Introduction
Lung cancer (both small cell and non-small cell lung
cancer) is the second most common type of cancer diagnosed in both
men and women (1). In 2019, ~13% of
all new cases of cancer were lung cancer, including >228,000 new
cases of lung cancer in the United States (1). Lung cancer led to >142,000 deaths
each year, making it, by far, the leading cause of
cancer-associated death amongst both men and women; accounting for
more deaths than colon, breast and prostate cancer-associated
deaths combined (1). Non-small cell
lung cancer (NSCLC) accounts for ~85% of all lung cancer cases
(1). In clinical treatments, NSCLCs
are relatively insensitive to chemotherapy, compared with small
cell carcinoma. Therefore, improving the efficiency of chemotherapy
against NSCLCs is of utmost importance for clinical lung cancer
treatments.
In previous studies, the clinical value of
traditional Chinese medicines have been assessed, due to the lower
incidence of side effects (2,3).
Increasing attention has been given to a fungal polysaccharide,
lentinan (LNT), due to its strong antitumor activity (4,5). It
was reported to effectively inhibit proliferation, differentiation,
growth and senescence of cells (6).
LNT has also been reported to effectively prevent development of
cancers caused by chemical or viral carcinogens (4,5).
Clinically, LNT enhanced the effects chemotherapy and improved the
survival of patients with several types of cancer, including
gastric, colon, breast and lung cancer (7). Current evidence has also shown LNT can
target small-cell lung cancer cells (7). Although it has a relatively weak
effect on cancer, the results of the present study have
demonstrated the effects of LNT, indicating its potential to act as
an adjuvant for use alongside chemotherapy.
Previously, a compound obtained from Rabdosia
rubescens, called oridonin, has been shown to possess potential
as an anticancer treatment. Studies have shown that oridonin can
suppress the growth of breast (8)
and pancreatic cancer (9). It was
also found to inhibit gene mutations induced by chemical
carcinogens (10) and may exert its
effects by blocking sodium pumps in cancer cells, decreasing
nutrient uptake (11), as well as
through regulation of the apoptotic pathways via modulation of
caspase activity/expression (12).
A previous study showed that oridonin enhances the anticancer
effects of LNT in SMMC-7721 human hepatoma cells (13) and HepG2 human hepatoblastoma cells
(14). However, its effect on lung
cancer have not been studied previously, to the best of our
knowledge.
Several traditional Chinese medicines have been
studied extensively (6,7,15).
Clinical findings from our hospital showed that the use of oridonin
enhanced the beneficial effects of LNT. In the present study, the
human fetal lung fibroblast cell line MRC-5, lung cancer cell line
A549, and Lewis lung carcinoma mouse model were used to evaluate
and validate the adjuvant effects of oridonin on the therapeutic
effects of LNT in lung cancer.
Materials and methods
Cell lines and cell culture
The Human fetal lung fibroblast cell line MRC-5, the
NSCLC cell line A549, and the Lewis lung carcinoma cells were
obtained from the Conservation Genetics CAS Kunming Cell Bank.
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
was used to culture the cells. The cells were cultivated in culture
flasks in a humidified incubator with 5% CO2 at 37˚C. In
the experiments, the cells were exposed to 0-20 µg/ml oridonin,
0-300 µg/ml LNT or a combination of both for 24 h.
MTT assay
The MTT assays were performed as described
previously (16). A549 cells
(4x104 cells/well) were seeded into 96-well plates in
100 µl supplemented media per a well the night before treatments.
Different concentration of LNT (Shanghai Yuanye Biological
Technology Co., Ltd.) and oridonin (Shanghai Yuanye Biological
Technology Co., Ltd.) were added to the plates and cultured for 72
h. Subsequently, the culture medium was discarded, and 100 µl fresh
culture media containing 0.5 mg/ml MTT was added (Nanjing Aoduofuni
Biology Technology, Co. Ltd.), and the plates were further
incubated for 4 h. The solutions were discarded and 100 µl DMSO was
added to dissolve the crystals, Finally, the optical density of
each well was measured at a wavelength of 540 nm using a microplate
reader (Imar; Bio-Rad Laboratories, Inc.) and the ratio of
suppression of viability induced by the different treatments was
calculated. Based on preliminary results, 300 µg/ml LNT was used
for the subsequent experiments as the high concentration group
(LNT-H), as it was the concentration that had the most potent
effect on the viability of cancer cells, whilst having little
effect on the viability of normal lung cells. As a comparison, a
low concentration group (LNT-L) was also included, in which cells
were treated with 100 µg/ml LNT.
Reverse transcription-quantitative
(RT-q)PCR
RT-qPCR was performed as described previously
(17). Total RNA was extracted from
A549 cells or lung tissues using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). A Takara Reverse
Transcription system was used to synthesize target cDNAs, and RT
was performed according to the manufacturer's protocol (Takara Bio,
Inc.). Primers were purchased from Beijing Genomics Institute. The
following primers were used for the RT-qPCR: GAPDH forward,
5'-GTCTCCTCTGACTTCAACAGCG and reverse, 5'-ACCACCCTGTTGCTGTAGCCAA;
caspase-3 forward, 5'-AGAGGGGATCGTTGTAGAAG and reverse,
5'-GTTGCCACCTTTCGGTTAAC; caspase-8 forward, 5'-GCATTAGGGACAGGAATGGA
and reverse, 5'-CCCCTGACAAGCCTGAATAA; caspase-9 forward,
5'-AGCCAGATGCTGTCCCATAC and reverse, 5'-CAGGAACCGCTCTTCTTGTC; Bax
forward, 5'-ACCAAGAAGCTGAGCGAGTGTC and reverse,
5'-TGTCCAGCCCATGATGGTTC; Bcl-2 forward, 5'-CTACGAGTGGGATGCGGGAGATG
and reverse, 5'-GGTTCAGGTACTCAGTCATCCACAG; Bcl-xL forward,
5'-GGATGGCCACTTACCTGA and reverse, 5'-CGGTTGAAGCGTTCCTG; p53
forward, 5'-TTGCTTTATCTGTTCACTTGTG and reverse,
5'-TCCTTCCACTCGGATAAG; p21 forward, 5'-GTGAGCGATGGAACTTCGACT and
reverse, 5'-CGAGGCACAAGGGTACAAGAC; NF-κB forward, 5'
TGTAAAACGACGGCCAGT and reverse, 5'CAGGAAACAGCTATGACC; and inhibitor
of NF-κB-α (IκB-α) forward, 5'-GGCTGAAAGAACATGGACTTG and reverse,
5'-GTACACCATTTACAGGAGGG-3' (Tiangen Biotech Co. Ltd.) A Takara one
step RT-PCR kit was adopted for PCR (Takara Bio, Inc.). The
reaction system included 0.8 µl cDNA template, 5 µl SYBR Premix
ExTaq II (2X) (Takara Bio, Inc.), 0.4 µl each forward and reverse
primers (10 µmol/l), and 3.4 µl dH2O. The thermocycling
conditions included pre-heating for 30 sec at 95˚C; followed by 39
cycles of degeneration at 95˚C for 5 sec, annealing at 50˚C for 30
sec and extension at 68˚C for 45 sec with a final extension step of
5 min at 65˚C.
