Introduction
Myocardial ischemia injury is a complicated
pathophysiological process. Although reperfusion remains the only
efficient strategy for the survival of ischemic myocardial tissue
by rescuing the cardiomyocytes and promoting myocardial remodeling,
reperfusion itself often conversely induces the apoptosis of
cardiomyocytes and exacerbates the damage to the myocardium, which
is usually known as myocardial oxygen-glucose
deprivation/reperfusion (OGD/R) injury (1,2).
Previous studies have testified that the cellular degradation,
particularly autophagy, serves an important role in the process of
myocardial ischemia/reperfusion injury (3,4).
Autophagy, the body's self-cleaning system, is reported to provide
a necessary source of energy for cardiomyocytes during the early
stage (5), which protects
cardiomyocytes against ischemia/reperfusion injury (6). By contrast, autophagy could also
aggravate the apoptotic and cytotoxic death of cardiomyocytes
during the late stage of ischemia and reperfusion (7). Therefore, autophagy is of great
interest among the mechanisms involved in OGD/R-induced injury of
cardiomyocytes.
Ozone is a molecule consisting of three atoms of
oxygen, the basic function of which is to absorb incoming UV
radiation and protect humans from the damages of UV (8). Ozone, as a superoxide involved in the
activation of a variety of antioxidant enzymes (9), is widely used in inhibiting
inflammatory injury (10,11) by downregulating the expression of
inflammatory mediators [interleukin-6 (IL-6), interleukin-8 (IL-8)
and tumor necrosis factor-α (TNF-α)]. Previous studies have further
reported that ozone has significant therapeutic effects on chronic
ischemic diseases (including limb and kidney damage) (12,13)
and myocardial ischemia/reperfusion injury through activating the
antioxidant system by induction of transient oxidative stress and
promoting the release of oxygen to tissues (9). Of note, it was reported that reactive
oxygen species (ROS) could act as an early inducer of autophagy
upon nutrient deprivation (14).
Chen et al (15,16) reported that O2- is the
primary ROS involved in autophagy induced by deprivation of
glucose, glutamine, pyruvate or serum (15,16).
However, the role of ozone and the molecular mechanism associated
with its effect on OGD/R-induced myocardial injury has not yet been
reported.
Autophagy is a catabolic process aimed at recycling
cellular components and damaged organelles in response to diverse
conditions of stress, including nutrient deprivation, viral
infection and genotoxic stress (17). The results of a previous study
suggested that oxidative stress acts as the converging point of
these stimuli, and ROS and reactive nitrogen species are regarded
as the main intracellular signal transducers of the autophagy
pathway (18). The present study
investigated the protective effects of ozone on OGD/R-induced
cardiomyocytes injury using pharmacologic analysis, and whether
ozone-induced tolerance to myocardial OGD/R injury was mediated by
inhibiting autophagy.
Materials and methods
Ozone preparation
An ozone-generating device (Shenyang Medicines &
Health Products; HUMARES® GmbH) was used to generate the
mixed gas. The operating procedure was as the follows: Adding 3 ml
of DMEM (Thermo Fisher Scientific, Inc.) into a centrifuge tube and
collecting 3 ml of ozone gas from an ozone-generating device into
sterile syringe, then pumping the ozone into DMEM through a sterile
plastic tube and mixing the gas and DMEM on the oscillator for 5
min. Subsequently, the mixed culture medium was used to cultivate
the cells during reperfusion.
Cell culture
The rat cardiomyocyte H9C2 cell line was obtained
from BeNa Culture Collection (Beijing Beina Chunglian Institute of
Biotechnology). Cells were grown in complete high-glucose DMEM,
supplemented with 10% fetal bovine serum (Biological Industries)
and antibiotics (100 µg/ml streptomycin and 100 U/ml penicillin;
Thermo Fisher Scientific, Inc.). The cells were incubated in a
humidified incubator at 37˚C with 5% CO2.
Establishment of an OGD/R injury
model
The OGD/R injury model was established as previously
described (19). In order to mimic
oxygen-glucose deprivation, the cells were incubated in serum- and
glucose-free DMEM (Thermo Fisher Scientific, Inc.) and placed in a
hypoxia chamber (95% N2 and 5% CO2 at 37˚C)
for 6 h. Next, the cells were returned to culture under normal
conditions (95% humidified air and 5% CO2 at 37˚C) with
complete high-glucose DMEM for 4 h to mimic the reperfusion.
Cell treatment
The cells were divided into the following groups:
Control group, in which cells were cultured in DMEM; Model group,
in which cells underwent OGD/R injury; OGD/R+O3 group, in which
cells were treated with different concentrations of ozone during
reperfusion at 37˚C for 4 h; OGD/R+O3+Rapa group, in which cells
were treated with 100 nmol Rapamycin (Sigma-Aldrich; Merck KGaA) at
37˚C for 2 h prior to establishing the model; OGD/R+O3+3-MA group,
in which cells were treated with 5 mmol 3-MA (Selleck Chemicals) at
37˚C for 2 h prior to establishing the model.
Cell Counting kit-8 (CCK-8) assay
The cells were incubated in 96-well plates (5x103
cells/well) for 24 h. Next, the cells were used to establish the
OGD/R injury model and the cells were treated with different
concentrations of ozone (10, 20, 40 and 60 µg/ml) when mimicking
reperfusion. Following culture, CCK-8 solution (Dojindo Molecular
Technologies, Inc.) was added and the plate was incubated in the
dark for 2 h, followed by measurement of the OD value at 450 nm
using a microplate absorbance reader (BioTek China).
Cell apoptosis assay
Cell apoptosis levels was detected using a Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis
detection kit (Vazyme Biotech Co., Ltd.). In brief, the cells were
incubated at a density of 3.5x105 cells/well in 6-well
plates and were collected and stained with 5 µl Annexin V-FITC and
5 µl PI solution in the dark for 15 min at room temperature after
oxygen-glucose deprivation/reperfusion. Subsequently, the stained
cells were analyzed using a flow cytometer (FACScan™; BD
Biosciences) and Modfit software 3.2 (Verity Software House,
Inc.).
Detection of lactate dehydrogenase
(LDH)
LDH activity was examined using an LDH detection kit
(cat. no. BC0680; Beijing Solarbio Science & Technology Co.,
Ltd.), according to the manufacturer's protocol. The cells were
lysed on ice using an ultrasonic cell breaker (Ningbo Scientz
Biotechnology Co., Ltd.). Next, the cells were exposed to reagent I
and reagent II and incubated in a 37˚C water bath for 15 min.
