Introduction
Ovarian cancer (OV) is the fifth most common type of
cancer affecting women worldwide, particularly women aged >65
years (1,2). OV is a type of gynecological cancer
that is associated with a high morbidity and mortality, severely
compromising women's health and quality of life (3,4).
Radical surgery and chemotherapy are considered as two important
methods for treating OV, but these methods do not appear to reduce
the high recurrence rate of OV or improve the prognosis of the
patient with OV (5). The specific
pathogenesis behind OV has not been fully elucidated and is
incompletely understood. Moreover, patients with OV are commonly
diagnosed at an advanced stage, whereby they have missed the
opportunity for effective and timely treatment (6). Therefore, the present study aimed to
identify potential biomarkers to improve the detection of early
stage OV.
Long non-coding RNAs (lncRNAs) are ncRNAs >200
nucleotides in length (7-9),
which serve important roles in biological processes such as cell
differentiation, proliferation, migration and metastasis (10). HOXD antisense growth-associated long
non-coding RNA (HAGLR) opposite strand lncRNA (HAGLROS) has been
reported to sponge microRNA (miRNA/miR)-15 to accelerate the
development of lung cancer and its expression is associated with
tumor growth in patients with lung cancer (11). Moreover, HAGLROS has been found to
be highly expressed in hepatocellular carcinoma cells, where it
promotes the proliferation and reduces the apoptosis of
hepatocellular carcinoma cells through regulation of
miR-5095/autophagy related 12 signaling (12). Accumulating evidence has indicated
that HAGLROS is significantly upregulated in OV and that it is
closely associated with disease stage, tumor size and a poor
prognosis for patients with OV (13). However, the exact effects of HAGLROS
on the progression of OV remain to be elucidated.
miRNAs are RNAs without protein-coding abilities;
however, they have been demonstrated to be associated with the
proliferation, division, differentiation and apoptosis of various
types of cells (14,15). miRNAs are often associated with the
development of multiple diseases, including cancer (16). It has been reported that, compared
with normal tissues, miR-26b-5p is downregulated in human papillary
thyroid cancer tissues (17).
miR-26b-5p can inhibit the proliferation, invasion and migration of
papillary thyroid cancer cells (17). Emerging evidence has also indicated
that miR-26b-5p inhibits the development of multiple myeloma by
targeting the Hedgehog signaling pathway (18). Therefore, the present study examined
whether HAGLROS exerted any effects on the development of OV by
regulating miR-26b-5p.
The present study aimed to evaluate the specific
role of HAGLROS in OV and the underlying mechanism of action
through which HAGLROS exerts its effects on OV cells. These
findings may provide a novel insight into treatment strategies for
OV.
Materials and methods
Cell culture
The normal human ovarian epithelial cell line
(IOSE-80) and OV cell lines (ES-2, SKOV3, OVCAR3, HEY and A2780)
were purchased from the American Type Culture Collection (ATCC).
The information regarding the histotypes of the cell lines is
available from the ATCC (https://www.lgcstandards-atcc.org/). The ES-2 cell
line was established from a surgical OV tumor specimen. The SKOV3
and OVCAR3 cell lines were derived from a metastatic site: Ascites.
The HEY cell line was derived from a peritoneal sample from a
patient with moderately differentiated papillary cystadenocarcinoma
of the ovary. The A2780 cell line was established from an ovarian
endometroid adenocarcinoma tumor from an untreated patient. Cells
were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.) and incubated at 37˚C in an incubator containing 5%
CO2.
Cell transfection
SKOV3 cells (1x106 cells per well) were
plated into 6-well plates and transfection was performed when cells
reached the logarithmic growth phase, with 85% confluence. Two
short hairpin (sh)RNAs targeting HAGLROS (shRNA-HAGLROS-1 or
shRNA-HAGLROS-2; 1 µg), a scrambled shRNA [shRNA-negative control
(NC); 1 µg], miR-26b-5p inhibitor (5'-AAGUUCAUUAAGUCCUAUCCA-3'; 50
nM) and miR-26b-5p inhibitor NC (inhibitor-NC;
5'-CAGUACUUUUGUGUAGUACAA-3'; 50 nM), miR-26b-5p mimic
(5'-UUCAAGUAAUUCAGGAUAGGU-3'; 50 nM) and mimic-NC
(5'-UUGUACUACACAAAAGUACUG-3'; 50 nM) were designed and synthesized
by Shanghai GenePharma Co., Ltd. Transfection experiments were
performed using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) at 37˚C for 48 h, in accordance with the
manufacturer's guidelines. Cells were harvested at 48 h after
transfection and the transfection efficiency was determined using
reverse transcription-quantitative PCR (RT-qPCR).
Cell viability assay
A Cell Counting Kit-8 (CCK-8; Shanghai YiSheng
Biotechnology Co., Ltd., Shanghai, China) was performed for the
detection of cell viability. The transfected SKOV3 cells were
plated into 96-well plates (3,000 cells/well) and incubated for 0,
24, 48 and 72 h. Then, CCK-8 solution was added to each well and
incubated for another 4 h at 37˚C. The optical density was measured
at 450 nm using a microplate reader (Bio-Rad Laboratories,
Inc.).
Colony formation assay
SKOV3 cells were seeded into 6-well plates (500
cells per well) and incubated for 24 h to allow for adherence. The
medium was changed every 3 days. After 2 weeks, cells were fixed
with methanol for 30 min at room temperature and stained with 0.2%
crystal violet solution for 5 min at room temperature. Images were
captured using a light microscope (magnification, x10; Olympus
Corporation).
Immunofluorescence staining
The transfected SKOV3 cells were fixed with 4%
paraformaldehyde at room temperature for 20 min, followed by the
addition of 0.1% Triton X-100 solution at room temperature for 10
min. The cells were blocked with 3% BSA solution at 37˚C for 90
min. Subsequently, a primary antibody against Ki67 (cat. no.