Western blotting
Western blotting was performed as described
previously (18). Total protein was
extracted from A549 cells or lung tissues using RIPA Buffer
(Invitrogen; Thermo Fisher Scientific, Inc.). The concentration of
proteins was determined using a BCA assay. A total of 30 µg of each
protein was loaded per lane on a 12% SDS-gel (Nanjing KeyGen
Biotech Co., Ltd.), resolved using SDS-PAGE and transferred to a
PVDF membrane. The membrane was blocked in 5% milk in TBS with 0.1%
Tween-20 at room temperature for 1 h. The membrane was subsequently
incubated with the primary antibodies at 4˚C overnight, followed by
incubation with the secondary antibodies: Goat anti-rabbit IgG
H&L (HRP; cat. no. ab6721; Abcam; 1:5,000) or goat anti-mouse
IgG H&L (HRP; cat. no. ab6789; Abcam; 1:5,000) at room
temperature for 1 h. Signals were visualized using ECL; β-actin was
used as the loading control. Antibodies included: Anti-caspase-3
antibody (E87; cat. no. ab32351; Abcam; 1:2,000); anti-caspase-8
antibody (E7; cat. no. ab32397; Abcam; 1:2,000); anti-caspase-9
antibody (E23; cat. no. ab32539; Abcam; 1:5,000); anti-β-actin
antibody (cat. no. ab8227; Abcam; 1:3,000); anti-Bax antibody (E63;
cat. no. ab32503; Abcam; 1:2,000); Anti-Bcl-2 antibody (EPR17509;
cat. no. ab182858; Abcam; 1:2,000); anti-Bcl-XL antibody (E18; cat.
no. ab32370; Abcam; 1:3,000); anti-p53 antibody (E26; cat. no.
ab32389; Abcam; 1:6,000); anti-p21 antibody (EPR362; cat. no.
ab109520; Abcam; 1:1,000); anti-NF-kB p65 (phospho S276) antibody
(EPR17622; cat. no. ab183559; Abcam, 1:2,000); and anti-IkB alpha
antibody (EP697; cat. no. ab76429; Abcam; 1:2,000).
Experimental animal model and Lewis
lung carcinoma in vivo experiments
A total of 120 C57BL/6J male mice (7-weeks old) were
purchased from the Animal Center of the Wuhan University, were fed
standard mouse chow and housed at 23±1˚C with a 50±5% humidity and
a 12 h light/dark cycle. The animal experiments were approved by
the Animal Ethics Committee of Wuhan University. The modeling
method was performed as described previously (19). Anesthesia was induced by placing the
animals into a clear plastic box containing 2-3% isoflurane in a
50-50% mixture of O2 and air. After induction, the
animals received a 50-50% mixture of O2 and air
administered via a face mask with spontaneous ventilation. The
method of euthanasia used at the endpoint was CO2
inhalation by using a gradual 10 to 30% vol/min displacement
rate.
Lewis lung carcinoma cells; mouse lung cancer cells
that are widely used as a model for metastasis and are useful for
studying the mechanisms of cancer chemotherapeutic agents, were
used in this study. A total of 0.2 ml tumor cell suspension
(5x103 cells) was subcutaneously injected into the right
axillary of each C57BL/6J mouse. There were two sets of animal
experiments, in each set, 10 mice without Lewis lung cancer were
used as the normal group (without any cancer or treatment). After
Lewis lung cancer was induced in mice after 21 days, the mice were
randomly divided into six groups (n=18 per a group) as follows:
Control group (with cancer, without treatment); LNT-L group (0.2
ml/day 100 µg/ml LNT); oridonin group (0.2 ml/day 20 µg/ml
oridonin); oridonin + LNT-L group (0.2 ml/day 20 µg/ml oridonin and
0.2 ml/day 100 µg/ml LNT); LNT-H group (0.2 ml/day 300 µg/ml LNT);
oridonin + LNT-H group (0.2 ml/day 20 µg/ml oridonin and 0.2 ml/day
300 µg/ml LNT). For the first set of animal experiments, after 10
days, the busts of the mice were measured with a mini tape measure
before and after the treatments, and an increase in bust size
>50% compared with the respective size before injection was
defined as significant lung cancer metastasis. All the mice were
euthanized followed by the collection of lung tissues. The method
of euthanasia used at end point was CO2 inhalation as
described above. The euthanasia chamber enabled animals to be
readily visible and provided a minimum purity for CO2 of
at least 99.0%. This allowed induction of unconsciousness with
minimal distress to the animals. The lung tissues were used for
mRNA and protein extraction for use in the RT-qPCR and the western
blotting experiments. For the second set of experiments, the mice
were fed until the endpoint to obtain the survival rate. The
criteria for the endpoint were: i) Tumor growth that impedes the
ability to ingest food or water; ii) tumor pain or distress that
could not be relieved with palliative measures; or iii) Solid
tumors estimated to exceed 20% of normal body weight. The death of
animals was recorded every 5 days.
Statistical analysis
All experiments were repeated at least three times.
