Introduction
Thyroid cancer is one of the most common malignant
tumors, accounting for approximately 95% of all endocrine cancer
cases and 3% of all other cancer cases (1). The age-standardized mortality rate
worldwide was 0.35/105 in 2015(2). According to the American Cancer
Society, in the United States, ~52,890 new cases of thyroid cancer
were estimated in 2020, the number of female patients was projected
to reach 40,170 cases, and the number of new cases in males was
estimated to reach 12,720(1).
Thyroid cancer is histologically classified into 5 types. Papillary
thyroid cancer (PTC) and follicular thyroid cancer (FTC) are the
most common well-differentiated thyroid carcinomas, and these types
of cancer generally have satisfactory prognosis and can be
efficiently treated by conventional therapies (3). However, anaplastic thyroid cancer
(ATC), poorly differentiated thyroid cancer (PDTC) and medullary
thyroid cancer (MTC) may differentiate into more aggressive
diseases that do not respond adequately to conventional therapies,
ultimately leading to poor patient survival (4). Surgery and endocrine therapies play
vital roles in the treatment of thyroid cancer. However, some
patients have increased tumor invasiveness, which causes poor
therapeutic efficacy and great pain (5). Therefore, an effective treatment or
specific therapeutic strategy is urgently needed for the improved
management of this malignancy.
Herbs (also known as botanicals), vitamins, minerals
and probiotics have been used as complementary and alternative
medicines in the treatment of cancer (6). Traditional Chinese medicine (TCM) has
been reported to be an effective method for treating malignant
tumors (7,8). TCM has been revealed to reduce the
production of drug resistance and provoke less damage and fewer
side effects in patients. Curcuma longa (C. Longa)
Linn is a Zingiberaceae plant, and its antitumor effects have been
reported in thyroid (9), lung
(10) and colorectal cancer cells
(11). Curcumin is a main phenolic
active compound in C. Longa Linn, and it has numerous
pharmacological activities such as anti-inflammatory (12), antibacterial (13) and, especially, anticancer activities
(14). The anticancer effects of
curcumin are due to targeting a wide range of cellular and
molecular pathways involved in cancer pathogenesis (15). For example, curcumin has been
revealed to induce endoplasmic reticulum stress-associated
apoptosis in human PTC cells via disruption of intracellular
calcium homeostasis (16) and to
affect PTC cells by targeting the JAK/STAT3 signaling pathway
(17). Thus, the identification of
additional mechanisms underlying the anticancer effect of curcumin
on PTC requires further investigation.
MicroRNAs (miRNAs or miRs) are a group of noncoding
RNAs that have been developed as novel biomarkers for the diagnosis
and treatment of thyroid carcinoma (18). In previous studies, a variety of
tumor-related miRNAs have been reported to participate in the
development and diagnosis of cancer, including PTC (19,20).
Studies have demonstrated that miRNAs can act as either oncogenes
or tumor suppressors in cancer. For instance, miR-301a-3p
suppressed the progression of hepatocellular carcinoma by targeting
VGLL4(21). miR-486-5p was revealed
to be highly expressed in PTC and promoted PTC cell invasion by
influencing epithelial-mesenchymal transition (EMT) via targeting
of KIAA1199(22). However,
miR-188-5p was demonstrated to be expressed at low levels in PTC,
and restoration of its expression suppressed PTC cell proliferation
(23). Interestingly, a previous
study reported that curcumin exerted anticancer effects by
regulating miRNAs (24), and caused
a strong and significant reduction in miR-221, miR-222 and
miR-21(25). Functional miRNAs have
been indicated to regulate target genes or proteins (26). The constitutive activation of Janus
kinase/signal transducer and activator of transcription (JAK/STAT)
has been revealed to play a vital role in the cell biological
behavior and immune response associated with cancer progression
(27,28). The JAK/STAT signaling pathway has
been demonstrated to be involved in the oncogenesis related to
thyroid cancer and is considered to be a potential prognostic
biomarker and therapeutic target (17,29).
In addition, emerging evidence has indicated that STAT3 is related
to proliferation, migration and invasion in PTC (30-32).
Interestingly, a recent study reported that curcumin inhibited the
viability and migration of PTC cells by targeting the JAK/STAT3
signaling pathway (17), which may
be a potential therapeutic target for PTC.
In the present study, the antitumor effects of
curcumin on a PTC cell line (TPC-1) were examined and the molecular
mechanisms underlying the curcumin-mediated effects in TPC-1 cells
were investigated. Our results revealed that the miR-301a-5p/STAT3
axis may be an important signaling pathway in mediating the
antitumor effects of curcumin on TPC-1 cells. The present study may
provide new insights into the antitumor effects of curcumin in
PTC.
Materials and methods
Cell lines and cell culture
A PTC cell line (TPC-1) was obtained from Shanghai
Huiying Biological Technology Co., Ltd. A human thyroid cancer cell
line (BCPAP-R, the BRAF mutation) and a human normal cell line
(Nthy-ori3-1) were obtained from the Chinese Academy of Medical
Sciences. The cells were cultured in Dulbecco's modified Eagle's
medium (DMEM; Sigma-Aldrich; Merck KGaA) supplemented with 10%
fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.) in an
incubator containing 5% CO2 at 37˚C (Thermo Fisher
Scientific, Inc.) (33).
MiRNAs, vectors, and cell
transfection
Transfections were performed using 50 nM miR-301a-3p
mimic (5'-CGAAACUGUUAUGAUAACGUGAC-3'), 100 nM miR-301a-3p inhibitor
(5'-GUCACGUUAUCAUAACAGUUCG-3'), 50 nM miR-301a-3p negative control
mimic (NC mimic; 5'-UUCUCCGAACGUGUCACGU-3') and 100 nM miR-301a-3p
inhibitor NC (5'-CAGUACUUUUGUGUAGUACAA-3'), which were obtained
from Shanghai GenePharma Co., Ltd. The STAT3 overexpression vector
(pc-STAT3; 2 µg/µl) was constructed to promote the expression of
STAT3 in PTC-1 cells, and the empty vector was used as a control
for overexpression vector pc-STAT3. Cell transfection with the
miRNA mimics, miRNA inhibitors or vectors was performed using
Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific, Inc.) at
37˚C for 4 h according to the manufacturer's protocol. Following
transfection for 48 h, subsequent experiments were performed. For
the curcumin (Sigma-Aldrich; Merck KGaA) treatments, the cells were
incubated with different concentrations of curcumin (0, 2.5, 5, 10,
20 and 40 µM) at 37˚C for 24 h.
Cell-Counting Kit-8 (CCK-8) assay
Cell viability was detected by the CCK-8 assay
(Beyotime Institute of Biotechnology) according to the
manufacturer's instructions, as previously described (17). Briefly, TPC-1 and BCPAP cells were
seeded into 96-well plates at a density of 1x104
cells/well, cultured overnight and treated for 24 h. After
treatment, 10 µl of CCK-8 solution was added to each well of the
96-well plate and incubated at 37˚C for 4 h. The optical density
(OD) was measured at 450 nm with a microplate reader (BioTek
Instruments, Inc). The half-maximal inhibitory concentrations
(IC50) were estimated based on the dose-response
curve.
Wound healing assay
TPC-1 and BCPAP cells were seeded in 6-well plates
(~70-80% confluency) and cultured at 37˚C for 48 h until they
reached full confluence. The full confluent monolayers were
scratched with 200-µl pipette tips. Then, the cells were cultured
in DMEM supplemented with 10% FBS (34). The wound area was recorded via light
microscopy observations at 0 and 24 h and images were captured
using the Lionheart™ FX automated live cell imager
(BioTek Instruments, Inc.).
Transwell assay
TPC-1 and BCPAP cell suspensions were counted and
then diluted to 5x104 cells/ml. Transwell chambers were
placed into a 24-well plate (Costar; Corning, Inc.), and the cell
suspension was seeded into the apical chambers; DMEM medium (0.5
ml) containing 10% FBS was added to the basolateral chambers, and
the plates were incubated in an incubator containing 5%
CO2 at 37˚C for 24 h. The migrating cells on the basal
side of the membranes were treated with paraformaldehyde (4%) at
37˚C for 15 min, and crystal violet (0.1%) was used to stain the
cells at 37˚C for 5 min, followed by washing with PBS. Images of
each chamber were captured using a light microscopy
Lionheart™ FX automated live cell imager, and the number
of cells in each field was used to obtain an average number. For
the cell invasion assay, the Transwell chambers were precoated with
Matrigel (BD Biosciences) at 37˚C for 15 min, and a similar
protocol was followed.