11882S; 1:1,000; Cell Signaling Technology; Boston, MA, USA) was
incubated with the cells at 4˚C overnight, followed by incubation
with DyLight™ 488-conjugated secondary antibody (cat.
no. 35553; 1:2,000; Invitrogen; Thermo Fisher Scientific, Inc.) at
37˚C for 1.5 h in the dark. Subsequently, DAPI was used to stain
the cells for 5 min at room temperature. After mounting, the cells
were observed under a fluorescence microscope (magnification, x200;
Olympus Corporation).
TUNEL staining
The transfected SKOV3 cells were fixed with 4%
paraformaldehyde at 4˚C for 20 min. Cell apoptosis was examined
with a TUNEL Apoptosis Detection kit (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37˚C for 1 h, according to the manufacturer's
instructions. Subsequently, cells were incubated with
diaminobenzene for 10 min and counterstained with hematoxylin
(Beijing Solarbio Science & Technology Co., Ltd.) for 30 sec at
room temperature. The apoptosis of TUNEL-positive cells was
observed under an optical microscope (magnification, x200; Olympus
Corporation) and cells were counted in five randomly selected
microscopic fields.
RT-qPCR assay
Total RNA was extracted from cells utilizing
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). cDNA was then synthesized using a SuperScript IV
First-Strand Synthesis system (Invitrogen; Thermo Fisher
Scientific, Inc.) in accordance with the manufacturer's protocol.
Subsequently, using cDNA as the template, the gene expression
levels were analyzed using qPCR, which was conducted using iTaq™
Universal One-Step iTaq™ Universal SYBR®
Green Supermix (Bio-Rad Laboratories, Inc.) on an ABI 7500
instrument (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The following thermocycling conditions were used: Initial
denaturation at 95˚C for 10 min; followed by 40 cycles of
denaturation at 95˚C for 15 sec and annealing at 60˚C for 1 min;
and a final extension of 10 min at 72˚C. Primers used in this study
were designed and synthesized by Shenggong Biological Engineering
(Shanghai) Co., Ltd. The following primers pairs were used: HAGLROS
forward, 5'-TCTACACCCAGAGAGGGACG-3' and reverse,
5'-GCCTACTTCCTCCCACACAA-3'; GAPDH forward,
5'-ACAACTTTGGTATCGTGGAAGG-3' and reverse,
5'-GCCATCACGCCACAGTTTC-3'; miR-26b-5p forward,
5'-TTCAAGTAATTCAGGATAGGT-3' and reverse, 5'-GTGCGTGTCGTGGAGTC-3';
U6 forward, 5'-GGAACGATACAGAGAAGATTAGC-3' and reverse,
5'-TGGAACGCTTCACGAATTTGCG-3'. GAPDH or U6 were used as internal
controls for normalization. The 2-ΔΔCq method was used
to calculate relative RNA expression (19).
Western blot analysis
Cellular proteins were isolated from cells using
RIPA buffer (Beyotime Institute of Biotechnology). The protein
quantification was conducted using a BCA kit. Subsequently, equal
amounts of proteins (40 µg/lane) were separated on 10% SDS-PAGE
gels and transferred to PVDF membranes (EMD Millipore), which were
then blocked with 5% skimmed milk for 2 h at 37˚C. Primary
antibodies against Bcl-2 (1:1,000; cat. no. 15017; Cell Signaling
Technology, Inc.), Bax (1:1,000; cat. no. 14796; Cell Signaling
Technology, Inc.), cleaved-caspase3 (1:1,000; cat. no. 9664; Cell
Signaling Technology, Inc.) and GAPDH (1:1,000; cat. no. 5174; Cell
Signaling Technology, Inc.) were incubated with the membranes at
room temperature overnight. Subsequently, the membranes were
incubated with the horseradish peroxidase-conjugated goat
anti-rabbit secondary antibody (1:5,000; cat. no. abs20002; Absin
Bioscience, Inc.) at 37˚C for 1 h. The bands were detected using an
ECL reagent (EMD Millipore). The grayscale values of the membranes
were semi-quantified using ImageJ software (version 1.52r; National
Institutes of Health). GAPDH was used as an internal control.
Luciferase reporter assay
The target miRNA of HAGLROS was predicted using the
DIANA database (http://carolina.imis.athena-innovation.gr/diana_tools/web/index.php?r=lncbasev2%2Findex),
a database that predicts the binding of lncRNAs and miRNAs
(20). A dual luciferase reporter
assay system (Promega Corporation) was used to verify the
interaction between the HAGLROS and miR-26b-5p. The full-length of
the wild-type (WT) HAGLROS 3'-untranslated region (3'-UTR)
(HAGLROS-WT) (Shanghai GenePharma Co., Ltd.) with the putative
binding sites of miR-26b-5p was inserted into firefly luciferase
reporter and then transfected into SKOV3 cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Constructs containing the mutated putative
binding sequence of miR-26b-5p located within the 3'-UTR of HAGLROS
(HAGLROS-MUT) were considered as mutated control and mutated
controls were also cloned into SKOV3 cells. After incubation for 48
h at 37˚C, firefly and Renilla luciferase activities were
assessed.
Statistical analysis
GraphPad Prism version 8.0 (GraphPad Software, Inc.)
was used to analyze the experimental data. All experiments were
performed three times and the data were presented as mean ± SD.
Comparisons between two groups were conducted using unpaired
student's t-tests and comparisons between multiple groups were
performed using one-way ANOVAs with Tukey's post hoc tests.
P<0.05 was considered to show statistically significant.
