Introduction
Aging is a complex process and is associated with
several alterations, including a decline in the activity of the
hypothalamic-pituitary-gonadal (HPG) axis, hormonal abnormalities,
cognitive impairments and depression (1). Aging is accompanied by a decrease in
pulsatile luteinizing hormone (LH) secretion due to a decline in
the pulsatile secretion of gonadotropin-releasing hormone (GnRH)/LH
(2-4).
A recent study describes that aging could be accompanied by
decreased expression of arcuate (ARC) nucleus kisspeptin (Kp) along
with neurokinin B and dynorphins (5). However, neuronal projections
expressing Kp from the hypothalamic anteroventral periventricular
nucleus (AVPV) play a pivotal role in the regulation of GnRH along
with Kp expressed in the ARC nucleus (6). Kps are a family of peptide products
known to stimulate GnRH secretion and play an important role in
fertility and reproduction by regulating the HPG axis. Kp acts
through G-protein coupled receptor 54 (GPR54) and numerous studies
reported the presence and functions of Kp or GPR54 in various types
of tissue and organs (7-9).
In the brain, the Kp-GPR54 system functions predominantly to
control the reproductive process (10). In the hypothalamus, the increased
GPR54 signaling initiates puberty, whereas its loss of function
delays pubertal onset (11,12).
In addition to its major function as an upstream
regulator of the HPG axis, the Kp-GPR54 system also triggers
several other signaling pathways. The role of the Kp-GPR54 system
in reproductive and non-reproductive functions was indicated in a
previous study (13). Numerous
studies reported that Kp induces phospholipase C enzyme activity
through GPR54 (14,15). Additionally, it has been shown that
mutation of GPR54 elicits prolonged activation of the ERK signaling
pathway in response to Kp (16).
Furthermore, loss of function of GPR54 is known to be associated
with hypogonadotropic hypogonadism (17). Therefore, the loss or gain of
function of the GPR54 receptor determines the specificity and
importance of appropriate signaling of the Kp-GPR54 system
(13).
The Kp-GPR54 system has been extensively
investigated in the hypothalamus. Certain studies suggest that
GPR54 is expressed in the hippocampus and amygdala (8,9).
However, its expression in other brain regions, such as the
medulla, pons, cerebellum, midbrain, frontal lobe and cortex
requires further investigation. Moreover, age-induced alterations,
if any, could elucidate the potential role of Kp-GPR54 in other
regions of the brain besides the hypothalamus. In aging, GnRH
administration has been shown to promote neurogenesis in the
hypothalamus, hippocampus and other brain regions. Additionally,
GnRH travels to other brain regions to affect the aging process
(18). Furthermore, GnRH regulates
GPR54 levels (19). Therefore, the
aim of the present study was to evaluate the expression of GPR54 in
different brain regions, including the hypothalamus, hippocampus,
medulla, pons, cerebellum, midbrain, frontal lobe and cortex in
adult (age, 3-4 months) and old (age, 20-24 months) male Wistar
rats.
Materials and methods
Animals
Male Wistar rats representing two different age
groups, adult (age, 3-4 months; n=8) and old (age, 20-24 months;
n=8) were procured from the Indian Council of Medical Research
Institute, National Institute of Nutrition (Hyderabad, India) for
use in the present study. The rats were housed at room temperature
(23-24˚C), in 12-h light/dark cycle and relative humidity of
55-60%. Food and water were provided ad libitum and cage
changes were at random intervals. The study was approved by the
Institutional Animal Ethics Committee (IAEC) of the University of
Hyderabad (approval no. UH/IAEC/NBVS/2019-I/03). All procedures
were performed according to IAEC guidelines
The rats were anesthetized with 290 mg/kg body
weight avertin via intraperitoneal injection and sacrificed by
decapitation. The brains were removed by dissection and the various
regions, including the hypothalamus, hippocampus, cortex, midbrain,
frontal lobe, medulla and pons and cerebellum were isolated
(20,21). All tissue samples were stored at
-80˚C until analysis.
RNA isolation and reverse
transcription
Total RNA was isolated from the hypothalamus and
other regions of the brain using TRIzol® (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Total
RNA was dissolved in nuclease-free water and quantified using a
NanoDrop™ Spectrophotometer (Thermo Fisher Scientific, Inc.). cDNA
was synthesized using an iScript cDNA Synthesis Kit (Bio-Rad
Laboratories, Inc.).
Quantitative (q)PCR
qPCR was performed using the SYBR™ Green (Applied
Biosystems; Thermo Fisher Scientific, Inc.) detection method to
assess GPR54, Kiss1 and GnRH1 mRNA expression
levels. qPCR was performed using 40 ng cDNA from each sample
(Adult, n=4; Old, n=4). By using the first-strand cDNA template
from each sample, the target and reference genes were amplified
separately. The primer sequences for GPR54, Kiss1,
GnRH1 and GAPDH were as follows: GPR54
forward, 5'-GCGGCCACAGATGTCACTTT-3' and reverse,
5'-AGGTGGGCAGCGGATAGA-3'; Kiss1 forward,
5'-GCTGCTGCTTCTCCTCTGTGT-3' and reverse, 5'-CTGTTGGCCTGTGGGTTCA-3';
GnRH1 forward, 5'-GCAGAACCCCAGAACTTCGA-3' and reverse,
5'-CAACGCCAAGGAGCTCAAGT-3'; GAPDH forward,
5'-TATCACTCTACCCACGGCAAG-3' and reverse,
5'-ATACTCAGCACCAGCATCACC-3'. Amplification was performed using an
Applied Biosystems Power SYBR-Green PCR Master Mix (2X; Thermo
Fisher Scientific, Inc.) and Applied Biosystems™ 7500 Real-Time PCR
System (Thermo Fisher Scientific, Inc.). The reaction set-up for
PCR was as follows: 1 µl 10-pmol forward primer, 1 µl 10-pmol
reverse primer, 10 µl Power SYBR-Green PCR Master Mix (2X) and 8 µl
(40 ng) cDNA in a total reaction volume of 20 µl. The standard
thermocycling conditions were as follows: Initial denaturation at
95˚C for 10 min, followed by 40 cycles of denaturation at 95˚C for
15 sec and 60˚C for 1 min. Single amplification of the product and
absence of primer-dimer formation was confirmed by dissociation
curves. Cycle threshold (Cq) values were taken from the exponential
phase of PCR amplification. The expression levels of target genes,
GPR54, Kiss1 and GnRH1 were normalized to
GAPDH expression (ΔCq=target gene Cq-GAPDH Cq) and the relative
quantity of target mRNA expression in each sample was equal to
2-ΔΔCq (22).