Subsequently, reagent III was added and the cells were incubated in
the water bath again for 15 min. Finally, reagent IV was added to
the mixture and incubated at room temperature for 3 min. The OD
value was measured at 560 nm using a light absorption microplate
reader (BioTek China).
Detection of malondialdehyde (MDA) The
MDA detection kit (cat
no. BC0020; Beijing Solarbio Science &
Technology Co., Ltd.) was used to detect the content of MDA,
according to the manufacturer's protocol. The cells were lysed and
then mixed with reagent I and reagent III. Subsequently, the
mixture was heated in boiling water for 30 min, and centrifuged at
10,000 x g at room temperature for 10 min. The OD value was
measured at 532, 450 and 600 nm using a light absorption microplate
reader (BioTek China).
Detection of superoxide dismutase
(SOD)
SOD activity was detected using the SOD detection
kit (cat. no. BC0170; Beijing Solarbio Science & Technology
Co., Ltd.), according to the manufacturer's protocol. The cells
were lysed on ice and the lysate was mixed with reagent I to IV,
and was then kept at room temperature for 30 min. The OD value was
measured at 560 nm using a light absorption microplate reader
(BioTek China).
Western blot analysis
Radio Immunoprecipitation Assay (RIPA; Beyotime
Institute of Biotechnology) was used to lyse the cells and the
total protein was extracted. The protein concentration was
determined by bicinchoninic acid protein assay (Beyotime Institute
of Biotechnology). Next, 25 µg proteins were loaded onto 15%
SDS-PAGE gels. Following electrophoresis, the proteins were
transferred onto nitrocellulose membranes (Shanghai Kang Lang
Biological Technology Co., Ltd.). The blots were blocked with 5%
skimmed milk for 1 h at room temperature. Subsequently, the
membrane was incubated with primary rabbit polyclonal antibodies
against BAX (1:5,000; cat. no. 50599-2-Ig), Bcl-2 (1:500; cat. no.
26593-1-AP), LC3B (1:300; cat. no. 18725-1-AP), Beclin-1 (1:1,000;
cat. no. 3495), Atg5 (1:500; cat. no. 10181-2-AP) (all Wuhan
Sanying Biotechnology), cleaved caspase-3 (1:1,000; cat. no. 9664;
Cell Signaling Technology, Inc.) and β-actin (1:10,000; mouse; cat.
no. AC004; ABclonal Biotechnology Co., Ltd.) overnight at 4˚C.
Subsequently, membranes were washed in TBS with 2% Tween-20 once
and incubated with goat anti-rabbit immunoglobulin G (IgG; 1:2,000;
cat. no. AS014; Abclonal Biotechnology Co., Ltd.) or goat
anti-mouse IgG (1:2,000; cat. no. AS003; Abclonal Biotechnology
Co., Ltd.) for 1 h at room temperature. Subsequently, membranes
were washed in TBST three times. Digital images of immunoblots were
obtained using ProteinSimple FluorChemQ2 and the densitometry of
the bands was analyzed using ImageJ software 1.53 (National
Institutes of Health).
Statistical analysis
Data analysis was performed by GraphPad Prism 8.0
(GraphPad Software, Inc.). Data are presented as the mean ±
standard deviation of at least three measurements. Data were
analyzed using one-way analysis of variance and Tukey's HSD test
(α=0.05). P<0.05 was considered to indicate a statistically
significant difference.
Results
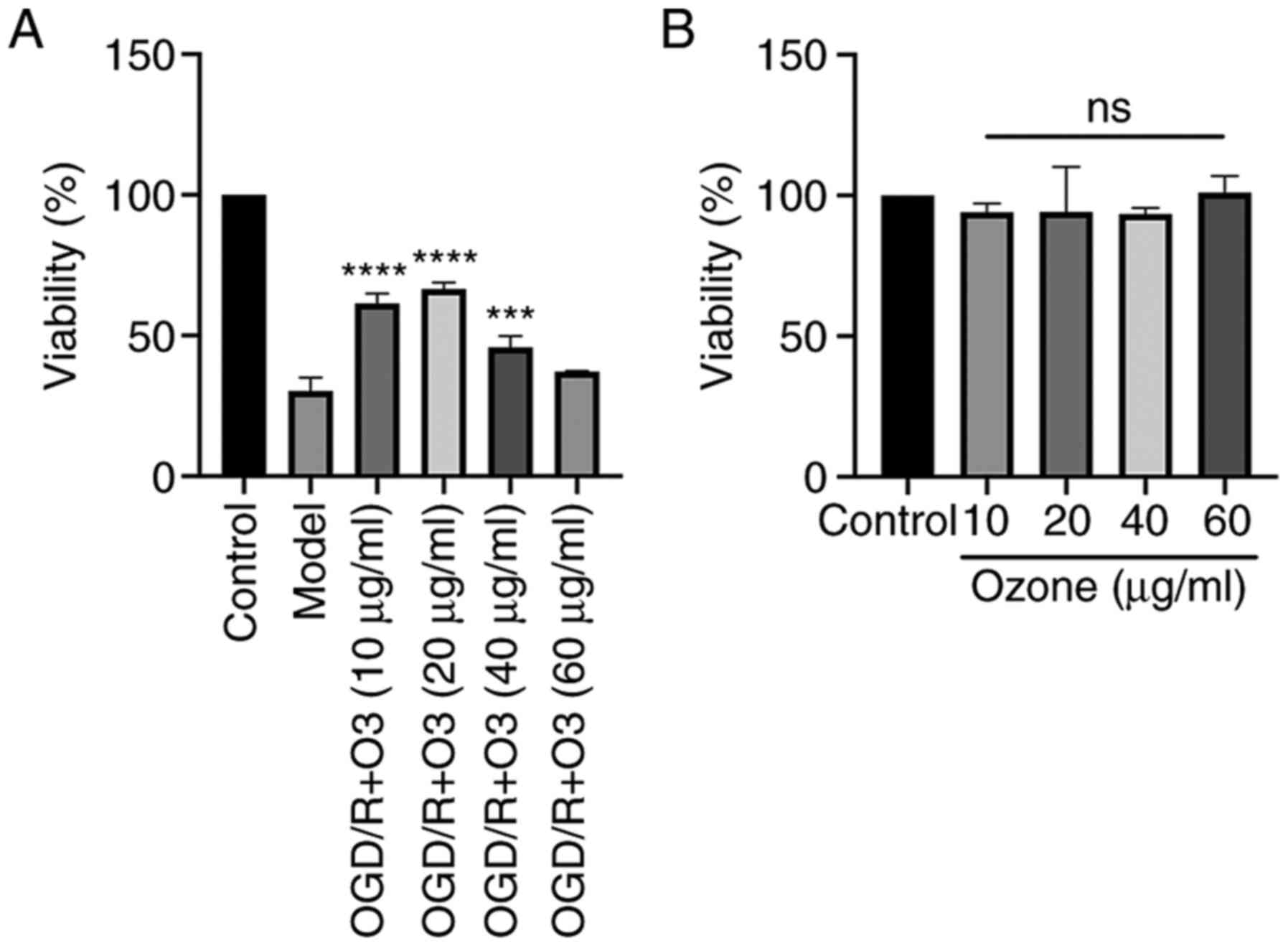
Ozone increases the viability of H9C2
cells undergoing OGD/R
H9C2 cells undergoing OGD/R were treated with
different doses of ozone and cell viability was measured by CCK-8
assay. As shown in Fig. 1A, the
cell viability was decreased following OGD/R treatment. The results
also demonstrated that the viability of the cells in ozone groups
with concentrations of 10, 20, 40 and 60 µg/ml were 61.5±3.48,
66.52±2.33, 45.85±3.93 and 37.19±0.19%, respectively. Compared with
the OGD/R group, the viability of the cells in the ozone groups
with ozone concentrations of 10, 20 and 40 µg/ml were significantly
increased (P<0.05), and the concentration of 20 µg/ml was the
most effective (Fig. 1A).