A one-way ANOVA followed by a post-hoc Tukey's test was used to
compare differences between multiple groups. The survival was
analyzed using the Kaplan-Meier (KM) method. SAS version 9.1
statistical software package (SAS Institute Inc.) was used for
statistical analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of oridonin and LNT on the
growth of MRC-5 and A549 cells
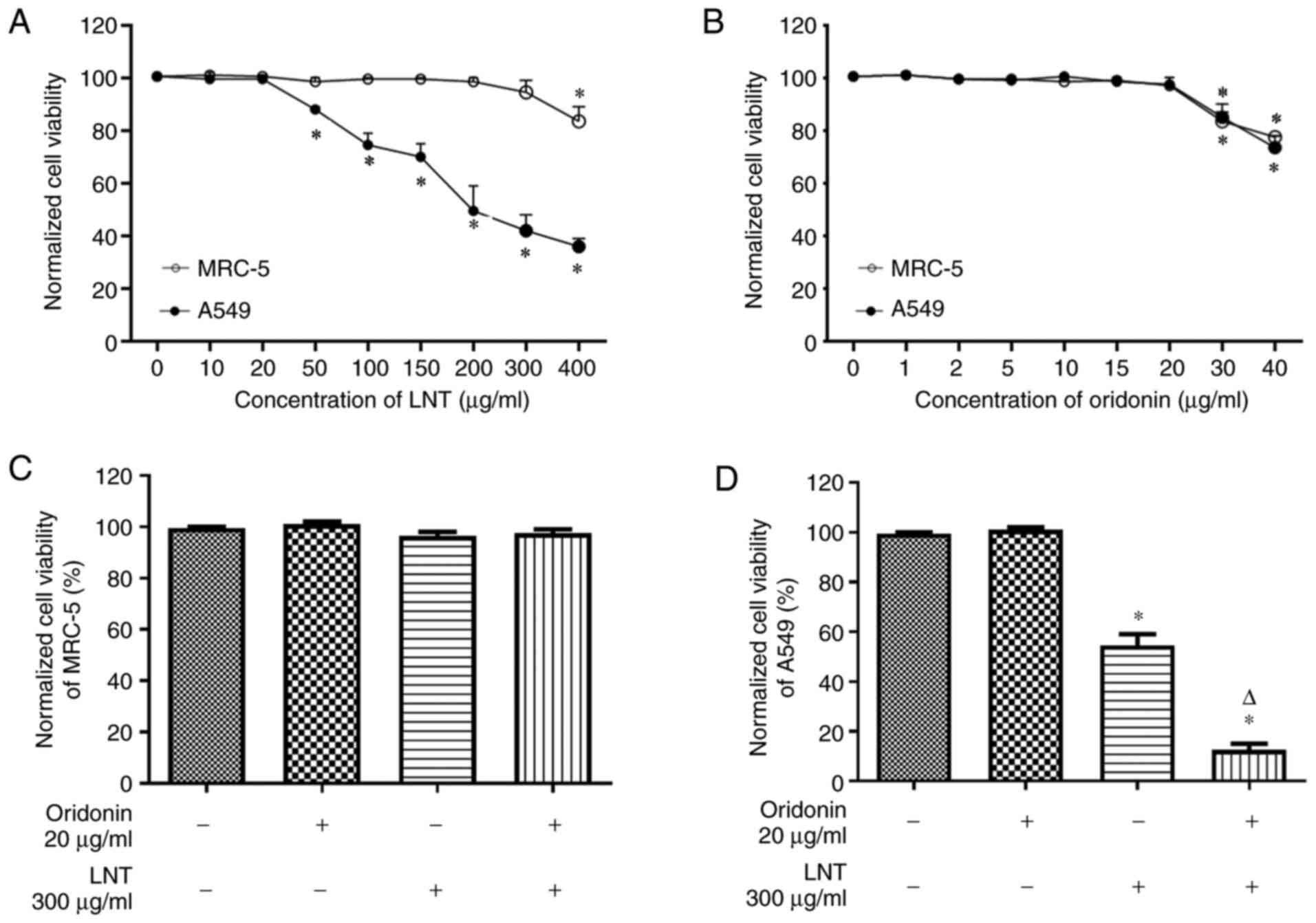
Cell viability was determined using MTT assays. The
viability of MRC-5 and A549 cells were not affected by 0-20 µg/ml
oridonin. Additionally, 0-300 µg/ml LNT did not affect the
viability of MRC-5 cells, but 50-400 µg/ml LNT significantly
inhibited the viability of A549 cells. Oridonin (0-20 µg/ml); LNT
(LNT-L, 100 µg/ml; LNT-H, 300 µg/ml); or the combination of both
(LNT-L, 20 µg/ml oridonin + 100 µg/ml LNT; LNT-H, 20 µg/ml oridonin
+ 300 µg/ml LNT) did not affect MRC-5 cell viability. When compared
with the 0 µg/ml oridonin group, oridonin had no effect on A549
cell viability; however, LNT significantly suppressed A549 cell
viability, and when combined with oridonin, the reduction in
viability was further increased (Fig.
1).
In subsequent studies, 20 µg/ml oridonin was used,
as it was the highest concentration that had no significant effect
on cell viability. Based on preliminary results, 300 µg/ml LNT was
used for the subsequent experiments as the high concentration group
(LNT-H), as it was the concentration that had the most potent
effect on the viability of cancer cells, whilst having little
effect on the viability of normal lung cells. As a comparison, a
low concentration group (LNT-L) was also included, in which cells
were treated with 100 µg/ml LNT.
