Introduction
Systemic sclerosis (SSc) is a connective tissue
disease manifested through an altered microvascularization which is
then followed by cutaneous and visceral fibrosis, in the context of
autoimmune alteration (1). The
etiopathogenesis of this autoimmune disease burdened by skin damage
and a high degree of viscera involvement is yet insufficiently
known (2-9).
Despite numerous studies, the therapeutic management remains
unsatisfactory (6,9-17).
Jacobsen et al highlight the presence of
antinuclear antibodies (ANAs) in 86% of the patients diagnosed with
SSc (18). Fabri and Hunzelmann
reported positive ANAs in a higher number of patients, more
specifically 90% (19). In a
review, Haustein revealed that 85% of SSc cases had a positive
immunological profile, and a dynamic autoimmune evaluation
identified the presence of autoantibodies in up to 98% of patients
with SSc (1). In another study,
Steen et al identified negativity for ANAs in 53% of the
enrolled patients (20). Normal ANA
titers do not rule out the disease's presence (1,21).
Monfort et al (22,23) and other authors (24-26)
identified an association between the cases of SSc with normal
levels of ANAs and neoplastic pathology in their studies in recent
years (22-26).
Consequently, they consider SSc cases presenting with normal ANA
levels as paraneoplastic SSc (22,23).
Depending on the extent of the skin involvement, there are two
subsets of SSc: Limited and diffuse (27). Each of the two subsets of this
disease has a characteristic immunological profile. Thus,
anticentromere antibodies (ACAs) are known to be characteristic of
the limited SSc subset and anti-Scl70 antibodies have specificity
for diffuse SSc (1). Anti-Scl70
antibodies are present in diffuse SSc and are associated with an
increased risk for interstitial pulmonary fibrosis, without having
increased renal involvement, a trait which is found in other
immunological models (19).
Statistics have shown that these autoantibodies are more common in
Japanese and Thai patients and less likely in the African-American
population (19). The presence of
ACAs is associated with a better prognosis and with higher survival
rates, by also taking into account the lower risk of impaired lung
and kidney function; this is in direct opposition to the presence
of anti-Scl70 antibodies that aggravate the prognosis (19). Each of these types of autoantibodies
can be found only singularly, and not in combination (1). Haustein notes that ACAs and anti-Scl70
antibodies are useful predictors for the two subsets of SSc and
directs the diagnosis to a specific subset from an early stage
(1). However, in a study conducted
by the University of Pittsburgh on a group of 397 patients, Steen
and colleagues found normal ACA titers in 57% of patients with
limited SSc and elevated titers of anti-Scl70 antibodies in only
33% of patients with diffuse SSc (20). These observations indicate an
inconsistent correlation between the immunological profile and the
SSc subset (28).
Patients and methods
We conducted an observational study on a group of 37
patients diagnosed with SSc according to the criteria developed and
reviewed in 2013 by the American College of Rheumatology
(ACR)/European League Against Rheumatism (EULAR) (29).
Ethical approval for this study was obtained from
the Ethics Review Committee of the Medical University of Iași
(24.06.2017), as well as from the Ethics Council of the ‘Sf. Maria’
Clinical Hospital in Bucharest (5213/04.04.2019). Patients were
hospitalized between February 2019 and March 2020, in the Internal
Medicine and Rheumatology Departments of the ‘Sf. Maria’ Clinical
Hospital in Bucharest, Romania.
We appreciated the extension of skin induration, as
being limited to the hands, face and feet or extended to the trunk
and abdomen (30), according to the
Le Roy criteria; a characteristic on which the patients were placed
into SSc subsets: Limited and diffuse forms (30). Blood samples were obtained for
autoantibody detection after each patient signed an informed
consent. Correlations were made between the autoantibody profile
and the limited and diffuse SSc clinical type. The enrolled
patients were evaluated clinically, biologically and with imaging
studies in order to identify the existence of a possible neoplasm.
