Introduction
Worldwide, myocardial infarction is one of the most
common and severe heart diseases (1). During myocardial infarction,
ischemia-reperfusion (I/R) can result in myocardial injury
(2). In order to investigate the
mechanisms of I/R injury, myocardial hypoxia-reoxygenation (H/R) is
an ideal in vitro model (3-5).
Myocardial H/R has been reported to have a complex association with
a number of molecules that ultimately damage the heart (6,7). In
the past decade, despite some advances in therapeutic drugs that
decrease myocardial I/R damage, new cardiac protective drugs are
still urgently needed to improve treatment outcomes and minimize
the injury caused by myocardial I/R.
Post-anesthesia adaptation is a form of cellular
protection (8). Tissues are better
able to resist I/R injury when they are treated with volatile
anesthetics (8,9). Propofol exerts a protective effect on
I/R injury as an anesthetic (10).
The mechanism of the protective effect was found to involve free
radical scavenging (11) or
inhibition of calcium channels (12). However, as a new type of anesthetic,
sevoflurane, is widely applied in cardiac surgery because it acts
faster, gives more stable recovery, and more efficient anesthesia
(13). In addition, sevoflurane
exhibits excellent cardioprotective performance in I/R injury
(14). To better understand and
utilize the protective effects of anesthetics, more research is
still needed. Therefore, the aim of the present study was to
determine whether sevoflurane exerts a better cardioprotective
effect than propofol on H/R injury in cardiomyocytes and the
associated mechanism.
A disintegrin and metalloproteinase (ADAM) is a
family of peptidase proteins regulating proteolysis and mediate
cell-cell and cell-matrix interactions (15). As one of the key members, ADAM8 was
originally revealed to encode a protein containing a
carboxy-terminal transmembrane domain (16). Subsequent research revealed that rat
heart transplantation-induced distal myocardial remodeling in I/R
injury or myocardial infarction was associated with the
upregulation of ADAM8(17).
According to RNA-seq analysis (GSE4386), ADAM8 is differently
expressed in the samples treated with sevoflurane and propofol.
Besides, GO enrichment suggested that ADAM8 is associated with
cellular process and cell activation. Based on the findings of the
aforementioned studies, the role of ADAM8 in cell viability was
further investigated, when cardiomyocytes are undergoing H/R
injury.
MicroRNAs (miRNAs/miRs) are non-coding RNAs that
mediate target gene expression by binding to the 3' untranslated
region (3'-UTR). Several miRNAs have been identified to be
associated with myocardial H/R injury. For example, a study
revealed that miR-101a-3p suppressed H9C2 cell proliferation and,
during H/R injury, promoted H9C2 cell apoptosis (18). Likewise, miR-142-p could promote
cell apoptosis in a cardiomyocyte model of H/R injury (19). Upregulation of miR-221-5p was
observed in myocardial hypertrophy and heart failure (20). The present study attempted to
explore the role of miR-221-5p in influencing H9C2 cell
proliferation and apoptosis, following H/R injury. Interestingly,
it was found that miR-221-5p could target ADAM8 and negatively
affected the protection of H9C2 cells subjected to H/R injury.
The present study aimed to determine the roles and
interactions of sevoflurane, propofol, ADAM8 and miR-221-5p in the
protection of myocardial tissue in the H/R model. The study
outcomes will provide more information on the therapeutic strategy
in the alleviation of the negative effects of I/R on the ischemic
myocardial tissue in clinical practice.
Materials and methods
Cell culture and H/R model
Rat embryo cardiomyocyte cell line (H9C2), obtained
from American Type Culture Collection, was cultured in DMEM medium
(Hyclone; Cytvia) with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin/streptomycin (Invitrogen;
Thermo Fisher Scientific, Inc.). The cells were cultured in an
incubator with 5% CO2 at 37˚C. The H/R model was
established by culturing H9C2 cells in an oxygen-deficient
condition (95% N2 and 5% CO2) for 6 h.
Subsequently, the cells were placed back to the normal condition
(95% O2 and 5% CO2) for another 6 h.
Bioinformatics analysis
GSE4386 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE4386)
downloaded from GEO comprised the mRNA microarray datasets
including sevoflurane and propofol samples. With P<0.05 and log
fold change (FC)>1, the upregulated genes in sevoflurane samples
were screened from GSE4386. Subsequently, STRING (https://string-db.org/) and Metascape (http://metascape.org/gp/index.html#/main/step1) were
used for biological process analysis of the upregulated genes.
Finally, Venny 2.1.0 (http://bioinfogp.cnb.csic.es/tools/venny/) was used to
examine the overlap of the common genes in STRING and
Metascape.
Cell transfection and cell
treatment
The ADAM8 overexpression vectors were obtained from
GeneCreate Biotech. Briefly, the full length of ADAM8 was
synthesized and subcloned into pcDNA3.1 vector (Invitrogen; Thermo
Fisher Scientific, Inc.). The empty vector pcDNA 3.1 was used as
the negative control. The small interfering RNA (siRNA) named
si-ADAM8 (5'-CGGCACCUGCAUGACAACGUA-3') and non-targeting siRNA
(si-NC, 5'-UUCUCCGAACGUGUCACGUTT-3') were designed and synthesized
by Guangzhou RiboBio Co., Ltd. miR-221-5p mimic (sense,
5'-AGCUACAUUGUCUGCUGGGUUUC-3'), mimic-NC
(5'-GAAAUGUACUUGAGCGUGGAGAC-3'), miR-221-5p inhibitor
(5'-ACAGAAAUCUACAUUGUAUGCCAGGU-3') and inhibitor-NC
(5'-CUAAAACCGGCCGUACGGCGUU-3') were synthesized by Guangzhou
RiboBio Co., Ltd. Cell transfection was carried out using
Lipofectamine™ 3000 Transfection Reagent (Thermo Fisher
Scientific, Inc.), following the manufacturer's instructions.
For the treatment of sevoflurane (S) and propofol
(P), H/R+S or H/R+P was generated by the addition of 2% sevoflurane
(Sigma-Aldrich; Merck KGaA) or 50 µmol/l propofol (Sigma-Aldrich;
Merck KGaA) to H9C2 cells cultured in 95% O2 and 5%
CO2 for 1 h before being treated as the H/R group.
Cell Counting Kit-8 (CCK-8) assay
Cell Counting Kit-8 (TransGen Biotech Co., Ltd.) was
used to measure the cell viability, following the manufacturer's
instruction. H9C2 cells (5x104 cells) were inoculated
into the well of a 96-well plate. Before the detection at 1, 4 or 7
h, 10 µl CCK-8 solution was added to the well and incubated in the
incubator at 37˚C for 1 h. After 1 h of incubation, the absorbance
at 450 nm was detected by a Microplate reader (BioTek Instruments,
Inc.).
Bromodeoxynucleoside uracil (BrdU)
cell proliferation assay
BrdU Cell Proliferation Assay kit (CST, US) was used
to evaluate H9C2 cell proliferation activity. H9C2 cells
(5x104 cells) were inoculated to the well in a 96-well
plate. After 48 h incubation, BrdU solution was added into the
96-well plate and incubated in an incubator for 1 h. Then, the
medium was removed, and 100 µl fixing/denaturing solution was added
into each well for a 30 min incubation at room temperature.