The effect of ozone on the viability of normal H9C2
cells was also analyzed. The results demonstrated that the
viability of the cells treated with ozone concentrations of 10, 20,
40 and 60 µg/ml were 94.12±3.02, 94.24±15.96, 93.43±2.13 and
101.01±5.9%, respectively. There was no significant difference
among the groups (Fig. 1B).
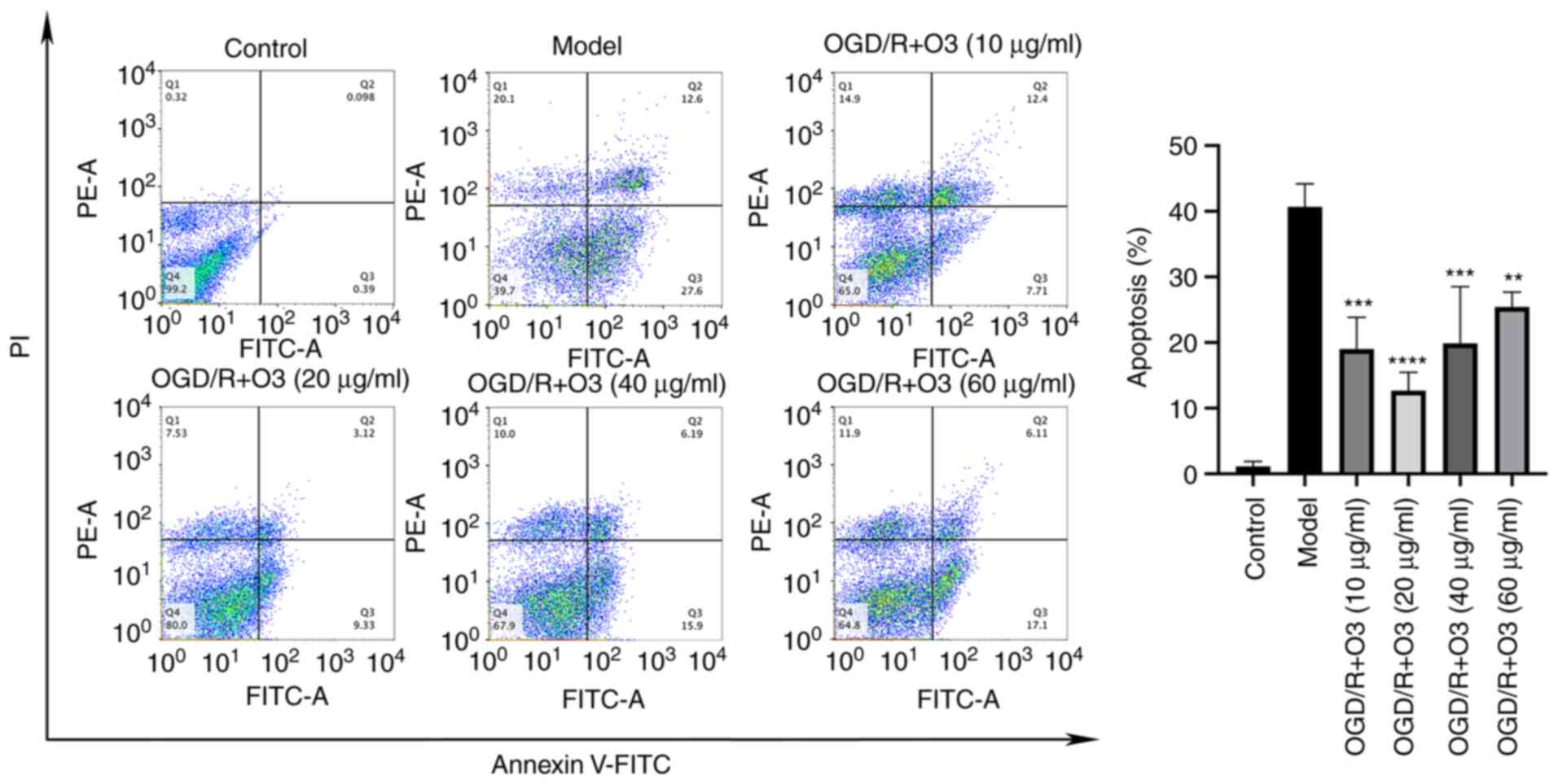
Ozone inhibits the apoptosis of H9C2
cells induced by OGD/R
To investigate the effects of different
concentrations of ozone on the apoptosis of H9C2 cells, an Annexin
V-FITC/PI apoptosis detection kit was used to detect the ratio of
apoptosis, and western blotting was performed to detect the
apoptosis-related proteins. The results of flow cytometric analysis
demonstrated that the rates of apoptosis in ozone groups with
concentrations of 10, 20, 40 and 60 µg/ml were 19.03, 12.70, 19.89
and 25.45%, respectively. When compared with that of OGD/R group
(40.67%), the rate of apoptosis in all ozone-treated groups was
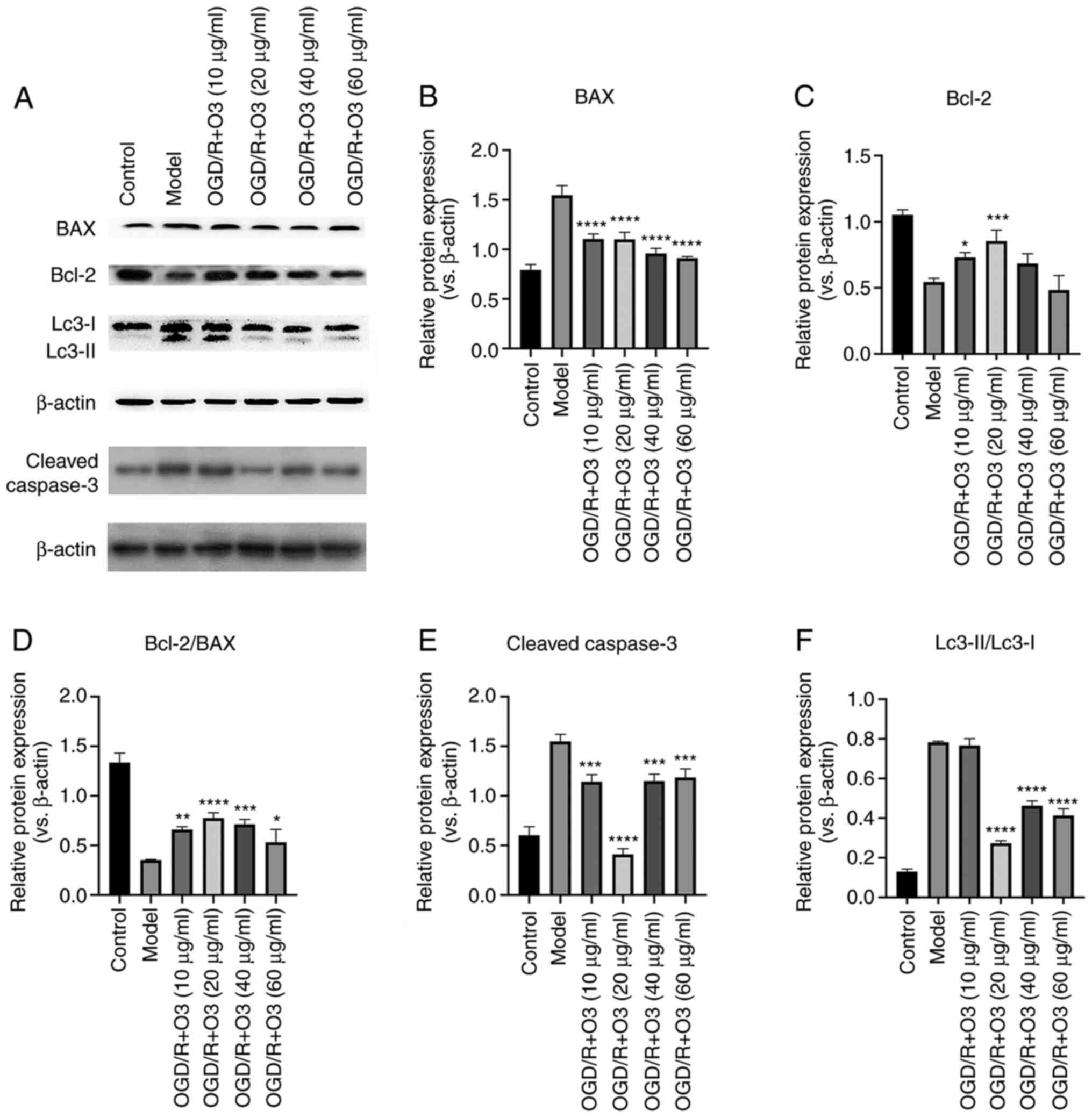
decreased (Fig. 2). The results of