Results
Interference of lncRNA HAGLROS
inhibits the proliferation of OV cells
The expression levels of HAGLROS were detected using
RT-qPCR in normal human ovarian epithelial cells (IOSE-80) and OV
cell lines (ES-2, SKOV3, OVCAR3, HEY and A2780). As presented in
Fig. 1A, the expression levels of
HAGLROS were notably upregulated in OV cells, compared with IOSE-80
cells. The highest expression levels of HAGLROS were found in SKOV3
cells, thus SKOV3 cells were selected for further experiments. The
aforementioned results also indicated that HAGLROS may act as a
tumor suppressor in OV. Therefore, HAGLROS was knocked down through
transfections with shRNA-HAGLROS-1 and shRNA-HAGLROS-2. HAGLROS
expression was significantly decreased following transfection and
shRNA-HAGLROS-2, which displayed a lower expression of HAGLROS, was
selected for the following experiments (Fig. 1B). Subsequently, it was observed
that the cell viability and colony formation abilities were
markedly suppressed by the knockdown of HAGLROS compared with the
shRNA-NC group in SKOV3 cells (Fig.
1C and D). Consistently, the
expression levels of Ki67, a proliferation-related protein, was
decreased in the HAGLROS-knockdown group, compared with those in
the shRNA-NC group (Fig. 1E).
Overall, these data provided evidence that knockdown of HAGLROS
inhibited the proliferation of OV cells.
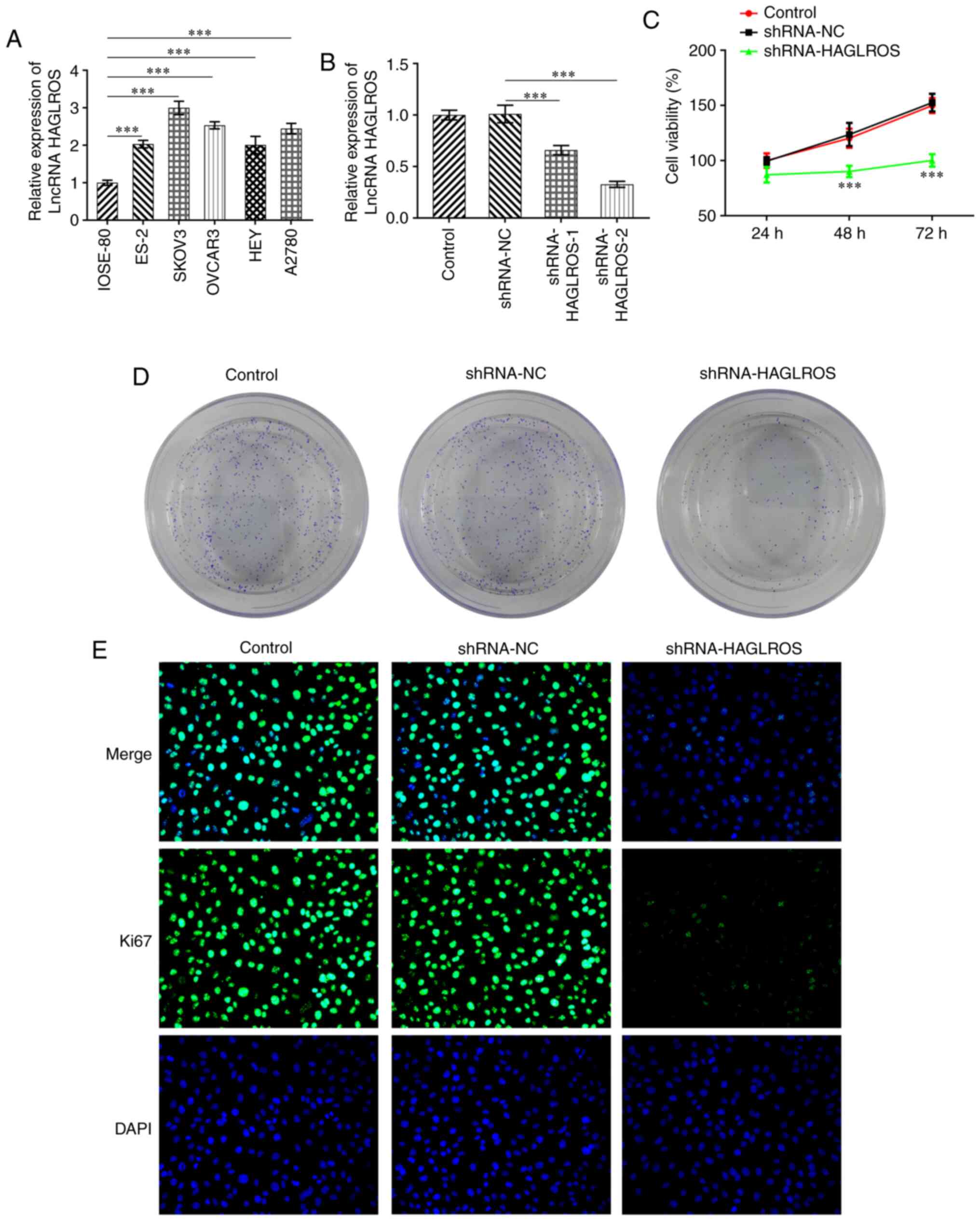 | Figure 1Interference of HAGLROS inhibits the
proliferation of OV cells. (A) RT-qPCR was used to detect the
expression levels of HAGLROS in several OV cell lines (ES-2, SKOV3,
OVCAR3, HEY and A2780) and the normal human ovarian epithelial cell
line IOSE-80. ***P<0.001. (B) RT-qPCR was used to
evaluate the transfection efficiency of HAGLROS knockdown plasmids.
***P<0.001. (C) Cell viability was examined using a
Cell Counting Kit-8 assay. ***P<0.001 vs. shRNA-NC.
(D) Cell proliferation was assessed using a colony formation assay.