Fixing/denaturing solution was removed, and the sample was
incubated with BrdU detection antibody for 1 h. Subsequently,
anti-mouse IgG was used to label BrdU detection antibody, and HRP
solution was used for coloration. Finally, the absorbance at 450 nm
was detected by a microplate reader (BioTek Instruments, Inc.).
Caspase-3 activity assay
Caspase-3 Activity Assay kit was used to measure
H9C2 cell apoptosis rate. After cells were digested by 2.5% trypsin
(Gibco; Thermo Fisher Scientific, Inc.), the lysis solution was
used for cell protein extraction, and the protein concentration was
at least 1 µg/µl. The sample (50 µl) was added to the 96-well plate
with 40 µl buffer solution and 10 Ac-DEVD-pNA, and then incubated
at 37˚C for 2 h. After the incubation, the absorbance at 405 nm was
detected by a microplate reader (BioTek Instruments, Inc.).
Total RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted using RNAiso Plus (Takara
Bio, Inc.) following standard instructions. The H9C2 cells in a
12-well plate were lysed by 1 ml RNAiso Plus and transferred to a
new 2-ml tube without RNase. Then, 200 µl chloroform was added to
the tube, shaken and mixed. The sample was incubated at room
temperature for 5 min and centrifuged at 10,000 x g, 4˚C for 15
min. The supernatant was transferred to a new 1.5-ml centrifuge
tube with 500 µl isopropanol. After 10 min incubation at room
temperature, the supernatant was centrifuged at 8,000 x g, and 4˚C
for 10 min. Absolute ethanol (1 ml) was used to clean the
precipitate and it was subsequently centrifuged at 6,000 x g, 4˚C
for 5 min, and the supernatant was removed. Finally, 40 µl
RNase-free was added to the tube to dissolve the precipitate and
stored at -80˚C. ReverTra Ace qPCR RT Master mix with gDNA Remover
(Toyobo Life Science) was used for RNA reverse transcription,
following the manufacturer's instructions. The relative mRNA
expression level was measured by RT-qPCR with QuansStudio 5
instrument (Thermo Fisher Scientific, Inc.). RT-qPCR was performed
in three repetitions by using AceQ® Universal SYBR qPCR
Master mix (Vazyme Biotech Co., Ltd.) following the manufacturer's
instructions. The primers used in RT-qPCR were designed by Oligo
7.0 software (https://www.oligo.net/downloads.html) and synthesized
by Sangon Biotech Co., Ltd. The sequences of the primers used in
the present study are listed in Table
I. Relative mRNA expression was computed using the
2-ΔΔCq method (21).
 | Table IPrimers' sequences used in the present
study. |
Table I
Primers' sequences used in the present
study.
| Primer | Sequences |
|---|
| ADAM8 | F:
5'-AAGCCTACCTCAGGGCTCTC-3' |
| | R:
5'-CTTTGGGGCATAAACAGGAA-3' |
| MicroRNA- | F:
5'-GCCGAGACCTGGCATACAAT-3' |
| 221-5p | R:
5'-CTCAACTGGTGTCGTGGA-3' |
| GAPDH | F:
5'-CTCATGACCACAGTCCATGC-3' |
| | R:
5'-TTCAGCTCTGGGATGACCTT-3' |
| U6 | F:
5'-TGCGGGTGCTCGCTTCGGCAGC-3' |
| | R:
5'-CCAGTGCAGGGTCCGAGGT-3' |
Western blotting assay
For protein extraction from cells, ice-cold
radioimmunoprecipitation lysis buffer (Beyotime Institute of
Biotechnology, Inc.) with 1 mM phenylmethylsulfonyl fluoride
(Beyotime Institute of Biotechnology, Inc.) was used. Protein
concentration was measured using Pierce™ Rapid Gold BCA
Protein Assay kit (Thermo Fisher Scientific, Inc.). A total of 20
µg/lane protein samples were separated by 12% SDS-PAGE in vertical
electrophoresis and transferred onto nitrocellulose membrane using
a transfer system (both from BioRad Laboratories, Inc.). After
blocking with 5% skimmed milk for 2 h at room temperature, the
membrane was incubated with anti-ADAM8 (cat. no. ab186432; Abcam)
or anti-GAPDH antibody (cat. no. ab9485; Abcam) at 4˚C overnight in
a 1:500 dilution. The anti-rabbit IgG antibody (cat. no. BM2006;
Boster Biological Technology) was incubated at room temperature in
a 1:10,000 dilution. DAB Horseradish peroxidase color development
kit (Beyotime Institute of Biotechnology, Inc.) was used for band
imprint display. ImageJ 1.8.0 software (National Institutes of
Health) was used for protein quantification.
Dual-luciferase reporter assay
TargetScan was first used to predict what miRNAs
might target ADAM8, and then, a dual-luciferase reporter assay was
performed to verify the association of the target. In brief, the
sequence of ADAM8 3'-UTR with potential target sites of miR-221-5p
was synthesized by GeneCreate Biotech and subcloned into pmiR-GLO
vector (Promega Corporation), and was named WT. The sequence of
ADAM8 3'-UTR was then synthesized, in which potential target sites
of miR-221-5p have been mutated, and the mutated sequence was
subcloned into the pmiR-GLO vector and was named Mut.
Co-transfection was performed in H9C2 cells using Lipofectamine
3000 Transfection Reagent (Thermo Fisher Scientific, Inc.) and the
co-transfection groups were: WT + miR-221-5p mimic, WT + mimic
control, Mut + miR-221-5p mimic, and Mut + mimic control. After 48
h of co-transfection, firefly and Renilla luciferase activities
were measured using a Dual-GLO Luciferase Assay System kit (Promega
Corporation) and a microplate reader (BioTek Instruments, Inc.)
following the manufacturer's instructions.
Data analysis
Data were analyzed using SPSS 22.0 software (IBM
Corp.). One-way ANOVA analysis followed by Dunnett post hoc test
was performed for multiple comparisons. An unpaired Student's
t-test was applied for comparisons between two groups.
*P<0.05 was considered to indicate a statistically
significant difference. Data are expressed as mean ± SD. Each
experiment had at least three replicates.