western blotting revealed that the expression of Bcl-2 was
increased in response to ozone treatment (10 and 20 µg/ml) when
compared with the control group. Furthermore, the expression of BAX
was attenuated in all ozone-treated groups. In addition, the ratio
of Bcl-2/BAX was increased and the expression of cleaved caspase-3
was decreased in all groups (Fig.
3A-E).
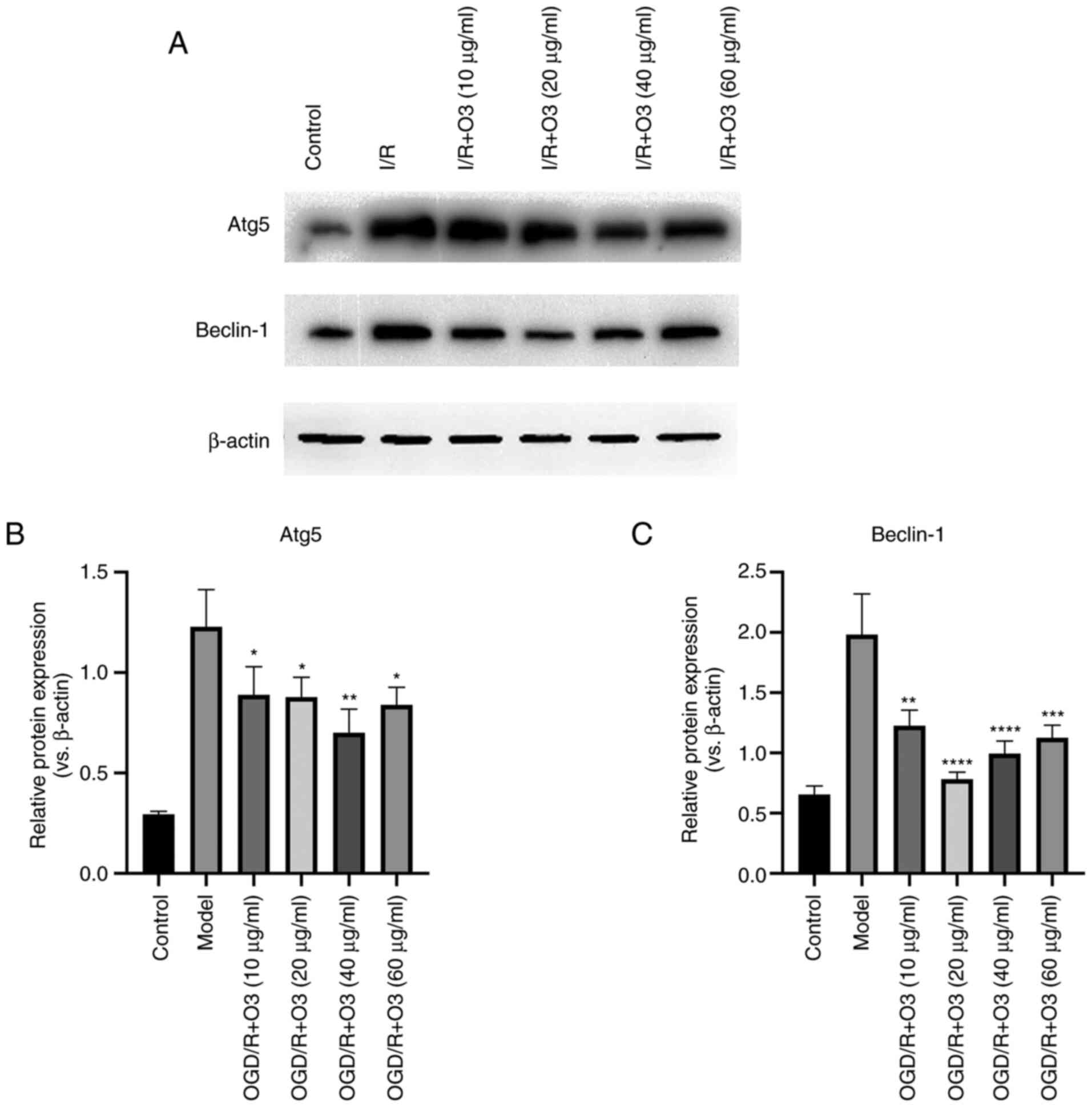
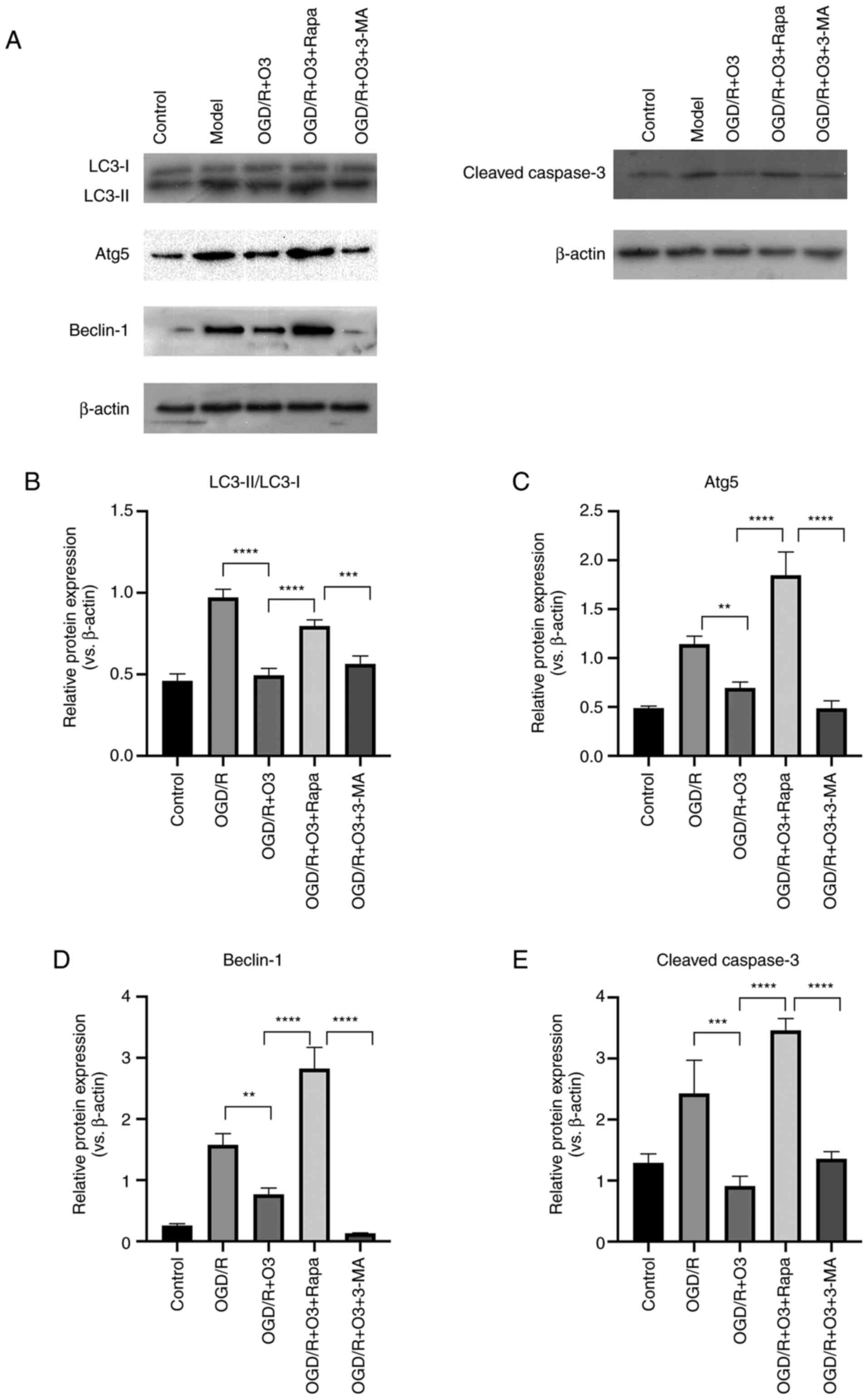
Ozone inhibits the autophagy of H9C2
cells undergoing OGD/R
In order to test the effects of different
concentrations of ozone on the autophagy of cardiomyocytes, the
expression of autophagy-related proteins was detected by western
blotting. The results demonstrated that the expression of
autophagy-related proteins (LC3-II/LC3-I, Atg5 and Beclin-1)
significantly increased following OGD/R. Compared with the model
group, incubation with 20, 40 and 60 µg/ml ozone significantly
decreased the expression of LC3-II/LC3-I and Beclin-1, and the
inhibition effects in the 20 µg/ml group were the most notably. In
addition, the expression of Atg5 was decreased following treatment
with all concentrations of ozone and 40 µg/ml was the most notably,
but there was no statistic difference between the 20 µg/ml and 40
µg/ml ozone groups (Figs. 3F and
4A-C). The aforementioned results
indicated that ozone treatment was able to attenuate OGD/R-induced
autophagy.
Rapamycin reverses the protective
effect of ozone on H9C2 cells undergoing OGD/R and 3-MA further
improves the effect
The results demonstrated that the expression of
autophagy-related proteins (LC3-II/LC3-I, Atg5 and Beclin-1) was
significantly decreased following incubation with the 20 µg/ml
ozone and that the autophagy inducer, rapamycin, reversed the
effect of ozone. Furthermore, the effect of a decrease in
apoptosis-related protein (cleaved casapase-3) expression was also
reversed by rapamycin. By contrast, pretreatment with 3-MA notably
decreased the expression of Beclin-1 but had no significant effect
on the ratio of LC3-II/LC3-I and Atg5 when compared with that of
the OGD/R+O3 group (Fig. 5).