Western blot assay
Proteins were extracted from the cells using RIPA
(Beyotime Institute of Biotechnology), and the concentration was
determined according to standard protocols of BCA protein assay
kits (Beyotime Institute of Biotechnology). The total protein in
the supernatants (40 µg/well) was separated by 10% SDS-PAGE and
then transferred to PVDF membranes. After blocking with 5% skim
milk for 1 h at room temperature, the membranes were incubated
overnight at 4˚C with the following antibodies: Rabbit anti-matrix
metallopeptidase (MMP)-2 (1:1,000; cat. no. 40994; Cell Signaling
Technology, Inc.), rabbit anti-MMP-9 (1:1,000; cat. no. 13667; Cell
Signaling Technology, Inc.), rabbit anti-E-cadherin (1:10,000; cat.
no. ab40772; Abcam), rabbit anti-N-cadherin (1:10,000; cat. no.
ab76011; Abcam), rabbit anti-vimentin (1:1,000; cat. no. ab92547;
Abcam), rabbit anti-fibronectin (1:1,000; cat. no. ab2413; Abcam),
rabbit p-anti-JAK1 (1:1,000; cat. no. ab215338; Abcam), rabbit
p-anti-JAK2 (1:1,000; cat. no. ab32101; Abcam), rabbit p-anti-JAK3
(1:5,000; cat. no. ab61102; Abcam), rabbit p-anti-STAT1 (1:1,000;
cat. no. ab109461; Abcam), rabbit p-anti-STAT2 (1:1,000; cat. no.
ab53132; Abcam), rabbit anti-JAK1 (1:1,000; cat. no. ab133666;
Abcam), rabbit anti-JAK2 (1:1,000; cat. no. ab108596; Abcam),
rabbit anti-JAK3 (1:5,000; cat. no. ab45141; Abcam), rabbit
anti-STAT1 (1:1,000; cat. no. ab109320; Abcam), rabbit anti-STAT2
(1:1,000; cat. no. ab233177; Abcam), rabbit anti-STAT3 (1:1,000;
cat. no. ab68153; Abcam) and rabbit β-actin (1:5,000; cat. no.
ab8226; Abcam). After 3 washes with TBS +0.1% Tween-20, the
immunoblots were incubated for 1 h at room temperature with
alkaline phosphatase-labeled goat anti-rabbit antibodies (1:1,000;
cat. no. 14708; Cell Signaling Technology, Inc.). The
immunoreactive bands were visualized using an enhanced
chemiluminescence reagent (Beyotime Institute of Biotechnology).
The blots were semiquantified by ImageJ software (version 1.47;
National Institutes of Health).
Real-time quantitative polymerase
chain reaction (RT-qPCR)
Total RNA was isolated from cells using
TRIzol® solution (Takara Biotechnology Co., Ltd.)
according to the manufacturer's protocol. First-strand cDNA was
synthesized from the total RNA using the PrimeScript™ RT
Reagent Kit (Takara Biotechnology Co., Ltd.) according to the
manufacturer's protocol. The expression level of the miR-301a-3p
miRNA was assessed using an RT-qPCR system (ABI Prism 7300; Applied
Biosystems; Thermo Fisher Scientific, Inc.) with 2X TaqMan Fast
qPCR Master Mix (Sangon Biotech, Co., Ltd.), according to the
manufacturer's protocol. Specific primers were designed and
synthesized by Sangon Biotech Co., Ltd. The relative miRNA levels
were calculated using the 2-ΔΔCq method (35) and normalized to the U6 level. All
the samples were analyzed in at least 3 parallel reactions. The
primer sequences were as follows: miR-301a-3p (Accession number:
NR_029842) forward, 5'-ACACTCCAGCTGGGCAGTGCAATAGTATTGTC-3' and
reverse, 5'-CTCAACTGGTGTCGTGGA-3'; and U6 (Accession number:
NR_004394) forward, 5'-CTCGCTTCGGCAGCACATA-3' and reverse,
5'-AACGATTCACGAATTTGCGT-3'. The following thermocycling conditions
were used: Initial denaturation at 95˚C for 3 min; 40 cycles at
95˚C for 5 sec, 60˚C for 15 sec and 72˚C for 30 sec.
miR-301a-3p differential analysis by
starBase
Differentially expressed miR-301a-3p between 509
thyroid carcinoma and 58 normal thyroid tissues was analyzed by
starBase (version 2.0; http://starbase.sysu.edu.cn/starbase2/) (36). Filters were set up as: miRNA,
has-miR-301a-3p; cancer, thyroid carcinoma; chart type, box plot;
data scale, log2-scale. The expression data of cancers
were downloaded from The Cancer Genome Atlas (TCGA) project via
Genomic Data Commons Data Portal (37). The data were grouped by cancer and
normal type.
Bioinformatics and luciferase reporter
assays
StarBase online software was used to predict the
targeted binding sites of miRNAs in mRNAs. Subsequently, the
targeted binding relationships between miR-301a-3p and STAT3 were
predicted. A pmirGLO Dual-Luciferase Expression Vector (Promega
Corporation) that included wild-type (WT) or mutant (MUT)
3'-untranslated region (UTR) binding sites of the STAT3 sequence,
named WT-STAT3 or MUT-STAT3, respectively, was constructed and
co-transfected with miR-301a-3p mimics, inhibitors and the
corresponding negative controls into 293T cells (Bank of Type
Culture Collection of the Chinese Academy of Sciences). A total of
48 h after transfection, the results were obtained using a
Dual-Luciferase Reporter Assay System (Promega Corporation),
according to the manufacturer's protocol. Firefly luciferase
activity was normalized to Renilla luciferase activity and
all experiments were performed in triplicate.
Statistical analysis
All the experiments were performed with three
independent biological replicates. The data are expressed as the
mean ± standard deviation (SD). The IC50 values in cells
were calculated following non-linear regression on the
dose-response curves. Multigroup comparisons were performed using
one-way analysis of variance (ANOVA) followed by Tukey's post hoc
test, and comparisons between two groups were performed using
unpaired t-tests. P<0.05 was considered to indicate a
statistically significant difference and the analyses were
conducted with SPSS 20.0 software (IBM Corp.).
Results
Curcumin suppresses PTC cell
viability
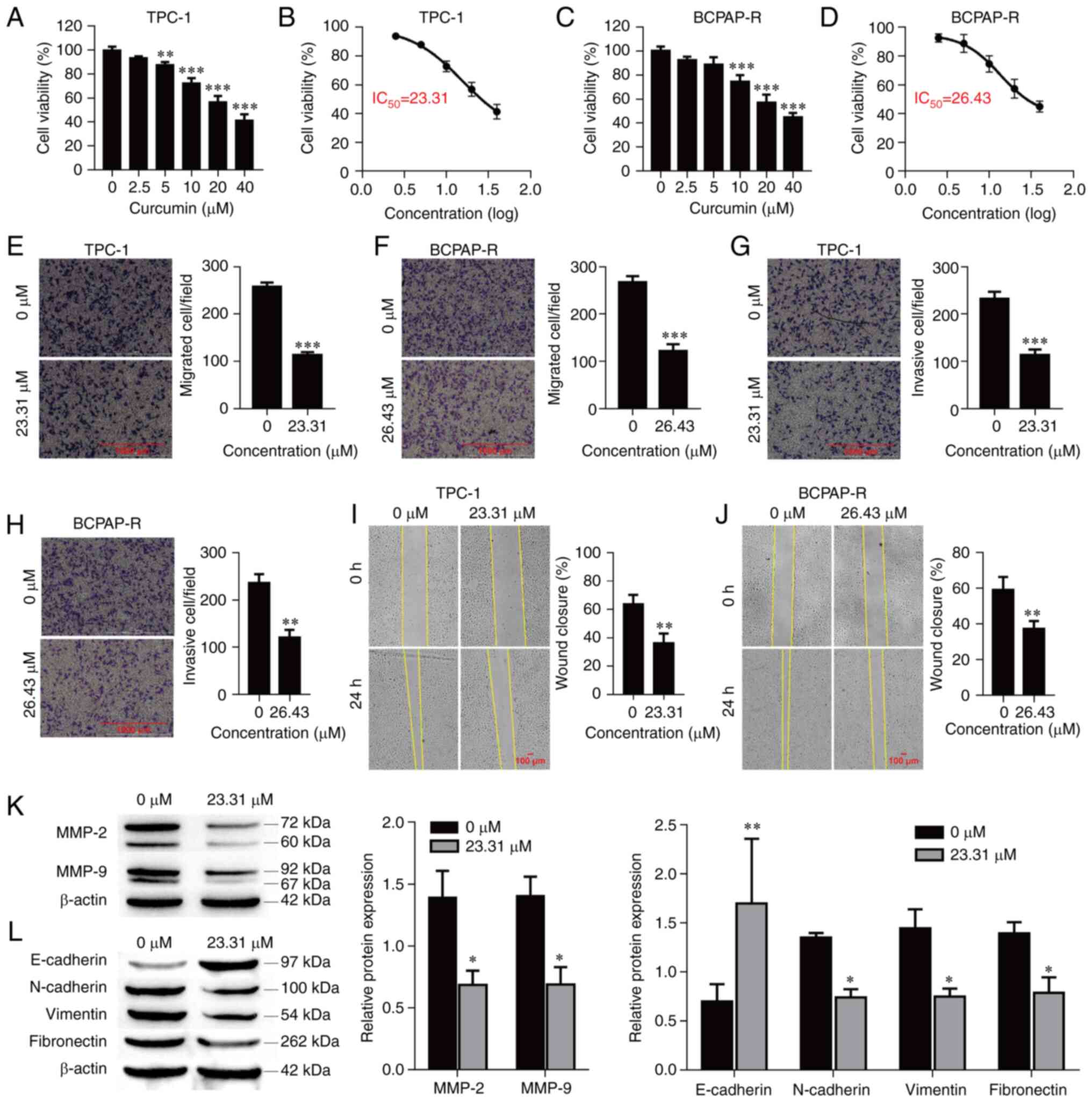
The antitumor effect of curcumin on PTC cells was
examined by CCK-8 assay. TPC-1 and BCPAP-R cells were treated with
different concentrations of curcumin (0, 2.5, 5, 10, 20 and 40 µM)
for 24 h. As demonstrated in Fig.
