Introduction
Limb fracture combined with traumatic brain injury
(TBI) is one of the most common multiple injuries and patients
often suffer from severe craniocerebral injury combined with long
bone fracture of the limbs (1).
Early active treatment of fractures has an important impact on the
prognosis of patients (2).
Brain injury not only increases the probability of
heterotopic ossification, but also results in excessive callus
formation and accelerated fracture healing (3,4). In
addition, Boes et al (5)
found that the growth rate of the callus and the rate of fracture
healing in rats with fracture combined with brain injury is faster
than those in rats with simple fracture and that the amount of
callus formed in rats with fracture combined with brain injury is
greater than that in rats with only a simple fracture.
Osteopontin (SPP1), also known as early T-lymphocyte
activating factor-1, is a potential proinflammatory factor related
to the inflammatory response (6).
SPP1 can bind to SPP1 receptors on the surface of synovial cells to
induce signal transduction, which affects the adhesion and
proliferation of synovial cells (7). SPP1 is overexpressed in the articular
cartilage, synovial fluid and synovium of patients with
osteoarthritis and its expression level is positively related to
the severity of the disease (8,9). The
expression of SPP1 is regulated through a number of mechanisms,
among which microRNAs (miRNAs/miRs) have been widely studied
(10). miRNA molecules are
non-encoding RNAs that have 18-22 nucleotides (11). These molecules inhibit the
translation of mRNA by targeting the binding sites at the
3'-untranslated region (UTR) of mRNA (11). miR-let-7a (11) and miR-539(12) are reported to regulate the
expression of SPP1. Bioinformatics prediction suggests that miR-433
(accession no. 000014; region, homo sapiens chromosome 14;
GRCh38.p13) is a potential upstream regulatory gene of
SPP1(13). miR-433 is a microRNA
closely related to several human diseases. The expression of
miR-433is increased in a fibrotic heart disease model (14) and inhibits breast cancer cell
proliferation by targeting Rap1a and regulating the mitogen
activated protein kinase (MAPK) signaling pathway (15). In addition, miR-433 plays important
roles in esophageal cancer and glioma (16,17),
but its function in the pathological process of brain injury
combined with fracture has not been fully defined. It may be
hypothesized that SPP1 expression is significantly increased and
miR-433 expression reduced in patients with tibial fracture
combined with brain injury. The negative regulation between the two
may affect fracture pathology and healing process.
In the present study, the expression of SPP1 in
tibial fracture callus and heterotopic ossification tissues in
craniocerebral injury were examined in order to investigate the
relationship between SPP1 and miR-433. The present study aimed to
provide a scientific basis for the clinical diagnosis and treatment
of TBI.
Materials and methods
Patients
A total of 26 patients with tibial fracture combined
with brain injury received treatment at Jinan Zhangqiu District
People's Hospital (China) between June 2016 and June 2018 and were
included as the TBI group. In addition, 26 patients with simple
tibial fracture were included as a control group. All patients
received immobilization treatment and callus was collected during
the operation. At the time of steel plate removal the ossified
tissue samples of patients with heterotopic ossification were
collected. Peripheral blood was collected from all patients on the
morning of the operation day. Patients with injuries to body parts
other than the brain and those diagnosed with severe infection,
tumor, rheumatoid arthritis, osteoarthritis or immune-related
diseases were excluded. All procedures performed in the current
study were approved by the Ethics Committee of Jinan Zhangqiu
District People's Hospital. Written informed consent was obtained
from all patients or their families.
Reverse transcription-quantitative
PCR
To examine gene expression, RT-qPCR was performed.
Tissue samples (100 mg) were ground into powder in liquid nitrogen
and lysed with 1 ml TRIzol® reagent following the
manufacturer's manual (Thermo Fisher Scientific, Inc.). Plasma (100
µl) was directly lysed with 1 ml TRIzol® reagent. Total
RNA was extracted using the phenol chloroform method (18). The concentration and quality of RNA
was measured using ultraviolet spectrophotometry (Nanodrop ND2000;
Thermo Fisher Scientific, Inc.). cDNA was obtained by reverse
transcription from 1 µg RNA and stored at -20˚C. Reverse
transcription of mRNA was performed at 42˚C for 40 min using
TIANScript II cDNA First Strand Synthesis kit (Tiangen Biotech.
Co., Ltd.), and reverse transcription of miRNA was carried out at
42˚C using miRcute miRNA cDNA First Strand Synthesis Kit (Tiangen
Biotech. Co., Ltd.) according to the manufacturer's
instructions.
A SuperReal PreMix (SYBR Green) qRT-PCR kit (Tiangen
Biotech. Co., Ltd.) was used to determine the mRNA expression level
of SPP1 using β-actin as an internal reference. The sequences of
the SPP1 primers were 5'-GTTATGAAACGAGTCAGCTG-3' (forward) and
5'-TTAATTGACCTCAGAAGATG-3' (reverse). The sequences of the β-actin
primers were 5'-AGCGGGAAATCGTGCGTG-3' (forward) and
5'-GAGGGTACATGGTGGTGCC-3' (reverse). The reaction system (20 µl)
was composed of 10 µl SYBR Premix EXTaq, 0.5 µl upstream primer,
0.5 µl downstream primer, 2 µl cDNA and 7 µl ddH2O. The
PCR conditions were as follows: Initial denaturation at 95˚C for 5
min; 30 cycles of denaturation at 95˚C for 30 sec and annealing at
58˚C for 30 sec followed by elongation at 72˚C for 30 sec (iQ5;
Bio-Rad Laboratories, Inc.). The 2-ΔΔCq method (19) was used to calculate the relative
expression of SPP1 mRNA against β-actin. Each sample was tested in
triplicate.
The expression of miR-433 was determined by miRcute
miRNA RT-PCR Kit (Tiangen Biotech. Co., Ltd.), using U6 as internal
reference. The sequences of the miR-433 primers were
5'-GGATCATGATGGGCTCCT-3' (forward), and 5'-CAGTGCGTGTCGTGGAGT-3'
(reverse). The sequences of the U6 primers were
5'-GCTTCGGCAGCACATATACTAAAAT-3' (forward) and
5'-CGCTTCACGAATTTGCGTGTCAT-3' (reverse). The reaction system (20
µl) contained 10 µl qRT-PCR-Mix, 0.5 µl upstream primer, 0.5 µl
downstream primer, 2 µl cDNA and 7 µl ddH2O. The
reaction protocol was as follows: Initial denaturation at 95˚C for
5 min; 40 cycles of 95˚C for 10 sec and 60˚C for 20 sec; and
elongation at 72˚C for 20 sec. The 2-ΔΔCq method
(19) was used to calculate the
relative expression of miR-433 against U6. Each sample was tested
in triplicate.