All of the procedures in this study were performed in accordance
with the Declaration of Helsinki.
The results were introduced in an Excel file with
statistical analysis processing, followed by the use of Microsoft
Excel, SPSS version 24.0 (IBM Corp.). The results were presented as
a table. The quantitative data were characterized through
descriptive statistics; the qualitative data were characterized
through frequency distributions and contingency tables, and
comparisons between samples were made using the Chi-squared test.
All P-values were two-tailed; a P-value of 0.05 was considered
significant.
Results
We conducted an observational study on a group of 37
patients with SSc from the southeastern region of Romania.
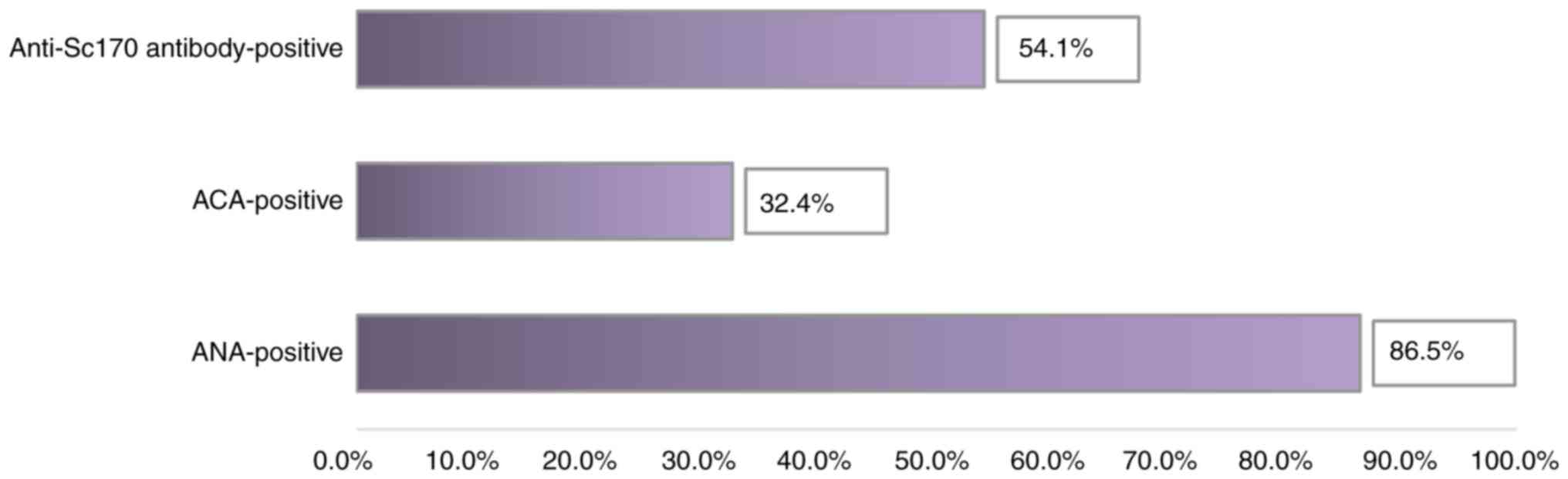
Following the analysis of the immune profile, we observed that most
patients diagnosed with SSc (86.5% of them) had elevated ANA
levels. Of these, the majority (54.1%) had high titers of
anti-Scl70 antibodies specific to diffuse SSc (Table I and Fig. 1).
 | Table IThe immunological profile of SSc:
Frequency distributions of the total group and by subsets. |
Table I
The immunological profile of SSc:
Frequency distributions of the total group and by subsets.