1A and C, curcumin
significantly reduced the viability of the PTC cells in a
concentration-dependent manner. The data indicated antitumor
activity, and based on the dose-response curve, the IC50
of curcumin was approximately 23.31 and 26.43 µM for the TPC-1 and
BCPAP-R cells, respectively, at 24 h (Fig. 1B and D). Hence, cells treated with 23.31 and
26.43 µM curcumin for 24 h were used for the following
experiments.
Curcumin inhibits cell migration,
invasion and EMT
To further explore the functions of curcumin in PTC
cells, its effects on the migration and invasion of TPC-1 and
BCPAP-R cells were examined. Curcumin treatment at a concentration
of 23.31 µM or 26.43 µM for 24 h significantly inhibited cell
migration and invasion compared with the control treatment
(Fig. 1E-H; P<0.001,
respectively). Similarly, the wound healing assay demonstrated that
curcumin significantly reduced cell migration (Fig. 1I and J; P<0.01, respectively). The protein
levels of MMP-2 and MMP-9 were evaluated in TPC-1 cells treated
with curcumin. Curcumin significantly reduced the protein
expression of MMP-2 and MMP-9 (Fig.
1K; P<0.01, respectively). In addition, EMT-related markers
(N-cadherin, E-cadherin, vimentin and fibronectin) were also
detected by western blot analysis. As revealed in Fig. 1L, compared with the control
treatment, curcumin treatment decreased the protein expression
levels of N-cadherin (P=0.047), vimentin (P=0.021) and fibronectin
(P=0.048) but increased the protein expression level of E-cadherin
(P<0.01). These data indicated that curcumin suppressed cell
migration and invasion as well as MMP-2, MMP-9 and EMT marker
expression in PTC cells.
Curcumin inhibits cell viability,
migration, invasion and EMT by upregulating miR-301a-3p
The expression of miR-301a-3p in thyroid carcinoma
was validated using starBase. As revealed in Fig. 2A, the expression of miR-301a-3p was
downregulated in thyroid carcinoma (P=0.043) and significantly
downregulated in TPC-1 and BCPAP-R cells compared with Nthy-ori-1
cells (Fig. 2B; P<0.001). Next,
to explore the potential mechanism by which curcumin drives the
progression of PTC cells, the expression of miR-301a-3p was
detected after treatment with different concentrations of curcumin
(0, 5, 10, 20 and 40 µM) for 24 h. As observed in Fig. 2C, curcumin markedly increased the
expression of miR-301a-3p in a concentration-dependent manner. To
further examine the function of miR-301a-3p in TPC-1 cells, cell
viability, migration and invasion were analyzed. As anticipated,
transfection of miR-301a-3p inhibitors significantly decreased the
expression of miR-301a-3p compared with transfection of the NC
group (Fig. 2D; P<0.001). The
cell viability of TPC-1 cells transfected with the miR-301a-3p
inhibitors was higher than that of the of the TPC-1 cells
transfected with NC (P=0.048), and the viability was decreased
after curcumin treatment (P<0.001) but increased after
miR-301a-3p inhibitor transfection (Fig. 2E; P<0.001). Moreover, the
addition of the miR-301a-3p inhibitors significantly increased the
migration and invasion abilities of the TPC-1 cells (P=0.027 and
P=0.021, respectively) compared with the NC group, and cell
migration and invasion were inhibited after curcumin treatment
(P<0.001, respectively); these effects were reversed by the
transfection of miR-301a-3p inhibitors (Fig. 2F and G; P<0.001 and P<0.01, respectively).
Similarly, the wound healing assay demonstrated that curcumin
significantly inhibited wound closure, which was enhanced by the