Western blotting
To evaluate the expression of proteins western
blotting was carried out. Before lysis, bone tissues (100 mg) were
ground into powder, which was lysed on ice with precooled
radio-immunoprecipitation assay (RIPA) lysis buffer (200 µl;
Beyotime Institute of Biotechnology) for 30 min. The mixture was
centrifuged at 8,000 x g and 4˚C for 15 min. A Bicinchoninic acid
protein concentration determination kit (RTP7102; Real-Times
Biotechnology Co., Ltd.) was used to measure protein concentration
in the supernatant. The samples were then mixed with 5x sodium
dodecyl sulfate loading buffer before denaturation in a boiling
water bath for 5 min. Afterwards, the samples (20 µg) were
subjected to 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis at 100 V. The resolved proteins were transferred to
polyvinylidene difluoride membranes on ice (100 V, 2 h) and blocked
with 5% skimmed milk at room temperature for 1 h. The membranes
were then incubated with rabbit anti-human SPP1 (1:1,000; cat. no.
ab8448; Abcam) or β-actin (1:5,000; cat. no. ab129348; Abcam)
polyclonal primary antibodies at 4˚C overnight. After washing with
phosphate-buffered saline with Tween-20 (0.1%) 3 times, each for 15
min, the membranes were incubated with goat anti-rabbit horseradish
peroxidase-conjugated secondary antibody (1:3,000; cat. no. ab6721;
Abcam) for 1 h at room temperature before washing with PBST 3
times, each for of 15 min. The membrane was developed with an
enhanced chemiluminescence detection kit (cat. no. ab65623; Abcam).
Image lab v3.0 software (Bio-Rad Laboratories, Inc.) was used to
acquire and analyze image signals. The relative quantity of SPP1
protein was measured against β-actin.
Enzyme-linked immunosorbent assay
(ELISA)
To measure the secretion of proteins ELISA was
employed. A Human SPP1 ELISA kit (cat. no. ab100618; Abcam) was
used to determine the concentration of SPP1 in the plasma according
to the manufacturer's protocols. The absorbance was read by a
microplate reader (Multiskan FC; Thermo Fisher Scientific, Inc.) at
450 nm.
Bioinformatics
miRanda (http://www.microma.org/rnicroma/home.do) was used to
predict genes that might regulate SPP1 according to the guide
provided by the online software.
Dual luciferase reporter assay
To identify the direct interaction between genes a
dual luciferase reporter assay was used. According to
bioinformatics results, wild-type (WT;
5'-AUAUUUGUUAUUCUCUCAUGAA-3') and mutant
(5'-AUAUUUGUUAUUCUCAGUACUA-3') regions of miR-433 in the 3'-UTR of
the SPP1 gene were chemically synthesized in vitro. Their
two ends were attached with Spe-1 and HindIII restriction sites,
and then cloned into pMIR-REPORT luciferase reporter plasmids
(Thermo Fisher Scientific, Inc.). Plasmids (0.8 µg) with WT or
mutant 3'-UTR sequences were co-transfected with agomiR-433 (100
nM; Sangon Biotech Co., Ltd.) into 293T cells (The Cell Bank of
Type Culture Collection of The Chinese Academy of Sciences) at 37˚C
and under 5% CO2 using Lipofectamine® 2000
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. For control, 293T cells were transfected with
agomiR-negative control (NC). After culture for 24 h, the cells
were lysed using a dual luciferase reporter assay kit (cat. no.
E1980; Promega Corporation) according to the manufacturer's manual
and luminescence intensity was measured using GloMax 20/20
luminometer (Promega Corporation). Using Renilla
luminescence activity as internal reference, the luminescence
values of each group of cells were measured.
Cell culture and transfection
For in vitro examination, human osteoblasts
hFOB1.19 (Cell Bank of Type Culture Collection of The Chinese
Academy of Sciences) were cultured in F12/DMEM medium supplemented
with 10% fetal bovine serum (FBS), 100 IU/ml penicillin and 100
IU/ml streptomycin under 37˚C, 5% CO2 and 70% humidity.
The cells were passaged every three days.
The day before transfection, hFOB1.19 cells
(3x105) in the logarithmic growth phase were seeded onto
24-well plates and cultured in antibiotic-free F12/DMEM medium
supplemented with 10% FBS until they reached 70% confluency. In the
first vial, 0.5 µg plasmids/agomiR (Sangon Biotech Co., Ltd.) was
mixed with 50 µl Opti MEM (Thermo Fisher Scientific, Inc.). In the
second vial, 1 µl Lipofectamine 2000 (Thermo Fisher Scientific,
Inc.) was mixed with 50 µl OptiMEM media. After a 5 min incubation,
the two vials were combined and left to incubate for an additional
20 min at room temperature. The mixtures were then added onto cells
in respective groups. Six hours later, the medium was replaced with
F12/DMEM medium containing 10% FBS. After cultured for a further 48
h, the cells were collected for further assays.
Statistical analysis
The results were analyzed using SPSS 18.0
statistical software (SPSS, Inc.). The data are presented as the
mean ± the standard deviation. Comparisons between two groups was
carried out using an unpaired Student's t-test. Comparison between
more than two groups was performed by one-way analysis of variance
followed by a Student-Newman-Keuls post-hoc test. P<0.05
indicated a statistically significant difference.
Results
TBI enhances the incidence of callus
formation and heterotopic ossification in patients with fracture
but does not alter fracture healing time
Among the 26 patients with fracture combined with
brain injury (TBI group), 16 were male and 10 were female (age
range, 20-59 years; median age, 40.6 years). Among the 26 patients
with simple tibial fracture (control group), 16 were male and 10
were female (age range, 18-61 years; median age, 40.2 years). None
of the above patients had history of hormone or traditional Chinese
medicine treatment, radiotherapy or chemotherapy. Of all the
patients, 16 cases had open fractures and 36 had closed fractures.