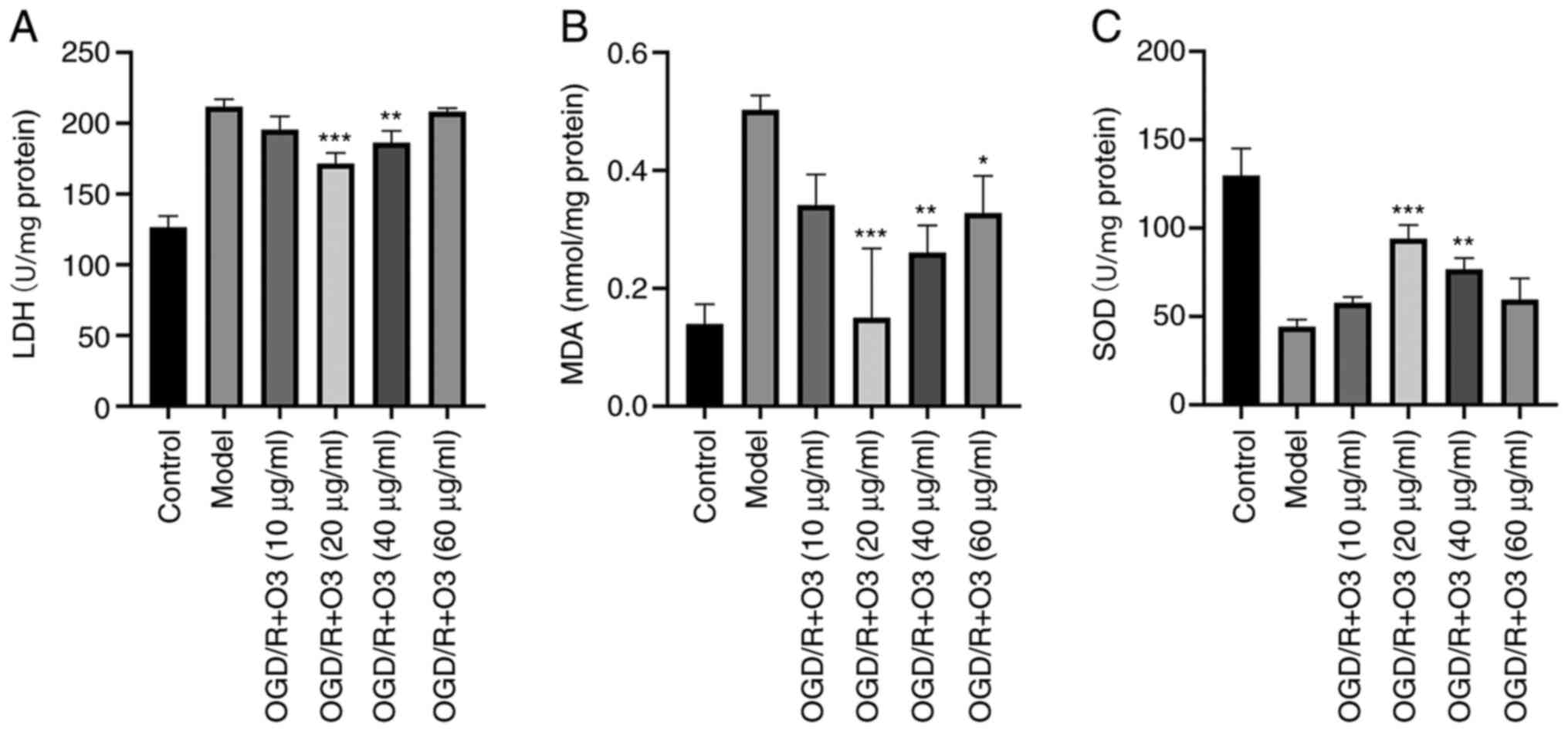
Ozone ameliorates the oxidative stress
induced by OGD/R
In order to elucidate whether ozone affected the
oxidative stress in H9C2 cells, the related indexes of oxidative
stress (MDA, LDH and SOD) were detected. The results demonstrated
that, compared with the normal cardiomyocytes, the cardiomyocytes
that underwent OGD/R had significantly higher contents of MDA and
higher LDH activity but lower SOD activity. In groups treated with
different concentrations of ozone (10, 20, 40 and 60 µg/ml ozone),
the contents of MDA were 0.34±0.05, 0.15±0.12, 0.26±0.05 and
0.33±0.06 nmol/mg protein, respectively, while that of the OGD/R
group was 0.5±0.02 nmol/mg protein. The activities of LDH were
195.46±9.35, 160.31±9.2, 186.31±8.3 and 202.78±9.68 U/mg protein,
respectively, while that in the OGD/R group was 211.76±5.3 U/mg
protein. Furthermore, the activities of SOD were 576.83±3.31,
94.09±7.64, 76.74±6.3 and 59.56±11.98 U/mg protein, respectively,
while the content in model group was 44.28±3.94 U/mg protein
(Fig. 6).
Discussion
The results of the present study suggested that
OGD/R significantly decreased cell viability and induced apoptosis
in cardiomyocytes. Ozone therapy is a novel strategy used in
cardio-protection in recent years (9,20). A
previous study reported that ozone can significantly ameliorate the
ischemia/reperfusion injury following cardio-pulmonary bypass and
that ozonated autohemotherapy was beneficial to patients with heart
failure with reduced ejection fraction (21). According to the results of the
present study, ozone significantly inhibited OGD/R-induced
oxidative stress and attenuated cardiomyocyte apoptosis by
ameliorating OGD/R-induced activation of autophagy. CCK-8 assay is
a highly sensitive method for determining the number of viable
cells in cytotoxicity experiments (22) compared with photomicrographs of the
cells in which the differences of the cells between ozone treated
groups are not clear. According to the results of CCK-8 kit
detection in the present study, ozone treatment increased the
viability of cardiomyocytes that had undergone OGD/R. Using an
isolated rat heart perfusion model, Ahmed et al (9) found that the cardiac function index of
rats in the ozone pretreatment group was significantly improved,
confirming that ozone had a protective effect on myocardium.