(E) Ki67 expression was determined using immunofluorescence
staining (magnification, x200). HAGLROS, HOXD antisense
growth-associated long non-coding RNA opposite strand lncRNA;
lncRNA, long non-coding RNA; NC, negative control; OV, ovarian
cancer; RT-qPCR, reverse transcription-quantitative PCR; shRNA,
short hairpin RNA. |
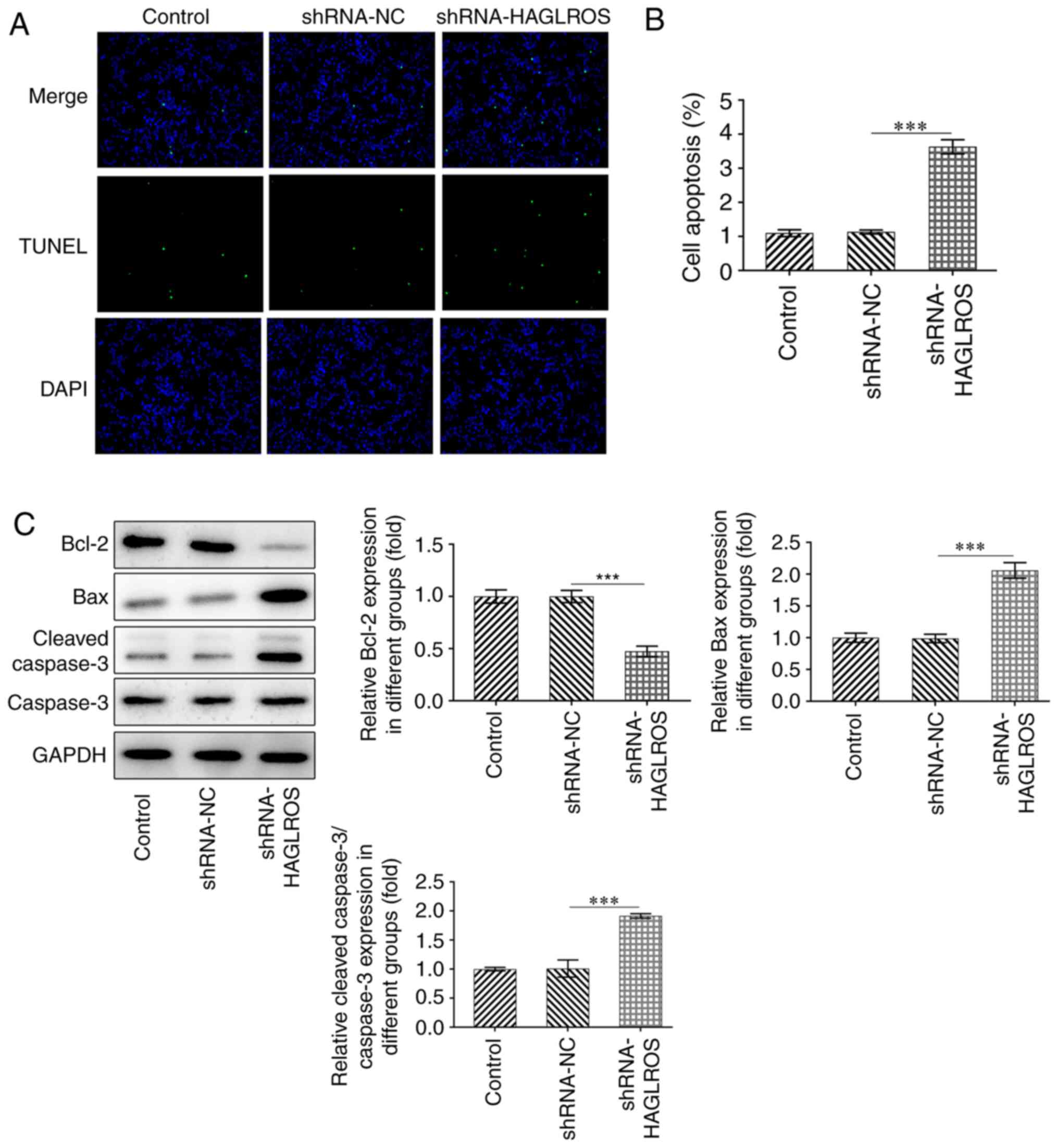
Interference of HAGLROS promotes the
apoptosis of OV cells
The apoptosis of OV cells was detected using a TUNEL
assay to confirm whether knockdown of HAGLROS could affect the
apoptosis of OV cells. The TUNEL assay results demonstrated that
the apoptosis of OV cells in the shRNA-HAGLROS group was notably
increased compared with OV cells in the shRNA-NC group (Fig. 2A and B). Furthermore, the expression levels of
the anti-apoptotic protein Bcl-2 were downregulated, while those of
the pro-apoptotic proteins Bax and cleaved-caspase3 were
upregulated after HAGLROS knockdown, compared with the shRNA-NC
group (Fig. 2C). These results
suggested that interference of HAGLROS promoted the apoptosis of OV
cells.