transfection of miR-301a-3p inhibitors (Fig. 2H; P<0.001).
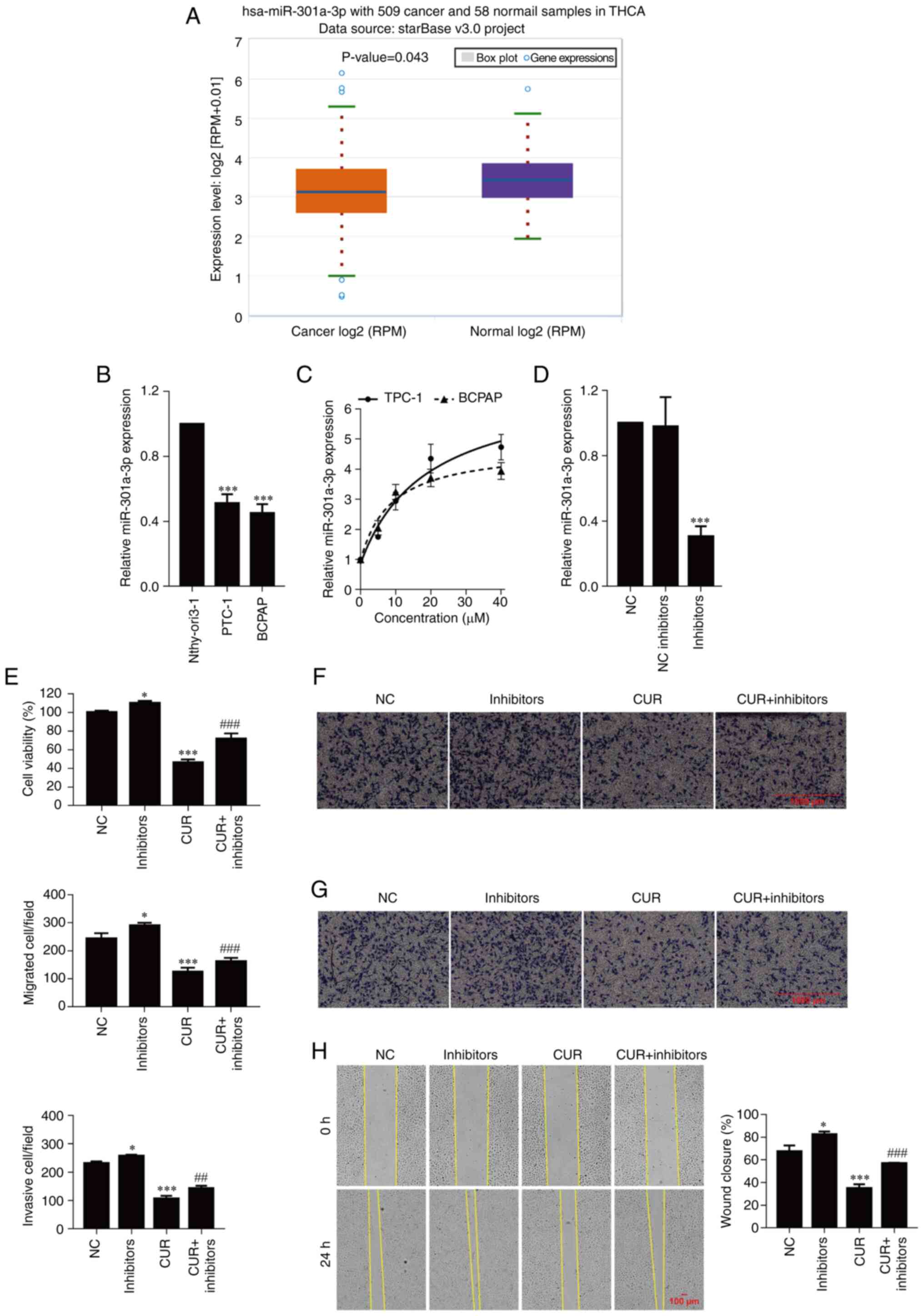 | Figure 2Curcumin inhibits cell viability,
migration, and invasion by upregulating miR-301a-3p. (A) Validation
of miR-301a-3p expression in THCA using starBase. (B) The
expression of miR-301a-3p was determined by real-time quantitative
PCR. ***P<0.001, vs. Nthy-ori3-1 cells. (C and D) The
expression of miR-301a-3p was determined by real-time quantitative
PCR. (E) Cell viability was examined by CCK-8 assay. (F) Cell
migration was determined by Transwell cell migration assays and
analyzed at a magnification of x20. (G) Cell invasion was
determined by Transwell cell invasion assays and analyzed at a
magnification of x20. (H) Percentange (%) of wound closure in wound
healing assays was analyzed at a magnification of x20. The data are
expressed as the mean ± SD. *P<0.05 and
***P<0.001, vs. NC; ##P<0.01 and
###P<0.001, vs. CUR. miR, microRNA; THCA, thyroid
carcinoma; NC, negative control; inhibitors, miR-301a-3p
inhibitors; CUR, curcumin (23.31 µM). |
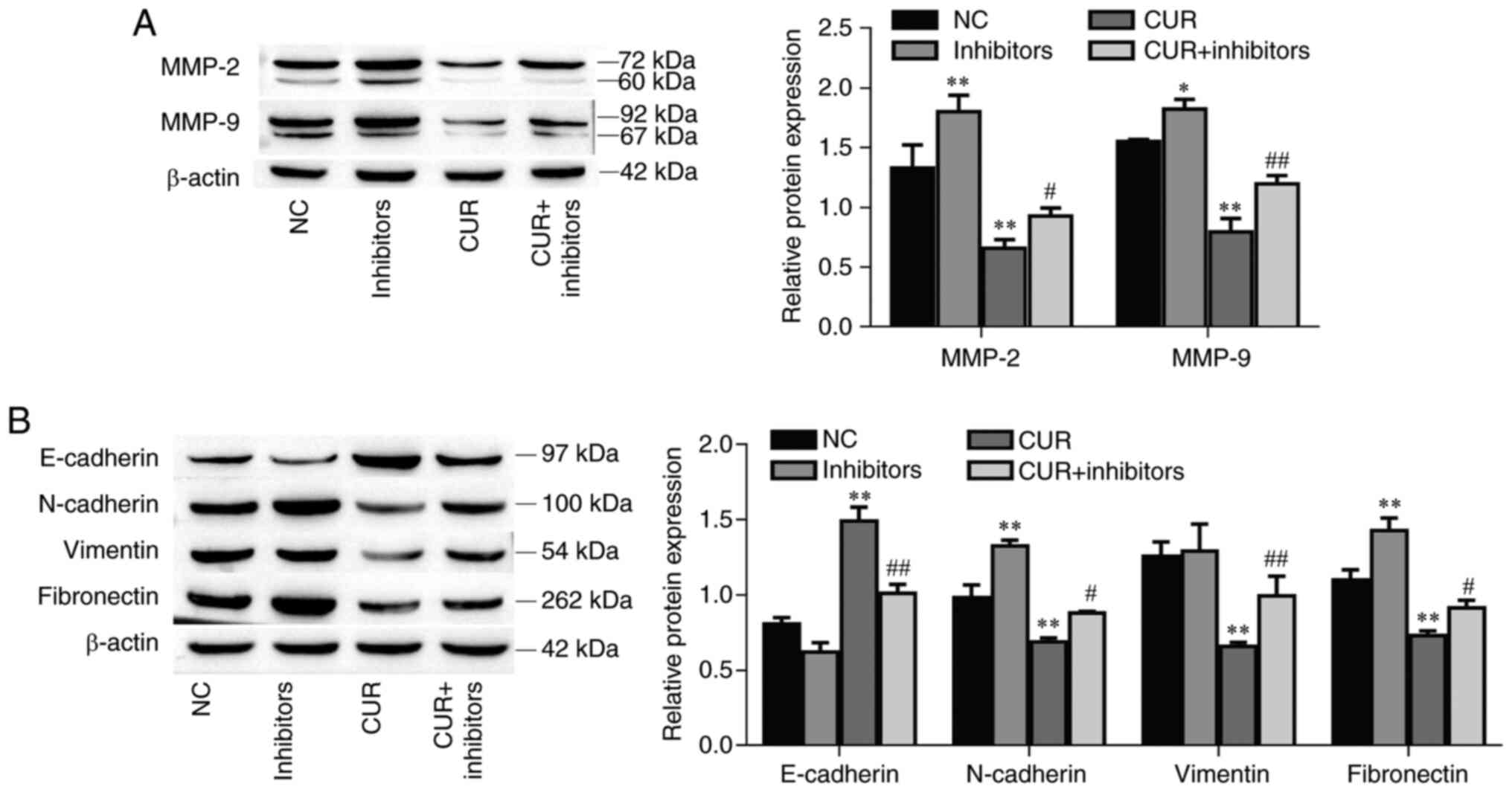
The protein expression levels of MMP-2 and MMP-9
were increased in the miR-301a-3p inhibitor group compared with the
NC group (P<0.01 and P=0.033, respectively). Furthermore, the
MMP-2 and MMP-9 protein expression levels were decreased after
curcumin treatment (P<0.01, respectively) but increased after
miR-301a-3p inhibitor transfection (Fig. 3A; P=0.029 and P<0.01,
respectively). In addition, the EMT-related markers N-cadherin and
fibronectin were increased in the miR-301a-3p inhibitor group
compared with the NC group (P<0.01, respectively); moreover,
these markers were decreased after curcumin treatment (P<0.01,
respectively), and this effect was reversed by the transfection of
miR-301a-3p inhibitors (Fig. 3B;
P=0.032 and P=0.040, respectively). Conversely, compared with the
control group, E-cadherin protein expression was decreased in the
miR-301a-3p inhibitor group (P=0.038); however, E-cadherin protein
expression increased after curcumin treatment (P<0.01), and this
effect was reversed by the transfection of miR-301a-3p inhibitors
(P<0.01). Interestingly, miR-301a-3p inhibitor transfection did
not affect vimentin expression (P=0.954), however curcumin
significantly decreased vimentin expression (P<0.01) and was
reversed by the addition of miR-301a-3p inhibitors (P<0.01).
Collectively, these data indicated that curcumin suppressed TPC-1
cell migration and invasion by upregulating miR-301a-3p
expression.
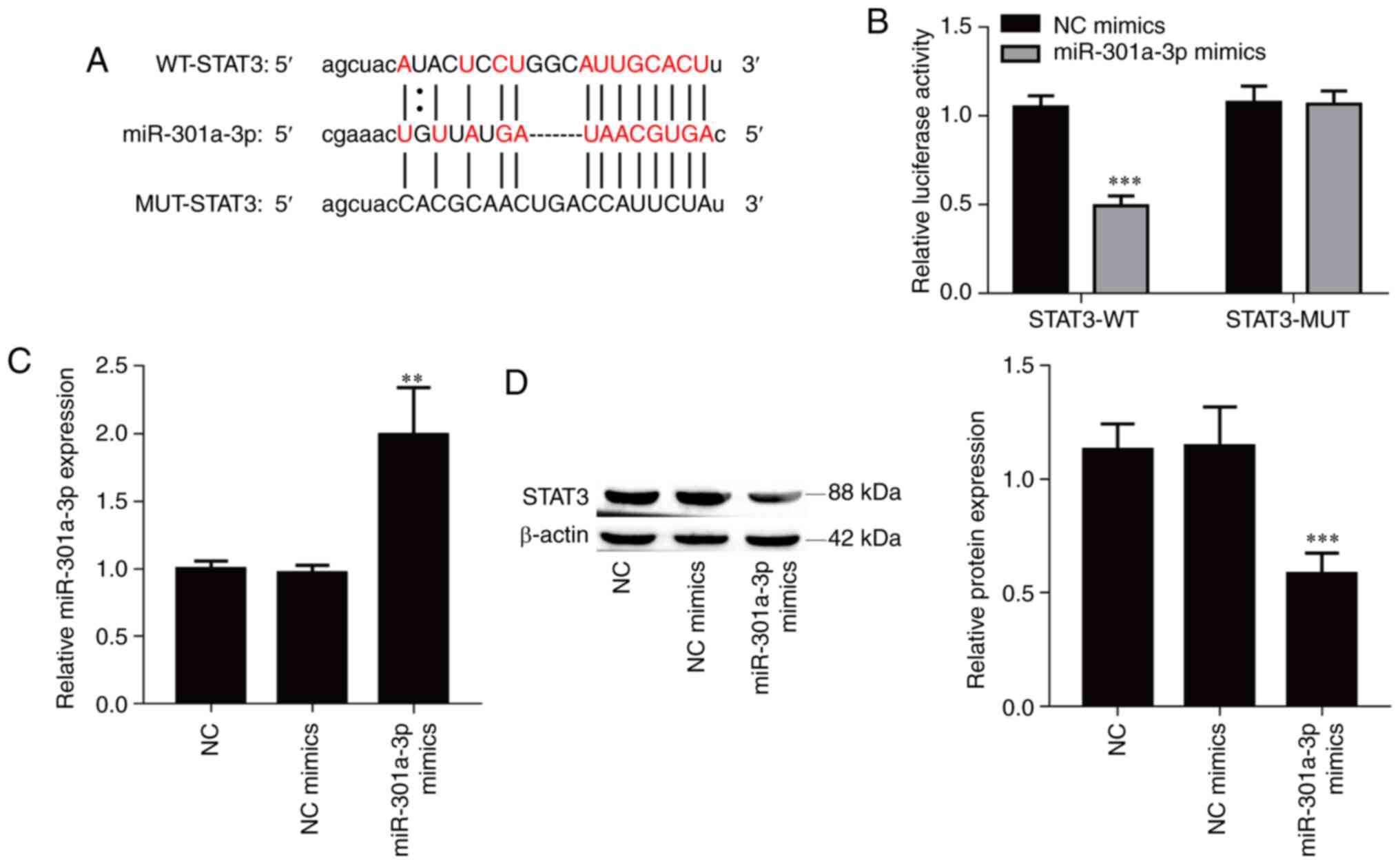
miR-301a-3p directly targets
STAT3
The bioinformatics software starBase was used to
identify potential targets of miR-301a-3p, and STAT3 was selected
due to its role in cancer metastasis. To confirm the interaction
between miR-301a-3p and STAT3, a luciferase reporter vector
containing the WT or MUT 3'UTR of STAT3 was constructed (Fig. 4A). Overexpression of miR-301a-3p
significantly suppressed the luciferase activity of the WT reporter
vector (Fig. 4B; P<0.001) but
did not affect the luciferase activity of the mutant reporter
vector in 293T cells. Furthermore, transfection of miR-301a-3p
mimics resulted in a significant increase of the expression of
miR-301a-3p in TPC-1 cells compared with the NC mimics group
(Fig. 4C; P<0.01), and
overexpression of miR-301a-3p also significantly suppressed the
protein expression level of STAT3 in TPC-1 cells (Fig. 4D; P<0.001). Collectively, the
aforementioned results indicated that miR-301a-3p directly targeted
the STAT3 gene.