Effect of oridonin and LNT on the mRNA
and protein expression levels of apoptosis associated genes and
proteins in A549 cells
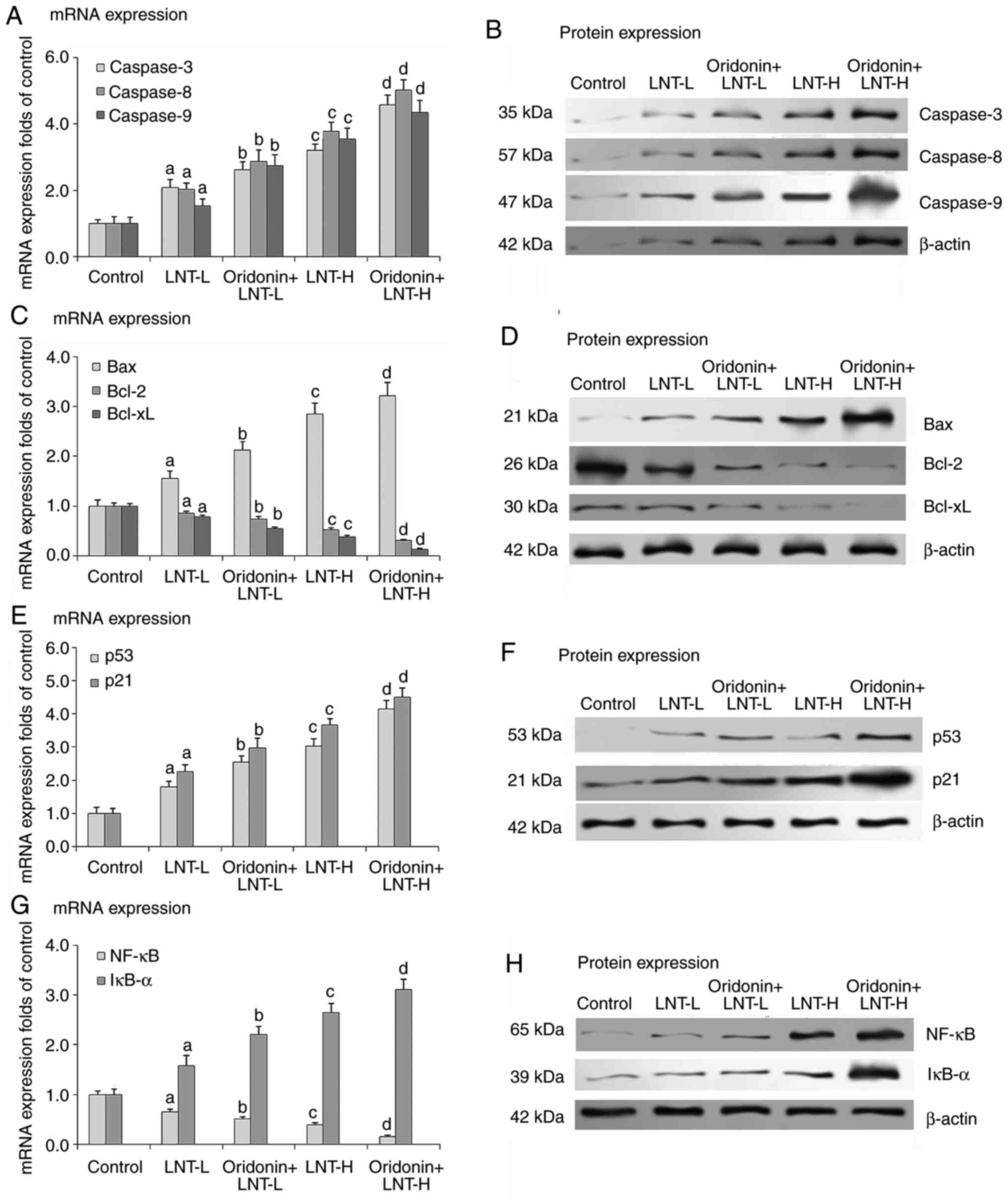
At both concentrations assessed, LNT increased the
mRNA and protein expression levels of caspase-3, caspase-8 and
caspase-9 in A549 cells, with a significantly larger increase in
the LNT-H group compared with the control group. Compared with the
LNT groups, LNT with oridonin further increased the mRNA and
protein expression levels of caspase-3, caspase-8 and caspase-9
when combined with LNT in A549 cells (Fig. 2A and B). Additionally, compared with the groups
that received no treatment, LNT with LNT at both concentrations
assessed increased the mRNA and protein expression of Bax in A549
cells, and the increase was greater in the LNT-H group. Compared
with the LNT groups, LNT with oridonin further increased the mRNA
and protein expression levels of Bax in LNT treated A549 cells.
Conversely, compared with the no treatment group, LNT at both
concentrations assessed decreased the mRNA and protein expression
levels of Bcl-2 and Bcl-xL in A549 cells, and the decrease was
greater in the LNT-H group. Compared with the LNT groups, oridonin
combined with LNT further decreased the mRNA and protein expression
levels of Bcl-2 and Bcl-xL in the A549 cells (Fig. 2C and D). These results suggested that LNT may
increase apoptosis of cancer cells, and that oridonin may augment
these effects.
Effect of oridonin and LNT on the mRNA
and protein expression levels of the p53/p21 pathway proteins in
A549 cells
It was hypothesized that the effect of LNT on the
viability of the cells was associated with p53/p21 signaling, thus,
their expression was assessed at the mRNA and protein level.
Compared with the no treatment group, LNT at both concentrations
assessed increased the mRNA and protein expression levels of p53
and p21 in A549 cells. Compared with the LNT groups, LNT with
oridonin further increased the mRNA and protein expression levels
of p53 and p21 when combined with LNT in A549 cells (Fig. 2E and F). These results suggested that LNT
exerted its effects via modulation of the p53/p21 pathway.
Effect of oridonin and LNT on the mRNA
and protein expression levels of NF-κB and IκB-α in A549 cells
Compared with the no treatment group, LNT at both
concentrations tested, increased the mRNA and protein expression
levels of NF-κB in A549 cells, and the increase was greater
in the LNT-H group. Compared with the LNT groups, LNT with oridonin
further increased the mRNA and protein expression levels of
NF-κB in LNT treated A549 cells. Conversely, compared with
the no treatment group, both concentrations of LNT assessed
decreased the mRNA and protein expression levels of IκB-α in A549
cells, and the decrease was greater in the LNT-H group. Compared
with the LNT groups, LNT with oridonin further decreased the mRNA
and protein expression levels of IκB-α when combined with LNT
(Fig. 2G and H). These results suggested the involvement
of NF-κB and IκB-α signaling in the effects of oridonin and
LNT.
Effects of oridonin and LNT on lung
tumor metastasis in mice
A mouse model of lung cancer metastasis was
established, and the mice were treated with either a low or high
dose of LNT (LNT-L and LNT-H, respectively), oridonin, oridonin +
LNT-L, LNT-H, or oridonin + LNT-H. Mice without cancer or any
treatments and mice with lung cancer that received no treatments
were used as controls. As demonstrated in Table I, after 10 days, oridonin alone had
no effect on short term lung cancer metastasis when compared with
the control. LNT treatment decreased the metastasis with a higher
inhibitory rate in LNT-H group compared with the LNT-L group.
