Introduction
Human cytomegalovirus is a herpesvirus, which is
widespread in people, and most infected individuals exhibit no
specific clinical characteristics. According to statistics, the
infection rate of this virus in adults in the West is between 60
and 70% (1). However, children are
the main vulnerable group of the virus. The body function of
children is not fully developed and their immune function is weaker
than that of normal adults, suggesting that more attention should
be paid to children infected with cytomegalovirus with regard to
diagnosis and treatment (2).
Cytomegalovirus often leads to pathological changes in the lung and
liver of children, and its infection is one of the primary risk
factors for hearing damage in children, which can lead to dysplasia
of brain and neuron, and even death (3-5).
Therefore, it is imperative to evaluate the severity of illness and
efficacy on children and to implement effective treatments.
MicroRNA is a non-coding single-stranded small RNA
molecule in human body, which affects the development and
progression of some diseases and can be used as a potential
indicator of various diseases (6-8).
Previous findings have shown that there are some exogenous
microRNAs stemming from viruses and microorganisms in the human
body that participate in the development and progression of some
diseases (9). miR-UL112-3p is a
microRNA encoded by human cytomegalovirus, which is involved in
viral transcriptional activation and immune evasion, and is related
to the regulation of cell cycle, proliferation and apoptosis
(10). One study by Pan et
al (11) has found another
microRNA encoded by cytomegalovirus that may be used as a
therapeutic indicator for hepatitis B. Therefore, we suspected that
miR-UL112-3p can also be used to evaluate the efficacy on
children.
Ganciclovir is a common antiviral drug in clinical
practice, with relatively good efficacy on cytomegalovirus
infection in children (12).
However, there are some side effects in the treatment process,
causing symptoms such as myelosuppression (13). The liver function of children
infected with cytomegalovirus is often damaged. However, findings
of recent studies have shown that ganciclovir can improve the liver
function of patients with hepatitis C virus (HCV) liver fibrosis,
and can alleviate hepatitis Caused by cytomegalovirus infections in
children (14,15). However, few studies are available on
the effect of small-dose ganciclovir on the liver function of
children infected with cytomegalovirus, the specific efficacy and
safety thereof. and its effects on miR-UL112-3p.
Therefore, the aim of the current study was to
examine the efficacy of small-dose ganciclovir and
conventional-dose ganciclovir on children infected with
cytomegalovirus, and analyze the changes of the liver function and
miR-UL112-3p expression in children before and after treatment, to
provide a basis and direction for the clinical application of
ganciclovir.
Materials and methods
Patients
A total of 141 children infected with
cytomegalovirus admitted to the Affiliated Hospital of Weifang
Medical University from May 2015 to August 2017 were enrolled, of
which 74 children were treated with small-dose ganciclovir as an
observation group (Obs group), and the rest were treated with
conventional-dose ganciclovir as the control group (Con group). The
Obs group comprised 43 males and 31 females, with an average age of
(3.15±0.85) years, and the Con group consisted of 38 males and 29
females, with an average age of (3.06±0.72) years.
This study was approved by the Medical Ethics
Committee of the Affiliated Hospital of Weifang Medical University
(SD-2015-265), and written informed consent was obtained from legal
guardiansand/or parent(s) of the children after the guardians
understood the study.
Inclusion and exclusion criteria
The inclusion criteria of the study were: i)
Patients confirmed with cytomegalovirus infections according to
virology and pathology, ii) patients <18 years old, iii)
patients without allergic reaction to drugs used in this study, iv)
patients with detailed clinical data, and v) patients willing to
receive the therapy and follow-up.
The exclusion criteria included: i) Patients who had
received antiviral treatment within one month before admission, ii)
patients with other comorbid liver or kidney diseases, or other
viral infectious diseases, and iii) patients with congenital immune
diseases.
Main kits and instruments
PCR instrument (Applied Biosystems 7500),
electrophoresis apparatus (Bio-Rad Mini-PROTEAN), ultraviolet
spectrophotometer (Beckman Coulter, DU 700), fully automatic
biochemical analyser (Abbott Aeroset 2000), total RNA extraction
kit (EasyPure miRNA Kit) and reverse transcription + PCR kit
(TransScript miRNA First-Strand cDNA Synthesis SuperMix; TransGen
Biotech Co., Ltd.; nos. ER601-01, AT351-01). Primers were designed
and synthesized by Shanghai Sangon Biotech Co., Ltd. (Table I).
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene | Upstream primer | Downstream
primer |
|---|
|
miR-UL112-3p |
5'-AAGTGACGGTGAGATCCAGGCT-3' |
5'-CCTCCGGATCACATGGTTACTCA-3' |
| U6 |
5'-CTCGCTTCGGCAGCACA-3' |
5'-AACGCTTCACGAATTTGCGT-3' |
Treatment methods
Children in the Obs group were treated with
small-dose ganciclovir as follows: They were given ganciclovir
mixed with 5% glucose solution through intravenous drip at 3 mg/kg
(12 h/time) for a course of 6 weeks. By contrast, children in the
Con group were treated with conventional-dose ganciclovir as
follows: They were given ganciclovir mixed with 5% glucose solution
through intravenous drip at 5 mg/kg (12 h/time) for a course of 6
weeks (16).
Basis for efficacy evaluation
The efficacy on the two groups was analyzed, and the
number of patients with markedly effective treatment, effective
treatment or ineffective treatment was counted, separately.
Treatment with the following outcomes was determined as markedly
effective: Disappearance of clinical symptoms and signs, and
retraction of liver and spleen to normal. Treatment with the
following outcomes was determined as effective: Relief of clinical
symptoms and signs, and significant retraction of liver and spleen.
Treatment with the following outcomes was determined as
ineffective: No significant relief of the disease or aggravation
for the patient (17). The total
number of cases with effective treatment were calculated as: The
number of cases with markedly effective treatment + the number of
cases with effective treatment.