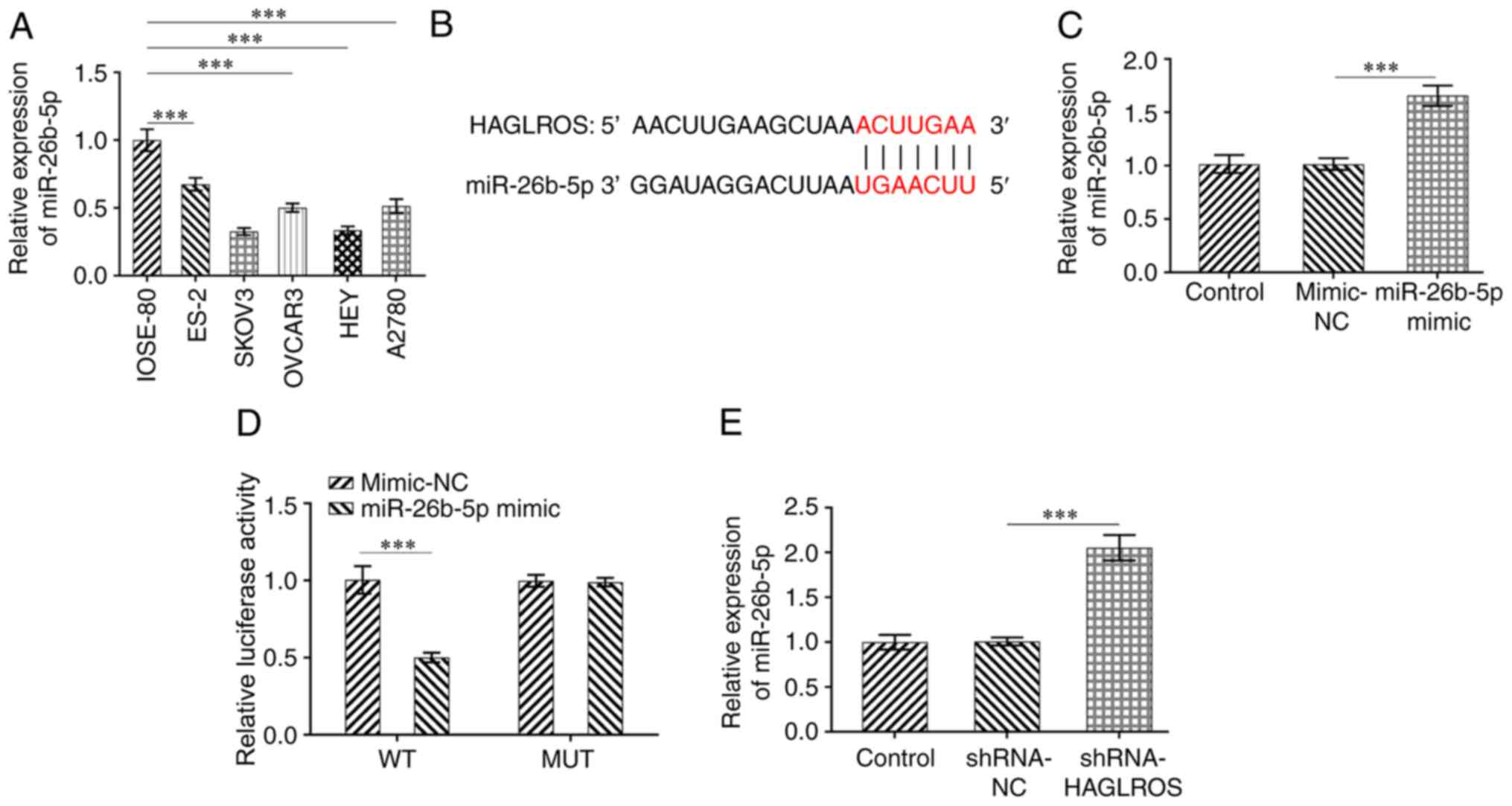
HAGLROS is directly targeted by
miR-26b-5p
The DIANA database (http://carolina.imis.athenainnovation.gr/diana_tools/web/index.php?r=lncbasev2%2Findex)
predicted that HAGLROS could target miR-26b-5p. The expression of
miR-26b-5p was determined using RT-qPCR in a normal human ovarian
epithelial cell line and OV cell lines. It was found that
miR-26b-5p was downregulated in OV cell lines compared with normal
human ovarian epithelial cells (Fig.
3A). The binding sites between HAGLROS and miR-26b-5p are
presented in Fig. 3B. Significantly
upregulated miR-26b-5p expression levels were observed after
transfection with miR-26b-5p mimic as compared to the mimic-NC
group (Fig. 3C). Dual luciferase
reporter assay results suggested that the luciferase activity in
the miR-26b-5p mimic + HAGLROS-WT group was weaker compared with
other groups, indicating the regulatory relationship between
HAGLROS and miR-26b-5p (Fig. 3D).
Additionally, HAGLROS-knockdown markedly increased the expression
levels of miR-26b-5p in SKOV3 cells as compared with the shRNA-NC
group (Fig. 3E). Thus, these
results suggested that HAGLROS may bind directly to miR-26b-5p.
