Introduction
Heat stroke (HS) is a condition characterized by a
core body temperature of >40˚C and neurological abnormalities. A
less severe form of HS without neurological abnormalities is termed
heat-induced illness. HS is recognized as a serious and prevalent
disorder that may lead to secondary systemic inflammation and
multiple organ dysfunction syndrome (MODS) (1,2).
Epidemiological studies have shown that the average case fatality
rate of HS in China is 10-15% and that the mortality rate of HS is
as high as 40% (3). Therefore,
early identification of critically ill patients and timely and
effective interventions are crucial to improving the survival rate
of patients with HS. However, there is currently no method for the
evaluation of the severity and prognosis of HS, as current methods
are sensitive but not specific and typically rely on comprehensive
scores based on vital signs and routine biochemical tests (4,5), which
may occur at later stages, meaning that treatment is initiated too
late.
In recent years, many studies have shown that
exosomes play an important role in the pathogenesis of various
conditions, including pathogenic immune responses, inflammation,
tumors and infection (6,7). Exosomes are vesicle-like structures
measuring 30-150 nm in diameter and containing multiple bioactive
molecules, including proteins, nucleic acids and lipids. According
to previous studies, the levels and contents of plasma exosomes
vary between healthy people and patients with various illnesses,
such as sepsis, cardiogenic shock and alcoholic hepatitis (8-10),
and the expression profiles of exosomal cargo are highly
disease-related and disease-specific. Moreover, compared with
freely-circulating substances in the plasma, exosomal contents,
which are protected by the bilayer membrane of the exosome, are
more stable and easier to transport within the circulatory system
without being degraded. Therefore, the time window for their
detection is wider (11-13).
Taken together, these findings suggest that plasma exosomes and
their contents can be used as novel and more reliable biomarkers of
diseases (14).
Few studies have been conducted on exosomes and
their relationship with HS. A previous study in dogs found that the
plasma level of histone H3 significantly increased after HS
occurrence and was correlated with multiple organ injury (5). Other nuclear proteins or other histone
monomers may also be released from the nucleus to the extracellular
space which serve as danger signals in stressful conditions, as
demonstrated in numerous disease models, such as sepsis, trauma and
HS (15-17).
In our previous study, proteomic analysis of HS induced exosomes
was performed and histone H3 identified among the top 10 most
upregulated proteins in HS-exosomes, with the highest fold change
(14.62) among histone monomers and HMGB1(18). The aim of the present study was to
investigate whether the level of histone H3 in plasma exosomes in
patients with HS could be considered as an early clinical indicator
for disease severity, organ dysfunction and mortality risk. The
study also aimed to investigate changes in plasma exosome levels of
histone H3 in patients with HS and to assess their correlation with
organ dysfunction and disease severity.
Materials and methods
Patient recruitment and clinical data
collection
Between June 2016 and June 2019, 44 patients who
were admitted to the intensive care unit (ICU) of the General
Hospital of the Southern Theatre Command of the People's Liberation
Army (PLA) of China, a tertiary hospital, within 24 h of HS
occurrence were prospectively enrolled. Patients were diagnosed
with HS according to the Expert Consensus on Diagnosis and
Treatment of Heat Stroke in China (2019) (19), promulgated by the Expert Group on
Prevention and Treatment of Heat Stroke and Critical Care Committee
of the PLA of China. The diagnostic criteria were as follows: A
medical history of i) exposure to high temperature and high
humidity or ii) high intensity exercise; clinical manifestations of
i) central nervous system dysfunction (e.g., coma, convulsion,
delirium or abnormal behavior), ii) core body temperature >40˚C
and iii) multiple (≥2) organ dysfunction that could not be
explained by other etiologies. Patients with malignant tumors,
chronic liver or kidney diseases, chronic cardiac insufficiency
(New York grades 3-4), chronic pulmonary insufficiency, underlying
central nervous system disease or metabolic disorders were
excluded. Patients were categorized as survivors (n=36) and
non-survivors (n=8). Healthy subjects from the physical examination
center (15) were enrolled as the
control group. Baseline demographic characteristics were recorded
on ICU admission (day 1). Body temperature was measured using an
ear thermometer on day 1. Blood samples were collected on day 1 and
after 4 days of treatment (day 4). Biochemical parameters (lactate,
albumin, alanine aminotransferase, aspartate aminotransferase, urea
nitrogen, creatine kinase, creatine kinase-myocardial band,
creatinine, cardiac troponin I, fibrin degradation product, fibrin,
international normalized ratio, myoglobin, partial pressure of
arterial oxygen, procalcitonin, platelet, prothrombin time, total
bilirubin, white blood cell count and D-dimer levels were assessed)
reflecting organ function were collected from patients at the
Laboratory of the General Hospital of the Southern Theatre Command
of the People's Liberation Army (PLA) of China. Acute Physiology
and Chronic Health Enquiry (APACHE) II (20) and Sequential Organ Failure
Assessment (SOFA) (21) scores were
also recorded. Written informed consent was obtained from all
patients or their representatives. This study was approved by the
Medical Ethics Review Committee of the General Hospital of the
Southern Theater Command of the PLA of China.
Blood sample collection and exosome
isolation
A 10 ml volume of blood was collected from the
peripheral vein of each patient using ethylenediaminetetraacetic
acid anticoagulant tubes. Blood collection tubes were left standing
vertically at 22-27˚C for 30 min. Whole blood was then centrifuged
at 2,500 x g at 4˚C for 10 min to isolate the plasma. Each plasma
sample was diluted with the equivalent volume of phosphate-buffered
saline (PBS) and subjected to three rounds of centrifugation at 4˚C
(2,500 x g for 30 min, 12,000 x g for 45 min and 110,000 x g for 2
h). Finally, the precipitate (exosomes) was resuspended in 50-200
µl of PBS and stored at -80˚C, as previously described (12).
Observation of exosome morphology
using transmission electron microscopy
Exosome samples were obtained from a healthy
individual, a patient with mild HS (HS without multiple organ
failure), and a patient with a severe HS (HS complicated with
multiple organ failure) (randomly selected) and prepared for
transmission electron microscopy (TEM) evaluation. Exosomes were
precipitated in 50-100 µl 2% paraformaldehyde (diluted in PBS at
4˚C). A 5 µl volume of exosome suspension was added to a
formvar-coated copper grid (Mecalab Ltée), incubated for 30 min,
washed in 100 µl of PBS, fixed in 2% paraformaldehyde for 10 min
and then stained with 2% uranyl acetate dissolved in 50% ethanol
for 15 min (all at 4˚C). The samples were then visualized using a
Philips CM10 transmission electron microscope (model no. JEM-2100F;
Philips Healthcare).