Determination methods
Venous blood (5 ml) was sampled from each child, and
placed in a coagulation promoting tube, and centrifuged at 3,000 x
g and 4˚C for 10 min for serum collection, and the miR-UL112-3p
expression in the serum was detected using a reverse transcription
quantitative PCR (RT-qPCR) assay. Total RNA was extracted from the
collected serum with the EasyPure miRNA Kit, and its concentration,
purity and integrity were detected by an ultraviolet
spectrophotometer and 1% agarose gel electrophoresis. DNA was
extracted using a TIANamp genomic DNA kit (Tianjian Biotechnology
Co., Ltd.), and the CMV-DNA level was quantified using a CMV-DNA
diagnostic kit (Daan Gene Co., Ltd.). Reverse transcription was
carried out to the total RNA using the TransScript®
miRNA RT Enzyme Mix and 2X TS miRNA Reaction Mix according to the
protocol. PCR amplification was carried out, and the PCR system
consisted of 20 µl total volume containing 1 µl cDNA, 0.4 µl
upstream primers, 0.4 µl downstream primers,10 µl 2X
TransTaq® Tip Green qPCR SuperMix, 0.4 µl Passive
Reference Dye (X50), and ddH2O added to adjust the
volume. The PCR conditions were: 94˚C for 30 sec, followed by 40
cycles at 94˚C for 5 sec and 60˚C for 30 sec. Three repeated wells
were set for each sample, and the experiment was repeated three
times. In the study, U6 was taken as an internal reference
and data were analyzed using 2-ΔΔct (18). The total bilirubin (TB), alanine
aminotransferase (ALT), and aspartate aminotransferase (AST) were
detected using the full automatic biochemical analyzer.
Outcome measures
Primary outcome measures: The Obs group and the Con
group were compared in terms of efficacy and changes of liver
function indexes (TB, ALT, and AST) and miR-UL112-3p before and
after treatment, and Pearson's correlation analysis was carried out
to analyze the correlation of serum miR-UL112-3p expression and TB,
ALT and AST levels in all children before treatment.
Secondary outcome measures: The clinical baseline
data of the two groups were compared (sex, age, course of disease,
family size, place of residence, mother's pregnancy age, mother's
pregnancy condition, family history of cytomegalovirus infections,
and clinical symptoms), and total adverse reactions including
gastrointestinal reactions, leucopenia, thrombocytopenia,
dizziness, and pruritus of the two groups were also compared after
treatment. Additionally, a receiver operating characteristic (ROC)
curve was drawn to analyze the predictive value of miR-UL112-3p for
cytomegalovirus infections before treatment, andthe AUC, 95% CI,
cut-off point, Youden index, specificity and sensitivity of
miR-UL112-3p for predicting efficacy in the two groups were
evaluated.
Statistical analysis
The collected data were analyzed using SPSS20.0
(Cabit Information Technology Co., Ltd.), and visualized into
required figures using GraphPad Prism 7 (Softhead Software
Technology Co., Ltd.). Enumeration data were expressed as usage
(%), and analyzed through the Chi-square test, and expressed by
χ2. Measurement data were expressed as the mean ±
standard deviation (mean ± SD). The relationship among the factors
was carried out using the two-way ANOVA, and Bonferroni post hoc
test was used for correlation. Pearson's correlation analysis was
employed to analyze the correlation between serum miR-UL112-3p
expression and TB, ALT and AST levels in all children before
treatment. A ROC curve was drawn to evaluate the predictive value
of miR-UL112-3p for cytomegalovirus infections before treatment.
P<0.05 suggests a significant difference between groups.
Results
Clinical data
There was no significant difference between the two
groups with regard to factors such as sex, age, course of disease,
family size, place of residence, mother's pregnancy age, mother's
pregnancy condition, family history of cytomegalovirus infections,
and clinical symptoms (jaundice, hepatomegaly, and splenomegaly)
(all P>0.05) (Table II).
 | Table IIClinical data. |
Table II
Clinical data.
| Variable | Obs group (n=74) | Con group (n=67) | t/χ2 | P-value |
|---|
| Sex | | | 0.028 | 0.867 |
|
Male | 43 (58.11) | 38 (56.72) | | |
|
Female | 31 (41.89) | 29 (43.28) | | |
| Age (years) | 3.15±0.85 | 3.06±0.72 | 0.675 | 0.501 |
| Course of disease
(month) | 8.26±4.34 | 8.15±4.18 | 0.153 | 0.879 |
| No. of family
members | | | 0.556 | 0.456 |
|
>3 | 19 (25.68) | 21 (31.34) | | |
|
≤3 | 55 (74.32) | 46 (68.66) | | |
| Place of
residence | | | 0.151 | 0.698 |
|
Urban
area | 60 (81.08) | 56 (83.58) | | |
|
Rural
area | 14 (18.92) | 11 (16.42) | | |
| Mother' pregnant
age | 26.16±4.63 | 25.44±4.37 | 0.947 | 0.345 |
| Pregnancy | | | 0.794 | 0.373 |
|
Multiparity | 49 (66.22) | 49 (73.13) | | |
|
Primiparity | 25 (33.78) | 18 (26.87) | | |
| Mother's abnormal
pregnancy history | | | 0.059 | 0.808 |
|
Yes | 11 (14.86) | 9 (13.43) | | |
|
No | 63 (85.14) | 58 (86.57) | | |
| Clinical
symptoms | | | | |
|
Jaundice | 48 (64.86) | 36 (53.73) | 1.810 | 0.179 |
|
Hepatomegaly | 32 (43.24) | 25 (37.31) | 0.513 | 0.474 |
|
Splenomegaly | 9 (12.16) | 8 (11.94) | 0.002 | 0.968 |
Efficacy
There was no significant difference between the two
groups in the number of patients with markedly effective treatment,
those with effective treatment, and those without effective
treatment, and the total number of patients with markedly effective
or effective treatment (all P>0.05) (Table III).
 | Table IIIEfficacy. |
Table III
Efficacy.
| Items | Obs group
(n=74) | Con group
(n=67) |
χ2-value | P-value |
|---|
| Patients with
markedly effective treatment | 19 (25.68) | 21 (31.34) | 0.556 | 0.456 |
| Patients with
effective treatment | 42 (56.75) | 36 (53.73) | 0.130 | 0.718 |
| Patients without
effective treatment | 13 (17.57) | 10 (14.93) | 0.180 | 0.672 |
| Patients with
markedly effective or effective treatment | 61 (82.43) | 57 (85.07) | | |
Changes of the liver function before
and after treatment
Comparison of the changes of TB, ALT, and AST
between the two groups showed that before treatment, there were no
significant differences between the Obs group and the Con group in
TB, ALT, and AST level [(116.77±47.18) vs. (124.51±41.56);
(175.53±77.13) vs. (185.37±71.48); (244.43±97.20) vs.