Among the patients in the TBI group, 8 cases had cerebral contusion
and laceration, 12 cases had intracranial hematoma, 3 cases had
diffuse axonal injury and 3 cases had brain stem injury. To
evaluate the differences between the two groups, fracture healing
time, the ratio of callus diameter to tibial shaft diameter and
heterotopic ossification incidence were compared. The data showed
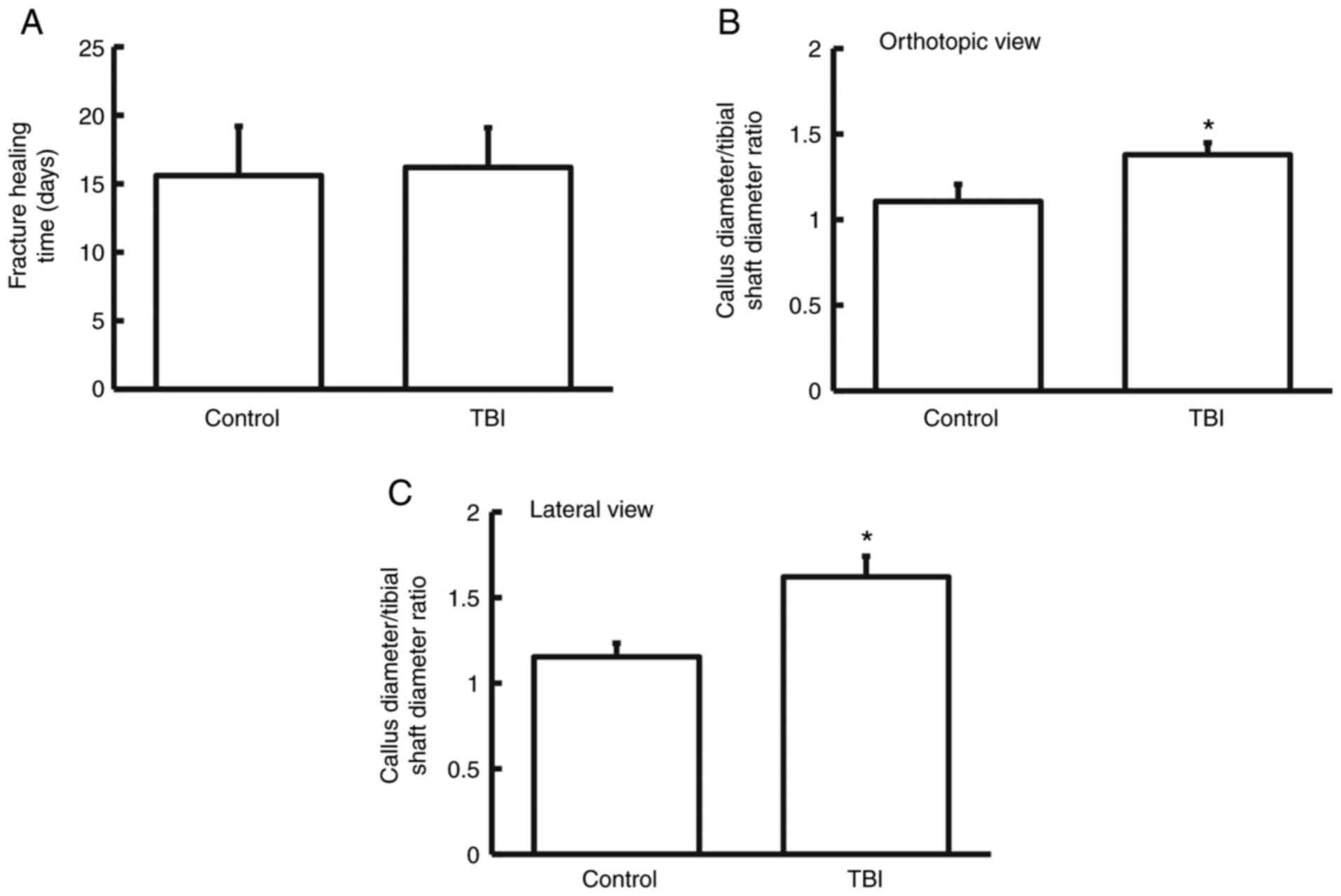
that the fracture healing time in the TBI group was not different
from that in the control group (P>0.05; n=3; Fig. 1A). The ratios of callus diameter to
tibial shaft diameter in the TBI group were significantly higher
than those of the control group in both orthotopic view and lateral
view (P<0.05 for both; n=3) (Fig.
1B and C). In addition,
heterotopic ossification incidence in the TBI group was
significantly higher than that of control group (P<0.05; n=3;
Table I). These results suggest
that TBI enhances the incidence of callus formation and heterotopic
ossification in patients with fractures but that it does not alter
fracture healing time.
 | Table IHeterotopic ossification status. |
Table I
Heterotopic ossification status.
| Groups | With heterotopic
ossification | Without heterotopic
ossification | Incidence of
heterotopic ossification |
|---|
| Control | 3 | 23 | 11.5% |
| TBI | 6 | 20 | 23.0%a |
SPP1 mRNA expression is elevated in
patients who have a tibial fracture in combination with
craniocerebral injury in comparison with controls
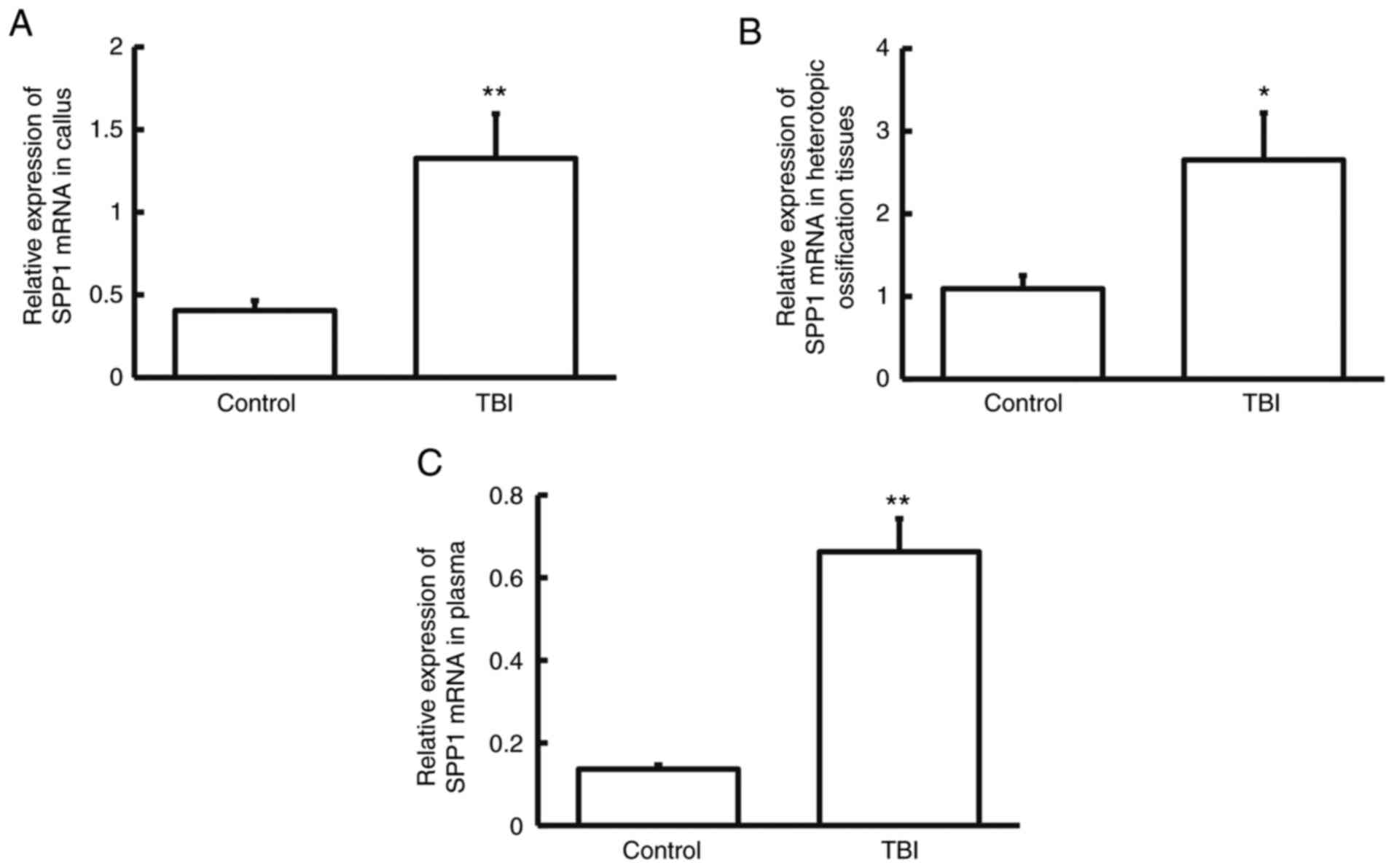
The expression of SPP1 mRNA was measured using
RT-qPCR. The data indicated that SPP1 mRNA levels in callus,
heterotopic ossification tissues and plasma from patients in the
TBI group were significantly higher than those from their
respective control groups (P<0.05; n=3; Fig. 2A-C). The result indicates that SPP1
mRNA expression is elevated in patients who have a tibial fracture
in combination with craniocerebral injury.