Nanoparticle tracking analysis
To determine the level and size distribution of the
isolated exosomes, nanoparticle tracking analysis (NTA) was
performed using the NanoSight NS3000 (Malvern Panalytical; Spectris
plc), according to the manufacturer's instructions. Briefly, the
exosome samples were diluted to a final ratio of 1:5,000 in sterile
PBS, and each sample was analyzed three times, each for 60 sec,
using the NanoSight's automatic analysis settings.
Western blot analysis
Proteins extracted from the plasma exosomes using
RIPA lysis buffer (Biosharp Life Sciences; cat. no. BL504A) with a
protease inhibitor (Biosharp Life Sciences; cat. no. BL612A) and
phenylmethanesulfonyl (Biosharp Life Sciences; cat. no. BL507A) at
a ratio of 100:1:1. The protein concentration were determined using
a BCA test kit (Biosharp Life Sciences; cat. no. BL521A). Protein
(10 µg/lane) was loaded and isolated using 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and electro-transferred
to a 0.2 µm nitrocellulose membrane. After blocking the nonspecific
binding sites with bovine serum albumin (Gibco; Thermo Fisher
Scientific, Inc.) for 1 h at 25˚C, the membrane was incubated with
primary antibodies (diluted 1:1,000) at 4˚C overnight and then
incubated with HRP-conjugated secondary antibodies (Goat Anti-Mouse
IgG H&L; cat. no. ab6789; 1:5,000) at 25˚C for 1 h. The
antibodies used were the following: Anti-CD63 (cat. no. ab216130),
anti-CD9 (cat. no. ab223052), anti-CD81 (cat. no. ab155760),
anti-Tsg-101 (cat. no. ab30871) and anti-GAPDH (cat. no. ab8245)
and were all obtained from Abcam.
Histone H3 assay
Histone H3 levels in the plasma exosomes were
measured using a commercial ELISA kit (Human Histone H3 ELISA kit;
cat. no. MM-51796H; Jiangsu Enzyme Free Industrial Co., Ltd.). The
protocol provided by the manufacturer was followed without
modifications.
Statistical analysis
A kurtosis test was used to check the data for
normality. Normally distributed data are presented as the mean ±
standard deviation, while non-normally distributed data were
presented as medians ± interquartile ranges. Each statistical
analysis involving two groups was performed using a two-tailed
Student's t-test for normally distributed data, Kruskal-Wallis H
test for non-normal data and Fisher's exact test for categorical
data. Mixed two-way ANOVA with both within-subjects (day) and
between-subjects (patient group) factors with a post hoc test
(simple main effects with a Bonferroni correction) was utilized to
compare more than two groups. For categorical data, McNemar's test
was used for comparisons within groups. Correlations were analyzed
using the Spearman's rank correlation test. The discriminated
ability of survivors and non-survivors by plasma exosome histone H3
was examined by receiver operator characteristic (ROC) curve
analysis with comparisons to plasma alanine aminotransferase (ALT)
and aspartate aminotransferase (AST). A P-value of <0.05 was
considered statistically significant. All tests were performed
using GraphPad software version 7.0 (GraphPad Software, Inc.).
Results
Comparison of baseline characteristics
between healthy controls and patients with HS
Blood samples from 44 patients clinically diagnosed
with HS and from 15 healthy controls were collected. All patients
with HS and healthy controls were male and had few underlying
diseases. The mean patient age was comparable between healthy
controls and HS patients 25±6 (range 17-40) vs. 21±4 (range 16-38)
years]. Clinical and laboratory data are summarized in Table I, Table
II and Table III. The overall
28-day mortality rate in patients with HS was 18.2% (8/44). The
duration of ICU stay was significantly longer in non-survivors than
in survivors (median: 10 vs. 7 days; P<0.001). Body temperatures
on admission were similar between the HS survivor and non-survivor
groups (38.58±1.62 and 39.11±1.81˚C, respectively) and were higher
in these groups than in healthy controls (36.42±0.46˚C; both
P<0.001). The majority of the patients were cooled prior to
admission and the cooling strategies are presented in Table II. On day 4, significant resolution
of hyperthermia was observed in both survivors and non-survivors
(both P<0.001 vs. day 1; Table
I).
 | Table IComparison of patient characteristics
measured on day 1 (admission) and day 4. |
Table I
Comparison of patient characteristics
measured on day 1 (admission) and day 4.
| | Healthy controls
(15) | Survivors (36) | Non-survivors
(8) | |
|---|
| Characteristic | Day 1 | Day 4 | Day 1 | Day 4 | Day 1 | Day 4 | P-value |
|---|
| Temperature
(˚C) | 36.42±0.46 | 36.78±0.26 |
38.58±1.62b |
36.80±0.79d |
39.11±1.81b |
36.61±0.94e | <0.001 |
| APACHE II
score | 0.47±0.83 | 0.40±0.83 |
9.11±7.46b |
6.33±8.21c |
16.13±530b |
15.75±3.20d | <0.001 |
| SOFA score | 0.6±0.63 | 0.33±0.49 |
5.94±2.30a |
7.42±6.99d |
10.88±4.32b |
16.5±2.98d,f | <0.001 |
 | Table IIComparison of patient characteristics
measured at a single time point. |
Table II
Comparison of patient characteristics
measured at a single time point.
| Characteristic | Healthy controls
(15) | Survivors (36) | Non-survivors
(8) | P-value |
|---|
| Median length of
ICU stay/days (IQR) | N/A | 7 (5-8.65) | 10 (3-17.45) | <0.001 |
| Cooling
strategy | | | | |
|
Alcohol
wipe, n (%) | N/A | 33 (91.2) | 8(100) | 0.643 |
|
Ice pack, n
(%) | N/A | 36(100) | 8(100) | 0.987 |
|
Water bath,
n (%) | N/A | 1 (2.8) | 2(25) | <0.01 |
|
Cooled
saline infusion, n (%) | N/A | 30 (83.3) | 7 (87.5) | 0.345 |
|
Cooled
saline gastric lavage, n (%) | N/A | 1 (2.8) | 1 (12.5) | <0.05 |
|
CRRT with
cooled dialysate, n (%) | N/A | 2 (5.6) | 2(25) | <0.01 |
| Time lag between HS
onset and ICU admission | N/A | 1.28±0.14 | 1.25±0.3 | 0.931 |
 | Table IIIComparison of clinical and laboratory
values of patients with heat stroke and healthy controls according
to day of admission and outcome status. |
Table III
Comparison of clinical and laboratory
values of patients with heat stroke and healthy controls according
to day of admission and outcome status.