(251.76±94.42)], while after treatment, the two groups showed
decreased TB, ALT, and AST levels, and the Obs group showed
significantly lower TB, ALT and AST levels than the Con group
[(33.25±13.73) vs. (48.35±16.24); (93.47±38.36) vs. (128.87±41.27);
(113.96±32.78) vs. (138.75±38.42)] Fig.
1.
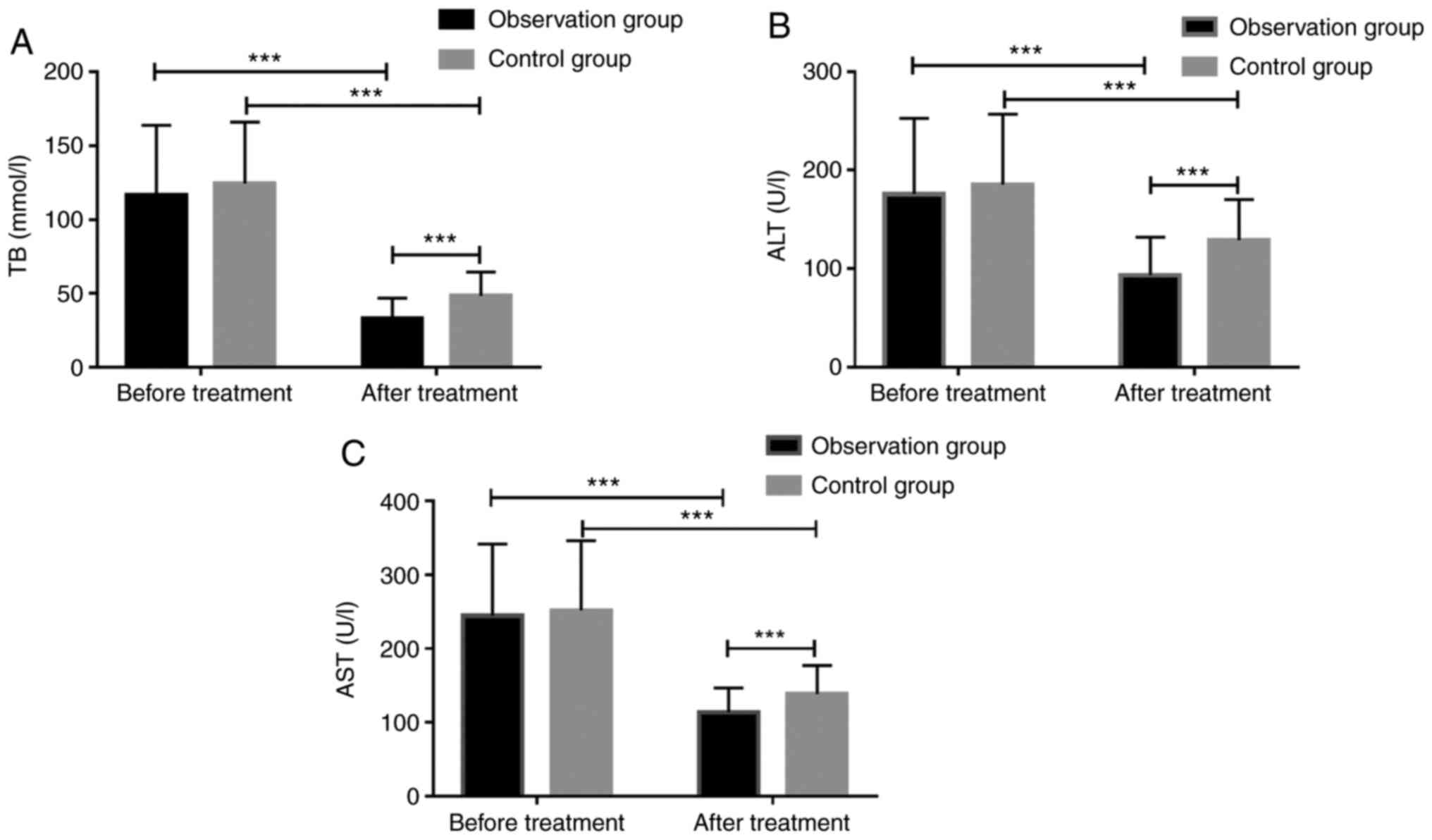 | Figure 1Changes of the liver function before
and after treatment. (A) Before treatment, there was no significant
difference in TB level between the two groups (P>0.05), while
after treatment, both groups showed decreased TB level, and the TB
level in the Obs group was significantly lower than that of the Con
group (P<0.001). (B) Before treatment, there was no significant
difference in ALT level between the two groups (P>0.05), while
after treatment, both groups showed a lower ALT level, and the ALT
level in the Obs group was significantly lower than that of the Con
group (P<0.001). (C) Before treatment, there was no significant
difference in AST level between the two groups (P>0.05), while
after treatment, both groups showed a lower AST level, and the AST
level in the Obs group was significantly lower than that of the Con
group (P<0.001). ***P<0.001. |
Adverse reactions
The comparison between the two groups in adverse
reactions showed that there was no significant difference between
the Obs group and the Con group in total adverse reactions
including gastrointestinal reactions, leucopenia, thrombocytopenia,
dizziness, and pruritus (all P>0.05). Table IV.