Oridonin augmented the suppression of LNT against lung cancer
metastasis at both doses. These results suggested that oridonin may
serve as an adjuvant, to augment the effects of LNT on lung tumor
metastasis in mice.
 | Table IInhibitory effect of oridonin and LNT
on lung tumor metastasis in mice. |
Table I
Inhibitory effect of oridonin and LNT
on lung tumor metastasis in mice.
| Group | Total, n | Lung tumor
metastasis, n | Inhibitory rate,
% |
|---|
| Normal | 10 | 0 | 0 |
| Control | 18 | 18 | 100 |
| Oridonin | 18 | 18 | 100 |
| LNT-L | 18 | 15 | 16.7 |
| Oridonin +
LNT-L | 18 | 13 | 27.8 |
| LNT-H | 18 | 9 | 50.0 |
| Oridonin +
LNT-H | 18 | 7 | 61.1 |
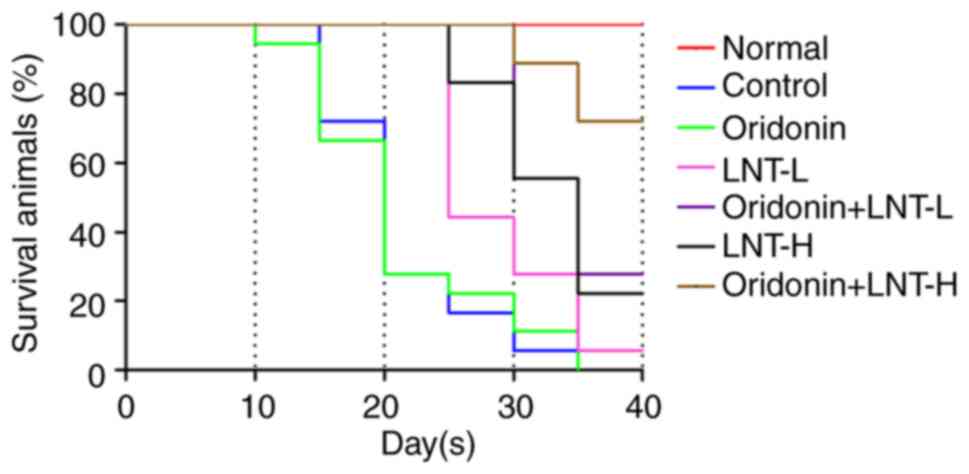
Effects of oridonin and LNT on the
overall survival of mice with lung cancer
A survival assay was used to assess the effects of
oridonin and LNT. The results revealed that when compared with the
control, oridonin alone had almost no effect on the survival of the
animals. Both LNT-L and LNT-H improved the survival, and LNT-H
exhibited improved effects on outcomes compared with LNT-L. Compare
with LNT groups, oridonin combined with LNT notably improved
survival at both doses of LNT assessed, and the survival was
greatest in the mice treated with a high dose of LNT combined with
oridonin (Fig. 3). These results
suggested that the combined use of both oridonin and LNT most
notably improved survival.
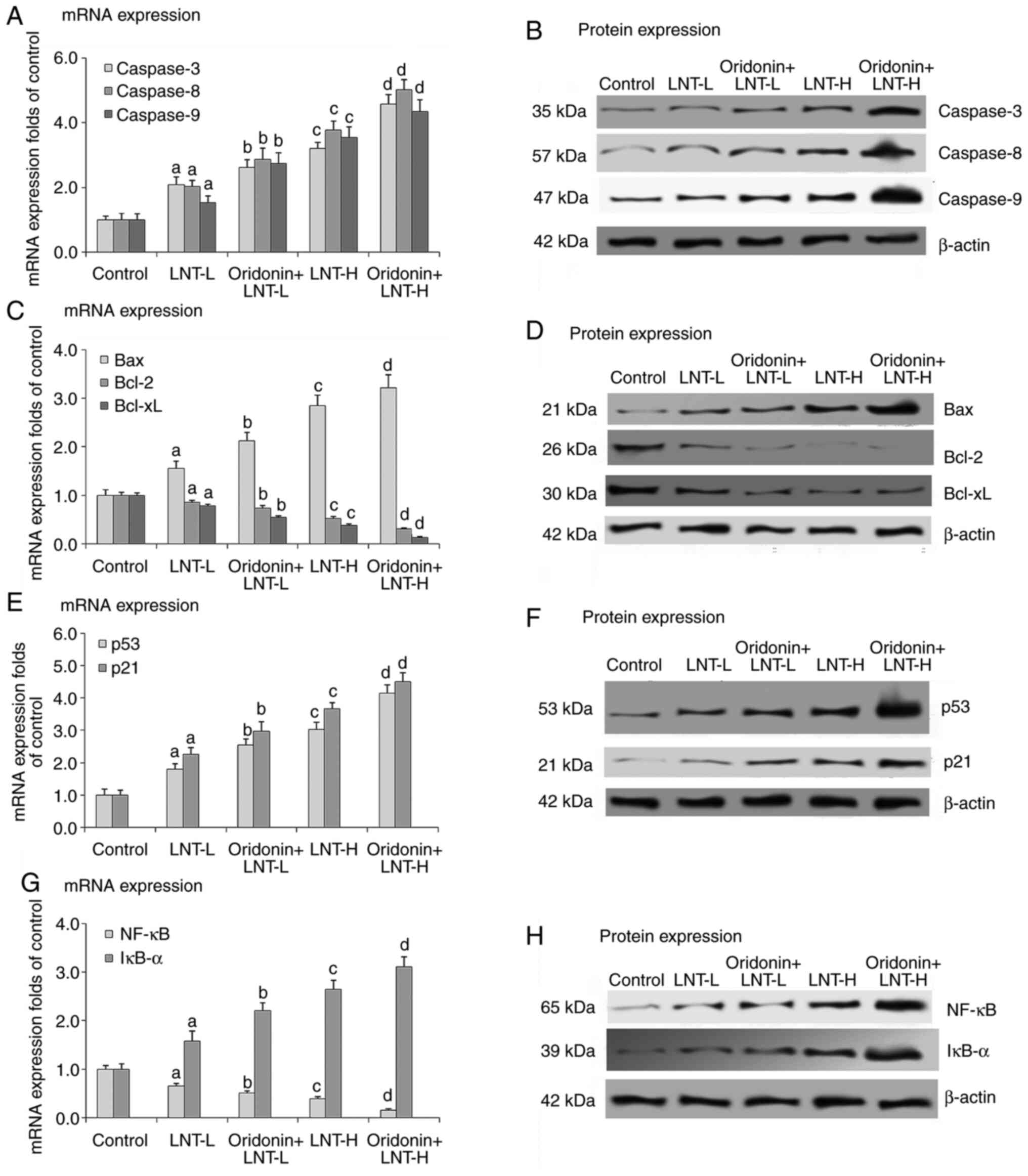
Effects of oridonin and LNT on the
mRNA and protein expression levels of caspase-3, caspase-8 and
caspase-9 in the mice lung cancer tissues
The mRNA and protein expression levels of caspase-3,
caspase-8, and caspase-9 were significantly decreased in the lung
tissue samples of all treatment groups compared with the control.