| | Healthy controls
(n=15) | Survivors
(n=36) | Non-survivors
(n=8) | |
|---|
|
Characteristics | Day 1 | Day 4 | Day 1 | Day 4 | Day 1 | Day 4 | P value |
|---|
| Hemodynamic
data | | | | | | | |
|
HR
(beats/min) | 74.3±7.74 | 73.80±4.93 |
86.67±26.79a | 91.28±18.49 | 91.38±35.50 | 95.13±18.34 | 0.0092 |
|
MAP
(mmHg) | 77.6±6.99 | 77.53±7.62 | 75.53±15.45 | 77.08±16.48 | 76.13±19.79 | 65.00±14.32 | 0.3998 |
|
Vasoactive
drug, n (%) | 0 (0) | 0 (0) | 6
(16.7)b | 5
(13.9)e | 3
(37.5)i,c | 4(50)o,f,j | <0.001 |
|
Lactate
(µmol/l) | 1.07±0.47 | 0.91±0.48 | 1.91±1.93 |
2.11±2.28d |
3.39±1.97b,g |
3.78±1.84d | 0.0012 |
| Ventilatory
data | | | | | | | |
|
PaO2/FiO2 | 378.7±72.25 | 394.3±31.99 | 319.7±53.59 | 294.0±80.74 |
313.1±71.52c |
302.9±72.66d | 0.3158 |
|
MV, n
(%) | 0 (0) | 0 (0) | 7
(19.4)a | 12
(33.3)e | 5
(62.5)h,c | 7
(87.5)n,f,j | <0.001 |
| Inflammation
data | | | | | | | |
|
WBC
(x109 cells/l) | 9.42±3.14 | 6.60±1.42 | 11.34±5.48 |
7.08±3.01g | 11.72±5.92 | 7.80±3.64 | <0.001 |
|
PCT
(ng/ml) | 0.34±0.34 | 0.38±0.29 | 2.88±3.63 |
5.50±16.56g | 3.27±2.32 |
16.92±27.79e | 0.0211 |
| Hepatic data | | | | | | | |
|
ALT
(U/l) | 25.51±13.7 | 19.9 ±8.94 | 414.6±1437 | 727.4.4±1294 |
1832±2463a |
1788±1190d | 0.0013 |
|
AST
(U/l) | 22.4±13.8 | 24.07±9.04 |
354.9±1323c | 329.9±442.8 |
3905±5177c |
2261±4160f | <0.001 |
|
TBil
(µmol/l) | 9.29±4.45 | 14.29±4.79 | 36.28±73.123 | 55.70±72.45 | 39.81±28.63 |
225.2±218.3f,o,l | <0.001 |
|
ALB
(g/l) | 40.79±5.36 | 43.03±3.31 | 39.26±3.63 | 40.69±5.73 | 35.94±3.77 | 37.00±1.90 | 0.0046 |
| Renal data | | | | | | | |
|
Cr
(µmol/l) | 95.4±27.28 | 70.8±12.2 | 125.1±55.72 | 114.9±79.13 | 162.4±100.44 |
190.0±87.00f,m | <0.001 |
|
BUN
(mmol/l) | 5.51±2.19 | 5.13±1.51 | 6.84±5.30 | 7.29±5.09 | 6.76±2.52 |
16.05±9.97f,o,k | <0.001 |
|
Urine output
(ml/d) | 2680±727.2 | 2521±530.7 | 2570±1131 | 1863±1488 | 1939±1044 |
122.9±107.1f,n,j | <0.001 |
|
CRRT, n
(%) | 0 (0) | 0 (0) | 12
(33.3)a | 6 (16.7) | 6(75)e | 8(100)d,n | <0.001 |
| Coagulation
data | | | | | | | |
|
PT (s) | 13.39±0.93 | 13.53±0.96 | 19.76±12.16 | 18.01±7.71 |
24.73±7.69a |
24.50±7.03d | 0.0024 |
|
INR | 1.02±0.09 | 1.087±0.11 | 1.80±1.72 | 1.70±1.26 |
2.18±0.87i,e |
3.08±1.27e | 0.0031 |
|
Fib
(g/l) | 3.53±0.66 | 3.78±0.76 |
2.23±0.67b | 3.39±1.90 |
2.01±0.53h |
2.13±1.42d | <0.001 |
|
PLT
(x109/l) | 219.6±65.05 | 195.7±74.14 |
139.0±57.47b | 154.8±74.85 |
85.13±40.83c |
80.00±18.31f | <0.001 |
|
D-dimer | 1.46±1.32 | 0.56±0.35 |
6.46±7.55c | 3.93±5.80 |
16.14±6.00c |
16.46±5.85o,f | <0.001 |
|
FDP | 6.8±2.88 | 4.19±0.98 | 32.55±64.79 | 27.73±41.21 |
133.2±276.3g,b | 86.90±52.09 | 0.0044 |
| Rhabdomyo lysis
data | | | | | | | |
|
CK
(µg/l) | 54.3±23.52 | 63.6±24.85 | 1582±2432 | 1042±1680 |
7999±7799i,c |
5185±2444f,n | <0.001 |
|
MYO
(µg/l) | 48.15±24.91 | 25.39±24.85 |
8876±1024b |
285.0±634.8a |
1050±516.7g | 896.9±1299 | <0.001 |
| Cardiac data | | | | | | | |
|
CK-MB | 2.54±1.45 | 1.73±1.11 | 14.51±18.07 | 9.12±20.50 |
51.55±76.75a |
47.26±102.4d | 0.0028 |
|
cTnI | 12.76±9.94 | 4.74±2.69 | 424.9±935.1 | 65.13±115.3 | 459.6±398.0 |
1349±3497d,m | 0.0257 |
| CNS data | | | | | | | |
|
GCS
score | 15±0 | 15±0 | 12.03±4.05 |
11.86±3.74d |
8.0±4.50g,c |
7.38±3.46m,f | <0.001 |
Based on the results of routine biochemical
examinations performed on day 1, few of the routine biochemical
parameters reflecting organ dysfunction were significantly
different between non-survivors and survivors. These were
hemodynamic instability (as measured by an increased need for
vasoactive drugs and increased lactate levels), respiratory
impairment (an increased need for mechanical ventilation), need for
continuous renal replacement therapy and presence of coagulation
abnormalities [prolonged international normalized ratio (INR),
increased fibrinogen degradation product (FDP) levels and decreased
fibrinogen levels], rhabdomyolysis (increased creatine kinase and
myoglobin levels) and central nervous dysfunction [decreased
Glasgow coma scale (GCS) score]. Meanwhile, there were no
significant differences between survivors and non-survivors in
terms of heart rate (HR), mean arterial pressure,
PaO2/FiO2 ratio, liver injury indices [total
bilirubin (TBil) level and ALT]/AST ratio], renal function
[creatinine levels, blood urea nitrogen (BUN) levels, and urine
output], prothrombin and D-dimer levels, cardiac injury index
[cardiac troponin-I (cTnI)], and levels of the inflammatory marker
procalcitonin. Myoglobin levels were significantly higher in HS
survivors than in healthy controls but were lower in non-survivors
than in survivors. Notably, TBil levels, renal parameters, and
D-dimer and cTnI levels did not appear to be significantly
different between non-survivors and survivors until day 4. On day
1, both the APACHE II and SOFA scores, which were used to assess
overall disease severity, were not significantly different between
non-survivors and survivors, whereas SOFA scores were higher among
non-survivors only on day 4. Hemodynamic indices, mechanical
ventilation (MV) ratios, TBil levels, and renal parameters in
non-survivors deteriorated from day 1 to day 4, while the remaining
indicators of organ function remained unchanged. Among survivors,
most variables were not significantly different between day 1 and
4, except for white blood cell count and procalcitonin levels
(Table I, Table II and Table III).