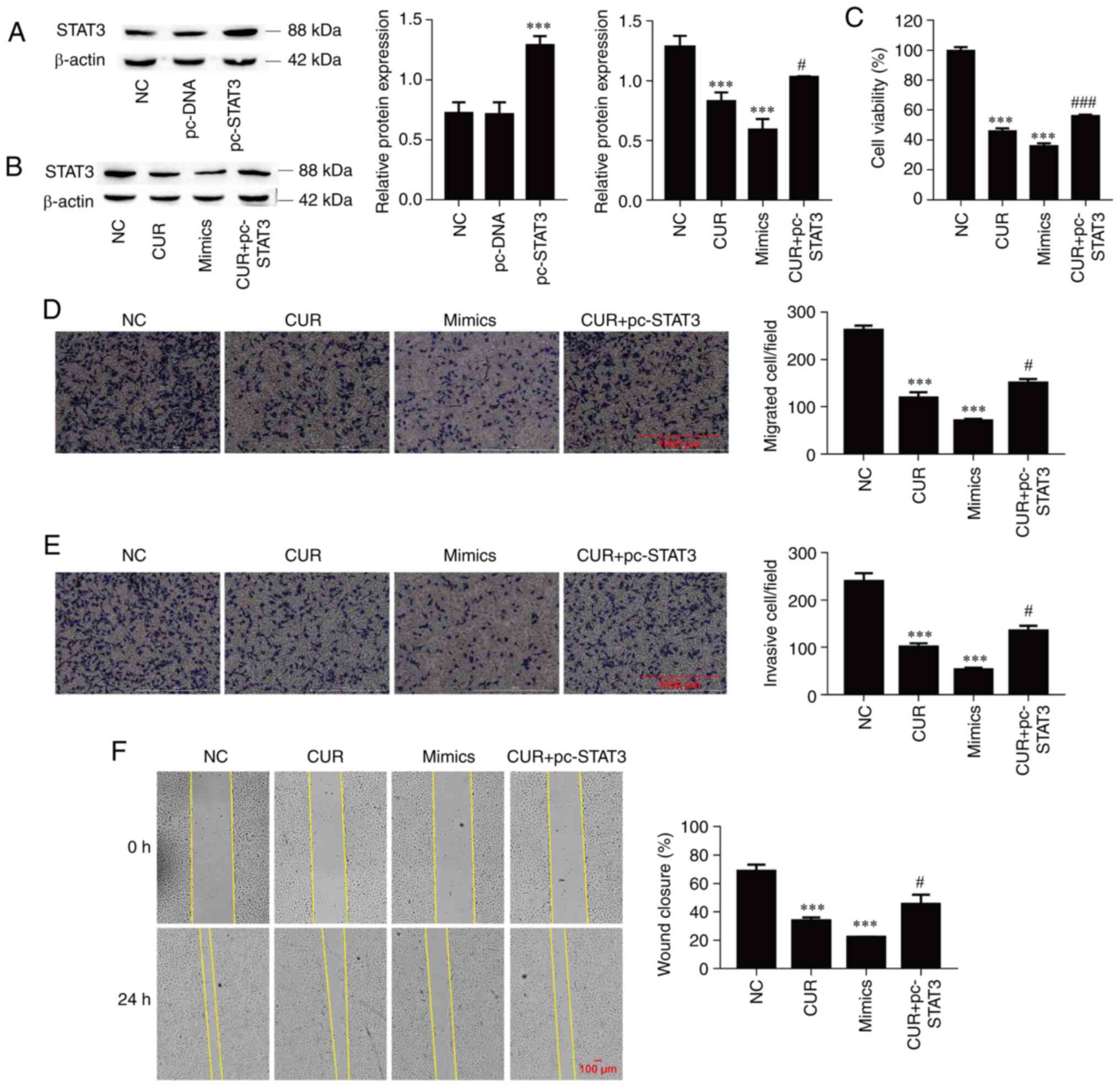
Curcumin inhibits cell viability,
migration and invasion by regulating the miR-301a-3p/STAT3
axis
The next aim was to determine the functional
interaction of miR-301a-3p and STAT3 during the curcumin-mediated
inhibition of TPC-1 cell viability, migration and invasion.
Firstly, the successful overexpression of STAT3 was confirmed
through the transfection of pc-STAT3 vector (Fig. 5A; P<0.001). Subsequently, the
expression of STAT3 was significantly suppressed by curcumin
treatment (P<0.001), which was significantly reversed by STAT3
overexpression in TPC-1 cells (P=0.040), and miR-301a-3p
overexpression also significantly suppressed STAT3 protein
expression (Fig. 5B; P<0.001).
Overexpression of miR-301a-3p significantly decreased cell
viability compared with NC group (P<0.001); in addition, cell
viability was also significantly suppressed by curcumin treatment
(P<0.001), and this effect was significantly reversed by STAT3
overexpression in TPC-1 cells (Fig.
5C; P<0.001). Moreover, cell migration and invasion were
inhibited by miR-301a-3p overexpression and curcumin treatment
(P<0.001, respectively), and the overexpression of STAT3
alleviated the inhibitory effect of curcumin on cell migration and
invasion (Fig. 5D and E; P=0.018 and P=0.028, respectively).
Similarly, the wound healing assay demonstrated that the
miR-301a-3p mimics and curcumin significantly reduced cell
migration (P<0.001), and the overexpression of STAT3 reversed
the inhibitory effect of curcumin on cell migration (Fig. 5F; P=0.047). These data indicated
that curcumin suppressed TPC-1 cell migration and invasion by
regulating the miR-301a-3p/STAT3 axis.
Curcumin inhibits MMP-2, MMP-9 and EMT
marker expression and suppresses the JAK/STAT pathway by regulating
the miR-301a-3p/STAT3 axis
The protein expression levels of MMP-2, MMP-9 and
EMT-related markers (N-cadherin, vimentin and fibronectin) were
decreased in the miR-301a-3p mimic and curcumin groups compared
with the NC group (P<0.001, respectively), and the
overexpression of STAT3 reversed the inhibitory effect of curcumin
on protein expression (Fig. 6A and
B; P<0.05). Conversely, the
E-cadherin protein expression was increased after the miR-301a-3p
and curcumin treatments (P<0.001), but the overexpression of
STAT3 reversed the effect of curcumin on the promotion of protein
expression (P=0.028). STAT3 is one of the pivotal proteins involved
in the JAK/STAT signaling pathway (38). Therefore, the effects of curcumin on
the JAK/STAT signaling pathway were investigated. As demonstrated
in Fig. 6C, the levels of
phosphorylated JAK1, JAK2, JAK3, STAT1 and STAT2 were significantly
suppressed by curcumin treatment (P<0.001, respectively), which
was effectively reversed by the overexpression of STAT3 in TPC-1
cells (P<0.05), and the overexpression of miR-301a-3p also
significantly suppressed the levels of phosphorylated JAK1, JAK2,
JAK3, STAT1 and STAT2 (P<0.001, respectively). Collectively,
these data indicated that curcumin inhibited MMP-2, MMP-9 and
EMT-related marker expression, and suppressed the JAK/STAT pathway
by regulating the miR-301a-3p/STAT3 axis.