SPP1 protein expression is increased
in patients who have a tibial fracture in combination with
craniocerebral injury in comparison with controls
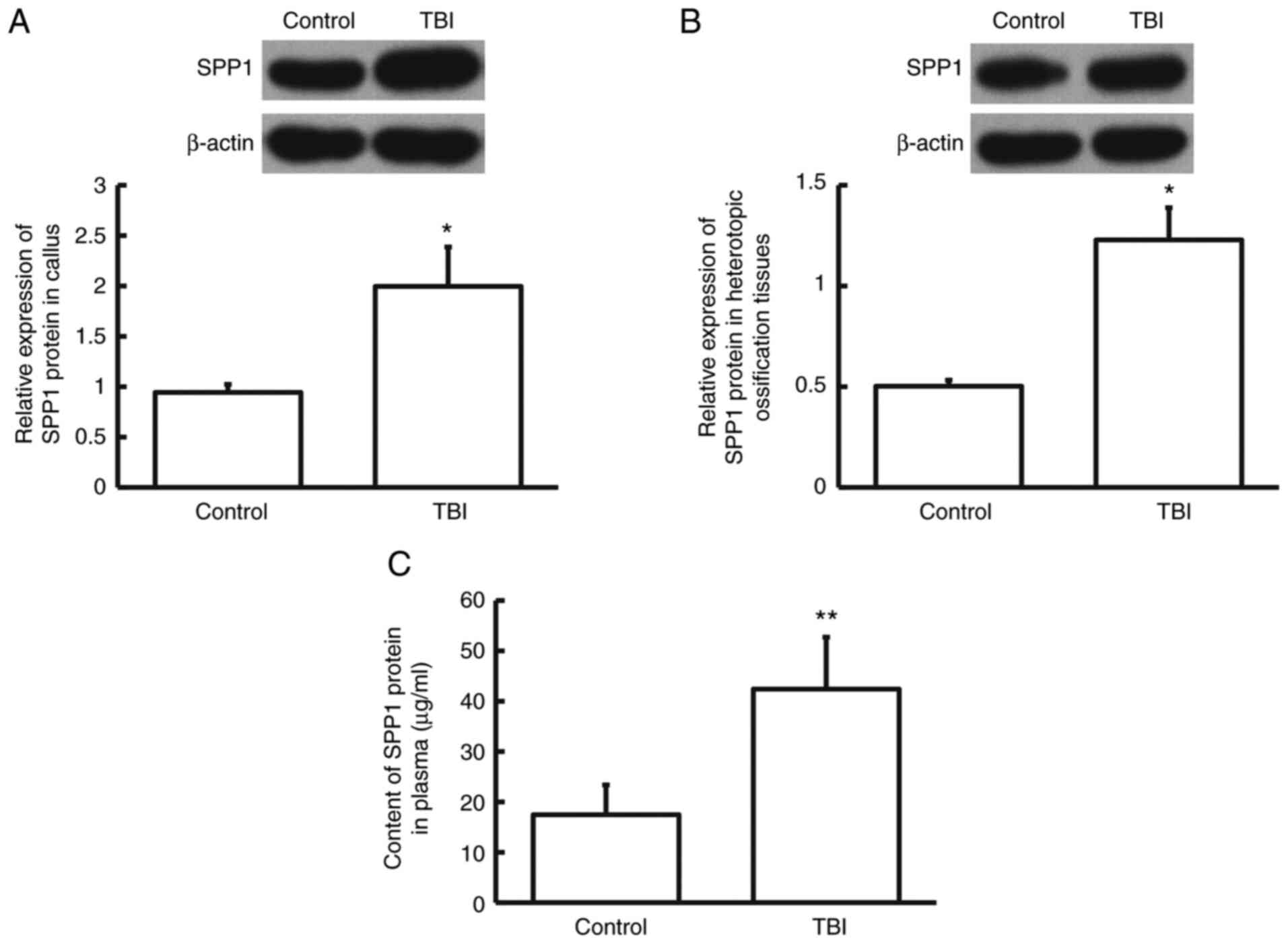
The protein expression levels of SPP1 were assessed
using western blotting. The data indicated that SPP1 protein levels
in callus, heterotopic ossification tissues and plasma from
patients in the TBI group were significantly higher than those from
control group (P<0.05; n=3; Fig.
3A-C). The result suggests that SPP1 protein expression is
increased in patients who have tibial fracture in combination with
craniocerebral injury.