 | Table IVAdverse reactions. |
Table IV
Adverse reactions.
| Adverse
reactions | Obs group
(n=74) | Con group
(n=67) |
χ2-value | P-value |
|---|
| Gastrointestinal
reactions | 3 (4.05) | 7 (10.45) | 2.182 | 0.140 |
| Leucopenia | 4 (5.41) | 5 (7.46) | 0.249 | 0.618 |
|
Thrombocytopenia | 5 (6.76) | 7 (10.45) | 0.615 | 0.433 |
| Dizziness | 2 (2.70) | 1 (1.49) | 0.247 | 0.619 |
| Pruritus | 4 (5.41) | 5 (7.46%) | 0.249 | 0.617 |
| Total adverse
reactions | 18 (24.32) | 25 (37.31) | 2.799 | 0.094 |
miR-UL112-3p level and ROC
The comparison between the two groups in the changes
of miR-UL112-3p before and after treatment reflected that before
treatment, there was no significant difference in miR-UL112-3p
expression between the Obs group and the Con group (1.57±0.62) vs.
(1.53±0.60), while after treatment, both groups showed decreased
miR-UL112-3p expression, and the Obs group showed significantly
lower miR-UL112-3p expression than the Con group (1.02±0.39) vs.
(1.21±0.35). We divided the patients in each group into an
effective group and an ineffective group, separately. It was found
that in the Obs group, the miR-UL112-3p expression in the effective
group was significantly lower than that in the ineffective group
(1.44±0.59) vs. (2.18±0.39), and in the Con group, the miR-UL112-3p
expression in the effective group was also significantly lower than
that in the ineffective group (1.41±0.48) vs. (2.02±0.32). The ROC
curve of the Obs group presented that the area under the curve
(AUC), specificity, and sensitivity of miR-UL112-3p before
treatment in predicting efficacy were 0.866, 73.77 and 84.62%, and
its 95% CI, cut-off point, and Youden index were 0.777-0.955,1.754
and 58.39%, respectively,and the AUC, specificity, and sensitivity
of the ROC of the Con group were 0.837, 75.44 and 90.00%, and its
95% CI, cut-off point, and Youden index were 0.736-0.938, 1.679 and