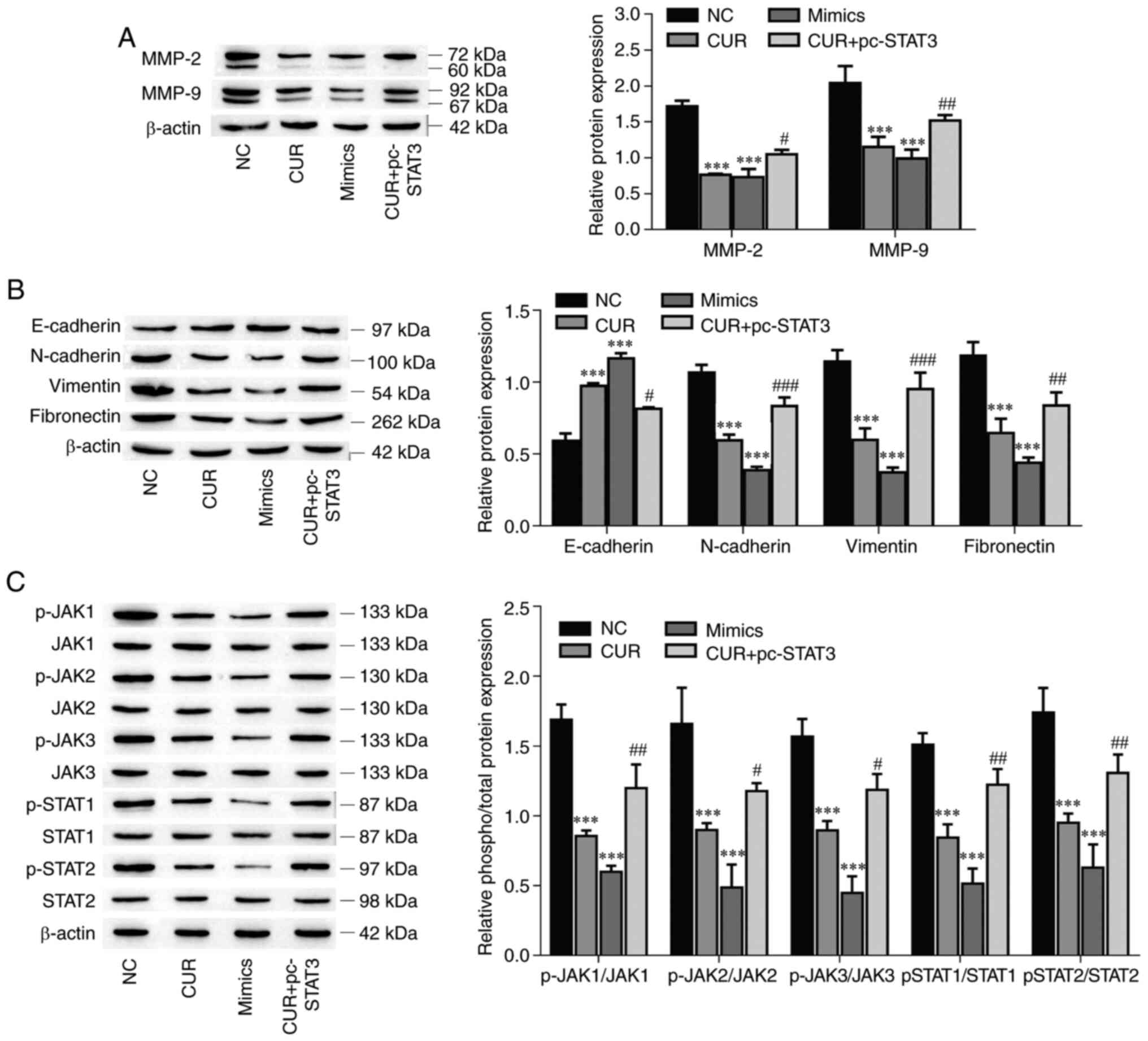 | Figure 6Curcumin inhibits MMP-2, MMP-9 and
EMT, and suppresses the JAK/STAT pathway by regulating the
miR-301a-3p/STAT3 axis. (A) The expression of MMP-2 and MMP-9 was
determined by western blotting. (B) The expression of EMT-related
markers was determined by western blot analysis. (C) The levels of
phosphorylated and total protein components of the JAK/STAT
signaling pathway were determined by western blotting. The data are
expressed as the mean ± SD. ***P<0.01, vs. NC;
#P<0.05, ##P<0.01 and
###P<0.001, vs. CUR. EMT, epithelial-mesenchymal
transition; JAK/STAT, Janus kinase/signal transducer and activator
of transcription; miR, microRNA; NC, negative control; CUR,
curcumin (23.31 µM); mimics, miR-301a-3p mimics; pc-STAT3,
overexpression of STAT3. |
Discussion
Curcumin has been revealed to serve as a potent
antitumor drug for the treatment of multiple cancers, including PTC
(14,39). It has already been suggested that
curcumin suppresses the survival, invasion and migration of cancer
cells (39). In the present study,
the IC50 of curcumin was approximately 23.31 and 26.43
µM in TPC-1 and BCPAP-R cells at 24 h, respectively. Previous
studies have reported that 20-50 µM of curcumin could significantly
promote the apoptosis of PTC cells (17,40-42).
For example, Khan et al determined that 20 and 10 µM of
curcumin significantly decreased BCPAP-R and TPC-1 cell viability,
respectively (17); Schwertheim
et al reported that 50, 25 and 25 µM curcumin significantly
inhibited TPC-1, BHT-101 and FTC-133 cell viability, respectively
(40). Thus, 23.31 µM (TPC-1 cells)
and 26.43 µM (BCPAP-R cells) curcumin were used in experiments.
Curcumin has been demonstrated to possess antitumor,
anti-inflammatory, antioxidant and antimicrobial properties
(43-45).
Typically, curcumin is involved in multiple anticancer processes
(46). Results of a previous study
have demonstrated that miRNAs may act as protective regulators of
curcumin antitumor activity (47).
The present study revealed that miR-301a-3p was expressed at lower
levels in PTC and was upregulated by curcumin. miR-301a-3p has been
revealed to regulate multiple biological processes, including cell
proliferation, differentiation, biological development and numerous
metabolic processes in organisms (48,49).
Furthermore, it was demonstrated that inhibition of miR-301a-3p
reversed the inhibitory effects of curcumin on the viability,
migration and invasion of TPC-1 cells. Thus, miR-301a-3p was
identified as a protective regulator in PTC cells. The
curcumin-mediated regulation of miRNA expression, including the
activation/inactivation of miRNA transcription, the regulation of
associated signaling pathways and the methylation/demethylation of
miRNAs, is complex (50). Spadotto
et al revealed that extensive methylation of complexes led
to downregulated miR-301a expression (51). Curcumin has been demonstrated to
cause epigenetic modulation of miR-203 expression by demethylating
the miR-203 promoter in bladder cancer (52). Curcumin could increase miR-301a-3p
expression via decreased methylation of the complex and/or
miR-301a-3p promoter in PTC.
Curcumin has been revealed to inhibit cell
proliferation, migration and invasion by activating the JAK2/STAT3
signaling pathway, as it has been reported for different human
malignancies, such as retinoblastoma and ovary and thyroid cancers
(17,53,54).
Our study further demonstrated that curcumin inhibited the JAK/STAT
signaling pathway by upregulating miR-301a-3p expression and that
miR-301a-3p directly targeted STAT3. These observations indicated
that miR-301a-3p was a suppressor of STAT3 protein expression and
the JAK/STAT signaling pathway in TPC-1 cells. STAT3 is considered
to be one of the most common oncogenes in human cancers and plays a
key role in PTC progression (17,55).
It was further demonstrated that curcumin and miR-301a-3p mimics
reduced the levels of phosphorylated JAK1, JAK2, JAK3, STAT1 and
STAT2 via the targeting of STAT3 by miR-301a-3p, whereas the
overexpression of STAT3 abolished the suppressive effect of
curcumin on this signaling pathway. Thus, the miR-301a-3p/STAT3
axis was identified as an efficient regulator of JAK/STAT signaling
pathway in TPC-1 cells.
It has been reported that curcumin regulates EMT in
human cancers, including PTC (41,56,57).
Notably, EMT is considered to be a critical event in cancer cell
migration and invasion, and curcumin has been reported to inhibit
PTC cell migration and invasion (25,58,59),
indicating that curcumin inhibits PTC cell migration and invasion
by inhibiting EMT. In the present study, it was demonstrated that
curcumin inhibited EMT, migration and invasion of PTC cells.
MiR-301a-3p inhibitors increased EMT and reversed the inhibitory
effects of curcumin in EMT of TPC-1 cells. These observations
indicated that curcumin inhibited EMT by upregulating miR-301a-3p
expression. It has been reported that curcumin suppressed EMT
through the miR-200c/EPM5 axis and the ERK5/AP-1 and TET1-NKD-Wnt
signaling pathways (60-62).
Furthermore, our results indicated that the targeting of STAT3 by
miR-301a-3p and the overexpression of STAT3 also reversed the
inhibitory effects of curcumin on EMT in TPC-1 cells. Thus, it was
revealed that curcumin inhibited EMT by regulating the
miR-301a-3p/STAT3 axis.
In our study, it was demonstrated that curcumin
inhibited STAT3 expression. Furthermore, the overexpression of
STAT3 reversed the inhibitory effects of curcumin on the viability,
migration and invasion of TPC-1 cells. It has been reported that
suppression of STAT3 activity or expression inhibits human cancer
progression (55,63,64).
Curcumin has been demonstrated to promote PTC cell apoptosis by
increasing DNA damage, endoplasmic reticulum stress and the
PI3K/Akt signaling pathway (16,42,59).
These observations demonstrated that targeting STAT3 is a potential
therapeutic target for PTC. In the present study, it was revealed
that curcumin inhibited the viability of TPC-1 cells by regulating
the miR-301a-3p/STAT3 axis. An increasing number of studies has
reported that curcumin leads to cell cycle arrest at the G2/M phase
in PTC cells (40,42,65).
Therefore, it was theorized that curcumin inhibited the viability
of PTC cells by affecting the G2/M phase of the cell cycle. It has
been reported that curcumin promotes PTC cell apoptosis and
inhibits PTC cell migration and invasion (16,25,40-42,57-59,65).
A recent study reported that curcumin mediated BCPAP-R and TPC-1
cell apoptosis and stemness by targeting the JAK/STAT3 signaling
pathway (17). Notably, the present
study revealed that curcumin downregulated STAT3 expression by
upregulating miR-301a-3p expression in TPC-1 cells. Previous
studies have demonstrated that STAT3 promotes tumour progression in
PTC (66-68).
The results of the present study provided strong evidence that
curcumin decreases the expression of STAT3 and suppresses the
viability, migration and invasion of PTC cells via upregulating
miR-301a-3p.
However, the potential role and underlying mechanism
of curcumin in PTC was only investigated in vitro, and
several limitations should also be considered. Curcumin affects
miRNAs that have been reported to be involved in human cancer
progression (15). Curcumin may
affect the expression of additional miRNAs in PTC cells (40). In future studies, microarray assays
may be used to profile the miRNAs that could be regulated by
curcumin in TPC-1 cells. In addition, the present study only
selected STAT3 as a target of miR-301a-3p and TPC-1 cells as the
cell model for further study, but miR-301a-3p may also target other
genes. Consequenlty, future studies may investigate more targets of
miR-301a-3p to further reveal the underlying antitumor mechanisms
of the antitumor effects of curcumin on additionl types of PTC
cells. Moreover, whether the use of curcumin with chemotherapeutic
reagents currently used to treat PTC could potentiate the antitumor
effects and reduce drug toxicity will be investigated in our future
studies.