Western blotting
Tissue samples were Dounce homogenized in lysis
buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% Nonidet P-40 and
0.5% sodium deoxycholate) supplemented with protease inhibitors to
a final concentration of 10% homogenate. Lysates were sonicated and
centrifuged at 10,000 x g for 5 min at 4˚C, clear supernatants were
collected and the protein concentration was estimated using the
Bradford Method (23). Each sample
(50 µg protein/lane) was separated by 12% SDS-PAGE (24) and electrophoretically transferred
onto a nitrocellulose membrane. Blocking was performed with 5%
non-fat milk for 1 h at room temperature and washed with PBS with
0.05% tween 20 (PBST) three times each 5 min. The membrane was
probed with the following primary antibodies: Anti-GPR54 (dilution,
1:1,000; cat. no. GTX100374; GeneTex, Inc.) and anti-Kp (dilution
1:1,000; cat. no. GTX337642; GeneTex, Inc.) antibodies for 12 h at
4˚C, washed three times at 5 min each with PBS followed by
anti-rabbit horseradish peroxidase-conjugated secondary antibody
for 2 h at room temperature (dilution, 1:2,000; cat. no. SC2357;
Santa Cruz Biotechnology, Inc.). The blots were washed with PBS and
developed using ECL™ Prime (Cytiva) and an imaging system
(Chemidoc; Bio-Rad Laboratories, Inc.). Densitometry was performed
using ImageJ 1.49v Software (National Institutes of Health).
Statistical analysis
Data were analyzed using unpaired Student's t-test
for comparison between the two groups. Results are expressed as the
mean ± standard error of the mean and P<0.05 was considered to
indicate a statistically significant difference. SigmaPlot 11.0
software (Systat Software, Inc.) was used for statistical
analysis.
Results
Aging-associated decreases in GPR54,
Kiss1 and GnRH1 mRNA levels are observed in the hypothalamus
Neuronal projections expressing Kp from the
hypothalamic AVPV region play a pivotal role in the regulation of
GnRH along with Kp expressed in the ARC nucleus (6). Age-dependent decline in Kp expression
levels has been observed in the hypothalamic ARC nucleus of the
brain (5). However, it remains
unclear whether AVPV Kp levels also alter with aging. Furthermore,
it is essential to determine the steady-state levels of the Kp
target receptor, GPR54, to understand the Kp-GPR54 mediated
signaling mechanism.
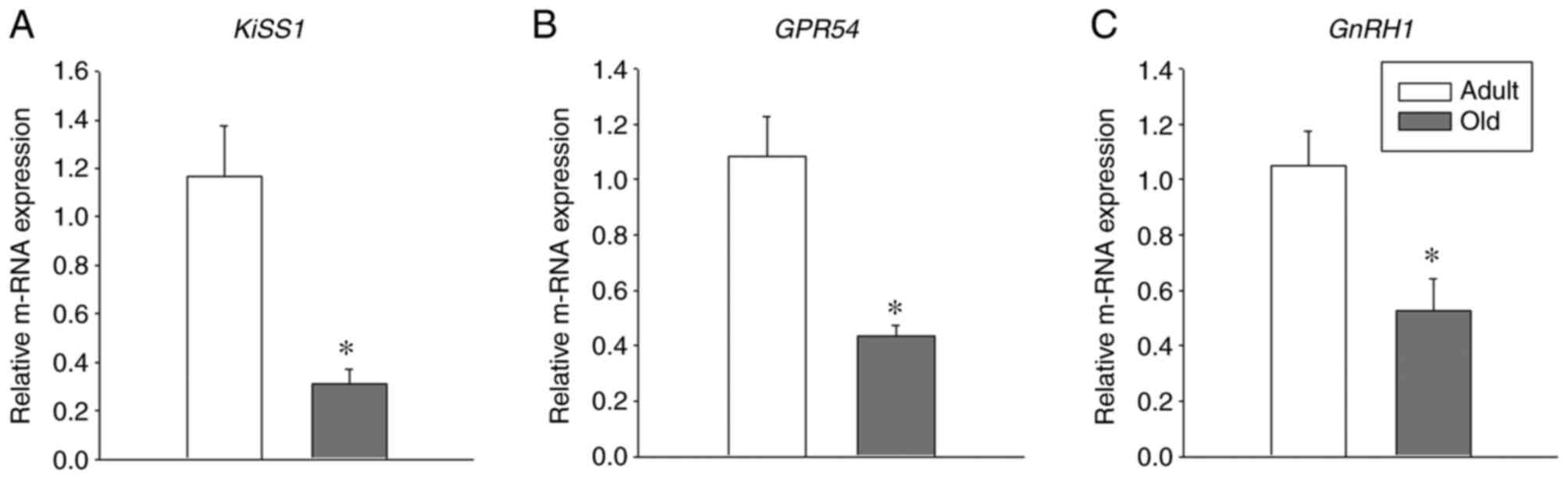
To investigate the age-associated changes of
GPR54, Kiss1 and GnRH in the hypothalamus, the
hypothalamic brain region from adult and old-aged rats was isolated
in the present study. qPCR was performed from total cDNA to
estimate the relative mRNA levels of GPR54, Kiss1 and
GnRH. Notably, a significant decrease was observed in the
mRNA levels of GPR54, Kiss1 and GnRH in
old-age rats when compared with adult rats (Fig. 1; P<0.05). These results indicate
the important role of GPR54, Kiss1 and GnRH1
in the aging process.