Expression of miR-433 is reduced in
patients who have a tibial fracture in combination with
craniocerebral injury in comparison with controls
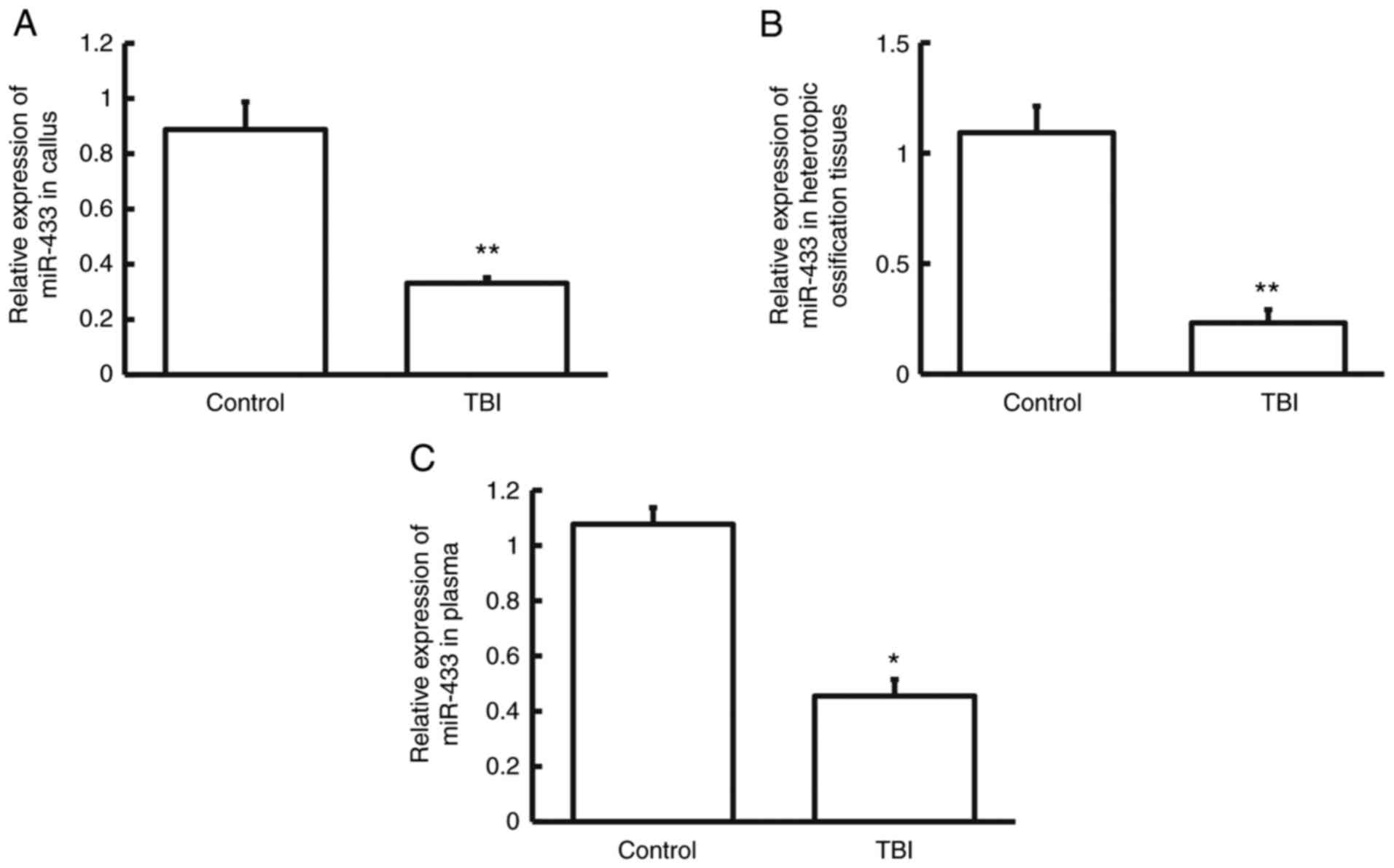
To examine whether the expression of miR-433 is
altered in these patients, RT-qPCR was performed. The data showed
that miR-433 levels in callus, heterotopic ossification tissues and
plasma from patients in TBI group was significantly lower than
those from the control group (P<0.05; Fig. 4A-C; n=3). The result indicates that
expression of miR-433 is decreased in patients who have tibial
fracture in combination with craniocerebral injury.
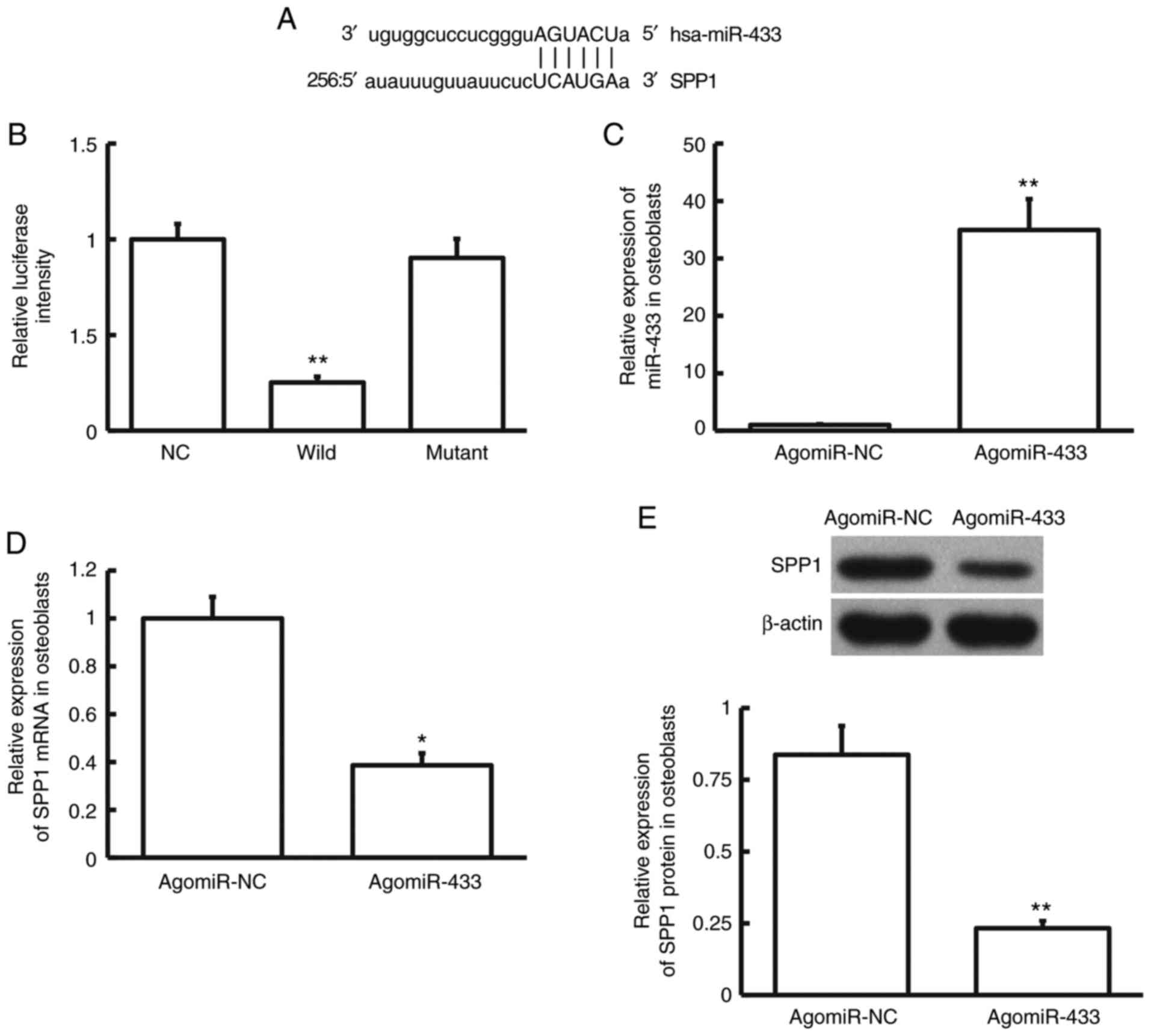
miR-433 regulates the expression of
SPP1 mRNA and protein by directly binding to the 3'-UTR of SPP1
mRNA
Bioinformatics prediction is a powerful tool for the
study of the function of miRNAs (20). To predict genes that may regulate
SPP1, miRanda was used. miR-433 was identified as a potential gene
that could regulate SPP1 (Fig. 5A).