| | SSc limited | SSc diffuse | Total | |
|---|
| Increased
autoantibodies | n | % | n | % | n | % | Chi-square | P-value |
|---|
| ANAs | | | | | | | | |
|
No | 1 | 7.1 | 4 | 17.4 | 5 | 13.5 | 0.782 | 0.362 (NS) |
|
Yes | 13 | 92.9 | 19 | 82.6 | 32 | 86.5 | | |
| ACAs | | | | | | | | |
|
No | 4 | 28.6 | 21 | 91.3 | 25 | 67.6 | 15.629 | 0.000
(SS) |
|
Yes | 10 | 71.4 | 2 | 8.7 | 12 | 32.4 | | |
| Anti-Scl70
antibodies | | | | | | | | |
|
No | 11 | 78.6 | 6 | 26.1 | 17 | 45.9 | 9.653 | 0.002
(SS) |
|
Yes | 3 | 21.4 | 17 | 73.9 | 20 | 54.1 | | |
| Total | | 14 | 100.0 | 23 | 100.0 | 37 | 100.0 | |
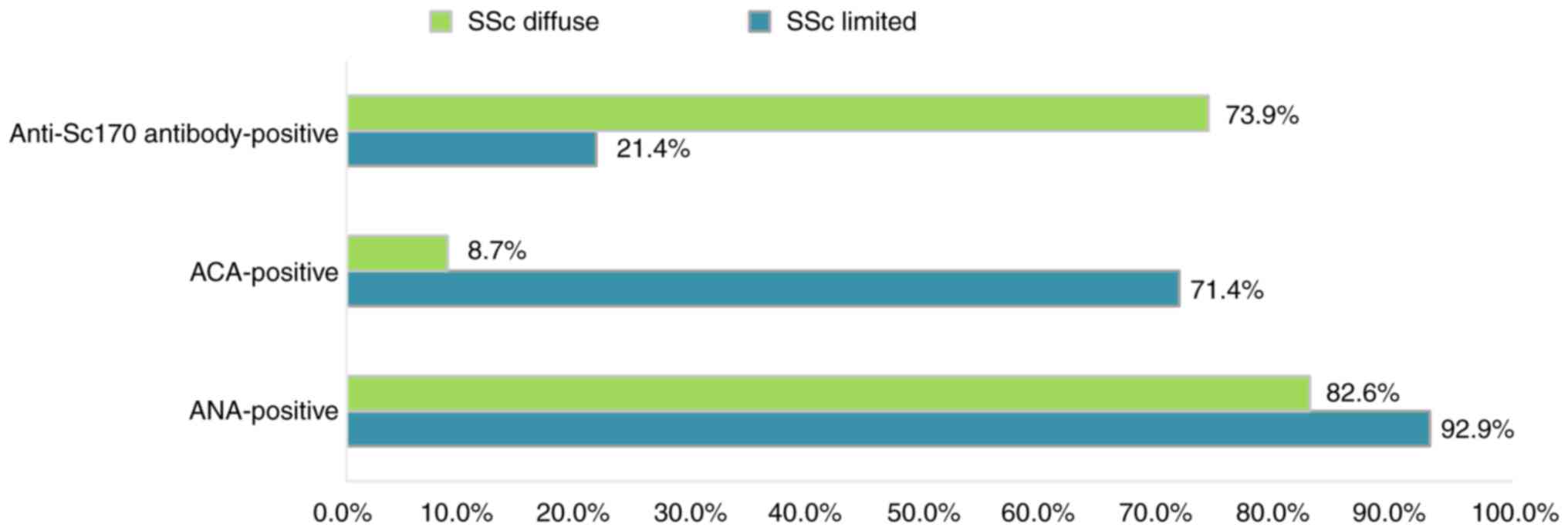
The distribution of SSc cases with positive ANAs did
not register significant differences between the diffuse and
limited subset of SSc. Therefore, the percentage of patients with
limited SSc who had positive ANAs (92.9%) was slightly higher than
that of the patients with diffuse SSc with only 82.6%. Relative to
the entire group, we noted that almost 2/3 of the patients with
positive ANA levels were part of the subset with diffuse SSc. Of
the patients with normal ANA titers, the majority (17.4%) were from
the diffuse subset of SSc (Table
I). This result suggests that the proportion of patients
without elevations in the ANA titer is low and is more common in
the diffuse subset of SSc.