Aging-associated decreases in GPR54
and Kp protein levels are observed in the hypothalamus
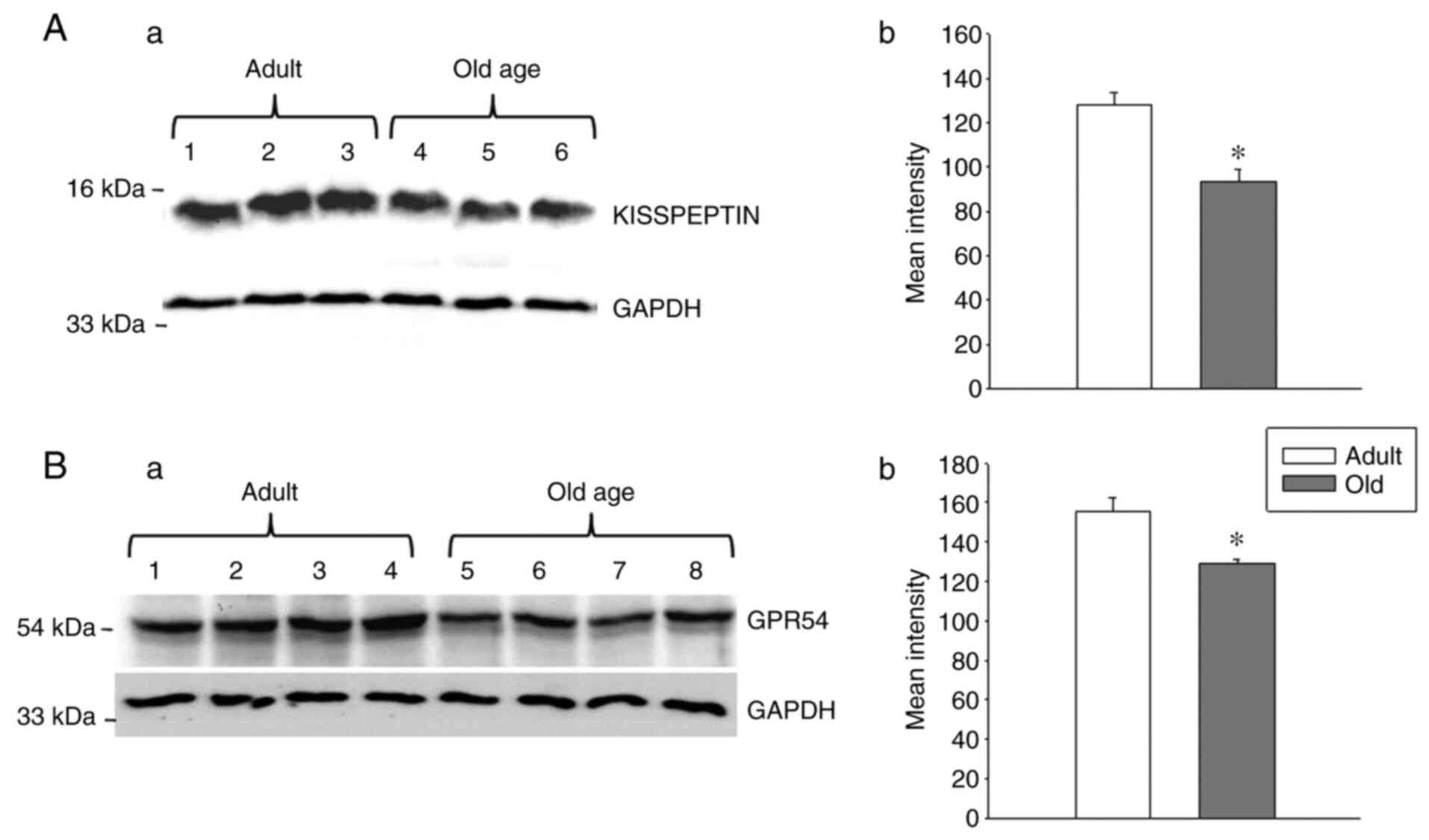
As an aging-associated decrease was observed in
GPR54 and Kiss1 mRNA levels in the hypothalamus, the
present study investigated whether GPR54 and Kp protein levels were
also altered during the aging process. To examine this, western
blotting was conducted to analyze the protein levels of GPR54 and
Kp. A decrease in GPR54 and Kp protein levels was observed in the
hypothalamus of the old-age rats when compared with that of the
adult rats (Fig. 2; P<0.05).
These results indicate the strong association between GPR54 and Kp
levels at the mRNA and protein levels.
Aging-associated alterations in GPR54
expression are observed in the extra-hypothalamic brain region
The Kp-GPR54 system has been evaluated extensively
in the hypothalamus. However, few studies reported the expression
of GPR54 in the amygdala and hippocampus. Furthermore, it has been
shown that the Kp-GPR54 system modulates real-time synaptic
transmission in the hippocampus, likely through signaling pathways
and their associated trophic factors and tyrosine kinase receptors
(25). This indicates that the
Kp-GPR54 system has a selective function in other brain regions
besides the hypothalamus.
Aging is characterized by diminished neurogenesis
and GnRH administration is known to reverse this age-induced effect
in the hypothalamus and hippocampus. Furthermore, GnRH
administration appeared to exert similar effects in other brain
regions (18), which indicates that
GnRH travels within the brain to promote neurogenesis.
Additionally, GnRH is also known to regulate GPR54 levels (19). In the present study, an
age-dependent reduction of GnRH levels was observed in the
hypothalamus region. However, whether expression of GPR54 regulates
the aging process in extra-hypothalamic regions requires further
investigation. To date, to the best of our knowledge, no studies
have documented the expression of GPR54 in other regions of the
brain.
To evaluate whether GPR54 is expressed in other
regions of the brain, extra-hypothalamic brain regions, such as the
hippocampus, frontal lobe, cortex, midbrain, cerebellum, medulla
and pons were isolated and the mRNA and protein levels of GPR54
were evaluated.