Treatment with LNT at both concentrations assessed increased the
mRNA and protein expression levels of caspase-3, caspase-8 and
caspase-9 in the mice lung cancer tissue samples, and the effect
was more potent in the mice treated with a high dose of LNT.
Oridonin further increased the mRNA and protein expression levels
of caspase-3, caspase-8 and caspase-9 in the lung cancer tissues of
the LNT treated animals (Fig. 4A
and B).
Effects of oridonin and LNT on the
mRNA and protein expression levels of Bax, Bcl-2 and Bcl-xL in the
mice lung cancer tissues
Compared with the control, the mRNA and protein
expression levels of Bax were significantly increased, whereas
those of Bcl-2 and Bcl-xL were significantly decreased in the lung
cancer tissue samples in treatment groups. Compared with the
control, LNT at both concentrations assessed increased the mRNA and
protein expression levels of Bax, and the effect of LNT-H was more
potent than that of LNT-L. Compared with LNT groups, LNT with
oridonin further increased the mRNA and protein expression levels
of Bax in the lung cancer tissue samples of mice treated with LNT.
Conversely, compared with the control, LNT at both concentrations
assessed decreased the mRNA and protein expression levels of Bcl-2
and Bcl-xL and the decrease was greater in the LNT-H group.
Compared with the LNT groups, LNT with oridonin further decreased
the mRNA and protein expressions of Bcl-2 and Bcl-xL (Fig. 4C and D).
Effects of oridonin and LNT on the
mRNA and protein expression levels of p53 and p21 in mice lung
cancer tissues
The mRNA and protein expression levels of p53 and
p21 in lung tissue samples were significantly decreased in the mice
with cancer compared with non-cancerous control mice. Treatment
with LNT at both concentrations increased the mRNA and protein
expression levels of p53 and p21 in the lung cancer tissues, and
the increase was greater in the LNT-H treated group. Oridonin
further increased the mRNA and protein expression levels of p53 and
p21 in the lung cancer tissues when combined with LNT-H (Fig. 4E and F).
Effects of oridonin and LNT on the
mRNA and protein expression levels of NF-κB and IκB-α in lung
tissues
The mRNA and protein expression levels of
NF-κB were significantly increased, whereas those of IκB-α
were significantly decreased in lung tissue samples of mice with
cancer compared with those without. LNT at both concentrations
tested decreased the mRNA and protein expression levels of NF-κB,
and the decrease was greater in the LNT-H group. Oridonin further
decreased the mRNA and protein expression levels of NF-κB in
the lung cancer tissue samples of the LNT treated animals.
Conversely, LNT at both concentrations assessed increased the mRNA
and protein expression levels of IκB-α, and the decrease was larger
in the LNT-H treated group. Oridonin further increased the mRNA and
protein expression levels of IκB-α in the LNT treated mice with
lung cancer (Fig. 4G and H). The in vivo results were similar
to those observed in the A549 cells, confirming the effects and
mechanisms of oridonin and LNT on lung cancer.
Discussion
Oridonin has been reported to increase the
anticancer effects of LNT in HepG2 human hepatoblastoma cells
(14), and also to enhance the
in vitro anticancer effects of LNT in SMMC-7721 human
hepatoma cells through regulation of the expression of genes
associated with apoptosis (13).
However, there are no studies that have investigated the effects of
LNT on lung cancer, to the best of our knowledge. Lung cancer cells
differ from liver cancer due to their differing natures of the
cells they are derived from; however, it was hypothesized that LNT
would exhibit beneficial effects for the treatment of lung cancer,
similar to its effects in liver cancer cells. In addition, oridonin
was also shown to inhibit human pancreatic cancer migration
(9). The aim of the present study
was to assess the effects of the combined treatment of oridonin and
LNT in vivo.
The results showed that the A549 lung cancer cell
line was considerably more sensitive than the normal human fetal
lung fibroblast cell line MRC-5 to LNT. This suggested that LNT can
be used as a potential cancer medicine with few side effects. LNT
has been previously shown to exhibit a suppressive effect on cell
growth in certain cancer cell lines (20), as well as an immunomodulatory effect
in patients with lung cancer (21).
A retrospective study showed that LNT improved the quality of life
of patients with multiple types of cancers, including: Lung cancer
(3,469 cases); gastric cancer (3,039 cases); colorectal cancer
(1,646 cases); ovarian cancer (183 cases); cervical cancer (130
cases); pancreatic cancer (15 cases); cardiac cancer (15 cases);
nasopharyngeal cancer (14 cases); duodenal cancer (1 case);
Non-Hodgkin lymphoma (70 cases); and 110 cancer cases with no
classifying patient information (4). LNT significantly promoted the efficacy
of chemotherapy and radiation therapy during these cancer treatment
regimens (4). An in vivo
study also showed that LNT exhibited therapeutic potential for
colitis-associated cancer. Additionally, it has been shown that 36
µg/ml (0.1 mmol/l) oridonin inhibited breast cancer growth and
metastasis through inhibition of the Notch signaling pathway
(8).
In the present study, MTT assays were used to assess
the viability of lung cancer cells. MTT assays are widely used in
cancer pharmacological studies (22). The results showed that 0-300 µg/ml
oridonin had little effect on both MRC-5 and A549, indicating that
lung cancer cells may have a lower sensitivity to oridonin than
breast cancer cells. The survival rate is a critical indicator of
the effectiveness of cancer therapies in different types of cancer
(23). Thus, the clinical treatment
with LNT and oridonin were mimicked using a mouse model, and
survival of the mice was assessed as described previously (24). The in vivo experiments
performed in the present study showed that oridonin alone failed to
suppress the migration of lung cancer cells, and did not affect the
survival of mice with lung cancer. However, oridonin, when combined
with LNT, promoted the effects of LNT with regard to both the
viability of A549 cells and the metastasis and the survival of
mice. These results suggest that oridonin may activate pathways
that facilitate the actions of LNT. However, in the survival
experiments, mice were anesthetized, and this procedure may
potentially affect the induced cancer (23,25),
which may have potentially affected the results.
To explore the underlying mechanisms modulated by
LNT, the expression of several potential targets of LNT in both
A549 and lung tissue samples were assessed. A previous study showed
that proliferation and apoptosis affect cancer cell viability
(26). Thus, it was hypothesized
that apoptosis may be involved in the effects of oridonin and LNT.