ACAs, known to be specific for the limited type of
SSc (1), were identified in 32.4%
of the patients from the investigated group. Of these, most
patients (71.4%) belonged to the limited subset. Within the
subgroup with limited SSc, increased ACA titers were present in a
significant percentage of patients (71.4%). Contrary to
expectations, more than 1/4 of the patients from the subset with
limited SSc had normal ACA titers (Fig.
2).
Anti-Scl70 antibodies that are specific for the
diffuse forms of SSc (1) were found
in 54.1% of patients in the entire analyzed group, and in 73.9% of
patients with diffuse SSc. We noted that a fairly large share
(26.1%) of patients with diffuse SSc did not show increases in
anti-Scl70 antibodies. Surprisingly, in the subgroup of patients
with limited SSc, several isolated cases with anti-Scl70-positive
antibodies were identified. Similar observations were made by Steen
et al (20) but our study
identified smaller discrepancies between the subset of SSc and the
autoantibodies specific to each subset, as compared to Steen et
al American study which enrolled a very large number of
patients with SSc (20).
Discussion
Following the patient group analysis from the
south-eastern region of Romania, most of the SSc patients enrolled
in this study had high ANA titers; however a low percentage of SSc
cases with normal ANA values was also identified, suggesting that a
normal ANA titer does not rule out presence of the disease. Our
results are similar to those of other authors who revealed the
existence of a small percentage of SSc cases with normal ANA levels
(12-14).
Haustein also observed ANA positivity during the course of the
disease in a large group of patients in an American clinic. At the
start of the study, ANAs were positive in 85% of the enrolled
patients and in dynamics, ANA tested positive in up to 96% of the
patients studied (1). Starting from
Haustein's observation of ANA positivization following disease
progression in a patient with present SSc criteria, it can be
stated that the immunological profile could be negative in the
early stages of immunopathy.
Given the results of our study and by analyzing the
results of other authors, it can be appreciated that the
immunological profile of SSc is not always associated with the
extent of skin damage reflected by the subset of SSc, as noted by
Steen et al (20). This
finding suggests the need to study the levels of ANAs during the
disease progression in order to discover a possible increase.
Monfort and his collaborators classified ScS with normal ANA titers
as cases of paraneoplastic SSc (22,23).
Among patients with negative ANA in our group, we did not identify
any neoplastic process that could support this hypothesis.
Referring to the type of ANA identified, the present study recorded
high levels of ACAs in almost 3/4 of patients with limited SSc, but
we also identified some limited SSc with elevated titers of
anti-Scl70 antibodies, with the knowledge that these autoantibodies
are characteristic of diffuse SSc (1). Similarly, in the subset of patients
diagnosed with diffuse SSc, 3/4 of them had high titers of
anti-Scl70 antibodies, while a small number of cases had elevated
ACA levels, which are known to be characteristic of the limited SSc
subset (1).
As a peculiarity of our study, the percentage of
patients having an immunological profile inconsistent with the
subset of SSc, was lower than that found by other authors (12). Thus, only a few isolated cases of
patients with diffuse SSc showed positive ACAs and cases of limited
SSc with positive anti-Scl70 antibodies were also reported as
isolated cases. Therefore, our study found a more balanced
consistency between the disease subset and the autoantibodies
specific for each subset.
In conclusion, the necessity for other studies
regarding SSc cases with negative ANAs and on subsets of SSc with
an atypical immunological profile remains high.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CB, EN, AIH, MLD and MC were involved in the
conception of the study and had major contribution in the writing
and revising of the manuscript. LGS, IAP, CO, SC and ML assisted in
the acquisition, analysis and interpretation of the data. All
authors have read and approved the final manuscript for
publication.