Dual luciferase reporter assay showed that the luciferase intensity
of cells co-transfected with agomiR-433 and pMIR-REPORT-wild-type
luciferase reporter plasmids was significantly lower than that in
the NC group (P<0.01; n=3). By contrast, the luciferase
intensity of cells co-transfected with agomiR-433 and
pMIR-REPORT-mutant luciferase reporter plasmids was not
significantly different from that in the NC group (P>0.05; n=3;
Fig. 5B). After transfection with
agomiR-433, the expression of miR-433 in hFOB1.19 cells was
significantly higher than that of cells transfected with agomiR-NC
(P<0.01; n=3; Fig. 5C).
Moreover, expression of SPP1 mRNA and protein in hFOB1.19 cells
transfected with agomiR-433 was significantly lower than that in
hFOB1.19 cells transfected with agomiR-NC (P<0.05; n=3; Fig. 5D and E). These results suggest that miR-433
regulates the expression of SPP1 mRNA and protein by directly
binding with the 3'-UTR of SPP1 mRNA.
Discussion
In the present study, the expression of SPP1 in
callus, heterotopic ossification tissue and blood of patients with
tibial fracture was examined after craniocerebral injury, the
expression of miR-433 upstream of SPP1 measured in various
specimens and the regulation of SPP1 by miR-433 in osteoblasts
assessed in cells. The mechanism of miR-433 regulation of SPP1 in
the process of tibial fracture recovery after craniocerebral injury
was also preliminarily discussed.
Fracture healing is a complex process with
histological and biochemical changes and it is affected by many
factors such as patients' general condition, fracture type and
severity, blood supply and mechanics (21). The natural process of fracture
healing is generally divided into three connected parts: i) The
initial stage of hematoma and inflammation, ii) the callus
formation stage; and iii) the bone plate formation and remoulding
stage (21). Heterotopic
ossification is an important secondary disease after fracture and
occurs where a bone structure is formed outside of the bone system
(22). Heterotopic ossification is
characterized by the rapid formation of calcified bone in soft
tissues, causing swelling and pain around joints, as well as joint
movement disorders (22). The
formation of heterotopic ossification occurs in response to a
particular stimulus and the interaction between osteoblasts and the
microenvironment (23).
SPP1 can promote adhesion of tumor cells and
degradation of their extracellular matrix by binding with CD44 and
integrin, thereby inhibiting tumor cell apoptosis (24). In addition, SPP1 promotes
angiogenesis and inhibits immune functions of the body, leading to
tumor invasion and metastasis (25). SPP1 is secreted by osteoblasts,
osteocytes and osteoclasts and plays important roles in the
mineralization and absorption of bone matrix. SPP1 is abundant in
areas of endochondral ossification and intramembranous ossification
and can be observed in the cytoplasm of osteoblasts and osteoblasts
in woven bones. SPP1 protein contains a region rich in aspartic
acid and it can combine with hydroxyapatite in tissues through this
region to exert its roles (26-28).
After the initiation of bone matrix mineralization, the level of
SPP1 in osteoblasts begins to increase, suggesting that SPP1 exerts
its functions in the termination of mineralization of osteoblasts
(29). The present study results
suggested that the expression of SPP1 in callus, heterotopic
ossification tissue and plasma of patients with tibial fracture and
craniocerebral injury was significantly higher than that of
patients with simple tibial fracture, suggesting that the
expression of SPP1 might be affected by craniocerebral injury.
However, there was no significant difference in healing time
between the two groups of patients, suggesting that SPP1 might not
be related to the healing of patients.
Endogenous, small, noncoding miRNAs in cells may
cleave SPP1 mRNA and inhibit its translation (30). In this manner miRNAs promote
up-regulation or down-regulation of mRNA expression to mediate the
activity of protein-coding genes, playing important roles in the
emergence and progression of diseases (31,32).
Using bioinformatics prediction, it was hypothesized that miR-433
was closely related to SPP1 and could be an upstream miRNA that
regulated SPP1. miR-433 is a gene that has received increasing
attention. Studies show that miR-433 may be a new target for tumor
prevention, diagnosis and treatment (33,34).
miR-433 inhibits the invasion and migration of ovarian cancer cells
by targeting the expression of Notch1(35). In addition, miR-433 suppresses the
proliferation, invasion and migration of glioma cells by inhibiting
the expression of cAMP-response element binding protein (17). It has also been reported that
miR-433 is related to the occurrence and progress of gastric
cancer, liver cancer and myeloproliferative diseases (36-38).
Yang et al (36) and Guo
et al (37) indicated that
CpG islands in the upstream promoter region of miR-433 were
hypermethylated and that miR-433 expression was induced by
5-azacytidine, a DNA methyltransferase inhibitor. MicroRNA-433-3p
promotes osteoblast differentiation through targeting Dickkopf WNT
signaling pathway inhibitor 1(39)
or mediates estrogen related receptor-γ-suppressed osteoblast
differentiation via direct targeting of Runt-related transcription
factor 2 mRNA in C3H10T1/2 cells (40). In addition, miR-433 dampens
glucocorticoid receptor signaling, impacting circadian rhythm and
osteoblastic gene expression (41).
miR-433 is also associated with a new form of dominant X-linked
chondrodysplasia, suggesting that it has connections with
bone-related diseases (42). In the
present study, miR-433 expression in patients with TBI was lower
than that in patients with simple tibial fracture. In consideration
of our previous results regarding the expression of SPP1 mRNA and
protein, it was hypothesized that the up-regulation of SPP1 could
be caused by the abnormal down-regulation of miR-433. To identify
direct binding between miR-433 and SPP1 mRNA a dual luciferase
reporter assay was carried out. The result indicated that miR-433
was able to directly bind to the 3'-UTR of SPP1 mRNA to regulate
the expression of SPP1. Moreover, human osteoblast line hFOB1.19
was transfected with agomiR-433 The results suggested that
up-regulation of miR-433 inhibited SPP1 expression in osteoblasts,
indicating a potential regulatory relationship between the two
molecules in patients with tibial fracture and TBI.