In summary, curcumin suppressed the cell viability,
migration, invasion and EMT of TPC-1 cells. Moreover, curcumin
treatment increased miR-301a-3p expression and inhibited STAT3
expression. Overexpression of miR-301a-3p inhibited cell viability,
migration, invasion, and EMT and the JAK/STAT signaling pathway by
targeting STAT3, and miR-301a-3p inhibitors and STAT3
overexpression reversed the curcumin-induced cell viability,
migration, invasion and EMT of TPC-1 cells. Collectively, curcumin
played an anticancer role in TPC-1 cells by regulating
miR-301a-3p/STAT3, indicating that curcumin is a promising
oncotherapeutic agent. These findings may provide a possible
strategy for the clinical treatment of PTC.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Scientific
Research Fund of Yunnan Education Department (grant no.
2019J1262).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YiL, DK, YZ and JM conceived and designed the
research. YiL, DK, YZ, SL, Yal, LD, NZ and JM performed the
experiments and analyzed the data. YiL, DK and JM wrote the
manuscript. YiL, SL and YaL interpreted the data. YZ, SL and JM
reviewed/edited the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang J, Yu F, Shang Y, Ping Z and Liu L:
Thyroid cancer: Incidence and mortality trends in China, 2005-2015.
Endocrine. 68:163–173. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Laha D, Nilubol N and Boufraqech M: New
therapies for advanced thyroid cancer. Front Endocrinol (Lausanne).
11(82)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kumar A and Bal CS: Differentiated thyroid
cancer. Indian J Pediatr. 70:707–713. 2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rao SN, Zafereo M, Dadu R, Busaidy NL,
Hess K, Cote GJ, Williams MD, William WN, Sandulache V, Gross N, et
al: Patterns of treatment failure in anaplastic thyroid carcinoma.
Thyroid. 27:672–681. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Berretta M, Della Pepa C, Tralongo P,
Fulvi A, Martellotta F, Lleshi A, Nasti G, Fisichella R, Romano C,
De Divitiis C, et al: Use of complementary and alternative medicine
(CAM) in cancer patients: An Italian multicenter survey.
Oncotarget. 8:24401–24414. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Han YG, Ma LG, Zhao L, Feng W and Zheng X:
Rosmarinic inhibits cell proliferation, invasion and migration via
up-regulating miR-506 and suppressing MMP2/16 expression in
pancreatic cancer. Biomed Pharmacother. 115(108878)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhao J, Fang Z, Zha Z, Sun Q, Wang H, Sun
M and Qiao B: Quercetin inhibits cell viability, migration and
invasion by regulating miR-16/HOXA10 axis in oral cancer. Eur J
Pharmacol. 847:11–18. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Perna A, De Luca A, Adelfi L, Pasquale T,
Varriale B and Esposito T: Effects of different extracts of
curcumin on TPC1 papillary thyroid cancer cell line. BMC Complement
Altern Med. 18(63)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhou JJ, Zheng JY, Cheng XQ, Xin GZ, Wang
SL and Xie T: Chemical markers' knockout coupled with
UHPLC-HRMS-based metabolomics reveals anti-cancer integration
effects of the curcuminoids of turmeric (Curcuma longa L.)
on lung cancer cell line. J Pharm Biomed Anal.
175(112738)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Buhrmann C, Popper B, Kunnumakkara AB,
Aggarwal BB and Shakibaei M: Evidence that calebin a, a component
of Curcuma longa suppresses NF-B mediated proliferation, invasion
and metastasis of human colorectal cancer induced by TNF-β
(lymphotoxin). Nutrients. 11(2904)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Huang W, Li X, Wang D, Sun Y, Wang Q, Bu Y
and Niu F: Curcumin reduces LPS-induced septic acute kidney injury
through suppression of lncRNA PVT1 in mice. Life Sci.
254(117340)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Huang G, Yan Y, Xu D, Wu J, Xu C, Fu L and
Lin B: Curcumin-loaded simplenanoMOFs@CMFP. A biological
preserving paste with antibacterial properties and long-acting,
controllable release. Food Chem. 337(127987)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang CY, Zhang L, Yu HX, Bao JD, Sun Z
and Lu RR: Curcumin inhibits invasion and metastasis in K1
papillary thyroid cancer cells. Food Chem. 139:1021–1028.
2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mirzaei H, Masoudifar A, Sahebkar A, Zare
N, Sadri Nahand J, Rashidi B, Mehrabian E, Mohammadi M, Mirzaei HR
and Jaafari MR: MicroRNA: A novel target of curcumin in cancer
therapy. J Cell Physiol. 233:3004–3015. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang L, Cheng X, Xu S, Bao J and Yu H:
Curcumin induces endoplasmic reticulum stress-associated apoptosis
in human papillary thyroid carcinoma BCPAP cells via disruption of
intracellular calcium homeostasis. Medicine (Baltimore).
97(e11095)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Khan AQ, Ahmed EI, Elareer N, Fathima H,
Prabhu KS, Siveen KS, Kulinski M, Azizi F, Dermime S, Ahmad A, et
al: Curcumin-mediated apoptotic cell death in papillary thyroid
cancer and cancer stem-like cells through targeting of the
JAK/STAT3 signaling pathway. Int J Mol Sci. 21(438)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Celano M, Rosignolo F, Maggisano V, Pecce
V, Iannone M, Russo D and Bulotta S: MicroRNAs as biomarkers in
thyroid carcinoma. Int J Genomics. 2017(6496570)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Aragon Han P, Weng CH, Khawaja HT,
Nagarajan N, Schneider EB, Umbricht CB, Witwer KW and Zeiger MA:
MicroRNA expression and association with clinicopathologic features
in papillary thyroid cancer: A systematic review. Thyroid.
25:1322–1329. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang N, Hu X, Du Y and Du J: The role of
miRNAs in colorectal cancer progression and chemoradiotherapy.
Biomed Pharmacother. 134(111099)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hu J, Ruan J, Liu X, Xiao C and Xiong J:
MicroRNA-301a-3p suppressed the progression of hepatocellular
carcinoma via targeting VGLL4. Pathol Res Pract. 214:2039–2045.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Jiao X, Ye J, Wang X, Yin X, Zhang G and
Cheng X: KIAA1199, a target of micoRNA-486-5p, promotes papillary
thyroid cancer invasion by influencing epithelial-mesenchymal
transition (EMT). Med Sci Monit. 25:6788–6796. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhou P, Irving A, Wu H, Luo J, Aguirre J,
Costa M, Khamsuree M, Gerads N and Liu WB: Validation of
microRNA-188-5p inhibition power on tumor cell proliferation in
papillary thyroid carcinoma. Cell Transplant.
29(963689720918300)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Jiao DM, Yan L, Wang LS, Hu HZ, Tang XL,
Chen J, Wang J, Li Y and Chen QY: Exploration of inhibitory
mechanisms of curcumin in lung cancer metastasis using a
miRNA-transcription factor-target gene network. PLoS One.
12(e0172470)2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Allegri L, Rosignolo F, Mio C, Filetti S,
Baldan F and Damante G: Effects of nutraceuticals on anaplastic
thyroid cancer cells. J Cancer Res Clin Oncol. 144:285–294.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Carthew RW and Sontheimer EJ: Origins and
mechanisms of miRNAs and siRNAs. Cell. 136:642–655. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Morgan EL and Macdonald A: Manipulation of
JAK/STAT signalling by high-risk HPVs: Potential therapeutic
targets for HPV-associated malignancies. Viruses.
12(977)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Qing X, Tan GL, Liu HW, Li W, Ai JG, Xiong
SS, Yang MQ and Wang TS: LINC00669 insulates the JAK/STAT
suppressor SOCS1 to promote nasopharyngeal cancer cell
proliferation and invasion. J Exp Clin Cancer Res.
39(166)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Couto JP, Daly L, Almeida A, Knauf JA,
Fagin JA, Sobrinho-Simões M, Lima J, Máximo V, Soares P, Lyden D
and Bromberg JF: STAT3 negatively regulates thyroid tumorigenesis.
Proc Natl Acad Sci USA. 109:E2361–E2370. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yang L, Tan Z, Li Y, Zhang X, Wu Y, Xu B
and Wang M: Insulin-like growth factor 1 promotes proliferation and
invasion of papillary thyroid cancer through the STAT3 pathway. J
Clin Lab Anal. 34(e23531)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang T, He L, Wang Z, Dong W, Sun W, Qin
Y, Zhang P and Zhang H: Calcitriol enhances Doxorubicin-induced
apoptosis in papillary thyroid carcinoma cells via regulating
VDR/PTPN2/p-STAT3 pathway. J Cell Mol Med. 24:5629–5639.
2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kong D, Li A, Liu Y, Cui Q, Wang K, Zhang
D, Tang J, Du Y, Liu Z, Wu G and Wu K: SIX1 Activates STAT3
signaling to promote the proliferation of thyroid carcinoma via
EYA1. Front Oncol. 9(1450)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhou Y, Liu S, Luo Y, Zhang M, Jiang X and
Xiong Y: LncRNA MAPKAPK5-AS1 promotes proliferation and migration
of thyroid cancer cell lines by targeting miR-519e-5p/YWHAH. Eur J
Histochem. 64(3177)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sun Y, Zhang L and Zhang S:
MicroRNA-124-3p inhibits tumourigenesis by targeting
mitogen-activated protein kinase 4 in papillary thyroid carcinoma.