Results
Sevoflurane exhibits better protective
effects than propofol on hypoxia-reoxygenation injury in
cardiomyocytes
In order to explore the protective effects of
sevoflurane or propofol on hypoxic cardiomyocytes, cell viability,
proliferation and apoptosis were detected by CCK-8, BrdU and
caspase-3 activity assays. H9C2 cells with no additional treatment
were cultured as the control group. On the other hand, after being
treated with H/R injury, sevoflurane (H/R+S) or propofol (H/R+P)
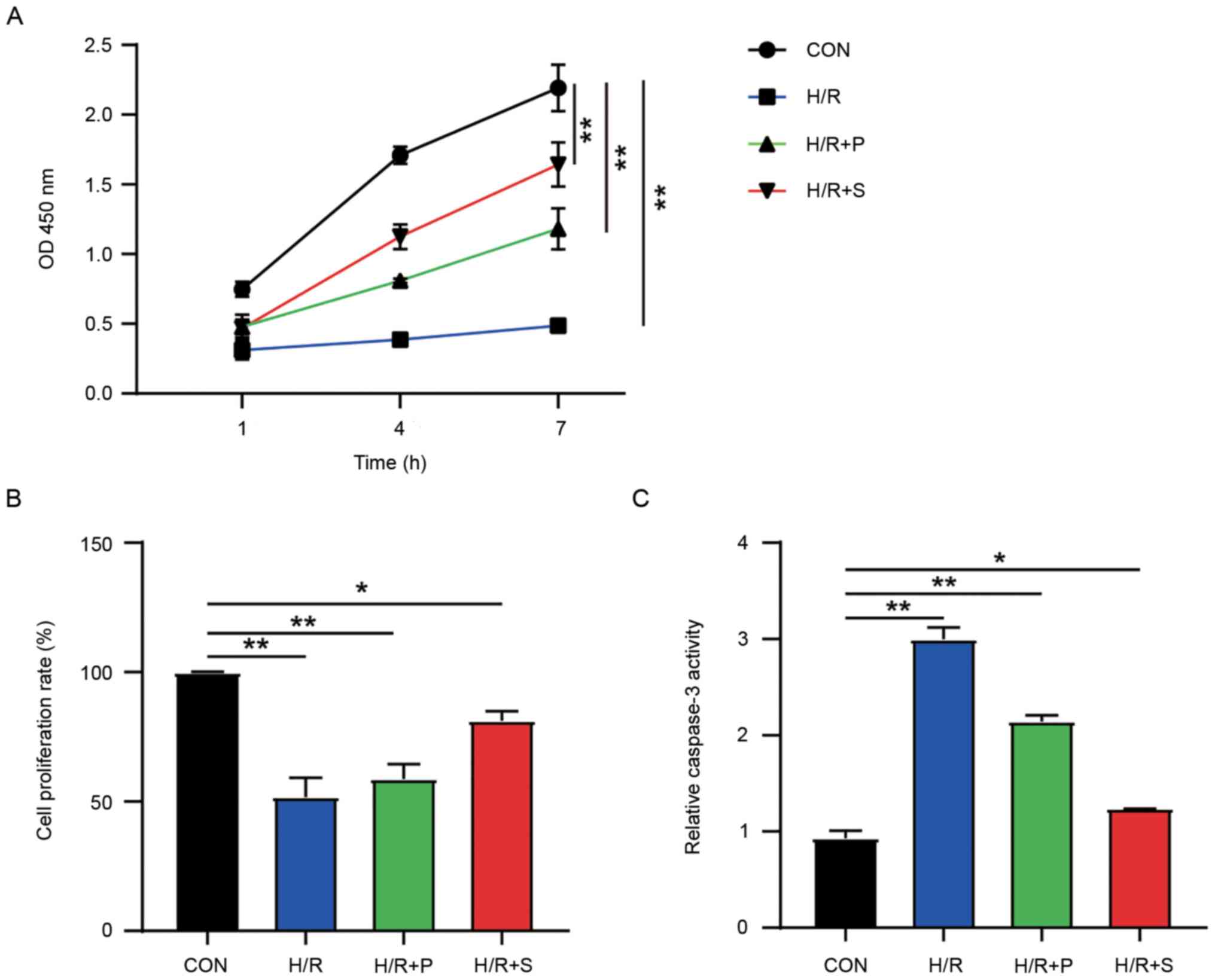
were added. The CCK-8 assay (Fig.
1A) showed a notable inhibition in the viability of H9C2 cells
treated with H/R injury, indicating a severe injury to the cell
viability. In spite of the severe injury, both sevoflurane and
propofol could restore the effect of H/R injury on cell viability,
and sevoflurane had a better effect on the improvement of H9C2 cell
injury than propofol. The BrdU assay (Fig. 1B) revealed that the cell
proliferation rate was almost 50% restrained in the H/R group
compared with that of the control group. Cell proliferation
activity in both H/R+S (25% restrained compared with the control
group) and H/R+P group (40% restrained compared to the control
group) was improved, with a better effect in the H/R+S group, again
indicating that the effect of sevoflurane was improved compared
with propofol in improving H9C2 cell H/R injury. Caspase-3 activity
assay (Fig. 1C) was used to
determine cell apoptosis, which indicated that H/R treatment
significantly accelerated the apoptosis of H9C2 cells by nearly
three times the normal level. Sevoflurane treatment restored cell
apoptosis to a level almost the same as the control group while
propofol treatment only restored cell apoptosis to twice the level
of that in the control group, indicating that sevoflurane treatment
could dramatically compromise the effect of H/R injury on cell
apoptosis, better than propofol treatment (Fig. 1C). The aforementioned results thus,
suggest that cardiomyocytes with H/R injury could be better
protected by sevoflurane compared with propofol.
Sevoflurane-rather than
propofol-induced ADAM8 upregulation
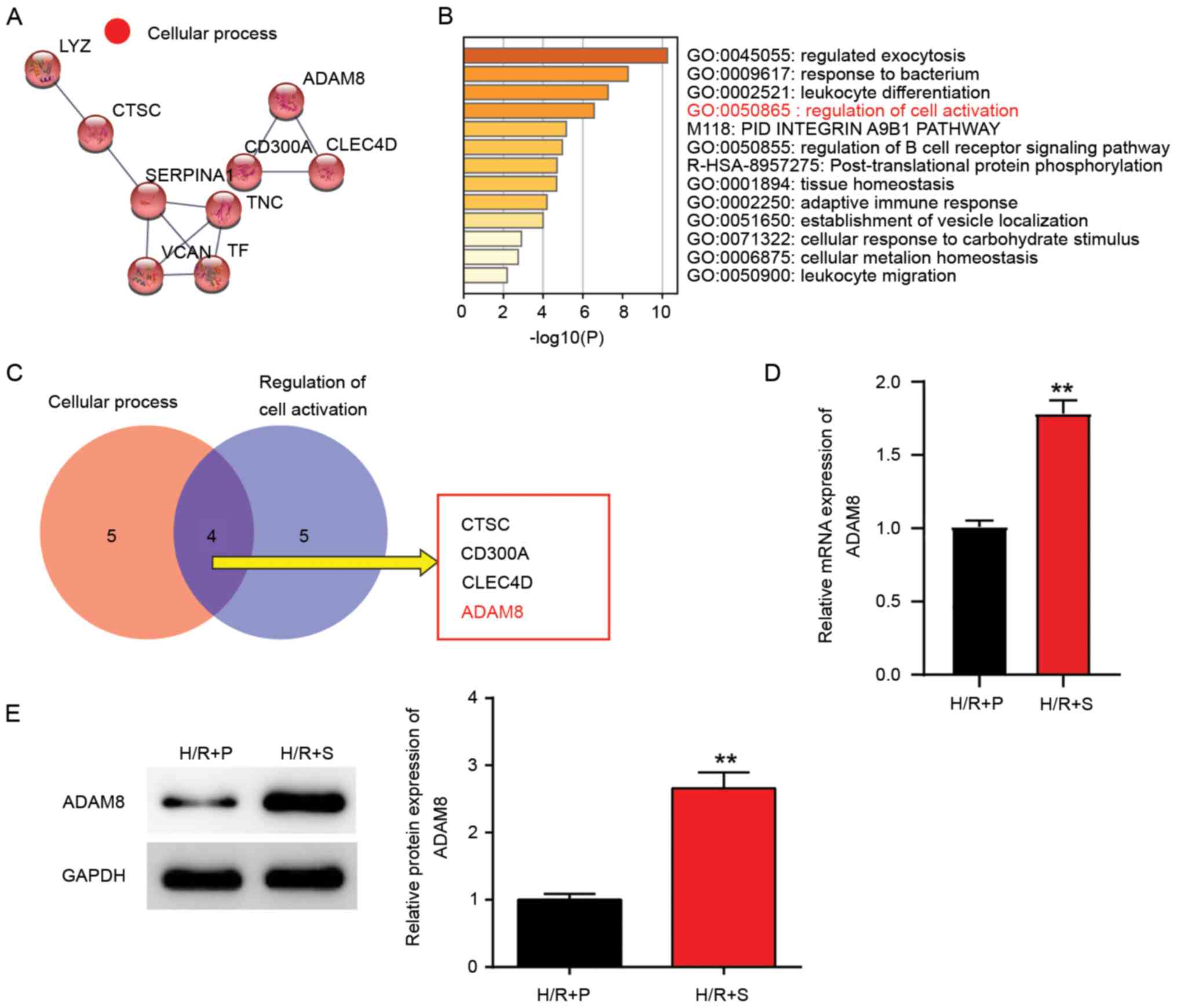
In order to identify the key genes associated with
sevoflurane and propofol, GSE4386 downloaded from the GEO datasets
was used. A total of 37 upregulated genes with P<0.05 and log