65.44%, respectively. The AUC of the two groups was >0.8,
indicating that miR-UL112-3p has good predictive value in
therapeutic effect diagnosis (Fig.
2).
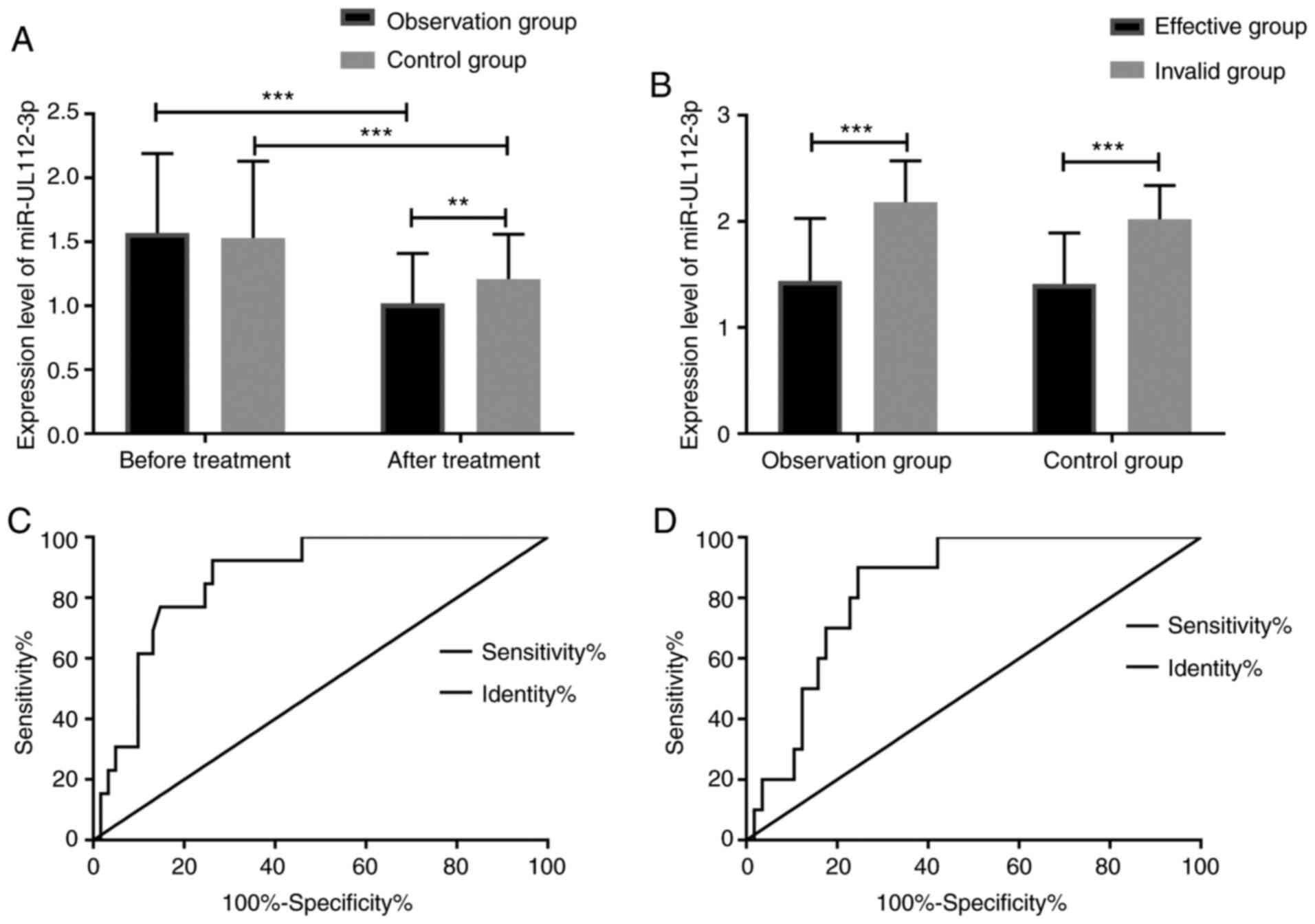 | Figure 2miR-UL112-3p changes and ROC curve for
efficacy prediction. (A) Comparison of miR-UL112-3p expression
between the observation group and the control group before and
after treatment. Before treatment, there was no significant
difference in miR-UL112-3p expression between the Obs group and the
Con group (P>0.05), while after treatment, both groups showed a
decreased miR-UL112-3p expression, and the decrease in the Obs
group was significant (P=0.003). (B) Before treatment, in the Obs
group, the effective group showed a significantly lower
miR-UL112-3p expression than the ineffective group (P<0.001),
and in the Con group, the effective group also showed a
significantly lower miR-UL112-3p expression than the ineffective
group (P<0.001). (C) The AUC of miR-UL112-3p in predicting
efficacy of the Obs group was 0.866, and the 95% CI thereof was
0.777-0.955. When the cut-off point was >1.754, the best
specificity and sensitivity were 73.77 and 84.62%, respectively,
and the Youden index was 58.39%. (D) The AUC of miR-UL112-3p of the
Con group in predicting efficacy was 0.837, and the 95% CI of it
was 0.736-0.938. When the cut-off point was >1.679, the best
specificity and sensitivity were 75.44 and 90.00%, respectively,
and the Youden index was 65.44%. **P<0.01,
***P<0.001. |
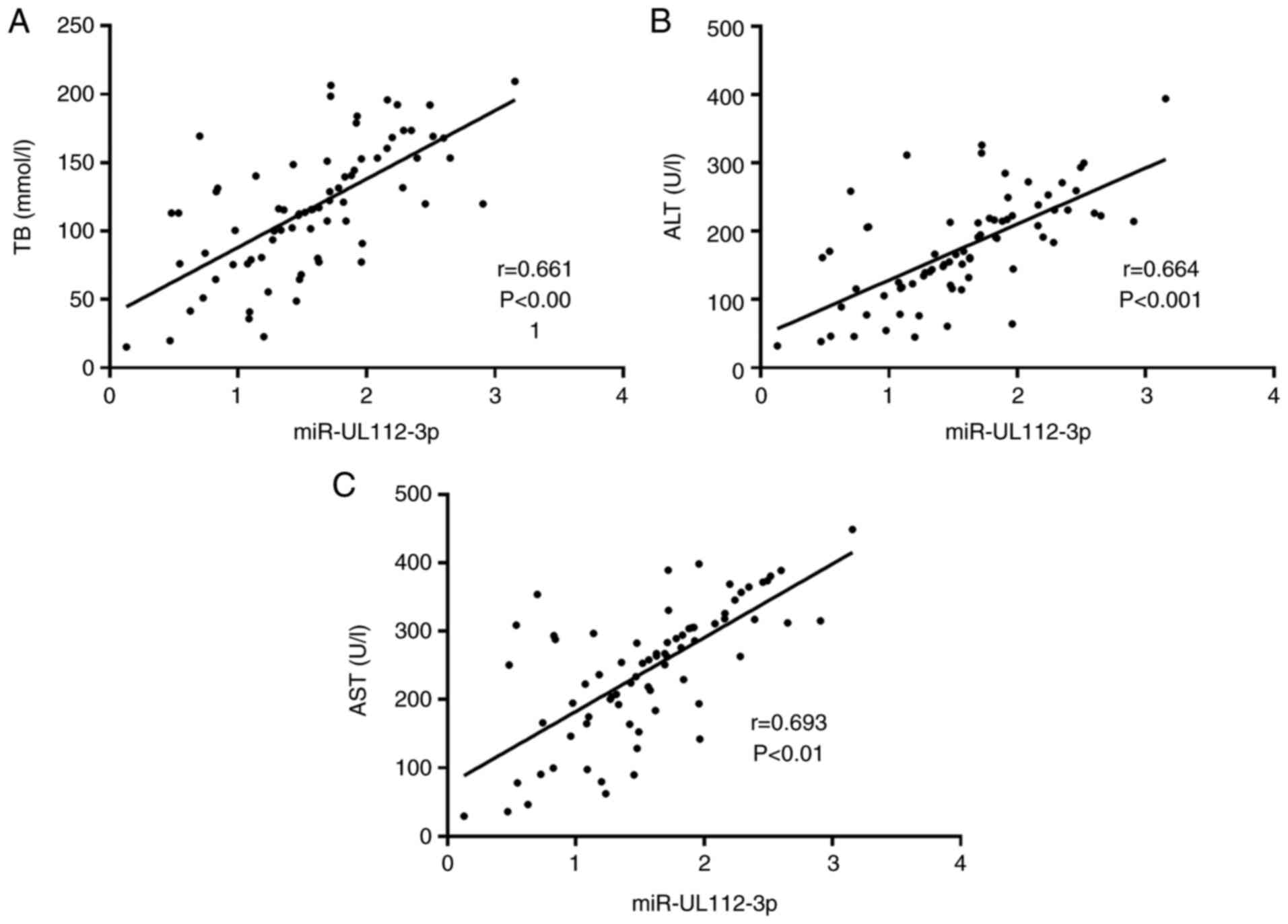
Relationship between the miR-UL112-3p
expression and liver function indexes
Pearson's correlation analysis was carried out to
analyze the relationship between the miR-UL112-3p expression and
liver function indexes (TB, ALT, and AST) in all children in the
Obs group before treatment, and it was found that miR-UL112-3p was
positively correlated with TB, ALT and AST, respectively (Fig. 3).