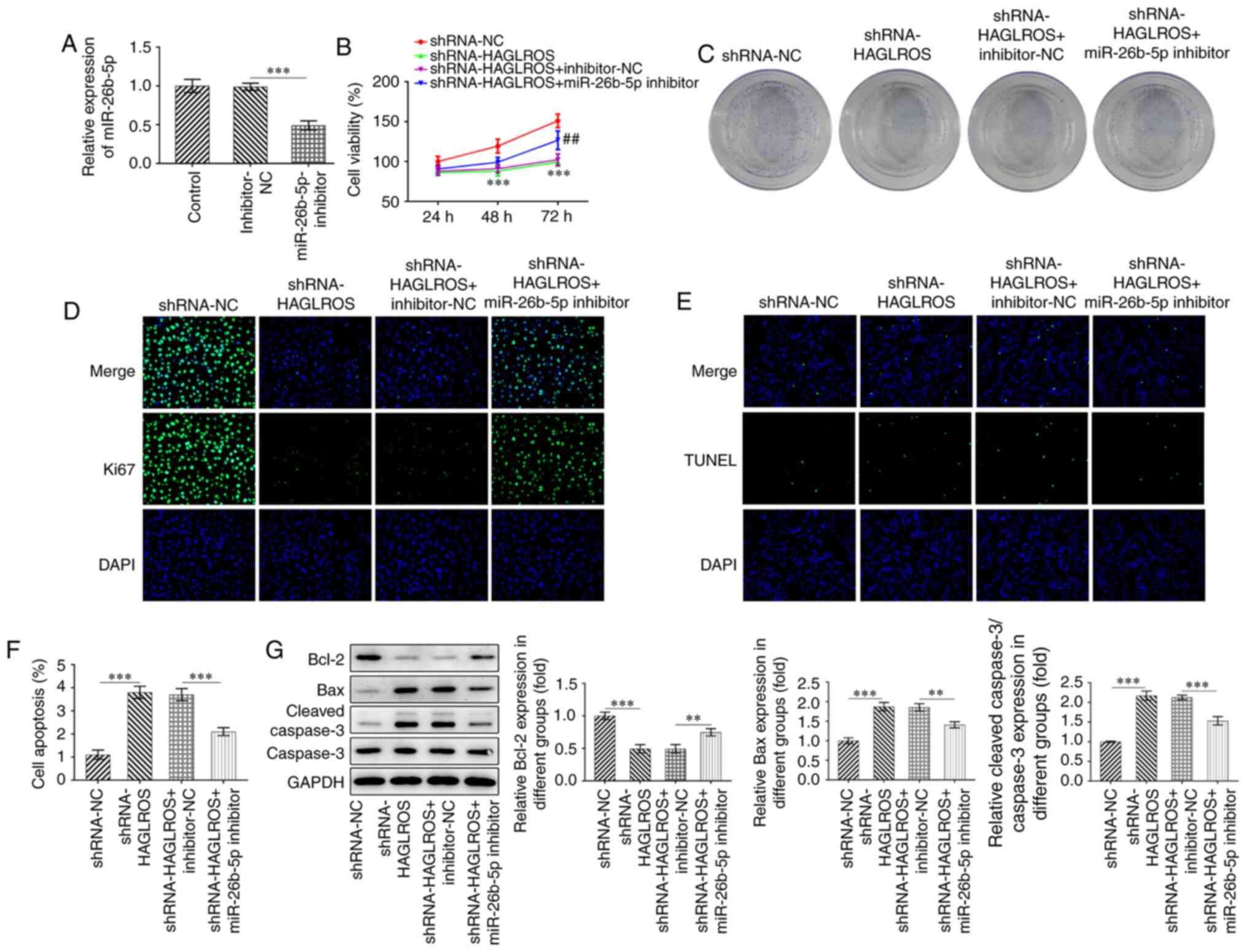
Interference of HAGLROS suppresses the
proliferation and promotes the apoptosis of OV cells by regulating
miR-26b-5p
As the regulatory relationship between HAGLROS and
miR-26b-5p was identified, it was subsequently investigated whether
interference of HAGLROS could inhibit the proliferation and promote
the apoptosis of OV cells by targeting miR-26b-5p. First, the
miR-26b-5p inhibitor was used to decrease the expression levels of
miR-26b-5p (Fig. 4A). The
proliferative (Fig. 4B) and colony
formation (Fig. 4C) abilities of
SKOV3 cells transfected with shRNA-HAGLROS were found to be
markedly decreased, which was reversed by the miR-26b-5p inhibitor.
Consistently, the expression of Ki67 was notably downregulated in
the shRNA-HAGLROS compared with the shRNA-NC group, whereas
miR-26b-5p-knockdown attenuated this effect (Fig. 4D). Additionally, the TUNEL staining
(Fig. 4E and F) results indicated that the impact of
HAGLROS knockdown on the apoptosis of SKOV3 cells was partially
counteracted by the miR-26b-5p inhibitor. Meanwhile,
co-transfection with shRNA-HAGLROS and miR-26b-5p inhibitor led to
a significant upregulation of Bcl-2 protein expression levels, as
well as a downregulation of Bax and cleaved caspase-3 protein
expression levels (Fig. 4G).
Collectively, these findings demonstrate that interference of
HAGLROS inhibits the proliferation and promotes the apoptosis of
SKOV3 cells by regulating miR-26b-5p.
Discussion
Accumulating evidence has indicated that lncRNAs are
involved in tumorigenesis and have the potential to be used as
diagnostic and prognostic biomarkers in a wide range of cancer
types (21,22). The major finding of the present
study was that HAGLROS was notably upregulated in OV tissues and
cells, and that HAGLROS-knockdown inhibited the proliferation and
promoted the apoptosis of OV cells by sponging miR-26b-5p.
lncRNAs, which can serve as critical tumor promoters
or suppressors, can affect the occurrence and progression of
multiple types of diseases (23,24).
HAGLROS is abnormally expressed in the serum of patients with
pneumonia and it has been found to decrease apoptosis and autophagy
in lipopolysaccharide-induced WI-38 cells (25). Previous studies have also reported
the importance of HAGLROS in the development of various types of
cancer (11,26). For instance, HAGLROS, which was
found to be upregulated in gastric cancer, is an indicator for the
overall survival of patients with gastric cancer (27). High expression levels of HAGLROS has
also been closely associated with the short overall survival time
of patients with non-small cell lung cancer (28). Moreover, HAGLROS has been found to
be elevated in patients with OV at advanced stages and its
expression is considered to be associated with a poor prognosis for
patients with OV (13). Therefore,
HAGLROS is of great clinical significance for the diagnosis and
treatment of OV. In the present study, HAGLROS was highly expressed
in OV cells, which was in line with the findings of the previous
study (13). Furthermore, OV cells
had presented with reduced proliferation and enhanced apoptosis
after knockdown of HAGLROS.
Mechanically, it is known that lncRNAs function as
competing endogenous RNAs to regulate tumor progression by sponging
specific miRNAs (29,30). miRNAs are important in gene
regulation and cellular processes in various types of diseases
(31). In the present study, a
binding site for miR-26b-5p was identified in the sequence of
HAGLROS and the dual-luciferase reporter assay confirmed that
HAGLROS could directly target miR-26b-5p.
miR-26 consists of four highly conserved small
non-coding RNAs that share similarity in structures and sequences
(32). miR-26b, a member of the
miR-26 family, has been considered as an effective marker in the
diagnosis and treatment of breast cancer, gastric cancer and OV
(33,34). With regards to the functions of
miR-26b-5p in cancer, accumulating evidence has suggested that
miR-26b-5p may inhibit cancer progression in prostate cancer,
thyroid cancer and head and neck squamous cell carcinoma (35,36).