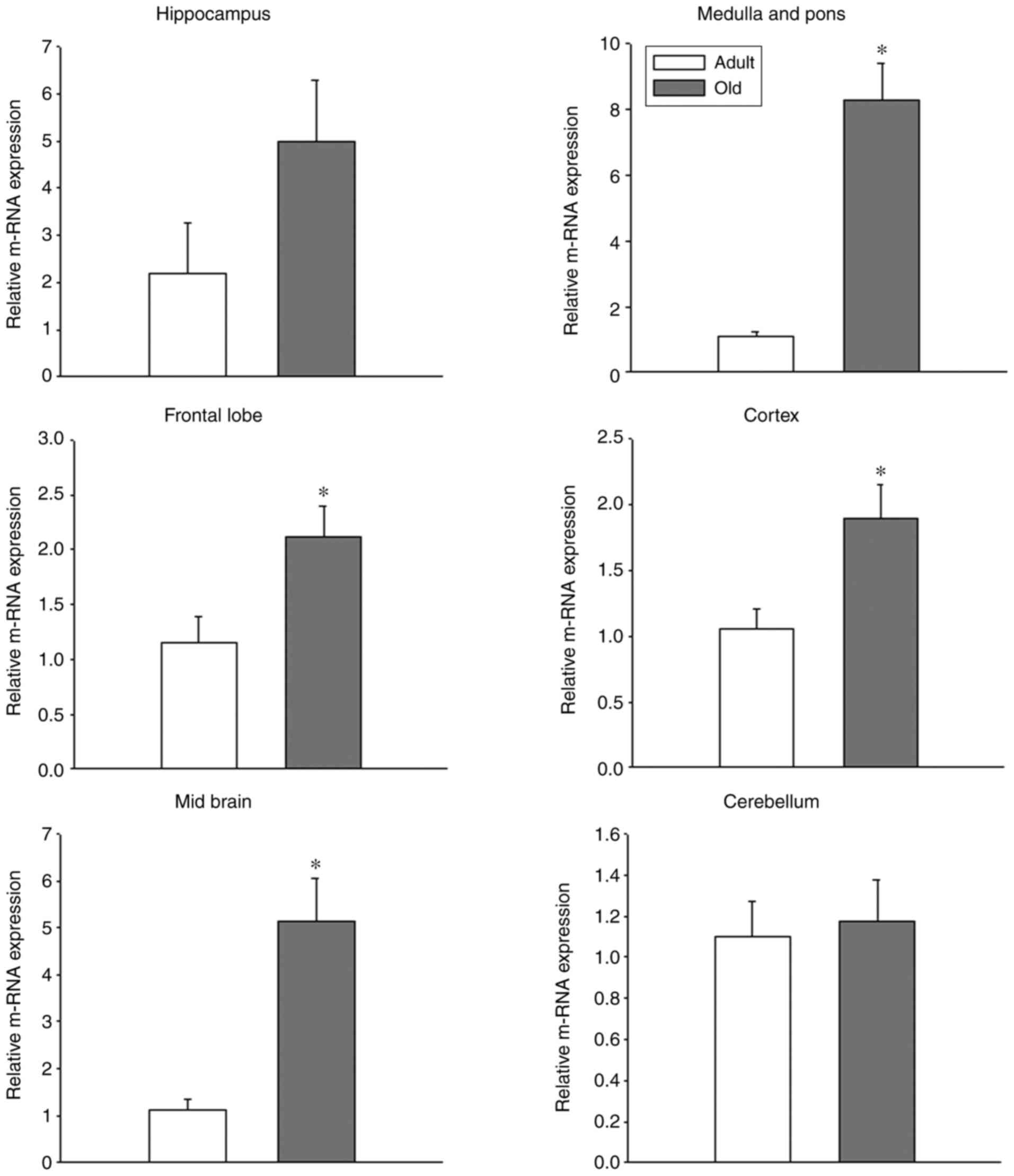
In the hippocampus, GPR54 mRNA was observed in adult
and old-age rats; however, the difference between adult and old-age
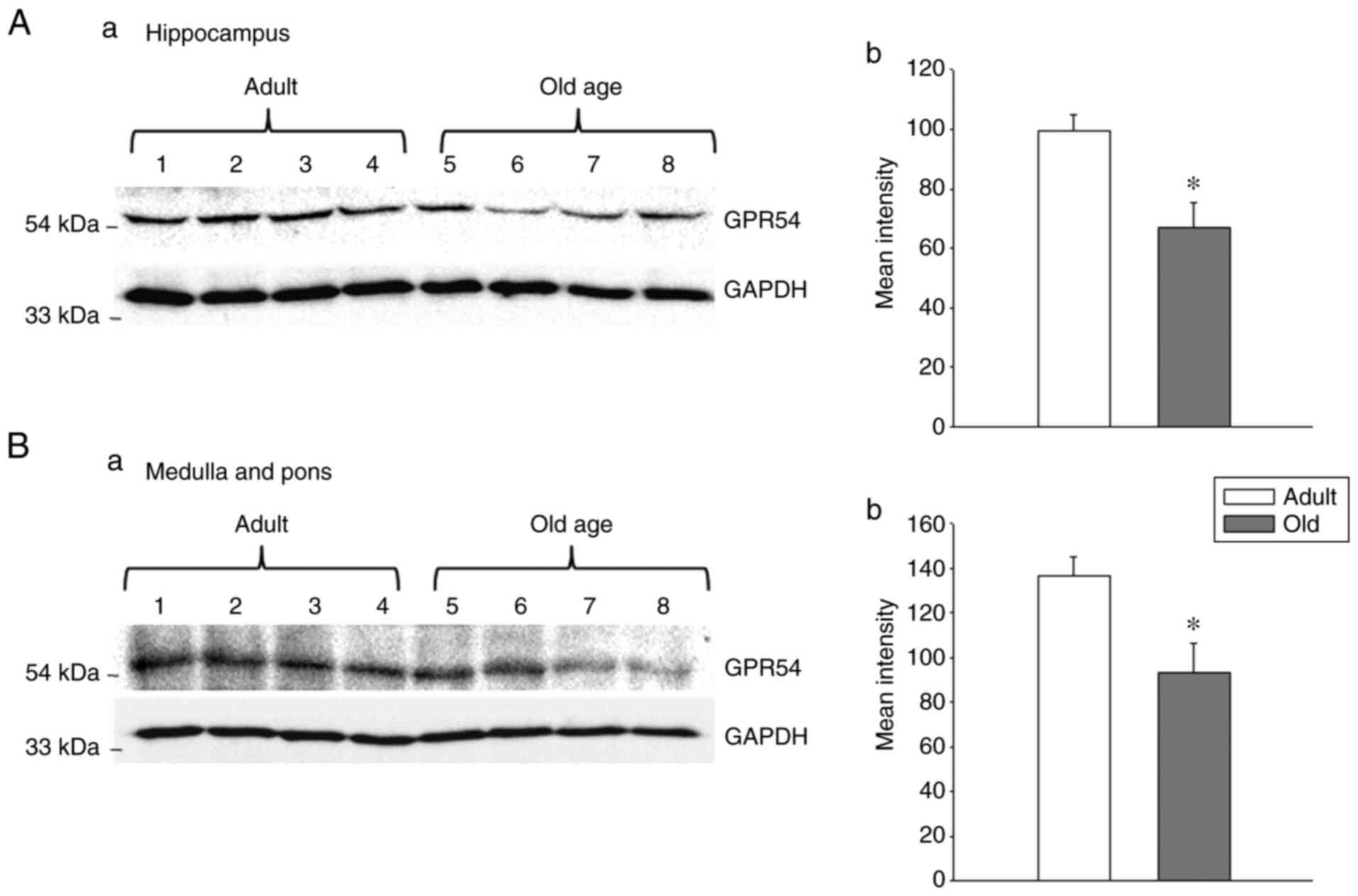
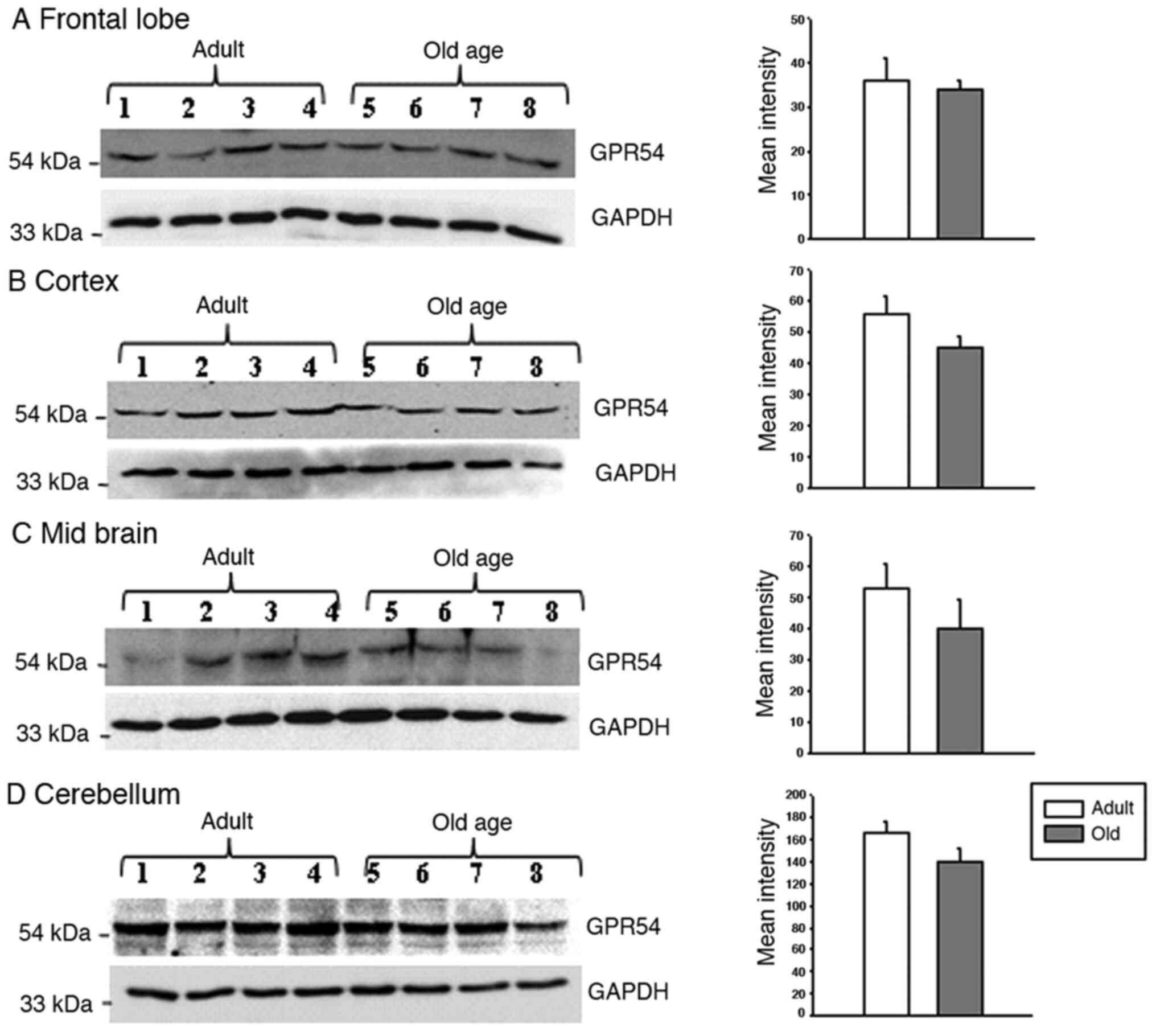
rats was not statistically significant (Fig. 3). Indeed, the protein levels of
GPR54 were significantly decreased in old-age rats when compared
with adult rats (Fig. 4A;
P<0.05). In the medulla and pons, the relative mRNA expression
of GPR54 was significantly increased in old-age rats when compared
with that in adult rats (Fig. 3;
P<0.05). By contrast, the protein levels were decreased in
old-age rats compared with that in adult rats (Fig. 4B; P<0.05). In the frontal lobe,
the relative mRNA expression of GPR54 was significantly increased
in old-age rats when compared with that in adult rats (Fig. 3; P<0.05). Although GPR54 protein
expression was observed in the adult and old-age rat groups, the
difference between adult and old age rats was not statistically
significant (Fig. 5).
GPR54 mRNA levels were significantly increased in
the cortex of old-age rats when compared to that in adult rats
(Fig. 3; P<0.05); however, the
same tendency was not observed in GPR54 protein expression levels
where the difference between adult and old-age rats was not
statistically significant (Fig. 5).
In the midbrain, similar findings to the frontal lobe and cortex
were observed, that is to say, a significant increase in GPR54 mRNA
levels was observed in old-age rats when compared with adult rats
(Fig. 3; P<0.05) and no
significant difference in GPR54 protein levels was observed between
the two groups (Fig. 5). Although
GPR54 mRNA and protein expression was observed in adult and old-age
rats in the cerebellum, the difference was not statistically
significant (Figs. 3 and 5).