fold change (FC) of >1 were screened out and uploaded to STRING
and Metascape for biological process analysis (Fig. 2A and B). Then, the Venny 2.1.0 analysis result
showed that CTSC, CD300A, CLEC4D and ADAM8 were associated with
cellular process and the regulation of cell activation (Fig. 2C). A review of the literature
revealed that ADAM8 upregulation was associated with I/R injury in
rat hearts after cardiac arrest (17), therefore, the function of ADAM8 was
explored in cardiomyocytes under H/R injury. Next, both the mRNA
and protein expression levels of ADAM8 were examined in
cardiomyocytes with the treatment of H/R+S and H/R+P. The RT-qPCR
assay (Fig. 2D) showed that ADAM8
mRNA expression was ~75% higher in the H/R+S group compared with
the H/R+P group. Likewise, western blotting (Fig. 2E) showed that the protein expression
of ADAM8 in the H/R+S group was much higher (50% higher vs. H/R+P
group), in agreement with the result of mRNA expression analysis.
Taken together, the higher expression of ADAM8 in the H/R+S group
indicated that sevoflurane might cause the healing of the H/R
injury in cardiomyocytes by co-activating ADAM8.
Sevoflurane exerts improved protective
effect than propofol by regulating ADAM8
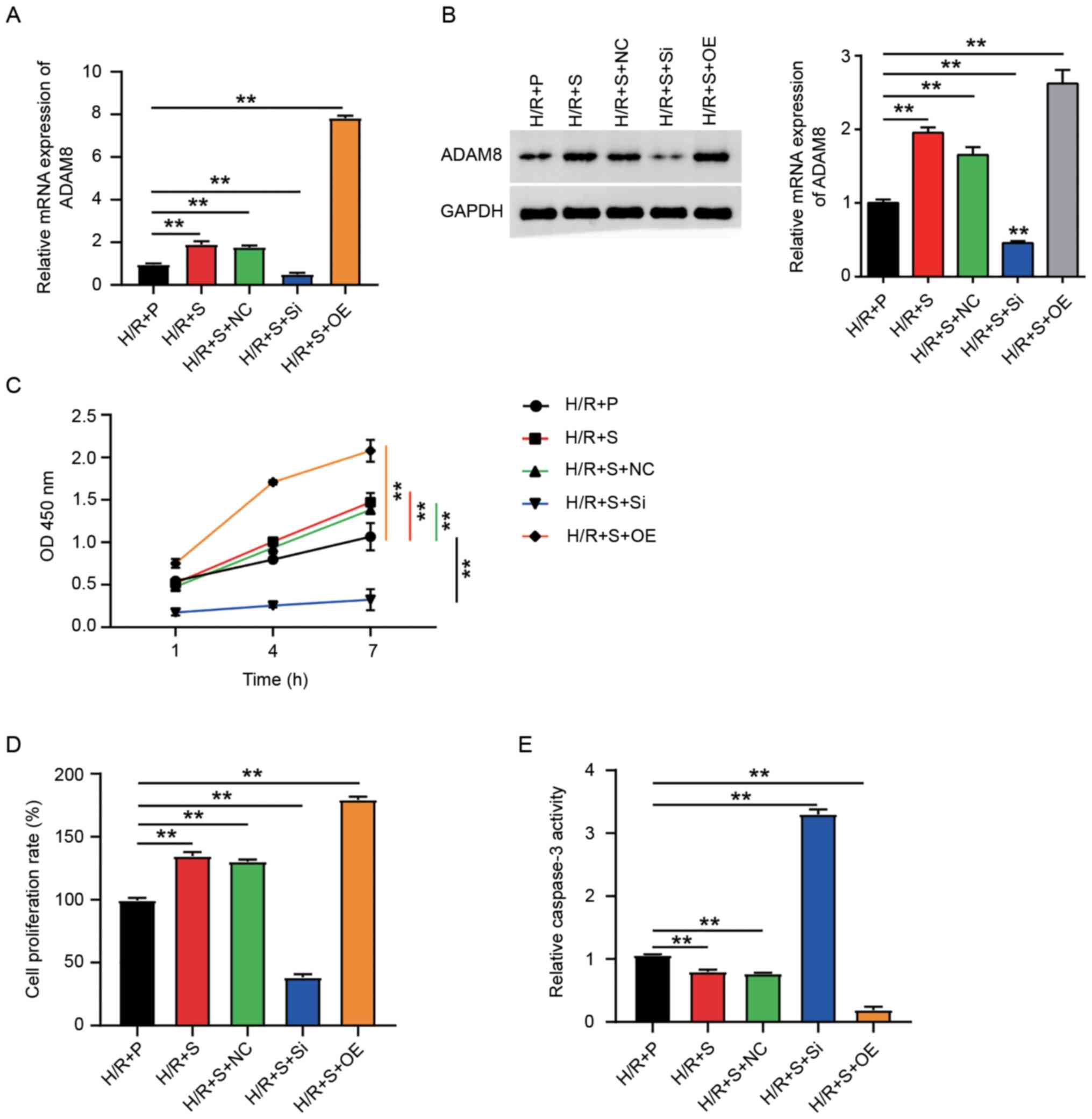
According to the aforementioned results, ADAM8 could
be involved in sevoflurane-mediated healing of H/R injury in
cardiomyocytes. Therefore, the association between ADAM8 and
sevoflurane was further explored. After successfully transfecting
H9C2 cells with si-ADAM8 and ADAM8-overexpression vector (Fig. S1A), ADAM8 mRNA and protein levels
were successfully upregulated by the vector overexpressing ADAM8
and downregulated by si-ADAM8 in H9C2 cells treated with H/R+S
(Fig. 3A and B). It is suggested that the
ADAM8-overexpression vector and si-ADAM8 could successfully
transfect H9C2 cells for use in the subsequent verifications
(CCK-8, BrdU and caspase-3 activity assays). CCK-8 assay result
(Fig. 3C) showed that ADAM8
overexpression could dramatically increase cell viability while
si-ADAM8 decreased cell viability. Likewise, the BrdU assay
(Fig. 3D) demonstrated that
compared with the H/R+P group, ADAM8 overexpression increases cell
proliferation activity by ~75%, while si-ADAM8 decreased cell
proliferation activity by ~60%. Caspase-3 activity assay was
carried out to investigate the effect of ADAM8 on cell apoptosis.