Characterization of plasma exosomes
isolated from healthy subjects and patients with HS
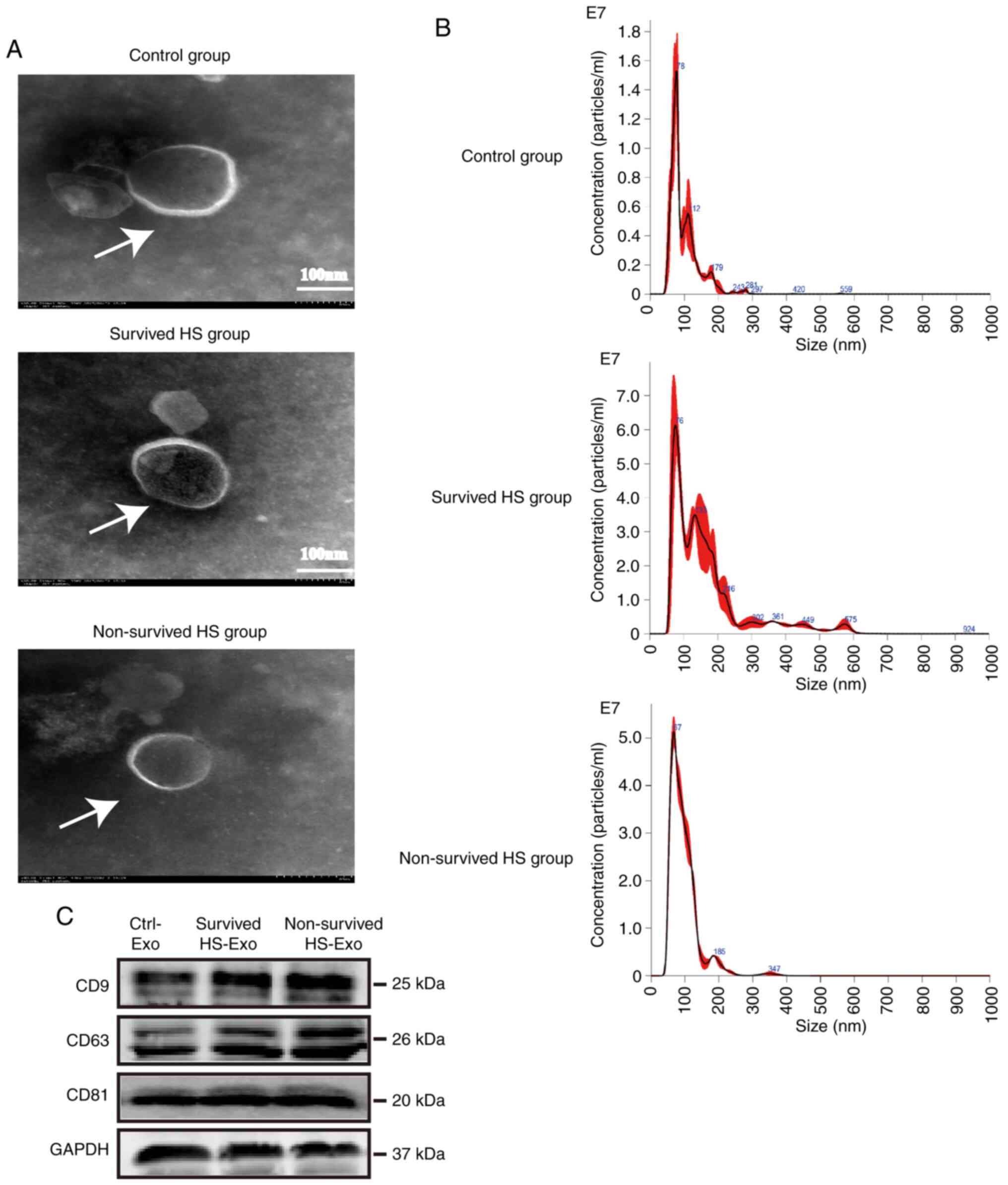
TEM was performed for the isolated plasma exosome
samples obtained from healthy controls and surviving and
non-surviving patients with HS. Double-membrane vesicle-like
structures of ~100 nm in diameter were observed in the samples from
each group (Fig. 1A). According to
the NTA results, the level of plasma exosomes was higher in both
the survived and non-survived HS group than in the control group
(6.10x109 and 3.37x109 vs.
0.66x109 particles/ml), while the diameter distribution
was comparable between the two groups, the peak value was 76 nm in
the control group vs. 78 nm for the survived HS group and 67 nm for
the non-survived HS group; the proportion of particles with a
diameter of 30-200 nm was 94.7% in the control group vs. 78.2% for
the survived HS group and 97.1% for the non-survived HS group
(Fig. 1B). The proportion of
particles with a diameter >200 nm was much higher in the HS
group. It can be hypothesized that this may be due to the increased
presence of contaminant microparticles/microvesicles in the HS
group as a result of increased cell injury/death. Western blot
analysis indicated that the characteristic exosomal surface markers
CD9, CD63 and CD81(22) were
positively expressed in both the control and survived and
non-survived HS groups (Fig. 1C).
Therefore, the characteristics of the extracted vesicles were
consistent with those of plasma exosomes.