Discussion
Kp was initially recognized as a metastasis
suppressor (26). Subsequently, a
strong association between the Kp-GPR54 system and the HPG axis was
identified in the control of reproduction. Kp mediates pulsatile
secretion of gonadotropins, LH and follicle stimulating hormone,
via GnRH (6). During old age, such
pulsatile LH secretion is reduced due to the deterioration of the
GnRH/LH pulse generator (3,4). A previous study indicated that this is
accompanied by a decrease in the expression of Kp, dynorphin and
neurokinin B during the aging process (5). The role of the Kp-GPR54 system has
been extensively investigated in the hypothalamus. Although certain
studies state that GPR54 is also expressed in the hippocampus and
amygdala (8,9), its association with aging has not been
elucidated. Notably, in a recent study, the functional importance
of the Kp-GPR54 system was evaluated in rat brain hippocampus and
neuronal cell lines (27).
Therefore, it is considered important to evaluate GPR54 expression
in extra-hypothalamic regions. Furthermore, to the best of our
knowledge, there are no studies on GPR54 expression and its
association with aging in extra-hypothalamic brain regions, such as
the midbrain, frontal lobe, cortex, cerebellum, medulla and
pons.
Aging leads to the loss of various physiological
functions and ultimately decreases life span (1). In addition to its role in regulating
sex hormones, GnRH was known to restore aging-impaired neurogenesis
(18). Previously, it was
demonstrated that GnRH treatment induces neurogenesis in the
hypothalamus, hippocampus and other brain regions (18). Kps are the upstream regulator of
GnRH and controlled regulation of the Kp-GPR54 system could be
important for aging. Notably, Kp expression was controlled by
oestradiol, while GPR54 expression was regulated by GnRH (19). Thus, in line with existing
literature, the present study aimed to examine the expression of
Kp, GPR54 and GnRH in different brain regions of rats to elucidate
the molecular changes that occur during aging. To the best of our
knowledge, this is the first study describing the expression of
GPR54 and its association with aging in various brain regions.
To evaluate the age-associated changes of Kp, GPR54
and GnRH in the hypothalamus, the hypothalamus of adult and old-age
rats was examined in the present study. Numerous studies have
described the presence and pivotal role of Kp and GPR54 in the
hypothalamus (28-31).
Furthermore, in several species, including humans, peripheral
administration of Kp is known to stimulate GnRH release in the
hypothalamus (1,29,32-38).
All of these studies demonstrate that the Kp-GPR54 system plays an
important role in pubertal maturation, reproduction and GnRH
release. In the present study, hypothalamic GPR54 mRNA
expression was demonstrated to be significantly reduced in old-age
rats when compared with adult rats. Accordingly, hypothalamic GPR54
protein levels were also significantly decreased in the old-age
rats. Similarly, hypothalamic Kp mRNA and protein levels were
observed to be decreased in old-age rats when compared with adult
rats. A previous study has shown that Kp regulates GnRH mRNA
expression (35). Various lines of
evidence indicate that GnRH levels are decreased in old age
(1,36,37).
Furthermore, hypothalamic GnRH1 mRNA levels were identified
to be significantly decreased in old-age rats when compared to
adult rats in the present study. GnRH protein levels were not
evaluated, as it is a well-established fact that aging diminishes
GnRH secretion in rodents and mammals (1,36,37).
Decreased hypothalamic Kp levels may be responsible for reduced
levels of hypothalamic GnRH1. In the present study, it was
hypothesized that decreased expression of GPR54 in the
hypothalamus during aging may be due to decreased GnRH expression.
However, further studies are required to elucidate the functional
importance of the Kp-GPR54 system in aging.
Initially, Lee et al (9) identified GPR54 mRNA expression
across rat brain regions through Northern blot analysis and in
situ hybridization techniques. The authors observed that
GPR54 mRNA was predominant in limbic brain regions,
including the hypothalamus, hippocampus and periaqueductal grey
regions. However, a limitation of the study was the lack of a
detailed quantitative description of GPR54 protein
expression (9). GnRH neurons
co-expressing GPR54 have been confirmed by in situ
hybridization and feedback regulation of gonadotropin secretion by
the Kp-GPR54 system was also demonstrated (39). However, in situ hybridization
of GPR54 mRNA was identified to be similar among juvenile
and adult mice (40). By contrast,
GnRH neurons expressing GPR54 were found to increase from the
postnatal period to adulthood in mice (41).
As GnRH induces neurogenesis in hypothalamic and
extra-hypothalamic regions during aging and as it regulates GPR54
expression, the present study investigated GPR54 expression in
extra-hypothalamic regions, including the frontal lobe, cortex,
midbrain, cerebellum, hippocampus, medulla and pons. In the
hippocampus, GPR54 protein levels were significantly decreased in
old-age rats when compared with that in adult rats. However, the
difference in GPR54 mRNA expression between those groups was not
identified to be statistically significant. A previous study
identified that age-impaired neurogenesis in the hippocampus was
prevented by GnRH treatment (18).
In the present study, it was hypothesized that decreased GPR54
expression in the hippocampus could be attributed to the decreased
synthesis of GnRH in the hypothalamus. In the medulla and pons,
GPR54 mRNA levels were significantly increased in old-age rats when
compared to that of adult rats. In contrast to the mRNA levels,
GPR54 protein levels were decreased in the old-age rats when
compared to the adult rats. The decrease in GPR54 protein
expression could be due to GnRH control, as GnRH is known to travel
to other brain regions, not just the hypothalamus and hippocampus
(19). In the frontal lobe, the
GPR54 mRNA levels were significantly increased in old-age rats when
compared to that of adult rats. However, the difference in GPR54
protein expression between old age rats and adult rats was not
statistically significant. Similar results were observed in the
cortex and midbrain.
Various factors are known to affect mRNA and protein
expression in aging. For example, perturbed translational control
(42,43). It is evident that aging is
accompanied by a loss of translational control that leads to the
shutdown of protein synthesis of specific mRNAs (42,43).
In general, it preserves the protein homeostasis that prevents the
production of excessive quantities of undesirable proteins for
normal cellular function (42,43).
Regulation of protein synthesis is a common determinant of
longevity (42,43). Therefore, reducing translational
factors that reduce mRNA translation could be an adaptive response
to increase longevity or it could be associated with an
age-associated decline in cellular functions (42,43).
In the present study, GPR54 mRNA and protein expression in the
cerebellum were not observed to be significantly different between
old-age and adult rats. The present study hypothesized that this
could be due to a deficiency in translation of GPR54 in these
particular regions, however, further studies are required to
confirm this hypothesis.