The injury and healing of fractures involves the
cooperation between multiple signaling pathways and the
participation of many kinds of cells. For example, bone marrow
mesenchymal stem cells promote tibial fracture healing in rabbits
through JAK-STAT signaling pathway (43). Hyperhomocysteinemia inhibits tibial
fracture healing in rats through PI3K/AKT signaling pathway
(44). In addition, Qiao et
al (45) report that
intermittent hypoxia training and remote ischemic preconditioning
significantly enhance fracture healing; however, intermittent
hypoxia training exhibited superior bone formation and healing
effects compared with remote ischemic preconditioning, suggesting
that the interaction of multiple signal pathways and various
physiological conditions in the human body have an impact on the
condition of the fracture.
Due to practical limitations, it was not possible to
match the patient's injuries precisely and individual differences
in this study are larger than desirable. Therefore, future work is
planned to continue to expand the number of cases as a follow-up
study, in addition to the study of cell signaling pathway in order
to gather more conclusive evidence for the findings of the present
study.
In conclusion, the present study demonstrates that
miR-433 expression in patients with tibial fracture and TBI is
significantly lower than in patients with simple tibial fractures.
As miR-433 directly targets SPP1 to regulate its expression, the
expression of SPP1 mRNA is enhanced, leading to increased SPP1
protein expression in bone tissues and blood. This may play a
biological role in the healing of tibial fractures.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by a grant from the
Hospital-level Science and Technology Development Plan Project Fund
of Zhangqiu District People's Hospital of Jinan City (grant no.
2018-17).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZH and ML contributed to the design of the study.
ZH, FS, YC, XD and BZ performed the experiments. ZH and FS analyzed
the data. ZH, FS and ML interpreted results and prepared the
manuscript. All authors have read and approved the final
manuscript. ZH, FS, YC and XD confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
All procedures performed in the current study were
approved by the Ethics Committee of Jinan Zhangqiu District
People's Hospital. Written informed consent was obtained from all
patients or their families.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li Z, Zhao J, Luo W, Li K, Lei S and Wang
Y: Nrp-1 expression in healing process of traumatic brain injury
combined with tibial fracture. Zhong Nan Da Xue Xue Bao Yi Xue Ban.
42:154–160. 2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
2
|
Kobylecki C, Glasse H, Amin J, Gregson CL,
Lyell V and Henderson EJ: Fracture risk assessment in atypical
parkinsonian syndromes. Mov Disord Clin Pract. 8:385–389.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Brady RD, Grills BL, Church JE, Walsh NC,
McDonald AC, Agoston DV, Sun M, O'Brien TJ, Shultz SR and McDonald
SJ: Closed head experimental traumatic brain injury increases size
and bone volume of callus in mice with concomitant tibial fracture.
Sci Rep. 6(34491)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jodoin M, Rouleau DM, Therrien E, Chauny
JM, Sandman E, Larson-Dupuis C, Leduc S, Gosselin N and De Beaumont
L: Investigating the incidence and magnitude of heterotopic
ossification with and without joints involvement in patients with a
limb fracture and mild traumatic brain injury. Bone Rep.
11(100222)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Boes M, Kain M, Kakar S, Nicholls F,
Cullinane D, Gerstenfeld L, Einhorn TA and Tornetta P III:
Osteogenic effects of traumatic brain injury on experimental
fracture-healing. J Bone Joint Surg Am. 88:738–743. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pang X, Gong K, Zhang X, Wu S, Cui Y and
Qian BZ: Osteopontin as a multifaceted driver of bone metastasis
and drug resistance. Pharmacol Res. 144:235–244. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen G, Zhang X, Li R, Fang L, Niu X,
Zheng Y, He D, Xu R and Zhang JZ: Role of osteopontin in synovial
Th17 differentiation in rheumatoid arthritis. Arthritis Rheum.
62:2900–2908. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gao SG, Li KH, Zeng KB, Tu M, Xu M and Lei
GH: Elevated osteopontin level of synovial fluid and articular
cartilage is associated with disease severity in knee
osteoarthritis patients. Osteoarthritis Cartilage. 18:82–87.
2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gattorno M, Gregorio A, Ferlito F, Gerloni
V, Parafioriti A, Felici E, Sala E, Gambini C, Picco P and Martini
A: Synovial expression of osteopontin correlates with angiogenesis
in juvenile idiopathic arthritis. Rheumatology (Oxford).
43:1091–1096. 2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Song SZ, Lin S, Liu JN, Zhang MB, Du YT,
Zhang DD, Xu WH and Wang HB: Targeting of SPP1 by microRNA-340
inhibits gastric cancer cell epithelial-mesenchymal transition
through inhibition of the PI3K/AKT signaling pathway. J Cell
Physiol. 234:18587–18601. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yu H, Sun H, Wang Z and Liu Y: MicroRNA
let-7a up-regulates OPN expression in a mouse model of allergic
rhinitis. J Laryngol Otol. 131:955–960. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Liu Y, Qian K, Li C, Ma Y and Chen X:
Roles of microRNA-539 and osteopontin in rheumatoid arthritis. Exp
Ther Med. 15:2681–2687. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jiang K, Teng GD and Chen YQ: MicroRNA-23
suppresses osteogenic differentiation of human bone marrow
mesenchymal stem cells by targeting the MEF2C-mediated MAPK
signaling pathway. J Gene Med. 22(e3216)2020.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Tao L, Bei Y, Chen P, Lei Z, Fu S, Zhang
H, Xu J, Che L, Chen X, Sluijter JP, et al: Crucial role of miR-433
in regulating cardiac fibrosis. Theranostics. 6:2068–2083.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhang T, Jiang K, Zhu X, Zhao G, Wu H,
Deng G and Qiu C: miR-433 inhibits breast cancer cell growth via
the MAPK signaling pathway by targeting Rap1a. Int J Biol Sci.
14:622–632. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shi Q, Wang Y, Mu Y, Wang X and Fan Q:
MiR-433-3p inhibits proliferation and invasion of esophageal
squamous cell carcinoma by targeting GRB2. Cell Physiol Biochem.