Firstly, the caspase signaling pathway was assessed. Caspases
(cysteine-aspartic proteases or cysteine-dependent
aspartate-directed proteases) are a family of protease enzymes that
serve essential roles in programmed cell death and inflammation
(27). A previous study showed that
co-treatment with paclitaxel and LNT enhanced cell apoptosis rates
by inducing caspase-3 activation (28). In the present study, it was shown
that LNT reduced A549 cell viability by increasing the expression
of the apoptosis executioner caspase-3, and oridonin further
promoted this increase in expression. Moreover, it was also shown
that there was a potential negative feedback of the caspase
signals, as the expression of the apoptosis initiators of caspases,
caspases 8 and 9, increased with alongside caspase-3.
LNT has been reported to exert synergistic apoptotic
effects when combined with paclitaxel in A549 cells (28). The apoptosis-inducing effects of LNT
have also been reported in a study using the human bladder cancer
cell line T24(29). In hepatoma
cells, oridonin was shown to promote the effects of LNT through
regulating the expression of apoptotic genes (13). To confirm that the effects observed
in the present study were mediated by apoptosis (28), the activity of the Bax signaling
pathway, an apoptosis regulatory pathway, was assessed. Bcl2 family
members act as anti- or pro-apoptotic regulators in cancer cells.
Bcl-xL acts as an anti-apoptotic protein by preventing the release
of mitochondrial contents, such as cytochrome c, which leads
to caspase activation and ultimately, programmed cell death
(30). In the present study, the
expression of Bax, Bcl-2 and Bcl-xL were affected by LNT and
oridonin, suggesting that their effects were mediated by regulation
of apoptosis.
The expression of the Bcl2 family of genes is
regulated by the tumor suppressor p53 and has been shown to be
involved in p53-mediated apoptosis (31,32).
In was hypothesized that p53 and p21 may be the upstream targets of
LNT and oridonin. Hence, the expression of p53 and p21 in A549 and
lung tissues were determined, and the results showed that their
expression was increased by treatment with LNT and oridonin. NF-κB
is a protein complex that controls transcription of DNA, cytokine
production and cell survival, whereas its inhibitor, IκBα,
functions to inhibit the transcriptional activity of NF-κB
transcription factors (33). In the
present study, it was shown that NF-κB and IκBα were involved in
the effects of LNT and oridonin on cancer development, and their
expression was altered in the treated cells, and may have served a
role in the decrease in cell viability observed in the treated
cells. In the lung tissues, the results were similar to that of the
in vitro experiments.
In conclusion, it was shown that oridonin enhanced
the antitumor effects of LNT. Additionally, several potential
regulatory mechanisms by which oridonin and LNT exerted their
effects were determined. However, there are other mechanisms that
may also be involved, such as cancer stem cells, which might be
targeted by oridonin and LNT (34).
Thus, more work is required to confirm these results and obtain a
more in-depth understanding of the specific mechanisms modulated by
oridonin and LNT. Several compounds derived from traditional
Chinese medicines have been explored for their potential clinical
use in the treatment of various diseases (35-37).
In cancer treatment, although traditional medicines are not able to
cure cancer alone, when applied in combination with traditional
treatment approaches, they may reduce the adverse effects caused by
chemotherapy or radiotherapy, thus improving therapeutic outcomes
and quality of life for patients, or may act as adjuvants (38). In addition, some ion channels might
also be involved in the drug action of cancer cell proliferation
(39).
In the present study, a constituent compound of a
traditional herbal medicine was shown to augment the effects of a
more traditional treatment. These results support the notion of the
further study of oridonin and LNT as a novel cancer drug regimen,
and contributes to the application of traditional medicines as
clinical treatments.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the Program of Wuhan
Health Commission (grant no. WX20C34).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YG, JC, and ZC made substantial contributions to
conception and design, acquisition of data, and the analysis and
interpretation of data. JC and ZC were involved in drafting the
manuscript and revising it critically for important intellectual
content. YG and JC confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the Animal
Ethics Committee of Wuhan University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Liu H, Xiong Y, Wang H, Yang L, Wang C,
Liu X, Wu Z, Li X, Ou L, Zhang R and Zhu X: Effects of water
extract from epimedium on neuropeptide signaling in an
ovariectomized osteoporosis rat model. J Ethnopharmacol.
221:126–136. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang C, Chen G, Wong J, Liu H, Xong Y,
Wang P, Yang L, Zhu X and Zhang Rl: Effect of herba epimedium
extract on bone mineral density and microstructure in
ovariectomised rat. J Pharm Biomed Sci. 6:275–278. 2016.
|
|
4
|
Zhang M, Zhang Y, Zhang L and Tian Q:
Mushroom polysaccharide lentinan for treating different types of
cancers: A review of 12 years clinical studies in China. Prog Mol
Biol Transl Sci. 163:297–328. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tian Y, Yi W, Bai L, Zhang P, Si J, Hou X,
Deng Y and Hou J: Lentinan in-situ coated tungsten oxide nanorods
as a nanotherapeutic agent for low power density photothermal
cancer therapy. Int J Biol Macromol. 137:904–911. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Meng X, Liang H and Luo L: Antitumor
polysaccharides from mushrooms: A review on the structural
characteristics, antitumor mechanisms and immunomodulating
activities. Carbohydr Res. 424:30–41. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kidd PM: The use of mushroom glucans and
proteoglycans in cancer treatment. Altern Med Rev. 5:4–27.
2000.PubMed/NCBI
|
|
8
|
Xia S, Zhang X, Li C and Guan H: Oridonin
inhibits breast cancer growth and metastasis through blocking the
Notch signaling. Saudi Pharm J. 25:638–643. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gui Z, Luo F, Yang Y, Shen C, Li S and Xu
J: Oridonin inhibition and miR200b-3p/ZEB1 axis in human pancreatic
cancer. Int J Oncol. 50:111–120. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang W, Huang Q and Hua ZC: Oridonin: A
promising anticancer drug from China. Front Biol. 5:540–545.