The results (Fig. 3E) revealed that
ADAM8 overexpression inhibited cell apoptosis by ~70% relative to
the H/R+P group, while si-ADAM8 increased the rate of cell
apoptosis by more than three-time level in the H/R+P group, and a
much higher caspase-3 activity compared with that in the H/R+P
group. The aforementioned results suggest the important role of
ADAM8 in sevoflurane-mediated improvement in cardiomyocyte survival
from H/R injury.
miR-221-5p targets ADAM8 in the
regulation of hypoxia-reoxygenation injury in cardiomyocytes
The subsequent focus of the study was on the
upstream of ADAM8 to further investigate the regulatory mechanisms
of ADAM8 in the protection from H/R injury in cardiomyocytes and
examined the epigenetic regulation of ADAM8 by miRNA. TargetScan
was used to identify the potential miRNAs that targeted ADAM8, and
the top 4 miRNAs binding to ADAM8 were predicted with Centext score
percentile rank (Fig. 4A). The
present study focused on exploring the association between
miR-221-5p and ADAM8, since the miR-221-5p was the top
most-predicted miRNA. The sequence of miR-221-5p matched with the
position 274-281 of ADAM8 3'-UTR (Fig.
4B). After detecting the transfection efficiency of the
miR-221-5p mimic and inhibitor (Fig.
S1B), dual-luciferase reporter assay was used to verify whether
miR-221-5p could bind to ADAM8 3'-UTR. It was shown that the
co-transfection of ADAM8 wild type and miR-221-5p mimic could
significantly suppress the luciferase activity by ~60% compared
with the control group, whereas ADAM8 mutant type and miR-221-5p
mimic failed to decrease the luciferase activity (Fig. 4C), confirming the target association
between miR-221-5p and ADAM8 3'-UTR. Given that miR-221-5p could
target ADAM8, the expression level of miR-221-5p was assessed in
H/R+S- or H/R+P-treated H9C2 cells. The result depicted that the
expression of miR-221-5p in the H/R+S group was only 30% of that in
the H/R+P group, which was completely in contrast to the expression
of ADAM8 (Fig. 4D). Western
blotting assay was performed after the transfections of si-ADAM8,
miR-221-5p inhibitor and si-ADAM8 plus miR-221-5p inhibitor.
Silencing miR-221-5p could dramatically enhance the protein
expression of ADAM8 in cells treated with H/R+S (Fig. 4E). Although si-ADAM8 was capable of
decreasing the protein expression of ADAM8, the protein expression
level of ADAM8 could be upregulated on inhibiting miR-221-5p. Taken
together, sevoflurane is able to decrease miR-221-5p expression and
further release the inhibition of ADAM8; thus, playing a role in
H/R injury in cardiomyocytes.
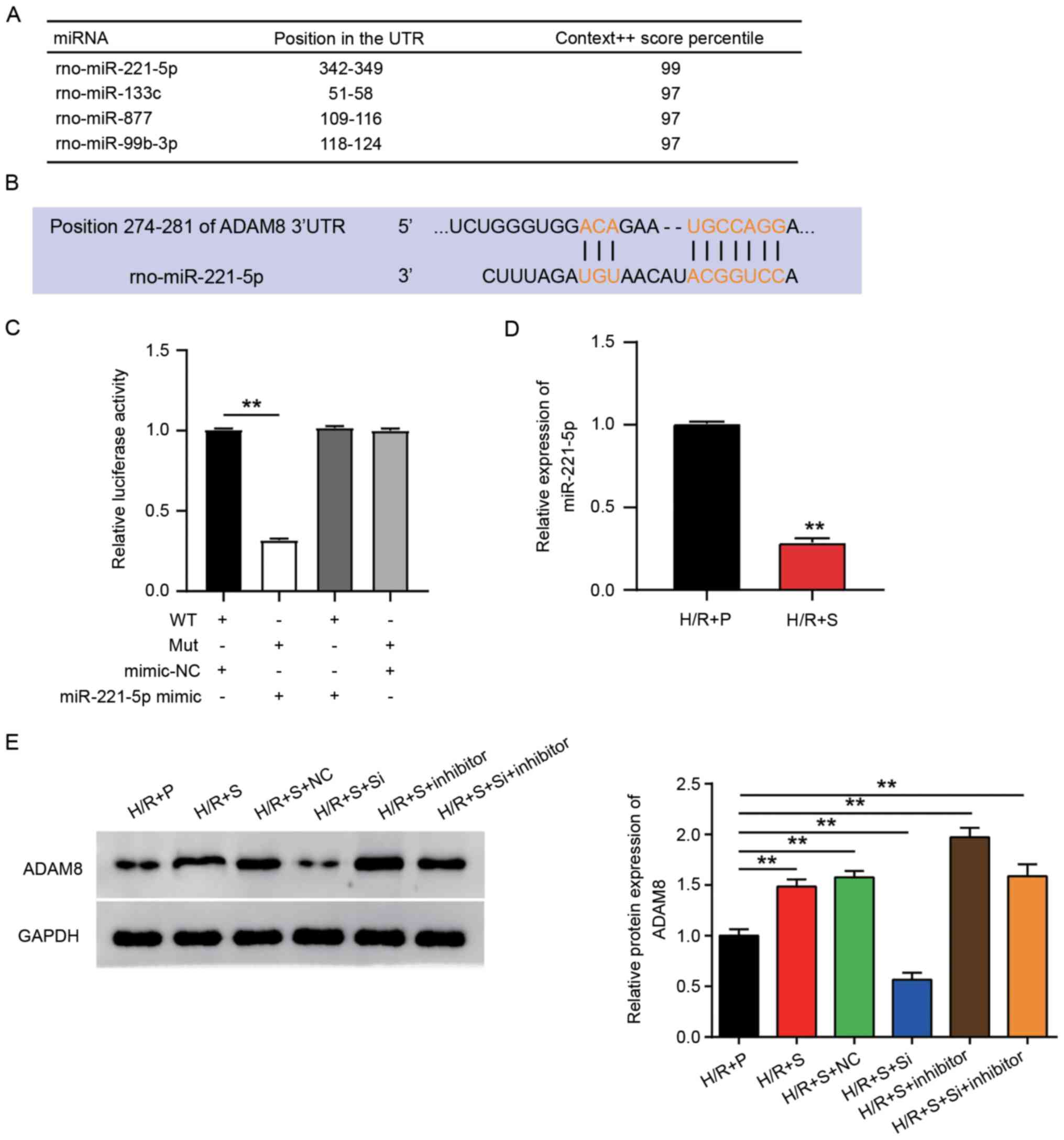 | Figure 4miR-221-5p targets ADAM8 in the
regulation of H/R injury in cardiomyocytes. (A) TargetScan was
applied to predict the potential miRNAs that might target ADAM8.