Enrichment of histone H3 in plasma
exosomes in patients with HS
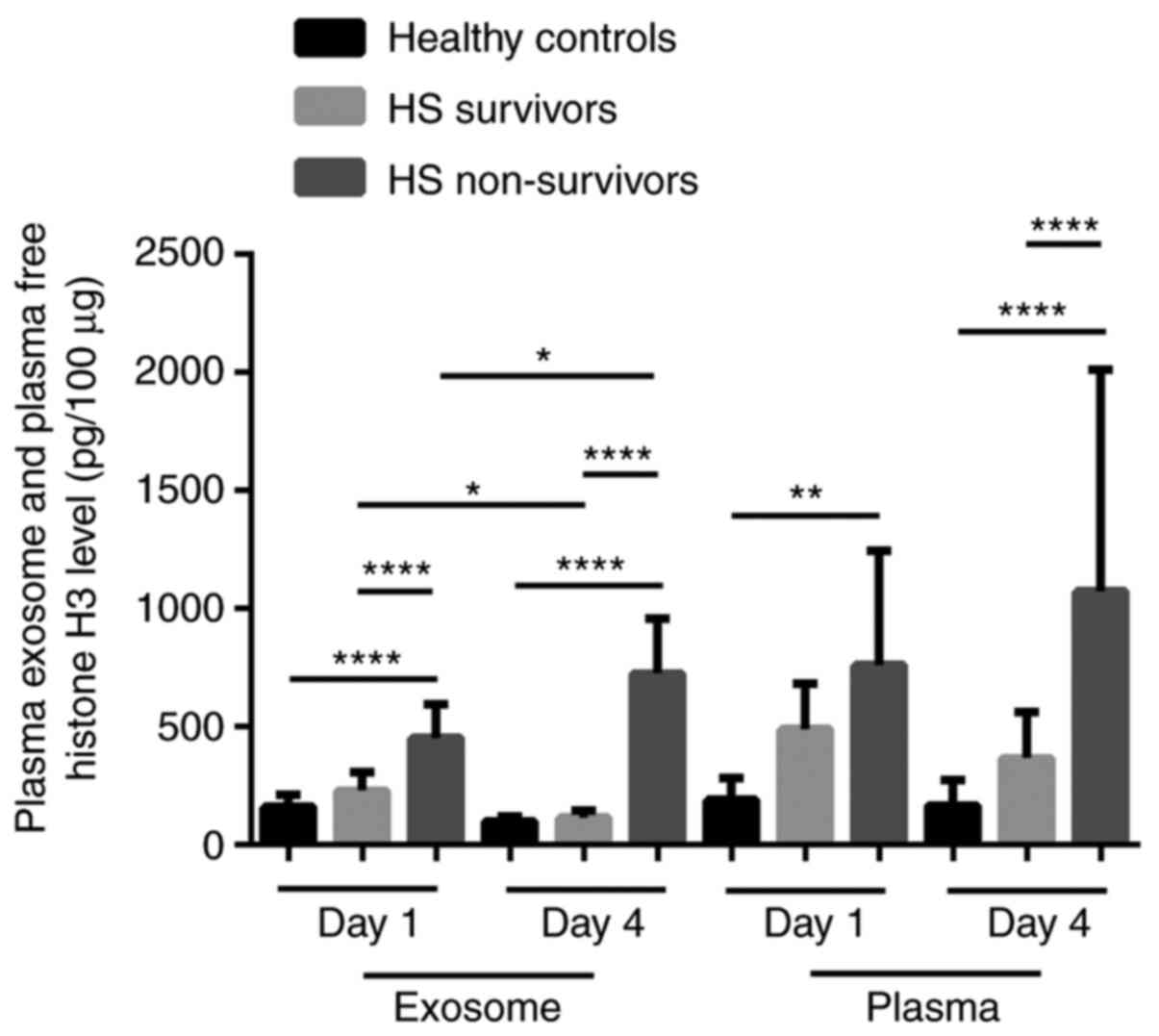
The levels of histone H3 in exosomes and free
histone H3 in plasma were detected by ELISA. Histone H3 levels in
plasma exosomes on days 1 and 4 after admission were significantly
higher in HS non-survivors than in both HS survivors and healthy
controls (all P<0.001). On admission, a 2.0-fold increase in
histone H3 levels was observed in non-survivors in comparison with
survivors. However, there were no significant changes in plasma
exosome levels of histone H3 between survivors and healthy controls
(P>0.05). Additionally, on day 4, histone H3 levels in plasma
exosomes significantly increased in non-survivors (P<0.001) but
decreased, though not significantly in survivors (P>0.05),
compared with levels on day 1. Moreover, levels of free histone H3
in plasma on days 1 and 4 were significantly higher in HS
non-survivors than in healthy controls (all P<0.001). However,
no significant changes in plasma exosomal levels of histone H3 were
observed between survivors and healthy controls (P>0.05) on both
days 1 and 4. On day 4, levels of free histone H3 in plasma were
elevated in non-survivors (P<0.001) but remained unchanged in
survivors (P>0.05), compared with levels on day 1 (Fig. 2).
Correlation of histone H3 levels in
plasma exosomes with organ function and disease severity in HS
patients
The histone H3 levels in plasma exosomes on ICU
admission were significantly positively correlated with numerous
organ function parameters, including HR, lactate levels, ALT
levels, creatinine levels, BUN levels, prothrombin levels, INR, and
D-dimer levels, as well as APACHE II and SOFA scores on days 1 and
4; AST, TBil, myoglobin, and troponin-I levels on day 1; and FDP
levels on day 4. By contrast, the plasma exosomal levels of histone
H3 were negatively correlated with PaO2/FiO2
ratios, urine output, fibrinogen levels, platelet counts, and GCS
scores on both days 1 and 4 and with albumin levels on day 4
(Table IV). However, all
correlations between plasma exosomal levels of histone H3 and organ
function indicators on day 1 were poor (all r<0.6), except for
that with D-dimer levels (r=0.78), whereas the correlations between
plasma exosomal levels of histone H3 and organ function indicators
improved on day 4.
 | Table IVCorrelation between day 1 plasma
exosome histone H3 levels and laboratory indicators on days 1 and
4. |
Table IV
Correlation between day 1 plasma
exosome histone H3 levels and laboratory indicators on days 1 and
4.