Cell Biochem Funct. 38:1017–1024. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
StarBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42 (Database Issue):D92–D97. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang Z, Jensen MA and Zenklusen JC: A
Practical guide to the cancer genome atlas (TCGA). Methods Mol
Biol. 1418:111–141. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Owen KL, Brockwell NK and Parker BS:
JAK-STAT signaling: A double-edged sword of immune regulation and
cancer progression. Cancers. 11(2002)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Giordano A and Tommonaro G: Curcumin and
cancer. Nutrients. 11(2376)2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Schwertheim S, Wein F, Lennartz K, Worm K,
Schmid KW and Sheu-Grabellus SY: Curcumin induces G2/M arrest,
apoptosis, NF-κB inhibition, and expression of differentiation
genes in thyroid carcinoma cells. J Cancer Res Clin Oncol.
143:1143–1154. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhang CY, Zhang L, Yu HX, Bao JD and Lu
RR: Curcumin inhibits the metastasis of K1 papillary thyroid cancer
cells via modulating E-cadherin and matrix metalloproteinase-9
expression. Biotechnol Lett. 35:995–1000. 2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhang L, Cheng X, Gao Y, Bao J, Guan H, Lu
R, Yu H, Xu Q and Sun Y: Induction of ROS-independent DNA damage by
curcumin leads to G2/M cell cycle arrest and apoptosis in human
papillary thyroid carcinoma BCPAP cells. Food Funct. 7:315–325.
2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Liu L, Fu Y, Zheng Y, Ma M and Wang C:
Curcumin inhibits proteasome activity in triple-negative breast
cancer cells through regulating p300/miR-142-3p/PSMB5 axis.
Phytomedicine. 78(153312)2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Nozari E, Moradi A and Samadi M: Effect of
atorvastatin, curcumin, and quercetin on miR-21 and miR-122 and
their correlation with TGFβ1 expression in experimental liver
fibrosis. Life Sci. 259(118293)2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sharifi-Rad J, Rayess YE, Rizk AA, Sadaka
C, Zgheib R, Zam W, Sestito S, Rapposelli S, Neffe-Skocińska K,
Zielińska D, et al: Turmeric and its major compound curcumin on
health: Bioactive effects and safety profiles for food,
pharmaceutical, biotechnological and medicinal applications. Front
Pharmacol. 11(01021)2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ashrafizadeh M, Rafiei H, Mohammadinejad
R, Afshar EG, Farkhondeh T and Samarghandian S: Potential
therapeutic effects of curcumin mediated by JAK/STAT signaling
pathway: A review. Phytother Res. 34:1745–1760. 2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wang N, Feng T, Liu X and Liu Q: Curcumin
inhibits migration and invasion of non-small cell lung cancer cells
through up-regulation of miR-206 and suppression of PI3K/AKT/mTOR
signaling pathway. Acta Pharm. 70:399–409. 2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Zhang L, Zhang Y, Zhu H, Sun X, Wang X, Wu
P and Xu X: Overexpression of miR-301a-3p promotes colorectal
cancer cell proliferation and metastasis by targeting deleted in
liver cancer-1 and runt-related transcription factor 3. J Cell
Biochem. 120:6078–6089. 2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Xia X, Zhang K, Luo G, Cen G, Cao J, Huang
K and Qiu Z: Downregulation of miR-301a-3p sensitizes pancreatic
cancer cells to gemcitabine treatment via PTEN. Am J Transl Res.
9:1886–1895. 2017.PubMed/NCBI
|
|
50
|
Zhou S, Zhang S, Shen H, Chen W, Xu H,
Chen X, Sun D, Zhong S, Zhao J and Tang J: Curcumin inhibits cancer
progression through regulating expression of microRNAs. Tumour
Biol. 39(1010428317691680)2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Spadotto V, Giambruno R, Massignani E,
Mihailovic M, Maniaci M, Patuzzo F, Ghini F, Nicassio F and Bonaldi
T: PRMT1-mediated methylation of the microprocessor-associated
proteins regulates microRNA biogenesis. Nucleic Acids Res.
48:96–115. 2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Saini S, Arora S, Majid S, Shahryari V,
Chen Y, Deng G, Yamamura S, Ueno K and Dahiya R: Curcumin modulates
microRNA-203-mediated regulation of the Src-Akt axis in bladder
cancer. Cancer Prev Res (Phila). 4:1698–1709. 2011.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Li Y, Sun W, Han N, Zou Y and Yin D:
Curcumin inhibits proliferation, migration, invasion and promotes
apoptosis of retinoblastoma cell lines through modulation of
miR-99a and JAK/STAT pathway. BMC Cancer. 18(1230)2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Kim MJ, Park KS, Kim KT and Gil EY: The
inhibitory effect of curcumin via fascin suppression through
JAK/STAT3 pathway on metastasis and recurrence of ovary cancer
cells. BMC Women's Health. 20(256)2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Wu Z, Liu H, Sun W, Du Y, He W, Guo S,
Chen L, Zhao Z, Wang P, Liang H and Deng J: RNF180 mediates STAT3
activity by regulating the expression of RhoC via the proteasomal
pathway in gastric cancer cells. Cell Death Dis.
11(881)2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Bahrami A, Majeed M and Sahebkar A:
Curcumin: A potent agent to reverse epithelial-to-mesenchymal
transition. Cell Oncol. 42:405–421. 2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zhang L, Cheng X, Gao Y, Zhang C, Bao J,
Guan H, Yu H, Lu R, Xu Q and Sun Y: Curcumin inhibits metastasis in
human papillary thyroid carcinoma BCPAP cells via down-regulation
of the TGF-β/Smad2/3 signaling pathway. Exp Cell Res. 341:157–165.
2016.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Tan C, Zhang L, Cheng X, Lin XF, Lu RR,
Bao JD and Yu HX: Curcumin inhibits hypoxia-induced migration in K1
papillary thyroid cancer cells. Exp Biol Med (Maywood).
240:925–935. 2015.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Xu X, Qin J and Liu W: Curcumin inhibits
the invasion of thyroid cancer cells via down-regulation of
PI3K/Akt signaling pathway. Gene. 546:226–232. 2014.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Wang H, Cai XL and Ma L: Curcumin modifies
epithelial-mesenchymal transition in colorectal cancer through
regulation of miR-200c/EPM5. Cancer Manag Res. 12:9405–9415.
2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Zhang T, Zhao L, Zhang T, Wu W, Liu J,
Wang X, Wan Y, Geng H, Sun X, Qian W and Yu D: Curcumin negatively
regulates cigarette smoke-induced renal cell carcinoma
epithelial-mesenchymal transition through the ERK5/AP-1 pathway.
Onco Targets Ther. 13:9689–9700. 2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Lu Y, Zhang R, Zhang XJ, Zhang B and Yao
Q: Curcumin may reverse 5-fluorouracil resistance on colonic cancer
cells by regulating TET1-NKD-Wnt signal pathway to inhibit the EMT
progress. Biomed Pharmacother. 129(110381)2020.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Huang Q, Zhong Y, Dong H, Zheng Q, Shi S,
Zhu K, Qu X, Hu W, Zhang X and Wang Y: Revisiting signal transducer
and activator of transcription 3 (STAT3) as an anticancer target
and its inhibitor discovery: Where are we and where should we go?
Eur J Med Chem. 187(111922)2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Sasidharan Nair V, Toor SM, Ali BR and
Elkord E: Dual inhibition of STAT1 and STAT3 activation
downregulates expression of PD-L1 in human breast cancer cells.
Expert Opin Ther Targets. 22:547–557. 2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Esposito T, Lucariello A, Hay E, Contieri
M, Tammaro P, Varriale B, Guerra G, De Luca A and Perna A: Effects
of curcumin and its adjuvant on TPC1 thyroid cell line. Chem Biol
Interact. 305:112–118. 2019.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Shiraiwa K, Matsuse M, Nakazawa Y, Ogi T,
Suzuki K, Saenko V, Xu S, Umezawa K, Yamashita S, Tsukamoto K and
Mitsutake N: JAK/STAT3 and NF-κB signaling pathways regulate cancer
stem-cell properties in anaplastic thyroid cancer cells. Thyroid.
29:674–682. 2019.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Wen J, Wang H, Dong T, Gan P, Fang H, Wu
S, Li J, Zhang Y, Du R and Zhu Q: STAT3-induced upregulation of
lncRNA ABHD11-AS1 promotes tumour progression in papillary thyroid
carcinoma by regulating miR-1301-3p/STAT3 axis and PI3K/AKT
signalling pathway. Cell Prolif. 52(e12569)2019.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Mancikova V, Montero-Conde C,
Perales-Paton J, Fernandez A, Santacana M, Jodkowska K,
Inglada-Pérez L, Castelblanco E, Borrego S, Encinas M, et al:
Multilayer OMIC data in medullary thyroid carcinoma identifies the
STAT3 pathway as a potential therapeutic target in
RETM918T tumors. Clin Cancer Res. 23:1334–1345.
2017.PubMed/NCBI View Article : Google Scholar
|