The top 4 miRNAs are listed. (B) The location to which miR-221-5p
binds ADAM8. (C) Dual-luciferase reporter assay was performed to
verify the target association between miR-221-5p and ADAM8. One-way
ANOVA and Unpaired t-test were used for the statistical analysis.
**P<0.001. (D) H9C2 cells were subjected to H/R
injury and treated with sevoflurane or propofol, and then mRNA
expression of miR-221-5p was determined by reverse
transcription-quantitative PCR assay. Unpaired t-test was used for
the statistical analysis. **P<0.001. (E) H9C2 cells
were subjected to H/R injury and treated with sevoflurane or
propofol, and then protein expression of miR-221-5p was determined
by western blotting assay. One-way ANOVA was used for the
statistical analysis. Data are presented as mean ± SD.
**P<0.001. miR/miRNA, microRNA; H/R,
hypoxia-reoxygenation; +S, with sevoflurane treatment; +P, with
propofol treatment; NC, negative control; si-, small interfering
RNA; WT, wild type; mut, mutant. |
Inhibitory effect of miR-221-5p in the
protection of hypoxia-reoxygenation injury in cardiomyocytes when
treated with sevoflurane or propofol
Since miR-221-5p could target and inhibit the
expression level of ADAM8, the activity of H9C2 cells treated with
sevoflurane or propofol was examined after silencing miR-221-5p by
an inhibitor. After transfections with si-ADAM8, miR-221-5p
inhibitor and si-ADAM8 plus miR-221-5p inhibitor in H9C2 cells
treated with H/R+S, respectively, CCK-8, BrdU, and caspase-3
activity assay were conducted. CCK-8 assay (Fig. 5A) showed that miR-221-5p inhibitor
could increase cell viability relative to the H/R+P group. However,
when miR-221-5p inhibitor and si-ADAM8 were added, cell viability
suppressed by si-ADAM8 increased to the same level as that in the
H/R+S+NC group. Similar to the CCK-8 assay, the BrdU assay also
suggested (Fig. 5B) that miR-221-5p
inhibitor could increase cell proliferation activity by ~80%
relative to the H/R+P group. However, in the
H/R+S+si-ADAM8+miR-221-5p inhibitor group, cell proliferation
activity was compromised to a level the same as that in the
H/R+S+NC group. A caspase-3 activity assay was conducted to explore
the effect of the miR-221-5p inhibitor on cell apoptosis and
observed (Fig. 5C) a dramatic
inhibition, by ~70%, in cell apoptosis in the miR-221-5p inhibitor
group relative to the H/R+P group. In spite of this, the negative
effect of the miR-221-5p inhibitor on cell apoptosis could be
relieved by si-ADAM8 co-transfection. These results indicate that
the viability of sevoflurane or propofol-treated H9C2 cells can be
enhanced by inhibiting miR-221-5p for the protection from H/R
injury in cardiomyocytes.
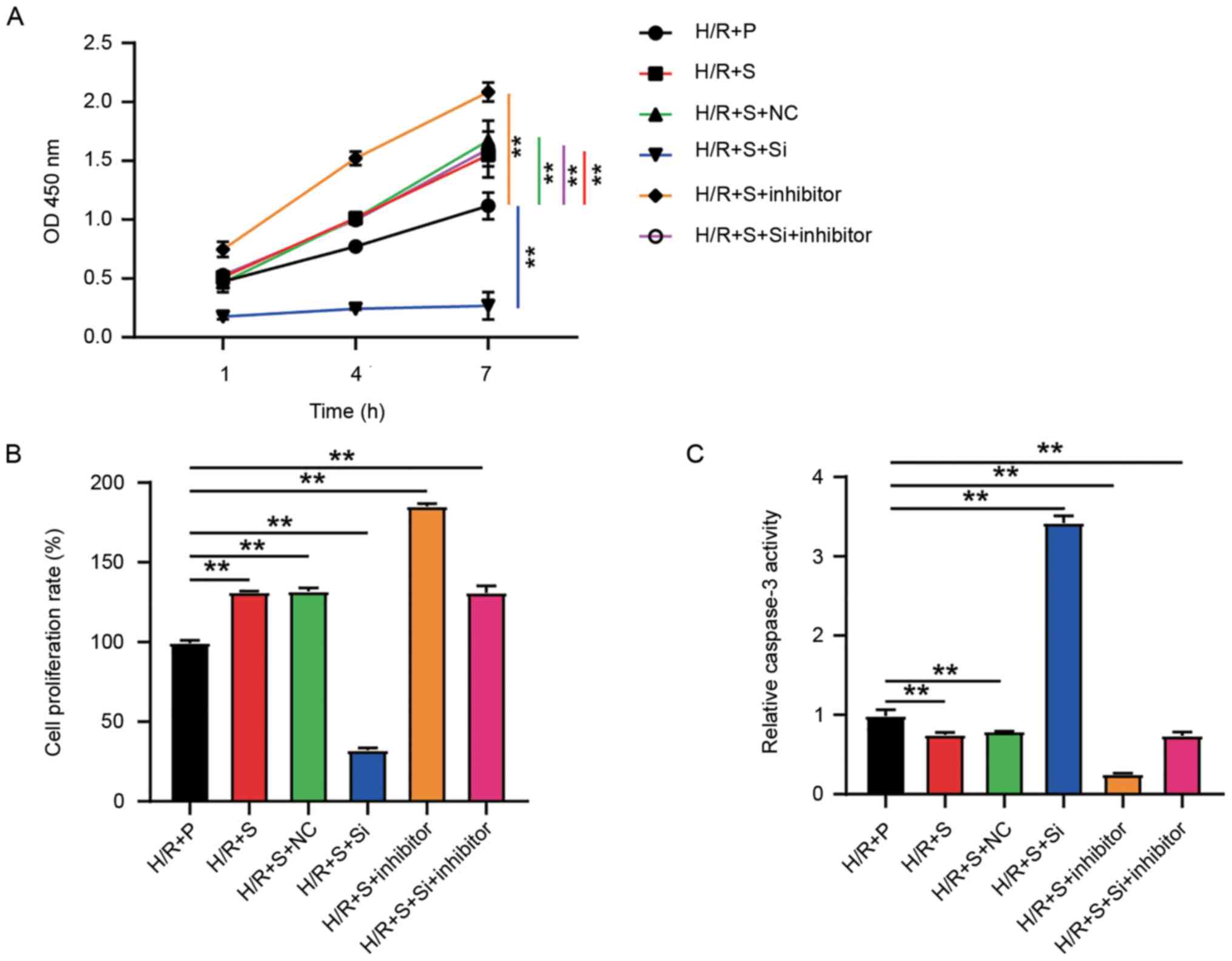 | Figure 5miR-221-5p exhibits an inhibitory
effect on the protection of H/R injury in cardiomyocytes treated
with sevoflurane or propofol. (A) Cell viability was detected by
Cell Cycle Kit-8 assay after H9C2 cells were treated with H/R+P,
and after the transfections with si-ADAM8, miR-221-5p inhibitor or
si-ADAM8 plus miR-221-5p inhibitor in H9C2 cells treated with
H/R+S, respectively. (B) Cell proliferation activity was measured
by BrdU assay after H9C2 cells were treated with H/R+P and after
the transfections with si-ADAM8, miR-221-5p inhibitor or si-ADAM8
plus miR-221-5p inhibitor in H9C2 cells treated with H/R+S,
respectively. (C) Cell apoptosis was determined by caspase-3
activity assay after H9C2 cells were treated with H/R+P and after
the transfections with si-ADAM8, miR-221-5p inhibitor or si-ADAM8
plus miR-221-5p inhibitor in H9C2 cells treated with H/R+S,
respectively. Data are presented as mean ± SD.