However, the protective effect of ozone on cardiomyocytes is
associated with its concentration. A previous study demonstrated
that the ‘therapeutic window’ of ozone ranging between 10 and 80
µg/ml ensures a small and precise oxidative stress which is able to
elicit medical efficacy and no toxicity (23). The present study indicated that the
protective effect of ‘20 µg/ml ozone’ reached the peak, and then
slowly decreased when the concentration of ozone increased, but the
protective effect still existed when the concentration was up to 60
µg/ml. Notably, the effect of ozone on the viability of
cardiomyocytes is associated with the state of the cells. The
results of the present study demonstrated that the activity of the
cells did not change when normal cardiomyocytes were treated with a
certain concentration of ozone, which demonstrates that ozone in a
certain concentration range would not damage normal cells as it is
non-toxic. Therefore, we hypothesized that ozone in a certain
concentration range is not toxic to normal cardiomyocytes, but that
it could produce a protective effect on myocardial OGD/R
injury.
Cell apoptosis is an important consequence of
ischemia/reperfusion injury and may be induced by the activation of
either extrinsic or intrinsic pathways. A previous study has
demonstrated that myocardial OGD/R was associated with apoptosis
(6). Among the apoptosis-related
proteins, Bcl-2 family and cleaved caspase-3 are key factors
regulating apoptosis (24). Bcl-2
may promote cell survival, but BAX may promote cell death by
apoptosis. The ratio of Bcl-2/BAX is the key factor that decides
whether apoptosis happens to cells following injuries. In line with
the results of a previous study (25), the present study demonstrated that
incubation with a low concentration of ozone (20 µg/ml)
significantly upregulated the ratio of Bcl-2/BAX and downregulated
the expression of cleaved caspase-3 when compared with high
concentration (60 µg/ml) ozone and the OGD/R group. Additionally,
the results of flow cytometry further demonstrated that incubation
with low concentration ozone significantly attenuated the apoptosis
rate when compared with the OGD/R group. Based on the
aforementioned results, we hypothesized that ozone may decrease the
cardiomyocyte apoptosis caused by OGD/R.
Autophagy is a catabolic process aimed to remove
damaged proteins or organelles. It is an important mechanism for
proteasomal degradation and production of survival signals
(26). However, recent studies have
reported that the protective function of autophagy is changing
(27,28). Additionally, it was demonstrated
that autophagy induced cell survival during the process of
myocardial ischemic injury, but that it caused cell death during
the process of reperfusion injury (28). LC3, Atg5 and Bclin-1 are
autophagy-related proteins and several studies have used them to
examine autophagy (23,29). The results of the present study
suggested that treating H9C2 cells with ozone at concentrations of
10, 20, 40 or 60 µg/ml attenuated the expression of LC3-II/LC3-I,
Atg5 and Beclin-1. In addition, treatment with the autophagy
inducer, rapamycin, reversed the effect of ozone on decreasing the
expression of autophagy-related proteins and apoptosis-related
protein. By contrast, the autophagy inhibitor, 3-MA, only decreased
the expression of Beclin-1 compared with OGD/R+O3 group, which may
be due to the fact that the autophagy during reperfusion is
Beclin-1-dependent (30).
Therefore, autophagy may be the main target for ozone treatment to
prevent myocardial injury induced by OGD/R.
A previous study demonstrated that there is an
association between oxidative stress and cell autophagy; therefore,
we hypothesized that the internal mechanism of the anti-oxidative
stress effect produced by ozone may be associated with autophagy
(26). It is easy to induce
oxidative stress following OGD/R (31). Oxidative stress is a result of
imbalance between the generation of ROS and the antioxidant defense
systems (32). LDH and MDA are the
important indicators of cellular oxidative stress, and SOD is a
protective indicator (33,34). The results of the present study
demonstrated that 20, 40 and 60 µg/ml ozone decreased LDH and MDA
levels and increased SOD release, and that 20 µg/ml ozone was the
most effective. The results demonstrated that ozone significantly
decreased cellular oxidative stress of cardiomyocytes under OGD/R
conditions, suggesting that ozone may exert an anti-autophagy
effect on OGD/R-treated cardiomyocytes by blocking oxidative
stress.
The present study has certain limitations. It was
verified that ozone had no effect on the viability of normal cells
and was non-toxic, so groups treated with ozone only have no
significance. Moreover, the purpose of the current experiments was
to demonstrate that the protective effect of ozone on H9C2 cells
suffering from OGD/R injury was due to autophagy, so groups treated
with rapamycin and 3-MA only were not used. Adding these groups
will make the experimental design more comprehensive, therefore
they will be adopted in future studies. Additionally, the level of
oxidative stress of cardiomyocytes when treated with autophagy
inhibitors and autophagy inducers needed to be detected to further
demonstrate the correlation between autophagy and oxidative stress.
Furthermore, the protective molecular pathways were not
investigated in the present study. All these limitations require
further investigation in future studies.
In conclusion, ozone induced tolerance against
myocardial OGD/R damage through inhibition of autophagy.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Jiangsu Provincial
Medical Youth Talent (grant no. QNRC2016273) and General Project of
Jiangsu Health Committee (grant no. H2018047).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LX, ZZ and ZW designed the study. LX and LZ
performed the experiments. LZ and PL analyzed the data. LX and LZ
confirm the authenticity of all the raw data. LX and LZ drafted the
manuscript. All authors approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Granger DN and Kvietys PR: Reperfusion
injury and reactive oxygen species: The evolution of a concept.
Redox Biol. 6:524–551. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Huang L, Guo B, Liu S, Miao C and Li Y:
Inhibition of the LncRNA Gpr19 attenuates ischemia-reperfusion
injury after acute myocardial infarction by inhibiting apoptosis
and oxidative stress via the miR-324-5p/Mtfr1 axis. IUBMB Life.
72:373–383. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Kuma A, Hatano M, Matsui M, Yamamoto A,
Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T and Mizushima N: The
role of autophagy during the early neonatal starvation period.
Nature. 432:1032–1036. 2004.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gurusamy N, Lekli I, Gorbunov NV,
Gherghiceanu M, Popescu LM and Das DK: Cardioprotection by
adaptation to ischaemia augments autophagy in association with
BAG-1 protein. J Cell Mol Med. 13:373–387. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wu ZQ, Cui SY, Zhu L and Zou ZQ: Study on
the Mechanism of mTOR-Mediated Autophagy during Electroacupuncture
Pretreatment against Cerebral Ischemic Injury. Evid Based
Complement Alternat Med. 2016(9121597)2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gurusamy N, Lekli I, Mukherjee S, Ray D,
Ahsan MK, Gherghiceanu M, Popescu LM and Das DK: Cardioprotection
by resveratrol: A novel mechanism via autophagy involving the
mTORC2 pathway. Cardiovasc Res. 86:103–112. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Matsui Y, Kyoi S, Takagi H, Hsu CP,
Hariharan N, Ago T, Vatner SF and Sadoshima J: Molecular mechanisms
and physiological significance of autophagy during myocardial
ischemia and reperfusion. Autophagy. 4:409–415. 2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Elvis AM and Ekta JS: Ozone therapy: A
clinical review. J Nat Sci Biol Med. 2:66–70. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ahmed LA, Salem HA, Mawsouf MN, Attia AS
and Agha AM: Cardioprotective effects of ozone oxidative
preconditioning in an in vivo model of ischemia/reperfusion injury
in rats. Scand J Clin Lab Invest. 72:345–354. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Merin O, Attias E, Elstein D, Schwalb H,
Bitran D, Zimran A and Silberman S: Ozone administration reduces
reperfusion injury in an isolated rat heart model. J Card Surg.