There are only a small number of studies regarding
the function of GPR54 across extra-hypothalamic brain regions,
therefore this is less well understood. In one study, the presence
of the Kp-GPR54 system in the dentate gyrus of the hippocampus has
been shown to modulate the synaptic excitability via the MAP kinase
pathway (25,44). Another study described the presence
of GPR54 in all three layers of the dentate gyrus and in the CA3
pyramidal cell layer region, indicating the role of GPR54 in the
proper functioning of the hippocampus (41). In our recent study, Kp was
demonstrated to preserve mitochondrial function by inducing
autophagy/mitophagy in the hippocampus of aging rat brains and
neuronal cell lines (27). The
mechanistic details of Kp-induced autophagy/mitophagy were also
demonstrated. The silencing of GPR54 was shown to diminish the
effects of Kp-induced autophagy/mitophagy. Thus, as Kp protects
mitochondrial health in the hippocampus of rat brains, it could
also be used as a therapeutic target in hippocampus-associated
impairments, such as memory, cognitive aging and other diseases
associated with mitochondrial dysfunction.
The present study supports previous findings on
GPR54 expression outside the hypothalamic regions. In addition, the
altered expression of GPR54 among adults and old-age rats suggests
a role of GPR54 in the aging process, although whether it is a
cause or effect remains unknown. Thus, further investigations are
required to investigate the functional significance of GPR54 in
different brain regions with respect to aging.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by a National
Post-Doctoral Fellowship grant (to UM; grant. no. PDF/2016/000191)
from the Department of Science and Technology, Science and
Engineering Research Board, Government of India and New Delhi.
Department of Biotechnology, Government of India (to NBVS; grant
no. BT/PR10319/BRB/10/1267/2013).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
UM performed the research. NKT, VRT and MF helped UM
with the experiments. UM and NBVS designed the research. UM, KM and
NBVS analyzed the data. UM, NKT, KM, SV and NBVS discussed and
interpreted the data. UM, NKT and NBVS wrote the paper. NBVS and UM
can confirm the authenticity of all the raw data. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Institutional Animal
Ethics Committee (IAEC) of the University of Hyderabad (approval
no. UH/IAEC/NBVS/2019-I/03). All procedures were performed
according to IAEC guidelines
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Veldhuis JD: Aging and hormones of the
hypothalamo- pituitary axis: Gonadotropic axis in men and
somatotropic axes in men and women. Ageing Res Rev. 7:189–208.
2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Steiner RA, Bremner WJ, Clifton DK and
Dorsa DM: Reduced pulsatile luteinizing hormone and testosterone
secretion with aging in the male rat. Biol Reprod. 31:251–258.
1984.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Scarbrough K and Wise PM: Age-related
changes in pulsatile luteinizing hormone release precede the
transition to estrousacyclicity and depend upon estrous cycle
history. Endocrinology. 126:884–890. 1990.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wise PM, Dueker E and Wuttke W:
Age-related alterations in pulsatile luteinizing hormone release:
Effects of long-term ovariectomy, repeated pregnancies and
naloxone. Biol Reprod. 39:1060–1066. 1988.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kunimura Y, Iwata K, Ishigami A and Ozawa
H: Age- related alterations in hypothalamic kisspeptin, neurokinin
B, and dynorphin neurons and in pulsatile LH release in female and
male rats. Neurobiol Aging. 50:30–38. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pinilla L, Aguilar E, Dieguez C, Millar RP
and Tena-Sempere M: Kisspeptins and reproduction: Physiological
roles and regulatory mechanisms. Physiol Rev. 92:1235–1316.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mead EJ, Maguire JJ, Kuc RE and Davenport
AP: Kisspeptins: A multifunctional peptide system with a role in
reproduction, cancer and the cardiovascular system. Br J Pharmacol.
151:1143–1153. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kotani M, Detheux M, Vandenbogaerde A,
Communi D, Vanderwinden JM, Le Poul E, Brézillon S, Tyldesley R,
Suarez-Huerta N, Vandeput F, et al: The metastasis suppressor gene
KiSS-1 encodes kisspeptins, the natural ligands of the orphan G
protein-coupled receptor GPR54. J Biol Chem. 276:34631–34636.
2001.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lee DK, Nguyen T, O'Neill GP, Cheng R, Liu
Y, Howard AD, Coulombe N, Tan CP, Tang-Nguyen AT, George SR and
O'Dowd BF: Discovery of a receptor related to the galanin
receptors. FEBS Lett. 446:103–107. 1999.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Oakley AE, Clifton DK and Steiner RA:
Kisspeptin signaling in the brain. Endocr Rev. 30:713–743.
2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shahab M, Mastronardi C, Seminara SB,
Crowley WF, Ojeda SR and Plant TM: Increased hypothalamic GPR54
signaling: A potential mechanism for initiation of puberty in
primates. Proc Natl Acad Sci. 102:2129–2134. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Seminara SB, Messager S, Chatzidaki EE,
Thresher RR, Acierno JS Jr, Shagoury JK, Bo-Abbas Y, Kuohung W,
Schwinof KM, Hendrick AG, et al: The GPR54 gene as a regulator of
puberty. N Engl J Med. 349:1614–1627. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Castano JP, Martínez-Fuentes AJ,
Gutiérrez-Pascual E, Vaudry H, Tena-Sempere M and Malagón MM:
Intracellular signaling pathways activated by kisspeptins through
GPR54: Do multiple signals underlie function diversity? Peptides.
30:10–15. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ohtaki T, Shintani Y, Honda S, Matsumoto
H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, et
al: Metastasis suppressor gene KiSS-1 encodes peptide ligand of a
G-protein-coupled receptor. Nature. 411:613–617. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Muir AI, Chamberlain L, Elshourbagy NA,
Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM,
Chambers JK, Murdock P, et al: AXOR12, a novel human G protein-
coupled receptor, activated by the peptide KiSS-1. J Biol Chem.
276:28969–28975. 2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Teles MG, Bianco SD, Brito VN, Trarbach
EB, Kuohung W, Xu S, Seminara SB, Mendonca BB, Kaiser UB and
Latronico AC: A GPR54-activating mutation in a patient with central
precocious puberty. N Engl J Med. 358:709–715. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
De Roux N, Genin E, Carel JC, Matsuda F,
Chaussain JL and Milgrom E: Hypogonadotropic hypogonadism due to
loss of function of the KiSS1-derivedpe ptidereceptor GPR54.