**P<0.001. One-way ANOVA was used for the statistical
analysis. H/R, hypoxia-reoxygenation; +S, hypoxia-reoxygenation
injury with sevoflurane treatment; +P, hypoxia-reoxygenation injury
with propofol treatment; si-, small interfering RNA; NC, negative
control. |
Discussion
Firstly, the effects of anesthetics (sevoflurane and
propofol) on cardiomyocytes (H9C2 cells) with H/R injury were
investigated and found that sevoflurane had a better protective
effect compared with propofol on these cardiomyocytes. Then, the
in-depth mechanism underlying the improved cardioprotective effect
of sevoflurane on H9C2 cells exposed to H/R injury was explored.
miR-221-5p/ADAM8 axis was found to participate in the regulation of
sevoflurane in protecting the cardiomyocytes suffering from H/R
injury. ADAM8 was found to improve cell activity, while miR-221-5p
had an opposite effect on cardiomyocytes with anesthetics treatment
and H/R injury. Overall, it was demonstrated that when
cardiomyocytes are suffering from H/R injury, sevoflurane is able
to regulate the miR-221-5p/ADAM8 axis and exert better protective
effects than propofol.
Until now, multiple studies have shown that
sevoflurane is helpful in myocardial protection. For instance,
studies have assessed the cardioprotective effect of sevoflurane
postconditioning in diabetic rats (22) and the protective role of sevoflurane
against the early stage of I/R injury (23). The present study attempted to
explore the function of sevoflurane in myocardial H/R cases.
Consistent with the previous studies, the present study results
provide new evidence of the important role of sevoflurane in
protecting cardiomyocytes from I/R injury. It could be confirmed
that sevoflurane could protect H9C2 cells from H/R injury by
stimulating cell viability and proliferation, while hindering cell
apoptosis. In addition to sevoflurane, propofol was also proved to
play a protective role in cardiomyocytes in previous studies.
Propofol plays a cardioprotective role in myocardial I/R injury
through MAPK/ERK signaling pathway (24) and showed a cardioprotective effect
on immature hearts at clinically relevant concentrations (25). In the present study, it was also
observed that propofol exerted a protective effect on H9C2 cells
exposed to H/R injury and that sevoflurane was more protective than
propofol in the case of H/R injury in cardiomyocytes. This is in
agreement with a previous study (26), which demonstrated that sevoflurane
provides greater protection than propofol to the myocardium in
patients undergoing off-pump coronary artery bypass surgery and
thus provides a reference value in the protection of cardiomyocytes
with I/R injury.
ADAM8 was originally identified to be an
immune-specific factor (16). In
2019, Schick et al (27)
demonstrated the close association of ADAM8 with vascular disease
markers, and since then, ADAM8 has been used as an important
biomarker in cardiovascular diseases. In the present study, ADAM8
was also determined as a biomarker involved in cardiomyocyte
injury. Further investigation revealed that ADAM8 expression is
suppressed by propofol treatment (28). Similarly, hypoxia could
significantly induce, and propofol could effectively inhibit ADAM8
in another study (29). These two
reports are concurrent with the present results that indicated a
lower ADAM8 expression level in H9C2 cells treated with propofol
than sevoflurane. This indicated that the better cardioprotective
effect of sevoflurane than propofol might rely on the increase in
ADAM8 expression level. As expected, the present study results
showed that ADAM8 was able to enhance the protective effect of
sevoflurane rather than propofol by facilitating cell viability,
cell proliferation, and impeding cell apoptosis of H9C2 cells
subjected to H/R injury. Another research demonstrated the
promotional effect of ADAM8 on cell growth and is in accordance
with the present study data showing that knockdown of ADAM8 notably
inhibited cell proliferation (30).
In short, the present study results provide evidence that the
prominent protective effect of sevoflurane was promoted by ADAM8
overexpression, which contributed significantly to cell viability
and proliferation; thus, enhancing the activity and survival of
cardiomyocytes exposed to H/R injury.
To elaborate on the potential factors that might
participate in ADAM8-mediated protection of cardiomyocytes with H/R
injury, the relevant epigenetic mechanism was examined. Using
TargetScan and dual-luciferase reporter assay, ADAM8 was predicted
and determined as a novel target of miR-221-5p, which exerted a
contrasting effect to that of ADAM8. miR-221-5p was earlier found
to be expressed at a high level in cardiac hypertrophy and heart
failure (20). It was hypothesized
that miR-221-5p would attenuate the cardioprotective effect of
sevoflurane or propofol in H9C2 cells subjected to H/R injury. As
expected, the present findings collectively suggested that
knockdown of miR-221-5p promoted cell viability and proliferation
and inhibited cell apoptosis, which could strengthen the protective
effect of sevoflurane on cardiomyocytes with heart diseases.
However, the present study did not further
investigate the other participants in the downstream signaling
pathway of ADAM8. In addition, the present study was restricted to
in vitro experiments; in vivo experiments would
provide more validation if carried out in the future. Moreover,
more clinical analysis and practice are required to verify the
present study data and to get a deeper understanding of the
mechanisms of sevoflurane, propofol, miR-221-5p and ADAM8 in the
recovery and protection of myocardial tissue with H/R injury.
Overall, the present study results show the
mechanisms of improved cardioprotective effect by sevoflurane
compared with propofol on cardiomyocytes suffering H/R injury
through the miR-221-5p/ADAM8 axis. The present study also revealed
that sevoflurane protected cardiomyocytes via the upregulation of
ADAM8 and downregulation of miR-221-5p. The present study provides
novel evidence for the therapeutic strategy against cardiovascular
diseases induced by myocardial I/R injury.
Supplementary Material
Transfection efficiency of si-ADAM8,
ADAM8-overexpression vector, miR-221-5p mimic and miR-221-5p
inhibitor. (A) Transfection efficiency of si-ADAM8 and
ADAM8-overexpression vector. (B) The transfection efficiency of
miR-221-5p mimic and miR-221-5p inhibitor. **P<0.001,
##P<0.001. si, small interfering RNA; si-NC, negative
control of siRNA; OE-NC, pcDNA 3.1 empty vector; ADAM8-OE, ADAM8
overexpression vector; miR, microRNA.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DX performed the experiments and data analysis. HFD
conceived and designed the study. HF designed the methodology. HFD
and HF confirm the authenticity of all the raw data. DX wrote the
manuscript. HFD reviewed and edited the manuscript. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Androulakis AE, Andrikopoulos GK, Richter
DJ, Tentolouris CA, Avgeropoulou CC, Adamopoulos DA, Toutouzas PK,
Trikas AG, Stefanadis CI and Gialafos JE: The role of carotid
atherosclerosis in the distinction between ischaemic and
non-ischaemic cardiomyopathy. Eur Heart J. 21:919–926.