2010.
|
|
11
|
Wang ZN, Wo XD and Zhou YL: Molecule
mechanisms of Oridonin on tumors chemoprevention and therapy. China
Medical Herald. 28:14–16. 2008.(In Chinese).
|
|
12
|
Yang J, Jiang H, Wang C, Yang B, Zhao L,
Hu D, Qiu G, Dong X and Xiao B: Oridonin triggers apoptosis in
colorectal carcinoma cells and suppression of microRNA-32
expression augments oridonin-mediated apoptotic effects. Biomed
Pharmacother. 72:125–134. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xu T, Jin F, Wu K, Ye Z and Li N: Oridonin
enhances in vitro anticancer effects of lentinan in SMMC-7721 human
hepatoma cells through apoptotic genes. Exp Ther Med. 14:5129–5134.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sun Z, Han Q, Duan L, Yuan Q and Wang H:
Oridonin increases anticancer effects of lentinan in HepG2 human
hepatoblastoma cells. Oncol Lett. 15:1999–2005. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang H, Mo S, Yang L, Wang P, Sun K, Xiong
Y, Liu H, Liu X, Wu Z, Ou L, et al: Effectiveness associated with
different therapies for senile osteopo-rosis: A network
Meta-analysis. J Tradit Chin Med. 40:17–27. 2020.PubMed/NCBI
|
|
16
|
Li R, Xiao C, Liu H, Huang Y, Dilger JP
and Lin J: Effects of local anesthetics on breast cancer cell
viability and migration. BMC Cancer. 18(666)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li X, Peng B, Zhu X, Wang P, Xiong Y, Liu
H, Sun K, Wang H, Ou L, Wu Z, et al: Changes in related circular
RNAs following ERβ knockdown and the relationship to rBMSC
osteogenesis. Biochem Biophys Res Commun. 493:100–107.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu X, Liu H, Xiong Y, Yang L, Wang C,
Zhang R and Zhu X: Postmenopausal osteoporosis is associated with
the regulation of SP, CGRP, VIP, and NPY. Biomed Pharmacother.
104:742–750. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Amikishieva AV, Ilnitskaya SI, Nikolin VP,
Popova NA and Kaledin VI: Depressive-like psychoemotional state
versus acute stresses enhances Lewis lung carcinoma metastasis in
C57BL/6J mice. Exp Oncol. 33:222–225. 2011.PubMed/NCBI
|
|
20
|
Qian Y, Wang D, Fan M, Xu Y, Sun X and
Wang J: Effects of intrinsic metal ions of lentinan with different
molecular weights from Lentinus edodes on the antioxidant capacity
and activity against proliferation of cancer cells. Int J Biol
Macromol. 120:73–81. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang XE, Wang YH, Zhou Q, Peng M, Zhang J,
Chen M, Ma LJ and Xie GM: Immunomodulatory effect of lentinan on
aberrant T subsets and cytokines profile in non-small cell lung
cancer patients. Pathol Oncol Res. 26:499–505. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu H, Dilger JP and Lin J: Effects of
local anesthetics on cancer cells. Pharmacol Ther.
212(107558)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li R, Liu H, Dilger JP and Lin J: Effect
of propofol on breast cancer cell, the immune system, and patient
outcome. BMC Anesthesiol. 18(77)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li R, Huang Y, Liu H, Dilger JP and Lin J:
Comparing volatile and intravenous anesthetics in a mouse model of
breast cancer metastasis. Cancer Res. 78(2162)2018.
|
|
25
|
Liu H: A clinical mini-review: Clinical
use of Local anesthetics in cancer surgeries. G Med Sci. 1:030–034.
2020.
|
|
26
|
Liu H, Dilger JP and Lin J: The role of
transient receptor potential melastatin 7 (TRPM7) in cell
viability: A potential target to suppress breast cancer cell cycle.
Cancers (Basel). 12(131)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Van Opdenbosch N and Lamkanfi M: Caspases
in cell death, inflammation, and disease. Immunity. 50:1352–1364.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu W, Gu J, Qi J, Zeng XN, Ji J, Chen ZZ
and Sun XL: Lentinan exerts synergistic apoptotic effects with
paclitaxel in A549 cells via activating ROS-TXNIP-NLRP3
inflammasome. J Cell Mol Med. 19:1949–1955. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bao L, Wang Y, Ma R, Ren X, Cheng R and B
A: Apoptosis-inducing effects of lentinan on the proliferation of
human bladder cancer T24 cells. Pak J Pharm Sci. 28:1595–1600.
2015.PubMed/NCBI
|
|
30
|
Siddiqui WA, Ahad A and Ahsan H: The
mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update.
Arch Toxicol. 89:289–317. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wawryk-Gawda E, Chylińska-Wrzos P,
Lis-Sochocka M, Chłapek K, Bulak K, Jędrych M and Jodłowska-Jędrych
B: P53 protein in proliferation, repair and apoptosis of cells.
Protoplasma. 251:525–533. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang X, Simpson ER and Brown KA: p53:
Protection against tumor growth beyond effects on cell cycle and
apoptosis. Cancer Res. 75:5001–5007. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
DiDonato JA, Mercurio F and Karin M: NF-κB
and the link between inflammation and cancer. Immunol Rev.
246:379–400. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu H: A prospective for the potential
effect of Local Anesthetics on Stem-Like cells in colon cancer.
Biomed J Sci & Tech Res. 25:18927–18930. 2020.
|
|
35
|
Wu Z, Ou L, Wang C, Yang L, Wang P, Liu H,
Xiong Y, Sun K, Zhang R and Zhu X: Icaritin induces MC3T3-E1
subclone14 cell differentiation through estrogen receptor-mediated
ERK1/2 and p38 signaling activation. Biomed Pharmacother. 94:1–9.
2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chen G, Wang C, Wang J, Yin S, Gao H,
Xiang LU, Liu H, Xiong Y, Wang P, Zhu X, et al: Antiosteoporotic
effect of icariin in ovariectomized rats is mediated via the
Wnt/β-catenin pathway. Exp Ther Med. 12:279–287. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liu H, Xiong Y, Zhu X, Gao H, Yin S, Wang
J, Chen G, Wang C, Xiang L, Wang P, et al: Icariin improves
osteoporosis, inhibits the expression of PPARΥ, C/EBPα,
FABP4 mRNA, N1ICD and jagged1 proteins, and increases Notch2 mRNA
in ovariectomized rats. Exp Ther Med. 13:1360–1368. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Xiang Y, Guo Z, Zhu P, Chen J and Huang Y:
Traditional Chinese medicine as a cancer treatment: Modern
perspectives of ancient but advanced science. Cancer Med.
8:1958–1975. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Liu H, Dilger JP and Lin J: Lidocaine
suppresses viability and migration of human breast cancer cells:
TRPM7 as a target for some breast cancer cell lines. Cancers
(Basel). 13(234)2021.PubMed/NCBI View Article : Google Scholar
|