22:339–342. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Di Filippo C, Marfella R, Capodanno P,
Ferraraccio F, Coppola L, Luongo M, Mascolo L, Luongo C, Capuano A,
Rossi F, et al: Acute oxygen-ozone administration to rats protects
the heart from ischemia reperfusion infarct. Inflamm Res.
57:445–449. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tylicki L, Nieweglowski T, Biedunkiewicz
B, Chamienia A, Debska-Slizien A, Aleksandrowicz E,
Lysiak-Szydlowska W and Rutkowski B: The influence of ozonated
autohemotherapy on oxidative stress in hemodialyzed patients with
atherosclerotic ischemia of lower limbs. Int J Artif Organs.
26:297–303. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Foglieni C, Fulgenzi A, Belloni D,
Sciorati C, Ferrero E and Ferrero ME: Ozonated autohemotherapy:
Protection of kidneys from ischemia in rats subjected to unilateral
nephrectomy. BMC Nephrol. 12(61)2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Filomeni G, Desideri E, Cardaci S, Rotilio
G and Ciriolo MR: Under the ROS…thiol network is the principal
suspect for autophagy commitment. Autophagy. 6:999–1005.
2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Al-Salam S and Hashmi S: Myocardial
Ischemia Reperfusion Injury: Apoptotic, Inflammatory and Oxidative
Stress Role of Galectin-3. Cell Physiol Biochem. 50:1123–1139.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen Y, Azad MB and Gibson SB: Superoxide
is the major reactive oxygen species regulating autophagy. Cell
Death Differ. 16:1040–1052. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gustafsson AB and Gottlieb RA: Eat your
heart out: Role of autophagy in myocardial ischemia/reperfusion.
Autophagy. 4:416–421. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kroemer G, Mariño G and Levine B:
Autophagy and the integrated stress response. Mol Cell. 40:280–293.
2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen J, Jiang Z, Zhou X, Sun X, Cao J, Liu
Y and Wang X: Dexmedetomidine Preconditioning Protects
Cardiomyocytes Against Hypoxia/Reoxygenation-Induced Necroptosis by
Inhibiting HMGB1-Mediated Inflammation. Cardiovasc Drugs Ther.
33:45–54. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
El-Sawalhi MM, Darwish HA, Mausouf MN and
Shaheen AA: Modulation of age-related changes in oxidative stress
markers and energy status in the rat heart and hippocampus: A
significant role for ozone therapy. Cell Biochem Funct. 31:518–525.
2013.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Buyuklu M, Kandemir FM, Set T, Bakırcı EM,
Degirmenci H, Hamur H, Topal E, Kucukler S and Turkmen K:
Beneficial Effects of Ozone Therapy on Oxidative Stress, Cardiac
Functions and Clinical Findings in Patients with Heart Failure
Reduced Ejection Fraction. Cardiovasc Toxicol. 17:426–433.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li Y, Xia J, Jiang N, Xian Y, Ju H, Wei Y
and Zhang X: Corin protects H2O2-induced
apoptosis through PI3K/AKT and NF-κB pathway in cardiomyocytes.
Biomed Pharmacother. 97:594–599. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sagai M and Bocci V: Mechanisms of Action
Involved in Ozone Therapy: Is healing induced via a mild oxidative
stress? Med Gas Res. 1(29)2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhu L, Hao J, Cheng M, Zhang C, Huo C, Liu
Y, Du W and Zhang X: Hyperglycemia-induced Bcl-2/Bax-mediated
apoptosis of Schwann cells via mTORC1/S6K1 inhibition in diabetic
peripheral neuropathy. Exp Cell Res. 367:186–195. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cai HA, Tao X, Zheng LJ, Huang L, Peng Y,
Liao RY and Zhu YM: Ozone alleviates ischemia-reperfusion injury by
inhibiting mitochondrion-mediated apoptosis pathway in SH-SY5Y
cells. Cell Biol Int. 44:975–984. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Filomeni G, De Zio D and Cecconi F:
Oxidative stress and autophagy: The clash between damage and
metabolic needs. Cell Death Differ. 22:377–388. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Du J, Li Y and Zhao W: Autophagy and
myocardial ischemia. In: Autophagy: Biology and Diseases. Le W
(ed). Springer, Singapore. Adv Exp Med Biol. 1207:217–222.
2020.
|
|
28
|
Shi B, Ma M, Zheng Y, Pan Y and Lin X:
mTOR and Beclin1: Two key autophagy-related molecules and their
roles in myocardial ischemia/reperfusion injury. J Cell Physiol.
234:12562–12568. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Galluzzi L and Green DR:
Autophagy-Independent Functions of the Autophagy Machinery. Cell.
177:1682–1699. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Pattingre S, Tassa A, Qu X, Garuti R,
Liang XH, Mizushima N, Packer M, Schneider MD and Levine B: Bcl-2
antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell.
122:927–939. 2005.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kurian GA, Rajagopal R, Vedantham S and
Rajesh M: The Role of Oxidative Stress in Myocardial Ischemia and
Reperfusion Injury and Remodeling: Revisited. Oxid Med Cell Longev.
2016(1656450)2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sinha N and Dabla PK: Oxidative stress and
antioxidants in hypertension-a current review. Curr Hypertens Rev.
11:132–142. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yang F, Pei R, Zhang Z, Liao J, Yu W, Qiao
N, Han Q, Li Y, Hu L, Guo J, et al: Copper induces oxidative stress
and apoptosis through mitochondria-mediated pathway in chicken
hepatocytes. Toxicol In Vitro. 54:310–316. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Huang Z, Ji H, Shi J, Zhu X and Zhi Z:
Engeletin Attenuates Aβ1-42-Induced Oxidative Stress and
Neuroinflammation by Keap1/Nrf2 Pathway. Inflammation.
43:1759–1771. 2020.PubMed/NCBI View Article : Google Scholar
|