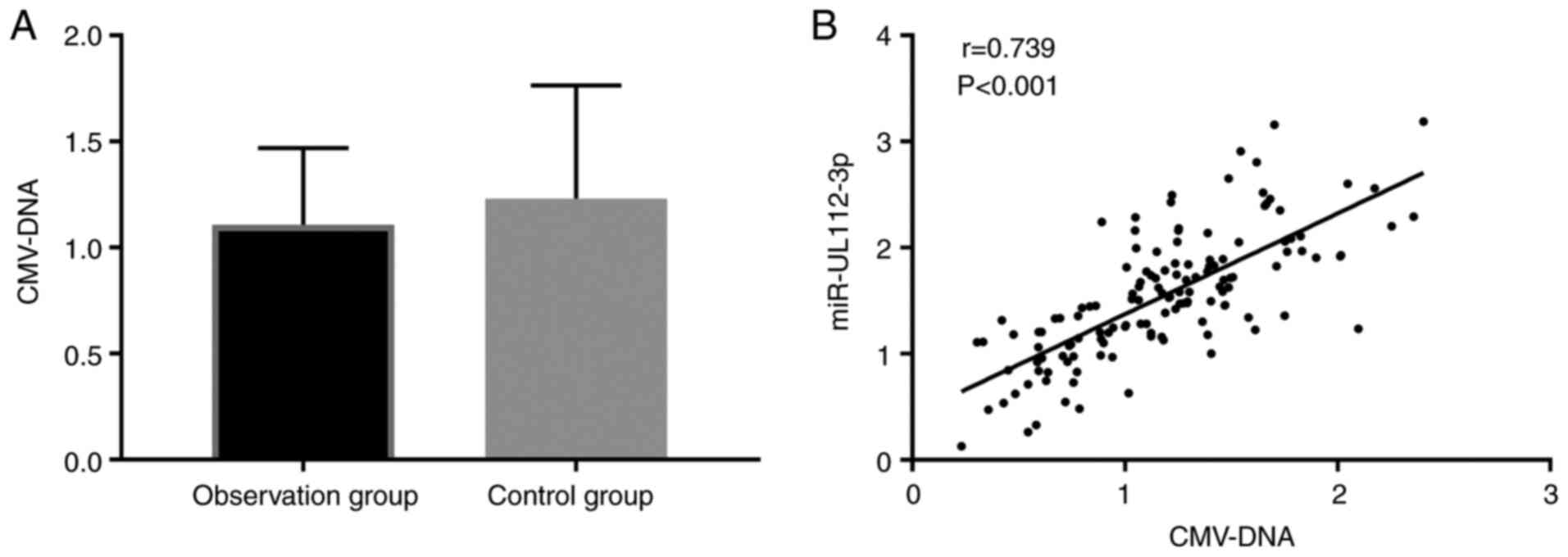
CMV-DNA level and its correlation with
miR-UL112-3p in the two groups
We compared the CMV-DNA level between the two groups
after treatment, finding that the CMV-DNA level in the Obs group
was lower than that in the Con group (1.11±0.36) vs. (1.23±0.53),
but the difference was not significant (P>0.05). We also
analyzed the correlation between the level of CMV-DNA and that of
miR-UL112-3p in children, finding a positive correlation between
them (Fig. 4).
Discussion
Cytomegalovirus infections, after infecting human
body, remain in the precursors of myeloplasts and monocytes, and
carry out replicative infection. This infection type can
downregulate HLA-I and upregulate Fas ligand to protect itself and
promote apoptosis of activated T cells, thus escaping from the
immune process (19). Ganciclovir
is effective in the treatment of cytomegalovirus infections;
however, it results in unavoidable adverse reactions. Previous
studies have reported that half-dose ganciclovir is an effective
and safe treatment for children with CMV infections after
hematopoietic stem cell transplantation with low CMV antigen level
(16,20). Therefore, this study explored
whether reducing the dose of ganciclovir affected its efficacy on
cytomegalovirus infections in children.
Firstly, we compared the clinical efficacy on the
Obs group and that on the Con group, finding that there was no
significant difference between the two groups in the number of
patients with markedly effective treatment, those with effective
treatment, and those without effective treatment, suggesting that
the efficacy of small-dose ganciclovir is relatively close to that
of conventional-dose ganciclovir, and the dose difference has less
influence on the efficacy. We also compared the changes of the
liver function between the two groups, finding that before
treatment, there was no significant difference between the groups
in TB, ALT, and AST levels, while after treatment, TB, ALT, and AST
levels in the two groups decreased, and those in the Obs group were
significantly lower than those in the Con group. Children infected
with cytomegalovirus often have higher TB, ALT and AST due to liver
damage (21,22). However, with inhibition and control
on the virus in children via small-dose ganciclovir, their liver
function slowly recovers. It has been previously reported that
ganciclovir may affect the liver and kidney function of patients
(23). We suspected that small-dose
ganciclovir would exert less effect on the liver, making it more
effective in improving the liver function.
Furthermore, the adverse reactions in the two groups
were compared, showing that the children suffered from adverse
reactions including gastrointestinal reactions, leucopenia,
thrombocytopenia, dizziness, and pruritus, which have been
mentioned in previous studies (24), and there was no significant
difference in adverse reactions between the two groups. Although
the total adverse reaction rate of the Obs group was lower than
that of the Con group, the difference was insignificant.
Subsequently, the miR-UL112-3p expression between
the two groups was compared, indicating that before treatment,
there was no significant difference between the two groups, while
after treatment, both groups showed decreased miR-UL112-3p
expression, and the miR-UL112-3p expression in the Obs group was
significantly lower than that in the Con group. The increase of
miR-UL112-3p expression is often caused by the infection due to
lyses of cells by viruses after infection, and ganciclovir can
exert an antiviral effect, thus inhibiting the infection of the
virus and lowering the miR-UL112-3p expression. We compared
miR-UL112-3p expression between the effective group and the
ineffective group, and found that in the Obs group and the Con
group, the miR-UL112-3p expression in the effective group was
significantly lower than that in the ineffective group. This
findings suggests prediction of the efficacy on children by
analyzing the miR-UL112-3p expression in children prior to
treatment. Therefore, we drew a ROC curve of miR-UL112-3p
expression in the Obs group and the Con group before treatment, and
it was found that the AUC, specificity, and sensitivity of
miR-UL112-3p in the ROC curve of the Obs group were 0.866, 73.77
and 84.62%, respectively, while the AUC, specificity, and
sensitivity of the ROC of the Con group were 0.837, 75.44 and
90.00%, respectively. We also found that the AUC of the ROC curve
of miR-UL112-3p in each group was >0.8, and the AUC of
miR-UL112-3p in the Obs group was >0.85, indicating that
miR-UL112-3p has a good predictive value. It indicates that
miR-UL112-3p is a potential prediction indicator for efficacy on
children infected with cytomegalovirus.