Moreover, a previous study revealed that the expression of
miR-26b-5p was reduced in bladder cancer tissues and cells compared
with that in adjacent bladder tissues, and that miR-26b-5p acted as
a tumor suppressor in bladder cancer (31). Additionally, miR-26b-5p has been
reported to play a protective effect on cis-diamine
dichloroplatinum-induced ovarian granulosa cells through targeting
mitogen-activated protein kinase kinase kinase 9(37). In the present study, a series of
functional experiments indicated a regulatory association between
HAGLROS and miR-26b-5p. The results suggested that HAGLROS was
inversely associated with the expression of miR-26b-5p. It was also
identified that the miR-26b-5p inhibitor abolished the
anti-proliferative and pro-apoptotic effects of HAGLROS in OV
cells.
In conclusion, to the best of our knowledge, the
present results provided the first evidence that HAGLROS is notably
upregulated in OV cells and that interference of HAGLROS inhibited
the proliferation and promoted the apoptosis of OV cells through
regulating miR-26b-5p. As such, these findings may provide
researchers with valuable additional insights into an in-depth
understanding of the underlying mechanisms of action for the
HAGLROS/miR-26b-5p axis in OV and indicate the potential value of
HAGLROS as a promising biomarker for the diagnosis and treatment of
OV. The specific signaling pathway or specific signaling proteins
regulated by HAGLROS and miR-26b-5p, the expression levels of
HAGLROS in the different histotypes of OV and different OV cell
lines should be investigated in further experiments, the lack of
which are limitations of the present study.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ and MM searched the literature, designed the
experiments and performed the experiments. LZ analyzed, interpreted
the data and wrote the manuscript. MM revised the manuscript. LZ
and MM confirmed the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sullivan R: Cancer research in the UK: A
policy review of the junior academic clinical faculty. Mol Oncol.
1:366–373. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rooth C: Ovarian cancer: Risk factors,
treatment and management. Br J Nurs. 22 (Suppl 17):S23–S30.
2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lin X, Feng D, Li P and Lv Y: LncRNA
LINC00857 regulates the progression and glycolysis in ovarian
cancer by modulating the Hippo signaling pathway. Cancer Med.
9:8122–8132. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jelovac D and Armstrong DK: Recent
progress in the diagnosis and treatment of ovarian cancer. CA
Cancer J Clin. 61:183–203. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lou Y, Jiang H, Cui Z, Wang X, Wang L and
Han Y: Gene microarray analysis of lncRNA and mRNA expression
profiles in patients with highgrade ovarian serous cancer. Int J
Mol Med. 42:91–104. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang M, Song Y and Yu L: LncRNA PTCSC3
suppressed cervical carcinoma cell invasion and proliferation via
regulating miR-574-5p. Am J Transl Res. 11:7186–7194.
2019.PubMed/NCBI
|
|
8
|
Yuan L, Ma T, Liu W, Chen Y, Yuan Q, Ye M,
Yu L, Li J, Niu Y and Nan Y: LINC00994 promoted invasion and
proliferation of gastric cancer cell via regulating miR-765-3p. Am
J Transl Res. 11:6641–6649. 2019.PubMed/NCBI
|
|
9
|
Yin X, Zhang J, Li C, Zhang Z, Jin T, Song
L, Zhang R, Wang W, Tao Y and Wang X: LncRNA HOXA11-AS
accumulation-induced microRNA-761 downregulation regulates cell
growth by targeting TRIM29 in papillary thyroid cancer. Am J Transl
Res. 11:6826–6837. 2019.PubMed/NCBI
|
|
10
|
Lei X, Yang S, Yang Y, Zhang J, Wang Y and
Cao M: Long noncoding RNA DLX6-AS1 targets miR-124-3p/CDK4 to
accelerate Ewing's sarcoma. Am J Transl Res. 11:6569–6576.
2019.PubMed/NCBI
|
|
11
|
Wang WL, Yu DJ and Zhong M: LncRNA HAGLROS
accelerates the progression of lung carcinoma via sponging
microRNA-152. Eur Rev Med Pharmacol Sci. 23:6531–6538.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wei H, Hu J, Pu J, Tang Q, Li W, Ma R, Xu
Z, Tan C, Yao T, Wu X, et al: Long noncoding RNA HAGLROS promotes
cell proliferation, inhibits apoptosis and enhances autophagy via
regulating miR-5095/ATG12 axis in hepatocellular carcinoma cells.
Int Immunopharmacol. 73:72–80. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yang M, Zhai Z, Zhang Y and Wang Y:
Clinical significance and oncogene function of long noncoding RNA
HAGLROS overexpression in ovarian cancer. Arch Gynecol Obstet.
300:703–710. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Han G, Qiu N, Luo K, Liang H and Li H:
Downregulation of miroRNA-141 mediates acquired resistance to
trastuzumab and is associated with poor outcome in breast cancer by
upregulating the expression of ERBB4. J Cell Biochem: Feb 11, 2019
(Epub ahead of print).
|
|
15
|
Yu FQ, Wang Z, Wang XW, Wang SL, Li XD,
Huang QS and Lin JH: MicroRNA-885-5p promotes osteosarcoma
proliferation and migration by downregulation of cell division
cycle protein 73 homolog expression. Oncol Lett. 17:1565–1572.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dejene SB, Ohman AW, Du W, Randhawa D,
Bradley A, Yadav N, Elias KM, Dinulescu DM and Setlur SR: Defining
fallopian tube-derived miRNA cancer signatures. Cancer Med.