| | Day 1 | Day 4 |
|---|
| Laboratory
indicators | R-value | P-value | R-value | P-value |
|---|
| Hemodynamic
data | | | | |
|
HR
(beats/min) | 0.2968 | 0.0225 | 0.5417 | <0.001 |
|
MAP
(mmHg) | -0.3292 | 0.0109 | -0.2744 | 0.0354 |
|
Lactate
(µmol/l) | 0.3443 | 0.0076 | 0.3903 | 0.0022 |
| Ventilatory
data | | | | |
|
PaO2/FiO2 | -0.2493 | 0.0569 | -0.3503 | 0.0065 |
| Inflammatory
data | | | | |
|
WBC
(x109 cells/l) | -0.0005 | 0.9971 | 0.08678 | 0.5134 |
|
PCT
(ng/ml) | 0.3667 | 0.0043 | 0.4608 | <0.001 |
| Hepatic data | | | | |
|
ALT
(U/l) | 0.3674 | 0.0042 | 0.4426 | <0.001 |
|
AST
(U/l) | 0.3832 | 0.0027 | 0.2844 | 0.0291 |
|
TBil
(µmol/l) | 0.2526 | 0.0536 | 0.5034 | <0.001 |
|
ALB
(g/l) | -0.3465 | 0.0072 | -0.3632 | 0.0075 |
| Renal data | | | | |
|
Cr
(µmol/l) | 0.3693 | 0.0040 | 0.6858 | <0.001 |
|
BUN
(mmol/l) | 0.1343 | 0.3107 | 0.5436 | <0.001 |
|
Urine output
(ml/day) | -0.2157 | 0.1009 | -0.7205 | <0.001 |
| Coagulation
data | | | | |
|
PT (s) | 0.4661 | <0.001 | 0.4916 | <0.001 |
|
INR | 0.4056 | 0.0014 | 0.6214 | <0.001 |
|
Fib
(g/l) | -0.4179 | 0.0010 | -0.4945 | <0.001 |
|
PLT
(x109/l) | -0.4627 | <0.001 | -0.5376 | <0.001 |
|
D-Dimer | 0.6767 | <0.001 | 0.7310 | <0.001 |
|
FDP | 0.2662 | <0.001 | 0.6268 | <0.001 |
| Rhabdomyolysis
data | | | | |
|
CK
(µg/l) | 0.4830 | <0.001 | 0.7936 | <0.001 |
|
MYO
(µg/l) | 0.4611 | <0.001 | 0.4851 | <0.001 |
| Cardiac data | | | | |
|
CK-MB | 0.3649 | 0.0053 | 0.2486 | <0.001 |
|
cTnI | 0.3232 | 0.0126 | 0.1850 | 0.1606 |
| CNS data | | | | |
|
GCS
score | -0.6999 | <0.001 | -0.7474 | <0.001 |
|
APACHE II
score | 0.6909 | <0.001 | 0.7738 | <0.001 |
|
SOFA
score | 0.6888 | <0.001 | 0.8111 | <0.001 |
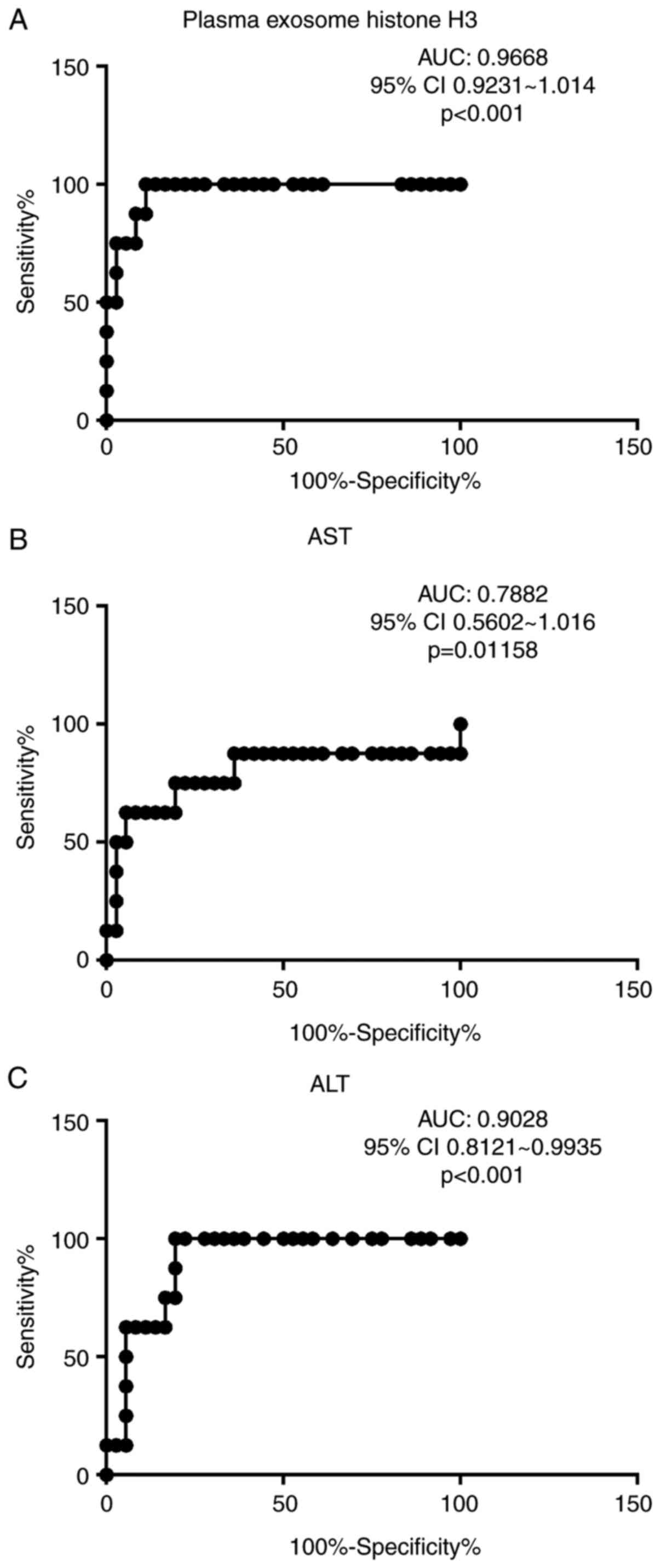
The area under the receiver operating characteristic
(ROC) curve of plasma exosomal levels of histone H3 for
discriminating between non-survivors and survivors was 0.9668 [95%
confidence interval (CI), 0.9231-1.014; P<0.001]. At a cutoff
value of 307 pg/100 µg, the sensitivity and specificity for
predicting mortality risk were 95 and 91.67%, respectively
(Fig. 3). The area under the ROC of
exosome histone H3 was higher than both plasma AST (0.7882; 95% CI
0.5602-1.016; P=0.01158) and similar with ALT (0.9028; 95% CI,
0.8121-0.9935; P<0.001; Fig.
3).
Discussion
The present study aimed to evaluate changes in
plasma exosomal levels of histone H3 in HS patients and their
correlation with organ function and disease severity. The mortality
of patients with severe HS was as high as 18.2% and non-survivors
were characterized by a prolonged ICU stay, more severe
hyperthermia on admission and a higher incidence of subsequent
multiple organ dysfunction. Histone H3 was enriched in the plasma
exosomes of patients with HS and was expressed at higher levels in
non-survivors than in survivors. The abundance of histone H3 in
plasma exosomes also decreased during the course of the disease in
survivors but increased in non-survivors. There was a significant
correlation between plasma exosomal levels of histone H3 with both
organ dysfunction (as assessed by SOFA scores) and disease severity
(as assessed by APACHE II scores) and the histone H3 levels in
plasma exosomes were significantly different between non-survivors
and survivors (area under the ROC curve: 0.9250). The sensitivity
and specificity for predicting mortality risk were optimized at a
histone H3 cutoff value of 307 pg/100 µg.
Despite the early introduction of intensive cooling
strategies to treat HS, the mortality of patients with severe HS in
the present study remained as high as 18.2% and was often
associated with MODS. Such a high mortality rate may be partly due
to the fact that this study was conducted in a tertiary hospital,
which receives a high number of critically ill patients transported
from local clinics and emergency departments.