In a recent study, it was mentioned that the level
of serum miR-US25-1-5p in children with cytomegalovirus infections
was positively correlated with the levels of elevated serum
γ-glutamyltranspeptidase, direct bilirubin, and total bile acid
(25). Finally, we carried out
Pearson's correlation analysis to explore the correlation between
miR-UL112-3p and liver function indexes in all children before
treatment, and it was found that miR-UL112-3p was positively
correlated with TB, ALT and AST levels, respectively. Furthermore,
miR-UL112-3p is an important miRNA with which cytomegalovirus can
escape from the host immune system (26). The increase of miR-UL112-3p
expression reflects the activity of cytomegalovirus, and it affects
the liver function of patients. In a study by Zhang et al
(25) it was identified that
miR-UL112-3p expression in infants infected with cytomegalovirus
positively correlated with the serum level of bilirubin, indicating
that the expression of miR-UL112-3p in infants infected with
cytomegalovirus is significantly correlated with the liver
function, which also supports our conclusions. Moreover, we
verified that the expression of miR-UL112-3p was positively
correlated with ALT and AST levels. We also compared the HMV-DNA
level in the two groups. It was found that the HMV-DNA level in the
Obs group was slightly lower than that in the Con group, but the
difference was not significant. Moreover, we found that the level
of HMV-DNA in patients was positively correlated with the level of
miR-UL112-3p.
However, our study has some limitations. First,
subjects enrolled in our study were all patients, and no healthy
individual was enrolled; thus, we did not study the differences
between the treated children and healthy individuals. Secondly, we
identified a particular correlation between miR-UL112-3p and ALT
and AST in children through correlation analysis. However, this
specific relationship remains to be determined. In addition, we
have not tested the CMV-DNA of patients before treatment, thus, the
extent of CMV-DNA reduction by treatment with ganciclovir remain to
be clarified. Although ganciclovir has always been the first choice
for the treatment of cytomegalovirus infections, it only shows
moderate antiviral activity, which is not sufficient to completely
inhibit virus replication (27).
New therapies such as letermovir are under development (28,29),
and we hope to compare the efficacy of ganciclovir and those of
other therapeutic schemes or combination therapy in subsequent
studies.
In conclusion, small-dose ganciclovir can ameliorate
the liver function of children, and decrease the expression of
miR-UL112-3p. The AUC, specificity, and sensitivity of miR-UL112-3p
in the ROC curve of the Obs group were 0.866, 73.77 and 84.62%,
respectively, while the AUC, specificity, and sensitivity of the
ROC of the Con group were 0.837, 75.44 and 90.00%, respectively.
However, the results of the present study remain to be verified and
compared against new treatments.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QW, WZ, BW and TH conceived and designed the study,
and drafted the manuscript. QW, BW, GQ, FL and DL collected,
analyzed and interpreted the experimental data. WZ and TH revised
the manuscript for important intellectual content. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Affiliated Hospital of Weifang Medical University. Signed written
informed consents were obtained from the patients and/or
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sun H, Li S, Yan Y, Chen Z, Wang Y, Hao C
and Ji W: Associations between patient clinical characteristics and
the presence of cytomegalovirus DNA in the bronchoalveolar lavage
fluid of children with recurrent wheezing. BMC Infect Dis.
18(458)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Neant N, Klifa R, Bouazza N, Moshous D,
Neven B, Leruez-Ville M, Blanche S, Treluyer JM, Hirt D and Frange
P: Model of population pharmacokinetics of cidofovir in
immunocompromised children with cytomegalovirus and adenovirus
infection. J Antimicrob Chemother. 73:2422–2429. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gentile I, Zappulo E, Riccio MP, Binda S,
Bubba L, Pellegrinelli L, Scognamiglio D, Operto F, Margari L,
Borgia G and Bravaccio C: Prevalence of congenital cytomegalovirus
infection assessed through viral genome detection in dried blood
spots in children with autism spectrum disorders. In Vivo.
