: Manumycin A is a
potent inhibitor of mammalian thioredoxin reductase-1 (TrxR-1). ACS
Med Chem Lett. 9:318–322. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Xie L, Luo Z, Zhao Z and Chen T:
Anticancer and antiangiogenic iron(II) complexes that target
thioredoxin reductase to trigger cancer cell apoptosis. J Med Chem.
60:202–214. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Bertelli M, Kiani AK, Paolacci S, Manara
E, Kurti D, Dhuli K, Bushati V, Miertus J, Pangallo D, Baglivo M,
et al: Hydroxytyrosol: A natural compound with promising
pharmacological activities. J Biotechnol. 309:29–33.
2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Britton J, Davis R and O'Connor KE:
Chemical, physical and biotechnological approaches to the
production of the potent antioxidant hydroxytyrosol. Appl Microbiol
Biotechnol. 103:5957–5974. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Romana-Souza B, Saguie BO, Pereira de
Almeida Nogueira N, Paes M, Dos Santos Valença S, Atella GC and
Monte-Alto-Costa A: Oleic acid and hydroxytyrosol present in olive
oil promote ROS and inflammatory response in normal cultures of
murine dermal fibroblasts through the NF-κB and NRF2 pathways. Food
Res Int. 131(108984)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Sun T, Chen Q, Zhu SY, Wu Q, Liao CR, Wang
Z, Wu XH, Wu HT and Chen JT: Hydroxytyrosol promotes autophagy by
regulating SIRT1 against advanced oxidation protein productinduced
NADPH oxidase and inflammatory response. Int J Mol Med.
44:1531–1540. 2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Avola R, Graziano AC, Pannuzzo G, Bonina F
and Cardile V: Hydroxytyrosol from olive fruits prevents
blue-light-induced damage in human keratinocytes and fibroblasts. J
Cell Physiol. 234:9065–9076. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Peng S, Zhang B, Yao J, Duan D and Fang J:
Dual protection of hydroxytyrosol, an olive oil polyphenol, against
oxidative damage in PC12 cells. Food Funct. 6:2091–2100.
2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Long LH, Hoi A and Halliwell B:
Instability of, and generation of hydrogen peroxide by, phenolic
compounds in cell culture media. Arch Biochem Biophys. 501:162–169.
2010.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Fabiani R, Fuccelli R, Pieravanti F, De
Bartolomeo A and Morozzi G: Production of hydrogen peroxide is
responsible for the induction of apoptosis by hydroxytyrosol on
HL60 cells. Mol Nutr Food Res. 53:887–896. 2009.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Fabiani R, Sepporta MV, Rosignoli P, De
Bartolomeo A, Crescimanno M and Morozzi G: Anti-proliferative and
pro-apoptotic activities of hydroxytyrosol on different tumour
cells: The role of extracellular production of hydrogen peroxide.
Eur J Nutr. 51:455–464. 2012.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Rosignoli P, Fuccelli R, Sepporta MV and
Fabiani R: In vitro chemo-preventive activities of hydroxytyrosol:
The main phenolic compound present in extra-virgin olive oil. Food
Funct. 7:301–307. 2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Fan QZ, Zhou J, Zhu YB, He LJ, Miao DD,
Zhang SP, Liu XP and Zhang C: Design, synthesis, and biological
evaluation of a novel indoleamine 2,3-dioxigenase 1 (IDO1) and
thioredoxin reductase (TrxR) dual inhibitor. Bioorg Chem.
105(104401)2020.PubMed/NCBI View Article : Google Scholar
|