Proc Natl Acad Sci. 100:10972–10976. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang G, Li J, Purkayastha S, Tang Y,
Zhang H, Yin Y, Li B, Liu G and Cai D: Hypothalamic programming of
systemic ageing involving IKK-β, NF-κB and GnRH. Nature.
497:211–216. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Richard N, Galmiche G, Corvaisier S,
Caraty A and Kottler ML: KiSS-1 and GPR54 genes are co-expressed in
rat gonadotrophs and differentially regulated in vivo by oestradiol
and gonadotrophin-releasing hormone. J Neuroendocrinol. 20:381–393.
2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Spijker S: Dissection of rodent brain
regions. Mol Cell Neurosci. 57:23–26. 2011.
|
|
21
|
Heffner TG, Hartman JA and Seiden LS: A
Rapid method for the regional dissection of the rat brain.
Pharmacol Biochem Behav. 13:453–456. 1980.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Arai AC, Xia YF, Suzuki E, Kessler M,
Civelli O and Nothacker HP: Cancer metastasis-suppressing peptide
metastin upregulates excitatory synaptic transmission in
hippocampal dentate granule cells. J Neurophysiol. 94:3648–3652.
2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Welch DR, Chen P, Miele ME, McGary CT,
Bower JM, Stanbridge EJ and Weissman BE: Microcell-mediated
transfer of chromosome 6 into metastatic human C8161 melanoma cells
suppresses metastasis but does not inhibit tumorigenicity.
Oncogene. 9:255–262. 1994.PubMed/NCBI
|
|
27
|
Mattam U, Talari NK, Paripati AK,
Krishnamoorthy T and Sepuri NBV: Kisspeptin preserves mitochondrial
function by inducing mitophagy and autophagy in aging rat brain
hippocampus and human neuronal cell line. Biochim Biophys Acta Mol
Cell Res. 1868(118852)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gottsch ML, Cunningham MJ, Smith JT, Popa
SM, Acohido BV, Crowley WF, Seminara S, Clifton DK and Steiner RA:
A role for kisspeptins in the regulation of gonadotropin secretion
in the mouse. Endocrinology. 145:4073–4077. 2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kinoshita M, Tsukamura H, Adachi S, Matsui
H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H
and Maeda K: Involvement of central metastin in the regulation of
preovulatory luteinizing hormone surge and estrous cyclicity in
female rats. Endocrinology. 146:4431–4436. 2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Clarkson J and Herbison AE: Postnatal
development of kisspeptin neurons in mouse hypothalamus; sexual
dimorphism and projections to gonadotropin-releasing hormone
neurons. Endocrinology. 147:5817–5825. 2006.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mikkelsen JD and Simonneaux V: The
neuroanatomy of the kisspeptin system in the mammalian brain.
Peptides. 30:26–33. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Navarro VM, Castellano JM,
Fernández-Fernández R, Barreiro ML, Roa J, Sanchez-Criado JE,
Aguilar E, Dieguez C, Pinilla L and Tena-Sempere M: Developmental
and hormonally regulated messenger ribonucleic acid expression of
KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and
potent luteinizing hormone-releasing activity of KiSS-1 peptide.
Endocrinology. 145:4565–4574. 2004.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Matsui H, Takatsu Y, Kumano S, Matsumoto H
and Ohtaki T: Peripheral administration of metastin induces marked
gonadotropin release and ovulation in the rat. Biochem Biophys Res
Commun. 320:383–388. 2004.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Thompson EL, Patterson M, Murphy KG, Smith
KL, Dhillo WS, Todd JF, Ghatei MA and Bloom SR: Central and
peripheral administration of kisspeptin-10 stimulates the
hypothalamic- pituitary-gonadal axis. J Neuroendocrinol.
16:850–858. 2004.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Dhillo WS, Chaudhri OB, Patterson M,
Thompson EL, Murphy KG, Badman MK, McGowan BM, Amber V, Patel S,
Ghatei MA and Bloom SR: Kisspeptin-54 stimulates the
hypothalamic-pituitary gonadal axis in human males. J Clin
Endocrinol Metab. 90:6609–6615. 2005.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sano A and Kimura F: Electrical activity
of the pulse generator of gonadotropin-releasing hormone in
26-month-old female rats. Neuroendocrinology. 72:199–207.
2000.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bonavera JJ, Swerdloff RS, Leung A, Lue
YH, Baravarian S, Superlano L, Sinha-Hikim AP and Wang C: In the
male brown-Norway (BN) male rat, reproductive aging is associated
with decreased LH-pulse amplitude and area. J Androl. 18:359–365.
1997.PubMed/NCBI
|
|
38
|
Novaira HJ, Sonko ML and Radovick S:
Kisspeptin induces dynamic chromatin modifications to control GnRH
gene expression. Mol Neurobiol. 53:3315–3325. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Irwig MS, Fraley GS, Smith JT, Acohido BV,
Popa SM, Cunningham MJ, Gottsch ML, Clifton DK and Steiner RA:
Kisspeptin activation of gonadotropin releasing hormone neurons and
regulation of KiSS-1mRNA in the male rat. Neuroendocrinology.
80:264–272. 2004.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Han SK, Gottsch ML, Lee KJ, Popa SM, Smith
JT, Jakawich SK, Clifton DK, Steiner RA and Herbison AE: Activation
of gonadotropin-releasing hormone neurons by kisspeptin as a
neuroendocrine switch for the onset of puberty. J Neurosci.
25:11349–11356. 2005.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Herbison AE, de Tassigny XD, Doran J and
Colledge WH: Distribution and postnatal development of Gpr54gene
expression in mouse brain and gonadotropin-releasing hormone
neurons. Endocrinology. 151:312–321. 2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gonskikh Y and Polacek N: Alterations of
the translation apparatus during aging and stress response. Mech
Ageing Dev. 168:30–36. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Anisimova AS, Alexandrov AI, Makarova NE,
Gladyshev VN and Dmitriev SE: Protein synthesis and quality control
in aging. Aging (Albany NY). 10:4269–4288. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Arai AC and Orwig N: Factors that regulate
KiSS1 gene expression in the hippocampus. Brain Res.
1243:10–18. 2008.PubMed/NCBI View Article : Google Scholar
|