2000.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Li J, Zhang H and Zhang C: Role of
inflammation in the regulation of coronary blood flow in ischemia
and reperfusion: Mechanisms and therapeutic implications. J Mol
Cell Cardiol. 52:865–872. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lin Y, Huang S, Chen Y, Wu Z, Liang Z, Zou
M and Chen C: Helix B surface peptide protects cardiomyocytes from
hypoxia/reoxygenation-induced autophagy through the PI3K/Akt
pathway. J Cardiovasc Pharmacol. 76:181–188. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sun J, Li YZ, Ding YH, Wang J, Geng J,
Yang H, Ren J, Tang JY and Gao J: Neuroprotective effects of gallic
acid against hypoxia/reoxygenation-induced mitochondrial
dysfunctions in vitro and cerebral ischemia/reperfusion injury in
vivo. Brain Res. 1589:126–139. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hwang IC, Kim JY, Kim JH, Lee JE, Seo JY,
Lee JW, Park J, Yang HM, Kim SH, Cho HJ and Kim HS: Therapeutic
potential of a novel necrosis inhibitor, 7-amino-indole, in
myocardial ischemia-reperfusion injury. Hypertension. 71:1143–1155.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chen X, Li X, Zhang W, He J, Xu B, Lei B,
Wang Z, Cates C, Rousselle T and Li J: Activation of AMPK inhibits
inflammatory response during hypoxia and reoxygenation through
modulating JNK-mediated NF-κB pathway. Metabolism. 83:256–270.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liang S, Aiqun M, Figtree G and Ping Z:
GAPDH-silence preserves H9C2 cells from acute hypoxia and
reoxygenation injury. Int J Biol Macromol. 81:375–386.
2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yu J, Wu J, Xie P, Maimaitili Y, Wang J,
Xia Z, Gao F, Zhang X and Zheng H: Sevoflurane postconditioning
attenuates cardiomyocyte hypoxia/reoxygenation injury via restoring
mitochondrial morphology. PeerJ. 4(e2659)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cope DK, Impastato WK, Cohen MV and Downey
JM: Volatile anesthetics protect the ischemic rabbit myocardium
from infarction. Anesthesiology. 86:699–709. 1997.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang Y, Chen Z, Feng N, Tang J, Zhao X,
Liu C, Xu H and Zhang M: Protective effect of propofol
preconditioning on ischemia-reperfusion injury in human hepatocyte.
J Thorac Dis. 9:702–710. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Navapurkar VU, Skepper JN, Jones JG and
Menon DK: Propofol preserves the viability of isolated rat
hepatocyte suspensions under an oxidant stress. Anesth Analg.
87:1152–1157. 1998.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li YC, Ridefelt P, Wiklund L and
Bjerneroth G: Propofol induces a lowering of free cytosolic calcium
in myocardial cells. Acta Anaesthesiol Scand. 41:633–638.
1997.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lu Y, Bu M and Yun H: Sevoflurane prevents
hypoxia/reoxygenation-induced cardiomyocyte apoptosis by inhibiting
PI3KC3-mediated autophagy. Hum Cell. 32:150–159. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Takahashi Y, Shibata T, Sasaki Y, Hirai H,
Takemura S, Minamiyama Y, Sakaguchi M and Suehiro S: Pre-ischemic
administration of landiolol prevents ischemia-reperfusion injury in
the rat heart. Osaka City Med J. 53:9–16. 2007.PubMed/NCBI
|
|
15
|
Wolfsberg TG, Straight PD, Gerena RL,
Huovila AP, Primakoff P, Myles DG and White JM: ADAM, a widely
distributed and developmentally regulated gene family encoding
membrane proteins with a disintegrin and metalloprotease domain.
Dev Biol. 169:378–383. 1995.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yoshiyama K, Higuchi Y, Kataoka M,
Matsuura K and Yamamoto S: CD156 (human ADAM8): Expression, primary
amino acid sequence, and gene location. Genomics. 41:56–62.
1997.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Vuohelainen V, Raitoharju E, Levula M,
Lehtimaki T, Pelto-Huikko M, Honkanen T, Huovila A, Paavonen T,
Tarkka M and Mennander A: Myocardial infarction induces early
increased remote ADAM8 expression of rat hearts after cardiac
arrest. Scand J Clin Lab Invest. 71:553–562. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu J, Wang J, Ning Y and Chen F: The
inhibition of miR-101a-3p alleviates H/R injury in H9C2 cells by
regulating the JAK2/STAT3 pathway. Mol Med Rep. 21:89–96.
2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Huang P, Lin Q and Liu X: MicroRNA-142-5p
inhibits autophagy in cardiomyocytes in a mouse model of
hypoxia/reoxygenation. FEBS Open Bio: May 11, 2020 (Epub ahead of
print).
|
|
20
|
Shah R, Ziegler O, Yeri A, Liu X, Murthy
V, Rabideau D, Xiao CY, Hanspers K, Belcher A, Tackett M, et al:
MicroRNAs associated with reverse left ventricular remodeling in
humans identify pathways of heart failure progression. Circ Heart
Fail. 11(e004278)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xie P, Yang L, Talaiti A, Wu JJ, Yu J, Yu
T, Wang HY, Huang B, Wu Q, Maimaitili Y, et al:
Deferoxamine-activated hypoxia-inducible factor-1 restores
cardioprotective effects of sevoflurane postconditioning in
diabetic rats. Acta Physiol (Oxf). 221:98–114. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ohsumi A, Marseu K, Slinger P, McRae K,
Kim H, Guan Z, Hwang DM, Liu M, Keshavjee S and Cypel M:
Sevoflurane attenuates ischemia-reperfusion injury in a rat lung
transplantation model. Ann Thorac Surg. 103:1578–1586.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yan HJ, Qi GQ and Ma Y: Effect of propofol
on myocardial ischemia-reperfusion injury through MAPK/ERK pathway.
Eur Rev Med Pharmacol Sci. 23:11051–11061. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shirakawa M, Imura H and Nitta T: Propofol
protects the immature rabbit heart against ischemia and reperfusion
injury: Impact on functional recovery and histopathological
changes. Biomed Res Int. 2014(601250)2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Conzen PF, Fischer S, Detter C and Peter
K: Sevoflurane provides greater protection of the myocardium than
propofol in patients undergoing off-pump coronary artery bypass
surgery. Anesthesiology. 99:826–833. 2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Schick D, Babendreyer A, Wozniak J, Awan
T, Noels H, Liehn E, Bartsch JW, Vlacil AK, Grote K, Zayat R, et
al: Elevated expression of the metalloproteinase ADAM8 associates
with vascular diseases in mice and humans. Atherosclerosis.
286:163–171. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yu X, Shi J, Wang X and Zhang F: Propofol
affects the growth and metastasis of pancreatic cancer via ADAM8.
Pharmacol Rep. 72:418–426. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gao Y, Yu X, Zhang F and Dai J: Propofol
inhibits pancreatic cancer progress under hypoxia via ADAM8. J
Hepatobiliary Pancreat Sci. 26:219–226. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Yang Z, Bai Y, Huo L, Chen H, Huang J, Li
J, Fan X, Yang Z, Wang L and Wang J: Expression of A disintegrin
and metalloprotease 8 is associated with cell growth and poor
survival in colorectal cancer. BMC Cancer. 14(568)2014.PubMed/NCBI View Article : Google Scholar
|