Currently, the methods for accurately assessing the
severity and prognosis of HS are limited (1). Circulating biochemical markers have
been proposed to indicate organ failure, facilitate an accurate
diagnosis and indicate prompt treatment in patients with HS. These
biomarkers include high-mobility group box protein 1(15), neutrophil gelatinase-associated
lipocalin (also known as 24p3, uterocalin, or neu-related
lipocalin) (23), cTnI (24), the ratio of urinary heat shock
protein 72 to urinary creatinine (25), histones (5) and cryptdin-2 peptide (an intestinal
α-defensin) (26). However, these
biomarkers are still at the experimental stage and have not been
clinically evaluated or approved. As novel diagnostic biomarkers,
exosomes and their contents have gained attention for several
reasons (27). Exosomes are
superior to freely-circulating substances in plasma as they contain
highly specific cell, organ and disease-related substances, due to
their bioactive roles in sorting and transporting exosomal cargo in
response to different stimuli or insults (27). Moreover, the active components
within the exosome's double-membrane surface are protected from
degradation, allowing for the preservation of biological substances
in various environments and thereby providing a wider time window
for detection (6). Conversely, the
diagnostic value of active components directly exposed to plasma is
significantly reduced, as a result of chemical instability,
resulting in a short half-life. This narrows the time window for
detection and increases the rate of false negatives. Furthermore,
efforts to discover valuable and novel serum or plasma biomarkers
are often impeded by the abundance of background blood components
(13). In this regard, isolated
exosomes could be preferred as alternative biomarkers, as they have
bioactive roles within the cell and are more stable and resistant
to degradation, which could increase the sensitivity of
diagnosis.
Serum histones have been identified as biomarkers of
the severity of HS in dogs (5). In
the present study, the value of plasma exosomal levels of histone
H3 as a potential prognostic indicator for severe HS were
clinically verified. Compared with extracellular forms of histones,
which are passively released as a consequence of cell death and
destruction of chromatin induced by severe injury (28), the current authors hypothesized that
the release of exosomes may be a relatively early event that occurs
in the initial phase of injury through an active mode independent
of cell death. In a previous experiment from our team (data not
shown), histones in HS hepatic exosomes were not indicated to be
derived from dead cells and inhibition of apoptosis or necrosis did
not significantly affect the number of exosomes released by the HS
hepatocytes nor the levels of the exosomal histone H3. This would
render exosomal histone levels more sensitive to detection than
many other proposed markers. In the present study, experiments were
not performed to determine the cell origin of plasma exosomes. In
our previous study, proteomic analysis of HS stimulated hepatocyte
exosomes suggested that histone H3 was among the top 10 most
upregulated proteins with a fold change of 14.62. It could be
plausible to hypothesize that the exosome H3 comes from the liver
(18).
Histones are highly conserved intranuclear proteins
that traditionally serve to maintain the structural conformation
and stability of chromatin. They have also been recognized to
function as endogenous damage-associated molecular pattern
molecules upon their release into the extracellular space under
different types of pathological stress, such as injury or infection
(28), to promote inflammation and
tissue damage (16). Specifically,
histones trigger sterile inflammation, resulting in cell death and
organ injury, by interacting with toll-like receptors (TLRs),
including TLR2, TLR4 and TLR9. The administration of a sublethal
dose of histones to mice resulted in an intra-alveolar hemorrhage
in the lung along with neutrophil margination and accumulation
(17). Xu et al (29) also suggested that extracellular
histones could be released and act as major mediators of
endothelial dysfunction and organ failure in response to
inflammatory processes such as sepsis. Moreover, in concanavalin A
and acetaminophen liver toxicity models, histones were released and
shown to be critical mediators of liver cell death through TLR2 or
TLR4(30). Conversely, in animal
models of acute organ injury, neutralization of circulating
histones was a protective factor against mortality (31).
In the present study, the elevation of exosomal
histone levels in patients with HS and its correlation with disease
severity also suggest that histones may be potential targets for
alleviating HS-induced injuries. There are currently three
therapeutic strategies that antagonize the deleterious effects of
histones: Blocking the release of histones, neutralizing
circulating histones and inducing competing intracellular signal
transduction (32). These
strategies have been shown to be beneficial in reducing acute organ
injury related to sepsis, trauma and toxicity, among others, in
animal models (33,34). However, the drugs administered in
these strategies are directed to target circulating histones or
neutrophil extracellular trap-associated histones, whereas
interfering with intracellular signaling pathways may disrupt DNA
structure or function, which could cause catastrophic adverse
effects (35). The results of the
present study suggest that the blockade of histones in exosomes may
provide new therapeutic targets. Inhibition of the production of
exosomes by GW4869 has been shown to confer protection against
cardiac injury from sepsis (36).
The present study has a number of limitations. There
is currently no standard method for isolating exosomes. The current
preferred method is based on ultracentrifugation, which has limited
accessibility and reproducibility in clinical practice. The
possibility of acquiring purer exosomes remains the main obstacle
in research as well as in clinical practice (6). Ultracentrifugation applied in the
present study was not able to 100% exclude contaminant
microparticles/microvesicles or apoptotic bodies. Purer EV
preparations may be obtained by using density gradients or size
exclusion chromatography, which may be less accessible and more
expensive in clinical practice (6).
This study had a relatively small sample size, which makes it
difficult to generalize the results to different populations.
Furthermore, patients were enrolled from only one hospital, which
might lead to selection bias. Mechanisms by which H3 localize to
exosomes is a subject of investigation in future experiments.
In conclusion, the present study demonstrated that
histone H3 in plasma exosomes may be an innovative and effective
marker for risk stratification and prognosis in patients with
severe HS and thus, may have potential clinical applications.
Further studies with larger sample sizes are required to validate
these findings.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by grants from the National
Natural Science Foundation of China (grant no. 81671896), the PLA
Logistics Research Project of China (grant nos. CWH17L020 and
18CXZ032), the Natural Science Foundation of Guangdong Province
(grant no. 2019A1515012088) and the Guangdong Provincial Science
and Technology Plan Project (grant no. 2017A020215055).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HT and LS contributed to the study design and
confirmed the authenticity of all the raw data. YL collected and
interpreted the patient data and mainly contributed to writing the
manuscript. XS performed the statistical analyses. ZL obtained
ethics approval from the hospital and performed the
characterization of exosomes. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Medical Ethics Review
Committee of the General Hospital of the Southern Theater Command
of the PLA. Written informed consent was obtained from all patients
or their representatives.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Epstein Y and Yanovich R: Heatstroke. N
Engl J Med. 380:2449–2459. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hifumi T, Kondo Y, Shimizu K and Miyake Y:
Heat stroke. J Intensive Care. 6(30)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Leon LR and Helwig BG: Heat stroke: Role
of the systemic inflammatory response. J Appl Physiol (1985).
109:1980–1988. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Leon LR and Bouchama A: Heat stroke. Compr
Physiol. 5:611–647. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bruchim Y, Ginsburg I, Segev G, Mreisat A,
Avital Y, Aroch I and Horowitz M: Serum histones as biomarkers of
the severity of heatstroke in dogs. Cell Stress Chaperones.
22:903–910. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gurunathan S, Kang MH, Jeyaraj M, Qasim M
and Kim JH: Review of the isolation, characterization, biological
function, and multifarious therapeutic approaches of exosomes.