8:6709–6716. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhou A, Pan H, Sun D, Xu H, Zhang C, Chen
X, Li L and Wang T: miR-26b-5p inhibits the proliferation,
migration and invasion of human papillary thyroid cancer in a
β-catenin-dependent manner. Onco Targets Ther. 13:1593–1603.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jia CM, Tian YY, Quan LN, Jiang L and Liu
AC: miR-26b-5p suppresses proliferation and promotes apoptosis in
multiple myeloma cells by targeting JAG1. Pathol Res Pract.
214:1388–1394. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Paraskevopoulou MD, Vlachos IS, Karagkouni
D, Georgakilas G, Kanellos I, Vergoulis T, Zagganas K, Tsanakas P,
Floros E, Dalamagas T and Hatzigeorgiou AG: DIANA-LncBase v2:
Indexing microRNA targets on non-coding transcripts. Nucleic Acids
Res. 44:D231–D238. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xu HY, Wang LY and Jiang XL: Silencing of
lncRNA DLEU1 inhibits tumorigenesis of ovarian cancer via
regulating miR-429/TFAP2A axis. Mol Cell Biochem. 476:1051–1061.
2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Du LJ, Mao LJ and Jing RJ: Long noncoding
RNA DNAH17-AS1 promotes tumorigenesis and metastasis of non-small
cell lung cancer via regulating miR-877-5p/CCNA2 pathway. Biochem
Biophys Res Commun. 533:565–572. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang XJ, Qi GT, Zhang XM, Wang L and Li
FF: lncRNA RHPN1-AS1 promotes the progression of endometrial cancer
through the activation of ERK/MAPK pathway. J Obstet Gynaecol Res.
47:533–543. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yao HP, Chen R, Yang YX and Jiang J:
LncRNA BBOX1-AS1 aggravates the development of ovarian cancer by
sequestering miR-361-3p to augment PODXL expression. Reprod Sci.
28:736–744. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu MH, Han T, Shi SM and Chen EQ: Long
noncoding RNA HAGLROS regulates cell apoptosis and autophagy in
lipopolysaccharides-induced WI-38 cells via modulating miR-100/
NF-kappa B axis. Biochem Biophys Res Commun. 500:589–596.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhou KF, Xu J, Yin XF and Xia JN: Long
noncoding RNA HAGLROS promotes cell invasion and metastasis by
sponging miR-152 and upregulating ROCK1 expression in osteosarcoma.
Comput Math Method Med. 2020(7236245)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen JF, Wu P, Xia R, Yang J, Huo XY, Gu
DY, Tang CJ, De W and Yang F: STAT3-induced lncRNA HAGLROS
overexpression contributes to the malignant progression of gastric
cancer cells via mTOR signal-mediated inhibition of autophagy. Mol
Cancer. 17(6)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chen Y, Shen T, Ding X, Cheng L, Sheng L
and Du X: HAGLROS is overexpressed and promotes non-small cell lung
cancer migration and invasion. Jpn J Clin Oncol. 50:1058–1067.
2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liu XH, Sun M, Nie FQ, Ge YB, Zhang EB,
Yin DD, Kong R, Xia R, Lu KH, Li JH, et al: Lnc RNA HOTAIR
functions as a competing endogenous RNA to regulate HER2 expression
by sponging miR-331-3p in gastric cancer. Mol Cancer.
13(92)2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chen P, Zhao X, Wang H, Zheng M, Wang Q
and Chang W: The down-regulation of lncRNA PCAT18 promotes the
progression of gastric cancer via MiR-107/PTEN/PI3K/AKT signaling
pathway. Onco Targets Ther. 12:11017–11031. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wu K, Mu XY, Jiang JT, Tan MY, Wang RJ,
Zhou WJ, Wang X, He YY, Li MQ and Liu ZH: miRNA26a5p and miR26b5p
inhibit the proliferation of bladder cancer cells by regulating
PDCD10. Oncol Rep. 40:3523–3532. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lu J, Zhang W, Ding Y, Li X and Song J:
Expression of miR-26b in ovarian carcinoma tissues and its
correlation with clinicopathology. Oncol Lett. 17:4417–4422.
2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li D, Wei Y, Wang D, Gao H and Liu K:
MicroRNA-26b suppresses the metastasis of non-small cell lung
cancer by targeting MIEN1 via NF-kappaB/MMP-9/VEGF pathways.
Biochem Biophys Res Commun. 472:465–470. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu J, Tu F, Yao W, Li X, Xie Z, Liu H, Li
Q and Pan Z: Conserved miR-26b enhances ovarian granulosa cell
apoptosis through HAS2-HA-CD44-Caspase-3 pathway by targeting HAS2.
Sci Rep. 6(21197)2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kato M, Goto Y, Matsushita R, Kurozumi A,
Fukumoto I, Nishikawa R, Sakamoto S, Enokida H, Nakagawa M,
Ichikawa T and Seki N: MicroRNA-26a/b directly regulate La-related
protein 1 and inhibit cancer cell invasion in prostate cancer. Int
J Oncol. 47:710–718. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Fukumoto I, Kikkawa N, Matsushita R, Kato
M, Kurozumi A, Nishikawa R, Goto Y, Koshizuka K, Hanazawa T,
Enokida H, et al: Tumor-suppressive microRNAs (miR-26a/b,
miR-29a/b/c and miR-218) concertedly suppressed
metastasis-promoting LOXL2 in head and neck squamous cell
carcinoma. J Hum Genet. 61:109–118. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liu SN, Li L, Li MH and Zhang JJ: Effect
of miR-26b-5p on cis-diamine dichloroplatinum-induced ovarian
granulosa cell injury by targeting MAP3K9. In Vitro Cell Dev Biol
Anim. 56:213–221. 2020.PubMed/NCBI View Article : Google Scholar
|