31:467–473. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Riga M, Korres G, Chouridis P, Naxakis S
and Danielides V: Congenital cytomegalovirus infection inducing
non-congenital sensorineural hearing loss during childhood; a
systematic review. Int J Pediatr Otorhinolaryngol. 115:156–164.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Palma S, Roversi MF, Bettini M, Mazzoni S,
Pietrosemoli P, Lucaccioni L, Berardi A and Genovese E: Hearing
loss in children with congenital cytomegalovirus infection: An
11-year retrospective study based on laboratory database of a
tertiary paediatric hospital. Acta Otorhinolaryngol Ital. 39:40–45.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ghorbani S, Mahdavi R, Alipoor B, Panahi
G, Nasli Esfahani E, Razi F, Taghikhani M and Meshkani R: Decreased
serum microRNA-21 level is associated with obesity in healthy and
type 2 diabetic subjects. Arch Physiol Biochem. 124:300–305.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gong YY, Luo JY, Wang L and Huang Y:
MicroRNAs regulating reactive oxygen species in cardiovascular
diseases. Antioxid Redox Signal. 29:1092–1107. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Belarbi Y, Mejhert N, Gao H, Arner P,
Rydén M and Kulyté A: MicroRNAs-361-5p and miR-574-5p associate
with human adipose morphology and regulate EBF1 expression in white
adipose tissue. Mol Cell Endocrinol. 472:50–56. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ding M, Wang X, Wang C, Liu X, Zen K, Wang
W, Zhang CY and Zhang C: Distinct expression profile of HCMV
encoded miRNAs in plasma from oral lichen planus patients. J Transl
Med. 15(133)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liang Q, Wang K, Wang B and Cai Q:
HCMV-encoded miR-UL112-3p promotes glioblastoma progression via
tumour suppressor candidate 3. Sci Rep. 7(44705)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pan Y, Wang N, Zhou Z, Liang H, Pan C, Zhu
D, Liu F, Zhang CY, Zhang Y and Zen K: Circulating human
cytomegalovirus-encoded HCMV-miR-US4-1 as an indicator for
predicting the efficacy of IFNα treatment in chronic hepatitis B
patients. Sci Rep. 6(23007)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mukhopadhyay R, Roy S, Venkatadri R, Su
YP, Ye W, Barnaeva E, Mathews Griner L, Southall N, Hu X, Wang AQ,
et al: Efficacy and mechanism of action of low dose emetine against
human cytomegalovirus. PLoS Pathog. 12(e1005717)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Aryal S, Katugaha SB, Cochrane A, Brown
AW, Nathan SD, Shlobin OA, Ahmad K, Marinak L, Chun J, Fregoso M,
et al: Single-center experience with use of letermovir for CMV
prophylaxis or treatment in thoracic organ transplant recipients.
Transpl Infect Dis. 21(e13166)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li J, Pan CW, Zhou GY, Zhuge L, Fang PP,
Jin LX, Lin W, Lin XZ and Zheng Y: Studies on the interference of
ganciclovir to HCV liver fibrosis. Eur Rev Med Pharmacol Sci.
20:4343–4347. 2016.PubMed/NCBI
|
|
15
|
Kuwahara-Ota S, Chinen Y, Mizuno Y,
Takimoto-Shimomura T, Matsumura-Kimoto Y, Tanba K, Tsukamoto T,
Mizutani S, Shimura Y, Kobayashi T, et al: Human herpesvirus-6
pneumonitis in a patient with follicular lymphoma following
immunochemotherapy with rituximab. Infect Drug Resist. 11:701–705.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ju HY, Kang HJ, Hong CR, Lee JW, Kim H,
Park KD, Shin HY, Park JD, Choi EH, Lee HJ and Ahn HS: Half-dose
ganciclovir preemptive treatment of cytomegalovirus infection after
pediatric allogeneic hematopoietic stem cell transplantation.
Transpl Infect Dis. 18:396–404. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liu LW, Qian JH, Zhu TW, Zhang YH and Zhu
JX: A 5-year retrospective clinical study of perinatal
cytomegalovirus infection. Zhongguo Dang Dai Er Ke Za Zhi.
18:99–104. 2016.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
18
|
Yin CM, Suen WC, Lin S, Wu XM, Li G and
Pan XH: Dysregulation of both miR-140-3p and miR-140-5p in synovial
fluid correlate with osteoarthritis severity. Bone Joint Res.
6:612–618. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kasmapour B, Kubsch T, Rand U, Eiz-Vesper
B, Messerle M, Vondran FWR, Wiegmann B, Haverich A and Cicin-Sain
L: Myeloid dendritic cells repress human cytomegalovirus gene
expression and spread by releasing interferon-unrelated soluble
antiviral factors. J Virol. 92:e01138–17. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Piret J and Boivin G: Clinical development
of letermovir and maribavir: Overview of human cytomegalovirus drug
resistance. Antiviral Res. 163:91–105. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ye B and Zhao H: Early abnormal liver
enzyme levels may increase the prevalence of human cytomegalovirus
antigenaemia after hematopoietic stem cell transplantation. J Int
Med Res. 45:673–679. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Nolan N, Halai UA, Regunath H, Smith L,
Rojas-Moreno C and Salzer W: Primary cytomegalovirus infection in
immunocompetent adults in the United States-A case series. IDCases.
10:123–126. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ando G, Taguchi K, Enoki Y, Yokoyama Y,
Kizu J and Matsumoto K: Evaluation of the expression time of
ganciclovir-induced adverse events using JADER and FAERS. Biol
Pharm Bull. 42:1799–1804. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Imai S, Yamada T, Kasashi K, Ishiguro N,
Kobayashi M and Iseki K: Construction of a flow chart-like risk
prediction model of ganciclovir-induced neutropaenia including
severity grade: A data mining approach using decision tree. J Clin
Pharm Ther. 44:726–734. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang J, Huang Y, Wang Q, Ma Y, Qi Y, Liu
Z, Deng J and Ruan Q: Levels of human cytomegalovirus miR-US25-1-5p
and miR-UL112-3p in serum extracellular vesicles from infants with
HCMV active infection are significantly correlated with liver
damage. Eur J Clin Microbiol Infect Dis. 39:471–481.
2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Landais I, Pelton C, Streblow D,
DeFilippis V, McWeeney S and Nelson JA: Human cytomegalovirus
miR-UL112-3p targets TLR2 and modulates the TLR2/IRAK1/NFκB
signaling pathway. PLoS Pathog. 11(e1004881)2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Britt WJ and Prichard MN: New therapies
for human cytomegalovirus infections. Antiviral Res. 159:153–174.
2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hahn F, Hutterer C, Henry C, Hamilton ST,
Strojan H, Kraut A, Schulte U, Schütz M, Kohrt S, Wangen C, et al:
Novel cytomegalovirus-inhibitory compounds of the class
pyrrolopyridines show a complex pattern of target binding that
suggests an unusual mechanism of antiviral activity. Antiviral Res.
159:84–94. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Andronova VL: Modern ethiotropic
chemotherapy of human cytomegalovirus infection: Clinical
effectiveness, molecular mechanism of action, drug resistance, new
trends and prospects. Part 1. Vopr Virusol. 63:202–211.
2018.PubMed/NCBI View Article : Google Scholar : (In Russian).
|