Ethics approval and consent to
participate
This study was approved by the Ethics Review
Committees of the Medical University of Iași (24.06.2017) and of
the Ethics Council of the Clinical Hospital ‘St. Maria’ in
Bucharest (5213/04.04.2019) and was performed in accordance with
the Declaration of Helsinki. All patients provided informed consent
and approved the publication of data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Haustein UF: Systemic
sclerosis-scleroderma. Dermatol Online J. 8(3)2002.PubMed/NCBI
|
|
2
|
Juche A, Siegert E, Mueller-Ladner U,
Riemekesten G, Günther C, Kötter I, Henes J, Blank N, Voll RE,
Ehrchen J, et al: Reality of inpatient vasoactive treatment with
prostacyclin derivatives in patients with acral circulation
disorders due to systemic sclerosis in Germany. Z Rheumatol.
79:1057–1066. 2020.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
3
|
Bobeica C, Niculet E, Craescu M, Halip AI,
Popescu IA, Draganescu ML, Onisor C, Stefanescu B and
Gheuca-Solovastru L: Etiological factors of systemic sclerosis in
the southeast region of Romania. Exp Ther Med.
21(79)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gheorghe I, Tatu AL, Lupu I, Thamer O,
Cotar AI, Pircalabioru GG, Popa M, Cristea VC, Lazar V and
Chifiriuc MC: Molecular characterization of virulence and
resistance features in Staphylococcus aureus clinical strains
isolated from cutaneous lesions in patients with drug adverse
reactions. Rom Biotechnol Lett. 22:12321–12327. 2017.
|
|
5
|
Tatu AL and Cristea VC: Unilateral
blepharitis with fine follicular scaling. J Cutan Med Surg.
21(442)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Horodinschi RN, Stanescu AMA, Bratu OG,
Pantea Stoian A, Radavoi DG and Diaconu CC: Treatment with statines
in elderly patients. Medicina (Kaunas). 55(721)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tatu AL and Nwabudike LC: Reply to: Kubiak
K et al. Endosymbiosis and its significance in dermatology.
J Eur Acad Dermatol Venereol. 32:e346–e347. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gheorghe G, Toth PP, Bungau S, Behl T,
Ilie M, Pantea Stoian A, Bratu OG, Bacalbasa N, Rus M and Diaconu
CC: Cardiovascular risk and statin therapy considerations in women.
Diagnostics (Basel). 10(483)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bobeică C, Tatu AL, Crăescu M and
Solovăstru L: Dinamics of digital ulcers in systemic sclerosis. Exp
Ther Med. 20:61–67. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mazilu L, Stanculescu DL, Gheorghe AD,
Suceveanu AP, Parepa IR, Stoian AP, Pop CS, Bratu O and Suceveanu
AI: Chemotherapy and other factors affecting quality of life in
non-small cell lung cancer (NSCLC) patients. Rev Chim. 70:33–35.
2019.
|
|
11
|
Nwabudike LC and Tatu AL: Magistral
prescription with silver nitrate and peru balsam in
difficult-to-heal diabetic foot ulcers. Am J Ther. 25:e679–e680.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tatu AL, Clatici VG and Nwabudike LC:
Rosacea-like demodicosis (but not primary demodicosis) and
papulopustular rosacea may be two phenotypes of the same disease-a
microbioma, therapeutic and diagnostic tools perspective. J Eur
Acad Dermatol Venereol. 33:e46–e47. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Crismaru I, Pantea Stoian A, Bratu OG,
Gaman MA, Stanescu AMA, Bacalbasa N and Diaconu CC: Low-density
lipoprotein cholesterol lowering treatment: The current approach.