46:2187–2196. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sun S, Wang X, Xu X, Di H, Du J, Xu B,
Wang Q and Wang J: MiR-433-3p suppresses cell growth and enhances
chemosensitivity by targeting CREB in human glioma. Oncotarget.
8:5057–5068. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rolfs F, Piersma SR, Dias MP, Jonkers J
and Jimenez CR: Feasibility of phosphoproteomics on leftover
samples after RNA extraction with guanidinium thiocyanate. Mol Cell
Proteomics. 20(100078)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhao D, Chen H and Wang B: Assessing the
regulatory functions of LncRNA SNHG11 in gastric cancer cell
proliferation and migration. Front Cell Dev Biol.
9(620476)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Einhorn TA and Gerstenfeld LC: Fracture
healing: Mechanisms and interventions. Nat Rev Rheumatol. 11:45–54.
2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Dey D, Wheatley BM, Cholok D, Agarwal S,
Yu PB, Levi B and Davis TA: The traumatic bone: Trauma-induced
heterotopic ossification. Transl Res. 186:95–111. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Micha D, Voermans E, Eekhoff ME, van Essen
HW, Zandieh-Doulabi B, Netelenbos C, Rustemeyer T, Sistermans EA,
Pals G and Bravenboer N: Inhibition of TGFβ signaling decreases
osteogenic differentiation of fibrodysplasia ossificans progressiva
fibroblasts in a novel in vitro model of the disease. Bone.
84:169–180. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lin YH and Yang-Yen HF: The
osteopontin-CD44 survival signal involves activation of the
phosphatidylinositol 3-kinase/Akt signaling pathway. J Biol Chem.
276:46024–46030. 2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang KX and Denhardt DT: Osteopontin: Role
in immune regulation and stress responses. Cytokine Growth Factor
Rev. 19:333–345. 2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shao H, Wu R, Cao L, Gu H and Chai F:
Trelagliptin stimulates osteoblastic differentiation by increasing
runt-related transcription factor 2 (RUNX2): A therapeutic
implication in osteoporosis. Bioengineered. 12:960–968.
2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang J, Gao Z and Gao P: MiR-133b
modulates the osteoblast differentiation to prevent osteoporosis
via targeting GNB4. Biochem Genet, Mar 9, 2021 (Online ahead of
print).
|
|
28
|
He HP and Gu S: The
PPAR-γ/SFRP5/Wnt/β-catenin signal axis regulates the
dexamethasone-induced osteoporosis. Cytokine.
143(155488)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Foster BL, Ao M, Salmon CR, Chavez MB,
Kolli TN, Tran AB, Chu EY, Kantovitz KR, Yadav M, Narisawa S, et
al: Osteopontin regulates dentin and alveolar bone development and
mineralization. Bone. 107:196–207. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ma C, Wei F, Xia H, Liu H, Dong X, Zhang
Y, Luo Q, Liu Y and Li Y: MicroRNA-10b mediates TGF-β1-regulated
glioblastoma proliferation, migration and epithelial-mesenchymal
transition. Int J Oncol. 50:1739–1748. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhao X, Mohan R, Özcan S and Tang X:
MicroRNA-30d induces insulin transcription factor MafA and insulin
production by targeting mitogen-activated protein 4 kinase 4
(MAP4K4) in pancreatic β-cells. J Biol Chem. 287:31155–31164.
2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chen K and Rajewsky N: The evolution of
gene regulation by transcription factors and microRNAs. Nat Rev
Genet. 8:93–103. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Li J, Chen M and Yu B: miR-433 suppresses
tumor progression via Smad2 in non-small cell lung cancer. Pathol
Res Pract. 215(152591)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhao YF, Li MX, Chang GZ and He XH: ERK on
apoptosis of gastric cancer cells induced by microRNA-433. Zhonghua
Yi Xue Za Zhi. 98:3954–3957. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
35
|
Liang T, Guo Q, Li L, Cheng Y, Ren C and
Zhang G: MicroRNA-433 inhibits migration and invasion of ovarian
cancer cells via targeting Notch1. Neoplasma. 63:696–704.
2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yang Z, Tsuchiya H, Zhang Y, Hartnett ME
and Wang L: MicroRNA-433 inhibits liver cancer cell migration by
repressing the protein expression and function of cAMP response
element-binding protein. J Biol Chem. 288:28893–28899.
2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Guo LH, Li H, Wang F, Yu J and He JS: The
tumor suppressor roles of miR-433 and miR-127 in gastric cancer.
Int J Mol Sci. 14:14171–14184. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lin X, Rice KL, Buzzai M, Hexner E, Costa
FF, Kilpivaara O, Mullally A, Soares MB, Ebert BL, Levine R and
Licht JD: miR-433 is aberrantly expressed in myeloproliferative
neoplasms and suppresses hematopoietic cell growth and
differentiation. Leukemia. 27:344–352. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tang X, Lin J, Wang G and Lu J:
MicroRNA-433-3p promotes osteoblast differentiation through
targeting DKK1 expression. PLoS One. 12(e0179860)2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kim EJ, Kang IH, Lee JW, Jang WG and Koh
JT: MiR-433 mediates ERRγ-suppressed osteoblast differentiation via
direct targeting to Runx2 mRNA in C3H10T1/2 cells. Life Sci.
92:562–568. 2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Smith SS, Dole NS, Franceschetti T,
Hrdlicka HC and Delany AM: MicroRNA-433 dampens glucocorticoid
receptor signaling, impacting circadian rhythm and osteoblastic
gene expression. J Biol Chem. 291:21717–21728. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Simon D, Laloo B, Barillot M, Barnetche T,
Blanchard C, Rooryck C, Marche M, Burgelin I, Coupry I, Chassaing
N, et al: A mutation in the 3'-UTR of the HDAC6 gene abolishing the
post-transcriptional regulation mediated by hsa-miR-433 is linked
to a new form of dominant X-linked chondrodysplasia. Hum Mol Genet.
19:2015–2027. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wang P and Zhang Z: Bone marrow-derived
mesenchymal stem cells promote healing of rabbit tibial fractures
via JAK-STAT signaling pathway. Exp Ther Med. 19:2310–2316.
2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Liu S, Huang Y, Tian S, Zhang W, Xu Y and
Ge J: Hyperhomocysteinemia inhibits tibial fracture healing in rats
through PI3K/AKT signaling pathway. Exp Ther Med. 19:2083–2088.
2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Qiao J, Zhou M, Li Z, Ren J, Gao G, Cao G,
Shen H and Lu S: Comparison of remote ischemic preconditioning and
intermittent hypoxia training in fracture healing. Mol Med Rep.
19:1867–1874. 2019.PubMed/NCBI View Article : Google Scholar
|