Cells. 8(307)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Barile L and Vassalli G: Exosomes: Therapy
delivery tools and biomarkers of diseases. Pharmacol Ther.
74:63–78. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Braga D, Barcella M, Herpain A, Aletti F,
Kistler EB, Bollen Pinto B, Bendjelid K and Barlassina C: A
longitudinal study highlights shared aspects of the transcriptomic
response to cardiogenic and septic shock. Crit Care.
23(414)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Real JM, Ferreira LRP, Esteves GH, Koyama
FC, Dias MVS, Bezerra-Neto JE, Cunha-Neto E, Machado FR, Salomão R
and Azevedo LCP: Exosomes from patients with septic shock convey
miRNAs related to inflammation and cell cycle regulation: New
signaling pathways in sepsis? Crit Care. 22(68)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Momen-Heravi F, Saha B, Kodys K, Catalano
D, Satishchandran A and Szabo G: Increased number of circulating
exosomes and their microRNA cargos are potential novel biomarkers
in alcoholic hepatitis. J Transl Med. 13(261)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cai S, Cheng X, Pan XY and Li J: Emerging
role of exosomes in liver physiology and pathology. Hepatol Res.
47:194–203. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yang X, Weng Z, Mendrick DL and Shi Q:
Circulating extracellular vesicles as a potential source of new
biomarkers of drug-induced liver injury. Toxicol Lett. 225:401–406.
2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ban LA, Shackel NA and McLennan SV:
Extracellular vesicles: A new frontier in biomarker discovery for
non-alcoholic fatty liver disease. Int J Mol Sci.
17(376)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yuana Y, Sturk A and Nieuwland R:
Extracellular vesicles in physiological and pathological
conditions. Blood Rev. 27:31–39. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tong HS, Tang YQ, Chen Y, Qiu JM, Wen Q
and Su L: Early elevated HMGB1 level predicting the outcome in
exertional heatstroke. J Trauma. 71:808–814. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Pisetsky DS: The translocation of nuclear
molecules during inflammation and cell death. Antioxid Redox
Signal. 20:1117–1125. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Abrams ST, Zhang N, Manson J, Liu T, Dart
C, Baluwa F, Wang SS, Brohi K, Kipar A, Yu W, et al: Circulating
histones are mediators of trauma-associated lung injury. Am J
Respir Crit Care Med. 187:160–169. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li Y, Zhu X, Wang G, Tong H, Su L and Li
X: Proteomic analysis of extracellular vesicles released from
heat-stroked hepatocytes reveals promotion of programmed cell death
pathway. Biomed Pharmacother. 129(110489)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Expert Group on Prevention and Treatment
of Heat Stroke and Critical Care Committee of PLA of China. Expert
consensus on diagnosis and treatment of heat stroke in China
(2019). Med J Chin PLA. 44:181–197. 2019.
|
|
20
|
Johnson M, Corbett M and Fitzgerald F:
Evaluation of prognostic stratification in medical intensive care
unit patients using clinical judgment compared with APACHE, a
severity of disease classification. Chest. 88(31S)1985.
|
|
21
|
Vincent JL, Mormo R, Takala J, Willatts S,
De Mendonça A, Bruining H, Reinhart CK, Suter PM and Thijs LG: The
SOFA (sepsis related organ failure assessment) score to describe
organ dysfunction/failure. On behalf of the working group on
sepsis-related problems of the european society of intensive care
medicine. Intensive Care Med. 22:707–710. 1996.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang G, Jin S, Ling X, Li Y, Hu Y, Zhang
Y, Huang Y, Chen T, Lin J, Ning Z, et al: Proteomic profiling of
LPS-induced macrophage-derived exosomes indicates their involvement
in acute liver injury. Proteomics. 26(e1800274)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Segev G, Daminet S, Meyer E, De Loor J,
Cohen A, Aroch I and Bruchim Y: Characterization of kidney damage
using several renal biomarkers in dogs with naturally occurring
heatstroke. Vet J. 206:231–235. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mellor PJ, Mellanby RJ, Baines EA,
Villiers EJ, Archer J and Herrtage ME: High serum troponin I
concentration as a marker of severe myocardial damage in a case of
suspected exertional heatstroke in a dog. J Vet Cardiol. 8:55–62.
2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bruchim Y, Avital Y, Horowitz M,
Mazaki-Tovi M, Aroch I and Segev G: Urinary heat shock protein 72
as a biomarker of acute kidney injury in dogs. Vet J. 225:32–34.
2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ji J, Gu Z, Li H, Su L and Liu Z:
Cryptdin-2 predicts intestinal injury during heatstroke in mice.
Int J Mol Med. 41:137–146. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Masyuk AI, Masyuk TV and LaRusso NF:
Exosomes in the pathogenesis, diagnostics and therapeutics of liver
diseases. J Hepatol. 59:621–625. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Allam R, Kumar SV, Darisipudi MN and
Anders HJ: Extracellular histones in tissue injury and
inflammation. J Mol Med (Berl). 92:465–472. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Xu J, Zhang X, Pelayo R, Monestier M,
Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F and Esmon CT:
Extracellular histones are major mediators of death in sepsis. Nat
Med. 15:1318–1321. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Xu J, Zhang X, Monestier M, Esmon NL and
Esmon CT: Extracellular histones are mediators of death through
TLR2 and TLR4 in mouse fatal liver injury. J Immunol.
187:2626–2631. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Allam R, Scherbaum CR, Darisipudi MN,
Mulay SR, Hägele H, Lichtnekert J, Hagemann JH, Rupanagudi KV, Ryu
M, Schwarzenberger C, et al: Histones from dying renal cells
aggravate kidney injury via TLR2 and TLR4. J Am Soc Nephrol.
23:1375–1388. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sato K, Meng F, Glaser S and Alpini G:
Exosomes in liver pathology. J Hepatol. 65:213–221. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Allam R, Darisipudi MN, Tschopp J and
Anders HJ: Histones trigger sterile inflflammation by activating
the NLRP3 inflflammasome. Eur J Immunol. 43:3336–3342.
2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Huang H, Evankovich J, Yan W, Nace G,
Zhang LM, Ross M, Liao X, Billiar T, Xu J, Esmon CT and Tsung A:
Endogenous histones function as alarmins in sterile inflflammatory
liver injury through toll-like receptor 9 in mice. Hepatology.
54:999–1008. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Silk E, Zhao H, Weng H and Ma D: The role
of extracellular histone in organ injury. Cell Death Dis.
8(e2812)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Essandoh K, Yang L, Wang X, Huang W, Qin
D, Hao J, Wang Y, Zingarelli B, Peng T and Fan GC: Blockade of
exosome generation with GW4869 dampens the sepsis-induced
inflammation and cardiac dysfunction. Biochim Biophys Acta.
1852:2362–2371. 2015.PubMed/NCBI View Article : Google Scholar
|