Lipids Health Dis. 19(85)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tatu AL, Elisei AM, Chioncel V, Miulescu M
and Nwabudike LC: Immunologic adverse reactions of β-blockers and
the skin. Exp Ther Med. 18:955–959. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Buzia OD, Fasie V, Mardare N, Diaconu C,
Gurau G and Tatu AL: Formulation, preparation, physico-chimical
analysis, microbiological peculiarities and therapeutic challenges
of extractive solution of Kombucha. Rev Chim. 69:720–724. 2018.
|
|
16
|
Tatu AL, Ionescu MA and Nwabudike LC:
Contact allergy to topical mometasone furoate confirmed by
rechallenge and patch test. Am J Ther. 25:e497–e498.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nwabudike LC, Miulescu M and Tatu AL: Case
series of an alternative therapy for generalised lichen planus:
Four case studies. Exp Ther Med. 18:943–948. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jacobsen S, Halberg P, Ullman S, Van
Venrooij WJ, Høier-Madsen M, Wiik A and Petersen J: Clinical
features and serum antinuclear antibodies in 230 Danish patients
with systemic sclerosis. Br J Rheumatol. 37:39–45. 1998.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fabri M and Hunzelmann N: Differential
diagnosis of scleroderma and pseudoscleroderma. J Dtsch Dermatol
Ges. 5:977–984. 2007.PubMed/NCBI View Article : Google Scholar : (In English,
German).
|
|
20
|
Steen VD, Powell DL and Medsger TA Jr:
Clinical correlations and prognosis based on serum autoantibodies
in patients with systemic sclerosis. Arthritis Rheum. 31:196–203.
1988.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bobeica C, Niculet E, Craescu M, Onisor C,
Bujoreanu F, Draganescu ML, Halip IA and Gheuca-Solovastru L:
Epidemiological profile of systemic sclerosis in the southeast
region of Romania. Exp Ther Med. 21(77)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Monfort JB, Mathian A, Amoura Z, Francès
C, Barbaud A and Senet P: Cancers associated with systemic
sclerosis involving anti-RNA polymerase III antibodies. Ann
Dermatol Venereol. 145:33–36. 2018.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
23
|
Monfort JB, Lazareth I and Priollet P:
Paraneoplastic systemic sclerosis: About 3 cases and review of
literature. J Mal Vasc. 41:365–370. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nwabudike LC and Tatu AL: Response
to-chronic exposure to tetracyclines and subsequent diagnosis for
non-melanoma skin cancer in a large Mid-Western US population. J
Eur Acad Dermatol Venereol. 32(e159)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tatu AL and Nwabudike LC: The treatment
options of male genital lichen sclerosus et atrophicus short title
for a running head: Treatments of genital lichen sclerosus
conference: 14th National Congress of Urogynecology (Urogyn)
Location: Eforie, Romania Date: SEP 07-09, 2017. Proceedings of the
14th National Congress of Urogynecology and the National Conference
of the Romania Association for the Study of Pain, pp262-264,
2017.
|
|
26
|
Nwabudike LC, Elisei AM, Buzia OD,
Miulescu M and Tatu AL: Statins. A review on structural
perspectives, adverse reactions and relations with son-melanoma
skin cancer. Rev Chim. 69:2557–2562. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Steen VD and Medsger TA Jr: The value of
the health assessment questionnaire and special patient-generated
scales to demonstrate change in systemic sclerosis patients over
time. Arthritis Rheum. 40:1984–1991. 1997.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Denton CP: Advances in pathogenesis and
treatment of systemic sclerosis. Clin Med (Lond). 16:55–60.
2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Masi AT and Medsger TA Jr: Progress in the
evolution of systemic sclerosis classification criteria and
recommendation for additional comparative specificity studies. J
Rheumatol. 42:8–10. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
LeRoy EC, Black C, Fleischmajer R,
Jablonska S, Krieg T, Medsger TA Jr, Rowell N and Wollheim F:
Scleroderma (systemic sclerosis): Classification, subsets and
pathogenesis. J Rheumatol. 15:202–205. 1988.PubMed/NCBI
|